Abstract
Background
Acute chest syndrome (ACS) is a frequent cause of hospitalization and mortality in children with sickle cell disease. Transfusion is often required to prevent respiratory failure and treatment with dexamethasone may reduce the length of admission and the need for transfusions. We performed a retrospective cohort study to evaluate risk factors for readmission and prolonged hospitalization after different treatments for ACS.
Procedure
We identified patients <22 years of age hospitalized with ACS at Johns Hopkins Hospital from January 1998 to April 2004 using the hospitals discharge database and by reviewing dictated summaries.
Results
We identified 65 patients with 129 episodes of ACS (mean age 12.5 years, range 1.2–21.9 years). Thirty-nine episodes were treated with corticosteroids and 51 with transfusions. Patients were readmitted within 14 days after 23 episodes (18%). Readmission was strongly associated with report of an inhaler or nebulizer at home [odds ratio (OR) 6.0, P < 0.05], diastolic BP at 48 hr (OR 1.8 per 10 mm increase, P<0.01), corticosteroids (OR 20, P < 0.005), or transfusion (OR 0.03, P < 0.05). Treatment with corticosteroids alone (P < 0.05) and older age (P < 0.001) were associated with longer hospitalization.
Conclusions
These results demonstrate a greatly elevated independent risk of readmission after ACS in children with asthma and after treatment with corticosteroids and a protective effect of transfusion. Although dexamethasone has documented efficacy for reducing the duration of ACS, the substantial risk of readmission for pain should limit its use.
Keywords: acute chest syndrome, cohort study, corticosteroids, sickle cell disease, transfusions
INTRODUCTION
Acute chest syndrome (ACS) refers to a new pulmonary infiltrate accompanied by fever and/or symptoms or signs of respiratory disease in a patient with sickle cell disease (SCD). The specific definition varies among studies [1,2]. It is a cause of frequent hospitalization and death and a common indication for transfusion and treatment with hydroxyurea [3,4]. The incidence is highest in young children with homozygous sickle cell (HbSS), with rates of 3–25 per 100 person-years in the Cooperative Study of SCD (CSSCD) [3]. Several studies suggest that the case fatality rate is lower in children (1.1–1.5%) than adults (4.3–9%), but ACS accounts for a significant proportion of mortality in both groups [2,3,5]. Certain characteristics at presentation (hemoptysis, productive cough, dyspnea, tachypnea, age >20 years, a prior vaso-occlusive event, or a platelet count <200,000/µl) and during hospitalization (fever, extensive radiographic abnormalities, pleural effusion, transfusion, or mechanical ventilation) were associated with prolonged admission in the National Acute Chest Syndrome Study and the CSSCD [2,4]. However, neither study assessed predictors of readmission nor the impact of more recently proposed therapies for ACS, including high-dose dexamethasone and pre-emptive transfusion [1,6]. Currently, the use of simple or exchange transfusion, antibiotics, oxygen, and bronchodilators for wheezing is the standard of care for severe (and perhaps moderate) ACS, based on improvement in oxygenation and other clinical parameters [2,7,8]. However, transfusion has not been evaluated in a clinical trial and was associated with a new red cell antibody in 2.4% of patients in a prospective study of ACS [2]. Although a small randomized controlled trial demonstrated that treatment with corticosteroids resulted in the rapid resolution of mild to moderate ACS, the risk factors for rebound painful vaso-occlusion and readmission in routine clinical practice have not been adequately assessed [1]. We hypothesized that the administration of corticosteroids during ACS would be associated with an increased risk of readmission after adjustment for potential confounders.
METHODS
We conducted a retrospective cohort study to characterize risk factors for readmission, and prolonged hospitalization after ACS. We included patients <22 years of age hospitalized with ACS at Johns Hopkins Hospital from January 1998 to April 2004. We identified cases using the hospitals discharge database (ICD-9 codes for SCD and ACS, pneumonia, asthma, respiratory symptoms or respiratory failure) and by reviewing dictated summaries. All treatment decisions including the criteria for transfusion, corticosteroids, and discharge were at the discretion of the attending physician.
Definitions
ACS was defined using the same criteria as a randomized controlled trial of dexamethasone for ACS; a new pulmonary infiltrate (on the final radiology report) and two or more of the following: chest, upper abdominal, or rib pain; dyspnea; fever; tachypnea; grunting; nasal flaring; or retractions [1]. ACS was classified as severe in patients with lethargy, marked respiratory distress, an oxygen saturation of <85% with supplemental oxygen, or extensive pulmonary infiltrates (involvement of one complete lung or three lobes) [1].
Tachypnea was defined as a respiratory rate >97.5 percentile for age [9,10].
A respiratory clinical severity score (RCSS) was used wherein normal respirations = 0, tachypnea = 1, and tachypnea and retractions=2 [11].
Blood pressure was standardized (Z-score) for age and sex, using normal ranges for HbSS.[12] A history of hypertension was defined as prior treatment with an antihypertensive agent or a diagnosis of hypertension listed in the admission or clinic note.
A diagnosis of asthma was defined as a history of asthma recorded in the admission or clinic note for the patient. Report of multidose inhaler or nebulizer at home was also recorded, and was considered sufficient for a probable diagnosis of asthma.
Fever was defined as a temperature ≥38.3°C.
Observations were immediately before treatment in patients that received corticosteroids or transfusion and otherwise at the time of diagnosis of ACS.
Readmission was defined as hospitalization for any reason within 14 days of discharge.
Duration of ACS was defined as the time from the diagnosis of ACS to either discharge or the resolution of tachypnea and discontinuation of supplemental oxygen.
The total duration of hospitalization was defined as the sum of hospital days after the diagnosis of ACS and during the readmission.
Medical Record Extraction
A single reviewer collected data from the paper and electronic records using a structured form; histories, progress notes and discharge summaries were carefully reviewed for evidence of readmissions. These data included baseline characteristics, comorbid conditions, medications, type and frequency of complications, severity of illness, laboratory and radiographic evaluation, treatments given, duration of hospitalization and readmissions within 14 days of discharge. Corticosteroids were converted into prednisone equivalents (dose in mg/kg/day) using published standards [13].
Statistical Analysis
We compared variables by ANOVA or t-test if normally distributed or the Kruskal–Wallis equality-of-populations rank test if not. We used univariate and multivariate linear regression to identify association between patient characteristics and the duration of hospitalization. We calculated odds ratios for readmission using logistic regression, with adjustment for clustering and robust estimates of errors. We used Kaplan–Meier estimates and Cox proportional hazards regression models to determine and compare time to readmission among groups. We performed all statistical analyses with Stata 9.2 (College Station, TX).
RESULTS
Demographics
We identified 65 patients with 129 episodes of ACS (mean age 12.8 years, range 1.2–21.9 years). Most episodes were in patients with HbSS (1 1 1) or HbSC (17). Thirty-eight patients had a single admission for ACS, 12 had two, 8 had three, and 7 had 4 or more admissions. Nineteen episodes were severe; males had more episodes (65%) than females. At diagnosis, patients had tachypnea (87%), fever (86%) and chest, upper abdominal, or rib pain (79%) and received antibiotics (99%), oxygen (79%), and bronchodilators (69%). Thirty-nine episodes were treated with corticosteroids (dexamethasone in 33 [0.3 mg/kg every 12 hr in 30, with 25 receiving exactly four doses], prednisone in 5 [1 mg/kg/day in 2 and 2 mg/kg/day in 3 for 1–6 days], and prednisolone 2 mg/kg/day for 3 days in 1) and 51 were treated with transfusions [13 with exchange mostly (62%) for severe ACS]. Corticosteroids (dexamethasone in 5, prednisone in 11, and prednisolone in 1) were gradually reduced in 17 patients over a mean of 6 days (range 2–19 days). Baseline characteristics including age, prior episodes of ACS, asthma, hydroxyurea use, and the percentage of hemoglobin F were different among treatment groups (Table I). Hemoglobin at the time of treatment was significantly lower in the transfused patients (Table II).
TABLE I.
Baseline Characteristics by Treatment (Mean ± SD)
| Parameter | No Transfusion/ No CS (N=61) |
CS only (N=17) |
Transfusion only (N=29) |
Transfusion/CS (N=22) |
|---|---|---|---|---|
| Age (years) | 13 ± 6 | 11 ± 4 | 14 ± 5 | 10 ± 4* |
| Prior ACS # | 3.3 ± 4.0 | 2.7 ± 2.0 | 4.8 ± 3.3 | 6.4 ± 4.9$ |
| Prior HTN | 7% | 12% | 10% | 27% |
| Asthma | 22% | 25% | 25% | 55%† |
| HbF % | 7.9 ± 6.8 | 8.8 ± 7.2 | 4.1 ± 4.1† | 5.3 ± 5.1 |
| Hydroxyurea | 11% | 19% | 48%‡ | 18%* |
P < 0.05 compared transfusion only
P < 0.01 compared to no transfusion/no CS and P < 0.05 compared to CS
P < 0.05 compared to no transfusion/no CS
P < 0.001 compared to no transfusion/no CS; CS indicates corticosteroids; ACS, acute chest syndrome; HTN, hypertension; HbF, fetal hemoglobin.
TABLE II.
Characteristics of Acute Chest Syndrome by Treatment (Mean ± SD)
| Parameter | No Transfusion/ No CS |
CS only | Transfusion only | Transfusion/CS |
|---|---|---|---|---|
| RR (min) | 27 ± 7 | 31 ± 14 | 30 ± 13 | 35 ± 11* |
| FIO2 (%) | 21 IQR 21–24 | 24 IQR 23–32$ | 27 IQR 23–39$ | 29 IQR 24–80$ |
| Involved lobes | 1.3 ± 0.5 | 1.4 ± 0.6 | 1.8 ± 0.7* | 1.7 ± 0.8† |
| Wheezing | 8% | 12% | 10% | 36%†© |
| Severe ACS | 3% | 12% | 21% | 41%$# |
| RCSS | 0.8 ± 0.5 | 1.0 ± 0.5 | 1.0 ± 0.6 | 1.3 ± 0.5#% |
| Hemoglobin (g/L) | 78 ± 18 | 70 ± 16 | 64 ± 19% | 64 ± 7% |
| Bronchodilators | 57% | 69% | 74% | 95%* |
P < 0.01 compared to no transfusion/no CS
P < 0.001 compared to no transfusion/no CS
P < 0.05 compared to no transfusion/no CS
P < 0.05 compared to CS only
P < 0.05 compared to transfusion only
P < 0.005 compared to no transfusion/no CS. CS indicates corticosteroid; RR, respiratory rate; FIO2, fraction inspired oxygen; IQR, interquartile range.
Severity of ACS Was Greater in Patients Treated With Corticosteroids and Transfusions
At the time of treatment, respiratory rate, fraction of inspired oxygen, number of involved lobes, and percentage of patients with severe ACS were significantly higher in the groups treated with transfusions or transfusions and corticosteroids (Table II). The RCSS and the percentage of patients with wheezing and treated with bronchodilators were higher in the group treated with corticosteroids and transfusion. Some of these patients were transfused for progressive respiratory distress after treatment with corticosteroids, while others received corticosteroids after transfusion. A history of asthma (5/6) or wheezing (5/6) was much more frequent in the patients treated with prednisone or prednisolone than with dexamethasone (11/32, P < 0.05, and 5/33, P < 0.001).
Risk of Readmission and Duration of Hospitalization Was Increased in Patients Treated With Corticosteroids
Patients were readmitted within 14 days of discharge after 23 episodes (18%) of ACS. The prevalence of readmission was highest after treatment with corticosteroids alone (59%) and lowest after treatment with transfusion alone (7%, P < 0.001). The prevalence of readmission in patients with a history of asthma after treatment with corticosteroids (50%) and transfusion (0%) was similar to the group as a whole. Patients with a history of asthma were also more likely to be readmitted after no other treatment (31%) and after treatment with corticosteroids and transfusion (33%) than patients without this history (Fig. 1 and Fig. 2). Readmission occurred more frequently in patients with wheezing or a history of asthma that received dexamethasone (42%), versus prednisone or prednisolone (17%, P = 0.6). However, this result was not statistically significant and most of the group treated with prednisone or prednisolone was also transfused (67%).
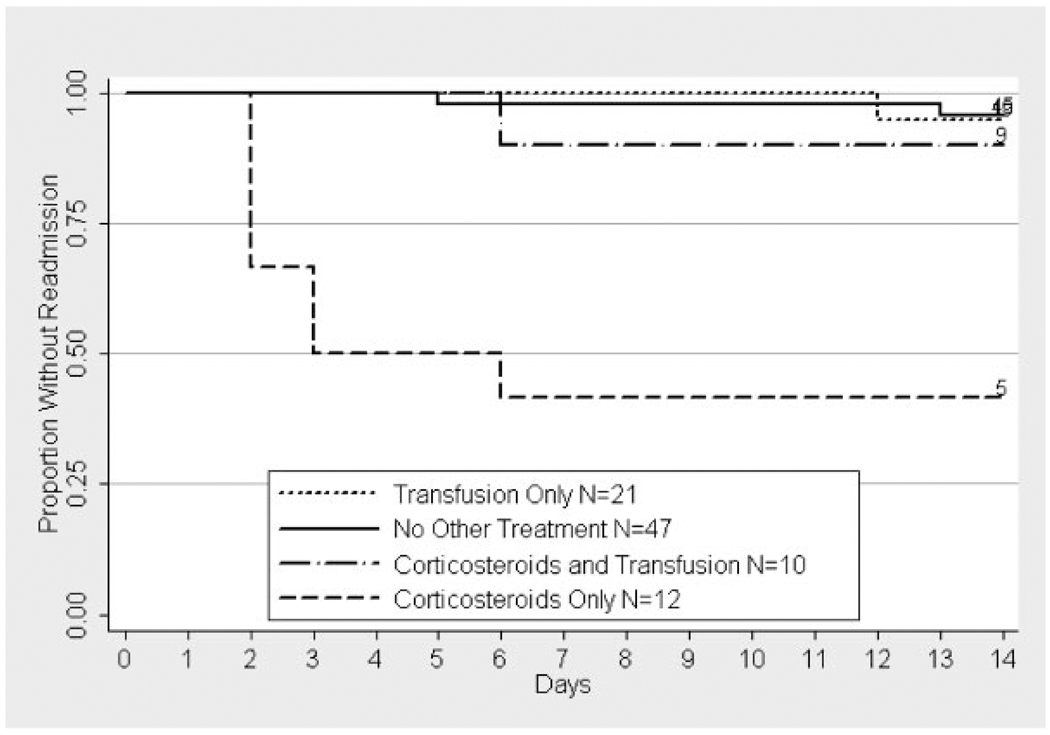
Fig. 1.
Kaplan–Meier estimate of time to readmission by treatment in patients without a history of asthma. The label includes the number of patients at risk on day 0 and the number of patients not readmitted by day 14. Treatment with corticosteroids alone was significantly different from no other treatment (P < 0.0001), transfusion (P < 0.005), but not corticosteroids and transfusion (P>0.5). The prevalence of readmission within 14 days of discharge was 58% (95% CI 28–85) in the group treated with corticosteroids alone, 4% (95% CI 1–15) in those receiving no other treatment, 5% (95% CI 1–24) in those receiving transfusion alone, and 10% (95% CI 0.3–45) in those treated with corticosteroids and transfusion.
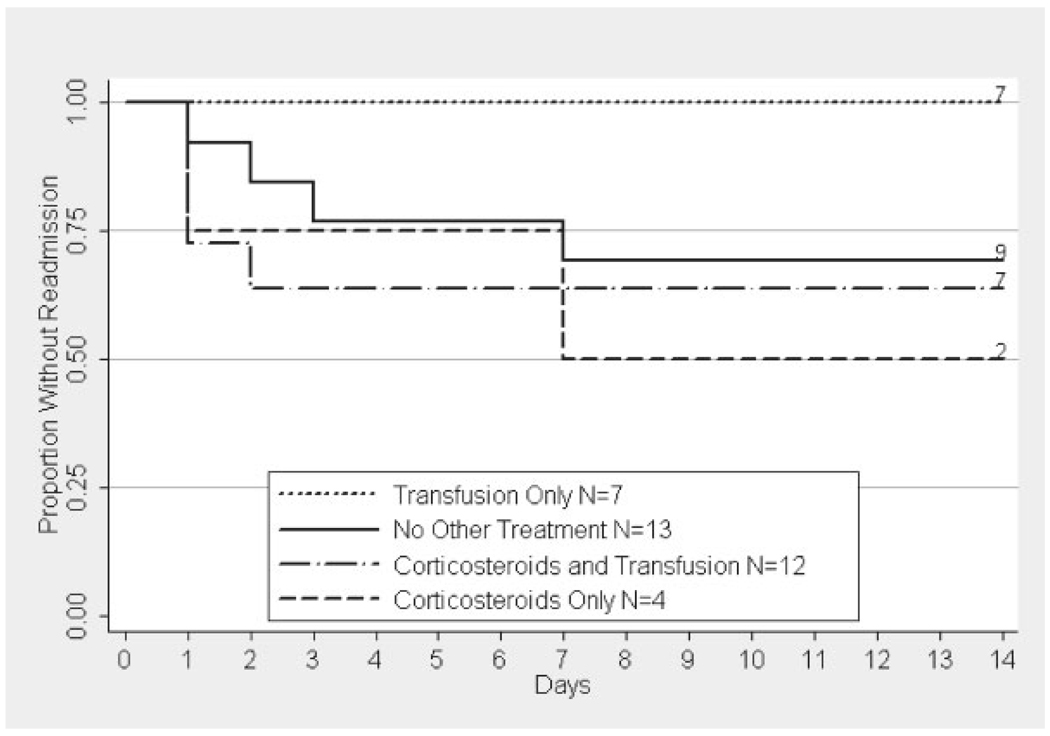
Fig. 2.
Kaplan–Meier estimate of time to readmission by treatment in patients with a history of asthma. The label includes the number of patients at risk on day 0 and the number of patients not readmitted by day 14. Treatment with corticosteroids alone was significantly different from transfusion (P < 0.05), but not corticosteroids and transfusion or no other treatment (P > 0.4). The prevalence of readmission within 14 days of discharge was 50% (95% CI 7–93) in the group treated with corticosteroids alone, 31% (95% CI 9–61) in those receiving no other treatment, 0% (95% CI 0–41) in those receiving transfusion alone, and 33% (95% CI 10–65) in those treated with corticosteroids and transfusion.
The mean time to readmission was shorter in patients treated with corticosteroids alone (3.6 days) or corticosteroids and transfusions (2.2 days) than transfusions alone (9 days) or no other treatment (5.2 days), but these differences were not statistically significant. Most readmissions were for painful crisis (13), painful crisis and fever (3), ACS (2), and hemorrhagic stroke (2). Patients with recurrent ACS had received neither corticosteroids nor transfusion. Both patients with hemorrhagic stroke had received dexamethasone and transfusions for ACS. Notably, those treated with corticosteroids alone were readmitted more frequently with pain than those receiving other treatments (53% vs. 6%, P < 0.0001).
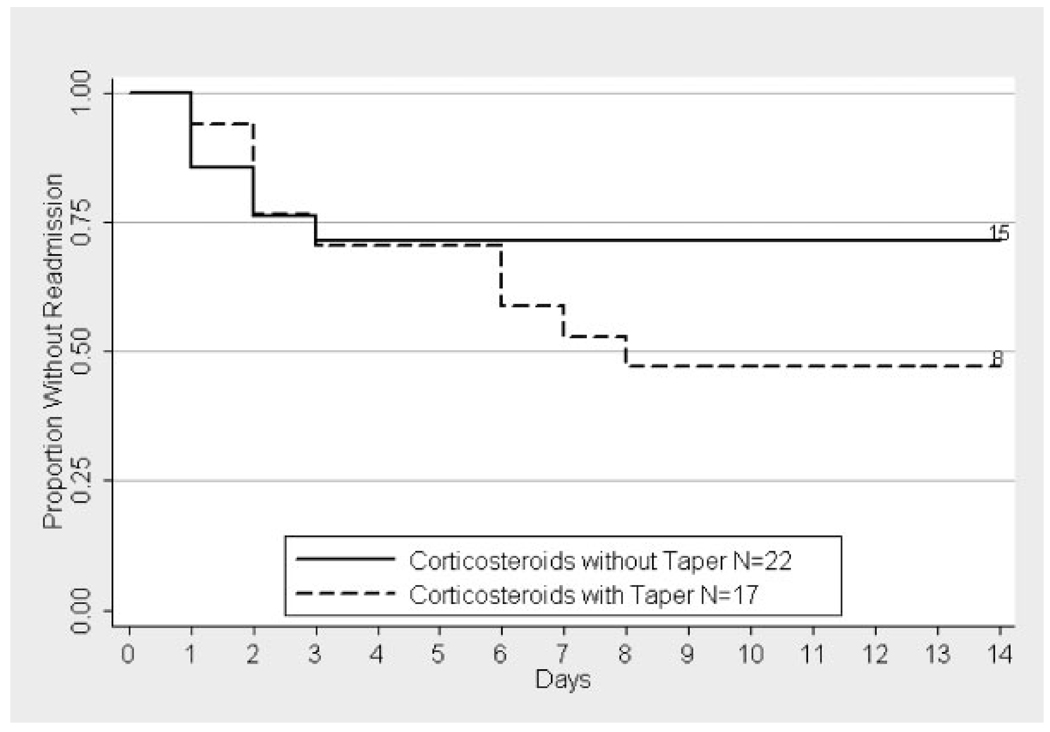
After adjustment for multiple confounders (listed in Table III), the risk of readmission had an inverse relationship with the number of previous admissions for ACS and a positive relationship with the presence of an inhaler or nebulizer at home or a history of asthma. Risk of readmission also increased with more severe disease as measured by the RCSS and with higher diastolic BP 48 hr after diagnosis (Table III). The risk of readmission was the greatest after treatment with corticosteroids alone and a taper of corticosteroids did not reduce this risk (Fig. 3). However, time to readmission was longer after treatment with corticosteroids and a taper (4.1 days) versus corticosteroids without a taper (1.7 days, P < 0.05). Severity of ACS (measured as RCSS or the percentage of severe episodes) was similar for the groups treated with or without a taper. Patients treated with lower doses of corticosteroids (≤2 mg/kg/day of prednisone equivalents) were readmitted less frequently than those treated with higher doses, but this difference was not statistically significant (17% vs. 42%, P=0.38). The frequency of readmission (7%) was markedly reduced after transfusion but not current treatment with hydroxyurea (Table III).
TABLE III.
Multivariate Odds of Readmission After Acute Chest Syndrome
| Variable | Odds ratio (95% CI) | P-value |
|---|---|---|
| Prior ACS (increase of 1) | 0.8 (0.6–0.95) | <0.05 |
| Home inhaler* | 6.0 (1.2–30) | <0.05 |
| History of asthma* | 3.8 (0.9–15) | 0.06 |
| RCSS (increase of 1) | 11 (2.1–53) | <0.005 |
| SBP at ACS (increase of 10 mg of Hg) | 1.7 (0.99–2.8) | 0.06 |
| DBP at 48 hr (increase of 10 mg of Hg) | 1.8 (1.2–2.8) | <0.01 |
| Corticosteroid treatment | 20 (2.6–152) | <0.005 |
| Transfusion | 0.03 (0.001–0.7) | <0.05 |
| Hydroxyurea | 2.0 (0.4–10) | NS |
CI, confidence interval; ACS, acute chest syndrome; RCSS, respiratory clinical severity score; SBP, systolic blood pressure; Hg, mercury; DBP, diastolic blood pressure.
Either home inhaler or history of asthma was included in the multivariate model.
Fig. 3.
Kaplan–Meier estimate of time to readmission by taper of corticosteroids. The label includes the number of patients at risk on day 0 and the number of patients not readmitted by day 14. Information on readmission was not available for one patient who was treated with both corticosteroids and transfusion. The difference between the two groups was not statistically significant.
Duration of ACS was similar for the four treatment groups, but total hospitalization after the diagnosis of ACS (including readmission) was longest after treatment with corticosteroids alone. We identified associations between treatment with corticosteroids alone and longer duration of hospitalization [on average 3.9 days longer (95% CI 0.7–7.0, P < 0.05) than the reference group (neither corticosteroids nor transfusions)] and with the number of involved lobes [1.3 days per additional lobe (95% CI−2.6 to−0.1, P < 0.05)] in the multivariate linear regression model after adjustment for severity. The older age groups (6–11, 12–17, and 18–21 years) also had longer hospitalizations by 2.7, 3.3, and 3.1 days (P < 0.001) than those 0–2 years of age. We did not identify significant associations with dyspnea, tachypnea, RCSS, platelet count at admission, fever, transfusion, or respiratory failure.
DISCUSSION
In our cohort, children and young adults with SCD were often readmitted after treatment with corticosteroids for ACS. The frequency of readmission after corticosteroids alone was higher (59%) than that seen in the one randomized controlled trial of dexamethasone for ACS (25%; note: two transfused patients were excluded in our reanalysis of the published data) [1]. The increased frequency of readmission in our study may reflect differences in the population (the randomized trial excluded patients with ACS that developed while hospitalized or severe ACS that were included in our cohort) or better outcomes in the randomized trial secondary to a placebo or Hawthorne effect [14]. The phenomenon of rebound pain was also seen after the treatment of painful vaso-occlusive events with high doses (15 mg/kg/day for 2 days) of methylprednisolone [15] and in a case series of 16 children with SCD treated with chronic corticosteroids [16]. Both the present study and the randomized trials of corticosteroids for ACS[1] and painful vaso-occlusive events [15] identified severe pain as the reason for most readmissions. In our cohort, a taper of corticosteroids increased the time to, but did not prevent, readmission.
In a mouse model of SCD, a significant decrease in adhesion molecule expression and vaso-occlusion was identified 24 hr after and a significant increase 72 hr after three doses of dexamethasone (1 mg/kg/day). In this model, corticosteroids may improve vaso-occlusion by inhibiting activation of nuclear factor κB and reducing the expression of adhesion molecules on endothelial cells [17].
The prevalence of readmission within 14 days of discharge was higher than expected for all patients and varied with baseline characteristics, disease severity, and treatment. Readmission was independently associated with a probable asthma (as measured by self report of an inhaler or nebulizer at home), more severe ACS (as measured by RCSS), elevated diastolic BP at 48 hr, and, most strongly, with treatment with corticosteroids. Readmission was less frequent in patients with many episodes of ACS and after transfusion. Asthma was associated with recurrent ACS in Jamaican children [18] and an increased rate of admission for ACS and painful crisis, but not prolonged hospitalization, in the infant cohort of the CSSCD [19]. However, most patients in our study who were treated with dexamethasone did not have a history of asthma or wheezing at the time of treatment, as recognized asthma exacerbations typically were treated with other corticosteroids (prednisone or prednisolone). Patients with a history of asthma, unless treated with transfusions alone, had a high prevalence of readmission.
Transfusion may decrease readmission by interrupting the vaso-occlusion and up-regulation of inflammatory mediators and adhesion molecules underlying ACS. In a small study of manual exchange transfusion for ACS, plasma levels of vascular cell adhesion molecule-1 (VCAM-1) decreased immediately and then increased to greater than the baseline level after 24 hr, without evidence of rebound pain [20]. Transfusion has well documented adverse effects in SCD, including acute and delayed hemolysis, hyperviscosity, allergic reactions, the transmission of infections, sensitization to red cell antigens, and iron overload [21].
Of note, two patients in our cohort, both treated with transfusions and corticosteroids, had primary hemorrhagic stroke. We have previously reported these patients in a case-control study of children with SCD that demonstrated a strong association between hemorrhagic stroke and antecedent transfusion or treatment with corticosteroids [22]. The randomized controlled trial of dexamethasone for ACS also reported one patient with infarctive stroke [1]. As stroke is well described as a complication of ACS [23], it is difficult to assess the relative importance of ACS versus transfusions and corticosteroids in these patients. However, this observation deserves further study, and may represent an additional risk of corticosteroids in this population.
Of the many risk factors for prolonged admission described in the National Acute Chest Syndrome Study [2], we only identified older age. This may reflect differences in the patient populations, as we only included patients <22 years of age and, on average, our patients had more mild disease, or our limited power to detect associations with 129 episodes of ACS. Other limitations include the non-random assignment of treatment and the type and dose of corticosteroid or transfusion. This could result in potential confounding by baseline characteristics or disease severity. To minimize this concern, we corrected for known confounders during analysis to provide an idea of how well these treatments perform in actual clinical practice. We were unable to identify a dose or taper of corticosteroids that was associated with a lower frequency of readmission; however, there were few patients that received these treatments. Other limitations were the retrospective collection of data by an unblinded reviewer and the absence of active follow-up of the patients, such that readmissions to other hospitals may have been missed. However, the same effort was made to identify readmissions irrespective of treatment and readmission is an objective outcome.
One important question not answered by the current study is the implication for use of corticosteroids for acute asthma exacerbations in patients with SCD. As all of the patients in our study had ACS, these data do not provide a basis for withholding corticosteroids in patients with SCD and asthma without ACS; however, the present study and other studies of the use of corticosteroids in SCD suggest that there could be a significant risk for rebound painful crisis and readmission in this group as well. Our results suggest a considerable risk of readmission after treatment with corticosteroids for moderate and severe ACS. This risk needs to be balanced against the risk of inadequate treatment of asthma, as asthmatic patients were more likely to be readmitted than those without asthma. Transfusion likely reduces this risk and the total duration of hospitalization. This substantial risk of readmission should limit the use of high-dose dexamethasone for ACS, until clearer indications, based on the overall utility of corticosteroids, can be established.
ACKNOWLEDGMENT
Sue Dixon for her assistance with the data collection. The contributions of the individual authors to this work were as follows. Designed research (JJS, CMT, GJK, JFC) performed research (JJS), analyzed data (JJS), and wrote the paper (JJS, CMT, JRK, JFC).
Grant sponsor: NIH; Grant number: K12RR01627; Grant sponsor: Doris Duke Charitable Foundation; American Society of Hematology.
Abbreviations
- ACS
acute chest syndrome
- BP
blood pressure
- OR
odds ratio
- SCD
sickle cell disease
- HbSS
homozygous sickle cell
- CSSCD
Cooperative Study of Sickle Cell Disease
- ICD-9
International Classification of Disease Version 9
- RCSS
respiratory clinical severity score.
Footnotes
Financial disclosure: None for JJS, CMT, JRK, GJK, or JFC.
REFERENCES
- 1.Bernini JC, Rogers ZR, Sandler ES, et al. Beneficial effect of intravenous dexamethasone in children with mild to moderately severe acute chest syndrome complicating sickle cell disease. Blood. 1998;92:3082–3089. [PubMed] [Google Scholar]
- 2.Vichinsky EP, Neumayr LD, Earles AN, et al. National Acute Chest Syndrome Study Group. Causes and outcomes of the acute chest syndrome in sickle cell disease. N Engl J Med. 2000;342:1855–1865. doi: 10.1056/NEJM200006223422502. [DOI] [PubMed] [Google Scholar]
- 3.Castro O, Brambilla DJ, Thorington B, et al. The Cooperative Study of Sickle Cell Disease. The acute chest syndrome in sickle cell disease: Incidence and risk factors. Blood. 1994;84:643–649. [PubMed] [Google Scholar]
- 4.Vichinsky EP, Styles LA, Colangelo LH, et al. Cooperative Study of Sickle Cell Disease. Acute chest syndrome in sickle cell disease: Clinical presentation and course. Blood. 1997;89:1787–1792. [PubMed] [Google Scholar]
- 5.Platt OS, Brambilla DJ, Rosse WF, et al. Mortality in sickle cell disease—Life expectancy and risk factors for early death. N Engl J Med. 1994;330:1639–1644. doi: 10.1056/NEJM199406093302303. [DOI] [PubMed] [Google Scholar]
- 6.Styles LA, Abboud MR, Larkin S, et al. Transfusion prevents acute chest syndrome predicted by elevated secretory phospholipase A2. British Journal of Haematology. 2007;136:343–344. doi: 10.1111/j.1365-2141.2006.06409.x. [DOI] [PubMed] [Google Scholar]
- 7.Mallouh AA, Asha M. Beneficial effect of blood transfusion in children with sickle cell chest syndrome. Am J Dis Child. 1988;142:178–182. doi: 10.1001/archpedi.1988.02150020080034. [DOI] [PubMed] [Google Scholar]
- 8.Emre U, Miller ST, Gutierez M, et al. Effect of transfusion in acute chest syndrome of sickle cell disease. J Pediatr. 1995;127:901–904. doi: 10.1016/s0022-3476(95)70025-0. [DOI] [PubMed] [Google Scholar]
- 9.Rusconi F, Castagneto M, Gagliardi L, et al. Reference values for respiratory rate in the first 3 years of life. Pediatrics. 1994;94:350–355. [PubMed] [Google Scholar]
- 10.Wallis LA, Healy M, Undy MB, et al. Age related reference ranges for respiration rate and heart rate from 4 to 16 years. Arch Dis Child. 2005;90:1117–1121. doi: 10.1136/adc.2004.068718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Emre U, Miller ST, Rao SP, et al. Alveolar-arterial oxygen gradient in acute chest syndrome of sickle cell disease. J Pediatr. 1993;123:272–275. doi: 10.1016/s0022-3476(05)81702-0. [DOI] [PubMed] [Google Scholar]
- 12.Pegelow CH, Colangelo L, Steinberg M, et al. Natural history of blood pressure in sickle cell disease: Risks for stroke and death associated with relative hypertension in sickle cell anemia. Am J Med. 1997;102:171–177. doi: 10.1016/s0002-9343(96)00407-x. [DOI] [PubMed] [Google Scholar]
- 13.Donohoue P. In: Oski’s Pediatrics Principles and Practice. 3rd edition. McMillan J, DeAngelis C, Feigin R, et al., editors. Philadelphia: Lippincott Williams & Wilkins; 1999. p. 1814. [Google Scholar]
- 14.De Amici D, Klersy C, Ramajoli F, et al. Impact of the Hawthorne Effect in a Longitudinal Clinical Study: The Case of Anesthesia. Control Clin Trials. 2000;21:103–114. doi: 10.1016/s0197-2456(99)00054-9. [DOI] [PubMed] [Google Scholar]
- 15.Griffin TC, McIntire D, Buchanan GR. High-dose intravenous methylprednisolone therapy for pain in children and adolescents with sickle cell disease. N Engl J Med. 1994;330:733–737. doi: 10.1056/NEJM199403173301101. [DOI] [PubMed] [Google Scholar]
- 16.Couillard S, Benkerrou M, Girot R, et al. Steroid treatment in children with sickle-cell disease. Haematologica. 2007;92:425–426. doi: 10.3324/haematol.10800. [DOI] [PubMed] [Google Scholar]
- 17.Belcher JD, Mahaseth H, Welch TE, et al. Critical role of endothelial cell activation in hypoxia-induced vasoocclusion in transgenic sickle mice. Am J Physiol Heart Circ Physiol. 2005;288:H2715–H2725. doi: 10.1152/ajpheart.00986.2004. [DOI] [PubMed] [Google Scholar]
- 18.Knight-Madden JM, Forrester TS, Lewis NA, et al. Asthma in children with sickle cell disease and its association with acute chest syndrome. Thorax. 2005;60:206–210. doi: 10.1136/thx.2004.029165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Boyd JH, Macklin EA, Strunk RC, et al. Asthma is associated with acute chest syndrome and pain in children with sickle cell anemia. Blood. 2006;108:2923–2927. doi: 10.1182/blood-2006-01-011072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liem RI, O’Gorman MR, Brown DL. Effect of red cell exchange transfusion on plasma levels of inflammatory mediators in sickle cell patients with acute chest syndrome. Am J Hematol. 2004;76:19–25. doi: 10.1002/ajh.20054. [DOI] [PubMed] [Google Scholar]
- 21.Wayne AS, Kevy SV, Nathan DG. Transfusion management of sickle cell disease. Blood. 1993;81:1109–1123. [PubMed] [Google Scholar]
- 22.Strouse JJ, Hulbert ML, DeBaun MR, et al. Primary hemorrhagic stroke in children with sickle cell disease is associated with recent transfusion and use of corticosteroids. Pediatrics. 2006;118:1916–1924. doi: 10.1542/peds.2006-1241. [DOI] [PubMed] [Google Scholar]
- 23.Ohene-Frempong K, Weiner SJ, Sleeper LA, et al. Cerebrovascular accidents in sickle cell disease: Rates and risk factors. Blood. 1998;91:288–294. [PubMed] [Google Scholar]