Abstract
Purpose
Ezrin is a membrane cytoskeletal linker protein and it is known to be associated with metastasis of primary osteosarcoma. The aim of this study is to determine the relationship between an ezrin expression and several key clinical parameters and to elucidate its potential prognostic value for patients with osteosarcoma.
Materials and Methods
Seventy patients with histologically confirmed osteosarcoma and who had no distant metastasis were enrolled between 1995 and 2005 at Yonsei Cancer Center, Severance Hospital, Korea. The clinical parameters were retrospectively reviewed and immunohistochemical staining (IHC) for ezrin was performed using the surgically resected specimens.
Results
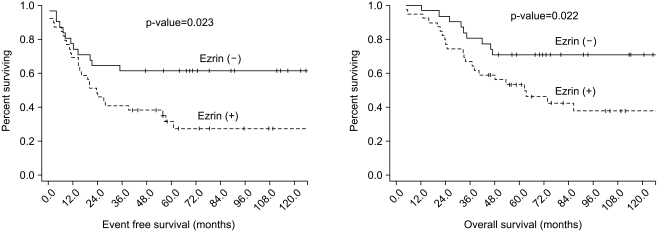
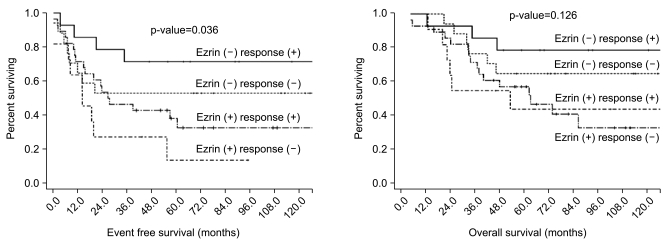
Of the 70 tumor specimens, 39 (55.7%) revealed an ezrin expression. More of an osteoblastic histology and an elevated initial ALP level were observed in the ezrin positive patients than in the ezrin negative patients (p=0.008 and 0.001, respectively). The proportion of patients who favorably responded to neoadjuvant chemotherapy (≥90% necrosis) was significantly higher in the group of ezrin positive patients than that in the group of ezrin negative patient (72.2% vs 45.2%, respectively, p=0.024). The ezrin positive patients showed more frequent recurrence than did the ezrin negative patients (64.1% vs 35.5%, respectively, p=0.017). The patients with an ezrin expression also demonstrated poorer survival than did those patients without ezrin expression (5-year EFS: 31.7% vs 61.3%, respectively, p=0.023, 5-year OS: 53.4% vs 71.0%, respectively, p=0.022). When comparing EFS according to both an ezrin expression and chemoresponsiveness, there were trends that the ezrin negative/chemoresponsive group showed the best 5-year EFS (71.4%), followed by the ezrin negative/chemoresistant group (52.9%), the ezrin positive/chemoresponsive group (38.1%) and the ezrin positive/chemoresistant group (13.6%). These trends were statistically significant (p=0.036).
Conclusion
The expression of ezrin by IHC staining was found in 55.7% of the patients with metastasis-free osteosarcoma. Immunoreactivity to ezrin is a negative prognostic factor for survival for the patients suffering with osteosarcoma. Identifying an ezrin expression might offer a valuable piece of information when treating patients with primary osteosarcoma.
Keywords: Osteosarcoma, Ezrin, Chemotherapy, Survival
Introduction
Primary osteosarcoma is the most common primary bone tumor that predominantly develops in adolescents and young adults. Even after the introduction of aggressive perioperative chemotherapy and wide excision of tumors, a substantial number of patients still experience local recurrence or distant metastases after curative resection of their primary tumors. The clinical outcome is extremely poor for these recurrent or metastatic patients (1). Thus, several attempts have been made to identify the prognostic factors for survival for the patients with osteosarcoma and to identify the patients with a high risk of developing recurrence from those patients with a low risk (2,3). There are currently very limited prognostic indicators; these include factors such as age, the tumor site, the histologic type, the tumor volume and an elevated serum alkaline phosphatase (ALP) level (4).
Ezrin is a membrane cytoskeletal linker protein which is produced by the expression of the Vil2 gene and ezin is a member of the ERM (Ezrin-Radixin-Moesin) family (5,6). Ezrin is involved in the interaction between cells or between cell and the extracellular matrix. It is related to the Rho mediated signal transduction pathway and to the Akt mediated apoptotic pathway. Ezrin is currently known to play an important role in the metastasis of various human cancers, including breast cancer, colorectal carcinoma, soft tissue sarcoma and serous ovarian carcinoma (7-10). Furthermore, ezrin has been suggested to be an importantly involved molecule in the metastasis of human osteosarcoma. In a previous pilot study, a high expression of ezrin was found to be associated with the early development of metastases in a mouse model (11). In addition, it has been noted that the disease-free interval is significantly shorter in pediatric patients with a high ezrin expression (11). However, there have been only a few reports regarding the expression of ezrin in osteosarcoma or its relationship with other clinicopathologic parameters (8,12,13).
The aim of this study is to determine the relationship between an ezrin expression and several key clinicopathologic features and to elucidate the potential value of an ezin expression for predicting the clinical outcome of patients with primary osteosarcoma.
Materials and Methods
1. The patients and tumor samples
Seventy patients who were histologically confirmed as having osteosarcoma and who had no clinical evidence of distant metastasis at the time of diagnosis were enrolled between 1995 and 2005 at Yonsei Cancer Center, Severance Hospital, Korea. All the patients underwent neoadjuvant chemotherapy and wide resection of tumor.
The clinicopathologic variables such as gender, age, the site of osteosarcoma, the histologic type, the initial serum ALP level, the type of surgery and the status of the resection margin were retrospectively reviewed on the basis of the medical records.
2. Immunohistochemistry
Immunohistochemical (IHC) staining was performed on the formalin-fixed, paraffin-embedded surgical specimens from all the patients. The slides of 70 primary surgical specimens were deparaffinized, rehydrated and then processed using the labeled streptavidin-biotin-peroxidase method. Antigen retrieval was performed by heating the slides in 10 mmol/L sodium citrate buffer (pH 6.0) for 15 minutes at 95℃. The slides were then incubated in 10% normal blocking serum for 30 minutes. After that, they were incubated overnight at 4℃ in appropriately diluted mouse monoclonal antibody that was reactive to ezrin (1 : 300; NeoMarkers, Fremont, CA). After washing with Tris buffer, the tissue slides were incubated with biotin-labelled secondary antibodies and then the slides were reacted with streptavidin-horseradish peroxidase with using the DAKO LSAB kit (DAKO, Glostrup, Denmark) at room temperature for 30 minutes at each step. Nova red (Vector Laboratory, Burlingame, CA) was used as the chromogen and hematoxylin was used as the nuclear counterstain. The ezrin expression was graded as negative (<10% of the cells stained), 1+ (10~33% of the cells stained), 2+ (34~67% of the cells stained), and 3+ (68~100% of the cells stained).
3. Statistical analysis
For survival analysis, overall survival (OS) was defined as the time interval between the date of surgery to the date of death or the last follow-up. Event-free survival (EFS) was defined as the time interval from surgery to progressive disease, death from any cause other than progression, or a second primary cancer.
The Statistical Package for Social Sciences (SPSS) version 12.0 for Windows (SPSS, Inc., Chicago, IL) was used for the statistical analysis. The correlation between the clinicopathologic variables and the ezrin expression was analyzed using independent sample t-tests for the continuous variables and the chi-square test for the discrete variables. Survival curves were calculated using the Kaplan-Meier method, and comparisons between the different groups were analyzed using the log-rank test. A multivariate analysis was done by Cox's regression to identify the prognostic factors for EFS and OS from the variables that proved significance on the univariate analysis. The accepted level of statistical significance was p<0.05.
Results
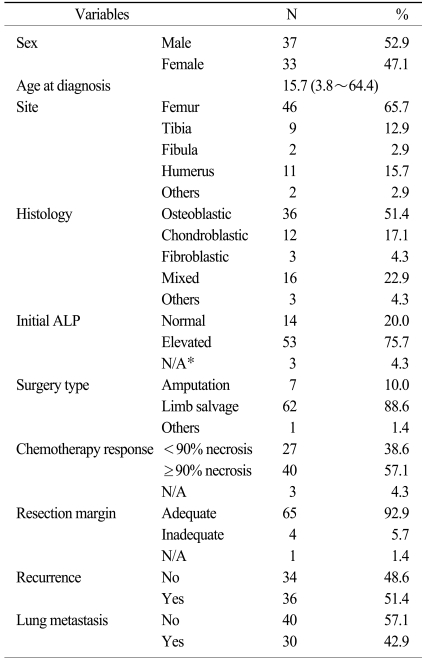
Table 1 shows the baseline clinical characteristics. The male-to-female ratio was 1.12. The median age at the time of diagnosis of osteosarcoma was 15.7 years (range: 3.8~64.4 years). The most prevalent primary site of osteosarcoma was the femur (65.7%) and the most common histologic subtype was osteoblastic osteosarcoma (51.4%). Most patients (88.6%) underwent a limb salvage operation and the surgical specimens revealed favorable responses (≥90% necrosis) in 40 patients (57.1%).
Table 1.
Baseline clinical characteristics
*not available.
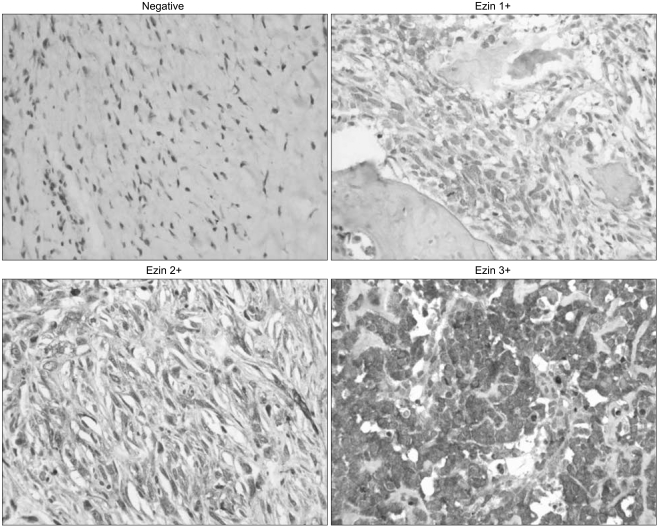
Of the 70 tumor specimens, 39 (55.7%) revealed the expression of ezrin, while 31 (44.3%) showed no expression of ezrin. Fig. 1 shows the representative expressions of ezrin based on the staining intensities. In the positive cases, ezrin was expressed in the cytoplasm of the osteosarcoma tumor cells.
Fig. 1.
Examples of ezrin immunoreactivity in osteosarcoma. Original magnification×200.
With the median follow-up of 59.9 months, among the 70 enrolled patients, 32 patients (45.7%) remained event-free, while 36 patients (51.4%) had recurrent disease and 2 patients (2.9%) died of postoperative infectious complications without any evidence of recurrence. Of the 36 recurred patients, 29 died of progressive disease and 8 survived until the time of analysis. After the curative resection of tumor, 68 patients underwent adjuvant chemotherapy, while the remaining 2 died of postoperative complications.
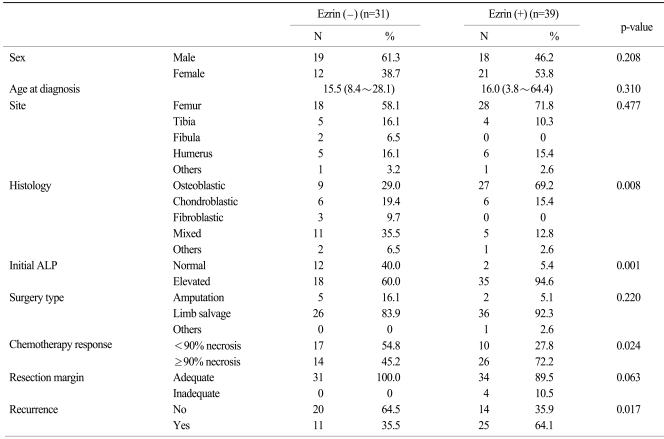
Table 2 shows the clinicopathologic parameters according to the expression of ezrin. There were no differences in gender, age, the site of tumor, the type of surgery and the status of the resection margin between the patients with or without an expression of ezrin. However, a more prevalent osteoblastic histology and a more elevated initial ALP level were observed in the ezrin positive patients than in the ezrin negative patients (p=0.008 and 0.001, respectively). The proportion of patients who favorably responded to neoadjuvant chemotherapy (≥90% necrosis) was significantly higher for the ezrin positive patients than that of the ezrin negative patient (72.2% vs 45.2%, respectively, p=0.024). In addition, the patients with ezrin positivity showed more frequent tumor recurrence than did those patients with ezrin negativity (64.1% vs 35.5%, respectively, p=0.017).
Table 2.
Key clinical parameters according to ezrin expression
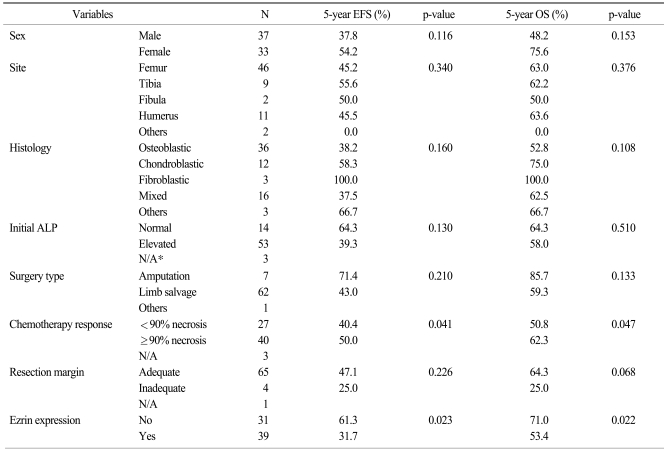
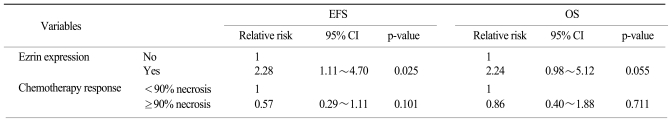
The 5-year OS was 61.1% and the 5-year EFS was 45.2% for all the patients. A poor histologic response to previous chemotherapy (<90% necrosis) and an expression of ezrin were negative prognostic factors on the univariate analysis (p=0.047 and 0.022, respectively), while gender, the tumor site, the histologic type, the initial ALP level, the type of surgery and the resection margin had no prognostic value (Table 3) (Fig. 2). On the Cox regression multivariate analysis, an ezrin expression showed independent significance for EFS (p=0.025), whereas its significance was marginal for OS (p=0.055) (Table 4).
Table 3.
5-year survival rate according to clinicopathologic variables
*not available.
Fig. 2.
Kaplan-Meier estimates of EFS and OS.
Table 4.
Cox's multivariate model for survival
Discussion
Osteosarcoma is a very heterogenous disease entity and there are multiple factors that have an influence on the prognosis of osteosarcoma. Some patients have shown a good response to neoadjuvant chemotherapy and they were eventually cured by the subsequent surgery, while others have shown resistance to chemotherapy and the disease rapidly progressed (14). The well known clinical prognostic factors are age, the tumor site, the histologic type, the tumor volume and an elevated serum alkaline phosphatase (ALP) level. Yet the molecular biomarkers of osteosarcoma are not well known and much remains to be investigated. There have been several reports on the expression patterns of molecular markers such as VEGF, ErbB-2, P-glycoprotein and ezrin, and their prognostic value for osteosarcoma (4). In this current study, ezrin was selected for IHC staining because ezrin is known to be involved in the early processes of metastasis and recurrence of osteosarcoma (11-13).
In our study, 55.7% of the osteosarcoma patients showed an expression of ezrin. This result was consistent with the results of previous reports that ranged from 40% to 60% (12,15,16). The patients with an ezrin expression also experienced a greater frequency of osteosarcoma recurrence after curative resection, and they subsequently had poorer EFS and OS than did those patients without an ezrin expression. The median EFS was 34.6 months for the patients with an ezrin expression, while the EFS curve did not reach the median for the patients without an ezrin expression because more than half of the patients without an ezrin expression survived. This negative prognostic value of an ezrin expression is similar with the reports on other solid malignancies (8,9,17).
In the three major previous studies regarding the relationship between the subtype of osteosarcoma and survival, the fibroblastic subtype and the chondroblastic subtype showed better survival than did the conventional osteoblastic subtype (18-20), but no satisfactory explanation was given about this difference in the prognosis. The present study demonstrated that the conventional osteoblastic histology is associated with a frequent expression of ezrin, as 27 out of 36 (75%) patients with an osteoblastic histology expressed ezrin according to IHC staining, while only 6 of 12 (50%) of the patients with an chondroblastic subtype showed a ezrin expression and none of the patients with a fibroblastic subtype showed an ezrin expression. Because our study's sample size was small, we cannot conclude a definite relationship between the histologic subtype and an ezrin expression. Yet we suggest the possibility that the more frequent ezrin expression in the osteoblastic subtype might be related to a bad prognosis for the osteoblastic subtype. An ezrin expression and more aggressive features for metastasis could be associated with a bad prognosis for this subtype.
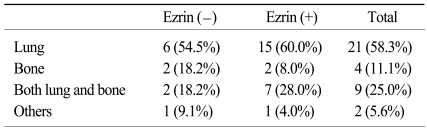
The concept of neoadjuvant chemotherapy was introduced in the late 1970s and this is now accepted as one of standard treatments for metastasis-free osteosarcoma (21,22). The advantages of neoadjuvant chemotherapy are the early treatment of potentially undetectable micrometastasis and the opportunity to assess the histologic response to neoadjuvant chemotherapy. Although the patients in the previously reported studies that responded favorably to neoadjuvant chemotherapy with ≥90% necrosis had a 70~87% 5-year OS, the patients with a poor response of <90% necrosis had less than 50.0% 5-year OS (2,22,23). However, the response to neoadjuvant chemotherapy is not always a good prognostic factor. A substantial number of good responders still had early recurrence at distant sites after curative resection. In this current study, among the 40 good responders, 10 patients (25.0%) recurred within 1 year after curative resection, and 8 of whom revealed ezrin immunoreactivity in the surgical specimen. In contrast, 10 of the 27 (37.0%) poor responders were able to achieve long term survival of more than 5 years after the surgery. Only 1 of these 10 long-term surviving poor responders showed ezrin immunoreactivity in their surgical specimen. The EFS curves, according to an ezrin expression and chemoresponsiveness, showed similar trends, which is consistent with these result (Fig. 3). The ezrin negative/ chemoresponsive group showed the best 5-year EFS (71.4%), followed by the ezrin negative/chemoresistant group (52.9%), the ezrin positive/chemoresponsive group (38.1%) and the ezrin positive/chemoresistant group (13.6%) (p=0.036). Overall survival according to an ezrin expression and chemoresponsiveness also showed similar trends, but it was not significant (p=0.126). That is to say, good responders with an ezrin expression might have a poorer outcome with early metastasis than the poor responders without an ezrin expression. Therefore, the good responders with an ezrin expression, who were more likely to recur early in spite of a favorable chemotherapy response, need more active tumor surveillance. Because lung and bone were the most prevalent sites of distant metastasis of osteosarcoma after the curative surgery (Table 5), regular follow-ups that include imaging of the lungs and bones could be helpful for the early detection of recurrence in this subset of patients.
Fig. 3.
EFS and OS according to ezrin expression and chemoresponsiveness.
Table 5.
Common sites of metastasis according to ezrin expression
Conclusion
The expression of ezrin by IHC staining was found in more than half (55.7%) of the patients with metastasis-free osteosarcoma. Immunoreactivity to ezrin is a negative prognostic factor for OS and EFS. Identifying an ezrin expression might offer a valuable piece of information when treating patients with primary osteosarcoma.
Footnotes
This study was supported by a faculty research grant of Yonsei University College of Medicine for 2007.
References
- 1.Meyers PA, Heller G, Healey J, Huvos A, Lane J, Marcove R, et al. Chemotherapy for nonmetastatic osteogenic sarcoma: the memorial sloan-kettering experience. J Clin Oncol. 1992;10:5–15. doi: 10.1200/JCO.1992.10.1.5. [DOI] [PubMed] [Google Scholar]
- 2.Bacci G, Longhi A, Versari M, Mercuri M, Briccoli A, Picci P. Prognostic factors for osteosarcoma of the extremity treated with neoadjuvant chemotherapy: 15-year experience in 789 patients treated at a single institution. Cancer. 2006;106:1154–1161. doi: 10.1002/cncr.21724. [DOI] [PubMed] [Google Scholar]
- 3.Kalifa C, Razafindrakoto H, Vassal G, Contesso G, Vanel D, Edeline V, et al. Chemotherapy in osteogenic sarcoma: the experience of the pediatric department of the gustave roussy institute. Cancer Treat Res. 1993;62:347–349. doi: 10.1007/978-1-4615-3518-8_42. [DOI] [PubMed] [Google Scholar]
- 4.Clark JC, Dass CR, Choong PF. A review of clinical and molecular prognostic factors in osteosarcoma. J Cancer Res Clin Oncol. 2008;134:281–297. doi: 10.1007/s00432-007-0330-x. [DOI] [PubMed] [Google Scholar]
- 5.Bretscher A, Edwards K, Fehon RG. ERM proteins and merlin: integrators at the cell cortex. Nat Rev Mol Cell Biol. 2002;3:586–599. doi: 10.1038/nrm882. [DOI] [PubMed] [Google Scholar]
- 6.Hunter KW. Ezrin, a key component in tumor metastasis. Trends Mol Med. 2004;10:201–204. doi: 10.1016/j.molmed.2004.03.001. [DOI] [PubMed] [Google Scholar]
- 7.Elliott BE, Meens JA, SenGupta SK, Louvard D, Arpin M. The membrane cytoskeletal crosslinker ezrin is required for metastasis of breast carcinoma cells. Breast Cancer Res. 2005;7:R365–R373. doi: 10.1186/bcr1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Weng WH, Ahlen J, Astrom K, Lui WO, Larsson C. Prognostic impact of immunohistochemical expression of ezrin in highly malignant soft tissue sarcomas. Clin Cancer Res. 2005;11:6198–6204. doi: 10.1158/1078-0432.CCR-05-0548. [DOI] [PubMed] [Google Scholar]
- 9.Elzagheid A, Korkeila E, Bendardaf R, Buhmeida A, Heikkila S, Vaheri A, et al. Intense cytoplasmic ezrin immunoreactivity predicts poor survival in colorectal cancer. Hum Pathol. 2008;39:1737–1743. doi: 10.1016/j.humpath.2008.04.020. [DOI] [PubMed] [Google Scholar]
- 10.Moilanen J, Lassus H, Leminen A, Vaheri A, Butzow R, Carpen O. Ezrin immunoreactivity in relation to survival in serous ovarian carcinoma patients. Gynecol Oncol. 2003;90:273–281. doi: 10.1016/s0090-8258(03)00262-2. [DOI] [PubMed] [Google Scholar]
- 11.Khanna C, Wan X, Bose S, Cassaday R, Olomu O, Mendoza A, et al. The membrane-cytoskeleton linker ezrin is necessary for osteosarcoma metastasis. Nat Med. 2004;10:182–186. doi: 10.1038/nm982. [DOI] [PubMed] [Google Scholar]
- 12.Kim MS, Song WS, Cho WH, Lee SY, Jeon DG. Ezrin expression predicts survival in stage IIB osteosarcomas. Clin Orthop Relat Res. 2007;459:229–236. doi: 10.1097/BLO.0b013e3180413dbf. [DOI] [PubMed] [Google Scholar]
- 13.Xu-dong S, Zan S, Shui-er Z, Li-na T, Wen-xi Y, Feng L, et al. Expression of ezrin correlates with lung metastasis in Chinese patients with osteosarcoma. Clin Invest Med. 2009;32:E180–E188. doi: 10.25011/cim.v32i2.6036. [DOI] [PubMed] [Google Scholar]
- 14.Sohn JH, Rha SY, Jeung H, Shin HC, Shin HJ, Goo YS, et al. Efficacy of pre- and postoperative chemotherapy in patients with osteosarcoma of the extremities. Cancer Res Treat. 2001;33:520–526. doi: 10.4143/crt.2001.33.6.520. [DOI] [PubMed] [Google Scholar]
- 15.Park HR, Jung WW, Bacchini P, Bertoni F, Kim YW, Park YK. Ezrin in osteosarcoma: comparison between conventional high-grade and central low-grade osteosarcoma. Pathol Res Pract. 2006;202:509–515. doi: 10.1016/j.prp.2006.01.015. [DOI] [PubMed] [Google Scholar]
- 16.Salas S, Bartoli C, Deville JL, Gaudart J, Fina F, Calisti A, et al. Ezrin and alpha-smooth muscle actin are immunohistochemical prognostic markers in conventional osteosarcomas. Virchows Arch. 2007;451:999–1007. doi: 10.1007/s00428-007-0474-8. [DOI] [PubMed] [Google Scholar]
- 17.Makitie T, Carpen O, Vaheri A, Kivela T. Ezrin as a prognostic indicator and its relationship to tumor characteristics in uveal malignant melanoma. Invest Ophthalmol Vis Sci. 2001;42:2442–2449. [PubMed] [Google Scholar]
- 18.Bentzen SM, Poulsen HS, Kaae S, Jensen OM, Johansen H, Mouridsen HT, et al. Prognostic factors in osteosarcomas. A regression analysis. Cancer. 1988;62:194–202. doi: 10.1002/1097-0142(19880701)62:1<194::aid-cncr2820620129>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 19.Uribe-Botero G, Russell WO, Sutow WW, Martin RG. Primary osteosarcoma of bone. Clinicopathologic investigation of 243 cases, with necropsy studies in 54. Am J Clin Pathol. 1977;67:427–435. doi: 10.1093/ajcp/67.5.427. [DOI] [PubMed] [Google Scholar]
- 20.Hauben EI, Weeden S, Pringle J, Van Marck EA, Hogendoorn PC. Does the histological subtype of high-grade central osteosarcoma influence the response to treatment with chemotherapy and does it affect overall survival? A study on 570 patients of two consecutive trials of the European Osteosarcoma Intergroup. Eur J Cancer. 2002;38:1218–1225. doi: 10.1016/s0959-8049(02)00037-0. [DOI] [PubMed] [Google Scholar]
- 21.Rosen G, Marcove RC, Caparros B, Nirenberg A, Kosloff C, Huvos AG. Primary osteogenic sarcoma. the rationale for preoperative chemotherapy and delayed surgery. Cancer. 1979;43:2163–2177. doi: 10.1002/1097-0142(197906)43:6<2163::aid-cncr2820430602>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 22.Provisor AJ, Ettinger LJ, Nachman JB, Krailo MD, Makley JT, Yunis EJ, et al. Treatment of nonmetastatic osteosarcoma of the extremity with preoperative and postoperative chemotherapy: a report from the Children's cancer group. J Clin Oncol. 1997;15:76–84. doi: 10.1200/JCO.1997.15.1.76. [DOI] [PubMed] [Google Scholar]
- 23.Souhami RL, Craft AW, Van der Eijken JW, Nooij M, Spooner D, Bramwell VH, et al. Randomised trial of two regimens of chemotherapy in operable osteosarcoma: a study of the European Osteosarcoma Intergroup. Lancet. 1997;350:911–917. doi: 10.1016/S0140-6736(97)02307-6. [DOI] [PubMed] [Google Scholar]