Abstract
Evidence from observational studies, prospective cohort studies and randomized clinical intervention studies indicate that moderate doses of long-chain n-3 polyunsaturated fatty acids (LC n-3 PUFA) significantly decrease risk of fatal coronary heart disease (CHD). Higher doses and longer duration of intervention may also protect from non-fatal CHD events. The exact mechanisms through which LC n-3 PUFA has an effect on CHD are not well established but may include a decrease in fasting and postprandial triacylglycerol levels, a decrease in arrhythmias, modulation of platelet aggregation and decreased synthesis of pro-inflammatory agents. The mechanistic relation between LC n-3 PUFA and inflammation has attracted great interest, and in vitro studies have revealed that these fatty acids decrease endothelial activation, affect eicosanoid metabolism (including epoxygenation pathways) and induce inflammatory resolution. However, the effects of LC n-3 PUFA on established biomarkers of inflammation and endothelial activation in vivo are not strong. Consequently we need new and more sensitive and systemic biomarkers to reveal the effects of LC n-3 PUFA on localized inflammatory processes.
Keywords: fish oil, LC n-3 PUFA, mechanisms, coronary heart disease, inflammation
Introduction
n-3 polyunsaturated fatty acids (n-3 PUFA or ω-3 fatty acids) are polyunsaturated fatty acids that have their first double bond at the third position when counted from the methyl end of the molecule. The simplest n-3 PUFA is α-linolenic acid (Figure 1). This fatty acid is an 18-carbon n-3 PUFA with three double bonds (18:3n-3) and its synthesis, as well as the synthesis of other n-3 PUFAs from n-6 fatty acids, requires the presence of the enzyme Δ15-desaturase. However, humans lack this enzyme and need to ensure adequate intake of n-3 PUFA by dietary means for example, through consumption of vegetables and nuts. α-Linolenic acid can however be converted to the longer-chain (LC) n-3 PUFA in the human body. The extent of this conversion is not precisely known and at best very limited (Goyens et al., 2005; 2006;). Thus most LC n-3 PUFA will also have to be obtained from the diet. These fatty acids, which predominantly are at least 20-carbon atoms long with five or more double bonds, primarily occur in fish and other seafoods. The main LC n-3 PUFA from these products are eicosapentaenoic acid (EPA; C20:5n-3) and docosahexaenoic acid (DHA: C22:6n-3) (Figure 1). The metabolism of n-3 and n-6 fatty acids is linked as their metabolic pathways compete for the same enzymes (desaturases and elongases). Thus downstream metabolism of linoleic and α-linolenic acid, producing LC derivatives like arachidonic acid (AA; 20:4n-6), EPA (20:5n-3) and DHA (22:6n-3), will influence each other (Figure 1).
Figure 1.
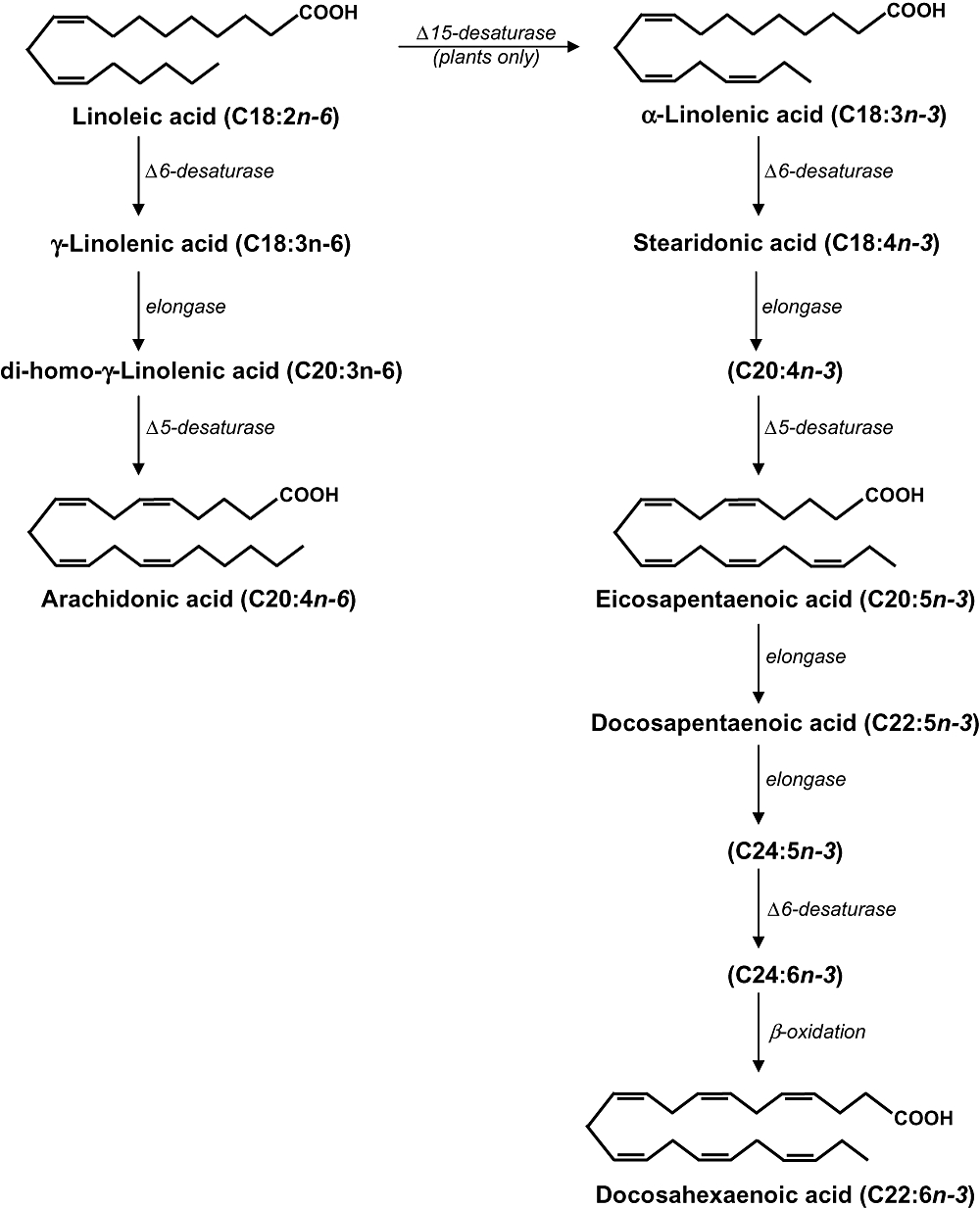
Biosynthesis pathways of n-6 and n-3 polyunsaturated fatty acids.
LC n-3 PUFA and heart disease: evidence from human studies
In the seventies, Bang and Dyerberg noted that Inuits from Greenland had very low levels of coronary heart disease (CHD). As the intake of fish and LC n-3 PUFA from sea animals in the Inuit population is very high, they suggested that high intake of LC n-3 PUFA may be associated with low prevalence of CHD (Bang et al., 1971; 1976; Dyerberg et al., 1978; Bang and Dyerberg, 1980). Since then many studies have been performed to investigate this association. The Zutphen study was the first prospective cohort study that showed that higher intake of fish was associated with less fatal CHD (Kromhout et al., 1985). Subsequently many observational studies found that higher intakes of fish or fish fatty acids were associated with a lower risk of CHD (Dolecek and Granditis, 1991; Rodriguez et al., 1996; Daviglus et al., 1997; Albert et al., 1998; Yuan et al., 2001; Hu and Willett, 2002; Hu et al., 2002). However, other studies reported no association or even a slightly increased risk of CHD in people with a higher fish intake (Ascherio et al., 1995; Salonen et al., 1995; Pietinen et al., 1997; Osler et al., 2003). High mercury concentrations in fish might explain the higher rates of fatal CHD in subjects with a high fish intake in Finland (Pietinen et al., 1997). A meta-analysis of 13 prospective observational studies indicated that people who ate at least one fish meal per week had approximately a 15% lower risk of dying of CHD compared with people who ate a fish meal less than once a month, and consumption of every additional 20 g of fish per day decreased the risk of fatal CHD by 7% (Whelton et al., 2004).
Studies suggest that LC n-3 PUFA in blood are the component responsible for the suspected protection against fatal heart disease. In three studies, a higher percentage of fish fatty acids in blood was associated with a lower risk of fatal heart disease, sudden death or cardiac arrest (Siscovick et al., 1995; 2000; Albert et al., 1998; 2002;). In the Cardiovascular Health Study, higher levels of LC n-3 PUFA in plasma phospholipids were associated with a lower risk of fatal CHD, but not with a lower risk of non-fatal myocardial infarction (Lemaitre et al., 2003). There was also no association between fish intake and risk of non-fatal CHD in the Physicians' Health Study (Ascherio et al., 1995). Thus observational studies suggest that a modest intake of fish is associated with a lower risk of fatal CHD, but not with non-fatal heart disease. However, as with all observational studies, other lifestyle factors may have confounded such associations. Furthermore, the beneficial effects of fish consumption on heart disease may depend on the type of fish consumed. Consumption of tuna or other broiled or baked fish two times per week or more was associated with lower risk of fatal ischaemic heart disease compared with consumption of less than once per month. However, intake of fried fish or fish sandwiches was not associated with a lower risk, but with trends towards higher risks (Mozaffarian et al., 2003).
By 2008 five randomized clinical intervention trials have been published on the direct effects of ω-3 fatty acid consumption on fatal CHD. The first GISSI-Prevenzione trial, a randomized open label study in 11 324 Italian patients with a recent myocardial infarction, demonstrated that patients had a 15% lower combined risk of mortality, non-fatal myocardial infarction and stroke upon supplementation for 3.5 years with 850 mg·day−1 of LC n-3 PUFA. The relative risk of cardiovascular death was also decreased by 30% and of sudden death by 45%. These effects were already evident just months after randomization. Additionally, the beneficial effects were seen in a population already protected with recommended secondary prevention that included anti-platelet therapy with acetylsalicylic acid (aspirin) and consumption of a ‘Mediterranean diet’. The GISSI-Prevenzione trial demonstrated that a significant protective effect could be obtained with doses much lower than those previously considered necessary for significant beneficial effects (GISSI Investigators, 1999). The second GISSI-HF study, a randomized double-blind placebo-controlled trial in 6975 Italian patients with chronic heart failure, revealed a moderate decrease in both all-cause mortality and admissions to hospital for cardiovascular reasons upon supplementation for on average 3.9 years with 1 g of LC n-3 PUFA daily. Again, the beneficial effects were seen in a population already treated with recommended therapies (Gissi-Hf, 2008). The diet and reinfarction trial 1 (DART) was carried out in 2033 English men with a recent myocardial infarction. Men who received advice to increase their fish intake to at least two meals per week, compared with no advice, had a 29% lower mortality rate during the 2 year follow-up (Burr et al., 1989). In contrast, the second DART trial of Burr et al. (Burr et al., 2003), in 3114 Welsh patients with stable angina without a myocardial infarction, showed no beneficial effect of n-3 PUFA intake during 9 year follow-up. In this trial, advice to eat fatty fish did not lower mortality, and intake of fish oil supplements was associated with a higher risk of cardiac and sudden death (Burr et al., 2003). However, methodological problems may have affected the outcome as compliance to advice was only shown for a subsample of patients. Unfortunately, both participants and providers were not masked, which implies that the intake of fish oil may have modified the behaviour of both patients and physicians towards intake of medication or diet and lifestyle. Furthermore, recruitment for DART-2 was interrupted for 1 year because of funding problems (Burr et al., 2005). The JELIS trial was performed in 18 645 Japanese men and women with hypercholesterolaemia treated with statins. Supplementation with 1.8 g EPA per day decreased major coronary events by 19% over 4.6 years. Non-fatal coronary events, rather than CHD death, were decreased (Yokoyama et al., 2007). This is consistent with the very low death rates from CHD in Japan because of a high background seafood consumption (Mozaffarian, 2007; 2008;). In summary, a modest intake of LC n-3 PUFA significantly decreases risk of fatal CHD risk. However, higher doses and longer duration of intervention, as reported for the JELIS study, has the potential to protect from non-fatal CHD events (Mozaffarian, 2008).
In 2006, two systematic reviews were published, assessing all available evidence from fish oil intervention studies. Hooper et al. (2006), based their review on 48 randomized clinical trials and 41 cohort studies, concluded that long-chain and shorter-chain ω-3 fats did not clearly affect total mortality, combined cardiovascular events or cancer. However, two main points of criticism on their approach were: (i) the pooling of results of studies addressing α-linolenic acid with reports on LC n-3 PUFA from fish and epidemiological evidence of protective effects from α-linolenic acid is not very convincing; and (ii) combination of fatal and non-fatal cardiovascular events (Geleijnse et al., 2006). In addition, their overall conclusion on LC n-3 PUFA from fish was quite heavily influenced by the results from the DART-2 study, which was troubled by methodological problems (Burr et al., 2003). Removal of DART-2 from the meta-analysis resulted in a protective effect for LC n-3 PUFA (Hooper et al., 2006). Indeed, several earlier meta-analyses have shown a protective effect of fish intake on stroke and on fatal CHD (Bucher et al., 2002; He et al., 2004; Whelton et al., 2004). The second review by Wang et al. (2006), based on 14 randomized clinical trials, 25 prospective cohort studies and 7 case–control studies, concluded that increased consumption of LC n-3 PUFA from fish or fish oil supplements, but not of α-linolenic acid, decreases rates of all-cause mortality, cardiac and sudden death, and possibly stroke. In addition, the benefits of fish oil were stronger in secondary compared with primary prevention settings, and adverse effects appeared to be minor (Wang et al., 2006).
Mechanisms of LC n-3 PUFA
The exact mechanisms through which LC n-3 PUFA influence CHD are not well established, but appear to include a decrease in fasting and postprandial triacylglycerol, decreased atherosclerosis, a decrease in arrhythmias, modulation of platelet aggregation and decreased synthesis of pro-inflammatory agents (de Roos et al., 2005b) (Table 1). These favourable effects have been primarily attributed to EPA, which is present in large amounts in fish oil. However, controlled studies in humans now demonstrate that DHA has equally important anti-arrhythmic and anti-atherogenic effects, although it is often present in lower amounts in oily fish and fish oil supplements (Mori and Woodman, 2006).
Table 1.
Overview of pathways and mechanisms through which long-chain n-3 polyunsaturated fatty acids could affect cardiovascular outcome
| Pathways | Mechanisms | |
|---|---|---|
| ↓ Plasma triacylglycerol | ↓ Triacylglycerol production | ↓ SREBP-1c activity |
| ↓ VLDL assembly and secretion | ||
| ↓ Activation of PPAR-α | ||
| ↓β-Oxidation in mitochondria and peroxisomes | ||
| ↓ Arrhythmias | Arrhythmogenesis | Action potential shortening |
| No effect on ventricular tachyarrhythmia | ||
| No effect on spontaneous atrial fibrillation | ||
| Prevention of post-operative atrial fibrillation | ||
| ↓ Heart rate | Better ventricular efficiency | |
| ↑ Platelet function | ↓ Production of the pro-aggregatory TxA2Antagonists of the TxA2/PGH2 receptor in vitro | |
| ↓ Inflammation | ↓ Endothelial activation | ↓ Expression of ICAM-1, VCAM-1 in vitro only |
| ↓ IL-6, IL-8 in vitro only | ||
| ↓ E-selectin in vitro only | ||
| ↓ NF-κB in vitro only | ||
| ↓↑ Production of TNF-α, IL-1 or IL-6 by activated mononuclear cells | ||
| Changes in eicosanoid metabolism | ↓ Production of PGE2 by inflammatory cells ex vivo | |
| ↓ TxB2 by inflammatory cells ex vivo | ||
| ↓ LTB4 by inflammatory cells ex vivo | ||
| ↓ 5-HETE by inflammatory cells ex vivo | ||
| ↓ LTE4 by inflammatory cells ex vivo | ||
| ↓ COX-2 expression and activity | ||
| Changes in epoxygenation pathways | ↓ Protein levels of soluble epoxide hydrolase | |
| ↑ Production of epoxy-EPA and DHA derivatives | ||
| ↑ Inflammatory resolution | ↓ Production of pro-inflammatory eicosanoids | |
| ↑ Production of lipoxins and aspirin-triggered lipoxins from AA | ||
| ↑ Production of E-series resolvins from EPA | Protects against pro-inflammatory gene expression | |
| Blocks transendothelial migration of polymorphonuclear leukocytes | ||
| ↓ Leukocyte rolling | ||
| ↓ Platelet aggregation | ||
| ↓ Neutrophil infiltration | ||
| ↑ Production of D-series resolvins from DHA | ↓ Neutrophil infiltration | |
| ↑ Production of protectins from DHA | ↓ Transmigration of neutrophils | |
AA, arachidonic acid; COX, cyclooxygenase; DHA, docosahexaenoic acid; EPA, eicosapentaenoic acid; HETE, hydroxyeicosatetraenoic acid; ICAM, intercellular adhesion molecule; IL, interleukin; LT, leukotriene; NF-κB, nuclear factor-κB; PG, prostaglandin; PPAR-α: peroxisome proliferator-activated receptor-α; SREBP-1c, sterol receptor element binding protein-1c; TNF-α, tumour necrosis factor-α; Tx, thromboxane; VCAM, vascular cell adhesion molecule; VLDL, very low-density lipoprotein.
Effects on triacylglycerol levels
Triacylglycerol-lowering properties are among the best-established in vivo actions of ω-3 fatty acids. LC n-3 PUFA decrease very low-density lipoprotein (VLDL) assembly and secretion, resulting in diminished triacylglycerol production, through a decreased sterol receptor element binding protein-1c activity (Jump and Clarke 1999; Davidson, 2006; Jump, 2008). Pharmacokinetic data obtained from obese male subjects with dyslipidaemia show that 4 g of fish oil per day decrease the production of VLDL apolipoprotein B, without a change in the pool size of low-density lipoprotein (LDL) apolipoprotein B, and also with no change in the fractional catabolic rate of VLDL apolipoprotein B (Chan et al., 2002). Although kinetic studies fail to show an effect of LC n-3 PUFA on VLDL clearance, VLDL particles enriched in LC n-3 PUFA are more susceptible to in vitro lipase-mediated conversion to LDL than controls (Lu et al., 1999; Park et al., 2004). Indeed, a rise in LDL seen upon fish oil supplementation results directly from the conversion of VLDL into LDL particles in obese male subjects (Chan et al., 2002). In addition, LC n-3 PUFA increase β-oxidation of other fatty acids in mitochondria and peroxisomes (Jump and Clarke 1999; Harris and Bulchandani, 2006; Jump, 2008), possibly through activation of peroxisome proliferator-activated receptor (PPAR)-α (Nakamura et al., 2004; Davidson, 2006). This occurs despite the evidence that LC n-3 PUFA are weak PPAR agonists (Krey et al., 1997; Jump, 2008).
Clinical trials clearly suggest beneficial effects of LC n-3 PUFA consumption on plasma triacylglycerol levels. A review of placebo-controlled human studies concludes that an average intake of 3–4 g·day−1 of LC n-3 PUFA decreases serum triacylglycerol concentrations by 25–30% in a dose-dependent manner. The same intake does not affect total cholesterol, but increases LDL concentrations by 5–10% and high-density lipoprotein (HDL) by 1–3% (Harris, 1997). The increase in LDL is mainly through a rise in amounts of the larger, more buoyant and potentially less atherogenic LDL particles, whereas the smaller, more dense and potentially more atherogenic LDL particles decrease (Calabresi et al., 2000; Stalenhoef et al., 2000; Durrington et al., 2001). Based on the triacylglycerol-lowering effects of LC n-3 PUFA, the American Food and Drug Administration has approved a prescription form of LC n-3 PUFA fatty acids. Lovaza™ (former Omacor) is prescribed as an adjunct to appropriate diet for the treatment of very high triacylglycerol levels (≥5.65 mmol·L−1 or ≥500 mg·dL−1) in adults. Each 1 g capsule of Lovaza™ contains approximately 465 mg EPA ethyl esters and 375 mg DHA ethyl esters. Clinical trials have shown that administration of 4 g·day−1 of Lovaza™ results in a decrease in triacylglycerol levels of 30–50% (Harris et al., 1997; Pownall et al., 1999; Stalenhoef et al., 2000; Bays, 2006). In addition, administration of Lovaza™ does not affect the efficacy of statins (McKenney et al., 2006). In patients with combined hyperlipidaemia, co-administration of Lovaza™ with statins was a safe and effective means of lowering serum triacylglycerol, despite the persistent high triacylglycerol levels when the patients received statins alone (Durrington et al., 2001; Davidson et al., 2007). Currently, also many types of non-prescription dietary supplements of LC n-3 PUFA are available; however, their efficacy, quality and safety are uncertain as they are not regulated by the same standards as pharmaceutical agents.
As the dose of LC n-3 PUFA required to achieve a lowering in triacylglycerol concentrations is higher than the doses required for a decrease in mortality from CHD, it is questionable whether the triacylglycerol-lowering effects of LC n-3 PUFA could affect CHD mortality. Thus, the major mechanisms underlying the beneficial effects of LC n-3 PUFA appear to be different, or in addition to, the effects on lowering plasma triacylglycerol concentrations (Deckelbaum et al., 2008). Indeed, the GISSI and JELIS trial discussed above showed only minor changes in plasma triacylglycerol levels (GISSI Investigators, 1999; Yokoyama et al., 2007).
Effects on atherosclerosis
Dietary intake of LC n-3 PUFA may have anti-atherosclerotic potential as supplementation with fish oil concentrate (6 g·day−1 for 3 months and then 3 g·day−1 for 21 months) in 223 patients with angiographically proven coronary artery disease modestly mitigated the course of coronary atherosclerosis (von Schacky et al., 1999). However, 12 randomized trials on the effect of fish oil on restenosis of carotid arteries after percutaneous transluminal coronary angioplasty or coronary artery bypass graft showed equivocal effects (Balk et al., 2006). Additionally, the only randomized intervention study on the effects of fish oil versus placebo on intima media thickness and the media of the carotid artery indicated worsening rather than improvement upon fish oil supplementation (Angerer et al., 2002). In animals, effects of physiological doses of LC n-3 PUFA on atherosclerosis development are again not convincing. One study assessing the effects of a low-fat high-cholesterol diet containing 1% (w·w−1) fish oil for 14 weeks in male apolipoprotein E knockout (apoE−/−) mice did not find an effect on atherogenesis, but this study lacked an appropriate control group (Xu et al., 2007). In a second study, 1% (w·w−1) dietary fish oil, compared with 1% (w·w−1) corn oil or an un-supplemented diet for 20 weeks, did not affect the size of atherosclerotic plaque in female apoE−/− mice, nor did it affect plasma lipids (Zampolli et al., 2006). However, the background diet in this study was a low-fat diet without added cholesterol, whereas a high-fat high-cholesterol diet may be required to exacerbate lesions and reveal any inhibitory effects of dietary compounds on atherogenesis. This is especially the case in the low-density lipoprotein receptor knockout (LDLR−/−) mouse model of diet-dependent diseases, characterized by high plasma cholesterol levels when fed a high-fat high-cholesterol diet (Breslow, 1996). Indeed, a high-fat high-cholesterol diet supplemented with low doses of fish oil (1% w·w−1) did inhibit atherosclerosis development in a study with LDLR−/− mice after 20 weeks of intervention (Zampolli et al., 2006). Much higher doses of dietary fish oil are causing a more consistent decrease in atherosclerosis development in both animal models. Wang et al. (Wang et al., 2004) found that a high-fat diet containing 20% (w·w−1) dietary fish oil, but no added cholesterol, after 10 weeks of intervention decreased atherosclerotic plaque size development in male apoE−/− mice compared with a high-fat diet containing 20% corn oil. Similarly, Casos et al. (2008) found that a low-fat high-cholesterol diet containing 5% (w·w−1) fish oil, compared with a diet containing 5% (w·w−1) corn oil, decreased atherosclerotic plaque development after 8, 12 and 20 weeks of intervention in male apoE−/− mice. In female LDLR−/− mice, a high-fat high-cholesterol diet containing 5% (w·w−1) menhaden oil, compared with 5% (w·w−1) olive oil, also decreased atherosclerotic lesion area (Saraswathi et al., 2007). Furthermore, a high-fat high-cholesterol diet containing 5% (w·w−1) EPA ethyl ester, compared with no EPA, for 12 weeks decreased plaque size in both male apoE−/− mice and LDLR−/− mice (Matsumoto et al., 2008). However, such high doses of fish oil or EPA intake would not be feasible, or indeed advisable, in humans. It should be noted that both models of murine atherosclerosis are far more aggressive than human disease, as disease develops in a few months rather than over a few decades. Thus any ability of dietary component to ameliorate disease would indicate an impressive anti-atherogenic capacity.
Effects on arrhythmias
Long-chain n-3 PUFA could decrease sudden death (Siscovick et al., 1995; GISSI Investigators, 1999; Albert et al., 1998; 2002;) through prohibiting cardiac arrhythmia. Animal models show that n-3 PUFA can reduce susceptibility to arrhythmia (McLennan et al., 1988; Billman et al., 1994; Leaf and Kang, 1996; Billman et al., 1999). Studies in dogs with ligated coronary arteries showed that intravenous infusion of n-3 PUFA prevented ventricular arrhythmia after an exercise programme (Billman et al., 1997; Billman et al., 1999). Dietary intake of fish oil also prevented ventricular fibrillation after electrophysiological stimulation in rats and marmoset monkeys (McLennan et al., 1988; 1993; McLennan, 1993). Furthermore, in cultured cardiomyocytes n-3 PUFA changed the conductance of ion channels in the membrane and thereby prevent occurrence of arrhythmia (Leaf et al., 1999). Thus, evidence from both in vivo and in vitro studies suggests that LC n-3 PUFA may decrease arrhythmias (Brouwer et al., 2006a; von Schacky, 2008). The majority of acute sudden deaths are caused by ventricular tachyarrhythmia (Huikuri et al., 2001), and three trials have used patients with implantable cardioverter defibrillators to test whether LC n-3 PUFA from fish oil can prevent ventricular tachycardia and ventricular fibrillation. These trials, however, did not show a strong protective effect of fish oil on life-threatening ventricular arrhythmia (Leaf et al., 2005; Raitt et al., 2005; Brouwer et al., 2006c). Similar to the lack of effects of LC n-3 PUFA on ventricular arrhythmias, three cross-sectional studies (Mozaffarian et al., 2004; Frost and Vestergaard, 2005; Brouwer et al., 2006b) did also not detect a consistent association between intake of fish fatty acids and spontaneous atrial fibrillation, the most common form of sustained arrhythmias. However, one experimental study (Calo et al., 2005) suggests that fish oil could prevent post-operative atrial fibrillation.
Increased heart rate is another independent risk factor for sudden death (Jouven et al., 2001), and LC n-3 PUFA from fish decrease heart rate (Geelen et al., 2005; Mozaffarian et al., 2005). In a cohort of 5073 men and women consumption of fish rich in LC n-3 PUFA was not only associated with lower heart rate, but also with lower systemic vascular resistance and a greater stroke volume. The reduction in heart rate may have been the result of better ventricular efficiency (Mozaffarian et al., 2006). Nevertheless the underlying mechanisms of the effects of LC n-3 PUFA on heart rate and sudden death are largely unknown. Animal and cell studies, as well as human studies, suggest that the underlying disease might determine whether or not fish oil can protect against fatal heart disease (Verkerk et al., 2006; Coronel et al., 2007; den Ruijter et al., 2007). Fish oil may be harmful in patients vulnerable to life-threatening arrhythmia based on re-entry, whereas it is probably protective in patients with a prior myocardial infarction. In patients with prior myocardial infarction and heart failure, arrhythmias may have been based on triggered activity and prolonged action potentials (den Ruijter et al., 2007; 2008;). These are the type of arrhythmias that are likely to be prevented by fish oil (Baartscheer et al., 2003; den Ruijter et al., 2007).
Effects on platelet function
Long-chain n-3 PUFA may decrease the risk of atherothrombosis by affecting platelet aggregation and haemostasis. The anti-thrombotic properties of EPA and DHA have been attributed to the incorporation into platelet phospholipids at the expense of the n-6 PUFA, such as AA (Smith, 2005). An important set of pathways clearly influenced by changes in the n-3/n-6 ratio are those for synthesis of eicosanoids. These include the cyclooxygenase (COX), lipoxygenase (LOX) and P450 epoxygenase pathways (Smith, 2005), for which EPA and DHA compete with AA as a substrate, inhibiting the production of the pro-aggregatory thromboxane A2 (TxA2) originating from AA (Figure 2). Indeed, the production of TxA2 from platelets stimulated by a variety of agonists decreased by between 60% and 80% after fatty acid supplementation both in vitro and in vivo (Siess et al., 1980; Goodnight, 1986; Kristensen et al., 1989; Weber, 1989; Christensen et al., 1997). Different biological effects of EPA and DHA are supported by experimental evidence. For example, incorporation of DHA into human platelets produced greater inhibition of platelet aggregation than either EPA alone or a combination of EPA and DHA (Croset and Lagarde, 1986). DHA was also more potent than EPA in inhibiting platelet TxA2 synthesis (Kramer et al., 1996). In addition DHA, and to some extent EPA, act as antagonists of the TxA2/prostaglandin H2 receptor in human platelets, thereby blocking the activation of platelets through the AA pathway (Swann et al., 1989).
Figure 2.
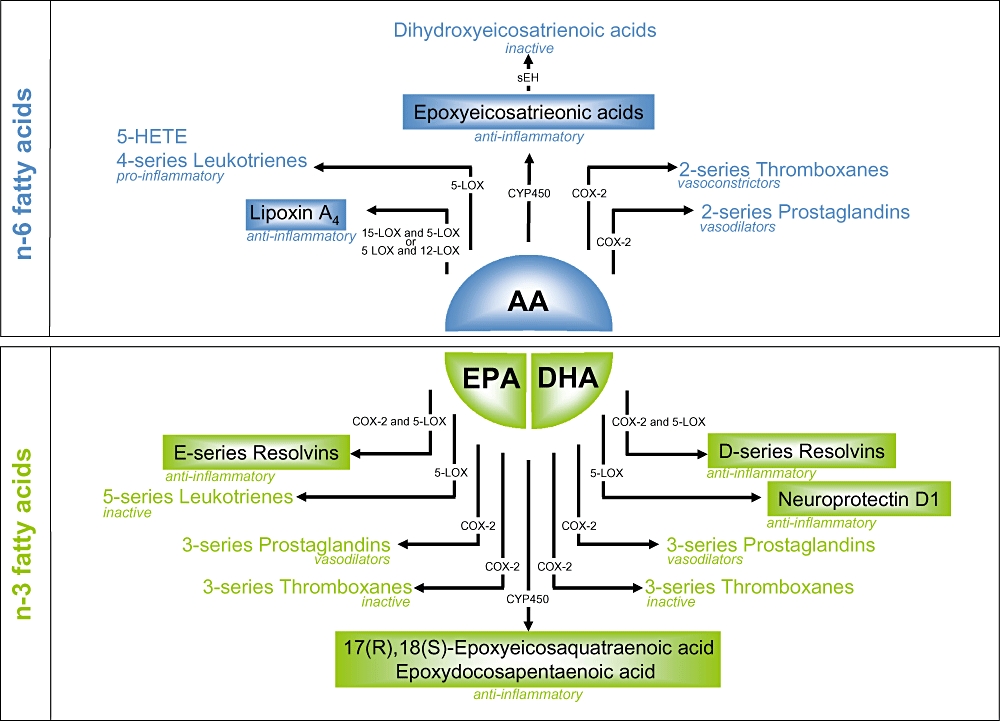
Pathways in eicosanoid metabolism leading to the generation of pro- and anti-inflammatory, or inactive eicosanoids, from arachidonic acid (an n-6 fatty acid – in blue) and eicosapentaenoic acid and docosahexaenoic acid (n-3 fatty acids – in green). 5-HETE, 5-hydroxyeicosatetraenoic acid; AA, arachidonic acid; COX, cyclooxygenase; DHA, docosahexaenoic acid; EPA, eicosapentaenoic acid; LOX, lipoxygenase; sEH, soluble epoxide hydrolase.
The effects of LC n-3 PUFA on haemostatic function and thrombogenesis in vivo are however unclear. Results of measurements of platelet aggregation after dietary LC n-3 PUFA intervention have yielded inconsistent findings, partly because of differences in study design, the source and quantity of these fatty acids given, and methodology (Mori et al., 1997; Hornstra, 2001). Relatively high doses of LC n-3 PUFA inhibited both platelet aggregation and platelet thromboxane release in response to collagen and adenosine diphosphate (ADP) (Hirai et al., 1980; Brox et al., 1983; Kristensen et al., 1989), and inhibited platelet aggregation by thrombin (Ahmed and Holub, 1984), and by adrenalin (Kristensen et al., 1987), or platelet-activating factor (Codde et al., 1987). In order to achieve significant reductions in platelet aggregation, doses of 3 g·day−1 appear to be necessary. At such high doses, lengthening of the bleeding time is also seen (Hornstra, 2001).
An emerging method to assess platelet function is to measure the change in markers of activation of the pro-coagulant processes after intervention. Large-scale studies are now increasingly turning to the technique to provide platelet-specific, consistent data, which are less susceptible to handling conditions than platelet aggregation techniques. The most widely used and validated markers are CD-62P (P-selectin) and the active conformation of the glycoprotein IIb/IIIa complex. In addition, future studies should not only examine ‘functional indices’ of platelet behaviour such as platelet aggregation, but also measures which are capable of showing the underlying changes induced in platelets after supplementation. For example, 3 g·day−1 of LC n-3 PUFA decreased the expression of platelet surface proteins important in supporting platelet–platelet and platelet–leukocyte interactions and tethering of the platelet to blood vessel walls (Vanschoonbeek et al., 2004). In addition, our group recently found that consumption of a moderate amount of fish oil (1 g·day−1) caused significant changes in levels of 65 platelet proteins (related to platelet structure, inflammation and thrombosis) in healthy volunteers after 3 weeks of intervention, without affecting platelet aggregation (B. de Roos, unpubl. results), indicating that changes in platelet function do indeed occur at lower dosage levels.
LC n-3 PUFA and inflammation: novel mechanisms in vitro and in vivo
The mechanistic connection between LC n-3 PUFA and inflammation has attracted great interest. This is mainly due to the multiple involvement of inflammatory processes, especially at the site of the vascular endothelium, in the development and progression of atherosclerosis (Ross, 1993; 1999;). Inflammation is now recognized as a prominent process in the development of atherosclerosis and CHD. Instigation of inflammation may well provide the link between hyperlipidaemia and atherogenesis (Libby, 2002). LC n-3 PUFA are believed to affect inflammatory processes mainly through two mechanistic pathways: endothelial activation and changes in eicosanoid production, or a combination of the two.
Endothelial activation
A number of inflammatory compounds are involved in vascular activation and atherogenesis. Various exogenous triggers for example, bacterial endotoxin (or lipopolysaccharide), can directly activate monocytes and macrophages. Activation results in the production and secretion of cytokines such as interleukin (IL)-1β and tumour necrosis factor-α (TNF-α) and other inflammatory mediators. This also results in the induction of adhesion molecule expression on the surface of endothelial cells and leukocytes. Adhesion molecules, such as vascular cell adhesion molecule-1 (VCAM-1), are involved in monocyte binding to the vascular endothelium (Libby, 2002). VCAM-1 is expressed by endothelial cells in response to for example cholesterol feeding, especially in areas of the vasculature prone to lesion formation (Cybulsky and Gimbrone, 1991). In addition to VCAM-1, P-selectin and E-selectin also contribute to leukocyte recruitment in atherosclerosis-susceptible mice (Dong et al., 1998). Transcriptional activation of VCAM-1 is, in part, regulated by nuclear factor-κB (NF-κB), upon exposure to oxidized lipoproteins or to the pro-inflammatory cytokines in endothelial cells (Collins and Cybulsky, 2001). The action of inflammatory cytokines, which initiate a cascade of inflammatory mediators, thus amplifies the initial inflammatory signal (Calder, 2006).
In vitro studies have shown that LC n-3 PUFA, and especially DHA, beneficially affect endothelial activation by decreasing monocyte and leukocyte rolling and adhesion by inhibiting expression of intercellular adhesion molecule (ICAM)-1, VCAM-1, IL-6, IL-8 and E-selectin. These functions are partially attributed to the inhibitory effects of DHA and EPA on the transcription factor NF-κB (De Caterina et al., 1994; 1998; 2000; Weber et al., 1995; Khalfoun et al., 1996). In humans, however, the effects of LC n–3 fatty acid consumption on biomarkers of inflammation are not convincing. A majority of intervention studies found no significant effect of fish oil supplementation or fish consumption on levels of plasma endothelial activation markers such as soluble adhesion molecules (s-ICAM, s-VCAM) and soluble P-selectin, pro-inflammatory cytokines (IL-1β, IL-6, IL-10), or the classic inflammation marker C-reactive protein (hsCRP), in healthy or diseased subjects (Thies et al., 2001; Grundt et al., 2003; Mori et al., 2003; Geelen et al., 2004; Jellema et al., 2004; Vega-Lopez et al., 2004; Seierstad et al., 2005; Fujioka et al., 2006; Lee et al., 2006; Browning et al., 2007; Kabir et al., 2007; Schiano et al., 2008; Yusof et al., 2008). A few studies report lowered plasma levels of s-VCAM-1 and IL-6 (Seierstad et al., 2005), TNF-α and IL-β1 (Accini et al., 2006), or VCAM-1 and E-selectin (Thies et al., 2001) upon consumption of LC n-3 PUFA. One study reports that ω-3 fatty acid supplementation in elderly men at risk of CHD increased VCAM (Berstad et al., 2003). The same group also reported that the same amount of LC n-3 PUFA in elderly men with hyperlipidaemia decreased levels of plasma s-ICAM (Hjerkinn et al., 2005). In addition, supplementation with LC n-3 PUFA decreased the production of TNF-α, IL-1 or IL-6 by activated mononuclear cells in some studies (Endres et al., 1989; Meydani et al., 1991; Abbate et al., 1996; Caughey et al., 1996; Kelley et al., 1999; Mori et al., 2003; Trebble et al., 2003), but other work using a wide range of supplementation doses failed to show such anti-inflammatory effects (Molvig et al., 1991; Cooper et al., 1993; Cannon et al., 1995; Schmidt et al., 1996; Blok et al., 1997; Yaqoob et al., 2000; Thies et al., 2001; Kew et al., 2003; Wallace et al., 2003; Kew et al., 2004; Miles et al., 2004). The discrepancy between the in vivo effects of LC n-3 PUFA are not clear, but may involve technical factors like treatment, dose, study design and choice of research subjects (Calder, 2001; de Roos et al., 2005b). Supplementation dose is, however, not related consistently to differential outcomes (Calder, 2006).
Eicosanoid metabolism
Eicosanoid metabolism is likely to play a key role in the anti-inflammatory effects of LC n-3 PUFA. Eicosanoids include prostaglandins, thromboxanes and leukotrienes that are key mediators of inflammatory responses (Figure 2). AA is the substrate for production of series 2-prostaglandins and thromboxanes and series 4-leukotrienes. When AA is substituted by LC n-3 PUFA – mainly EPA, the resulting eicosanoids are series 3-prostaglandins and thromboxanes and series 5-leukotrienes (Capdevila et al., 1981). EPA actually can, in contrast to DHA, function as a substrate for COX-1 and 5-LOX (Needleman et al., 1979; Lee et al., 1984; 1985;). Due to the smaller size of the substrate binding site of COX-1 compared with COX-2, the 22-carbon DHA can however be metabolized by COX-2 (Serhan et al., 2002; Massaro et al., 2006). The products of n-3 PUFA may be beneficial as they do not induce the same level of inflammation as those derived from AA. In humans, supplementation with fish oil has resulted in decreased production of prostaglandin E2 (PGE2), thromboxane B2, leukotriene B4, 5-hydroxyeicosatetraenoic acid and leukotriene E4 by inflammatory cells ex vivo (Lee et al., 1985; Endres et al., 1989; Meydani et al., 1991; von Schacky et al., 1993; Sperling et al., 1993; Caughey et al., 1996; Kelley et al., 1999; Trebble et al., 2003). In vitro, DHA decreased COX-2 activity and expression in human endothelial cells (Massaro et al., 2006) at concentrations of free fatty acids compatible with those found in healthy volunteers before and after intervention with fish oil (1.5–20 µmol·L−1) (Sublette et al., 2007; den Ruijter et al., 2008). The importance of this finding lies in the implication of COX-2 activation in inflammation (Pairet and Engelhardt, 1996), atherosclerotic plaque growth (Burleigh et al., 2002) and stability (Thies et al., 2003). The decreased COX-2 activity and expression was regulated through a decreased IL-1α-mediated NF-κB activation and an inhibition of p65 NF-κB subunit nuclear translocation (Massaro et al., 2006). However, the beneficial effects of LC n-3 fatty acids may not exclusively be mediated through eicosanoid production. For example, DHA exerts greater anti-inflammatory effects than EPA in the vascular endothelium. Being the direct precursor of eicosanoids EPA would be expected to have greater effects than DHA if eicosanoids played an important role. Also, these anti-inflammatory effects of fish oils are not altered by a COX blocker, eliminating an exclusive role of for example prostaglandins (De Caterina et al., 2000).
Long-chain n-3 PUFA could influence eicosanoid metabolism also via a different pathway. Preliminary data from our laboratory indicate that dietary fish oil directly affects hepatic levels of the enzyme soluble epoxide hydrolase (sEH), which regulates availability and metabolism of the cardioprotective and anti-inflammatory epoxyeicosatrienoic acids (EET) (de Roos et al., 2005a; Spector and Norris, 2007). EET are synthesized by the oxygenation of AA by cytochrome P450 NADPH-dependent epoxygenases. There are four regio-isomers of EET: 5,6-, 8,9-, 11,12- and 14,15-EET. EET inhibit expression of VCAM-1, ICAM-1 and E-selectin through an NF-κB-related mechanism (Node et al., 1999). sEH is responsible for the hydrolysis of the bioactive EETs to the less biologically active dihydroxyeicosatrienoic acids DHET (Spector et al., 2004). sEH is a therapeutic target for acute inflammation, thus the development of potent and stable inhibitors of sEH has attracted significant interest in recent years. The conversion of epoxides to their corresponding diols is blocked by sEH inhibitors by which the cardioprotective effects of EETs are better maintained (Yu et al., 2000; Imig et al., 2002; Zhao et al., 2004). A number of studies have provided evidence for the potential anti-inflammatory properties of sEH inhibitors. For example, the sEH inhibitor 12-(3-adamantan-1-yl-ureido)-dodecanoic acid (AUDA) decreased plasma levels of pro-inflammatory cytokines and nitric oxide in mice after endotoxin exposure. At the same time inflammatory resolution was enhanced through increased formation of lipotoxins (Schmelzer et al., 2005). After treatment of endothelial cells with AUDA, EET increased PPAR-γ transcription activity, which suppresses NF-κB-mediated inflammatory responses (Liu et al., 2005). Co-administration of AUDA and anti-inflammatory drugs decreased levels of the pro-inflammatory prostaglandin D2 and PGE2 to a greater extent than the sum of individual treatments. Furthermore, AUDA alone is more effective than anti-inflammatory drugs in decreasing levels of PGE2 in mice (Schmelzer et al., 2006). Therefore, regulation of sEH by fish oil could affect EET levels, thereby affecting inflammatory responses and eventually development of atherosclerosis. Such effects may be mediated through EPA, DHA or both. We recently found dietary fish oil (2% w·w−1), but not dietary DHA alone (2% w·w−1) decreased hepatic sEH protein levels over 10 weeks in apoE−/− mice, suggesting decreased degradation of anti-inflammatory EET (Y. Mavrommatis et al., unpubl. data).
In addition to a likely direct effect on sEH protein levels, dietary fish oil may also indirectly affect the epoxygenation pathway through substitution of AA in the phospholipids membranes. Epoxy-EPA and epoxy-DHA derivatives have bioactive properties, where the main EPA epoxide is 17(R),18(S)-epoxyeicosaquatraenoic acid (17,18-EEQ) and the main DHA epoxide is epoxydocosapentaenoic acids. Both 17,18-EEQ and epoxydocosapentaenoic acids induce vasodilation and decrease blood pressure with greater potency than EET (Lauterbach et al., 2002; Lu et al., 2002; Ye et al., 2002), and they may be responsible for some of the functional effects that n-3 fatty acids possess, either directly or by competing for CYP epoxygenases and decreasing availability of EET (Spector and Norris, 2007). Despite the important roles of EET analogs from EPA and DHA on blood pressure, their role in inflammation remains to be elucidated.
Another novel approach to the protective effects of LC n-3 PUFA against inflammatory pathways is based on the process of inflammatory resolution. At the site of inflammation, peripheral blood neutrophils initially release pro-inflammatory mediators, such as prostaglandins and leukotrienes, which activate and amply inflammation. But with time, sometimes within hours, PGE2 and prostaglandin D2 gradually cause a switch to resolution of inflammation through removal of leukocytes and cellular debris from an inflamed site, and production of the anti-inflammatory and pro-resolution lipoxins, resolvins and protectins (Serhan et al., 2008). This allows re-establishment of homeostasis and prevents inflammation from spreading, becoming chronic and resulting in disease. The potent anti-inflammatory and pro-resolving lipoxins and aspirin-triggered lipoxins are derived from the n-6 fatty acid AA, emphasizing that n-6 fatty acids are not just a precursor of pro-inflammatory eicosanoids (Serhan and Chiang, 2008). Indeed, endogenous aspirin-triggered lipoxins may offer new and potentially important mechanisms that underlie the clinical benefits of aspirin (Serhan et al., 2008). Resolvins are derived from EPA (E-series) and DHA (D-series) and are biologically active in low concentrations in vitro and in vivo (Serhan et al., 2000; 2002; Hong et al., 2003; Marcheselli et al., 2003). Indeed, significant amounts of resolvin E1, resolvin D3 occurred in colonic tissue, in addition to a reduction in inflammation and tissue injury in colitis, in fat-1 mice that express the humanized fat-1 gene from Caenorhabditis elegans. This gene allows them to endogenously produce n-3 fatty acids from the n-6 type (Hudert et al., 2006). This contrasts with the biological activity of the oxygenated products from LC n-3 PUFA, like thromboxanes and prostaglandins, which are either devoid of bioactivity or far less potent than their AA counterparts (Figure 2). Resolvin E1 protects against excessive pro-inflammatory gene expression in humans and blocks transendothelial migration of polymorphonuclear leukocytes, an integral step of atherogenesis (Serhan et al., 2000; Arita et al., 2005). Additionally, resolving E1 decreases leukocyte rolling by approximately 40% and inhibits platelet aggregation induced by ADP or thromboxane receptor agonist U46619. These anti-aggregatory effects were not present in collagen stimulated platelets, or when stimulated with DHA lipid mediators, suggesting a fatty acid- and agonist-specific anti-platelet action (Dona et al., 2008). Resolvin E2 also has potent anti-inflammatory action, mainly through decreased neutrophil infiltration (Tjonahen et al., 2006). Similarly to series E resolvins, D-series resolvins originating from DHA exert important anti-inflammatory action mainly by blocking neutrophil infiltration (Serhan et al., 2002). DHA also serves as substrate for the synthesis of protectins. Although there are a number of protectin isomers derived from DHA, protectin D1 is the most potent isomer and it is biosynthesized via a LOX-mediated pathway (Hong et al., 2003). Its principal activity is to decrease transmigration of neutrophils and synthetic protectin D1 decreases polymorphonuclear leukocytes infiltration in vivo (Hong et al., 2003), and at 10 nmol·L−1 it decreases human neutrophil transmigration by approximately 50% in vitro (Serhan et al., 2002; 2006;). Aspirin impinges on the endogenous production of lipoxins and resolvins. Its well-known ability to acetylate COX-2 and thereby inhibit the production of pro-inflammatory prostaglandins does not affect the generation of monohydroxy fatty acid species. Aspirin therefore can trigger the production of aspirin triggered lipoxins, as well as the production of the 18R-hydroxyeicosapentenoic acid precursor of resolvin E1. Because resolvin E1 is rapidly inactivated to 18-oxo-resolvin E1, synthetic resolvin analogues have already been synthesized and licensed for clinical development, and these pro-resolving agonists of resolution may be a useful approach to treat many diseases characterized by uncontrolled inflammation (Serhan and Chiang, 2008). The assessment of the impact of resolvin E1 production on human health so far is hampered by the difficulty of measuring the compound in human plasma, although one paper states to have detected resolvin E1 in plasma from six donors plasma of individuals taking aspirin and EPA (Arita et al., 2005). In addition, the generation of these agents is aspirin-dependent and is therefore unlikely to be physiologically relevant for dietary treatment only.
Conclusion
A large amount of new evidence from in vitro studies indicates that LC n-3 PUFA significantly affect mechanisms relating to inflammatory processes, such as endothelial activation, modification of eicosanoid metabolism – including epoxygenation pathways, and inflammatory resolution. Because inflammatory processes play such an important role in the development and progression of atherosclerosis, and thus myocardial infarction, it is not surprising to find that fish oil supplementation caused significant protective effects on mortality, non-fatal myocardial infarction and stroke, as well as cardiovascular and sudden death, in the GISSI-Prevenzione trial (GISSI Investigators, 1999). It may also explain why in the GISSI heart failure study the beneficial effects on mortality were much less pronounced (Gissi-Hf, 2008). Anti-inflammatory dietary compounds like fish oil may simply not be effective to any further extent when more permanent damage has been inflicted, like in heart failure patients.
However, it has so far been very difficult to provide evidence for the anti-inflammatory effects LC n-3 PUFA in humans in vivo. LC n-3 PUFA do not appear to affect the most extensively studied clinical marker of inflammation, CRP. There is, however, considerable debate regarding the usefulness of CRP as a risk biomarker of CHD and its potential causal role in atherogenesis. Various epidemiological studies have revealed a consistent, robust and significant association between increased serum or plasma CRP levels and the risk of future cardiovascular events (Scirica and Morrow, 2006). Nevertheless, the additional discriminative ability of elevated CRP beyond traditional risk predictors has been minimal (Danesh et al., 2004), partly because it correlates well with known risk factors such as smoking, low levels of HDL cholesterol and obesity (Lowe, 2005; Miller et al., 2005). Furthermore, CRP, an acute-phase reactant produced by the liver upon stimulation by IL-6, is a non-specific indicator of inflammation and thus may not directly participate in atherogenesis (Scirica and Morrow, 2006). Multiple in vitro and in vivo animal studies have identified several potential pro-inflammatory mechanisms by which CRP may promote atherosclerosis (Scirica and Morrow, 2006). However, in most of the initial studies, the recombinant CRP that was used was contaminated with bacterial lipopolysaccharide and the preservative azide, which are potent pro-inflammatory compounds themselves. Studies that used either local preparations of recombinant CRP or specific techniques to purify commercial CRP have not produced similar inflammatory reactions (Scirica and Morrow, 2006).
As well as CRP, LC n-3 PUFA also do not appear to affect plasma levels of soluble markers of endothelial activation and cytokines. On the other hand, such systemic markers may simply not enable the detection of a local inflammatory response, either in healthy or diseased subjects. Therefore, we may need to develop more specific and sensitive biomarkers to reveal similar or alternative pathways that are affected by dietary fatty acids. For that we will need to take into account that effects instigated by dietary intervention often produce relatively subtle effects (de Roos et al., 2008a). In one of our recent dietary intervention trials we assessed the effects of daily fish oil supplements on the serum proteome, using 2D, matrix-assisted laser desorption/ionization-mass spectrometry (MALDI-MS) and liquid chromatography-tandem mass spectrometry (LC-MS/MS). Serum levels of apolipoprotein A1, apolipoprotein L1, zinc-α-2-glycoprotein, haptoglobin precursor, α-1-anti-trypsin precursor, anti-thrombin III-like protein, serum amyloid P component and haemopexin were all down-regulated by fish oil supplementation. In addition, the decrease in serum apolipoprotein A1 was associated with a significant shift towards the larger, more cholesterol-rich, HDL2 particles. The alterations in serum proteins and HDL size imply that fish oil activates anti-inflammatory and lipid-modulating mechanisms believed to impede the early onset of CHD. These proteins are potential diagnostic markers to assess the mechanisms whereby fish oils protect against CHD in humans (de Roos et al., 2008b). Thus, determining changes in the plasma proteome upon dietary intervention offers the opportunity to systematically search for proteins that might be biomarkers of chronic diseases, and which may be altered by such treatments. Similarly, the lipidome could provide valuable insights into how fish oil consumption affects levels of bioactive lipid mediators that either are pro-inflammatory, or help to establish resolution of inflammation.
Acknowledgments
Work in BDR's laboratory is funded by the Scottish Government Rural and Environment Research and Analysis Directorate (RERAD).
Glossary
Abbreviations:
- 5-HETE
5-hydroxyeicosatetraenoic acid
- 17,18-EEQ
17(R),18(S)-epoxyeicosaquatraenoic acid
- AA
arachidonic acid
- ADP
adenosine diphosphate
- apoE−/−
apolipoprotein E knockout
- AUDA
12-(3-adamantan-1-yl-ureido)-dodecanoic acid
- CD-62P
P-selectin
- CHD
coronary heart disease
- COX
cyclooxygenase
- DART
diet and reinfarction trial
- DHA
docosahexaenoic acid
- DHET
dihydroxyeicosatrienoic acids
- EET
epoxyeicosatrienoic acids
- EPA
eicosapentaenoic acid
- HDL
high-density lipoprotein
- hsCRP
(high-sensitivity) C-reactive protein
- ICAM-1
intercellular adhesion molecule 1
- IL
interleukin
- LC-MS/MS
liquid chromatography-tandem mass spectrometry
- LC n-3 PUFA
long-chain n-3 polyunsaturated fatty acids
- LDL
low-density lipoprotein
- LDLR−/−
low-density lipoprotein receptor knockout
- LOX
lipoxygenase
- LTB4
leukotriene B4
- MALDI-MS
matrix-assisted laser desorption/ionization-mass spectrometry
- NF-κB
nuclear factor-κB
- PGH2
prostaglandin H2
- PPAR
peroxisome proliferator-activated receptor
- sEH
soluble epoxide hydrolase
- SREBP-1c
sterol receptor element binding protein-1c
- TNF-α
tumour necrosis factor-α
- TxA2
thromboxane A2
- VCAM-1
vascular cell adhesion molecule-1
- VLDL
very low-density lipoprotein
Conflict of interest
None.
References
- Abbate R, Gori AM, Martini F, Brunelli T, Filippini M, Francalanci I, et al. n-3 PUFA supplementation, monocyte PCA expression and interleukin-6 production. Prostaglandins Leukot Essent Fatty Acids. 1996;54:439–444. doi: 10.1016/s0952-3278(96)90028-9. [DOI] [PubMed] [Google Scholar]
- Accini R, Rosina M, Barmonti F, Della Noce C, Tonini A, Bernacchi F, et al. Effects of combined dietary supplementation on oxidative and inflammatory status in dyslipidemic subjects. Nutr Metab Cardiovasc Dis. 2006;16:121–127. doi: 10.1016/j.numecd.2005.05.006. [DOI] [PubMed] [Google Scholar]
- Ahmed AA, Holub BJ. Alteration and recovery of bleeding times, platelet aggregation and fatty acid composition of individual phospholipids in platelets of human subjects receiving a supplement of cod-liver oil. Lipids. 1984;19:617–624. doi: 10.1007/BF02534720. [DOI] [PubMed] [Google Scholar]
- Albert CM, Hennekens CH, O'Donnell CJ, Ajani UA, Carey VJ, Willett WC, et al. Fish consumption and risk of sudden cardiac death. JAMA. 1998;279:23–28. doi: 10.1001/jama.279.1.23. [DOI] [PubMed] [Google Scholar]
- Albert CM, Campos H, Stampfer MJ, Ridker PM, Manson JE, Willett WC, et al. Blood levels of long-chain n-3 fatty acids and the risk of sudden death. N Engl J Med. 2002;346:1113–1118. doi: 10.1056/NEJMoa012918. [DOI] [PubMed] [Google Scholar]
- Angerer P, Kothny W, Stork S, von Schacky C. Effect of dietary supplementation with omega-3 fatty acids on progression of atherosclerosis in carotid arteries. Cardiovasc Res. 2002;54:183–190. doi: 10.1016/s0008-6363(02)00229-8. [DOI] [PubMed] [Google Scholar]
- Arita M, Bianchini F, Aliberti J, Sher A, Chiang N, Hong S, et al. Stereochemical assignment, antiinflammatory properties, and receptor for the omega-3 lipid mediator resolvin E1. J Exp Med. 2005;201:713–722. doi: 10.1084/jem.20042031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ascherio A, Rimm EB, Stampfer MJ, Giovannucci EL, Willett WC. Dietary intake of marine n-3 fatty acids, fish intake, and the risk of coronary disease among men. N Engl J Med. 1995;332:977–982. doi: 10.1056/NEJM199504133321501. [DOI] [PubMed] [Google Scholar]
- Baartscheer A, Schumacher CA, Belterman CN, Coronel R, Fiolet JW. SR calcium handling and calcium after-transients in a rabbit model of heart failure. Cardiovasc Res. 2003;58:99–108. doi: 10.1016/s0008-6363(02)00854-4. [DOI] [PubMed] [Google Scholar]
- Balk EM, Lichtenstein AH, Chung M, Kupelnick B, Chew P, Lau J. Effects of omega-3 fatty acids on coronary restenosis, intima-media thickness, and exercise tolerance: a systematic review. Atherosclerosis. 2006;184:237–246. doi: 10.1016/j.atherosclerosis.2005.06.042. [DOI] [PubMed] [Google Scholar]
- Bang HO, Dyerberg J. The bleeding tendency in Greenland Eskimos. Dan Med Bull. 1980;27:202–205. [PubMed] [Google Scholar]
- Bang HO, Dyerberg J, Nielsen AB. Plasma lipid and lipoprotein pattern in Greenlandic West-coast Eskimos. Lancet. 1971;1:1143–1145. doi: 10.1016/s0140-6736(71)91658-8. [DOI] [PubMed] [Google Scholar]
- Bang HO, Dyerberg J, Hjoorne N. The composition of food consumed by Greenland Eskimos. Acta Med Scand. 1976;200:69–73. doi: 10.1111/j.0954-6820.1976.tb08198.x. [DOI] [PubMed] [Google Scholar]
- Bays H. Clinical overview of Omacor: a concentrated formulation of omega-3 polyunsaturated fatty acids. Am J Cardiol. 2006;98:71i–76i. doi: 10.1016/j.amjcard.2005.12.029. [DOI] [PubMed] [Google Scholar]
- Berstad P, Seljeflot I, Veierod MB, Hjerkinn EM, Arnesen H, Pedersen JI. Supplementation with fish oil affects the association between very long-chain n-3 polyunsaturated fatty acids in serum non-esterified fatty acids and soluble vascular cell adhesion molecule-1. Clin Sci (Lond) 2003;105:13–20. doi: 10.1042/CS20020349. [DOI] [PubMed] [Google Scholar]
- Billman GE, Hallaq H, Leaf A. Prevention of ischemia-induced ventricular fibrillation by omega 3 fatty acids. Proc Natl Acad Sci USA. 1994;91:4427–4430. doi: 10.1073/pnas.91.10.4427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billman GE, Kang JX, Leaf A. Prevention of ischemia-induced cardiac sudden death by n-3 polyunsaturated fatty acids in dogs. Lipids. 1997;32:1161–1168. doi: 10.1007/s11745-997-0149-2. [DOI] [PubMed] [Google Scholar]
- Billman GE, Kang JX, Leaf A. Prevention of sudden cardiac death by dietary pure omega-3 polyunsaturated fatty acids in dogs. Circulation. 1999;99:2452–2457. doi: 10.1161/01.cir.99.18.2452. [DOI] [PubMed] [Google Scholar]
- Blok WL, Deslypere JP, Demacker PN, van der Ven–Jongekrÿg J, Hectors MP, van der Meer JW, et al. Pro- and anti-inflammatory cytokines in healthy volunteers fed various doses of fish oil for 1 year. Eur J Clin Invest. 1997;27:1003–1008. doi: 10.1046/j.1365-2362.1997.2240775.x. [DOI] [PubMed] [Google Scholar]
- Breslow JL. Mouse models of atherosclerosis. Science. 1996;272:685–688. doi: 10.1126/science.272.5262.685. [DOI] [PubMed] [Google Scholar]
- Brouwer IA, Geelen A, Katan MB. n-3 Fatty acids, cardiac arrhythmia and fatal coronary heart disease. Prog Lipid Res. 2006a;45:357–367. doi: 10.1016/j.plipres.2006.02.004. [DOI] [PubMed] [Google Scholar]
- Brouwer IA, Heeringa J, Geleijnse JM, Zock PL, Witteman JC. Intake of very long-chain n-3 fatty acids from fish and incidence of atrial fibrillation. The Rotterdam Study. Am Heart J. 2006b;151:857–862. doi: 10.1016/j.ahj.2005.07.029. [DOI] [PubMed] [Google Scholar]
- Brouwer IA, Zock PL, Camm AJ, Bocker D, Hauer RN, Wever EF, et al. Effect of fish oil on ventricular tachyarrhythmia and death in patients with implantable cardioverter defibrillators: the Study on Omega-3 Fatty Acids and Ventricular Arrhythmia (SOFA) randomized trial. JAMA. 2006c;295:2613–2619. doi: 10.1001/jama.295.22.2613. [DOI] [PubMed] [Google Scholar]
- Browning LM, Krebs JD, Moore CS, Mishra GD, O'Connell MA, Jebb SA. The impact of long chain n-3 polyunsaturated fatty acid supplementation on inflammation, insulin sensitivity and CVD risk in a group of overweight women with an inflammatory phenotype. Diabetes Obes Metab. 2007;9:70–80. doi: 10.1111/j.1463-1326.2006.00576.x. [DOI] [PubMed] [Google Scholar]
- Brox JH, Killie JE, Osterud B, Holme S, Nordoy A. Effects of cod liver oil on platelets and coagulation in familial hypercholesterolemia (type IIa) Acta Med Scand. 1983;213:137–144. doi: 10.1111/j.0954-6820.1983.tb03705.x. [DOI] [PubMed] [Google Scholar]
- Bucher HC, Hengstler P, Schindler C, Meier G. N-3 polyunsaturated fatty acids in coronary heart disease: a meta-analysis of randomized controlled trials. Am J Med. 2002;112:298–304. doi: 10.1016/s0002-9343(01)01114-7. [DOI] [PubMed] [Google Scholar]
- Burleigh ME, Babaev VR, Oates JA, Harris RC, Gautam S, Riendeau D, et al. Cyclooxygenase-2 promotes early atherosclerotic lesion formation in LDL receptor-deficient mice. Circulation. 2002;105:1816–1823. doi: 10.1161/01.cir.0000014927.74465.7f. [DOI] [PubMed] [Google Scholar]
- Burr ML, Fehily AM, Gilbert JF, Rogers S, Holliday RM, Sweetnam PM, et al. Effects of changes in fat, fish, and fibre intakes on death and myocardial reinfarction: diet and reinfarction trial (DART) Lancet. 1989;2:757–761. doi: 10.1016/s0140-6736(89)90828-3. [DOI] [PubMed] [Google Scholar]
- Burr ML, Ashfield-Watt PA, Dunstan FD, Fehily AM, Breay P, Ashton T, et al. Lack of benefit of dietary advice to men with angina: results of a controlled trial. Eur J Clin Nutr. 2003;57:193–200. doi: 10.1038/sj.ejcn.1601539. [DOI] [PubMed] [Google Scholar]
- Burr ML, Dunstan FD, George CH. Is fish oil good or bad for heart disease? Two trials with apparently conflicting results. J Membr Biol. 2005;206:155–163. doi: 10.1007/s00232-005-0784-1. [DOI] [PubMed] [Google Scholar]
- Calabresi L, Donati D, Pazzucconi F, Sirtori CR, Franceschini G. Omacor in familial combined hyperlipidemia: effects on lipids and low density lipoprotein subclasses. Atherosclerosis. 2000;148:387–396. doi: 10.1016/s0021-9150(99)00267-1. [DOI] [PubMed] [Google Scholar]
- Calder PC. N-3 polyunsaturated fatty acids, inflammation and immunity: pouring oil on troubled waters of another fishy tale? Nutr Res. 2001;21:309–341. [Google Scholar]
- Calder PC. n-3 polyunsaturated fatty acids, inflammation, and inflammatory diseases. Am J Clin Nutr. 2006;83:1505S–1519S. doi: 10.1093/ajcn/83.6.1505S. [DOI] [PubMed] [Google Scholar]
- Calo L, Bianconi L, Colivicchi F, Lamberti F, Loricchio ML, De Ruvo E, et al. N-3 Fatty acids for the prevention of atrial fibrillation after coronary artery bypass surgery: a randomized, controlled trial. J Am Coll Cardiol. 2005;45:1723–1728. doi: 10.1016/j.jacc.2005.02.079. [DOI] [PubMed] [Google Scholar]
- Cannon JG, Fiatarone MA, Meydani M, Gong J, Scott L, Blumberg JB, et al. Aging and dietary modulation of elastase and interleukin-1 beta secretion. Am J Physiol. 1995;268:R208–R213. doi: 10.1152/ajpregu.1995.268.1.R208. [DOI] [PubMed] [Google Scholar]
- Capdevila J, Parkhill L, Chacos N, Okita R, Masters BS, Estabrook RW. The oxidative metabolism of arachidonic acid by purified cytochromes P-450. Biochem Biophys Res Commun. 1981;101:1357–1363. doi: 10.1016/0006-291x(81)91597-7. [DOI] [PubMed] [Google Scholar]
- Casos K, Saiz MP, Ruiz-Sanz JI, Mitjavila MT. Atherosclerosis prevention by a fish oil-rich diet in apoE(-/-) mice is associated with a reduction of endothelial adhesion molecules. Atherosclerosis. 2008;201:306–317. doi: 10.1016/j.atherosclerosis.2008.02.033. [DOI] [PubMed] [Google Scholar]
- Caughey GE, Mantzioris E, Gibson RA, Cleland LG, James MJ. The effect on human tumor necrosis factor alpha and interleukin 1 beta production of diets enriched in n-3 fatty acids from vegetable oil or fish oil. Am J Clin Nutr. 1996;63:116–122. doi: 10.1093/ajcn/63.1.116. [DOI] [PubMed] [Google Scholar]
- Chan DC, Watts GF, Barrett PH, Beilin LJ, Redgrave TG, Mori TA. Regulatory effects of HMG CoA reductase inhibitor and fish oils on apolipoprotein B-100 kinetics in insulin-resistant obese male subjects with dyslipidemia. Diabetes. 2002;51:2377–2386. doi: 10.2337/diabetes.51.8.2377. [DOI] [PubMed] [Google Scholar]
- Christensen JH, Korup E, Aaroe J, Toft E, Moller J, Rasmussen K, et al. Fish consumption, n-3 fatty acids in cell membranes, and heart rate variability in survivors of myocardial infarction with left ventricular dysfunction. Am J Cardiol. 1997;79:1670–1673. doi: 10.1016/s0002-9149(97)00220-8. [DOI] [PubMed] [Google Scholar]
- Codde JP, Vandongen R, Mori TA, Beilin LJ, Hill KJ. Can the synthesis of platelet-activating factor, a potent vasodilator and pro-aggregatory agent, be altered by dietary marine oils? Clin Exp Pharmacol Physiol. 1987;14:197–202. doi: 10.1111/j.1440-1681.1987.tb00375.x. [DOI] [PubMed] [Google Scholar]
- Collins T, Cybulsky MI. NF-kappaB: pivotal mediator or innocent bystander in atherogenesis? J Clin Invest. 2001;107:255–264. doi: 10.1172/JCI10373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper AL, Gibbons L, Horan MA, Little RA, Rothwell NJ. Effect of dietary fish oil supplementation on fever and cytokine production in human volunteers. Clin Nutr. 1993;12:321–328. doi: 10.1016/0261-5614(93)90027-2. [DOI] [PubMed] [Google Scholar]
- Coronel R, Wilms-Schopman FJ, den Ruijter HM, Belterman CN, Schumacher CA, Opthof T, et al. Dietary n-3 fatty acids promote arrhythmias during acute regional myocardial ischemia in isolated pig hearts. Cardiovasc Res. 2007;73:386–394. doi: 10.1016/j.cardiores.2006.10.006. [DOI] [PubMed] [Google Scholar]
- Croset M, Lagarde M. In vitro incorporation and metabolism of icosapentaenoic and docosahexaenoic acids in human platelets – effect on aggregation. Thromb Haemost. 1986;56:57–62. [PubMed] [Google Scholar]
- Cybulsky MI, Gimbrone MA., Jr. Endothelial expression of a mononuclear leukocyte adhesion molecule during atherogenesis. Science. 1991;251:788–791. doi: 10.1126/science.1990440. [DOI] [PubMed] [Google Scholar]
- Danesh J, Wheeler JG, Hirschfield GM, Eda S, Eiriksdottir G, Rumley A, et al. C-reactive protein and other circulating markers of inflammation in the prediction of coronary heart disease. N Engl J Med. 2004;350:1387–1397. doi: 10.1056/NEJMoa032804. [DOI] [PubMed] [Google Scholar]
- Davidson MH. Mechanisms for the hypotriglyceridemic effect of marine omega-3 fatty acids. Am J Cardiol. 2006;98:27i–33i. doi: 10.1016/j.amjcard.2005.12.024. [DOI] [PubMed] [Google Scholar]
- Davidson MH, Stein EA, Bays HE, Maki KC, Doyle RT, Shalwitz RA, et al. Efficacy and tolerability of adding prescription omega-3 fatty acids 4 g/d to simvastatin 40 mg/d in hypertriglyceridemic patients: an 8-week, randomized, double-blind, placebo-controlled study. Clin Ther. 2007;29:1354–1367. doi: 10.1016/j.clinthera.2007.07.018. [DOI] [PubMed] [Google Scholar]
- Daviglus ML, Stamler J, Orencia AJ, Dyer AR, Liu K, Greenland P, et al. Fish consumption and the 30-year risk of fatal myocardial infarction. N Engl J Med. 1997;336:1046–1053. doi: 10.1056/NEJM199704103361502. [DOI] [PubMed] [Google Scholar]
- De Caterina R, Cybulsky MI, Clinton SK, Gimbrone MA, Jr, Libby P. The omega-3 fatty acid docosahexaenoate reduces cytokine-induced expression of proatherogenic and proinflammatory proteins in human endothelial cells. Arterioscler Thromb. 1994;14:1829–1836. doi: 10.1161/01.atv.14.11.1829. [DOI] [PubMed] [Google Scholar]
- De Caterina R, Bernini W, Carluccio MA, Liao JK, Libby P. Structural requirements for inhibition of cytokine-induced endothelial activation by unsaturated fatty acids. J Lipid Res. 1998;39:1062–1070. [PubMed] [Google Scholar]
- De Caterina R, Liao JK, Libby P. Fatty acid modulation of endothelial activation. Am J Clin Nutr. 2000;71:213S–223S. doi: 10.1093/ajcn/71.1.213S. [DOI] [PubMed] [Google Scholar]
- Deckelbaum RJ, Leaf A, Mozaffarian D, Jacobson TA, Harris WS, Akabas SR. Conclusions and recommendations from the symposium, Beyond Cholesterol: prevention and treatment of coronary heart disease with n-3 fatty acids. Am J Clin Nutr. 2008;87:2010S–2012S. doi: 10.1093/ajcn/87.6.2010S. [DOI] [PubMed] [Google Scholar]
- Dolecek TA, Granditis G. Dietary polyunsaturated fatty acids and mortality in the Multiple Risk Factor Intervention Trial (MRFIT) World Rev Nutr Diet. 1991;66:205–216. doi: 10.1159/000419291. [DOI] [PubMed] [Google Scholar]
- Dona M, Fredman G, Schwab JM, Chiang N, Arita M, Goodarzi A, et al. Resolvin E1, an EPA-derived mediator in whole blood, selectively counterregulates leukocytes and platelets. Blood. 2008;112:848–855. doi: 10.1182/blood-2007-11-122598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong ZM, Chapman SM, Brown AA, Frenette PS, Hynes RO, Wagner DD. The combined role of P- and E-selectins in atherosclerosis. J Clin Invest. 1998;102:145–152. doi: 10.1172/JCI3001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durrington PN, Bhatnagar D, Mackness MI, Morgan J, Julier K, Khan MA, et al. An omega-3 polyunsaturated fatty acid concentrate administered for one year decreased triglycerides in simvastatin treated patients with coronary heart disease and persisting hypertriglyceridaemia. Heart. 2001;85:544–548. doi: 10.1136/heart.85.5.544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dyerberg J, Bang HO, Stoffersen E, Moncada S, Vane JR. Eicosapentaenoic acid and prevention of thrombosis and atherosclerosis? Lancet. 1978;2:117–119. doi: 10.1016/s0140-6736(78)91505-2. [DOI] [PubMed] [Google Scholar]
- Endres S, Ghorbani R, Kelley VE, Georgilis K, Lonnemann G, van der Meer JW, et al. The effect of dietary supplementation with n-3 polyunsaturated fatty acids on the synthesis of interleukin-1 and tumor necrosis factor by mononuclear cells. N Engl J Med. 1989;320:265–271. doi: 10.1056/NEJM198902023200501. [DOI] [PubMed] [Google Scholar]
- Frost L, Vestergaard P. n-3 Fatty acids consumed from fish and risk of atrial fibrillation or flutter: the Danish Diet, Cancer, and Health Study. Am J Clin Nutr. 2005;81:50–54. doi: 10.1093/ajcn/81.1.50. [DOI] [PubMed] [Google Scholar]
- Fujioka S, Hamazaki K, Itomura M, Huan M, Nishizawa H, Sawazaki S, et al. The effects of eicosapentaenoic acid-fortified food on inflammatory markers in healthy subjects – a randomized, placebo-controlled, double-blind study. J Nutr Sci Vitaminol (Tokyo) 2006;52:261–265. doi: 10.3177/jnsv.52.261. [DOI] [PubMed] [Google Scholar]
- Geelen A, Brouwer IA, Zock PL, Katan MB. Antiarrhythmic effects of n-3 fatty acids: evidence from human studies. Curr Opin Lipidol. 2004;15:25–30. doi: 10.1097/00041433-200402000-00006. [DOI] [PubMed] [Google Scholar]
- Geelen A, Brouwer IA, Schouten EG, Maan AC, Katan MB, Zock PL. Effects of n-3 fatty acids from fish on premature ventricular complexes and heart rate in humans. Am J Clin Nutr. 2005;81:416–420. doi: 10.1093/ajcn.81.2.416. [DOI] [PubMed] [Google Scholar]
- Geleijnse JM, Brouwer IA, Feskens EJ. Risks and benefits of omega 3 fats: health benefits of omega 3 fats are in doubt. BMJ. 2006;332:915–916. doi: 10.1136/bmj.332.7546.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GISSI Investigators. Dietary supplementation with n-3 polyunsaturated fatty acids and vitamin E after myocardial infarction: results of the GISSI-Prevenzione trial. Gruppo Italiano per lo Studio della Sopravvivenza nell'Infarto miocardico. Lancet. 1999;354:447–455. [PubMed] [Google Scholar]
- Gissi-Hf I. Effect of n-3 polyunsaturated fatty acids in patients with chronic heart failure (the GISSI-HF trial): a randomised, double-blind, placebo-controlled trial. Lancet. 2008;372:1223–1230. doi: 10.1016/S0140-6736(08)61239-8. [DOI] [PubMed] [Google Scholar]
- Goodnight SH. The antithrombotic effects of fish oil. In: Simopoulos AP, Kifer RR, Martin RT, editors. Health Effects of Polyunsaturated Falty Acids in Seafood. Orlando: Academic Press; 1986. pp. 135–149. [Google Scholar]
- Goyens PL, Spilker ME, Zock PL, Katan MB, Mensink RP. Compartmental modeling to quantify alpha-linolenic acid conversion after longer term intake of multiple tracer boluses. J Lipid Res. 2005;46:1474–1483. doi: 10.1194/jlr.M400514-JLR200. [DOI] [PubMed] [Google Scholar]
- Goyens PL, Spilker ME, Zock PL, Katan MB, Mensink RP. Conversion of alpha-linolenic acid in humans is influenced by the absolute amounts of alpha-linolenic acid and linoleic acid in the diet and not by their ratio. Am J Clin Nutr. 2006;84:44–53. doi: 10.1093/ajcn/84.1.44. [DOI] [PubMed] [Google Scholar]
- Grundt H, Nilsen DW, Mansoor MA, Hetland O, Nordoy A. Reduction in homocysteine by n-3 polyunsaturated fatty acids after 1 year in a randomised double-blind study following an acute myocardial infarction: no effect on endothelial adhesion properties. Pathophysiol Haemost Thromb. 2003;33:88–95. doi: 10.1159/000073852. [DOI] [PubMed] [Google Scholar]
- Harris WS. n-3 fatty acids and serum lipoproteins: human studies. Am J Clin Nutr. 1997;65:1645S–1654S. doi: 10.1093/ajcn/65.5.1645S. [DOI] [PubMed] [Google Scholar]
- Harris WS, Bulchandani D. Why do omega-3 fatty acids lower serum triglycerides? Curr Opin Lipidol. 2006;17:387–393. doi: 10.1097/01.mol.0000236363.63840.16. [DOI] [PubMed] [Google Scholar]
- Harris WS, Ginsberg HN, Arunakul N, Shachter NS, Windsor SL, Adams M, et al. Safety and efficacy of Omacor in severe hypertriglyceridemia. J Cardiovasc Risk. 1997;4:385–391. [PubMed] [Google Scholar]
- He K, Song Y, Daviglus ML, Liu K, Van Horn L, Dyer AR, et al. Accumulated evidence on fish consumption and coronary heart disease mortality: a meta-analysis of cohort studies. Circulation. 2004;109:2705–2711. doi: 10.1161/01.CIR.0000132503.19410.6B. [DOI] [PubMed] [Google Scholar]
- Hirai A, Hamazaki T, Terano T, Nishikawa T, Tamura Y, Kamugai A, et al. Eicosapentaenoic acid and platelet function in Japanese. Lancet. 1980;2:1132–1133. doi: 10.1016/s0140-6736(80)92558-1. [DOI] [PubMed] [Google Scholar]
- Hjerkinn EM, Seljeflot I, Ellingsen I, Berstad P, Hjermann I, Sandvik L, et al. Influence of long-term intervention with dietary counseling, long-chain n-3 fatty acid supplements, or both on circulating markers of endothelial activation in men with long-standing hyperlipidemia. Am J Clin Nutr. 2005;81:583–589. doi: 10.1093/ajcn/81.3.583. [DOI] [PubMed] [Google Scholar]
- Hong S, Gronert K, Devchand PR, Moussignac RL, Serhan CN. Novel docosatrienes and 17S-resolvins generated from docosahexaenoic acid in murine brain, human blood, and glial cells. Autacoids in anti-inflammation. J Biol Chem. 2003;278:14677–14687. doi: 10.1074/jbc.M300218200. [DOI] [PubMed] [Google Scholar]
- Hooper L, Thompson RL, Harrison RA, Summerbell CD, Ness AR, Moore HJ, et al. Risks and benefits of omega 3 fats for mortality, cardiovascular disease, and cancer: systematic review. BMJ. 2006;332:752–760. doi: 10.1136/bmj.38755.366331.2F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hornstra G. Influence of dietary fat type on arterial thrombosis tendency. J Nutr Health Aging. 2001;5:160–166. [PubMed] [Google Scholar]
- Hu FB, Willett WC. Optimal diets for prevention of coronary heart disease. JAMA. 2002;288:2569–2578. doi: 10.1001/jama.288.20.2569. [DOI] [PubMed] [Google Scholar]
- Hu FB, Bronner L, Willett WC, Stampfer MJ, Rexrode KM, Albert CM, et al. Fish and omega-3 fatty acid intake and risk of coronary heart disease in women. JAMA. 2002;287:1815–1821. doi: 10.1001/jama.287.14.1815. [DOI] [PubMed] [Google Scholar]
- Hudert CA, Weylandt KH, Lu Y, Wang J, Hong S, Dignass A, et al. Transgenic mice rich in endogenous omega-3 fatty acids are protected from colitis. Proc Natl Acad Sci USA. 2006;103:11276–11281. doi: 10.1073/pnas.0601280103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huikuri HV, Castellanos A, Myerburg RJ. Sudden death due to cardiac arrhythmias. N Engl J Med. 2001;345:1473–1482. doi: 10.1056/NEJMra000650. [DOI] [PubMed] [Google Scholar]
- Imig JD, Zhao X, Capdevila JH, Morisseau C, Hammock BD. Soluble epoxide hydrolase inhibition lowers arterial blood pressure in angiotensin II hypertension. Hypertension. 2002;39:690–694. doi: 10.1161/hy0202.103788. [DOI] [PubMed] [Google Scholar]
- Jellema A, Plat J, Mensink RP. Weight reduction, but not a moderate intake of fish oil, lowers concentrations of inflammatory markers and PAI-1 antigen in obese men during the fasting and postprandial state. Eur J Clin Invest. 2004;34:766–773. doi: 10.1111/j.1365-2362.2004.01414.x. [DOI] [PubMed] [Google Scholar]
- Jouven X, Zureik M, Desnos M, Guerot C, Ducimetiere P. Resting heart rate as a predictive risk factor for sudden death in middle-aged men. Cardiovasc Res. 2001;50:373–378. doi: 10.1016/s0008-6363(01)00230-9. [DOI] [PubMed] [Google Scholar]
- Jump DB. N-3 polyunsaturated fatty acid regulation of hepatic gene transcription. Curr Opin Lipidol. 2008;19:242–247. doi: 10.1097/MOL.0b013e3282ffaf6a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jump DB, Clarke SD. Regulation of gene expression by dietary fat. Annu Rev Nutr. 1999;19:63–90. doi: 10.1146/annurev.nutr.19.1.63. [DOI] [PubMed] [Google Scholar]
- Kabir M, Skurnik G, Naour N, Pechtner V, Meugnier E, Rome S, et al. Treatment for 2 mo with n 3 polyunsaturated fatty acids reduces adiposity and some atherogenic factors but does not improve insulin sensitivity in women with type 2 diabetes: a randomized controlled study. Am J Clin Nutr. 2007;86:1670–1679. doi: 10.1093/ajcn/86.5.1670. [DOI] [PubMed] [Google Scholar]
- Kelley DS, Taylor PC, Nelson GJ, Schmidt PC, Ferretti A, Erickson KL, et al. Docosahexaenoic acid ingestion inhibits natural killer cell activity and production of inflammatory mediators in young healthy men. Lipids. 1999;34:317–324. doi: 10.1007/s11745-999-0369-5. [DOI] [PubMed] [Google Scholar]
- Kew S, Banerjee T, Minihane AM, Finnegan YE, Muggli R, Albers R, et al. Lack of effect of foods enriched with plant- or marine-derived n-3 fatty acids on human immune function. Am J Clin Nutr. 2003;77:1287–1295. doi: 10.1093/ajcn/77.5.1287. [DOI] [PubMed] [Google Scholar]
- Kew S, Mesa MD, Tricon S, Buckley R, Minihane AM, Yaqoob P. Effects of oils rich in eicosapentaenoic and docosahexaenoic acids on immune cell composition and function in healthy humans. Am J Clin Nutr. 2004;79:674–681. doi: 10.1093/ajcn/79.4.674. [DOI] [PubMed] [Google Scholar]
- Khalfoun B, Thibault G, Bardos P, Lebranchu Y. Docosahexaenoic and eicosapentaenoic acids inhibit in vitro human lymphocyte-endothelial cell adhesion. Transplantation. 1996;62:1649–1657. doi: 10.1097/00007890-199612150-00020. [DOI] [PubMed] [Google Scholar]
- Kramer HJ, Stevens J, Grimminger F, Seeger W. Fish oil fatty acids and human platelets: dose-dependent decrease in dienoic and increase in trienoic thromboxane generation. Biochem Pharmacol. 1996;52:1211–1217. doi: 10.1016/0006-2952(96)00473-x. [DOI] [PubMed] [Google Scholar]
- Krey G, Braissant O, L'Horset F, Kalkhoven E, Perroud M, Parker MG, et al. Fatty acids, eicosanoids, and hypolipidemic agents identified as ligands of peroxisome proliferator-activated receptors by coactivator-dependent receptor ligand assay. Mol Endocrinol. 1997;11:779–791. doi: 10.1210/mend.11.6.0007. [DOI] [PubMed] [Google Scholar]
- Kristensen SD, Schmidt EB, Andersen HR, Dyerberg J. Fish oil in angina pectoris. Atherosclerosis. 1987;64:13–19. doi: 10.1016/0021-9150(87)90049-9. [DOI] [PubMed] [Google Scholar]
- Kristensen SD, Schmidt EB, Dyerberg J. Dietary supplementation with n-3 polyunsaturated fatty acids and human platelet function: a review with particular emphasis on implications for cardiovascular disease. J Intern Med Suppl. 1989;731:141–150. doi: 10.1111/j.1365-2796.1989.tb01448.x. [DOI] [PubMed] [Google Scholar]
- Kromhout D, Bosschieter EB, de Lezenne CC. The inverse relation between fish consumption and 20-year mortality from coronary heart disease. N Engl J Med. 1985;312:1205–1209. doi: 10.1056/NEJM198505093121901. [DOI] [PubMed] [Google Scholar]
- Lauterbach B, Barbosa-Sicard E, Wang MH, Honeck H, Kargel E, Theuer J, et al. Cytochrome P450-dependent eicosapentaenoic acid metabolites are novel BK channel activators. Hypertension. 2002;39:609–613. doi: 10.1161/hy0202.103293. [DOI] [PubMed] [Google Scholar]
- Leaf A, Kang JX. Prevention of cardiac sudden death by N-3 fatty acids: a review of the evidence. J Intern Med. 1996;240:5–12. doi: 10.1046/j.1365-2796.1996.449803000.x. [DOI] [PubMed] [Google Scholar]
- Leaf A, Kang JX, Xiao YF, Billman GE. n-3 fatty acids in the prevention of cardiac arrhythmias. Lipids. 1999;34(Suppl.):S187–S189. doi: 10.1007/BF02562284. [DOI] [PubMed] [Google Scholar]
- Leaf A, Albert CM, Josephson M, Steinhaus D, Kluger J, Kang JX, et al. Prevention of fatal arrhythmias in high-risk subjects by fish oil n-3 fatty acid intake. Circulation. 2005;112:2762–2768. doi: 10.1161/CIRCULATIONAHA.105.549527. [DOI] [PubMed] [Google Scholar]
- Lee KW, Blann AD, Lip GY. Effects of omega-3 polyunsaturated fatty acids on plasma indices of thrombogenesis and inflammation in patients post-myocardial infarction. Thromb Res. 2006;118:305–312. doi: 10.1016/j.thromres.2005.07.018. [DOI] [PubMed] [Google Scholar]
- Lee TH, Mencia-Huerta JM, Shih C, Corey EJ, Lewis RA, Austen KF. Effects of exogenous arachidonic, eicosapentaenoic, and docosahexaenoic acids on the generation of 5-lipoxygenase pathway products by ionophore-activated human neutrophils. J Clin Invest. 1984;74:1922–1933. doi: 10.1172/JCI111612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee TH, Hoover RL, Williams JD, Sperling RI, Ravalese J, III, Spur BW, et al. Effect of dietary enrichment with eicosapentaenoic and docosahexaenoic acids on in vitro neutrophil and monocyte leukotriene generation and neutrophil function. N Engl J Med. 1985;312:1217–1224. doi: 10.1056/NEJM198505093121903. [DOI] [PubMed] [Google Scholar]
- Lemaitre RN, King IB, Mozaffarian D, Kuller LH, Tracy RP, Siscovick DS. n-3 Polyunsaturated fatty acids, fatal ischemic heart disease, and nonfatal myocardial infarction in older adults: the Cardiovascular Health Study. Am J Clin Nutr. 2003;77:319–325. doi: 10.1093/ajcn/77.2.319. [DOI] [PubMed] [Google Scholar]
- Libby P. Inflammation in atherosclerosis. Nature. 2002;420:868–874. doi: 10.1038/nature01323. [DOI] [PubMed] [Google Scholar]
- Liu Y, Zhang Y, Schmelzer K, Lee TS, Fang X, Zhu Y, et al. The antiinflammatory effect of laminar flow: the role of PPARgamma, epoxyeicosatrienoic acids, and soluble epoxide hydrolase. Proc Natl Acad Sci USA. 2005;102:16747–16752. doi: 10.1073/pnas.0508081102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowe GD. Circulating inflammatory markers and risks of cardiovascular and non-cardiovascular disease. J Thromb Haemost. 2005;3:1618–1627. doi: 10.1111/j.1538-7836.2005.01416.x. [DOI] [PubMed] [Google Scholar]
- Lu G, Windsor SL, Harris WS. Omega-3 fatty acids alter lipoprotein subfraction distributions and the in vitro conversion of very low density lipoproteins to low density lipoproteins. J Nutr Biochem. 1999;10:151–158. doi: 10.1016/s0955-2863(98)00094-1. [DOI] [PubMed] [Google Scholar]
- Lu T, VanRollins M, Lee HC. Stereospecific activation of cardiac ATP-sensitive K(+) channels by epoxyeicosatrienoic acids: a structural determinant study. Mol Pharmacol. 2002;62:1076–1083. doi: 10.1124/mol.62.5.1076. [DOI] [PubMed] [Google Scholar]
- McKenney JM, Swearingen D, Di Spirito M, Doyle R, Pantaleon C, Kling D, et al. Study of the pharmacokinetic interaction between simvastatin and prescription omega-3-acid ethyl esters. J Clin Pharmacol. 2006;46:785–791. doi: 10.1177/0091270006289849. [DOI] [PubMed] [Google Scholar]
- McLennan PL. Relative effects of dietary saturated, monounsaturated, and polyunsaturated fatty acids on cardiac arrhythmias in rats. Am J Clin Nutr. 1993;57:207–212. doi: 10.1093/ajcn/57.2.207. [DOI] [PubMed] [Google Scholar]
- McLennan PL, Abeywardena MY, Charnock JS. Dietary fish oil prevents ventricular fibrillation following coronary artery occlusion and reperfusion. Am Heart J. 1988;116:709–717. doi: 10.1016/0002-8703(88)90328-6. [DOI] [PubMed] [Google Scholar]
- McLennan PL, Bridle TM, Abeywardena MY, Charnock JS. Comparative efficacy of n-3 and n-6 polyunsaturated fatty acids in modulating ventricular fibrillation threshold in marmoset monkeys. Am J Clin Nutr. 1993;58:666–669. doi: 10.1093/ajcn/58.5.666. [DOI] [PubMed] [Google Scholar]
- Marcheselli VL, Hong S, Lukiw WJ, Tian XH, Gronert K, Musto A, et al. Novel docosanoids inhibit brain ischemia-reperfusion-mediated leukocyte infiltration and pro-inflammatory gene expression. J Biol Chem. 2003;278:43807–43817. doi: 10.1074/jbc.M305841200. [DOI] [PubMed] [Google Scholar]
- Massaro M, Habib A, Lubrano L, Del Turco S, Lazzerini G, Bourcier T, et al. The omega-3 fatty acid docosahexaenoate attenuates endothelial cyclooxygenase-2 induction through both NADP(H) oxidase and PKC epsilon inhibition. Proc Natl Acad Sci USA. 2006;103:15184–15189. doi: 10.1073/pnas.0510086103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto M, Sata M, Fukuda D, Tanaka K, Soma M, Hirata Y, et al. Orally administered eicosapentaenoic acid reduces and stabilizes atherosclerotic lesions in ApoE-deficient mice. Atherosclerosis. 2008;197:524–533. doi: 10.1016/j.atherosclerosis.2007.07.023. [DOI] [PubMed] [Google Scholar]
- Meydani SN, Endres S, Woods MM, Goldin BR, Soo C, Morrill-Labrode A, et al. Oral (n-3) fatty acid supplementation suppresses cytokine production and lymphocyte proliferation: comparison between young and older women. J Nutr. 1991;121:547–555. doi: 10.1093/jn/121.4.547. [DOI] [PubMed] [Google Scholar]
- Miles EA, Banerjee T, Dooper MM, M'Rabet L, Graus YM, Calder PC. The influence of different combinations of gamma-linolenic acid, stearidonic acid and EPA on immune function in healthy young male subjects. Br J Nutr. 2004;91:893–903. doi: 10.1079/BJN20041131. [DOI] [PubMed] [Google Scholar]
- Miller M, Zhan M, Havas S. High attributable risk of elevated C-reactive protein level to conventional coronary heart disease risk factors: the Third National Health and Nutrition Examination Survey. Arch Intern Med. 2005;165:2063–2068. doi: 10.1001/archinte.165.18.2063. [DOI] [PubMed] [Google Scholar]
- Molvig J, Pociot F, Worsaae H, Wogensen LD, Baek L, Christensen P, et al. Dietary supplementation with omega-3-polyunsaturated fatty acids decreases mononuclear cell proliferation and interleukin-1 beta content but not monokine secretion in healthy and insulin-dependent diabetic individuals. Scand J Immunol. 1991;34:399–410. doi: 10.1111/j.1365-3083.1991.tb01563.x. [DOI] [PubMed] [Google Scholar]
- Mori TA, Woodman RJ. The independent effects of eicosapentaenoic acid and docosahexaenoic acid on cardiovascular risk factors in humans. Curr Opin Clin Nutr Metab Care. 2006;9:95–104. doi: 10.1097/01.mco.0000214566.67439.58. [DOI] [PubMed] [Google Scholar]
- Mori TA, Beilin LJ, Burke V, Morris J, Ritchie J. Interactions between dietary fat, fish, and fish oils and their effects on platelet function in men at risk of cardiovascular disease. Arterioscler Thromb Vasc Biol. 1997;17:279–286. doi: 10.1161/01.atv.17.2.279. [DOI] [PubMed] [Google Scholar]
- Mori TA, Woodman RJ, Burke V, Puddey IB, Croft KD, Beilin LJ. Effect of eicosapentaenoic acid and docosahexaenoic acid on oxidative stress and inflammatory markers in treated-hypertensive type 2 diabetic subjects. Free Radic Biol Med. 2003;35:772–781. doi: 10.1016/s0891-5849(03)00407-6. [DOI] [PubMed] [Google Scholar]
- Mozaffarian D. JELIS, fish oil, and cardiac events. Lancet. 2007;369:1062–1063. doi: 10.1016/S0140-6736(07)60504-2. [DOI] [PubMed] [Google Scholar]
- Mozaffarian D. Fish and n-3 fatty acids for the prevention of fatal coronary heart disease and sudden cardiac death. Am J Clin Nutr. 2008;87:1991S–1996S. doi: 10.1093/ajcn/87.6.1991S. [DOI] [PubMed] [Google Scholar]
- Mozaffarian D, Lemaitre RN, Kuller LH, Burke GL, Tracy RP, Siscovick DS. Cardiac benefits of fish consumption may depend on the type of fish meal consumed: the Cardiovascular Health Study. Circulation. 2003;107:1372–1377. doi: 10.1161/01.cir.0000055315.79177.16. [DOI] [PubMed] [Google Scholar]
- Mozaffarian D, Psaty BM, Rimm EB, Lemaitre RN, Burke GL, Lyles MF, et al. Fish intake and risk of incident atrial fibrillation. Circulation. 2004;110:368–373. doi: 10.1161/01.CIR.0000138154.00779.A5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mozaffarian D, Geelen A, Brouwer IA, Geleijnse JM, Zock PL, Katan MB. Effect of fish oil on heart rate in humans: a meta-analysis of randomized controlled trials. Circulation. 2005;112:1945–1952. doi: 10.1161/CIRCULATIONAHA.105.556886. [DOI] [PubMed] [Google Scholar]
- Mozaffarian D, Gottdiener JS, Siscovick DS. Intake of tuna or other broiled or baked fish versus fried fish and cardiac structure, function, and hemodynamics. Am J Cardiol. 2006;97:216–222. doi: 10.1016/j.amjcard.2005.08.025. [DOI] [PubMed] [Google Scholar]
- Nakamura MT, Cheon Y, Li Y, Nara TY. Mechanisms of regulation of gene expression by fatty acids. Lipids. 2004;39:1077–1083. doi: 10.1007/s11745-004-1333-0. [DOI] [PubMed] [Google Scholar]
- Needleman P, Raz A, Minkes MS, Ferrendelli JA, Sprecher H. Triene prostaglandins: prostacyclin and thromboxane biosynthesis and unique biological properties. Proc Natl Acad Sci USA. 1979;76:944–948. doi: 10.1073/pnas.76.2.944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Node K, Huo Y, Ruan X, Yang B, Spiecker M, Ley K, et al. Anti-inflammatory properties of cytochrome P450 epoxygenase-derived eicosanoids. Science. 1999;285:1276–1279. doi: 10.1126/science.285.5431.1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osler M, Andreasen AH, Hoidrup S. No inverse association between fish consumption and risk of death from all-causes, and incidence of coronary heart disease in middle-aged, Danish adults. J Clin Epidemiol. 2003;56:274–279. doi: 10.1016/s0895-4356(02)00600-5. [DOI] [PubMed] [Google Scholar]
- Pairet M, Engelhardt G. Distinct isoforms (COX-1 and COX-2) of cyclooxygenase: possible physiological and therapeutic implications. Fundam Clin Pharmacol. 1996;10:1–17. doi: 10.1111/j.1472-8206.1996.tb00144.x. [DOI] [PubMed] [Google Scholar]
- Park Y, Jones PG, Harris WS. Triacylglycerol-rich lipoprotein margination: a potential surrogate for whole-body lipoprotein lipase activity and effects of eicosapentaenoic and docosahexaenoic acids. Am J Clin Nutr. 2004;80:45–50. doi: 10.1093/ajcn/80.1.45. [DOI] [PubMed] [Google Scholar]
- Pietinen P, Ascherio A, Korhonen P, Hartman AM, Willett WC, Albanes D, et al. Intake of fatty acids and risk of coronary heart disease in a cohort of Finnish men. The Alpha-Tocopherol, Beta-Carotene Cancer Prevention Study. Am J Epidemiol. 1997;145:876–887. doi: 10.1093/oxfordjournals.aje.a009047. [DOI] [PubMed] [Google Scholar]
- Pownall HJ, Brauchi D, Kilinc C, Osmundsen K, Pao Q, Payton-Ross C, et al. Correlation of serum triglyceride and its reduction by omega-3 fatty acids with lipid transfer activity and the neutral lipid compositions of high-density and low-density lipoproteins. Atherosclerosis. 1999;143:285–297. doi: 10.1016/s0021-9150(98)00301-3. [DOI] [PubMed] [Google Scholar]
- Raitt MH, Connor WE, Morris C, Kron J, Halperin B, Chugh SS, et al. Fish oil supplementation and risk of ventricular tachycardia and ventricular fibrillation in patients with implantable defibrillators: a randomized controlled trial. JAMA. 2005;293:2884–2891. doi: 10.1001/jama.293.23.2884. [DOI] [PubMed] [Google Scholar]
- Rodriguez BL, Sharp DS, Abbott RD, Burchfiel CM, Masaki K, Chyou PH, et al. Fish intake may limit the increase in risk of coronary heart disease morbidity and mortality among heavy smokers. The Honolulu Heart Program. Circulation. 1996;94:952–956. doi: 10.1161/01.cir.94.5.952. [DOI] [PubMed] [Google Scholar]
- Ross R. The pathogenesis of atherosclerosis: a perspective for the 1990s. Nature. 1993;362:801–809. doi: 10.1038/362801a0. [DOI] [PubMed] [Google Scholar]
- Ross R. Atherosclerosis – an inflammatory disease. N Engl J Med. 1999;340:115–126. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- de Roos B, Duivenvoorden I, Rucklidge G, Reid M, Ross K, Lamers RJ, et al. Response of apolipoprotein E*3-Leiden transgenic mice to dietary fatty acids: combining liver proteomics with physiological data. FASEB J. 2005a;19:813–815. doi: 10.1096/fj.04-2974fje. [DOI] [PubMed] [Google Scholar]
- de Roos B, Mercer DK, Wainwright C, Thies F. Effect of long chain n-3 PUFA on endothelial activation, endothelial function and atheromatous plaque stability. Current Nutrition and Food. Science. 2005b;1:167–177. [Google Scholar]
- de Roos B, Duthie SJ, Polley AC, Mulholland F, Bouwman FG, Heim C, et al. Proteomic methodological recommendations for studies involving human plasma, platelets, and peripheral blood mononuclear cells. J Proteome Res. 2008a;7:2280–2290. doi: 10.1021/pr700714x. [DOI] [PubMed] [Google Scholar]
- de Roos B, Geelen A, Ross K, Rucklidge G, Reid M, Duncan G, et al. Identification of potential serum biomarkers of inflammation and lipid modulation that are altered by fish oil supplementation in healthy volunteers. Proteomics. 2008b;8:1965–1974. doi: 10.1002/pmic.200700457. [DOI] [PubMed] [Google Scholar]
- den Ruijter HM, Berecki G, Opthof T, Verkerk AO, Zock PL, Coronel R. Pro- and antiarrhythmic properties of a diet rich in fish oil. Cardiovasc Res. 2007;73:316–325. doi: 10.1016/j.cardiores.2006.06.014. [DOI] [PubMed] [Google Scholar]
- den Ruijter HM, Berecki G, Verkerk AO, Bakker D, Baartscheer A, Schumacher CA, et al. Acute administration of fish oil inhibits triggered activity in isolated myocytes from rabbits and patients with heart failure. Circulation. 2008;117:536–544. doi: 10.1161/CIRCULATIONAHA.107.733329. [DOI] [PubMed] [Google Scholar]
- Salonen JT, Nyyssonen K, Salonen R. Fish intake and the risk of coronary disease. N Engl J Med. 1995;333:937. doi: 10.1056/NEJM199510053331412. [DOI] [PubMed] [Google Scholar]
- Saraswathi V, Gao L, Morrow JD, Chait A, Niswender KD, Hasty AH. Fish oil increases cholesterol storage in white adipose tissue with concomitant decreases in inflammation, hepatic steatosis, and atherosclerosis in mice. J Nutr. 2007;137:1776–1782. doi: 10.1093/jn/137.7.1776. [DOI] [PubMed] [Google Scholar]
- von Schacky C. Omega-3 fatty acids: antiarrhythmic, proarrhythmic or both? Curr Opin Clin Nutr Metab Care. 2008;11:94–99. doi: 10.1097/MCO.0b013e3282f44bdf. [DOI] [PubMed] [Google Scholar]
- von Schacky C, Kiefl R, Jendraschak E, Kaminski WE. n-3 fatty acids and cysteinyl-leukotriene formation in humans in vitro, ex vivo, and in vivo. J Lab Clin Med. 1993;121:302–309. [PubMed] [Google Scholar]
- von Schacky C, Angerer P, Kothny W, Theisen K, Mudra H. The effect of dietary omega-3 fatty acids on coronary atherosclerosis. A randomized, double-blind, placebo-controlled trial. Ann Intern Med. 1999;130:554–562. doi: 10.7326/0003-4819-130-7-199904060-00003. [DOI] [PubMed] [Google Scholar]
- Schiano V, Laurenzano E, Brevetti G, De Maio JI, Lanero S, Scopacasa F, et al. Omega-3 polyunsaturated fatty acid in peripheral arterial disease: effect on lipid pattern, disease severity, inflammation profile, and endothelial function. Clin Nutr. 2008;27:241–247. doi: 10.1016/j.clnu.2007.11.007. [DOI] [PubMed] [Google Scholar]
- Schmelzer KR, Kubala L, Newman JW, Kim IH, Eiserich JP, Hammock BD. Soluble epoxide hydrolase is a therapeutic target for acute inflammation. Proc Natl Acad Sci USA. 2005;102:9772–9777. doi: 10.1073/pnas.0503279102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmelzer KR, Inceoglu B, Kubala L, Kim IH, Jinks SL, Eiserich JP, et al. Enhancement of antinociception by coadministration of nonsteroidal anti-inflammatory drugs and soluble epoxide hydrolase inhibitors. Proc Natl Acad Sci USA. 2006;103:13646–13651. doi: 10.1073/pnas.0605908103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt EB, Varming K, Moller JM, Bulow PI, Madsen P, Dyerberg J. No effect of a very low dose of n-3 fatty acids on monocyte function in healthy humans. Scand J Clin Lab Invest. 1996;56:87–92. doi: 10.1080/00365519609088592. [DOI] [PubMed] [Google Scholar]
- Scirica BM, Morrow DA. Is C-reactive protein an innocent bystander or proatherogenic culprit? The verdict is still out. Circulation. 2006;113:2128–2134. doi: 10.1161/CIRCULATIONAHA.105.611350. [DOI] [PubMed] [Google Scholar]
- Seierstad SL, Seljeflot I, Johansen O, Hansen R, Haugen M, Rosenlund G, et al. Dietary intake of differently fed salmon; the influence on markers of human atherosclerosis. Eur J Clin Invest. 2005;35:52–59. doi: 10.1111/j.1365-2362.2005.01443.x. [DOI] [PubMed] [Google Scholar]
- Serhan CN, Chiang N. Endogenous pro-resolving and anti-inflammatory lipid mediators: a new pharmacologic genus. Br J Pharmacol. 2008;153(Suppl. 1):S200–S215. doi: 10.1038/sj.bjp.0707489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serhan CN, Clish CB, Brannon J, Colgan SP, Chiang N, Gronert K. Novel functional sets of lipid-derived mediators with antiinflammatory actions generated from omega-3 fatty acids via cyclooxygenase 2-nonsteroidal antiinflammatory drugs and transcellular processing. J Exp Med. 2000;192:1197–1204. doi: 10.1084/jem.192.8.1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serhan CN, Hong S, Gronert K, Colgan SP, Devchand PR, Mirick G, et al. Resolvins: a family of bioactive products of omega-3 fatty acid transformation circuits initiated by aspirin treatment that counter proinflammation signals. J Exp Med. 2002;196:1025–1037. doi: 10.1084/jem.20020760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serhan CN, Gotlinger K, Hong S, Lu Y, Siegelman J, Baer T, et al. Anti-inflammatory actions of neuroprotectin D1/protectin D1 and its natural stereoisomers: assignments of dihydroxy-containing docosatrienes. J Immunol. 2006;176:1848–1859. doi: 10.4049/jimmunol.176.3.1848. [DOI] [PubMed] [Google Scholar]
- Serhan CN, Chiang N, Van Dyke TE. Resolving inflammation: dual anti-inflammatory and pro-resolution lipid mediators. Nat Rev Immunol. 2008;8:349–361. doi: 10.1038/nri2294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siess W, Roth P, Scherer B, Kurzmann I, Bohlig B, Weber PC. Platelet-membrane fatty acids, platelet aggregation, and thromboxane formation during a mackerel diet. Lancet. 1980;1:441–444. doi: 10.1016/s0140-6736(80)90995-2. [DOI] [PubMed] [Google Scholar]
- Siscovick DS, Raghunathan TE, King I, Weinmann S, Wicklund KG, Albright J, et al. Dietary intake and cell membrane levels of long-chain n-3 polyunsaturated fatty acids and the risk of primary cardiac arrest. JAMA. 1995;274:1363–1367. doi: 10.1001/jama.1995.03530170043030. [DOI] [PubMed] [Google Scholar]
- Siscovick DS, Raghunathan T, King I, Weinmann S, Bovbjerg VE, Kushi L, et al. Dietary intake of long-chain n-3 polyunsaturated fatty acids and the risk of primary cardiac arrest. Am J Clin Nutr. 2000;71:208S–212S. doi: 10.1093/ajcn/71.1.208S. [DOI] [PubMed] [Google Scholar]
- Smith WL. Cyclooxygenases, peroxide tone and the allure of fish oil. Curr Opin Cell Biol. 2005;17:174–182. doi: 10.1016/j.ceb.2005.02.005. [DOI] [PubMed] [Google Scholar]
- Spector AA, Norris AW. Action of epoxyeicosatrienoic acids on cellular function. Am J Physiol Cell Physiol. 2007;292:C996–C1012. doi: 10.1152/ajpcell.00402.2006. [DOI] [PubMed] [Google Scholar]
- Spector AA, Fang X, Snyder GD, Weintraub NL. Epoxyeicosatrienoic acids (EETs): metabolism and biochemical function. Prog Lipid Res. 2004;43:55–90. doi: 10.1016/s0163-7827(03)00049-3. [DOI] [PubMed] [Google Scholar]
- Sperling RI, Benincaso AI, Knoell CT, Larkin JK, Austen KF, Robinson DR. Dietary omega-3 polyunsaturated fatty acids inhibit phosphoinositide formation and chemotaxis in neutrophils. J Clin Invest. 1993;91:651–660. doi: 10.1172/JCI116245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stalenhoef AF, de Graaf J, Wittekoek ME, Bredie SJ, Demacker PN, Kastelein JJ. The effect of concentrated n-3 fatty acids versus gemfibrozil on plasma lipoproteins, low density lipoprotein heterogeneity and oxidizability in patients with hypertriglyceridemia. Atherosclerosis. 2000;153:129–138. doi: 10.1016/s0021-9150(00)00381-6. [DOI] [PubMed] [Google Scholar]
- Sublette ME, Bosetti F, DeMar JC, Ma K, Bell JM, Fagin-Jones S, et al. Plasma free polyunsaturated fatty acid levels are associated with symptom severity in acute mania. Bipolar Disord. 2007;9:759–765. doi: 10.1111/j.1399-5618.2007.00387.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swann PG, Venton DL, Le Breton GC. Eicosapentaenoic acid and docosahexaenoic acid are antagonists at the thromboxane A2/prostaglandin H2 receptor in human platelets. FEBS Lett. 1989;243:244–246. doi: 10.1016/0014-5793(89)80137-1. [DOI] [PubMed] [Google Scholar]
- Thies F, Miles EA, Nebe-von-Caron G, Powell JR, Hurst TL, Newsholme EA, et al. Influence of dietary supplementation with long-chain n-3 or n-6 polyunsaturated fatty acids on blood inflammatory cell populations and functions and on plasma soluble adhesion molecules in healthy adults. Lipids. 2001;36:1183–1193. doi: 10.1007/s11745-001-0831-4. [DOI] [PubMed] [Google Scholar]
- Thies F, Garry JM, Yaqoob P, Rerkasem K, Williams J, Shearman CP, et al. Association of n-3 polyunsaturated fatty acids with stability of atherosclerotic plaques: a randomised controlled trial. Lancet. 2003;361:477–485. doi: 10.1016/S0140-6736(03)12468-3. [DOI] [PubMed] [Google Scholar]
- Tjonahen E, Oh SF, Siegelman J, Elangovan S, Percarpio KB, Hong S, et al. Resolvin E2: identification and anti-inflammatory actions: pivotal role of human 5-lipoxygenase in resolvin E series biosynthesis. Chem Biol. 2006;13:1193–1202. doi: 10.1016/j.chembiol.2006.09.011. [DOI] [PubMed] [Google Scholar]
- Trebble T, Arden NK, Stroud MA, Wootton SA, Burdge GC, Miles EA, et al. Inhibition of tumour necrosis factor-alpha and interleukin 6 production by mononuclear cells following dietary fish-oil supplementation in healthy men and response to antioxidant co-supplementation. Br J Nutr. 2003;90:405–412. doi: 10.1079/bjn2003892. [DOI] [PubMed] [Google Scholar]
- Vanschoonbeek K, Feijge MA, Paquay M, Rosing J, Saris W, Kluft C, et al. Variable hypocoagulant effect of fish oil intake in humans: modulation of fibrinogen level and thrombin generation. Arterioscler Thromb Vasc Biol. 2004;24:1734–1740. doi: 10.1161/01.ATV.0000137119.28893.0b. [DOI] [PubMed] [Google Scholar]
- Vega-Lopez S, Kaul N, Devaraj S, Cai RY, German B, Jialal I. Supplementation with omega3 polyunsaturated fatty acids and all-rac alpha-tocopherol alone and in combination failed to exert an anti-inflammatory effect in human volunteers. Metabolism. 2004;53:236–240. doi: 10.1016/j.metabol.2003.09.012. [DOI] [PubMed] [Google Scholar]
- Verkerk AO, van Ginneken AC, Berecki G, den Ruijter HM, Schumacher CA, Veldkamp MW, et al. Incorporated sarcolemmal fish oil fatty acids shorten pig ventricular action potentials. Cardiovasc Res. 2006;70:509–520. doi: 10.1016/j.cardiores.2006.02.022. [DOI] [PubMed] [Google Scholar]
- Wallace FA, Miles EA, Calder PC. Comparison of the effects of linseed oil and different doses of fish oil on mononuclear cell function in healthy human subjects. Br J Nutr. 2003;89:679–689. doi: 10.1079/BJN1079/2002821. [DOI] [PubMed] [Google Scholar]
- Wang C, Harris WS, Chung M, Lichtenstein AH, Balk EM, Kupelnick B, et al. n-3 Fatty acids from fish or fish-oil supplements, but not alpha-linolenic acid, benefit cardiovascular disease outcomes in primary- and secondary-prevention studies: a systematic review. Am J Clin Nutr. 2006;84:5–17. doi: 10.1093/ajcn/84.1.5. [DOI] [PubMed] [Google Scholar]
- Wang HH, Hung TM, Wei J, Chiang AN. Fish oil increases antioxidant enzyme activities in macrophages and reduces atherosclerotic lesions in apoE-knockout mice. Cardiovasc Res. 2004;61:169–176. doi: 10.1016/j.cardiores.2003.11.002. [DOI] [PubMed] [Google Scholar]
- Weber C, Erl W, Pietsch A, Danesch U, Weber PC. Docosahexaenoic acid selectively attenuates induction of vascular cell adhesion molecule-1 and subsequent monocytic cell adhesion to human endothelial cells stimulated by tumor necrosis factor-alpha. Arterioscler Thromb Vasc Biol. 1995;15:622–628. doi: 10.1161/01.atv.15.5.622. [DOI] [PubMed] [Google Scholar]
- Weber PC. Clinical studies on the effects of n-3 fatty acids on cells and eicosanoids in the cardiovascular system. J Intern Med Suppl. 1989;225:61–68. doi: 10.1111/j.1365-2796.1989.tb01437.x. [DOI] [PubMed] [Google Scholar]
- Whelton SP, He J, Whelton PK, Muntner P. Meta-analysis of observational studies on fish intake and coronary heart disease. Am J Cardiol. 2004;93:1119–1123. doi: 10.1016/j.amjcard.2004.01.038. [DOI] [PubMed] [Google Scholar]
- Xu Z, Riediger N, Innis S, Moghadasian MH. Fish oil significantly alters fatty acid profiles in various lipid fractions but not atherogenesis in apo E-KO mice. Eur J Nutr. 2007;46:103–110. doi: 10.1007/s00394-006-0638-3. [DOI] [PubMed] [Google Scholar]
- Yaqoob P, Pala HS, Cortina-Borja M, Newsholme EA, Calder PC. Encapsulated fish oil enriched in alpha-tocopherol alters plasma phospholipid and mononuclear cell fatty acid compositions but not mononuclear cell functions. Eur J Clin Invest. 2000;30:260–274. doi: 10.1046/j.1365-2362.2000.00623.x. [DOI] [PubMed] [Google Scholar]
- Ye D, Zhang D, Oltman C, Dellsperger K, Lee HC, VanRollins M. Cytochrome p-450 epoxygenase metabolites of docosahexaenoate potently dilate coronary arterioles by activating large-conductance calcium-activated potassium channels. J Pharmacol Exp Ther. 2002;303:768–776. doi: 10.1124/jpet.303.2.768. [DOI] [PubMed] [Google Scholar]
- Yokoyama M, Origasa H, Matsuzaki M, Matsuzawa Y, Saito Y, Ishikawa Y, et al. Effects of eicosapentaenoic acid on major coronary events in hypercholesterolaemic patients (JELIS): a randomised open-label, blinded endpoint analysis. Lancet. 2007;369:1090–1098. doi: 10.1016/S0140-6736(07)60527-3. [DOI] [PubMed] [Google Scholar]
- Yu Z, Xu F, Huse LM, Morisseau C, Draper AJ, Newman JW, et al. Soluble epoxide hydrolase regulates hydrolysis of vasoactive epoxyeicosatrienoic acids. Circ Res. 2000;87:992–998. doi: 10.1161/01.res.87.11.992. [DOI] [PubMed] [Google Scholar]
- Yuan JM, Ross RK, Gao YT, Yu MC. Fish and shellfish consumption in relation to death from myocardial infarction among men in Shanghai, China. Am J Epidemiol. 2001;154:809–816. doi: 10.1093/aje/154.9.809. [DOI] [PubMed] [Google Scholar]
- Yusof HM, Miles EA, Calder PC. Influence of very long-chain n-3 fatty acids on plasma markers of inflammation in middle-aged men. Prostaglandins Leukot Essent Fatty Acids. 2008;78:219–228. doi: 10.1016/j.plefa.2008.02.002. [DOI] [PubMed] [Google Scholar]
- Zampolli A, Bysted A, Leth T, Mortensen A, De Caterina R, Falk E. Contrasting effect of fish oil supplementation on the development of atherosclerosis in murine models. Atherosclerosis. 2006;184:78–85. doi: 10.1016/j.atherosclerosis.2005.04.018. [DOI] [PubMed] [Google Scholar]
- Zhao X, Yamamoto T, Newman JW, Kim IH, Watanabe T, Hammock BD, et al. Soluble epoxide hydrolase inhibition protects the kidney from hypertension-induced damage. J Am Soc Nephrol. 2004;15:1244–1253. [PubMed] [Google Scholar]


