Abstract
Background and purpose:
We have previously shown that treatment with zinc plus cyclo-(His-Pro) (CHP) significantly stimulated synthesis of the insulin degrading enzyme and lowered plasma insulin and blood glucose levels, alongside improving oral glucose tolerance in genetically type 2 diabetic Goto-Kakizaki (G-K) rats and in aged obese Sprague-Dawley (S-D) rats. Thus, we postulated that zinc plus CHP (ZC) treatment might also improve body weight control in these rats. We therefore determined the effects of ZC treatment on body weights in both genetically diabetic, mature G-K rats and non-diabetic, obese S-D rats.
Experimental approach:
G-K rats aged 1.5–10 months and non-diabetic overweight or obese S-D rats aged 6–18 months were treated with 0–6 mg CHP plus 0–10 mg zinc·L−1 drinking water for 2–4 weeks, and changes in weight, serum leptin and adiponectin levels, food and water intakes were measured.
Key results:
The optimal dose of CHP (in combination with zinc) to reduce weight and plasma leptin levels and to increase plasma adiponectin levels was close to 0.1 mg·kg−1·day−1, in either mature G-K rats and aged overweight or obese S-D rats. Food and water intake significantly decreased in ZC treated rats in both aged S-D rats and mature G-K rats, but not in young S-D and G-K rats.
Conclusions and implications:
ZC treatment improved weight control and may be a possible treatment for overweight and obesity.
Keywords: Cyclo-(His-Pro), insulin degrading enzyme, zinc, leptin, adiponectin, obese S-D rats, diabetic G-K rats
Introduction
Hyperinsulinemia is one of the major causes of obesity, as insulin increases fat accumulation in adipocytes (Escriva et al., 2007). Hyperinsulinemia in obesity is caused mainly by decreased insulin clearance from the plasma (Meistas et al., 1983). Impaired digestion of insulin after uptake by cells induces accumulation of inactive insulin fragments in the cells, which is associated with significant impairment of insulin receptor signal transduction mechanisms, resulting in insulin resistance, hyperinsulinemia and obesity (Mora et al., 2003). Insulin levels in monocytes from obese patients were more than fourfold higher compared with those in these cells from normal subjects (Benzi et al., 1999). Similarly, plasma insulin levels in obese subjects are about 69% higher than in normal subjects (Meistas et al., 1983). Impaired insulin clearance in obese humans and a correlation of increased insulin clearance with weight loss has been demonstrated (Jimenez et al., 1987). Decreased insulin clearance in obese subjects is mainly due to the elevated free fatty acids which inhibit synthesis of the insulin degrading enzyme (IDE) (Hamel et al., 2003). Therefore, it appears that one of the plausible methods to treat obesity may be to reduce plasma and cellular insulin levels by stimulating IDE synthesis.
We have found that treatment of amyloid protein precursor transgenic mice with zinc plus cyclo-(His-Pro) (ZC) increased brain tissue IDE synthesis by at least 30% of control level (SA O'Barr et al., unpubl. data). The same ZC treatment decreased plasma insulin and blood glucose levels in genetically obese type 2 diabetic ob/ob mice (Hwang et al., 2003), and in both aged, non-obese, genetically type 2 diabetic Goto-Kakizaki (G-K) rats and insulin-resistant, overweight and obese Sprague-Dawley (S-D) rats (Song et al., 2003). The optimal dose for reducing blood glucose and plasma insulin levels was approximately 0.1 mg·kg−1·day−1 of Cyclo-(His-Pro) (CHP) plus 1.0 mg·kg−1·day−1 of zinc in either genetically type 2 diabetic G-K and non-diabetic obese S-D rats (Song et al., 2003) or genetically type 2 diabetic ob/ob mice (Hwang et al., 2003).
Obesity is generally associated with hypozincemia, as observed in Italian (Di Martino et al., 1993), Turkish (Ozata et al., 2002), Thai (Tungtrongchitr et al., 2003) and Taiwanese (Chen et al., 1988) adult populations, as well as in Turkish children (Yakinci et al., 1997). Zinc supplementation reduced sucrose-induced obesity in mice (Chen and Lin, 2000) and rats (Bock et al., 1995). These effects were previously explained by a reduction of food intake and an increased energy expenditure (Fried and Russell, 1977). However, excess zinc supplementation may promote obesity via increased lipogenesis by overstimulating gene expression of other zinc enzymes (Taneja et al., 1996; Chen et al., 1998), in addition to IDE (Perlman and Rosner, 1994). Plasma leptin levels are invariably elevated in obese animals, and may lead to leptin resistance. However, there is no clear evidence that zinc is essential for leptin signalling pathways or for enhancing the signal transduction of the leptin-receptor mediated system in the appetite controlling centres in the brain (Levenson, 2003) or in liver cells (Huang et al., 2004; 2007;). The current understanding of zinc effect on obesity is that zinc positively modulates secretion of insulin from pancreas (Huber and Gershoff, 1973) and leptin from adipose cells (Levenson, 2003). Based on these findings, it is clear that the mechanism of CHP to improve insulin sensitivity is to stimulate zinc uptake by cells (Rosenthal et al., 2003) and to enhance IDE synthesis (SA O'Barr et al., unpubl. data). This in turn helps to decrease plasma insulin levels (Song et al., 2003). Because plasma and cellular insulin levels are elevated in obese patients (Meistas et al., 1983; Benzi et al., 1999) and a reduction in plasma and cellular insulin levels are thought to improve body weight control (Mora et al., 2003), we hypothesized that ZC treatment might improve weight control apart from its effect in improving insulin sensitivity. In the present study, we determined the effect of ZC treatment on reducing body weight in mature, non-obese, genetically diabetic G-K rats and in aged, overweight and obese S-D rats.
Methods
Animal care and maintenance
All animal care and these experimental studies were conducted with the approval of the Institutional Animal Care and Use Committee of the VA Greater Los Angeles Healthcare System. Five 1.5-month-old male and five age-matched female stock of G-K rats were purchased from the University of South Florida, Comparative Medicine Department (Dr Robert V. Farese), Tampa, FL, USA and the colony was expanded at the animal facility of the VA Greater Los Angeles Healthcare System, Los Angeles, CA, USA. G-K rats were used at the age of 1.5, 3 or 10 months. These rats are genetically type 2 diabetic and express insulin resistance at birth. One-year-old retired breeder S-D rats were purchased from Charles River Laboratories (Indianapolis, IN, USA) and maintained in the animal care facility of the VA Greater Los Angeles Healthcare System. These S-D rats often developed overweight and obesity during normal aging, with or without expressing insulin resistance or diabetes. These overweight and obese animals mimic humans in developing obesity influenced by different environmental factors with unknown genetic influences. These animal models were selected because aged obese S-D rats develop hyperleptinemia and non-obese diabetic G-K rats are hyperinsulinemic. All animals were kept in a temperature-controlled room (24 ± 1°C) with a 12-h light cycle. Animals were fed either zinc adequate diet or zinc supplemented rat chow and offered water ad libitum with or without experimental agents.
Assessment of ZC treatment on the weight gain in G-K rats
The effects of ZC treatment on weight gain in 3- and 10-month-old diabetic G-K rats were compared with the control rats. Thirty rats were divided into three equal groups and given (to drink) distilled water (DW) containing either: (i) no additive; (ii) 10 mg·L−1 zinc only; or (iii) 10-mg zinc plus 1.0 mg CHP·L−1 (ZC) for 3 weeks. The age of these rats was determined to rule out the effects of growth and fat accumulation in nearly fully grown 3-month-old rats and compared with fully grown 10-month-old G-K rats. Weight gain was determined by dividing the increase in weight during the treatment period by the days of treatment. As CHP is known to be effective only in the control of zinc metabolism, we have not determined the effect of CHP alone on weight gain. In our previous study, we observed that acute treatment with high doses of ZC was effective in the improvement of oral glucose tolerance, after 1 week of ZC treatment in mature G-K rats (Song et al., 2003). Thus, we also tested in this study whether the decrease in weight gain would persist after the cessation of ZC or zinc treatment. In this study, all the rats were given their treatments in drinking water for 4 weeks and weight gains were measured over the time these rats were given plain drinking water for an additional 28 days.
Measurement of the optimal dose of CHP for body weight reduction in aged obese S-D rats
To determine the optimal dose of CHP necessary for the decrease in body weight (BW) in obese rats, aged obese S-D rats (12 months old weighing more than 650 g) were divided into four groups of 5–6 rats and given drinking water containing either: (i) no additive; (ii) 10-mg zinc plus 1 mg CHP·L−1; (iii) 10-mg zinc plus 3 mg CHP·L−1; and (iv) 10-mg zinc plus 6 mg CHP·L−1 for 15 days. Each rat was housed in a single cage and body weight and food and water intakes were measured every 2–4 days, at the time of replacing food. At the end of the study, rats were anaesthetized with halothane and blood was drawn from the ocular vein and collected in heparinized tubes. Based on the body weight change, volume of water intake, CHP concentration in the water and days of treatment, the optimal dose of CHP for the reduction of body weight in aged S-D rats was determined.
Effects of treatment with ZC on weights of aged S-D rats
Eighteen 6- to 18-month-old S-D rats were divided into three groups of six rats based on their baseline body weights, and treated with drinking water containing 3-mg CHP plus 10 mg zinc·L−1 (ZC) for 23 days. Because there are no standard criteria on defining overweight and obese rats (comparable to the body mass index in humans), we defined ‘overweight’ and ‘obese’ based on the body weight of aged rats. S-D rats weighing more than 1000 g are minimally active, which is similar to obese human subjects, and we classified these rats as obese. Rats weighing 650–850 g are generally active but these rats are much heavier than normal lean rats, and we classified these rats as overweight. Mature rats weigh approximately 350–450 g and were classified as lean normal rats. As we have not used rats with weights between 450 and 650 g and between 850 and 1000 g, we have not classified these rats.
Measurement of the optimal doses of CHP for plasma leptin levels and effects of the optimal dose of CHP on leptin and adiponectin levels in different animals
To determine the optimal dose of CHP necessary for the decrease in plasma leptin levels in overweight or obese aged S-D rats (12 months old weighing more than 650 g), cohorts were divided into four groups of 5–6 rats and given drinking water containing either: (i) no additive; (ii) 10-mg zinc plus 1 mg CHP·L−1; (iii) 10 mg zinc plus 3 mg CHP·L−1; and (iv) 10-mg zinc plus 6 mg CHP·L−1 for 15 days. In addition, fourteen 15-month-old male S-D rats weighing 650–850 g were divided into two groups of seven rats and given drinking water containing no additive or 3-mg CHP plus 10 mg zinc·L−1 for 4 weeks. In another study, 3-month-old G-K rats were given drinking water containing either no additive or 1-mg CHP plus 10 mg zinc·L−1 for 4 weeks. Although the CHP concentration in the drinking water was different between aged obese S-D rats and young lean G-K rats, the actual CHP intake was about 0.1 mg·kg−1·day−1 as calculated from the body weight and water intake of the two groups. Plasma levels of leptin and adiponectin were measured by using commercial elisa kits (ALPCO Diagnostics Co, Salem, NH).
Measurements of food and water intake
Each rat was housed in a single cage and body weight, food intake, and water consumption were measured every 2–4 days when replacing food. Food intake rate was determined by measuring the loss of food from the food container during the feeding period. Food intake value was determined by food intake divided by body weight and days of treatment. Similarly, water intake rate was also expressed as volume ingested per day and corrected for body weight (mL·kg−1·day−1).
Taste aversion test
In order to demonstrate that reduced water intake in rats treated with drinking water containing ZC is not due to the effect of taste aversion, twelve 15-month-old S-D rats weighing over 650–850 g were treated with drinking water containing 2 g·L−1 Equal Sweetener, and no additive or 10-mg zinc plus 3 mg CHP·L−1 (ZC) for 15 days using the modified method of Dess (1993). In another study, 6-month-old C57BL/6J mice (n= 14) were divided into two groups of seven mice. The first group of mice was treated with 32% sucrose solution (w/w) only, while the second group of mice was treated with 32% sucrose solution containing 3 mg CHP plus 10 mg zinc·L−1 for 21 days.
Statistical analysis
Analysis of variance (anova) and paired or unpaired t-tests were carried out using GraphPad InStat software (GraphPad Software Co, San Diego, CA, USA). All data are presented as means ± SEM. P values < 0.05 were considered to show statistically significant differences between means. To estimate sample size, we used previously published data (Song et al., 2003) to estimate differences between means, a desired power of 0.8, and a significance level of 0.05 for a two-tailed equal variances model to determine that a sample size of 5 or 6 per group would suffice to detect differences between the optimal treatment dose and control.
Materials
Animal diets
Zinc adequate diet (Modified RMH 2000 with 41 mg zinc·kg−1) and zinc supplemented rat chow (Prolab RMH 2500 containing 110 mg zinc·kg−1) were supplied by PMI International, LLC, Brentwood, MO, USA. CHP (Chemical Abstract Registry Number: 53109-32-31) was purchased from Hanchem Co, LTD, Daejeon, Korea. CHP is a metabolite of thyrotropin-releasing hormone (TRH) and is a cyclic dipeptide involved in inhibition of prolactin secretion (Jikihara et al., 1993). Zinc chloride was purchased from Sigma Co, St. Louis, MO, USA. Equal Sweetener (1 g contains 40 mg aspartame, 28.8 mg dextrose and 931.2 mg maltodextrin) was from Merisant US Inc., Chicago, IL, USA.
Results
ZC treatment reduced body weight and body weight gain in aged obese S-D rats and in mature G-K rats
To assess the effects of oral ZC on body weight, mature (10 months old) G-K rats were given drinking water containing no additive, 10 mg·L−1 zinc alone, or 1.0 mg CHP plus 10 mg zinc·L−1 (ZC) for 4 weeks. As CHP alone was not effective in the control of glucose and body weight in our previous study (Song et al., 2003), we have not included a CHP only control group in this study. As shown in Figure 1A, mature G-K rats treated either with only zinc or with ZC gained less weight than control rats receiving only plain drinking water (P < 0.001 with ZC treatment). When the treated G-K rats were switched to plain drinking water for 28 additional days, rats previously treated with zinc only gained weight to the same extent as the controls, but those previously treated with ZC maintained their reduction in weight (P < 0.05; Figure 1B). Younger (3 months old) rats exhibited much greater rates of weight gain than 10-month-old rats (Figure 2). In these rats, zinc treatment alone did not affect weight gain, but ZC treatment decreased weight gain, compared with controls (P < 0.001).
Figure 1.
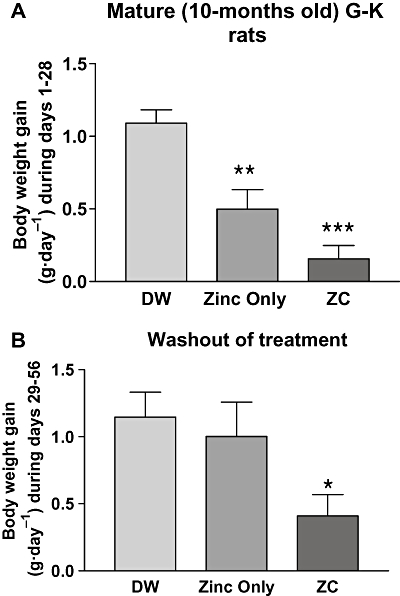
Weight gain in mature (10-month-old) Goto-Kakizaki (G-K) rats given drinking water containing zinc alone or CHP plus zinc. Rats were divided into three groups of 10 and given drinking water containing: (i) no additives (DW); (ii) 10-mg zinc only (Zinc); or (iii) 10-mg zinc plus 1.0 mg CHP·L−1 (zinc + CHP) for 4 weeks and the weight gain measured (A). All the rats used in the study (A) were then switched to a washout period where they all were given water with no additives for an additional 4 weeks (B). Only the rats which had been treated with zinc + CHP maintained their decreased rate of weight gain. *P < 0.05, **P < 0.01 and ***P < 0.001 compared with controls.
Figure 2.
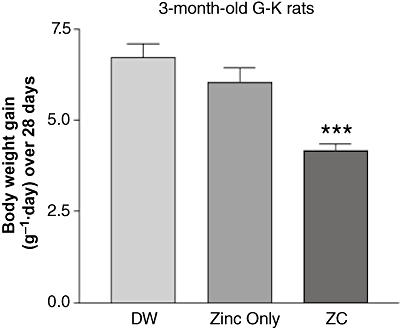
Thirty 3-month-old G-K rats were treated with drinking water containing: (i) no additives; (ii) 10 mg·L−1 zinc only; or (iii) 1.0-mg CHP plus 10 mg zinc·L−1 for 4 weeks. Only the rats on zinc + CHP showed a reduced weight gain. ***P < 0.001 compared with controls.
Determination of optimal dose of CHP for weight reduction
To establish the optimal dose of CHP for weight reduction, aged overweight (650–850 g) S-D rats were treated with different doses of CHP (0, 1, 3 or 6 mg·L−1) plus 10 mg·L−1 zinc in drinking water for 15 days. The optimal dose for the reduction of weight in these rats was 3 mg CHP with 10 mg zinc·L−1 drinking water (Figure 3). The optimal dose of CHP was calculated based on the amount of water intake, weight reduction and the duration of treatment and was found to be 0.1 mg CHP·kg−1·day−1 in essentially all the rat groups, based on the calculation described in the Methods.
Figure 3.
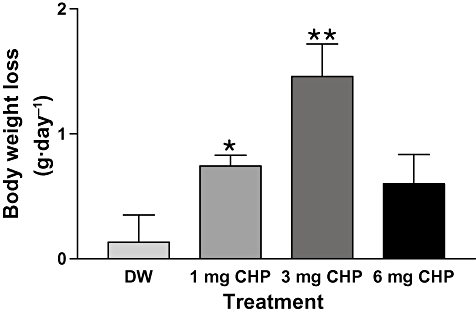
Body weight reduction in aged overweight S-D rats depends on CHP dose. Twenty-four 12-month-old S-D rats weighing 650–850 g were divided into four groups of six rats and treated with drinking water containing: (i) no additives; (ii) 1-mg CHP + 10 mg zinc·L−1; (iii) 3-mg CHP + 10 mg zinc·L−1; or (iv) 6-mg CHP + 10 mg zinc·L−1 for 15 days. The greatest weight reduction was seen in the rats taking water with 3-mg CHP + 10 mg zinc·L−1. *P < 0.05, **P < 0.01 versus controls.
Effect of initial weight on the rate of weight reduction induced by CHP plus zinc (ZC) treatment
To determine ZC effect on weight reduction, in relation to the initial weight of the animals, aged S-D rats (6–18 months old; n= 18) in three different groups were treated with 3-mg CHP plus 10 mg zinc·L−1 (ZC) drinking water for 23 days. As shown in Figure 4, the decrease in body weight was more marked (2.61 ± 0.35 g·day−1; P < 0.001) in the rats weighing over 1000 g than in those weighing 650–850 g (0.82 ± 0.02 g·day−1; P < 0.05). In contrast to the weight loss seen in overweight and obese rats, lean S-D rats (weighing 350–450 g) actually gained weight (Figure 4) although at a low rate (0.52 ± 0.02 g·day−1; P < 0.001).
Figure 4.
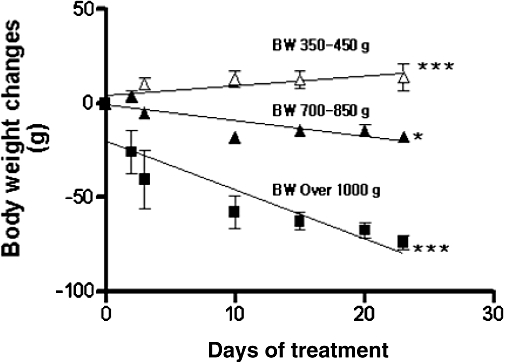
Effect of ZC (3-mg CHP plus 10 mg zinc·L−1) on body weight of aged overweight (650–850) obese (>1000 g) and lean (350–450 g) S-D rats depends on the initial body weight. Eighteen 6- to 18-month-old S-D rats were divided into three groups of six rats based on their body weights, and treated with drinking water containing 3 mg CHP plus 10 mg zinc·L−1. Both the obese and overweight groups lost weight over the 28 days of treatment but lean rats gained a small amount of weight. *P < 0.05, ***P < 0.001 from regression analyses.
Measurements of the optimal dose of CHP and treatment with ZC on plasma leptin and adiponectin levels in overweight S-D and diabetic G-K rats
The optimal dose of CHP in drinking water for overweight S-D rats was 3-mg CHP plus 10 mg zinc·L−1 as shown in Figure 5A. Plasma leptin levels in overweight 15-month-old S-D rats (650–850 g) treated with 3-mg CHP plus 10 mg zinc·L−1 (ZC) for 4 weeks decreased by about 20%, compared with controls (P < 0.01), and a similar effect was observed in non-obese diabetic G-K rats (P < 0.05) (Figure 5B). Both male and female lean diabetic G-K rats treated with ZC similarly decreased plasma leptin levels compared with controls (2.32 ± 0.13 ng·mL−1 vs. 1.80 ± 0.13 ng·mL−1 for males and 1.35 ± 0.08 ng·mL−1 vs. 1.12 ± 0.06 ng·mL−1 for females) although female rats weighed less (155.80 ± 4.56 g) than male rats (221.80 ± 6.47 g). Plasma adiponectin levels in overweight S-D rats treated with ZC for 4 weeks were increased by 11%, compared with controls (P < 0.001; Figure 6). In lean G-K rats, however, the plasma adiponectin levels were not affected by ZC treatment (Figure 6).
Figure 5.
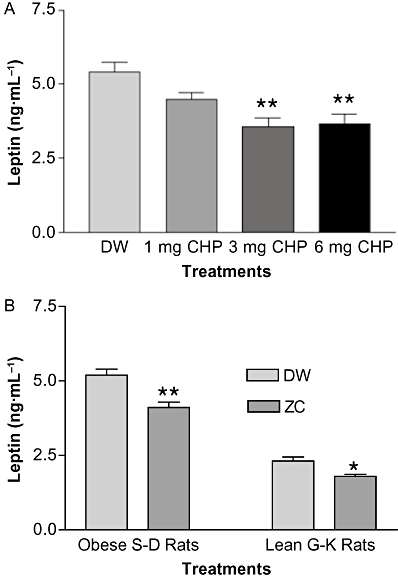
Optimal dose of CHP for the reduction of plasma leptin was determined (A). Overweight or obese aged S-D rats (650–850 g) were divided into four groups of 5–6 rats and treated with drinking water containing either: (i) no additive; (ii) 10-mg zinc plus 1 mg CHP·L−1; (iii) 10-mg zinc plus 3 mg CHP·L−1; and (iv) 10-mg zinc plus 6 mg CHP·L−1 for 15 days. In (B), plasma leptin levels in overweight non-diabetic S-D and lean diabetic G-K rats treated with ZC (zinc + CHP) were determined (B). Fourteen 15-month-old male S-D rats weighing 650–850 g were given drinking water containing no additive or 3-mg CHP plus 10 mg zinc·L−1 and 3-month-old G-K rats were given drinking water containing either no additive or 1-mg CHP plus 10 mg zinc·L−1 for 4 weeks. Rats with the ZC treatments showed reduced leptin level in both aged overweight S-D and lean diabetic G-K rats. *P < 0.05; **P < 0.01 versus controls.
Figure 6.
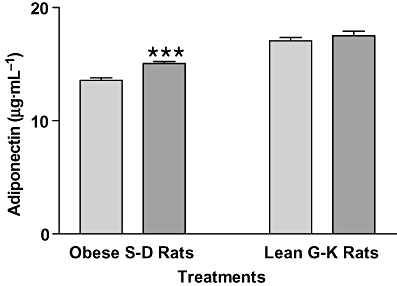
Plasma adiponectin levels in overweight non-diabetic S-D and lean diabetic G-K rats after ZC treatment. Fourteen 15-month-old male S-D rats weighing 650–850 g (These are overweight according to their criteria) were treated with drinking water containing either no additives or ZC (3-mg CHP plus 10 mg zinc·L−1) and 3-month-old G-K rats with drinking water containing either no additives or ZC (1-mg CHP plus 10 mg zinc·L−1) for 4 weeks, and plasma adiponectin levels were measured at the end of the 4-week treatment period. Only the S-D rats showed an increase in adiponectin levels at the end of the treatment. ***P < 0.001 versus controls.
ZC treatment decreased food intake in mature G-K and aged S-D rats
Thirty 1.5-month-old and thirty 10-month-old G-K rats were divided into three groups of 10 rats and given drinking water containing: (i) no additive; (ii) 10 mg·L−1 zinc only; and (iii) 10 mg zinc plus 3 mg CHP·L−1 (ZC) for 3 weeks. Food intake in all the groups of young rats was the same, regardless of different additives in the drinking water (Figure 7A). However, food intake in mature 10-month-old G-K rats significantly decreased when treated with zinc alone or ZC in drinking water and this decrease in food intake was the same with zinc or with ZC. When aged S-D rats were treated with ZC, food intake decreased in all the groups of rats whatever their initial body weight (Figure 7B). The percentage decreases of food intake were about 34% for obese rats, 35% for overweight rats and 31% for lean rats.
Figure 7.
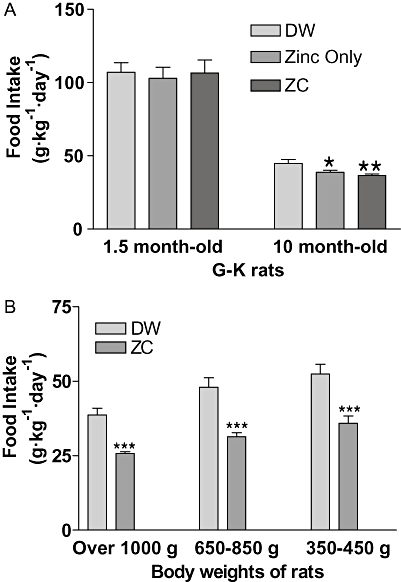
Food intake in relation to initial body weight of animals and ZC treatment in G-K and S-D rats. Thirty 1.5-month-old and thirty 10-month-old G-K rats were divided into three groups of 10 rats respectively, and treated with drinking water containing either no additives, 10 mg·L−1 zinc only or ZC (10-mg zinc plus 1 mg CHP·L−1) for 3 weeks and food intake measured for 3 weeks. No difference in food intake was shown in young rats due to ZC consumption, but in 10-month-old rats, treatment with zinc or with ZC reduced food intake (*P < 0.05; **P < 0.01), compared with controls (A). In (B) 6- to 18-month-old S-D rats (n= 18) were divided into three groups of six rats based on their body weights and treated with drinking water containing either no additives or ZC (3-mg CHP + 10 mg zinc·L−1) for 3 weeks. Clear reductions in food intake were observed in all groups of S-D rats receiving ZC water. ***P < 0.001 versus controls (B).
ZC treatment decreased water intake in aged S-D rats
Water intake in mature G-K rats decreased after ZC treatment (P < 0.01) or zinc treatment (P < 0.05) compared with controls (Figure 8A). However, ZC treatment induced a greater decrease than after zinc alone. No effect on water intake was exhibited in young G-K rats with zinc alone or with zinc plus CHP treatment. In S-D rats, water intake was decreased by ZC treatment in all weight groups (Figure 8B). The percentage of water intake decreases were about 17% for obese rats, 21% for overweight rats and 24% for lean rats.
Figure 8.
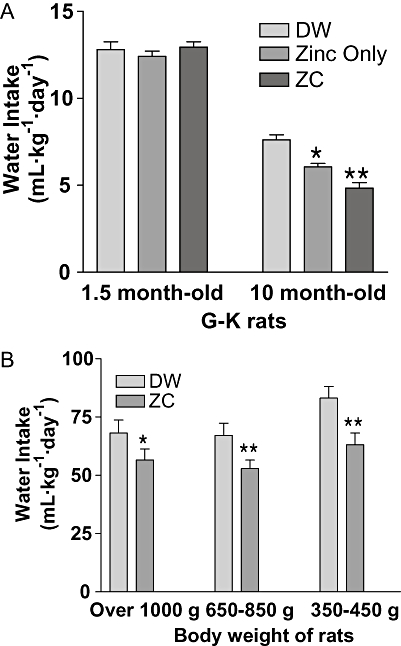
Water intakes in relation to initial body weight of animals and ZC treatments in G-K and S-D rats. In (A), 1.5-month-old (n= 30) and 10-month-old (n= 30) G-K rats were divided into three groups of 10 rats respectively, and treated with drinking water containing either no additives; 10 mg·L−1 zinc only; or ZC (10-mg zinc plus 1 mg CHP·L−1) for 3 weeks and water intake measured over this time. No difference was shown in water intake among the young rats, but a significant decrease was observed in 10-month-old G-K rats treated with zinc (*P < 0.05) or with ZC (**P < 0.01) compared with controls. In (B), 6- to 18-month-old S-D rats (n= 18) were divided into three groups of six rats based on their body weights and treated with drinking water containing either no additives or ZC (3-mg CHP + 10 mg zinc·L−1) for 3 weeks. All groups of S-D rats showed decreased water intake over the treatment period. *P < 0.05 and **P < 0.01 versus controls (B).
Taste aversion test
When S-D rats were given drinking water containing artificial sweetener (Equal Sweetener; 2 g·L−1) with or without ZC, rats treated with only sweetener gained body weight while the rats treated with ZC and sweetener containing water decreased their weight (P < 0.001) (Figure 9A). When 6-month-old C57BL/6J mice were treated with 32% sucrose solution (w/w), with ZC, their body weight gain was reduced compared with control mice, drinking 32% sucrose solution only (P < 0.01) (Figure 9B).
Figure 9.
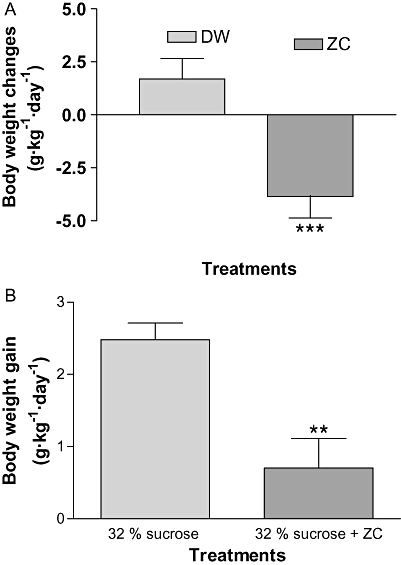
Taste aversion test in the control of body weight by ZC treatment. In (A), 15-month-old S-D rats weighing 650–850 g (n= 12) were given drinking water containing 2 g·L−1 Equal Sweetener, with either no additives (DW) or ZC (3-mg CHP plus 10 mg zinc·L−1) for 15 days. ZC still induced weight loss in the S-D rats in the presence of the artificial sweetener. In (B), 6-month-old C57BL/6J mice (n= 14) were divided into two groups of seven mice and treated with drinking water containing 32% sucrose (w/w), with either no additives (32% sucrose) or ZC (3-mg CHP plus 10 mg zinc·L−1) for 15 days. The body weight gain in the group of mice treated with ZC was less than that in the controls (32% sucrose only). ***P < 0.001; **P < 0.01 (t-test).
Discussion
In the present study, ZC treatment significantly decreased BW gain in lean G-K rats, and induced BW reduction in overweight or obese aged S-D rats. In parallel, plasma leptin levels were significantly decreased in both aged S-D and G-K rats but not in lean G-K rats. In contrast to plasma leptin levels, plasma adiponectin levels were significantly increased (P < 0.001) in aged overweight S-D rats, but not in lean, diabetic G-K rats. Plasma adiponectin levels exhibited a tendency to be lower in aged overweight S-D rats than in young G-K rats. These data (Figures 5 and 6) are consistent with the concept that adiponectin metabolism is generally related inversely to the leptin metabolism in animals and human forms of obesity (Bluher et al., 2006; Gannage-Yared et al., 2006). However, no clear-cut inverse relationship was shown in lean G-K rats. The optimal dose of CHP for decreasing body weight in overweight S-D rats weighing 650–850 g was 3 mg·L−1 CHP, and plasma leptin concentration was significantly decreased at this dose without an additional decrease at a higher dose. Based on the CHP concentration in the drinking water, body weight of animals, and water intake volume, the optimal dose of CHP were calculated as 0.117 mg·kg−1·day−1 for obese or overweight S-D rats, 0.118 mg·kg−1·day−1 for male G-K rats weighing 300 g and 0.095 mg·kg−1·day−1 for female G-K rats weighing 200 g. Thus, it appears that oral intake of about 0.1 mg CHP·kg−1·day−1 was most effective in reducing body weight and plasma leptin levels. This value is very similar to the optimal dose of CHP for lowering blood glucose and plasma insulin levels in diabetic G-K rats (Song et al., 2003) and in obese diabetic ob/ob mice (Hwang et al., 2003). Thus, treatment of both diabetic and obese animals with 0.1 mg CHP·kg−1·day−1 is effective for the control of blood glucose and body weight.
It has been reported that G-K rats have higher urinary zinc concentrations while fat and liver zinc concentrations are lower than Wistar control rats (Takita et al., 2004). As the zinc uptake rate of mature S-D rats increased with CHP treatment in a CHP concentration-dependent manner (Rosenthal et al., 2003), it is expected that zinc plus CHP treatment would improve zinc nutritional status to physiological levels, which should then help to improve BW control in overweight and obese subjects.. However, supplementing 10 mg·L−1 zinc in drinking water is equivalent to a daily zinc intake of about 1 mg·kg−1·day−1. This quantity of zinc is a physiologically appropriate dose based on the amount of zinc in rat chow (110 mg·kg−1 zinc in Prolab RMH 2500 diet), which is equivalent to a daily intake of zinc (per rat) of about 2.2 mg·kg−1 from the normal diet. Zinc doses with CHP in the drinking water were physiologically non-toxic doses (1–2 mg·kg−1 BW·day−1). Acinar cells of the pancreas started to degenerate only when mice were fed in excess of 240 mg zinc·kg−1·day−1 (Kelly et al., 1996). That was more than 200-fold higher than the dose used in the present study. Although the exact mechanism by which ZC treatment improves body weight control is not clearly understood, our studies point to the possibility that ZC treatment may improve leptin sensitivity as plasma leptin concentration decreased when food intake and body weight decreased and that ZC treatment reduces food and water intake. These effects may be due to the stimulation of zinc uptake in cells (Rosenthal et al., 2003), which may stimulate IDE synthesis (Perlman and Rosner, 1994).
Zinc deficiency results in hypoleptinemia in animals (Mangian et al., 1998) and in humans (Mantzoro et al., 1998), and supplementation with zinc increases leptin synthesis (Huang et al., 2004). Secretion of leptin is regulated by zinc in cultured rat adipocytes (Ott and Shay, 2001). Leptin deficiency is associated with hyperphagia, hyperinsulinemia and insulin resistance (Yildiz and Haznedaroglu, 2005).
Administration of leptin reversed all of these symptoms in rodents (Pocai et al., 2005). Thus, low zinc levels are associated with decreased synthesis of leptin and impaired control of body weight. Although leptin deficiency from a single homozygous gene mutation is associated with being overweight in humans (Stefan and Nicholls, 2005), mutation of leptin or its receptor is clinically rare. However, in most obese animal models and humans, plasma leptin levels are elevated (Gallou-Kabani et al., 2007; Vona-Davis et al., 2007), and age-related diet-induced obese animals and humans generally exhibit leptin resistance (Scarpace and Tumer, 2001).
Cyclo-(His-Pro) is a cyclic form of L-histidyl-proline which is distributed throughout the human body (Prasad, 1988). It is a stable metabolite of both TRH (Kagabu et al., 1998), and histidine-proline-rich glycoproteins, which are relatively abundant in the plasma (Morgan, 1978). This glycoprotein is responsible for zinc transport from the intestine to other tissue cells (Borza and Morgan, 1998). The CHP motif in the gut appears to facilitate zinc transport across the intestinal epithelium, perhaps in sequence with specific zinc transporters (Kambe et al., 2004). CHP is also found at concentrations as high as 3.5 ng·mg−1 protein in the anterior nucleus and 2.95 ng·mg−1 in the paraventricular nucleus of the hypothalamus and it has a cellular and subcellular localization distinct from that of TRH (Yamada and Wilber, 1989). More importantly, CHP stimulates intestinal zinc absorption and muscle cell zinc uptake (Rosenthal et al., 2003). Thus, CHP may be the effective agent that stimulates zinc metabolism and regulates metabolic activities in the control of body weight. The effects of CHP plus zinc (ZC) combinations on body weight was CHP dose dependent and related to plasma leptin levels and to adiponectin metabolism. Although the present study did not discover all the mechanisms by which ZC treatment reduced body weight, our data demonstrated that the reduction in body weight induced by ZC treatment was accompanied by decreased plasma leptin levels.
Treatment with CHP decreased food intake in rats, specifically fat intake and to some degree carbohydrate intake (Morley et al., 1981; Kow and Pfaff, 1991). In this study, food intake invariably decreased with ZC treatment, but CHP levels in the brains of obese rats are elevated compared with lean normal rats (Prasad et al., 1995), suggesting that there may be resistance to CHP action in obese animals. CHP reduces food intake by interacting with 5HT and fenfluramine in the brain, which are known anti-obesity agents (Kow and Pfaff, 1991) and intraventricular administration of CHP significantly reduced water intake (Ishihara et al., 1985). We also found decreased water intake in aged G-K and S-D rats after ZC treatment (Figure 8). In our study with young rats, no difference in water consumption was shown whether drinking water was treated or untreated with ZC.
When 15-month-old S-D rats weighing over 650–850 g were treated with drinking water containing 2 g·L−1 Equal Sweetener in drinking water containing ZC (10 mg zinc plus 3 mg CHP·L−1) for 15 days, the ZC treated rats significantly reduced their body weight compared with the rats treated with drinking water containing only sweetener. Furthermore, when 6-month-old C57BL/6J mice were treated with 32% sucrose (w/w), the body weight gain in the mice treated with ZC was significantly reduced compared with control. As there was no difference in the effects on body weight control, related to the treatment with sweetener treatment, these data suggest that dietary supplementation with ZC does not affect body weight through a taste aversion to ZC.
A vast diversity of phenotypes in human obesity indicates that this disorder is polyfactorial and not caused by a single gene mutation (Bouchard et al., 1990). There is thus no immediate expectation of being able to use an individual's genetic profile to assess their risk factors and their potential for developing obesity, or to diagnose a discrete molecular pathology and phenotype. However, environmental factors are likely to represent prime causative factors in modern obesity. Eating too many calories for one's energy needs combined with reduced energy expenditure could contribute to obesity. The present experimental data collectively suggest that zinc plus CHP treatment may help to reduce body weight gain and body weight in certain overweight and obese rodents by reducing energy intake and improving leptin sensitivity.
Acknowledgments
This research was supported financially by the NIH grant number NS 052456 and DVA merit review funding. We thank Dr Yan Ao and Karl Austin for their expert technical assistance.
Glossary
Abbreviations:
- CHP
cyclo-(His-Pro)
- elisa
enzyme-linked immuno-sorbent assay
- G-K
Goto-Kakizaki
- IDE
insulin degrading enzyme
- S-D
Sprague-Dawley
- TRH
thyrotropin-releasing hormone
- ZC
zinc plus cyclo-(His-Pro)
Conflict of interest
The patent right involved with this product is owned by the US Department of Veterans Affairs, although one author (M.K.S.) is the patent applicant.
References
- Benzi L, Ciccarone AM, Cecchetti P, DiCianni G, Caricato F, Trincavelli L, et al. Intracellular hyperinsulinemia: a metabolic characteristic of obesity with and without type 2 daibetes intracellular insulin in obesity and type 2 diabetes. Diabetes Res Clin Pract. 1999;46:231–237. doi: 10.1016/s0168-8227(99)00100-x. [DOI] [PubMed] [Google Scholar]
- Bluher M, Bullen JW, Lee JH, Kralish S, Fasshauer M, Kloting N, et al. Circulating adiponectin and expression of adiponectin receptors in human skeletal muscle: association with metabolic parameters and insulin resistance and regulated by physical training. J Clin Endocrinol Metab. 2006;91:2310–2316. doi: 10.1210/jc.2005-2556. [DOI] [PubMed] [Google Scholar]
- Bock BC, Kanarek RB, Aprille JR. Mineral content of the diet alters sucrose induced obesity in rats. Physiol Behav. 1995;57:659–668. doi: 10.1016/0031-9384(94)00312-2. [DOI] [PubMed] [Google Scholar]
- Borza DB, Morgan WT. Histidine-proline-rich glycoprotein as plasma pH sensor. J Biol Chem. 1998;273:5493–5499. doi: 10.1074/jbc.273.10.5493. [DOI] [PubMed] [Google Scholar]
- Bouchard C, Trembaly A, Despress JP, Nadeau A, Lupien PJ, Theeriault G, et al. The response to long-term overfeeding in identical twins. N Engl J Med. 1990;322:1477–1482. doi: 10.1056/NEJM199005243222101. [DOI] [PubMed] [Google Scholar]
- Chen MD, Lin PY. Zinc-induced hyperleptinemia relates to the amelioration of sucrose-induced obesity with zinc repletion. Obes Res. 2000;8:525–529. doi: 10.1038/oby.2000.65. [DOI] [PubMed] [Google Scholar]
- Chen MD, Lin PY, Lin WH, Cheng V. Zinc in hair and serum of obese individuals in Taiwan. Am J Clin Nutr. 1988;48:1307–1309. doi: 10.1093/ajcn/48.5.1307. [DOI] [PubMed] [Google Scholar]
- Chen MD, Liou SJ, Lin PY, Yang VC, Alexanders PS, Lin WH. Effects of zinc supplementation on the plasma glucose level and insulin activity in genetically obese (ob/ob) mice. Biol Trace Elem Res. 1998;61:303–311. doi: 10.1007/BF02789090. [DOI] [PubMed] [Google Scholar]
- Dess NK. Saccharin's aversive taste in rats: evidence and implications. Neurosci Biobehav Rev. 1993;17:359–372. doi: 10.1016/s0149-7634(05)80113-7. [DOI] [PubMed] [Google Scholar]
- Di Martino G, Matera MG, De Martino B, Vacca C, Di Martino S, Rossi F. Relationship between zinc and obesity. J Med. 1993;24:177–183. [PubMed] [Google Scholar]
- Escriva F, Gavete ML, Fermin Y, Perez Z, Gallardo N, Alvarez C, et al. Effect of age and moderate food restriction on insulin sensitivity in Wistar rats: role of adiposity. J Endocrinol. 2007;194:131–141. doi: 10.1677/joe.1.07043. [DOI] [PubMed] [Google Scholar]
- Fried SK, Russell CD. Diverse roles of adipose tissue in the regulation of systemic metabolism and energy balance. In: Bray GA, Bouchard C, James WP, editors. Handbook of Obesity. New York: Marcel Dekker; 1977. pp. 397–414. [Google Scholar]
- Gallou-Kabani C, Vige A, Gross MS, Rabes JP, Bolleau C, Larue-Achagiotis C, et al. C57BL/6J and A/J mice fed a high-fat diet delineate components of metabolic syndrome. Obesity. 2007;15:1996–2005. doi: 10.1038/oby.2007.238. [DOI] [PubMed] [Google Scholar]
- Gannage-Yared MH, Khalife S, Semaan M, Fares F, Jambart S, Halaby G. Serum adiponectin and leptin levels in relation to the metabolic syndrome, androgenic profile and somatotrophic axis in healthy non-diabetic elderly man. Eur J Endocrinol. 2006;155:167–176. doi: 10.1530/eje.1.02175. [DOI] [PubMed] [Google Scholar]
- Hamel FG, Upward JI, Bennett RG. In vitro inhibition of insulin-degrading enzyme by long-chain fatty acids and their coenzyme a thioesters. Endocrinology. 2003;144:2404–2408. doi: 10.1210/en.2002-0007. [DOI] [PubMed] [Google Scholar]
- Huang W, Dedousis N, Bhatt BA, O'Doberty RM. Impaired activation of phosphatidylinositol 3-kinase by leptin is a novel mechanisms of hepatic leptin resistance in diet-induced obesity. J Biol Chem. 2004;279:21695–21700. doi: 10.1074/jbc.M401546200. [DOI] [PubMed] [Google Scholar]
- Huang W, Dedousis N, O'Doberty RM. Hepatic steatosis and plasma dyslipidemia induced by a high-sucrose diet are corrected by an acute leptin infusion. J Appl Physiol. 2007;102:2260–2265. doi: 10.1152/japplphysiol.01449.2006. [DOI] [PubMed] [Google Scholar]
- Huber AM, Gershoff SN. Effect of zinc deficiency in rats on insulin release from the pancreas. J Nutr. 1973;103:1739–1744. doi: 10.1093/jn/103.12.1739. [DOI] [PubMed] [Google Scholar]
- Hwang IK, Go VL, Harris DM, Yip I, Kang KW, Song MK. Effects of cyclo (his-pro) plus zinc on glucose metabolism in genetically diabetic obese mice. Diabet Obes Metab. 2003;5:317–324. doi: 10.1046/j.1463-1326.2003.00281.x. [DOI] [PubMed] [Google Scholar]
- Ishihara H, Mori M, Kobayashi I, Kobayashi S. Intraventricular administration of cyclo (his-pro), metabolite of thryrotrophin-releasing hormone (TRH), decreases water intake in the rat. Proc Soc Exp Biol Med. 1985;178:623–628. doi: 10.3181/00379727-178-42052. [DOI] [PubMed] [Google Scholar]
- Jikihara H, Ikegami H, Koike K, Wada K, Morishige K, Kurachi H, et al. Intraventricular administration of histidyl-proline diketopeparazine [Cyclo(his-pro)] suppresses prolactin secretion and synthesis: a possible role of Cyclo (his-pro) as dopamine uptake blocker in rat hypothalamus. Endocrinology. 1993;132:953–958. doi: 10.1210/endo.132.3.7679984. [DOI] [PubMed] [Google Scholar]
- Jimenez J, Zuniga-Guajardo S, Zinman B, Angel A. Effects of weight loss in massive obesity on insulin and C-peptide dynamics: sequential changes in insulin production, clearance, and sensitivity. J Clin Endocrinol Metab. 1987;64:661–668. doi: 10.1210/jcem-64-4-661. [DOI] [PubMed] [Google Scholar]
- Kagabu Y, Mishiba T, Okino T, Yanagisawa T. Effects of thyrotropin-releasing hormone and its metabolites, Cyclo(his-pro) and TRH-OH, on growth hormone and prolactin synthesis in primary cultured pituitary cells of the common carp, Cyprinus carpio. Gen Comp Endocrinol. 1998;111:395–403. doi: 10.1006/gcen.1998.7124. [DOI] [PubMed] [Google Scholar]
- Kambe T, Yamaguchi-Iwai Y, Sasaki R, Nagao M. Overview of mammalian zinc transporters. Cell Mol Life Sci. 2004;61:49–68. doi: 10.1007/s00018-003-3148-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly EJ, Quaife CJ, Froelick GJ, Palmiter RD. Metallothionein I and II protect against zinc deficiency and zinc toxicity in mice. J Nutr. 1996;126:1782–1790. doi: 10.1093/jn/126.7.1782. [DOI] [PubMed] [Google Scholar]
- Kow LM, Pfaff DW. Cyclo (his-pro) potentiates the reduction of food intake induced by amphetamine, fenfluramine, or serotonin. Pharamcol Biochem Behav. 1991;38:365–369. doi: 10.1016/0091-3057(91)90292-a. [DOI] [PubMed] [Google Scholar]
- Levenson CW. Zinc regulation of food intake: new insights on the role of neuropeptide Y. Nutr Rev. 2003;61:247–249. doi: 10.1301/nr.2003.jul.247-249. [DOI] [PubMed] [Google Scholar]
- Mangian HF, Lee RG, Paul GL, Emmert JL, Shay NF. Zinc deficiency suppresses plasma leptin concentration in rats. J Nutr Biochem. 1998;9:47–51. [Google Scholar]
- Mantzoro CS, Prasad AS, Beck FWJ, Crabowski S, Kaplan J, Adair C, et al. Zinc may regulate serum leptin concentrations in humans. J Am Coll Nutr. 1998;17:270–275. doi: 10.1080/07315724.1998.10718758. [DOI] [PubMed] [Google Scholar]
- Meistas MT, Margolis S, Kowarski AA. Hyperinsulinemia of obesity is due to decreased clearance of insulin. Am J Physiol Endocrinol Metab. 1983;245:E155–E159. doi: 10.1152/ajpendo.1983.245.2.E155. [DOI] [PubMed] [Google Scholar]
- Mora MEV, Scarfone A, Calvani M, Grecco AV. Insulin clearance in obesity. J Am coll Nutr. 2003;22:487–493. doi: 10.1080/07315724.2003.10719326. [DOI] [PubMed] [Google Scholar]
- Morgan WT. Human serum histidine-proline-rich glycoprotein. Biochim Biophys Acta. 1978;533:319–333. doi: 10.1016/0005-2795(78)90098-3. [DOI] [PubMed] [Google Scholar]
- Morley JE, Levine AS, Prasad C. Histidyl-proline diketopiperazine decreases food intake in rats. Brain Res. 1981;210:475–478. doi: 10.1016/0006-8993(81)90930-6. [DOI] [PubMed] [Google Scholar]
- Ott ES, Shay NF. Zinc deficiency reduces leptin gene expression and leptin in rat adipocytes. Exp Biol Med. 2001;226:841–846. doi: 10.1177/153537020122600906. [DOI] [PubMed] [Google Scholar]
- Ozata M, Mergen M, Oktenli C, Aydin A, Sanisoglu SY, Bolu E, et al. Increased oxidative stress and hypozincemia in male obesity. Clin Biochem. 2002;35:627–631. doi: 10.1016/s0009-9120(02)00363-6. [DOI] [PubMed] [Google Scholar]
- Perlman RK, Rosner MR. Identification of zinc ligands of the insulin degrading enzyme. J Biol Chem. 1994;269:33140–33145. [PubMed] [Google Scholar]
- Pocai A, Morgan K, Buettner C, Gutierrez-Juarez R, Obici S, Rossetti L. Central leptin acutely reverses diet-induced hepatic insulin resistance. Diabetes. 2005;54:3182–3189. doi: 10.2337/diabetes.54.11.3182. [DOI] [PubMed] [Google Scholar]
- Prasad C. Cyclo (His-Pro): its distribution, origin and function in the human. Neurosci Biobehav Rev. 1988;12:19–22. doi: 10.1016/s0149-7634(88)80069-1. [DOI] [PubMed] [Google Scholar]
- Prasad C, Mizuma H, Brock JW, Porter JR, Svec F, Hilton C. A paradoxical elevation of brain cyclo (his-pro) levels in hyperphagic obese Zucker rats. Brain Res. 1995;699:149–153. doi: 10.1016/0006-8993(95)01022-n. [DOI] [PubMed] [Google Scholar]
- Rosenthal MJ, Hwang IK, Song MK. Effects of arachidonic acid and cyclo(his-pro) on zinc transport across small intestine and muscle tissues. Life Sci. 2003;70:337–348. doi: 10.1016/s0024-3205(01)01395-9. [DOI] [PubMed] [Google Scholar]
- Scarpace PJ, Tumer N. Peripheral and hypothalamic leptin resistance with age-related obesity. Physiol Behav. 2001;74:721–727. doi: 10.1016/s0031-9384(01)00616-3. [DOI] [PubMed] [Google Scholar]
- Song MK, Hwang IK, Rosenthal MJ, Harris DM, Yamaguchi DT, Yip I, et al. Anti-hyperglycemic activity of zinc plus cyclo (his-pro) in genetically diabetic Goto-Kakizaki and aged rats. Exp Biol Med. 2003;228:1338–1345. doi: 10.1177/153537020322801112. [DOI] [PubMed] [Google Scholar]
- Stefan M, Nicholls RD. What have rare genetic syndromes taught us about the pathophysiology of the common forms of obesity? Curr Diab Rep. 2005;4:143–150. doi: 10.1007/s11892-004-0070-0. [DOI] [PubMed] [Google Scholar]
- Takita S, Wakamoto Y, Kunitsugu I, Sugiyama S, Okuda M, Houbara T. Altered tissue concentration of mineral in spontaneousl diabetic rats (Goto-Kakizaki rats) J Toxicol Sci. 2004;29:195–199. doi: 10.2131/jts.29.195. [DOI] [PubMed] [Google Scholar]
- Taneja SK, Mahajan M, Arya P. Excess bioavailability of zinc may cause obesity in humans. Experientia. 1996;52:31–33. doi: 10.1007/BF01922412. [DOI] [PubMed] [Google Scholar]
- Tungtrongchitr R, Pongpaew P, Phonrat B, Tungtrongchitr A, Viroonudomphol D, Vudhivai N, et al. Serum copper, zinc, ceruloplasmin and superoxide dismutase in Thai overweight and obese. J Med Assoc Thai. 2003;86:543–551. [PubMed] [Google Scholar]
- Vona-Davis L, Howard-McNatt M, Rose DP. Adiposity, type 2 diabetes and the metabolic syndrome in breast cancer. Obes Rev. 2007;8:395–408. doi: 10.1111/j.1467-789X.2007.00396.x. [DOI] [PubMed] [Google Scholar]
- Yakinci C, Pac A, Kucukbay FZ, Tayfun M, Gul A. Serum zinc, copper, and magnesium levels in obese children. Acta Pediatr Jpn. 1997;39:339–341. doi: 10.1111/j.1442-200x.1997.tb03748.x. [DOI] [PubMed] [Google Scholar]
- Yamada M, Wilber JF. The distribution of histidyl-proline diketopiperazine [cyclo(His-Pro)] in discrete rat hypothalamic nuclei. Neuropeptides. 1989;13:221–223. doi: 10.1016/0143-4179(89)90074-7. [DOI] [PubMed] [Google Scholar]
- Yildiz BO, Haznedaroglu IC. Rethinking leptin and insulin action: therapeutic opportunities for diabetes. Int J Biochem Cell Biol. 2005;38:820–830. doi: 10.1016/j.biocel.2005.09.013. [DOI] [PubMed] [Google Scholar]


