Abstract
Background and purpose:
Endocannabinoids in tissues controlling energy homeostasis are altered in obesity, thus contributing to metabolic disorders. Here we evaluate endocannabinoid dysregulation in the small intestine of mice with diet-induced obesity (DIO) and in peripheral tissues of Zucker and lean rats following food deprivation and re-feeding.
Experimental approach:
Intestinal transit, evaluated using rhodamine-B-labelled dextran, and small intestinal endocannabinoid levels, measured by liquid chromatography mass spectrometry, were measured in mice fed normal or high-fat diets (HFDs). Endocannabinoid levels were measured also in various tissues of lean and Zucker rats fed ad libitum or following overnight food deprivation with and without subsequent re-feeding.
Key results:
After 8 weeks of HFD, baseline intestinal transit was increased in DIO mice and enhanced by cannabinoid CB1 receptor antagonism less efficaciously than in lean mice. Small intestinal anandamide and 2-arachidonoylglycerol levels were reduced and increased respectively. In Zucker rats, endocannabinoids levels were higher in the pancreas, liver and duodenum, and lower in the subcutaneous adipose tissue. Food deprivation increased endocannabinoid levels in the duodenum and liver of both rat strains, in the pancreas of lean rats and in adipose tissues of Zucker rats.
Conclusions and implications:
Reduced anandamide levels might account for increased intestinal motility in DIO mice. Regulation of endocannabinoid levels in rat peripheral tissues, induced by food deprivation and re-feeding, might participate in food intake and energy processing and was altered in Zucker rats. These data, together with previous observations, provide further evidence for dysregulation of peripheral endocannabinoids in obesity.
Keywords: 2-arachidonoylglycerol, anandamide, CB1, gastrointestinal motility, obesity, liver, pancreas, adipose tissue, insulin, hyperglycemia
Introduction
It is becoming generally accepted that the endocannabinoid system, and particularly its most studied components, the endocannabinoids anandamide (AEA) and 2-arachidonoylglycerol (2-AG), and the cannabinoid type-1 (CB1) receptors (nomenclature follows Alexander et al., 2008), are deeply involved in the control of food intake and energy homeostasis (see Matias and Di Marzo, 2007; Matias et al., 2007; Cota, 2008; Jesudason and Wittert, 2008; Pagano et al., 2008). Therefore, it is not surprising that there is increasing evidence for the involvement of these molecules also in obesity and related metabolic and cardiovascular disorders (see Bellocchio et al., 2007; Mendizábal and Adler-Graschinsky, 2007; Di Marzo, 2008a; Scheen and Paquot, 2008). This evidence is paralleled by the clinical development of CB1 receptor antagonists for the treatment of obesity (Van Gaal et al., 2005; Addy et al., 2008), dyslipidemia (Després et al., 2005), type 2 diabetes (Scheen et al., 2006) and atherosclerosis (Di Marzo, 2008b; Nissen et al., 2008). In fact, it was hypothesized (Di Marzo and Matias, 2005) that the high efficacy of CB1 receptor antagonists at reducing body weight in obese animals and humans, in a way also independent from food intake inhibition, is due to the occurrence of a general up-regulation of the endocannabinoid system in obesity, not only at the central (Di Marzo et al., 2001), but also at the peripheral (Engeli et al., 2005; Monteleone et al., 2005; Osei-Hyiaman et al., 2005; Matias et al., 2006; 2008a;) level.
Loss of hormone regulation over endocannabinoid levels is emerging as one of the main reasons for dysregulated endocannabinoid levels during obesity. In the hypothalamus, where leptin tonically down-regulates endocannabinoid biosynthesis, it was not surprising to observe elevated endocannabinoid levels in obese rodents with a malfunctioning leptin signalling system (Di Marzo et al., 2001). More intriguingly, it was recently shown that central leptin negatively controls endocannabinoid levels also in the white adipose tissue (WAT) (Buettner et al., 2008). In peripheral organs, however, also insulin seems to down-regulate endocannabinoid levels, or up-regulate endocannabinoid degradation, in lean animals, as shown so far in rat insulinoma cells (Matias et al., 2006), mouse 3T3L1 adipocytes (D'Eon et al., 2008) and human subcutaneous fat (Murdolo et al., 2007). Therefore, not only central leptin resistance, but also peripheral loss of sensitivity to insulin might result in elevated endocannabinoid levels. Accordingly, this phenomenon appears to precede the development of overt obesity and accompanies hyperglycemia in the liver (Osei-Hyiaman et al., 2005), pancreas (Starowicz et al., 2008), brown adipose tissue, soleus muscle and heart (Matias et al., 2008a) of mice fed for several weeks with high-fat diets (HFDs). In these mice, a striking redistribution of endocannabinoid tone in the various WAT depots, with decreased, unchanged and increased levels in the subcutaneous, mesenteric and epidydimal fat, respectively, was observed (Matias et al., 2006; D'Eon et al., 2008; Starowicz et al., 2008). By contrast, discrepant, and possibly species-specific, data exist regarding the dysregulation of CB1 receptors in the visceral and subcutaneous fat of rodents with HFD-induced obesity (DIO), with reports of either over-expression in rats (Yan et al., 2006) or no changes in mice (Starowicz et al., 2008).
In agreement with the clinical data with CB1 receptor antagonists, peripheral elevation of endocannabinoid tone has been reported also in obese humans, for example, in overweight/obese women with binge eating disorder (Monteleone et al., 2005) and in obese post-menopausal women (Engeli et al., 2005), in whom elevated blood levels of endocannabinoids were reported. Similar to observations in DIO mice (Matias et al., 2006; Starowicz et al., 2008), elevation of only 2-AG levels was observed in the visceral, but not subcutaneous, adipose tissue of overweight/obese humans (Matias et al., 2006). Two subsequent studies showed a positive correlation between 2-AG, but again not AEA, plasma levels and the amount of intra-abdominal fat (IAA) (measured by computer tomography) in obese humans (Blüher et al., 2006; Côtéet al., 2007). In these studies, plasma 2-AG levels also positively correlated with several cardiometabolic risk factors, such as decreased high density lipoprotein (HDL)-cholesterol and increased triacylglycerol levels, and indexes of insulin resistance. Accordingly, in overweight/non-obese patients with type 2 diabetes, both AEA and 2-AG plasma levels were found to be elevated as compared with non-diabetic matched controls (Matias et al., 2006), thus underlying again the potential link between insulin resistance and elevated endocannabinoid tone.
Dysregulated endocannabinoid levels (and hence CB1 receptor activity) might affect all those biological actions that are exerted through the endocannabinoid system in various organs. For example, in the adipose tissue, where CB1 receptor activation stimulates adipogenesis and lipogenesis (Matias et al., 2006; Bellocchio et al., 2008), the higher endocannabinoid tone in the visceral versus subcutaneous fat might contribute to accumulation of fat in the former depot at the expense of the latter. This phenomenon, given the proposed role of IAA in insulin resistance and atherogenic inflammation (Després et al., 2005), might in turn facilitate the development of type 2 diabetes and atherosclerosis. If CB1 receptor stimulation inhibits adiponectin expression and release, as shown so far in mouse and rat (Matias et al., 2006; Perwitz et al., 2006) mature/hypertrophic adipocytes, this scenario would be even worse, as this adipokine protects skeletal muscle against insulin resistance and counteracts atherogenic inflammation by inhibiting the release and effect of pro-inflammatory cytokines. In the liver, up-regulation of both CB1 receptors and endocannabinoid levels is known to facilitate excessive hepatic fatty acid and triglyceride production, which in turn contribute to insulin resistance and low-HDL/high-very low density lipoprotein (VLDL)-cholesterol, respectively, as well as to fatty liver (Osei-Hyiaman et al., 2005; 2008;). Generalized endocannabinoid level up-regulation, such as after administration of a potent inhibitor of AEA and 2-AG degradation, also directly enhances plasma triacylglycerol levels by impairing apolipoprotein E-mediated clearance of triacylglycerol-rich lipoproteins (Ruby et al., 2008). In the pancreas, the consequences of endocannabinoid overactivity might include excessive insulin release (Matias et al., 2006; Bermúdez-Silva et al., 2008), which, combined with reduced insulin sensitivity, might lead to further hyperglycemia and eventually to β-cell hypertrophy and death and, hence, type 2 diabetes (Di Marzo, 2008a). In the stomach, a recent study showed that DIO in mice is accompanied by reduced gastric AEA and CB1 expression levels and delayed gastric emptying (Di Marzo et al., 2008). As CB1 receptor activation also reduces gastric emptying, the authors concluded that in this organ a dysregulated endocannabinoid system was not responsible for the observed HFD-induced reduction in gastric emptying (which, however, if anything, would counteract food intake). However, a potential obesity-driven dysregulation of endocannabinoid levels in the small intestine and duodenum, where CB1 receptors control motility (and hence to some extent nutrient absorption) and satiety, respectively, has not yet been investigated.
Another issue that also remains to be investigated is how obesity affects the widely described food deprivation and re-feeding-induced increases and decreases, respectively, in AEA levels in the duodenum (Gómez et al., 2002; Petersen et al., 2006; Fu et al., 2007). In fact, in the hypothalamus, where endocannabinoids are negatively and positively controlled, respectively, by hormones the levels of which decrease (in the case of leptin) or increase (in the case of ghrelin and glucocorticoids) following food deprivation, which then undergo opposite changes following food consumption (Di Marzo et al., 2001; Malcher-Lopes et al., 2006; Matias and Di Marzo, 2007,Kola et al., 2008; for review), it is easy to explain why food deprivation causes an increase in 2-AG levels that is fully reversed by re-feeding (Kirkham et al., 2002). Furthermore, the well-established observation that these hormones become either ineffective (leptin) or over-produced (ghrelin and glucocorticoids) during obesity, also allows to explain the overproduction of endocannabinoids in the hypothalamus of obese animals (Di Marzo et al., 2001). By contrast, little is known of the mechanisms through which AEA levels are elevated in the duodenum following food deprivation and reduced following food consumption, apart from the suggestion that this phenomenon is not due to changes in biosynthetic and/or degradative enzymes (Fu et al., 2007), but rather to different availability of biosynthetic precursors (Petersen et al., 2006). Possibly more important from a clinical standpoint, it is not known whether similar physiologically relevant changes occur also in other peripheral tissues controlling energy homeostasis, such as the WAT depots, liver and pancreas, and if they are subject to pathogenic dysregulation following the development of obesity.
In order to provide an answer to some of the aforementioned open questions, we have investigated here the regulation and dysregulation of endocannabinoids in the small intestine of lean or DIO mice and in several peripheral tissues of lean or congenitally obese rats, following food deprivation and consumption. We have used DIO mice to investigate the role of potentially dysregulated endocannabinoid levels in the small intestine of obese mice because this model is more relevant to the human condition and also because we knew from previous data that these animals, apart from delayed gastric emptying (Di Marzo et al., 2008), undergo adaptive changes in their gastric endocannabinoid system (Aviello et al., 2008; Di Marzo et al., 2008). On the other hand, we used Zucker fa/fa rats to investigate the effect of food deprivation/re-feeding on endocannabinoid levels in the liver, pancreas and WAT depots, because similar studies had been performed in lean rats (Gómez et al., 2002; Kirkham et al., 2002; Petersen et al., 2006; Fu et al., 2007), and these animals exhibit a leptin signalling deficiency accompanied by obesity-related metabolic disorders, such as dyslipidaemia, fatty liver and glucose intolerance, which might be due to impaired hepatic, adipose and pancreatic function (Gary-Bobo et al., 2007).
Methods
Animals
All animal care and experimental procedures complied with the guidelines of the University of Naples Federico II. Male, 7 week old C57Bl/6J mice were purchased from Harlan Italy (Corezzana, MI). After 1 week acclimatization, animals were fed a diet containing 25.5% fat (49% of calories), 22% protein and 38.4% carbohydrate (TD97366, Harlan, Italy) for 8 and 14 weeks. Control mice received standard diet containing 5.7% fat (10.9% of calories), 18.9% protein and 57.3% carbohydrate (2018, Harlan, Italy). The fatty acid compositions of the standard and HFD have been reported previously (see ‘STD’ and ‘HFD1’ diets in Matias et al., 2008a), and show that the HFD used here contains significantly higher amounts of saturated and monounsaturated fats and lower amounts of both ω3- and ω6-polyunsaturated fatty acid precursors, that is, linoleic and α-linolenic acids, respectively, although the ratio between the latter fatty acids was similar in the two diets. Body weight was measured weakly. Mice were fed ad libitum, except for the 12 h period immediately preceding killing, which was after treatment for 3, 8 and 14 weeks. Fasting plasma glucose levels were determined in 12 h fasted animals using the glucose test kit with an automatic analyser (AQccu-Chek® Active, Roche, Nutley, NJ, USA) in blood samples obtained from tail vein. Measurements were made at time 0 and after 3, 8 and 14 weeks of dietary treatment.
Seven week old Wistar rats (Harlan, Corezzana, MI, Italy, ∼280 g body weight) and age-matched obese Zucker fa/fa rats (from Charles River, Italy, ∼320 g body weight) were given different feeding regimens, after 1 week of acclimatization.
At the end of the dietary treatments, the small intestine (from mice), and the liver, pancreas, duodenum and adipose (subcutaneous and visceral) tissues (from rats) were removed and immersed into liquid nitrogen, to be stored at −70° until extraction and purification of endocannabinoids.
Drug regimens in mice
Arachidonoylchloroethanolamide (ACEA; 0.125, 0.25, 0.5 and 1.0 mg·kg−1) and rimonabant (0.1, 0.2, 0.4 and 0.8 mg·kg−1) were given i.p. 30 min before the administration of the fluorescent marker. ACEA was purchased from Tocris Cookson (Bristol, UK), while rimonabant [5-(p-chlorophenyl)-1-(2,4-dichlorophenyl)-4-methyl-N-piperidinopyrazole-3-carboxamide hydrochloride] was a gift from Sanofi-Aventis Recherche, Montpellier, France. ACEA and rimonabant were dissolved in dimethyl sulphoxide (1 µL·10 g−1), which per se had no significant effect on intestinal transit.
Measurement of intestinal transit in mice
Transit was measured by evaluating the intestinal location of rhodamine-B-labelled dextran (Capasso et al., 2005; 2008a;). Animals were given fluorescent-labelled dextran (100 µL of 25 mg·mL−1 stock solution) via a gastric tube into the stomach. Twenty min after administration, the entire small intestine with its content was divided into 10 equal parts. The intestinal contents of each bowel segment were vigorously mixed with 2 mL of saline solution to obtain a supernatant containing the rhodamine. The supernatant was centrifuged at 800×g to sediment the intestinal chyme. The fluorescence in duplicate aliquots of the cleared supernatant was read in a multi-well fluorescence plate reader (LS55 Luminescence spectrometer, Perkin Elmer Instruments; excitation 530 ± 5 nm and emission 590 ± 10 nm) for quantification of the fluorescent signal in each intestinal segment. From the distribution of the fluorescent marker along the intestine, we calculated the geometric centre (GC) of small intestinal transit as follows:
 |
GC ranged from 1 (minimal motility) to 10 (maximal motility). This procedure yielded a non-radioactive measurement of intestinal transit.
Treatments in rats
Groups of five Wistar or Zucker rats were either fed ad libitum overnight and before death (‘ad lib’ groups, killed at 7.30 am), or kept without food overnight and then killed (‘fasted’ groups, killed at 7.30 am) or kept without food overnight until 7 am, then fed for 30 min and then killed.
Measurement of endocannabinoid levels
The extraction, purification and quantification of AEA and 2-AG from tissues require several biochemical steps as described previously (Di Marzo et al., 2001). First, tissues were homogenized (Dounce homgenizer) and extracted with chloroform/methanol/Tris-HCl 50 mmol·L−1 pH 7.5 (2:1:1, by vol.) containing internal standards ([2H]8 AEA and of [2H]5 2-AG, 10 pmol each). The lipid-containing organic phase was dried down, weighed and pre-purified by open bed chromatography on silica gel. Fractions were obtained by eluting the column with 9:1 (by vol.) chloroform/methanol. After lipid extraction and separation, the pre-purified lipids were then analysed by liquid chromatography-atmospheric pressure chemical ionization-mass spectrometry (LC-APCI-MS) by using a Shimadzu HPLC apparatus (LC-10ADVP) coupled to a Shimadzu (LCMS-2010) quadrupole MS via a Shimadzu APCI interface as previously described (Marsicano et al., 2002). The amounts of endocannabinoids in the tissues, quantified by isotope dilution with the above-mentioned deuterated standards, are expressed as pmol or nmol (g wet tissue)−1.
Statistical analyses
Data are expressed as the mean ± SEM. To determine statistical significance, Student's t-test was used for comparing a single treatment mean with a control mean, and a one-way anova followed by a Tukey-Kramer multiple comparisons test was used for analysis of more than two treatment means. anova followed by the Bonferroni's test was used for comparisons of endocannabinoid levels. P-values <0.05 were considered significant. The dose of ACEA that produced 50% inhibition of intestinal transit (ED50) or maximal inhibitory effect (Emax) were used to characterize its potency and efficacy respectively. The ED50 and Emax values were calculated by non-linear regression analysis using the equation for a sigmoid dose–response curve (GraphPad Prism).
Results
Experiments in DIO mice
After both 8 and 14 weeks of HFD, mice exhibited significantly increased body weight and blood fasting glucose levels with respect to mice fed with normal chow for the same periods of time (Aviello et al., 2008; Di Marzo et al., 2008; Matias et al., 2008a) and were considered to have DIO. These DIO mice also exhibited significantly higher intestinal transit than control mice at both 14 and, particularly, 8 weeks (Figure 1). AEA levels in the small intestine were reduced at both time points, although the effect was more marked after 8 weeks (Table 1). At this time point, but not after 14 weeks, 2-AG levels were significantly higher in DIO than in control mice given the standard diet (Table 1). The selective CB1 receptor antagonist, rimonabant, increased intestinal transit at both time points and it was less active in DIO than in lean mice after 8, but not 14, weeks of HFD (Figure 1). ACEA was tested only after 8 weeks of dietary treatment and inhibited intestinal motility with the same potency and efficacy in lean and DIO mice (lean mice: ED50 0.28 ± 0.10 mg·kg−1; Emax 49 ± 13%. DIO mice: 0.33 ± 0.12 mg·kg−1; Emax 48 ± 12%, n= 5).
Figure 1.
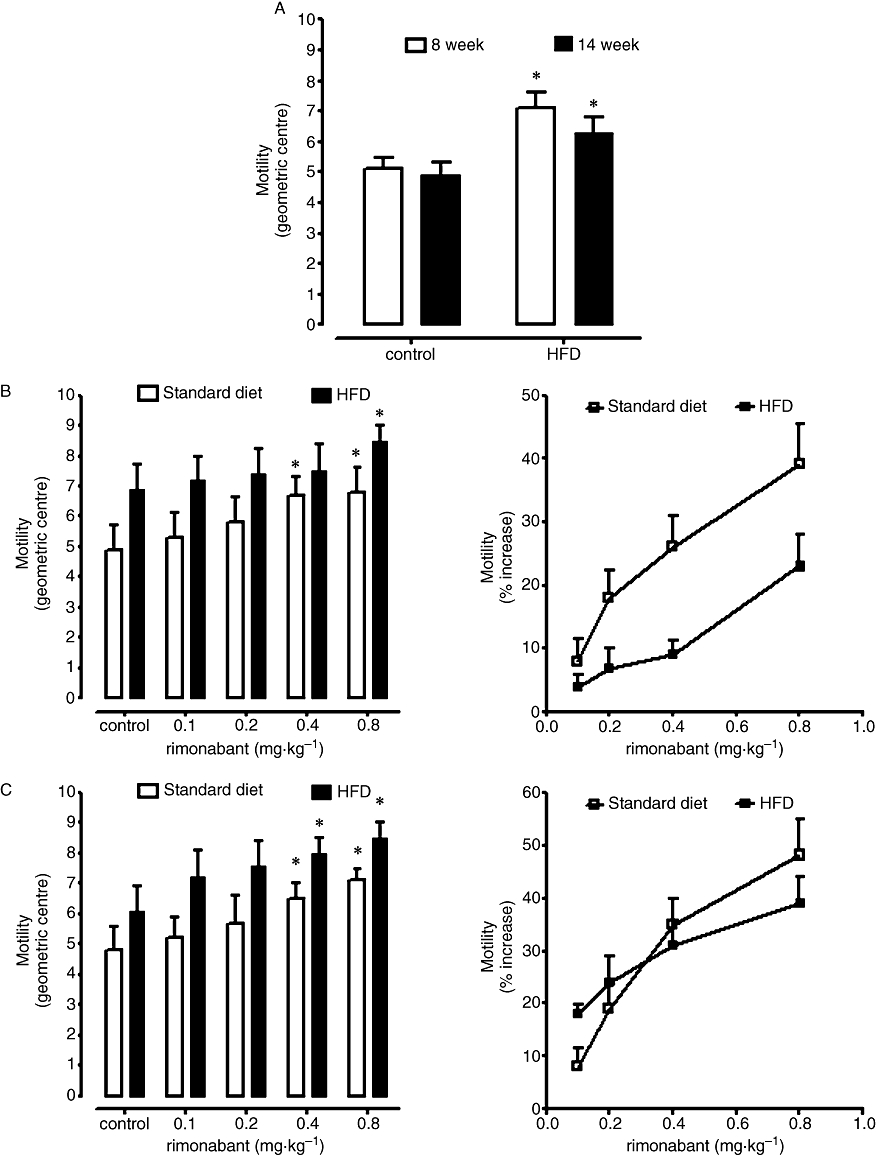
Effect of a standard diet and high-fat diet (HFD) on intestinal transit evaluated after 8 or 14 weeks of dietary treatment (A). (B,C) show the effect of rimonabant (0.1–0.8 mg·kg−1, i.p.) on intestinal transit in mice fed for 8 (B) or 14 weeks (C) a standard diet or an HFD. Results (mean ± SEM of 3–6 mice for each experimental group) are expressed as the geometric centre of the distribution of a fluorescent marker along the small intestine (A,B left panel and C right panel) or as percent of the increase of the corresponding control values (B,C right panels). *P < 0.05 versus corresponding control (A); *P < 0.05 versus corresponding control (B,C, left panels). A statistically significant difference (P < 0.05) was observed between the two dose–response curves reported in B, right panel.
Table 1.
Anandamide (AEA) and 2-arachidonoylglycerol (2-AG) levels in the small intestine of mice fed for up to 8 or 14 weeks with standard (STD) or high-fat diet (HFD)
|
8 weeks |
14 weeks |
|||
|---|---|---|---|---|
|
AEA |
2-AG |
AEA |
2-AG |
|
| (pmol·g−1wet tissue) | (nmol·g−1wet tissue) | (pmol·g−1wet tissue) | (nmol·g−1wet tissue) | |
| STD | 159.7 ± 26.2 | 7.1 ± 1.3 | 123.7 ± 7.2 | 10.9 ± 1.2 |
| HFD | 74.8 ± 9.0* | 16.3 ± 2.8* | 96.1 ± 9.8* | 12.3 ± 2.8 |
Data are means ± SE of four experiments.
P < 0.05 versus corresponding STD samples.
Experiments in lean wild-type and obese Zucker fa/fa rats
In agreement with previous data (Gómez et al., 2002; Petersen et al., 2006; Fu et al., 2007), overnight food deprivation (fasting) caused a significant increase of AEA levels in the duodenum of lean rats, and this effect was reversed by re-feeding after food deprivation (Table 2). For the first time, however, we report here that also 2-AG levels are increased by food deprivation and decreased by re-feeding in this tissue (Table 2). Furthermore, we found that a similar food deprivation-induced elevation of AEA and 2-AG levels occurred also in the liver and pancreas, although in this case re-feeding did not restore the baseline levels of the two compounds, except for AEA levels in the liver (Table 2). No significant changes were induced by these treatments in the two analysed WAT depots (Table 2).
Table 2.
Levels of anandamide and 2-arachidonoylglycerol (2-AG) in various peripheral tissues of wild-type and Zucker obese rats
| Endocannabinoid | Feeding status |
Tissue |
|||||
|---|---|---|---|---|---|---|---|
| Duodenum | Liver | Pancreas | Visceral adipose tissue | Subcutaneous adipose tissue | |||
| Wild type rats | Anandamide (pmol·g−1 wet tissue weight) | Ad lib | 2.1 ± 0.3 | 5.4 ± 0.8 | 14.7 ± 1.7 | 32.7 ± 2.4 | 52.5 ± 5.1 |
| Fasted | 7.8 ± 1.1*** | 37.9 ± 1.0*** | 47.9 ± 5.8** | 32.5 ± 2.7 | 64.8 ± 9.3 | ||
| Re-fed | 3.4 ± 0.3§§ | 10.7 ± 1.7*,§§§ | 28.2 ± 4.2*,§ | 35.9 ± 3.3 | 46.5 ± 7.3 | ||
| 2-AG (nmol·g−1 wet tissue weight) | Ad lib | 6.0 ± 1.2 | 2.8 ± 0.4 | 3.1 ± 0.5 | 1.5 ± 0.3 | 1.7 ± 0.3 | |
| Fasted | 12.8 ± 1.3** | 5.2 ± 0.4** | 5.3 ± 0.1** | 1.3 ± 0.2 | 2.5 ± 0.4 | ||
| Re-fed | 6.4 ± 0.2§§ | 4.6 ± 0.2** | 4.8 ± 0.7* | 1.9 ± 0.3 | 2.7 ± 0.3 | ||
| Zucker rats | Anandamide (pmol·g−1 wet tissue weight) | Ad lib | 4.3 ± 0.7# | 7.2 ± 0.3# | 6.7 ± 0.9 | 18.1 ± 4.4# | 11.2 ± 1.4### |
| Fasted | 37.4 ± 3.4***,## | 26.3 ± 5.9** | 9.6 ± 0.5# | 34.1 ± 5.1* | 24.9 ± 4.2**,## | ||
| Re-fed | 10.8 ± 2.0*,§§,# | 7.5 ± 0.7§§§ | 30.3 ± 5.0§ | 19.1 ± 2.5§,# | 9.9 ± 1.1§§,## | ||
| 2-AG (nmol·g−1 wet tissue weight) | Ad lib | 52.3 ± 5.1### | 5.0 ± 0.5## | 7.2 ± 0.8## | 1.3 ± 0.1 | 1.7 ± 0.3 | |
| Fasted | 236.8 ± 61.8**,## | 5.1 ± 0.7 | 6.8 ± 0.7 | 1.3 ± 0.1 | 1.3 ± 0.2# | ||
| Re-fed | 130.3 ± 28.6## | 5.5 ± 1.0 | 5.2 ± 0.6 | 1.2 ± 0.2 | 1.3 ± 0.2# | ||
For each strain of rats there were three treatment groups, those fed ad libitum (ad lib groups), those fasted overnight (fasted groups) and those fasted overnight and then re-fed for 30 min (re-fed groups). Data are means ± SD of separate determinations in 4–5 rats.
P < 0.05, 0.01 and 0.005 versus lean ‘ad lib’ in the same rat strain respectively;
P < 0.05, 0.01 and 0.005 versus ‘fasted’ in the same rat strain respectively;
P < 0.05, 0.01 and 0.005 versus corresponding group in lean rats respectively.
In obese Zucker fa/fa rats, basal AEA and/or 2-AG levels were higher than in lean rats in the duodenum, pancreas and liver (Table 2). Visceral adipose tissue AEA levels were slightly (∼1.5) lower in Zucker rats whereas the subcutaneous adipose tissue AEA levels were dramatically (∼5-fold) lower in these obese animals than in lean mice. 2-AG levels were unaltered in either the visceral or subcutaneous fat of Zucker or lean wild-type rats (Table 2). In contrast to its effects in lean rats, food deprivation induced a significant increase of AEA levels in both visceral and subcutaneous fat, and this effect was reversed by re-feeding (Table 2). Conversely, the effects of food deprivation and re-feeding observed in the duodenum, liver and pancreas of lean rats were different in Zucker rats (Table 2). In particular, in the duodenum, re-feeding did not any longer reduce 2-AG levels. Both AEA and 2-AG levels after food deprivation and re-feeding in this tissue were dramatically higher than in lean rats after the same treatments. In the liver, the effect of food deprivation on 2-AG levels was lost. In the pancreas, food deprivation did not affect any longer AEA and 2-AG levels, and re-feeding even increased AEA levels. On the other hand, the levels of AEA in this organ after food deprivation were lower than in lean rats after the same treatment.
Discussion
Here we have described new studies aimed at investigating the regulation and dysregulation of endocannabinoid levels in peripheral tissues directly involved in food intake and glucose and lipid processing following either prolonged feeding with an HFD leading to hyperglycemia and DIO in mice, or after food deprivation and re-feeding in lean and genetically obese rats.
Endocannabinoid dysregulation in the small intestine of DIO mice: possible impact on intestinal motility
In the first series of experiments carried out in this study, we have shown that an 8–14 week HFD causes significant changes in small intestine AEA and, after 8 weeks only, 2-AG levels. Interestingly, these two major endocannabinoids showed opposing changes, AEA levels being reduced, and 2-AG levels being increased after 8 weeks of HFD. Both AEA and 2-AG are ultimately derived from arachidonic acid, a polyunsaturated fatty acid produced from the elongation and desaturation of an essential fatty acid, linoleic acid, the amounts of which were lower in the HFD used in this study. Therefore, in view of the previous finding that higher levels of arachidonic acid in the incubation medium of isolated adipocytes increase the cellular amounts of 2-AG, but not AEA (Matias et al., 2008b), it is unlikely that the finding of increased levels of the former endocannabinoid might be due to changes in the availability of its biosynthetic precursor. Furthermore, the amounts of α-linolenic acid, which serves as precursor for ω3-polyunsaturated fatty acids that reduce the levels of both AEA and 2-AG (Matias et al., 2008b), were also significantly lower in the HFD than in the standard diet, and this should have led to higher amounts of both endocannabinoids after prolonged HFD. A recent report emphasized how the relative abundance of certain long chain fatty acids in the diet can influence small intestinal endocannabinoid levels after a relatively short period of time (1 week) (Artmann et al., 2008), whereas another study suggested that the effect of prolonged different HFDs on endocannabinoid tissue levels depends only in part on the composition of the diets (Matias et al., 2008a). Clearly, further studies are required in order to evaluate the impact of polyunsaturated fatty acids (and their precursors) in food on the tissue levels of endocannabinoids and their biosynthetic precursors.
Although it is not unusual to find opposing changes in AEA and 2-AG levels following various physiological or pathological stimuli (see Di Marzo and Maccarrone, 2008), the meaning of the present findings in the small intestine, where activation of CB1 receptors is well known to reduce intestinal transit (Izzo et al., 2001; Carai et al., 2006; Capasso et al., 2008b), is worthwhile being discussed. It is well-established that AEA, or agents capable of prolonging its lifespan by inhibiting its degradation, inhibit intestinal transit via CB1 receptor stimulation, whereas blockade of CB1 receptors by CB1 receptor antagonists causes the opposite effect, thus suggesting that endocannabinoids tonically inhibit motility (see Sanger, 2007; Izzo and Camilleri, 2008; Wright et al., 2008) for reviews). Therefore, the decrease of AEA levels observed here, which was greater after 8 weeks of HFD (∼85 pmol·g−1), might be seen as a possible underlying mechanism for the increased intestinal motility in DIO mice, which was observed particularly at this time point. In fact, the functional activity of small intestinal CB1 receptors per se, assessed by measuring the effect of an exogenous selective CB1 receptor agonist on intestinal motility, did not appear to change following 8 weeks of HFD, thus rendering changes in endocannabinoid levels a potentially important determinant in small intestinal CB1 receptor tone. The present finding that blockade of CB1 receptors by rimonabant increases intestinal transit less efficaciously in DIO than in lean mice is also suggestive of a lower CB1 receptor tone in the small intestine of DIO mice. In fact, rimonabant would be expected to be less active in the presence of lower constitutive or endocannabinoid-mediated CB1 receptor activity. On the other hand, the ∼100-fold higher increase of the small intestinal levels of 2-AG after 8 weeks of HFD (∼9.2 nmol·g−1) should have prevailed, at least at this time point, on the smaller molar reduction of AEA levels, even accounting for the somewhat reduced affinity of 2-AG for CB1 receptors. In this case, contrary to our findings, HFD should have been accompanied by reduced motility and rimonabant should have been more active in DIO mice. It is possible that the measured increase of 2-AG levels in DIO mice occurs at cellular sites different from those in which inhibition of intestinal motility by CB1 receptors is exerted [i.e. mostly at the level of cholinergic myenteric neurons (Sanger, 2007; Izzo and Camilleri, 2008; Wright et al., 2008)]. Therefore, further studies on the cellular origin of the 2-AG and AEA measured here need to be performed before making any further speculation on the endocannabinoid-mediated mechanisms whereby intestinal motility in DIO mice is reduced.
Our present results allow us to discard the possibility that the reduction by rimonabant of body weight in rodents is partly due to its increase of intestinal motility, an effect that is also observed in obese patients (Van Gaal et al., 2008) and that might lead to reduced nutrient adsorption. In fact, it is well-established that rimonabant, a CB1 receptor antagonist with multiple beneficial effects (Costa, 2007; Croci and Zarini, 2007; Di Marzo and Szallasi, 2008; Malfitano et al., 2008; Schäfer et al., 2008), is more effective at reducing body weight in obese versus lean rodents (Vickers et al., 2003), whereas the opposite was observed here with regard to increased intestinal transit. Furthermore, our present observation of opposing changes in intestinal AEA and 2-AG levels in DIO mice might be relevant to potential effects of the molecular targets of these compounds on either food intake-regulatory intestinal peptides (such as cholecystokinin, peptide YY and oxyntomodulin) or incretins (such as glucose-dependent insulinotropic polypeptide and glucagon-like peptide-1). Specific studies are now needed to investigate the intriguing possibility that intestinal polypeptides participate in some of the noxious effects of dysregulated CB1 receptors on glucose and lipid metabolism.
Effect of food deprivation/re-feeding on endocannabinoid levels in the duodenum, liver, pancreas and WAT of lean rats
The present finding of decreased small intestinal AEA levels following prolonged consumption of HFD in mice might be linked to the previous finding of increased AEA levels in the duodenum (the initial part of the small intestine) of rats following food deprivation (Gómez et al., 2002; Petersen et al., 2006; Fu et al., 2007). This finding might suggest that in this tissue, the levels of this endocannabinoid are always regulated in a way to adapt to transient shortage or chronic abundance of nutrients, not only by adjusting intestinal transit but, perhaps more importantly, by inhibiting or stimulating satiety, respectively, via the sensory and vagal afferents to the brainstem that originate from the duodenum (Gómez et al., 2002; Burdyga et al., 2004; Capasso and Izzo, 2008). In the series of experiments carried here in rats, we confirmed that food deprivation enhances, and re-feeding reduces, duodenal AEA levels. Furthermore, we also report for the first time that 2-AG levels are regulated in the same way in these animals. We have also observed here that food deprivation exerts in the liver and pancreas, but not in the WAT, of lean rats effects on endocannabinoid levels similar to those reported previously and here in the duodenum. In these two tissues, however, re-feeding only partially restored baseline AEA levels, without affecting 2-AG levels. These data are potentially relevant to what we know about the possible role played by CB1 receptors in these two organs in lean rodents. Our finding of an incomplete reversal of increased endocannabinoid tone following food deprivation in the pancreas might be partly explained by the previously reported negative control by both insulin and leptin over endocannabinoid levels in insulinoma β-cells (Matias et al., 2006). Reduced leptin and insulin levels in food deprivation would cause dis-inhibition of endocannabinoid levels in the endocrine pancreas. However, following food intake, leptin and insulin levels are elevated, and yet pancreatic 2-AG levels are not reduced, possibly because they are stimulated by other hormones released following food intake or by glucose (Matias et al., 2006), and are needed to further stimulate glucose-induced insulin release, necessary for new energy processing. Indeed, CB1 receptor stimulation in β-cells from human pancreatic islets and rat insulinoma cells enhances glucose-induced insulin release (Matias et al., 2006; Bermúdez-Silva et al., 2008). In hepatocytes from lean mice, CB1 receptors are coupled to reduced fatty acid oxidation and increased fatty acid synthesis (Osei-Hyiaman et al., 2005). Thus, elevated hepatic endocannabinoid levels following food deprivation might counterbalance the hepatic oxidation of fatty acids and elevate circulating free fatty acids, which might be needed for glucose-induced insulin secretion when fasting is terminated (Stein et al., 1996). On the other hand, following food intake, when easily utilizable ‘fuel’ becomes again available, 2-AG (but not AEA) levels remain elevated to make sure that at least some of this fuel is directed into fatty acid synthesis. Finally, the fact that there is no increase in endocannabinoid levels in WAT following food deprivation is in agreement with the adipogenic and pro-lipogenic role of the endocannabinoid system in this tissue (Bellocchio et al., 2008), as one would expect that during food deprivation some of the energy stored in fat is released via lipolysis, and such an event could not occur in the presence of increased CB1 receptor tone. However, as we did not compare here the expression or functional activity of CB1 receptors in rat tissues following food deprivation and re-feeding, the physiological relevance of the observed differences in endocannabinoid levels needs to be further investigated.
Endocannabinoid dysregulation in the duodenum, liver, pancreas and WAT of obese (fa/fa) rats
Another set of experiments carried out here consisted of repeating the food deprivation and feeding experiments in obese Zucker rats. First of all, we report for the first time that, similar to previous observations in DIO mice (Osei-Hyiaman et al., 2005; Starowicz et al., 2008), Zucker rats exhibit significantly higher endocannabinoid (2-AG and/or AEA) levels in the liver and pancreas and dramatically reduced AEA levels in the subcutaneous adipose tissue, with a small decrease of AEA in visceral fat and no changes of 2-AG in both WAT depots. The possible relevance of these findings to the development of fatty liver, hyperinsulinemia, insulin resistance and visceral fat accumulation has been discussed already for DIO mice (see Introduction; Di Marzo, 2008a,b;). Additionally, as already described above for the small intestine of DIO mice (where 2-AG levels are increased and AEA levels reduced as compared with mice fed a standard diet), Zucker rats exhibited dramatically higher levels of 2-AG in the duodenum, and slightly higher levels of AEA too. These alterations might be responsible, in part, for the hyperphagia typical of these animals, which is also due to impaired satiety mechanisms (Gómez et al., 2002; Burdyga et al., 2004). Furthermore, and perhaps more importantly, the pattern of food deprivation and re-feeding-induced effects on peripheral endocannabinoid levels was quite different in these obese and hyperglycaemic/dyslipidaemic rodents. In the liver, the potentially pro-lipogenetic action of food deprivation-induced enhanced AEA levels is maintained, thus possibly underlying in part also the development of fatty liver, which Zucker rats exhibit at a late stage in their life (Gary-Bobo et al., 2007). However, re-feeding still caused a reduction of hepatic AEA levels. In the pancreas, food deprivation did not change the basal levels of both endocannabinoids, and re-feeding even increased AEA levels. This indicates that the elevated or unaltered pancreatic basal levels of 2-AG or AEA, respectively, in Zucker rats are not subject to regulation during feeding behaviour, thus possibly resulting in dysregulation of insulin release from β-cells. In fact, the elevated AEA levels observed after re-feeding might be responsible in part for exaggerated post-prandial hyperinsulinemia in these animals. In the duodenum, endocannabinoid levels were increased by food deprivation to a greater extent than in lean mice, and AEA and 2-AG levels in food-deprived Zucker rats were ∼5 and almost 20-fold higher, respectively, than those measured in fasted lean rats. Furthermore, unlike those in lean rats, 2-AG levels were not reduced by re-feeding and duodenal levels of both endocannabinoids after re-feeding were again ∼3 and ∼20-fold higher than in lean rats. This phenomenon is again likely to be related to the typical hyperphagia of these animals, which are in a permanently ‘hungry’ state, but it might also result in dysregulation of intestinal polypeptides affecting metabolism (see above). On the other hand, although basal AEA levels are almost unaltered or markedly reduced in the visceral and subcutaneous adipose tissue of Zucker rats, respectively, they become stimulated by food deprivation and reduced by re-feeding. This ‘out-of-phase’ activation/inactivation of a pro-lipogenic and adipogenic system with respect to energy replenishment might impair the capability of the animal to store fat in the WAT after feeding, particularly in the ‘healthier’ subcutaneous depot, and thus contribute to ectopic fat accumulation outside the WAT. Also in the case of Zucker rats, however, further speculations on the pathological significance of the observed changes in peripheral tissue endocannabinoid levels following food deprivation and re-feeding will have to await a full evaluation of the expression or functional activity of CB1 receptors under the various experimental conditions used here.
What are the possible mechanisms of the alterations in basal and food deprivation/re-feeding-induced changes in endocannabinoid levels observed here in Zucker rats? These animals are characterized by systemic impairment of leptin signalling, which was suggested to inhibit tonically endocannabinoid levels in both the hypothalamus and WAT (Di Marzo et al., 2001; Buettner et al., 2008). However, while Zucker rats do exhibit elevated endocannabinoid levels in the hypothalamus (Di Marzo et al., 2001), they did not show here increased endocannabinoid levels in the WAT (if anything, the contrary effect was observed), thus suggesting that, at least in this species, leptin might not tonically control WAT endocannabinoid levels. Clearly, also the alterations in food deprivation/re-feeding-induced changes of endocannabinoid levels observed in Zucker rats cannot be due to alterations in leptin sensitivity. However, as mentioned above, insulin, which should have a more global action than leptin, was also suggested to control endocannabinoid levels (Matias et al., 2006; D'Eon et al., 2008), and Zucker rats are insulin-resistant. Therefore, at least the increased hepatic and pancreatic 2-AG and/or AEA levels can be ascribed in part to insulin resistance, which in fact might be responsible also for the fact that the fasting induced-elevation of endocannabinoid levels observed here in these same tissues in Zucker rats was significantly lower than in lean rats. Conversely, the finding of fasting induced-elevation of endocannabinoid levels in the WAT of Zucker, but not lean, rats clearly cannot be due to improved insulin sensitivity. Other possible reasons might be changes in hormones other than insulin and leptin that, like the latter, act on the WAT, and the plasma levels of which are still modulated by fasting and reduced by re-feeding in obese animals. For example, glucocorticoids, are not higher in the plasma of ad libitum fed Zucker rats compared with lean rats, but are elevated by fasting significantly more in the former animals (Doyon et al., 2006). Such hormonal variations might explain the stronger effects of food deprivation observed here in the duodenum and adipose tissues of these obese animals.
In conclusion, we have reviewed here the increasing evidence in favour of a dysregulation of endocannabinoid tone in obesity and hyperglycaemia, with particular emphasis on peripheral tissues involved in energy homeostasis, such as the proximal intestine, liver, pancreas and WAT. Furthermore, we have provided new data, obtained in two different animal models of obesity and in the corresponding lean controls, on endocannabinoid regulation and dysregulation during obesity and/or hyperglycemia, in these tissues. Our data, together with the previous findings discussed here, suggest that peripheral dysregulation of endocannabinoid levels in obesity might affect functions ranging from food intake and nutrient processing in the gastrointestinal tract to metabolic control by the liver, pancreas and WAT, also in relation to the regulation of these functions during energy depletion and replenishing.
Acknowledgments
This work was partly supported by a research grant by Sanofi-Aventis. The authors are grateful to Davide Castelluccio for technical assistance.
Glossary
Abbreviations:
- 2-AG
2-arachidonoylglycerol
- ACEA
arachidonoylchloroethanolamide
- AEA
anandamide
- DIO
high-fat diet-induced obesity
- GC
geometric centre
- HFD
high-fat diet
- IAA
intra-abdominal fat
- WAT
white adipose tissue
Conflict of interest
This work was partly supported by a research grant by Sanofi-Aventis.
References
- Addy C, Wright H, Van Laere K, Gantz I, Erondu N, Musser BJ, et al. The acyclic CB1R inverse agonist taranabant mediates weight loss by increasing energy expenditure and decreasing caloric intake. Cell Metab. 2008;7:68–78. doi: 10.1016/j.cmet.2007.11.012. [DOI] [PubMed] [Google Scholar]
- Alexander SPH, Mathie A, Peters JA. Guide to Receptors and Channels (GRAC) Br J Pharmacol. (3rd edn.) 2008;153:S1–S209. doi: 10.1038/sj.bjp.0707746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Artmann A, Petersen G, Hellgren LI, Boberg J, Skonberg C, Nellemann C, et al. Influence of dietary fatty acids on endocannabinoid and N-acylethanolamine levels in rat brain, liver and small intestine. Biochim Biophys Acta. 2008;1781:200–212. doi: 10.1016/j.bbalip.2008.01.006. [DOI] [PubMed] [Google Scholar]
- Aviello G, Matias I, Capasso R, Petrosino S, Borrelli F, Orlando P, et al. Inhibitory effect of the anorexic compound oleoylethanolamide on gastric emptying in control and overweight mice. J Mol Med. 2008;86:413–422. doi: 10.1007/s00109-008-0305-7. [DOI] [PubMed] [Google Scholar]
- Bellocchio L, Vicennati V, Cervino C, Pasquali R, Pagotto U. The endocannabinoid system in the regulation of cardiometabolic risk factors. Am J Cardiol. 2007;100:17P. doi: 10.1016/j.amjcard.2007.10.009. [DOI] [PubMed] [Google Scholar]
- Bellocchio L, Cervino C, Vicennati V, Pasquali R, Pagotto U. Cannabinoid type 1 receptor: another arrow in the adipocytes' bow. J Neuroendocrinol. 2008;20(Suppl. 1):130–138. doi: 10.1111/j.1365-2826.2008.01682.x. [DOI] [PubMed] [Google Scholar]
- Bermúdez-Silva FJ, Suárez J, Baixeras E, Cobo N, Bautista D, Cuesta-Muñoz AL, et al. Presence of functional cannabinoid receptors in human endocrine pancreas. Diabetologia. 2008;51:476–487. doi: 10.1007/s00125-007-0890-y. [DOI] [PubMed] [Google Scholar]
- Blüher M, Engeli S, Klöting N, Berndt J, Fasshauer M, Bátkai S, et al. Dysregulation of the peripheral and adipose tissue endocannabinoid system in human abdominal obesity. Diabetes. 2006;55:3053–3060. doi: 10.2337/db06-0812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buettner C, Muse ED, Cheng A, Chen L, Scherer T, Pocai A, et al. Leptin controls adipose tissue lipogenesis via central, STAT3-independent mechanisms. Nat Med. 2008;14:667–675. doi: 10.1038/nm1775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burdyga G, Lal S, Varro A, Dimaline R, Thompson DG, Dockray GJ. Expression of cannabinoid CB1 receptors by vagal afferent neurons is inhibited by cholecystokinin. J Neurosci. 2004;24:2708–2715. doi: 10.1523/JNEUROSCI.5404-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capasso R, Izzo AA. Gastrointestinal regulation of food intake: general aspects and focus on anandamide and oleoylethanolamide. J Neuroendocrinol. 2008;1:39–46. doi: 10.1111/j.1365-2826.2008.01686.x. [DOI] [PubMed] [Google Scholar]
- Capasso R, Matias I, Lutz B, Borrelli F, Capasso F, Marsicano G, et al. Fatty acid amide hydrolase controls mouse intestinal motility in vivo. Gastroenterology. 2005;129:941–951. doi: 10.1053/j.gastro.2005.06.018. [DOI] [PubMed] [Google Scholar]
- Capasso R, Borrelli F, Aviello G, Romano B, Scalisi C, Capasso F, et al. Cannabidiol, extracted from Cannabis sativa, selectively inhibits inflammatory hypermotility in mice. Br J Pharmacol. 2008a;154:1001–1008. doi: 10.1038/bjp.2008.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capasso R, Borrelli F, Cascio MG, Aviello G, Huben K, Zjawiony JK, et al. Inhibitory effect of salvinorin A, from Salvia divinorum, on ileitis-induced hypermotility: cross-talk between kappa-opioid and cannabinoid CB(1) receptors. Br J Pharmacol. 2008b;155:681–689. doi: 10.1038/bjp.2008.294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carai MA, Colombo G, Gessa GL, Yalamanchili R, Basavarajappa BS, Hungund BL. Investigation on the relationship between cannabinoid CB1 and opioid receptors in gastrointestinal motility in mice. Br J Pharmacol. 2006;148:1043–1050. doi: 10.1038/sj.bjp.0706824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa B. Rimonabant: more than an anti-obesity drug? Br J Pharmacol. 2007;150:535–537. doi: 10.1038/sj.bjp.0707139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cota D. Role of the endocannabinoid system in energy balance regulation and obesity. Front Horm Res. 2008;36:135–145. doi: 10.1159/000115362. [DOI] [PubMed] [Google Scholar]
- Côté M, Matias I, Lemieux I, Petrosino S, Alméras N, Després JP, et al. Circulating endocannabinoid levels, abdominal adiposity and related cardiometabolic risk factors in obese men. Int J Obes (Lond) 2007;31:692–699. doi: 10.1038/sj.ijo.0803539. [DOI] [PubMed] [Google Scholar]
- Croci T, Zarini E. Effect of the cannabinoid CB1 receptor antagonist rimonabant on nociceptive responses and adjuvant-induced arthritis in obese and lean rats. Br J Pharmacol. 2007;150:559–566. doi: 10.1038/sj.bjp.0707138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Eon TM, Pierce KA, Roix JJ, Tyler A, Chen H, Teixeira SR. The role of adipocyte insulin resistance in the pathogenesis of obesity-related elevations in endocannabinoids. Diabetes. 2008;57:1262–1268. doi: 10.2337/db07-1186. [DOI] [PubMed] [Google Scholar]
- Després JP, Golay A, Sjöström L. Rimonabant in Obesity-Lipids Study Group. Effects of rimonabant on metabolic risk factors in overweight patients with dyslipidemia. N Engl J Med. 2005;353:2121–2134. doi: 10.1056/NEJMoa044537. [DOI] [PubMed] [Google Scholar]
- Di Marzo V. The endocannabinoid system in obesity and type 2 diabetes. Diabetologia. 2008a;51:1356–1367. doi: 10.1007/s00125-008-1048-2. [DOI] [PubMed] [Google Scholar]
- Di Marzo V. Play an ADAGIO with a STRADIVARIUS: the right patient for CB1 receptor antagonists? Nat Clin Pract Cardiovasc Med. 2008b;5:610–612. doi: 10.1038/ncpcardio1319. [DOI] [PubMed] [Google Scholar]
- Di Marzo V, Matias I. Endocannabinoid control of food intake and energy balance. Nat Neurosci. 2005;8:585–589. doi: 10.1038/nn1457. [DOI] [PubMed] [Google Scholar]
- Di Marzo V, Maccarrone M. FAAH and anandamide: is 2-AG really the odd one out? Trends Pharmacol Sci. 2008;29:229–233. doi: 10.1016/j.tips.2008.03.001. [DOI] [PubMed] [Google Scholar]
- Di Marzo V, Szallasi A. Rimonabant in rats with a metabolic syndrome: good news after the depression. Br J Pharmacol. 2008;154:915–917. doi: 10.1038/bjp.2008.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Marzo V, Goparaju SK, Wang L, Liu J, Bátkai S, Járai Z, et al. Leptin-regulated endocannabinoids are involved in maintaining food intake. Nature. 2001;410:822–825. doi: 10.1038/35071088. [DOI] [PubMed] [Google Scholar]
- Di Marzo V, Capasso R, Matias I, Aviello G, Petrosino S, Borrelli F, et al. The role of endocannabinoids in the regulation of gastric emptying: alterations in mice fed a high-fat diet. Br J Pharmacol. 2008;153:1272–1280. doi: 10.1038/sj.bjp.0707682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyon C, Denis RG, Baraboi ED, Samson P, Lalonde J, Deshaies Y, et al. Effects of rimonabant (SR141716) on fasting-induced hypothalamic-pituitary-adrenal axis and neuronal activation in lean and obese Zucker rats. Diabetes. 2006;55:3403–3410. doi: 10.2337/db06-0504. [DOI] [PubMed] [Google Scholar]
- Engeli S, Böhnke J, Feldpausch M, Gorzelniak K, Janke J, Bátkai S, et al. Activation of the peripheral endocannabinoid system in human obesity. Diabetes. 2005;54:2838–2843. doi: 10.2337/diabetes.54.10.2838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu J, Astarita G, Gaetani S, Kim J, Cravatt BF, Mackie K, et al. Food intake regulates oleoylethanolamide formation and degradation in the proximal small intestine. J Biol Chem. 2007;282:1518–1528. doi: 10.1074/jbc.M607809200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gary-Bobo M, Elachouri G, Gallas JF, Janiak P, Marini P, Ravinet-Trillou C, et al. Rimonabant reduces obesity-associated hepatic steatosis and features of metabolic syndrome in obese Zucker fa/fa rats. Hepatology. 2007;46:122–129. doi: 10.1002/hep.21641. [DOI] [PubMed] [Google Scholar]
- Gómez R, Navarro M, Ferrer B, Trigo JM, Bilbao A, Del Arco I, et al. A peripheral mechanism for CB1 cannabinoid receptor-dependent modulation of feeding. J Neurosci. 2002;22:9612–9617. doi: 10.1523/JNEUROSCI.22-21-09612.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izzo AA, Camilleri M. Emerging role of cannabinoids in gastrointestinal and liver diseases: basic and clinical aspects. Gut. 2008;57:1140–1155. doi: 10.1136/gut.2008.148791. [DOI] [PubMed] [Google Scholar]
- Izzo AA, Fezza F, Capasso R, Bisogno T, Pinto L, Iuvone T, et al. Cannabinoid CB1-receptor mediated regulation of gastrointestinal motility in mice in a model of intestinal inflammation. Br J Pharmacol. 2001;134:563–570. doi: 10.1038/sj.bjp.0704293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jesudason D, Wittert G. Endocannabinoid system in food intake and metabolic regulation. Curr Opin Lipidol. 2008;19:344–348. doi: 10.1097/MOL.0b013e328304b62b. [DOI] [PubMed] [Google Scholar]
- Kirkham TC, Williams CM, Fezza F, Di Marzo V. Endocannabinoid levels in rat limbic forebrain and hypothalamus in relation to fasting, feeding and satiation: stimulation of eating by 2-arachidonoyl glycerol. Br J Pharmacol. 2002;136:550–557. doi: 10.1038/sj.bjp.0704767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kola B, Farkas I, Christ-Crain M, Wittmann G, Lolli F, Amin F, et al. The orexigenic effect of ghrelin is mediated through central activation of the endogenous cannabinoid system. PLoS ONE. 2008;3:e1797. doi: 10.1371/journal.pone.0001797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malcher-Lopes R, Di S, Marcheselli VS, Weng FJ, Stuart CT, Bazan NG, et al. Opposing crosstalk between leptin and glucocorticoids rapidly modulates synaptic excitation via endocannabinoid release. J Neurosci. 2006;26:6643–6650. doi: 10.1523/JNEUROSCI.5126-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malfitano AM, Laezza C, Pisanti S, Gazzerro P, Bifulco M. Rimonabant (SR141716) exerts anti-proliferative and immunomodulatory effects in human peripheral blood mononuclear cells. Br J Pharmacol. 2008;153:1003–1010. doi: 10.1038/sj.bjp.0707651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsicano G, Wotjak CT, Azad SC, Bisogno T, Rammes G, Cascio MG, et al. The endogenous cannabinoid system controls extinction of aversive memories. Nature. 2002;418:530–534. doi: 10.1038/nature00839. [DOI] [PubMed] [Google Scholar]
- Matias I, Di Marzo V. Endocannabinoids and the control of energy balance. Trends Endocrinol Metab. 2007;18:27–37. doi: 10.1016/j.tem.2006.11.006. [DOI] [PubMed] [Google Scholar]
- Matias I, Gonthier MP, Orlando P, Martiadis V, De Petrocellis L, Cervino C, et al. Regulation, function, and dysregulation of endocannabinoids in models of adipose and beta-pancreatic cells and in obesity and hyperglycemia. J Clin Endocrinol Metab. 2006;91:3171–3180. doi: 10.1210/jc.2005-2679. [DOI] [PubMed] [Google Scholar]
- Matias I, Gonthier MP, Petrosino S, Docimo L, Capasso R, Hoareau L, et al. Role and regulation of acylethanolamides in energy balance: focus on adipocytes and beta-cells. Br J Pharmacol. 2007;152:676–690. doi: 10.1038/sj.bjp.0707424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matias I, Petrosino S, Racioppi A, Capasso R, Izzo AA, Di Marzo V. Dysregulation of peripheral endocannabinoid levels in hyperglycemia and obesity: effect of high fat diets. Mol Cell Endocrinol. 2008a;286:S66–S78. doi: 10.1016/j.mce.2008.01.026. [DOI] [PubMed] [Google Scholar]
- Matias I, Carta G, Murru E, Petrosino S, Banni S, Di Marzo V. Effect of polyunsaturated fatty acids on endocannabinoid and N-acyl-ethanolamine levels in mouse adipocytes. Biochim Biophys Acta. 2008b;1781:52–60. doi: 10.1016/j.bbalip.2007.11.001. [DOI] [PubMed] [Google Scholar]
- Mendizábal VE, Adler-Graschinsky E. Cannabinoids as therapeutic agents in cardiovascular disease: a tale of passions and illusions. Br J Pharmacol. 2007;151:427–440. doi: 10.1038/sj.bjp.0707261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monteleone P, Matias I, Martiadis V, De Petrocellis L, Maj M, Di Marzo V. Blood levels of the endocannabinoid anandamide are increased in anorexia nervosa and in binge-eating disorder, but not in bulimia nervosa. Neuropsychopharmacology. 2005;30:1216–1221. doi: 10.1038/sj.npp.1300695. [DOI] [PubMed] [Google Scholar]
- Murdolo G, Kempf K, Hammarstedt A, Herder C, Smith U, Jansson PA. Insulin differentially modulates the peripheral endocannabinoid system in human subcutaneous abdominal adipose tissue from lean and obese individuals. J Endocrinol Invest. 2007;30:RC17–RC21. doi: 10.1007/BF03347440. [DOI] [PubMed] [Google Scholar]
- Nissen SE, Nicholls SJ, Wolski K, Rodés-Cabau J, Cannon CP, Deanfield JE, et al. Effect of rimonabant on progression of atherosclerosis in patients with abdominal obesity and coronary artery disease: the STRADIVARIUS randomized controlled trial. JAMA. 2008;299:1547–1560. doi: 10.1001/jama.299.13.1547. [DOI] [PubMed] [Google Scholar]
- Osei-Hyiaman D, DePetrillo M, Pacher P, Liu J, Radaeva S, Bátkai S, et al. Endocannabinoid activation at hepatic CB1 receptors stimulates fatty acid synthesis and contributes to diet-induced obesity. J Clin Invest. 2005;115:1298–1305. doi: 10.1172/JCI23057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osei-Hyiaman D, Liu J, Zhou L, Godlewski G, Harvey-White J, Jeong WI, et al. Hepatic CB(1) receptor is required for development of diet-induced steatosis, dyslipidemia, and insulin and leptin resistance in mice. J Clin Invest. 2008;118:3160–3169. doi: 10.1172/JCI34827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagano C, Rossato M, Vettor R. Endocannabinoids, adipose tissue and lipid metabolism. J Neuroendocrinol. 2008;1:124–129. doi: 10.1111/j.1365-2826.2008.01690.x. [DOI] [PubMed] [Google Scholar]
- Perwitz N, Fasshauer M, Klein J. Cannabinoid receptor signaling directly inhibits thermogenesis and alters expression of adiponectin and visfatin. Horm Metab Res. 2006;38:356–358. doi: 10.1055/s-2006-925401. [DOI] [PubMed] [Google Scholar]
- Petersen G, Sørensen C, Schmid PC, Artmann A, Tang-Christensen M, Hansen SH, et al. Intestinal levels of anandamide and oleoylethanolamide in food-deprived rats are regulated through their precursors. Biochim Biophys Acta. 2006;1761:143–150. doi: 10.1016/j.bbalip.2005.12.011. [DOI] [PubMed] [Google Scholar]
- Ruby MA, Nomura DK, Hudak CS, Mangravite LM, Chiu S, Casida JE, et al. Overactive endocannabinoid signaling impairs apolipoprotein E-mediated clearance of triglyceride-rich lipoproteins. Proc Natl Acad Sci USA. 2008;105:14561–14566. doi: 10.1073/pnas.0807232105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger GJ. Endocannabinoids and the gastrointestinal tract: what are the key questions? Br J Pharmacol. 2007;152:663–670. doi: 10.1038/sj.bjp.0707422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schäfer A, Pfrang J, Neumüller J, Fiedler S, Ertl G, Bauersachs J. The cannabinoid receptor-1 antagonist rimonabant inhibits platelet activation and reduces pro-inflammatory chemokines and leukocytes in Zucker rats. Br J Pharmacol. 2008;154:1047–1054. doi: 10.1038/bjp.2008.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheen AJ, Paquot N. Inhibitors of cannabinoid receptors and glucose metabolism. Curr Opin Clin Nutr Metab Care. 2008;11:505–511. doi: 10.1097/MCO.0b013e3282fcea11. [DOI] [PubMed] [Google Scholar]
- Scheen AJ, Finer N, Hollander P, Jensen MD, Van Gaal LF. Efficacy and tolerability of rimonabant in overweight or obese patients with type 2 diabetes: a randomised controlled study. Lancet. 2006;368:1660–1672. doi: 10.1016/S0140-6736(06)69571-8. [DOI] [PubMed] [Google Scholar]
- Starowicz KM, Cristino L, Matias I, Capasso R, Racioppi A, Izzo AA, et al. Endocannabinoid dysregulation in the pancreas and adipose tissue of mice fed with a high-fat diet. Obesity (Silver Spring) 2008;16:553–565. doi: 10.1038/oby.2007.106. [DOI] [PubMed] [Google Scholar]
- Stein DT, Esser V, Stevenson BE, Lane KE, Whiteside JH, Daniels MB, et al. Essentiality of circulating fatty acids for glucose-stimulated insulin secretion in the fasted rat. J Clin Invest. 1996;97:2728–2735. doi: 10.1172/JCI118727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Gaal LF, Rissanen AM, Scheen AJ, Ziegler O, Rössner S. Effects of the cannabinoid-1 receptor blocker rimonabant on weight reduction and cardiovascular risk factors in overweight patients: 1-year experience from the RIO-Europe study. Lancet. 2005;365:1389–1397. doi: 10.1016/S0140-6736(05)66374-X. [DOI] [PubMed] [Google Scholar]
- Van Gaal L, Pi-Sunyer X, Després JP, McCarthy C, Scheen A. Efficacy and safety of rimonabant for improvement of multiple cardiometabolic risk factors in overweight/obese patients: pooled 1-year data from the Rimonabant in Obesity (RIO) program. Diabetes Care. 2008;31(Suppl. 2):S229–240. doi: 10.2337/dc08-s258. [DOI] [PubMed] [Google Scholar]
- Vickers SP, Webster LJ, Wyatt A, Dourish CT, Kennett GA. Preferential effects of the cannabinoid CB1 receptor antagonist, SR 141716, on food intake and body weight gain of obese (fa/fa) compared to lean Zucker rats. Psychopharmacology (Berl) 2003;167:103–111. doi: 10.1007/s00213-002-1384-8. [DOI] [PubMed] [Google Scholar]
- Wright KL, Duncan M, Sharkey KA. Cannabinoid CB2 receptors in the gastrointestinal tract: a regulatory system in states of inflammation. Br J Pharmacol. 2008;153:263–270. doi: 10.1038/sj.bjp.0707486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan ZC, Liu DY, Zhang LL, Shen CY, Ma QL, Cao TB, et al. Exercise reduces adipose tissue via cannabinoid receptor type 1 which is regulated by peroxisome proliferator-activated receptor-delta. Biochem Biophys Res Commun. 2006;354:427–433. doi: 10.1016/j.bbrc.2006.12.213. [DOI] [PubMed] [Google Scholar]


