Abstract
Background and purpose:
We investigated the immunogenicity of a humanized anti-human Fas monoclonal antibody, R-125224, in cynomolgus monkeys to estimate its efficacy, as well as its toxicity in clinical situations.
Experimental approach:
R-125224 was intravenously administered to cynomolgus monkeys at single doses of 0.4, 1.2, 6 and 30 mg·kg−1, and the plasma concentrations of R-125224 and anti-R-125224 antibody (ARA) were measured. We conducted a competitive enzyme-linked immunosorbent assay to determine which part of R-125224 was recognized by ARA. We also examined the retention of radioactivity in mononuclear cells and granulocytes after the injection of [125I]-R-125224 to a collagen-induced arthritis monkey model.
Key results:
After i.v. administration of R-125224, the elimination of the plasma R-125224 concentrations was accelerated at around 10 days post-dose, and 10 of 12 monkeys were ARA positive. From an epitope analysis of ARA, the ARA produced in monkeys recognized the mouse-derived regions located in complementarity determining regions, but could not recognize the human IgG. After the injection of [125I]-R-125224 to a collagen-induced arthritis monkey model, a significantly longer retention of the radioactivity in mononuclear cells compared to granulocytes was observed.
Conclusions and implications:
In monkeys, the development of antibodies against R-125224 is rapid and highly frequent. Our hypothesis is that this highly frequent development of ARA might be due to the binding of R-125224 to immune cells, and its circulation in monkey blood might contribute to an increase in its chances of being recognized as an immunogen.
Keywords: pharmacokinetics, anti-Fas monoclonal antibody, Fas, immunogenicity, monkey, CDR grafting
Introduction
Recently, the availability of biopharmaceuticals has been increasing, and monoclonal antibodies have become one of the major therapeutic agents available for physicians to treat diseases such as cancer, autoimmune disorders and so on (Pendley et al., 2003). One of the primary issues of concern for the safety of these agents is their potential for immunogenicity. As a consequence of immunogenicity to biopharmaceuticals including antibody drugs, the biological effect decreases after the formation of neutralizing antibodies and, in rare cases, general immune effects such as anaphylaxis, allergic reactions or serum sickness, are observed (Schellekens, 2002). In order to reduce the immunogenicity, humanized antibody techniques have been applied to antibody drugs, such as complementarity determining region (CDR) grafting and full humanization using transgenic mice or pharge display libraries, and there are now more than 25 humanized antibody drugs on the market. Humanization of these drugs seems to have almost succeeded, but in some cases anti-idiotypic antibodies against the mouse-derived region of humanized antibody have been produced in human subjects (Pendley et al., 2003; Hwang and Foote, 2005). For example, anti-idiotypic antibodies against the anti-tumour necrosis factor (TNF)-α antibody infliximab were produced at a level of 10% in human subjects (Hwang and Foote, 2005).
Fas is a major member of a subgroup of the TNF receptor superfamily (Itoh et al., 1991). The binding of Fas with an endogenous ligand, Fas ligand (FasL), induces apoptosis in several types of cells. In particular, Fas-mediated apoptosis plays an important role in the immune system (Spanaus et al., 1998; Ruiz-Ruiz et al., 1999; Teh et al., 2000; Wang et al., 2000). Anti-Fas antibodies are known to induce apoptosis in a manner similar to that in the Fas–FasL system, and the Fas-mediated apoptosis-signalling pathway has been investigated using anti-Fas antibody (Inazawa and Yonehara, 1999; Kuwahara et al., 2001). In these investigations, anti-Fas antibody was found to cause apoptosis in human peripheral blood mononuclear cells, synovial lymphocytes or activated T lymphocytes, and hence would have a therapeutic effect on autoimmune disorders such as rheumatoid arthritis (RA) (Matsuno et al., 2002; Ogawa et al., 2003).
R-125224, a novel humanized anti-human Fas monoclonal antibody, is derived from m-HFE7A (anti-mouse Fas monoclonal antibody) and is produced by grafting the mouse CDRs to human IgG (Haruyama et al., 2002). In the humanizing of m-HFE7A, 8E10 was selected as the framework region of human IgG, which has L and H chains that belong to subgroups kIII and I, respectively, and then we selected h-HFE7A-δ and denominated it as R-125224. The h-HFE7A-δ variable domains comprise the framework of the human antibody 8E10 with mouse residues replacing the six CDRs, and additional mouse residues at positions 38, 66, 67, 69 and 71 of the H chain. In addition, residues at positions 81 and 103 of the L chain, and 44 and 75 of the H chain were replaced with human consensus residues, where 8E10 contains amino acid residues not commonly found in human antibodies.
R-125224 has a high affinity to human Fas and apoptosis-inducing activity, similar to m-HFE7A. Nakayama et al. (2006) have shown that R-125224 has unique in vitro cell selectivity of apoptosis induction, in that it induced apoptosis to activated human lymphocytes but not to human hepatocytes. Pharmacological studies revealed that R-125224 significantly suppressed osteoclastgenesis in vivo, and it is expected to have a therapeutic effect on autoimmune diseases such as RA (Ogawa et al., 2003). In addition, R-125224 not only binds to human cells expressing Fas, but also binds to monkey cells such as hepatocytes and lymphocytes as tightly as human cells, indicating cross-reactivity to cynomolgus monkey Fas (Saito et al., 2007a). Recently, in vivo Fas-specific biodistribution of 125I-labelled R-125224 ([125I]-R-125224) in cynomolgus monkeys was shown to occur (Saito et al., 2007a).
In the present study, we found that anti-R-125224 antibody (ARA) was frequently developed, which led to acceleration of the plasma half-lives of R-125224, after a single dose of R-125224 to cynomolgus monkeys, and we investigated the immunogenicity of R-125224 in cynomolgus monkeys, including an epitope analysis of ARA produced in cynomolgus monkeys. The epitope on R-125224 was proved to be mouse-derived regions, which was examined by competitive enzyme-linked immunosorbent assay (elisa). In addition, the possible cause of the highly frequent occurrence of anti-idiotype antibody production is also discussed in this article.
Methods
Pharmacokinetic study
Male cynomolgus monkeys, which were bred in the Institute of Laboratory Animals of Shude Guangdong in China, were used after an acclimatization period of 8 days. The animal experiments were conducted by contract with Primate Ltd. (Kumamoto, Japan) in accordance with their Institutional Guidelines for the Care and Use of Laboratory Animals. R-125224 was intravenously administered to the monkeys (n= 3 for each dose) at single doses of 0.4, 1.2, 6 and 30 mg·kg−1. Blood was collected before administration and at 1, 4 h, and 1, 2, 3, 7, 10, 14, 21 and 28 days after administration. Plasma fractions were obtained after centrifugation of the blood samples at 2000×g for 5 min at 4°C (TDL-5000B).
elisa for R-125224 determination
A 96-well plate was coated with Fas–AIC2 solution diluted with 0.05 M carbonate–bicarbonate buffer (pH 9.6), 100 µL per well, which corresponded to a Fas–AIC2 concentration of 0.704 µg·mL−1. After the plate was allowed to stand for 1 h at 37°C, the liquid was removed from the wells by suction and they were subsequently filled with blocking buffer (distilled water containing 50% Block Ace) and kept at 37°C for 1 h. The wells were emptied and washed six times each with 300 µL of phosphate-buffered saline (PBS) containing 0.5% Tween 20 (wash buffer). The plasma standards or plasma samples (100 µL) were added to the wells in triplicate and incubated for 1 h at 37°C. Then, the wells were washed in the same manner as described earlier, and 100 µL of anti-human IgG with horseradish peroxidase (HRPO), which was diluted 1:10 000 with PBS containing 0.2% Tween 20 and 10% Block Ace (assay buffer), was added to the wells, and the plate was incubated at 37°C for 1 h. After washing the wells, 100 µL of 3,3′,5,5′-tetramethylbenzidine (TMB) soluble reagent was added as a substrate of HRPO and incubated at room temperature for 8 min. Finally, 100 µL of TMB stop buffer was added to each well, and the absorbance was read at 450 nm using a spectrophotometer.
A calibration curve was constructed by plotting the absorbance at 450 nm (Y) against the plasma concentration (µg·mL−1, X). Each equation [equation (1)] for the calibration curve was obtained by a least square regression method using WinNonlin (Standard Network Edition, version 1.5, Pharsight, Co., Mountain View, CA, USA) with a weight of 1/Y.
| (1) |
where Emax is the response at infinite concentration, EC50 is the concentration necessary to produce a response of 50% of Emax and γ is the slope parameter.
We measured the plasma concentrations of R-125224 with quality control (QC) samples. The criteria for accuracy were 80–120% for QC samples, and 85–115% for standard curve.
elisa for ARA determination
A 96-well plate was coated with R-125224 solution prepared at 0.5 µg·mL−1 with 0.05 M carbonate–bicarbonate buffer, 100 µL per well. After incubation for 2 h at 37°C, the liquid was removed from the wells by suction and they were subsequently filled with blocking buffer and then kept for 1 h at 37°C. The wells were emptied and washed three times each with 300 µL of wash buffer. The solutions of plasma standards or plasma samples (100 µL) were added to the wells in duplicate and incubated for 1 h at 37°C. After being washed three times each with 300 µL of wash buffer, 100 µL of biotin-labelled R-125224 diluted 1:5000 with assay buffer, was added to the wells, and the plate was incubated at 37°C for 1 h. After being washed three times with 300 µL of wash buffer, 100 µL of streptavidin–HRPO conjugate, diluted 1:5000 with PBS containing 0.2% Tween 20 (0.2T–PBS), was added to the wells, and the plate was incubated for 1 h at 37°C. After the wells were washed using the same procedure as described earlier, 100 µL of TMB soluble reagent was added and incubated at room temperature for 10 min. Finally, 100 µL of TMB stop buffer was added to each well, and the absorbance was read at 450 nm.
Calculation of the plasma ARA concentrations was conducted in the same manner as described above ‘elisa for R-125224 determination’. The plasma concentrations of ARA were expressed as U·mL−1. The concentration of R-125224 to inhibit the 50% binding of standard ARA (µg·mL−1) to R-125224 immobilized on the plate was 0.015 µg·mL−1, and the plasma concentrations of ARA are expressed by the following equation.
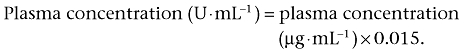 |
We measured the plasma concentrations of ARA with QC samples with the same criteria as the measurement of R-125224.
Epitope analysis of ARA developed against R-125224 in monkeys using a competitive elisa
The epitope on R-125224 recognized by ARA was analysed by a competitive elisa in which the binding of ARA to biotin-labelled R-125224 was in competition with unlabelled R-125224, Fab of R-125224, m-HFE7A and control human IgG. Immobilization of R-125224 and blocking with 50% Block Ace were performed using the same procedure as described above ‘elisa for ARA determination’. After the wells had been washed with wash buffer, 1% solution of plasma sample was added to the wells in duplicate and incubated for 1 h at 37°C. Biotin-labelled R-125224 and the competitor (R-125224, Fab of R-125224, m-HFE7A and control IgG) solutions were mixed. The final concentration of biotin-labelled R-125224 was prepared at 100 ng·mL−1, and those of R-125224, m-HFE7A and control IgG solutions were 50, 100, 200, 400, 800 and 2000 ng·mL−1. The final concentrations of the Fab of R-125224 solution were 33, 66, 132, 264, 528 and 1320 ng·mL−1, and each molar concentration corresponded to twofold R-125224 and m-HFE7A, with regard to the number of binding sites. After the addition of the mixture of biotin-labelled R-125224 and each competitor antigen solution to the wells, the plate was incubated for 1 h at 37°C. A mixture of biotin-labelled R-125224 solution and 0.2T–PBS was used as a control solution. After each well had been washed six times with 300 µL of wash buffer, 100 µL of streptavidin–HRPO conjugate, which was diluted 1:5000 with 0.2T–PBS, was added to the wells, and the plate was incubated for 1 h at 37°C. After the wells had been washed, 100 µL of TMB soluble reagent was added, and the plate was incubated at room temperature for 10 min. Finally, 100 µL of TMB stop buffer was added to each well, and the absorbance was read at 450 nm.
Determination of neutralizing antibody
Immobilization of Fas–AIC2 and blocking with 50% Block Ace were performed using the same procedure as described in the section on the elisa procedure for R-125224. Biotin-labelled R-125224 was diluted 1:5000 with assay buffer. ARA-positive monkey plasma (animal nos. 7–9 in the 6 mg·kg−1 dose group) was diluted with assay buffer containing the biotin-labelled R-125224 described earlier and prepared at ratios of 0.0001, 0.001, 0.002, 0.005, 0.01 and 0.025. The wells were emptied, and each well was washed six times with 300 µL of wash buffer. An aliquot of 100 µL of the mixture of biotin-labelled R-125224 and monkey plasma was added to the wells and incubated at 37°C for 1 h. Then, the same procedure as described earlier was followed.
In vivo binding of R-125224 to granulocytes and mononuclear cells
We speculated that R-125224 might bind to immune cells and circulate in monkey blood as a cell-bound form, which would increase its chance of being recognized as an immunogen. In order to evaluate the possibility of this hypothesis, we measured the in vivo binding of radiolabelled R-125224 to mononuclear cells and granulocytes. 125I-labelling of R-125224 was conducted following the method reported previously (Saito et al., 2007b). [125I]-R-125224 was >99.02% trichloroacetic acid (TCA) precipitable.
The animal experiments using [125I]-R-125224 were conducted by contract with Shin Nippon Biomedical Laboratories, Ltd. in accordance with their Institutional Guidelines for the Care and Use of Laboratory Animals. Female cynomolgus monkeys were obtained from the Guangxi Primate Center of China (Beijing, China). The ages and weight of the animals at time of receipt (initiation of acclimatization) were 3–5 years and 2.55–3.99 kg respectively. Each animal was given solid food once daily. Water was supplied to animals ad libitum via a self-administering watering system. The temperature and humidity in the room were maintained at about 26 ± 2°C and 50 ± 10%, respectively. Of 15 monkeys acclimatized, nine monkeys showing no abnormalities were selected and used for the development of collagen-induced arthritis after the second week of acclimatization. Twenty-four milligrams of bovine type-2 collagen in a vial was dissolved in 6 mL of 10 mM acetic acid in physiological saline. The solution was then mixed with an equal volume of complete Freund's adjuvant, and the mixture was emulsified by sonication. The emulsion was subcutaneously administered at the dorsal site of cynomolgus monkeys, 2 mL per head (first sensitization). The second and third sensitization was done 3 and 6 weeks after the first sensitization, respectively. During this period, clinical signs were observed daily, body weight was measured once a week and the elliptic area of the proximal interphalangeal joint was determined on the day before each sensitization and 2 weeks after the last sensitization. The animals were maintained under conventional housing conditions during the acclimatization period and during the experiments.
The dosing solution of [125I]-R-125224 (0.79 MBq·mL−1) was administered to nine monkeys intravenously via the cephalic vein at a dose of 0.4 mg·mL−1·kg−1. This experiment was conducted with three groups with tissue collection at 1, 24 and 168 h. Each group was given 0.79 MBq·kg−1 of [125I]-R-125224. After i.v. administration of [125I]-R-125224, blood was collected via the abdominal aorta, under anaesthesia with pentobarbital, using heparin-treated syringes at 1, 24 and 168 h post-dose. Blood and Ficoll-Paque were put into an ultracentrifuge tube at the ratio of 3:4, and the tube was centrifuged for 20 min at 400×g at room temperature. After the centrifugation, the buffy coat was collected from the tube, and saline was added to the buffy coat. This diluted buffy coat and Ficoll-Paque were put into an ultracentrifuge tube at the ratio of 3:4 and centrifuged for 20 min at 400×g and room temperature. The upper-to-middle layer was collected for the fraction of mononuclear cells, and the lower layer was collected for the fraction of granulocytes. Each fraction was suspended in 0.3 mL of physiological saline and centrifuged for 5 min at 300×g (05PR-22) at room temperature, and the washing process was conducted twice. After washing twice, the cell pellet was suspended in 0.3 mL of physiological saline (cell suspension), and 0.1 mL of the cell suspension was subjected to radioactivity measurement using a gamma counter. The cell suspension was diluted 50-fold with physiological saline, and 0.1 mL of the diluted cell suspension was used for counting the number of cells within a blood cell counting frame.
The radioactivity in the granulocytes and mononuclear cells was expressed as ng eq. of R-125224 per cellular concentration. After measurement of the total radioactivity, the remaining cell suspension was subjected to the measurement of the radioactivity in TCA. An aliquot of 1 mL each of physiological saline and 20% TCA solution were added to 0.1 mL of each of the leucocyte fractions, and the mixture was mixed well. Each mixture was then centrifuged for 15 min at 1800×g at 4°C. The supernatant was aspirated and the radioactivity in the precipitate was determined with a gamma counter.
Data analysis
The pharmacokinetic parameters of R-125224 were calculated by a non-compartmental approach using WinNonlin Professional Edition (version 1.5, Pharsight Co.). The area under the concentration–time curve (AUC) up to day 28 (AUC0–28 day) and the mean residence time up to the last quantifiable time (MRTlast) were calculated using the plasma concentrations up to day 28. Predicted AUC0–28 day (AUC0–28 day predicted) was calculated by extrapolation using the plasma concentrations up to day 7. The elimination half-life (t1/2), AUC up to day 7 (AUC0–7 day), the total body clearance (CLtotal) and the volume of distribution at steady state (Vss) were calculated using the plasma concentrations up to day 7 prior to ARA development. For the epitope analysis, the relative binding of ARA to biotin-labelled R-125224 in the presence of competitors (R-125224, Fab of R-125224, m-HFE7A and control human IgG) was determined and is expressed as the ratio to the control. The IC50 values (concentration at 50% inhibition) of each competitor were calculated according to the following equation (2).
| (2) |
where Emax and C represent the remaining reactivity (%) in the absence of a competitor and the competitor concentration (molar ratio of competitor to biotin-R-125224), respectively.
Statistical analysis
For the pharmacokinetic parameters, statistical analysis comparing AUC0–28 day predicted to AUC0–28 day was performed with paired t-test. Statistical analysis comparing the TCA precipitable ratios of 24 and 168 h to that of 1 h in blood and immune cells was conducted with Student's t-test.
Compounds and materials
R-125224, Fas–AIC2 (human Fas antigen), rabbit ARA standard and m-HFE7A were synthesized in Daiichi Sankyo Co., Ltd. (Tokyo, Japan). As a secondary antibody labelled with enzyme, goat anti-human kappa light chain conjugated with HRPO was purchased from Bethyl Laboratories, Inc. (Montgomery, TX, USA). Human IgG1 kappa myeloma purified protein (control human IgG) was purchased from Cosmo Bio Co., Ltd. (Tokyo, Japan). Biotin-labelled R-125224 was prepared using protein biotinylation module ECL (Amersham Pharmacia Biotech, Inc., Piscataway, NJ, USA). N-succinimidyl 4-[125I]-iodobenzoate ([125I]-PBI) reagent for 125I-labelling of R-125224 was purchased from Perkin Elmer Life Sciences, Inc. (Wellesley, MA, USA). Block Ace was purchased from Dainippon Pharma Co., Ltd. (Suita, Japan). TMB soluble reagent and TMB stop buffer were purchased from ScyTek Laboratories (West Logan, UT, USA). Ficoll-Paque was purchased from (Amersham Pharmacia Biotech).
Results
Pharmacokinetics of R-125224 and ARA development
The plasma concentrations of R-125224 and ARA after single i.v. administration of R-125224 at doses of 0.4, 1.2, 6 and 30 mg·kg−1 to cynomolgus monkeys (n= 3 for each dose) are shown in Figure 1. The elimination of R-125224 was accelerated after day 10 or 14 in monkey nos. 5–10 and 12, and, except for no. 10, those monkeys were ARA positive after day 10. The accelerated elimination of R-125224 in those monkeys was thought to be followed by the development of ARA. Of all 12 monkeys, 10 individuals were ARA positive, but no ARA was detected by elisa in plasma samples from nos. 10 and 11. The negative signal of ARA in the no. 10 sample might be due to an amount of R-125224 left in plasma that was sufficient to interfere with the detection of ARA, because our elisa detected only the antibodies that had two unoccupied binding sites. In the 6 mg·kg−1 dosing group, t1/2 calculated using plasma concentrations up to day 7 prior to ARA development was 6.34 ± 0.93 each day, and t1/2 after ARA development was 1.90 ± 0.67 each day. The pharmacokinetic parameters at each dose level are summarized in Table 1. The values of AUC0–7 day and AUC0–28 day were increased in a dose-dependent manner. The value of AUC0–28 day was significantly lower than the value for AUC0–28 day predicted at 6 mg·kg−1. In the other dosing groups, there were not significant differences between AUC0–28 day and AUC0–28 day predicted. The values of CLtotal calculated using plasma concentrations at days 0–7 ranged from 6.21 to 27.9 mL·day−1·kg−1 and were decreased in a dose-dependent manner.
Figure 1.
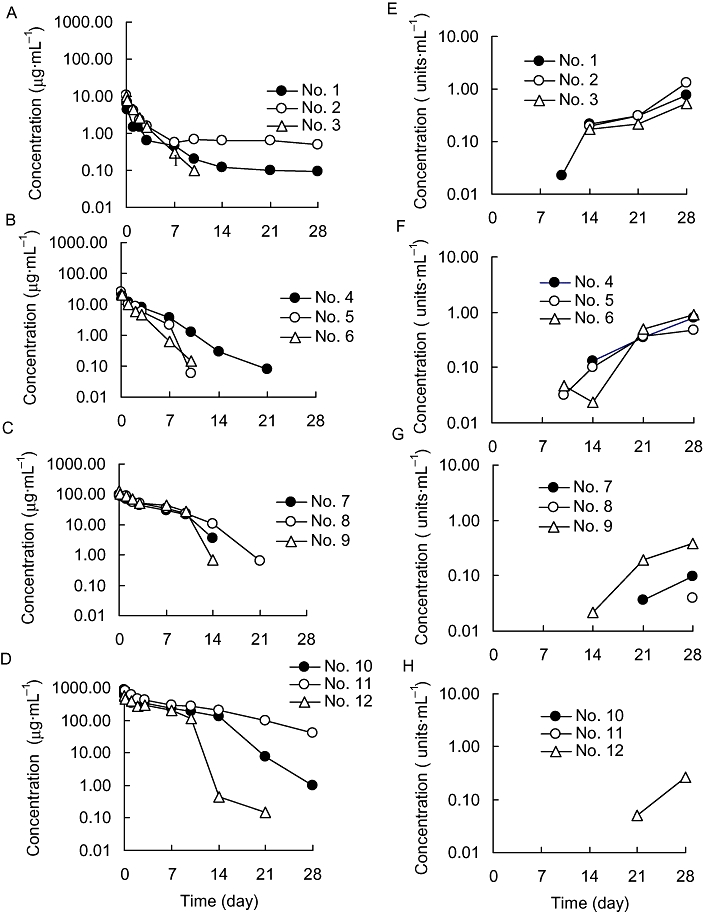
Plasma concentrations of R-125224 (A–D) and anti-R-125224 antibody (E–H) after i.v. administration to cynomolgus monkeys at a dose of 0.4 (A, E), 1.2 (B, F), 6 (C, G) and 30 mg·kg−1 (D, H).
Table 1.
Pharmacokinetic parameters of R-125224 after intravenous administration to cynomolgus monkeys at single doses of 0.4, 1.2, 6 and 30 mg·kg−1
| Dose (mg·kg−1) | AUC0–28 daya(µg·day·mL−1) | AUC0–28 day predictedb(µg·day·mL−1) | AUC0–7 dayb(µg·day·mL−1) | MRTlasta (day) | t1/2b (day) | CLtotalb(mL·day−1·kg−1) | Vssb(mL·kg−1) |
|---|---|---|---|---|---|---|---|
| 0.4 | 18.7 ± 8.8 | 14.5 ± 4.4 | 13.1 ± 4.3 | 5.33 ± 3.46 | 2.78 ± 1.20 | 27.9 ± 7.6 | 94.9 ± 65.8 |
| 1.2 | 54.5 ± 14.9 | 58.4 ± 18.5 | 48.9 ± 9.4 | 2.61 ± 0.89 | 2.60 ± 1.14 | 22.0 ± 6.9 | 67.2 ± 10.3 |
| 6 | 558 ± 69 | 676 ± 90* | 391 ± 48 | 4.75 ± 0.51 | 6.34 ± 0.93 | 8.40 ± 1.39 | 74.7 ± 10.3 |
| 30 | 4420 ± 1860 | 4670 ± 1370 | 2480 ± 550 | 6.53 ± 2.07 | 7.38 ± 1.40 | 6.21 ± 1.79 | 62.9 ± 9.0 |
Parameters were calculated using the plasma concentrations at days 0–28.
Parameters were calculated using the plasma concentrations at days 0–7.
Statistical analysis comparing AUC0–28 day predicted to AUC0–28 day were performed with paired t-test;
P < 0.05.
Epitope analysis of ARA
The binding of ARA to biotin-labelled R-125224 in the presence of competitors (non-labelled R-125224, m-HFE7A, Fab of R-125224 and control human IgG) was determined by a competitive elisa. We investigated ARA in the monkey plasma samples in the 0.4 and 1.2 mg·kg−1 dosing groups because their titre was higher than those of other plasma samples at higher doses; this made it easier to analyse the results. R-125224 and m-HFE7A have two binding sites in one molecule (bivalent). On the other hand, Fab of R-125224 has one in one molecule (monovalent). Considering this condition, the ratio of competitive antigen/biotin-R-125224 represented in Figure 2 is bivalent. As shown in Figure 2, the binding of the monkey ARA to biotin-labelled R-125224 was strongly inhibited by non-labelled R-125224, m-HFE7A and Fab of R-125224, but not by control human IgG. The IC50 values (molar ratio of competitor/biotin-labelled R-125224) of non-labelled R-125224, Fab of R-125224 and m-HFE7A were 0.496 ± 0.098, 1.02 ± 0.54 and 0.664 ± 0.15 respectively. The IC50 values of R-125224 and m-HFE7A were almost the same and were slightly higher than that of Fab. Taking into consideration the fact that the common residues among m-HFE7A, Fab of R-125224 and R-125224 are mouse-derived residues on CDRs of R-125224, the monkey ARA might recognize the mouse-derived regions as CDRs. In addition, these results prove that the human IgG framework is not recognized as an antigen in cynomolgus monkeys.
Figure 2.
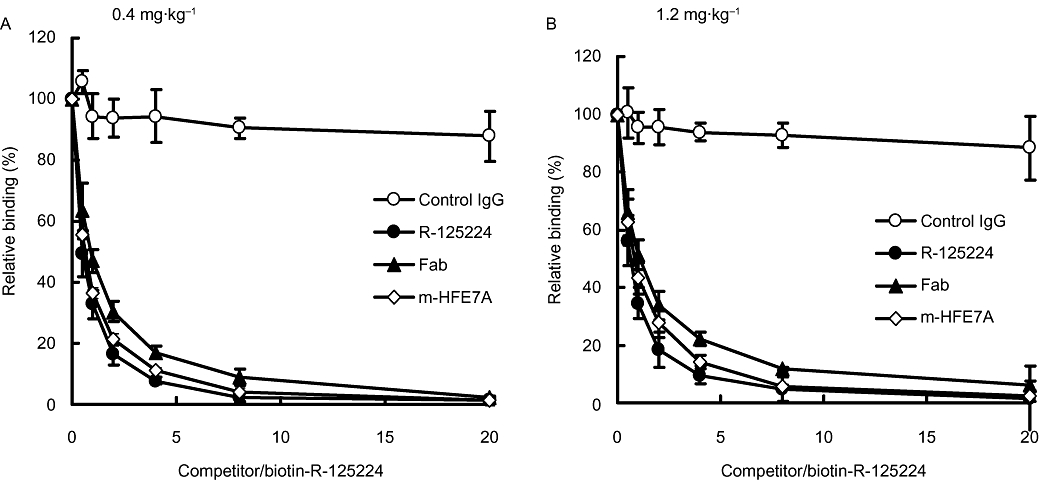
Relative binding of anti-R-125224 antibody and biotin-R-125224 in the presence of antigens (control human IgG, R-125224, Fab of R-125224 and m-HFE7A) at doses of 0.4 (A) and 1.2 mg·kg−1 (B). IC50 values (competitive antigen/biotin-R-125224) of R-125224, Fab of R-125224 and m-HFE7A were 0.496, 1.02, and 0.664 respectively.
Determination of neutralizing antibody
The effect of the monkey ARA on the binding of biotin-labelled R-125224 to Fas–AIC2 was examined by elisa. R-125224 binding activity (% control) plotted against the content of ARA-positive plasma (6 mg·kg−1 dosing group) is shown in Figure 3. The binding of biotin-labelled R-125224 to Fas–AIC2 was almost completely inhibited by ARA developed in monkeys at a concentration ratio of 0.005 (1/200 of plasma). These results indicate that the antibody that develops in monkeys is a neutralizing antibody.
Figure 3.
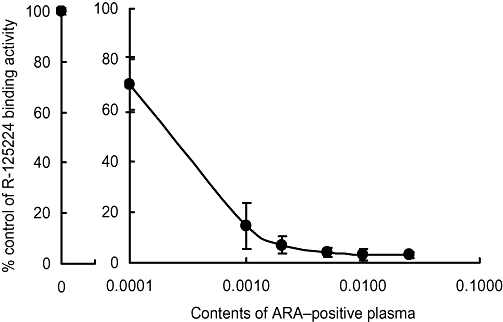
Binding activity of R-125224 and Fas antigen in the presence of anti-R-125224 antibody.
In vivo binding of R-125224 to granulocytes and mononuclear cells
In order to examine the affinity of R-125224 and immunocytes (granulocytes and mononuclear cells), we investigated the retention of the radioactivity in granulocytes, mononuclear cells and blood after i.v. administration of [125I]-R-125224 to a collagen-induced arthritis monkey model at a dose of 0.4 mg·kg−1 (Figure 4). In this experiment, we used an animal model of collagen-induced arthritis to enable us to collect the granulocytes and mononuclear cells more effectively. The concentrations of radioactivity in granulocytes and mononuclear cells at 1 h post-dose were 74.72 ± 36.33 and 384.74 ± 266.21 ng eq. of R-125224 per 108 cells, respectively, and that in blood at 1 h post-dose was 4316.16 ± 938.74 ng eq. of R-125224 mL−1. The concentrations of radioactivity in blood decreased in a time-dependent manner, and the concentrations at 24 and 168 h were 36.3 and 2.45% of that at 1 h, respectively. Similarly, the concentrations of radioactivity in granulocytes at 24 and 168 h were 48.2 and 11.0%, respectively. The apparent t1/2 of the radioactivity in blood and granulocytes was 33.1 and 57.0 h, respectively, indicating that they showed similar elimination profiles. On the other hand, the concentrations of radioactivity in mononuclear cells at 24 and 168 h were 101 and 63.3% of that at 1 h, respectively, and t1/2 in mononuclear cells was 236.8 h, which indicates these cells have a much longer retention time for radioactivity than blood or granulocytes. In order to distinguish the radioactivity derived from the free radioactive iodide related to [125I]-R-125224 and that of protein-bound 125I, we analysed the TCA-precipitable radioactivity in each cell fraction. As shown in Figure 5, the amount of radioactivity in the TCA-precipitable fraction of mononuclear cells and blood was more than 80% at 168 h post-dose, while that of granulocytes was around 80% at 1 h post-dose and decreased to around 60% at 24 and 168 h post-dose; these values at 24 and 168 h were significantly different from that at 1 h in granulocytes. Hence, mononuclear cells retain the radioactivity of protein-bound 125I for a relatively long period of time after i.v. doses of [125I]-R-125224. These results indicate that R-125224 is distributed to mononuclear cells and circulates in the blood for a considerable length of time after its injection.
Figure 4.
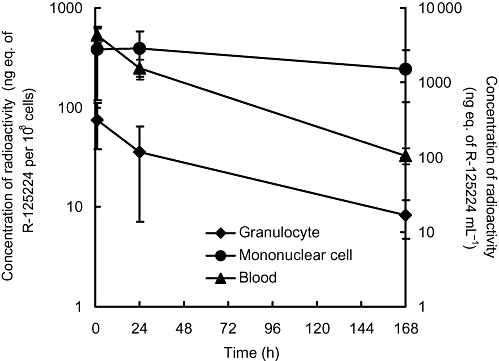
Concentrations of radioactivity in granulocytes, mononuclear cells and blood after i.v. administration of [125I]-R-125224 to cynomolgus monkeys at a dose of 0.4 mg·kg−1.
Figure 5.
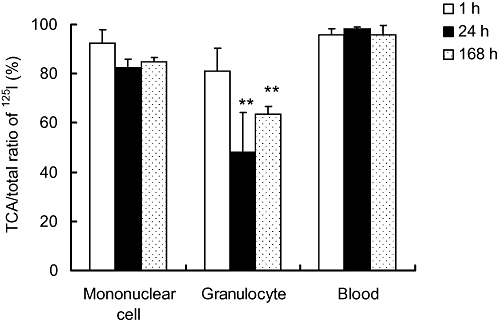
Trichloroacetic acid (TCA)-precipitable ratios to total radioactivity in mononuclear cells, granulocytes and blood. Statistical analysis comparing the values at 24 and 168 h to that at 1 h in blood and immune cells was performed with Student's t-test; **P < 0.05.
Discussion and conclusions
We think that a high incidence of antibody development against humanized antibody in monkeys is not typical and that the results obtained with R-125224 presented here represent one of the rare cases of high immunogenicity of humanized antibody in monkeys. In this study, we selected cynomolgus monkeys as an animal model that mimics human pharmacokinetics because R-125224 has cross-reactivity to cynomolgus monkey Fas (Saito et al., 2007a), and we expected R-125224 to have a long retention time similar to that of other humanized antibodies which show normal half-lives in monkeys. However, unexpectedly, in our study the number of cynomolgus monkeys, in which anti-idiotype antibodies developed against R-125224 after a single administration, was found to be extremely high.
The normal half-life values of humanized monoclonal antibodies have been reported to be within the range of 9–30 days in monkeys, in which anti-idiotype antibodies might not be produced (Davis et al., 1995; Zia-Amirhosseini et al., 1999; Benincosa et al., 2000). These humanized monoclonal antibodies were slowly eliminated in monkeys as host IgG, which might be due to the similarity of IgG between humans and monkeys. In some cases, however, anti-idiotype antibodies were developed against humanized monoclonal antibody in monkeys, as shown in previous reports and in this study. Among these cases, the humanized anti-Tac antibody (HAT), which was humanized by CDR grafting originating from mouse monoclonal antibody, was quite similar to that of R-125224 presented in this study (Hakimi et al., 1991).
Hakimi et al. (1991) demonstrated that anti-HAT antibody developed in 8 of 12 monkeys after multiple dosing and thata drastic change in serum concentration was observed after the development of the antibody. Schneider et al. (1993) examined the recognition site of the anti-HAT antibody, which developed in monkeys using variants of HAT, by use of a competitive elisa and found that the anti-HAT antibody from monkey serum recognized the mouse-derived region in CDRs. In another case, SGN-40, a humanized monoclonal anti-CD40 antibody, was shown to have a high incidence of rapid elimination due to the production of antibody against SGN-40 after multiple dosing to cynomolgus monkeys (Kelley et al., 2006). Antibody development against the monoclonal antibodies, HAT and SGN-40, in monkeys was similar to that of R-125224, but R-125224 seems to develop anti-idiotype antibody more easily than those antibodies, if one considers the antibodies produced after a single dose of R-125224.
Compared with other humanized antibodies, the number of amino acid residues derived from mouse antibody on R-125224 is not large, and the primary structure of R-125224 is unlikely to be the cause of the high incidence of ARA development. R-125224 is derived from m-HFE7A, a monoclonal mouse IgG anti-human Fas antibody and is designed by CDR grafting. In the humanization of m-HFE7A, five versions of humanized HFE7A were produced, which induced the same degree of apoptosis in WR19L12a cells, and h-HFE7A-δ (R-125224) was selected because the δ version has the smallest number of mouse residues transferred from m-HFE7A among the five h-HFE7As (Haruyama et al., 2002). Its variable domains comprised the framework of the human antibody 8E10 with mouse residues replacing the six CDRs (65 amino acids) and five additional mouse residues of the H chain (Haruyama et al., 2002). Carter et al. (1992) humanized the mouse monoclonal antibody, mumAb4D5 to produce humAb4D5 (Herceptin). Herceptin has six mouse-derived CDRs (49 amino acids) and seven amino acids at VH and VL. In another case, Avastin, the humanized anti-human VEGF monoclonal antibody A.4.6.1., has six mouse-derived CDRs (68 amino acids) and 10 additional mouse-specific residues (Presta et al., 1997). These antibodies showed minimal development of antibody in humans, and the values of their mouse-derived residues are almost consistent with that of R-125224. From this observation, the number of mouse-derived residues in R-125224 is not thought to be large and is unlikely to be the cause of the high incidence and rapid development of the anti-idiotype antibody.
As another possibility, we suggested that R-125224 might bind to immune cells and circulate in monkey blood as a cell-bound form, which would elevate its chance of being recognized as an immunogen. In order to evaluate this hypothesis, we measured the in vivo binding of R-125224 to mononuclear cells and granulocytes, which expressed Fas receptor (Liles et al., 1996), and this is likely to be one possible cause of the high immunogenicity of R-125224. This hypothesis was based on two results reported previously: firstly, one of the humanized anti-human l-selectin monoclonal antibodies, HuDREG55 which has cross-reactivity to monkeys, showed accelerated elimination that might be due to antibody development (Co et al., 1999), and secondly, R-125224 tightly bound to monkey lymphocytes in vitro (Haruyama et al., 2002). Co et al. reported the species cross-reactivity and pharmacokinetics of two humanized anti-human l-selectin monoclonal antibodies, HuDREG-55 and HuDREG-200, in rhesus monkeys. Interestingly, HuDREG-55, which had cross-reactivity to monkey l-selectin showed an accelerated clearance from serum. On the other hand, HuDREG-200, which had no cross-reactivity to monkeys, showed a normal half-life similar to IgG in monkeys. The authors explained these findings by hypothesizing that the shorter half-life of HuDREG-55 was probably related to the binding of HuDREG-55 to rhesus l-selectin expressed on leucocytes, and later to the development of antibodies against HuDREG-55. They suggested that the normal half-life of HuDREG-200 in rhesus monkeys was probably the result of a lack of cross-reactivity, allowing the antibodies to circulate freely in bloodstream without binding to the cells.
We evaluated the retention of radioactivity in granulocytes, mononuclear cells and blood in vivo after i.v. administration of [125I]-R-125224 in monkeys subjected to collagen-induced arthritis. According to the results, the elimination of radioactivity in mononuclear cells, which contain lymphocytes and monocytes, was significantly prolonged compared to that in granulocytes and blood (Figure 4). The TCA-precipitable radioactivity/total radioactivity ratio in mononuclear cells was more than 80%, which was higher than that of the granulocytes (Figure 5), indicating that the majority of radioactivity measured was derived from the protein-bound form in mononuclear cells. Although in this experiment we could not distinguish the R-125224 bound to the cell surface from that internalized to cells, the cell-bound form seems more likely because internalized proteins can be degraded rapidly in the cellular protein digestion system and are unlikely be detected as the major protein-bound form a week after dosing. Coffey et al. (2005) reported that murinized anti-mouse CD11a monoclonal antibody, muM17, was cleared in vivo by binding to peripheral blood mononuclear cells, lymphocytes and splenocytes, and by subsequent internalization and lysosomal degradation. From these observations, R-125224 is likely to circulate partially as a cell-bound form in blood. We expected granulocytes to have the same characteristics as mononuclear cells with regard to retention of R-125224, because the Fas receptor has been detected on the surface of both cells (Liles et al., 1996). The reason for the differences is unknown, although the affinity of R-125224 to granulocytes seemed to be less than that of mononuclear cells, as shown from the radioactivity concentrations measured 1 h post-dose. The long retention time of radioactivity observed in mononuclear cells might be derived from its high affinity to lymphocytes (Saito et al., 2007a), and indicates that R-125224 will remain in the blood for a long time in monkeys with collagen-induced arthritis. However, the association of the data showing R-125224 has a long retention time in mononuclear cells of these arthritic monkeys to our assumption that circulation of R-125224 as a cell-bound form would elevate its chance of being recognized as an immunogen in normal monkeys should be treated with caution (Iida et al., 1999; Murayama et al., 2000). In this experiment, we used monkeys with collagen-induced arthritis in order to collect blood cells more effectively, but disease states such as arthritis might change Fas expression levels in lymphocytes compared to normal monkeys as observed in humans (Hirano et al., 2003). To support our hypothesis, further experiments are needed to show that R-125224 has a long circulation time in normal monkeys as a cell-bound form, and has binding affinity to a subset of lymphocytes through the Fas-antigen or Fcγ-receptor.
Our results show that in monkeys, antibodies develop against R-125224, whose epitopes were the mouse-derived regions located in the CDRs of R-125224, and these antibodies develop rapidly with a high incidence after a single i.v. dose of R-125224. By referring to cases of other antibody drugs including many types of antibody production, it seems R-125224 belongs to the class which shows the highest incidence of antibody development in monkeys. Hence, we hypothesize that the highly frequent development of ARA might be due to the tight binding of R-125224 to immune cells and its circulation in monkey blood as a cell-bound form, resulting in the elevation of its chance of being recognized as an immunogen. Although further studies are needed to prove this concept, the study presented here provides one idea to explain the different frequencies of antibody production induced by therapeutic humanized antibodies.
Acknowledgments
We would like to thank Dr Shuichiro Ito for his fruitful discussions.
Glossary
Abbreviations:
- CDR
complementarity determining region
- HRPO
horseradish peroxidase
- [125I]-PBI
N-succinimidyl 4-[125I]-iodobenzoate
- TMB
3,3′,5,5′-tetramethylbenzidine
- TNF
tumour necrosis factor
Conflict of interest
The authors are employees of Daiichi Sankyo Co., Ltd.
References
- Benincosa LJ, Chow FS, Tobia LP, Kwok DC, Davis CB, Jusko WJ. Pharmacokinetics and pharmacodynamics of a humanized monoclonal antibody to factor IX in cynomolgus monkeys. J Pharmacol Exp Ther. 2000;292:810–816. [PubMed] [Google Scholar]
- Carter P, Presta L, Gorman CM, Ridgway JBB, Henner D, Wong WLT, et al. Humanization of an anti-p185HER2 antibody for human cancer therapy. Proc Natl Acad Sci USA. 1992;89:4285–4289. doi: 10.1073/pnas.89.10.4285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Co MS, Landolfe NF, Nagy JO, Tan JH, Vexler V, Vasquez M, et al. Properties and pharmacokinetics of two humanized antibodies specific for l-selectin. Immunotechnology. 1999;4:253–266. doi: 10.1016/s1380-2933(98)00024-4. [DOI] [PubMed] [Google Scholar]
- Coffey GP, Fox JA, Pippig S, Palmieri S, Reitz B, Gonzales M, et al. Tissue distribution and receptor-mediated clearance of anti-CD11A antibody in mice. Drug Metab Dispos. 2005;33:623–629. doi: 10.1124/dmd.104.002584. [DOI] [PubMed] [Google Scholar]
- Davis CB, Hepburn TW, Urbanski JJ, Kwok DC, Hart TK, Herzyk DJ, et al. Preclinical pharmacokinetic evaluation of the respiratory syncytial virus-specific reshaped human monoclonal antibody RSHZ19. Drug Metab Dispos. 1995;23(10):1028–1036. [PubMed] [Google Scholar]
- Hakimi J, Chizzonite R, Luke DR, Familletti PC, Bailon P, Kondas JA, et al. Reduced immunogenicity and improved pharmacokinetics of humanized anti-Tac in cynomolgus monkeys. J Immunol. 1991;147(4):1352–1359. [PubMed] [Google Scholar]
- Haruyama H, Ito S, Miyadai K, Takahashi T, Kawaida R, Takayama T, et al. Humanization of the mouse anti-Fas antibody HFE7A and crystal structure of the humanized HFE7A Fab fragment. Biol Pharm Bull. 2002;25:1537–1545. doi: 10.1248/bpb.25.1537. [DOI] [PubMed] [Google Scholar]
- Hirano T, Hiratsuka N, Iwahori T, Oka K, Wakasugi K. Fas/Fas-ligand expressions in peripheral-blood mononuclear cells of patients with myelodysplastic syndromes. Res Commun Mol Pathol Pharmacol. 2003;113(114):315–328. [PubMed] [Google Scholar]
- Hwang WYK, Foote J. Immunogenicity of engineered antibodies. Methods. 2005;36:3–10. doi: 10.1016/j.ymeth.2005.01.001. [DOI] [PubMed] [Google Scholar]
- Iida T, Ichimura H, Ui M, Shimada T, Akahata W, Igarashi T, et al. Sequential analysis of apoptosis induction in peripheral blood mononuclear cells and lymph nodes in the early phase of pathogenic and nonpathogenic SIVmac infection. AIDS Res Hum Retroviruses. 1999;15(8):721–729. doi: 10.1089/088922299310818. [DOI] [PubMed] [Google Scholar]
- Inazawa Y, Yonehara S. Fas-induced in vivo apoptosis in bone marrow anti-Fas mAb-induced elimination and successive proliferation of Fas-expressing cells especially those of myeloid lineage. Cell Struct Funct. 1999;24:151–159. doi: 10.1247/csf.24.151. [DOI] [PubMed] [Google Scholar]
- Itoh N, Yonehara S, Ishii A, Yonehara M, Mizushima S, Sameshima M, et al. The polypeptide encoded by the cDNA for human cell surface antigen Fas can mediate apoptosis. Cell. 1991;66:233–243. doi: 10.1016/0092-8674(91)90614-5. [DOI] [PubMed] [Google Scholar]
- Kelley SK, Gelzleichter T, Xie D, Lee WP, Darbonne WC, Qureshi F, et al. Preclinical pharmacokinetics, pharmacodynamics, and activity of a humanized anti-CD40 antibody (SGN-40) in rodents and non-human primates. Br J Pharmacol. 2006;148(8):1116–1123. doi: 10.1038/sj.bjp.0706828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuwahara H, Tani Y, Ogawa Y, Takaichi Y. Therapeutic effect of novel anti-human Fas antibody HFE7A on graft-versus-host disease model. Clin Immunol. 2001;99(3):340–346. doi: 10.1006/clim.2001.5028. [DOI] [PubMed] [Google Scholar]
- Liles WC, Kiener PA, Ledbetter JA, Aruffo A, Klebanoff SJ. Differential expression of Fas (CD95) and Fas ligand on normal human phagocytes: implications for the regulation of apoptosis in neutrophils. J Exp Med. 1996;184:429–440. doi: 10.1084/jem.184.2.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuno H, Yudoh K, Nakazawa F, Sawai T, Uzuki M, Nishioka K, et al. Antirheumatic effects of humanized anti-Fas monoclonal antibody in human rheumatoid arthritis/SCID mouse chimera. J Rheumatol. 2002;29:1609–1614. [PubMed] [Google Scholar]
- Murayama Y, Terao K, Inoue-Murayama M. Molecular cloning and characterization of cynomolgus monkey Fas. Hum Immunol. 2000;61:474–485. doi: 10.1016/s0198-8859(00)00100-2. [DOI] [PubMed] [Google Scholar]
- Nakayama J, Ogawa Y, Yoshigae Y, Onozawa Y, Yonemura A, Saito M, et al. A humanized anti-human Fas antibody, R-125224, induces apoptosis in type I activated lymphocytes but not in type II cells. Int Immunol. 2006;18:113–124. doi: 10.1093/intimm/dxh353. [DOI] [PubMed] [Google Scholar]
- Ogawa Y, Ohtsuki M, Uzuki M, Sawai T, Onozawa Y, Nakayama J, et al. Suppression of osteoclastogenesis in rheumatoid arthritis by induction of apoptosis in activated CD4+ T cells. Arthritis Rheum. 2003;48:3350–3358. doi: 10.1002/art.11322. [DOI] [PubMed] [Google Scholar]
- Pendley C, Schantz A, Wagner C. Immunogenicity of therapeutic monoclonal antibodies. Curr Opin Mol Ther. 2003;5(2):172–179. [PubMed] [Google Scholar]
- Presta LG, Chen H, O'Connor SJ, Chisholm V, Meng YG, Krummen L, et al. Humanization of anti-vascular endotherial growth factor monoclonal antibody for the therapy of solid tumors and other disorders. Cancer Res. 1997;57:4593–4599. [PubMed] [Google Scholar]
- Ruiz-Ruiz C, Robledo G, Font J, Izquierdo M, Lopez-Rivas A. Protein kinase C inhibits CD95 (Fas/APO-1)-mediated apoptosis by at least different mechanisms in Jurkat T cells. J Immunol. 1999;163:4737–4746. [PubMed] [Google Scholar]
- Saito M, Yoshigae Y, Nakayama J, Ogawa Y, Ohtsuki M, Kurihara A, et al. Tissue distribution of humanized anti-human Fas monoclonal antibody (R-125224) based on Fas antigen–antibody reaction in collagen-induced arthritis monkeys. Life Sci. 2007a;80:2005–2014. doi: 10.1016/j.lfs.2007.02.043. [DOI] [PubMed] [Google Scholar]
- Saito M, Yoshigae Y, Nakayama J, Ogawa Y, Ohtsuki M, Kurihara A, et al. In SCID mice with transplanted joint tissues from rheumatism patients, a model mice of human rheumatoid arthritis, anti-human Fas antibody (R-125224) distributed specifically to human synovium. Pharm Res. 2007b;24(2):310–317. doi: 10.1007/s11095-006-9148-5. [DOI] [PubMed] [Google Scholar]
- Schellekens H. Immunogenicity of therapeutic proteins: clinical implications and future prospects. Clin Ther. 2002;24:1720–1740. doi: 10.1016/s0149-2918(02)80075-3. [DOI] [PubMed] [Google Scholar]
- Schneider WP, Glaser SM, Kondas JA, Hakimi J. The anti-idiotype response by cynomolgus monkeys to humanized anti-Tac is primarily directed to complementarity-determining regions H1, H2, and L3. J Immunol. 1993;150(7):3086–3090. [PubMed] [Google Scholar]
- Spanaus KS, Schlapbach R, Fontana A. TNF-alpha and IFN-gamma render microglia sensitive to Fas ligand-induced apoptosis by induction of Fas expression and down-regulation of Bcl-2 and Bcl-xl. Eur J Immunol. 1998;28:4398–4408. doi: 10.1002/(SICI)1521-4141(199812)28:12<4398::AID-IMMU4398>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- Teh HS, Seebaran A, Teh SJ. TNF receptor 2-deficient CD8 T cells are resistant to Fas/Fas ligand-induced cell death. J Immunol. 2000;165:4814–4821. doi: 10.4049/jimmunol.165.9.4814. [DOI] [PubMed] [Google Scholar]
- Wang Y, Wu TR, Cai S, Welte T, Chin YE. Stat1 as a component of tumor necrosis factor alpha receptor 1-TRADD signaling complex to inhibit NF-kappaB activation. Mol Cell Biol. 2000;20:4505–4512. doi: 10.1128/mcb.20.13.4505-4512.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zia-Amirhosseini P, Minthorn E, Benincosa LJ, Hart TK, Hottenstein CS, Tobia LAP, et al. Pharmacokinetics and pharmacodynamics of SB-240563, a humanized monoclonal antibody directed to human interleukin-5, in monkeys. J Pharmacol Exp Ther. 1999;291:1060–1067. [PubMed] [Google Scholar]


