Abstract
Background and purpose:
Adding spironolactone to standard therapy in heart failure reduces morbidity and mortality, but the underlying mechanisms are not fully understood. We analysed the effect of canrenone, the major active metabolite of spironolactone, on myocardial contractility and intracellular calcium homeostasis.
Experimental approach:
Left ventricular papillary muscles and cardiomyocytes were isolated from male Wistar rats. Contractility of papillary muscles was assessed with force transducers, Ca2+ transients by fluorescence and Ca2+ fluxes by electrophysiological techniques.
Key results:
Canrenone (300–600 µmol·L−1) reduced developed tension, maximum rate of tension increase and maximum rate of tension decay of papillary muscles. In cardiomyocytes, canrenone (50 µmol·L−1) reduced cell shortening and L-type Ca2+ channel current, whereas steady-state activation and inactivation, and reactivation curves were unchanged. Canrenone also decreased the Ca2+ content of the sarcoplasmic reticulum, intracellular Ca2+ transient amplitude and intracellular diastolic Ca2+ concentration. However, the time course of [Ca2+]i decline during transients evoked by caffeine was not affected by canrenone.
Conclusion and implications:
Canrenone reduced L-type Ca2+ channel current, amplitude of intracellular Ca2+ transients and Ca2+ content of sarcoplasmic reticulum in cardiomyocytes. These changes are likely to underlie the negative inotropic effect of canrenone.
Keywords: spironolactone, canrenone, calcium, calcium channel, sarcoplasmic reticulum
Introduction
Spironolactone has beneficial effects in heart failure of both ischaemic and non-ischaemic aetiologies such that the addition of spironolactone to the standard therapy for heart failure reduces the mortality by 30% (Pitt et al., 1999). Therefore, since 2001, the American Heart Association has recommended the addition of spironolactone to the standard treatment of those patients (Hunt et al., 2001). The beneficial effect was initially attributed to the blockade of aldosterone receptor, but some studies have shown a direct effect of spironolactone and its derivative metabolites on cardiac excitation–contraction coupling (Coraboeuf and Deroubaix, 1974; Mugge et al., 1984; Vassallo et al., 1998; Cargnelli et al., 2001). Spironolactone blocks L-type Ca2+ channel currents (ICa-L) in smooth muscle cells (Dacquet et al., 1987), but this effect has not been studied in cardiomyocytes.
In humans, spironolactone is rapidly metabolized in the liver and does not appear in plasma or urine in measurable quantities (Sadée et al., 1973). The major active metabolite of spironolactone is canrenone. Canrenone has negative inotropic action on isolated cardiac papillary muscles (Coraboeuf and Deroubaix, 1974; Mugge et al., 1984). However, the mechanisms underlying the direct action of canrenone on cardiac contraction needs further clarification. Therefore, we decided to study the acute effect of canrenone in isolated preparations of cardiomyocytes and papillary muscles from the myocardium of normal rats.
Here we expanded the analysis of the effects of canrenone on isolated rat papillary muscles to both inotropic and lusitropic actions. Our results provided the first evidence of the action of canrenone on Ca2+ handling in cardiac muscle. We found that canrenone reduced amplitude of cardiac ICa-L and the Ca2+ content of sarcoplasmic reticulum (SR) with consequent reduction in amplitude of intracellular Ca2+ transients. These changes could contribute to the negative inotropic actions of canrenone on the heart.
Methods
Animals
All animal care and the protocols of this study conform to the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH Publication no. 96-23, revised, 1996) and were approved by the Ethics in Research Committee of the Federal University of São Paulo (CEP N° 640/01). A total of 65 male Wistar rats weighing 280–310 g were used. Animals were housed in light/dark cycles with food and water ad libitum.
Canrenone synthesis
Canrenone was obtained from potassium canrenoate (Sigma, St. Louis, MO, USA), as previously described (Megges et al., 1997). Canrenone purity was determined by three methods (Megges et al., 1997): thin layer chromatography, gas chromatography coupled to mass spectrometry and 1H and 13C-nuclear magnetic resonance, with results similar to those previously published (Megges et al., 1997). Canrenone was dissolved in Tween 20 (0.001%; USB, Cleveland, OH, USA) for use in papillary muscle preparations and in dimethyl sulphoxide (DMSO; 0.0083%; Sigma) for use in cardiomyocyte preparations. DMSO at this concentration had no significant effect on ICa-L (data not shown).
Papillary muscle mechanics
Myocardial contraction was evaluated in left ventricular papillary muscles, as described by others (Conrad et al., 1991). Briefly, under anaesthesia (1.2 mg·g−1 urethane, i.p.) the hearts were quickly removed and placed in oxygenated Krebs-Henseleit solution (in mM: 118 NaCl, 4.7 KCl, 1.25 CaCl2, 1.2 MgSO4, 1.2 KH2PO4, 11 glucose and 25 NaHCO3). Papillary muscle was dissected free and placed in a chamber containing Krebs-Henseleit solution oxygenated by a mixture of 95% O2 and 5% CO2 (pH 7.4). The muscle was connected to a Grass FTO3E force transducer (Astro-Med Inc., Grass Instrument Division, West Warwick, RI, USA) that was attached to a micromanipulator to allow variation of muscle length. Developed force was recorded with Acqknowledge 3.5.7 software (Biopac Systems Inc., Santa Barbara, CA, USA). Peak developed tension (DT, g·mm−2), maximum rate of tension increase (+dT/dt, g·mm−2·s−1) and decline (−dT/dt, g·mm−2·s−1), time to peak tension (TPT, ms) and time from peak tension to 50% relaxation (RT50, ms) were calculated. Developed force and its time derivatives were normalized for muscle cross-sectional area. The muscle cross-sectional area was estimated from the muscle weight and length by assuming a cylindrical shape and a specific gravity of 1.0.
Preparations were stimulated (rectangular voltage pulses, 5 ms duration, 10% above threshold at 0.2 Hz) throughout the experiment. The preparations were left contracting isotonically for 60 min under a low preload and then loaded to contract isometrically for 15 min. Then the muscle was slowly stretched to the Lmax, defined as the muscle length at which DT is maximum. The muscle was kept at this length for the rest of the experiment. Fifteen minutes later, perfusion with canrenone (150, 300 or 600 µM) or the vehicle alone (0.001% Tween 20) was started. Canrenone concentrations were chosen according to a previous study with right papillary muscles (Cargnelli et al., 2001). The mechanical behaviour of the electrically stimulated papillary muscle was evaluated before and at the end of 15 min of perfusion.
Cardiomyocyte isolation
Ventricular myocytes were isolated as reported previously (Santos et al., 1995). Briefly, under anaesthesia (1.2 mg·g−1 urethane, i.p.) the hearts were rapidly removed and attached to a modified Langendorff apparatus. The heart was perfused for 5 min at 10 mL·min−1 with Tyrode solution [in mM: 132 NaCl, 1 CaCl2, 1.8 MgCl2, 4.0 KCl, 10 HEPES (Sigma) and 5 glucose, pH 7.35] saturated with O2, followed by Ca2+-free Tyrode solution. When the heart stopped beating, collagenase II (Worthington, Lakewood, NJ, USA; 0.8 mg·mL−1) and bovine albumin (0.02%; Sigma) were added. Perfusion continued until the heart became flaccid (∼15–20 min). Then the enzyme was washed out with Ca2+-free Tyrode solution. Next, ventricular fragments were dispersed. The cell suspension was rinsed several times with Tyrode solution in which Ca2+ concentration was increased up to 1 mM.
Measurement of cell shortening and calcium transients
Myocytes were plated on collagen-treated perfusion chambers. The chamber was placed on an inverted microscope (Diaphot 300, Nikon Corp., Tokyo, Japan) equipped for epifluorescence measurement (RatioMaster, Photon Technology International, Monmouth Junction, NJ, USA). Myocytes were loaded with indo-1 acetoxymethyl ester (indo-1 AM; Molecular Probes, Eugene, OR, USA) for 15 min and superfused with Tyrode solution for 20 min. Excitation wavelength was 365 nm, and fluorescence emitted by the cell was recorded at 405 and 485 nm. Fluorescence ratios were converted to free intracellular Ca2+ ([Ca2+]i) according to the equation (Grynkiewicz et al., 1985):
where the indo-1 apparent dissociation constant (Kd) was 844 nM (Bassani et al., 1995a). Rmin, Rmax and β were determined in vivo (Bassani et al., 1994). Total Ca2+ concentration in the cytosol ([Ca2+]T) was calculated as:
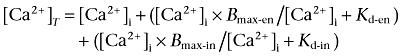 |
where Bmax-en and Kd-en are the maximal Ca2+ binding capacity (300 µM) and Kd (0.54 µM) of the endogenous Ca2+ binding sites (Bassani et al., 1994). Bmax-in and Kd-in are cytosolic indo-1 concentration and apparent dissociation constant (Bassani et al., 1998) assumed to be 50 µM (Bassani and Bassani, 2002) and 844 nM (Bassani et al., 1995a) respectively.
To estimate the Ca2+ content of the SR, electrical stimulation was stopped and the perfusion medium was changed to Na+- and Ca2+-free (0Na+-0Ca2+) Tyrode solution (Li+ replaced Na+ and 1 mM EGTA replaced Ca2+) to inhibit Na+–Ca2+ exchange (NCX) and remove residual Ca2+. Thirty seconds later, a 30 s perfusion with 10 mM caffeine (Sigma) in 0Na+-0Ca2+ Tyrode solution was started (Bassani et al., 1992; Bassani et al., 1995b). The total Ca2+ content of the SR ([Ca2+]SR) was considered to be the difference between [Ca2+]T at the peak of the [Ca2+]i transient evoked by caffeine and diastolic [Ca2+]T immediately before application of caffeine: [Ca2+]SR = [Ca2+]T(peak)−[Ca2+]T(dia).
The experimental protocol was as follows: cells were perfused with Tyrode solution and field-stimulated at 0.5 Hz (biphasic voltage pulses with amplitude 20% above threshold, 8 ms duration). After a steady state was attained: (i) [Ca2+]i transients were obtained before and after 5 min perfusion with 50 µM canrenone; and (ii) [Ca2+]SR was measured before and after 5 min perfusion with 50 µM canrenone. The concentration of canrenone for the experiments with isolated cardiomyocytes was based on previous experiments with smooth muscles cells (Dacquet et al., 1987) and cardiomyocytes (Caballero et al., 2003). We also analysed the time course of [Ca2+]i decline during transients evoked by 10 mM caffeine in Tyrode solution.
Cell shortening was measured with a video edge detection system (Centro de Engenharia Biomédica, UNICAMP, Campinas, SP, Brazil) simultaneously with [Ca2+]i measurement as previously described (Bassani et al., 1994; Ricardo et al., 2008).
Electrophysiological studies
Electrophysiological recordings were performed by using the whole-cell configuration of the patch-clamp technique (Hamill et al., 1981). Myocytes were continuously superfused with Tyrode solution containing 2 mM 4-aminopyridine (4-AP; Sigma) and 2 mM CaCl2. The glass microelectrodes had a tip resistance of 4–5 MΩ. Cells were internally dialysed with pipette solution containing in mM: 110 CsCl, 20 NaCl, 0.5 CaCl2, 5 ATP-Mg (Sigma), 0.1 GTP (Sigma), 10 EGTA (Sigma), 10 HEPES and 30 TEA-Cl (Sigma), pH 7.2. Transmembrane ionic currents were recorded (low-pass filtered at 1 KHz and sampled at 5 KHz) by using Axopatch 200 amplifier, Digidata 1200 interface and pClamp 6.0.4 software from Axon Instruments, USA. Current amplitude was taken as the difference between the peak and steady-state current and normalized to the cell capacitance.
For time course analysis of the effect of canrenone, ICa-L was elicited every 6 s by voltage-clamp steps from a holding potential of −70 mV to 0 mV for 250 ms. This protocol was done before, during and after (washout) 4 min of perfusion with 50 µM canrenone. For current–voltage relationship analysis, ICa-L was elicited by test potentials ranging from −50 to +60 mV, in 10 mV increments, for 200 ms before and during 4 min of perfusion with 50 µM canrenone. Before each pulse, a prepulse from the holding potential to −40 mV for 25 ms was used to inactivate Na+ and T-type Ca2+ channels. To investigate the effect of canrenone on the voltage dependence of the L-type calcium channel, activation and inactivation curves for ICa-L were determined as described previously (Nascimento et al., 2001). The activation curves were constructed from the current–voltage relationship by dividing the amplitude of ICa-L at each potential by the driving force. Steady-state inactivation curves were obtained with a classical double-pulse protocol. Preconditioning steps of 1.5 s from −60 to +60 mV in 10 mV intervals from a holding potential of −50 mV were applied before a fixed 500 ms step to 0 mV. The effects of canrenone on kinetics of reactivation of the L-type Ca2+ channel were studied by using a standard double-pulse protocol. Two depolarizing pulses to 0 mV with a varying inter-pulse interval were applied, from a holding potential of −40 mV, every 10 s.
Steady-state activation and inactivation curves were fitted with a Boltzmann function to the data of individual experiments: I/Imax = 1/[1 + exp(Vm−V½)/k], where Vm is the membrane potential, V½ the potential of half maximum activation/inactivation and k the slope factor (all in mV). The reactivation curve was fitted by a single exponential.
Statistical analysis
Results were expressed as mean ± SEM. Data were compared by Student's t-test or one-way analysis of variance followed by Student-Newman-Keuls post hoc analysis. In all cases, P < 0.05 was considered statistically significant.
Results
Effects of canrenone on contractility of papillary muscle
A clear negative inotropic effect was observed after 15 min of canrenone exposure as shown by changes in DT and +dT/dt. Indeed, DT decreased significantly at the end of perfusion with 150 µM (3.9 ± 0.5 to 3.6 ± 0.4 g·mm−2; P < 0.05; n = 7), 300 µM (4.4 ± 0.4 to 3.5 ± 0.3 g·mm−2; P < 0.01; n = 10) and 600 µM canrenone (4.0 ± 0.4 to 2.8 ± 0.3 g·mm−2; P < 0.001; n = 8), amounting to a relative reduction of DT values compared with those observed with vehicle perfusion (Table 1), when compared with basal levels. In addition, +dT/dt decreased significantly at the end of 15 min perfusion with 300 µM (53.8 ± 5.2 to 44.0 ± 4.2 g·mm−2·s−1; P < 0.001; n = 10) and 600 µM (50.5 ± 5.4 to 36.8 ± 4.0 g·mm−2·s−1; P < 0.001; n = 8) with significant reduction, compared with values obtained with vehicle (Table 1). TPT decreased with 600 µM canrenone (143 ± 2.5 to 135 ± 3.8 ms; P < 0.05; n = 8), but this reduction did not differ from that after vehicle perfusion (Table 1). Less influence of canrenone was noted in papillary muscle relaxation. After 600 µM canrenone −dT/dt decreased in absolute (27.1 ± 3.9 to 21.6 ± 3.0 g·mm−2·s−1; P < 0.05; n = 8) and relative values (Table 1), whereas the values for RT50 after canrenone did not differ from those obtained after vehicle perfusion (Table 1).
Table 1.
Effects of canrenone on contractile and relaxation properties of left ventricular papillary muscle from rats
| Tween (n = 9) | 150 µM CRN (n = 7) | 300 µM CRN (n = 10) | 600 µM CRN (n = 8) | |
|---|---|---|---|---|
| DT (%) | 96 ± 1 | 92 ± 1 | 80 ± 2† | 71 ± 2† |
| +dT/dt (%) | 99 ± 2 | 98 ± 5 | 82 ± 2† | 73 ± 2† |
| TPT (%) | 98 ± 2 | 102 ± 3 | 98 ± 1 | 96 ± 1 |
| −dT/dt (%) | 105 ± 6 | 93 ± 6 | 91 ± 4 | 80 ± 2* |
| RT50 (%) | 94 ± 4 | 100 ± 8 | 87 ± 3 | 87 ± 2 |
Contractile variables (mean ± SEM) were measured after 15 min exposure to vehicle (0.001% Tween 20) or canrenone (CRN) at the concentrations shown. Basal values in control, untreated papillary muscles were set to 100%.
+dT/dt, maximum rate of tension increase; −dT/dt, maximum rate of tension decline; DT, peak developed tension; RT50, time from peak tension to 50% relaxation; TPT, time to peak tension.
P < 0.01 compared with vehicle.
P < 0.001 compared with vehicle.
Effect of canrenone on cardiomyocyte shortening, [Ca2+]i transients and sarcoplasmic reticulum Ca2+ load
Canrenone (50 µM) reduced cardiomyocyte shortening amplitude from 5.2 ± 0.6% to 3.3 ± 0.5% (n = 14; P < 0.01; Figure 1A) of resting length. Reduction of shortening was accompanied by a reduction in the amplitude of the [Ca2+]i transient from 0.51 ± 0.04 to 0.33 ± 0.03 µM (n = 13; P < 0.001, Figure 1B). Canrenone also reduced diastolic Ca2+ concentration from 0.20 ± 0.01 to 0.18 ± 0.01 µM (n = 13; P < 0.01, Figure 1B). Canrenone did not affect the half-time for cell relaxation (t½-rel from 83 ± 4.8 to 88 ± 5.8 ms; n = 12) and calcium concentration decay at twitches (t½-Ca from 122 ± 6.5 to 115 ± 7.7 ms; n = 12). The time course of [Ca2+]i decline during transients evoked by caffeine in the presence of extracellular Na+, which relies mainly on Ca2+ extrusion via the NCX (Bassani et al., 1992; 1994;), was similar in the absence and presence of canrenone (t½-Ca = 1.5 ± 0.4 s, n = 5, and 1.9 ± 0.3 s, n = 5 respectively).
Figure 1.
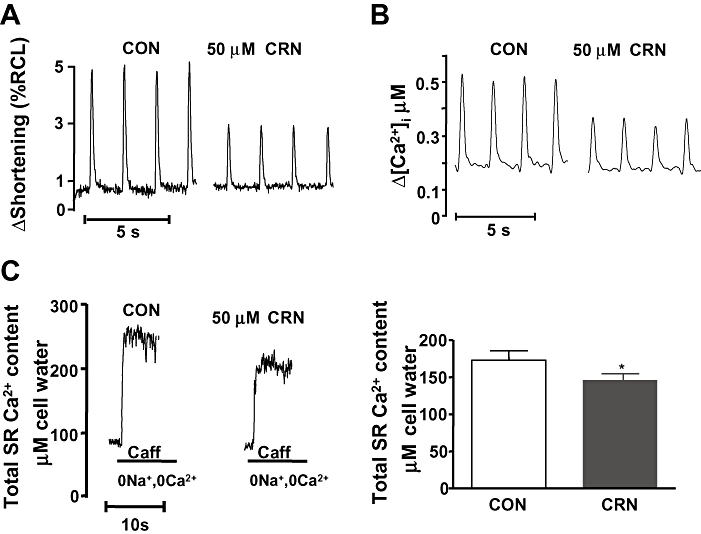
Effect of CRN on cardiomyocyte shortening and intracellular calcium transients. (A) Representative experimental record of changes in cardiomyocyte shortening (ΔShortening) as percentage of RCL during electrically induced twitches before and after 5 min of superfusion with 50 µM CRN. (B) Intracellular calcium transients (Δ[Ca2+]i) of cardiomyocytes during electrically induced twitches before and after 5 min of superfusion with 50 µM CRN. (C) Left: representative experimental record showing total SR Ca2+ content, estimated from caffeine-induced calcium release in a Na+- and Ca2+-free medium before and after 5 min of superfusion with 50 µM CRN. Right: total SR Ca2+ content, estimated from caffeine-induced calcium release, before and after 5 min of superfusion with 50 µM CRN (n = 6). *P < 0.05. 0Na+, Na+-free; 0Ca2+, Ca2+-free; [Ca2+]i, free intracellular Ca2+; Caff, caffeine; CON, control; CRN, canrenone; RCL, resting cell length; SR, sarcoplasmic reticulum.
Additionally, we studied the effect of canrenone on the [Ca2+]SR by measuring Ca2+ release induced by caffeine. We observed that canrenone decreased [Ca2+]SR by about 16% (n = 6; P < 0.01; Figure 1C).
Effect of canrenone on ICa-L
The amplitude of ICa-L decreased progressively during superfusion with canrenone (50 µM) over the 4 min superfusion period (Figure 2A,C; n = 5; P < 0.05) but did not recover significantly during the subsequent 4 min of canrenone washout (Figure 2A,C). In Figure 2B is shown the current–voltage relationship of ICa-L before and after 4 min of perfusion with canrenone. The current we measured was completely blocked by 1 µM nicardipine (Figure 2D) suggesting that it consisted of ICa-L without significant contamination by other currents.
Figure 2.
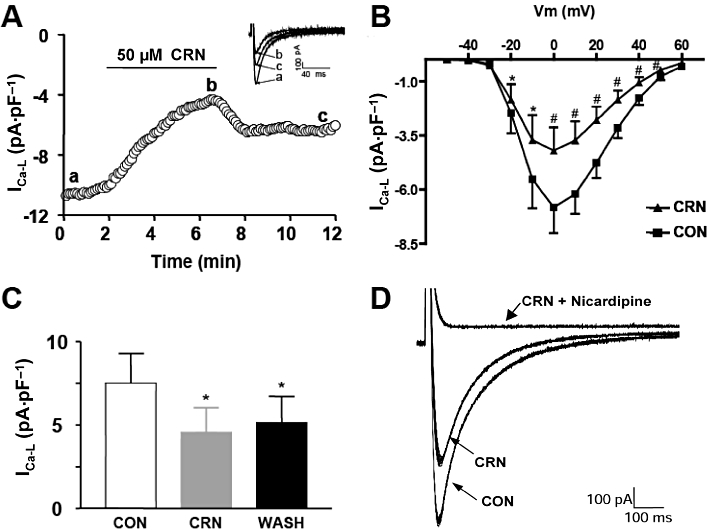
Effect of CRN on L-type Ca2+ channel current (ICa-L). (A) Representative experimental record showing time course changes in ICa-L current amplitude before (a), during (b) and after superfusion with CRN (c – washout). Inset shows current traces recorded at the indicated times (a, b and c). The current was elicited from a holding potential of −70 to 0 mV with a prepulse to −40 mV for 25 ms, activated every 6 s, in rat ventricular myocytes. (B) Current–voltage relationship of the peak ICa-L elicited by test potentials ranging from −50 to +60 mV, before and after 4 min of perfusion with CRN (n = 12). (C) Mean ± SEM values of maximal ICa-L amplitude before, during and after CRN perfusion (n = 5). (D) Representative experimental record showing ICa-L amplitude in control conditions and after sequential addition of CRN (50 µM) and nicardipine (1 µM). *P < 0.05 and #P < 0.001 CRN versus CON. CON, control; CRN, canrenone.
Figure 3 shows the normalized steady-state activation and inactivation curves in control conditions and in the presence of canrenone. Under control conditions, the analysis of steady-state activation curves revealed that V½ was −8.5 ± 1.7 mV and k was 5.0 ± 0.2 mV. In the presence of canrenone, V½ was −3.6 ± 1.7 mV and k was 7.0 ± 1.0 mV (n = 7, P > 0.05; Figure 3A). Analysis of steady-state inactivation curves also revealed similar parameters under control conditions (V½ was −32.2 ± 0.3 mV and k was 5.8 ± 0.2 mV) and in the presence of canrenone (V½ was −35.1 ± 1.2 mV and k was 7.3 ± 0.6 mV (n = 5, P > 0.05; Figure 3B).
Figure 3.
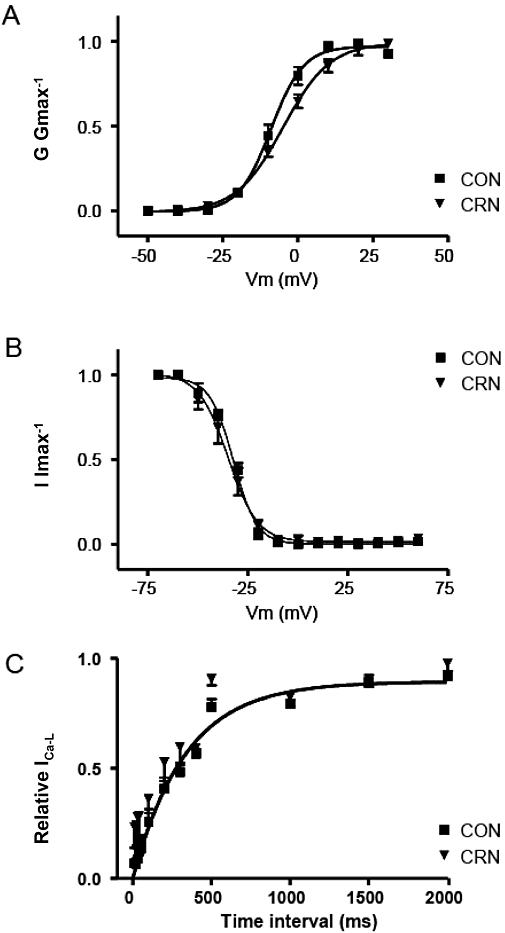
Lack of effect of CRN on voltage-dependent characteristics of L-type Ca2+ channel current (ICa-L) in rat cardiomyocytes. (A) The steady-state activation curves were calculated as normalized conductance values from the current-voltage curves before and 2 min after exposure to CRN (50 µM; n = 7). (B) Steady-state inactivation curve, ICa-L at the test step to 0 mV was normalized to maximum current and plotted against the potential of a 1 s inactivating conditioning prepulse between −60 and +60 mV before and 2 min after exposure to CRN (n = 5). (C) Time constant of recovery from inactivation of ICa-L. Depolarizing pulses were applied every 6 s at a holding potential of −40 mV. The recovery from inactivation was fitted by a single exponential (n = 5). Recovery was complete in about 1 s. CON, control; CRN, canrenone.
The canrenone effect on the time constant of reactivation of L-type Ca2+ channels was also studied. The time constant in control conditions was 229 ± 26.8 ms and 182 ± 11 ms (n = 5, P > 0.05; Figure 3C) in the presence of 50 µM canrenone. In all experiments reactivation was complete about 1 s. Thus, canrenone did not affect the steady-state activation and inactivation, and reactivation curves of the L-type Ca2+ channel.
Discussion and conclusions
We have demonstrated for the first time that the acute negative cardiac inotropic effect of canrenone is related to a decreased amplitude of ICa-L and of [Ca2+]I transients, together with a decreased [Ca2+]SR. We also observed that canrenone reduced diastolic [Ca2+]i. The observation that canrenone modulated cardiac contractility is in agreement with many earlier findings that aldosterone antagonists produce cardiac effects that may not depend on mineralocorticoid receptor antagonism (Tanz and Kerby, 1961; Baskin et al., 1973; Coraboeuf and Deroubaix, 1974; Sorrentino et al., 1996; 2000; Vassallo et al., 1998; Cargnelli et al., 2001; Barbato et al., 2002; Sugiyama et al., 2004).
The negative inotropic effect of canrenone, indicated by the reduction in DT and +dT/dt, was previously observed in papillary muscles of humans (Mugge et al., 1984), guinea pigs (Mugge et al., 1984) and rats (Cargnelli et al., 2001). This effect was also seen with other spironolactone metabolites, such as sodium canrenoate (Coraboeuf and Deroubaix, 1974) and potassium canrenoate (Baskin et al., 1973; Vassallo et al., 1998). The acute negative inotropic effect of canrenone represents a non-genomic effect of this drug on cardiac contractility. Moreover, another study showed that spironolactone produces a negative effect on the isolated working heart of the rat (Moreau et al., 1996).
We also studied the acute action of canrenone on cardiac lusitropism. We observed that only the highest concentration of canrenone reduced −dT/dt in papillary muscles preparations whereas the RT50 remained unchanged in all concentrations studied. In addition, we have not observed any change on [Ca2+]i transient kinetics induced by canrenone at lower concentrations. The effect observed on −dT/dt with the highest tested canrenone concentration might still be due to a direct effect of canrenone on relaxation. Alternatively, it is possible that the effect is secondary to reduced after load, that is, it may be due to the reduction of DT produced by the canrenone-treated muscle (Konishi et al., 1992).
During the cardiac action potential, Ca2+ enters the cardiomyocyte mainly through L-type calcium channels triggering the release of additional calcium by the activation of ryanodine-sensitive calcium channels of the SR, the so-called Ca2+-induced Ca2+ release (Fabiato, 1983). This process results in a rapid increase of [Ca2+]i, which activates myofilament shortening and cellular contraction. A reduced amplitude of the Ca2+ transient may result from lower amplitude of ICa-L, decreased [Ca2+]SR or both. Our experiments showed that canrenone exerts an acute inhibition of the ICa-L, causing a reduced Ca2+ influx. We consider that this reduced Ca2+ influx is the primary cause for the reduced [Ca2+]SR and diastolic [Ca2+]i at steady state, which we found after exposure of cardiomyocytes to canrenone. As a result, the amplitude of the [Ca2+]i transient was reduced in the presence of canrenone and this was responsible for the depressed contractility induced by canrenone both in the cardiomyocyte and in the isolated papillary muscle preparation. Our results at the cellular level corroborated the previously described negative inotropic action of canrenone at the muscle level.
Alternatively, the effects of canrenone on diastolic Ca2+ concentration could be attributed to changes in rapid and slow Ca2+ transport systems. However, we did not find evidence of increased Ca2+ transport by SR Ca2+ ATPase or NCX, as canrenone did not change the time course of [Ca2+]i decline during twitches and during transients evoked by caffeine in the presence of extracellular Na+ respectively. Additionally, we did not observe any visible effect of canrenone on the decay of the [Ca2+]I transients induced by caffeine in 0Na+-0Ca2+ Tyrode solution, which rules out major effects of canrenone on the slow Ca2+ transport systems, such as mitochondrial Ca2+ uniporter and sarcolemmal Ca2+ ATPase (Bassani et al., 1992; 1994;). Thus, our present data do not identify important effects of canrenone on the mechanisms that promote cytosolic Ca2+ removal, particularly the rapid transporters, namely SR Ca2+ ATPase and NCX (Bassani et al., 1992; 1994; Bassani and Bassani, 2002).
The positive inotropic effect of canrenone at low concentrations observed by Vassallo et al. (1998) was previously reported by Coraboeuf and Deroubaix (1974). These authors suggested that this positive inotropic effect was a consequence of the increased coronary flow induced by sodium canrenoate. They concluded that the negative inotropic effect of sodium canrenoate at low concentrations is overtaken by the increase in the ventricular pressure induced by the increased coronary flow. The increase in the coronary flow is a consequence of the vascular dilatation induced by canrenone (Sorrentino et al., 2000) through L-type calcium channel blockade in smooth muscle cell (Dacquet et al., 1987).
Coraboeuf and Deroubaix (1974) also reported a dose-dependent increase in amplitude and duration of the plateau phase of the action potential induced by sodium canrenoate. The authors considered that these effects could be caused by either an increase in depolarizing currents (sodium and calcium) or a reduction in repolarizing currents (chloride and potassium). The blockade of the ICa-L by canrenone reported by us excludes this current as a possible contributor to the effect of canrenone on the action potential. Coraboeuf and Deroubaix (1974) also provided evidence against a role for sodium and chloride currents in the action potential changes induced by canrenone. They concluded that the increase in amplitude and duration of the action potential plateau induced by sodium canrenoate is due to potassium current blockade. Gomez et al. (2005) demonstrated that spironolactone and canrenoic acid block IKs, IKur and Ito currents from cultured cells expressing hERG, Kv1.5 and Kv4.3. In our study, calcium current was recorded under conditions that suppress repolarizing currents (4-AP in the extracellular solution and K+ replaced by Cs+ in the intracellular solution). Therefore, our data do not exclude a possible effect of canrenone on potassium currents (Ito1, IKslow and IKur) from rat ventricular cardiomyocytes.
The previously described aldosterone action on ICa-L occurs only after 6 h of aldosterone exposure (Benitah and Vassort, 1999). Therefore, we considered that the acute effect of canrenone on ICa-L may be independent of its effect on aldosterone receptors. However, the precise pathways linking canrenone with the inhibition of the ICa-L are currently unknown and warrant further investigation.
Our results on ICa-L kinetics indicate that canrenone action on ICa-L may be indirect, rather than direct on the channel as canrenone did not alter any of the kinetic parameters of ICa-L studied by us. The decrease in ICa-L amplitude induced by canrenone reported by us was in agreement with results from a previous study (Dacquet et al., 1987) that described ICa-L inhibition by spironolactone in smooth muscle cells from rat portal vein. Moreover, the percentage of reduction in the ICa-L caused by spironolactone – 42% (Dacquet et al., 1987) – was similar to the one found by us with canrenone – 40%. Dacquet et al. (1987) also demonstrated that spironolactone action on ICa-L was reversible. We observed no significant recovery of ICa-L amplitude after washout, but we cannot conclude that this action of canrenone on ICa-L was irreversible, as our period of washout was not as long as that used by Dacquet et al. (1987).
Our finding that canrenone reduces the [Ca2+]SR induced by caffeine in a 0Na+-0Ca2+ solution is in accordance with those from a previous study (Dacquet et al., 1987), showing that spironolactone reduced the transient contractions induced by noradrenaline and acetylcholine in rat portal vein strips in a Ca2+-free solution.
We are aware that our results obtained under acute administration of canrenone to normal animals should not be extrapolated to disease models and may not reflect canrenone's effect after its chronic administration. Another limitation of the present work was that canrenone was studied at the cellular level at a single concentration. However, we and others (Cargnelli et al., 2001) have shown that increasing canrenone concentration in multicellular preparations produced greater negative inotropic effect.
In summary, we have demonstrated for the first time that canrenone reduces the amplitude of cardiac ICa-L and intracellular Ca2+ stores in cardiomyocytes. These effects reduce the amplitude of intracellular Ca2+ transients which, in turn, contributes to the negative inotropic action of canrenone on the heart.
Acknowledgments
The authors are indebted to Dr Rosana Almada Bassani and Dr Roberto M Saraiva for helpful discussions and to Luiz Fernando Rodrigues Junior, Ednei Luiz Antonio and Danilo Bocalini for technical support. This work was supported by grants from the Fundação de Amparo à Pesquisa do Estado de São Paulo to PJFT (99/04533-4), ATF (05/604578), MEMO (03/14076-7), and from Conselho Nacional de Desenvolvimento Científico e Tecnológico to JWMB (300632/2005-3) and PJFT (300.692/80-3). ARC received a fellowship from Coordenação de Aperfeiçoamento de Pessoal de Nível Superior.
Glossary
Abbreviations:
- +dT/dt
maximum rate of tension increase
- −dT/dt
maximum rate of tension decline
- Bmax-en
maximal Ca2+ binding capacity of the endogenous Ca2+ binding sites
- Bmax-in
maximal Ca2+ binding capacity of indo-1
- [Ca2+]i
free intracellular Ca2+
- [Ca2+]SR
total Ca2+ content of the SR
- [Ca2+]T
total Ca2+ concentration in the cytosol
- [Ca2+]T(peak)
[Ca2+]T at the peak of the [Ca2+]i transient
- [Ca2+]T(dia)
[Ca2+]T immediately before application of caffeine
- DT
peak developed tension
- ICa-L
L-type Ca2+ channel current
- k
slope factor
- Kd
indo-1 apparent dissociation constant
- Kd-en
Kd of the endogenous Ca2+ binding sites
- Kd-in
Kd
- Lmax
muscle length at which DT is maximum
- NCX
Na+–Ca2+ exchange
- RT50
time from peak tension to 50% relaxation
- SR
sarcoplasmic reticulum
- t½-rel
half-time for cell relaxation
- t½-Ca
half-time for calcium concentration decay at twitches
- TPT
time to peak tension
- Vm
membrane potential
- V½
potential of half maximum activation/inactivation
Conflict of interest
None.
References
- Barbato JC, Mulrow PJ, Shapiro JI, Franco-Saenz R. Rapid effects of aldosterone and spironolactone in the isolated working rat heart. Hypertension. 2002;40:130–135. doi: 10.1161/01.hyp.0000025879.29822.24. [DOI] [PubMed] [Google Scholar]
- Baskin SI, Akera T, Puckett CR, Brody SL, Brody TM. Effect of potassium canrenoate on cardiac functions and (Na ++ K +)-activated ATPase. Proc Soc Exp Biol Med. 1973;143:495–498. doi: 10.3181/00379727-143-37351. [DOI] [PubMed] [Google Scholar]
- Bassani JWM, Bassani RA, Bers DM. Relaxation in rabbit and rat cardiac cells: species-dependent differences in cellular mechanisms. J Physiol. 1994;476:279–293. doi: 10.1113/jphysiol.1994.sp020130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassani JWM, Bassani RA, Bers DM. Calibration of indo-1 and resting intracellular [Ca]i in intact rabbit cardiac myocytes. Biophys J. 1995a;68:1453–1460. doi: 10.1016/S0006-3495(95)80318-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassani JWM, Yuan W, Bers DM. Fractional SR Ca release is regulated by trigger Ca and SR Ca content in cardiac myocytes. Am J Physiol. 1995b;268:C1313–C1319. doi: 10.1152/ajpcell.1995.268.5.C1313. [DOI] [PubMed] [Google Scholar]
- Bassani RA, Bassani JWM. Contribution of Ca(2+) transporters to relaxation in intact ventricular myocytes from developing rats. Am J Physiol Heart Circ Physiol. 2002;282:H2406–H2413. doi: 10.1152/ajpheart.00320.2001. [DOI] [PubMed] [Google Scholar]
- Bassani RA, Bassani JWM, Bers DM. Mitochondrial and sarcolemmal Ca2+ transport reduce [Ca2+]i during caffeine contractures in rabbit cardiac myocytes. J Physiol. 1992;453:591–608. doi: 10.1113/jphysiol.1992.sp019246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassani RA, Shannon TR, Bers DM. Passive Ca2+ binding in ventricular myocardium of neonatal and adult rats. Cell Calcium. 1998;23:433–442. doi: 10.1016/s0143-4160(98)90100-2. [DOI] [PubMed] [Google Scholar]
- Benitah JP, Vassort G. Aldosterone upregulates Ca(2+) current in adult rat cardiomyocytes. Circ Res. 1999;85:1139–1145. doi: 10.1161/01.res.85.12.1139. [DOI] [PubMed] [Google Scholar]
- Caballero R, Moreno I, Gonzalez T, Arias C, Valenzuela C, Delpon E, et al. Spironolactone and its main metabolite, canrenoic acid, block human ether-a-go-go-related gene channels. Circulation. 2003;107:889–895. doi: 10.1161/01.cir.0000048189.58449.f7. [DOI] [PubMed] [Google Scholar]
- Cargnelli G, Trevisi L, Debetto P, Luciani S, Bova S. Effects of canrenone on aorta and right ventricle of the rat. J Cardiovasc Pharmacol. 2001;37:540–547. doi: 10.1097/00005344-200105000-00006. [DOI] [PubMed] [Google Scholar]
- Conrad CH, Brooks WW, Robinson KG, Bing OH. Impaired myocardial function in spontaneously hypertensive rats with heart failure. Am J Physiol. 1991;260:H136–H145. doi: 10.1152/ajpheart.1991.260.1.H136. [DOI] [PubMed] [Google Scholar]
- Coraboeuf E, Deroubaix E. Effect of a spirolactone derivative, sodium canrenoate, on mechanical and electrical activities of isolated rat myocardium. J Pharmacol Exp Ther. 1974;191:128–138. [PubMed] [Google Scholar]
- Dacquet C, Loirand G, Mironneau C, Mironneau J, Pacaud P. Spironolactone inhibition of contraction and calcium channels in rat portal vein. Br J Pharmacol. 1987;92:535–544. doi: 10.1111/j.1476-5381.1987.tb11354.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabiato A. Calcium-induced release of calcium from the cardiac sarcoplasmic reticulum. Am J Physiol. 1983;245:C1–C14. doi: 10.1152/ajpcell.1983.245.1.C1. [DOI] [PubMed] [Google Scholar]
- Gomez R, Nunez L, Caballero R, Vaquero M, Tamargo J, Delpon E. Spironolactone and its main metabolite canrenoic acid block hKv1.5, Kv4.3 and Kv7.1 + minK channels. Br J Pharmacol. 2005;146:146–161. doi: 10.1038/sj.bjp.0706302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grynkiewicz G, Poenie M, Tsien RY. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985;260:3440–3450. [PubMed] [Google Scholar]
- Hamill OP, Marty A, Neher E, Sakmann B, Sigworth FJ. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981;391:85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Hunt SA, Baker DW, Chin MH, Cinquegrani MP, Feldman AM, Francis GS, et al. ACC/AHA guidelines for the evaluation and management of chronic heart failure in the adult: executive summary a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee to Revise the 1995 Guidelines for the Evaluation and Management of Heart Failure) Circulation. 2001;104:2996–3007. doi: 10.1161/hc4901.102568. Developed in collaboration with the International Society for Heart and Lung Transplantation; Endorsed by the Heart Failure Society of America. [DOI] [PubMed] [Google Scholar]
- Konishi T, Nakamura Y, Kato I, Kawai C. Dependence of peak dP/dt and mean ejection rate on load and effect of inotropic agents on the relationship between peak dP/dt and left ventricular developed pressure–assessed in the isolated working rat heart and cardiac muscles. Int J Cardiol. 1992;35:333–341. doi: 10.1016/0167-5273(92)90231-q. [DOI] [PubMed] [Google Scholar]
- Megges R, Weiland J, Undeutsch B, Buchting H, Schon R. The nitration of canrenone with acetic anhydride/nitric acid. Steroids. 1997;62:762–766. doi: 10.1016/s0039-128x(97)00073-1. [DOI] [PubMed] [Google Scholar]
- Moreau D, Chardigny JM, Rochette L. Effects of aldosterone and spironolactone on the isolated perfused rat heart. Pharmacology. 1996;53:28–36. doi: 10.1159/000139412. [DOI] [PubMed] [Google Scholar]
- Mugge A, Schmitz W, Scholz H. Negative inotropic effects of aldosterone antagonists in isolated human and guinea-pig ventricular heart muscle. Klin Wochenschr. 1984;62:717–723. doi: 10.1007/BF01725704. [DOI] [PubMed] [Google Scholar]
- Nascimento JH, Salle L, Hoebeke J, Argibay J, Peineau N. cGMP-mediated inhibition of cardiac L-type Ca(2+) current by a monoclonal antibody against the M(2) ACh receptor. Am J Physiol Cell Physiol. 2001;281:C1251–C1258. doi: 10.1152/ajpcell.2001.281.4.C1251. [DOI] [PubMed] [Google Scholar]
- Pitt B, Zannad F, Remme WJ, Cody R, Castaigne A, Perez A, et al. The effect of spironolactone on morbidity and mortality in patients with severe heart failure. N Engl J Med. 1999;341:709–717. doi: 10.1056/NEJM199909023411001. Randomized Aldactone Evaluation Study Investigators. [DOI] [PubMed] [Google Scholar]
- Ricardo RA, Bassani RA, Bassani JWM. Osmolality- and Na+-dependent effects of hyperosmotic NaCl solution on contractile activity and Ca2+ cycling in rat ventricular myocytes. Pflugers Arch. 2008;455:617–626. doi: 10.1007/s00424-007-0322-3. [DOI] [PubMed] [Google Scholar]
- Sadée W, Dagcioglu M, Schroder R. Pharmacokinetics of spironolactone, canrenone and canrenoate-k in humans. J Pharmacol Exp Ther. 1973;185:686–695. [PubMed] [Google Scholar]
- Santos PE, Barcellos LC, Mill JG, Masuda MO. Ventricular action potential and L-type calcium channel in infarct-induced hypertrophy in rats. J Cardiovasc Electrophysiol. 1995;6:1004–1014. doi: 10.1111/j.1540-8167.1995.tb00377.x. [DOI] [PubMed] [Google Scholar]
- Sorrentino R, Cirino G, Calignano A, Mancuso F, Sorrentino L, Andriuoli G, et al. Increase in the basal tone of guinea pig thoracic aorta induced by ouabain is inhibited by spironolactone canrenone and potassium canrenoate. J Cardiovasc Pharmacol. 1996;28:519–525. doi: 10.1097/00005344-199610000-00007. [DOI] [PubMed] [Google Scholar]
- Sorrentino R, Autore G, Cirino G, Bianca REV, Calignano A, Vanasia M, et al. Effect of spironolactone and its metabolites on contractile property of isolated rat aorta rings. J Cardiovasc Pharmacol. 2000;36:230–235. doi: 10.1097/00005344-200008000-00013. [DOI] [PubMed] [Google Scholar]
- Sugiyama A, Satoh Y, Takahara A, Ando K, Wang K, Honsho S, et al. Electropharmacological effects of a spironolactone derivative, potassium canrenoate, assessed in the halothane-anesthetized canine model. J Pharmacol Sci. 2004;96:436–443. doi: 10.1254/jphs.fpj04025x. [DOI] [PubMed] [Google Scholar]
- Tan RD, Kerby CF. The inotropic action of certain steroids upon isolated cardiac tissue; with comments on steroidal cardiotonic structure-activity relationships. J Pharmacol Exp Ther. 1961;131:56–64. [PubMed] [Google Scholar]
- Vassallo PF, Stefanon I, Rossoni LV, Franca A, Vassallo DV. Small doses of canrenone block the effects of ouabain on the mechanical activity of the heart and vessels of the rat. J Cardiovasc Pharmacol. 1998;32:679–685. doi: 10.1097/00005344-199811000-00001. [DOI] [PubMed] [Google Scholar]


