Abstract
Understanding the dynamics of porcine reproductive and respiratory syndrome virus (PRRSV) vertical transmission is important to enhance the accuracy of monitoring protocols for endemically infected breeding herds. The objectives of this study were to determine the prevalence of PRRSV within infected litters, to quantify viremia, and to identify specific attributes of infected individuals. Eight gilts were intramuscularly inoculated with 101 TCID50 of a mildly virulent PRRSV strain (MN-30100) at 90 d of gestation. All inoculated gilts transmitted the virus in utero. The proportion of PRRSV PCR-positive piglets and the level of viremia in the piglets were higher at 4 d of age than at birth or at weaning. No specific attributes were associated with PRRSV infection in the piglets. This is the first report, that we are aware of, documenting the efficient in utero transmission of an extremely low dose of a mildly virulent strain of PRRSV. The results support the sampling of piglets late during lactation as a tool to monitor PRRSV shedding from sow-herds.
Résumé
Il est important de comprendre la dynamique de la transmission verticale du virus reproducteur et respiratoire porcin (PRSSV) afin d’améliorer la précision des protocoles de surveillance des troupeaux reproducteurs infectés de manière endémique. Les objectifs de la présente étude étaient de déterminer la prévalence du PRRSV parmi les portées infectées, de quantifier la virémie, et d’identifier des caractéristiques spécifiques des individus infectés. Huit truies ont été inoculées par voie intramusculaire avec 101 TCID50 d’une souche légèrement virulente de PRRSV (MN-30100) au 90e jour de gestation. Toutes les truies inoculées ont transmis le virus in utero. La proportion de porcelets positifs par PCR pour PRRSV et le degré de virémie chez les porcelets étaient plus élevés à 4 jours d’âge qu’au moment de la naissance ou au sevrage. Aucune caractéristique spécifique n’était associée à l’infection par le PRRSV chez les porcelets. À notre connaissance, la présente publication serait la première documentant la transmission in utero efficace d’une dose extrêmement faible d’une souche légèrement virulente du PRRSV. Les résultats indiquent que la prise d’échantillons chez des porcelets tardivement durant la lactation serait appropriée pour surveiller l’excrétion du PRRSV dans les troupeaux de truies.
(Traduit par Docteur Serge Messier)
Porcine reproductive and respiratory syndrome (PRRS) is an economically significant viral disease of swine, estimated to cost US pork producers approximately 560 million dollars in direct losses per year (1). This disease was first reported in 1989 (2,3). Clinical signs of porcine reproductive and respiratory syndrome virus (PRRSV) infection may include anorexia, lethargy, dyspnea, hyperthermia, reproductive failure, small weak-born pigs, reduction in average daily gain (ADG), and increase in mortality rates (2–4). This virus can be shed in saliva (5), semen (6), nasal secretions (7), urine (5), mammary secretions (8), and feces (7). The first report of PRRSV transplacental transmission described intranasal inoculation of 9 sows at 93 d of gestation with lung homogenates from clinically affected pigs (9). After the introduction of PRRSV into a susceptible population of pigs, an epidemic phase of the disease with clinical signs in all production stages is usually observed. In most cases, the infection becomes endemic in a short period of time and clinical signs are observed only in weaned groups of susceptible pigs (2) or in naïve gilts introduced for replacement (10).
The presence of subpopulations of PRRSV-naïve and positive adult swine co-existing within endemically infected herds can perpetuate the infection in the population by the continuous transmission of the virus to the piglets (11). The challenge for swine practitioners before making decisions regarding the duration of herd closure, flow of weaned pigs, or biosecurity measures, is to determine whether viral transmission has stopped in the sow-herd, at which time the herd can be defined as stable (12). An improved understanding of the dynamics of PRRSV vertical transmission is required to refine monitoring protocols for endemically infected breeding herds. Specific objectives of this study were to compare the prevalence of viremic litters and piglets at birth, 4 d after birth, and at weaning, to quantify PRRSV in serum of infected piglets, and to identify specific attributes of viremic piglets and litters.
Twelve gilts were obtained from a herd known to be free of PRRSV based on 10 y of diagnostic testing. At approximately 80 d of gestation, gilts were transported to the isolation facilities of the College of Veterinary Medicine at the University of Minnesota. After arrival, pigs were confirmed to be PRRSV naïve using enzyme-linked immunosorbent assay (ELISA) (HerdChek PRRS Antibody 2XR Test Kit; IDEXX Laboratories, Westbrook, Maine, USA) and reverse transcriptase — polymerase chain reaction (RT-PCR) (Taqman RT-PCR kit; Perkin-Elmer Applied Biosystems, Foster City, California, USA). Each individual was housed in an isolation room provided with a conventional farrowing crate, independent ventilation system, and slurry pit to prevent cross-contamination of pathogens between rooms. All protocols and procedures of pig management and care were approved by the University of Minnesota Institutional Animal Care and Use Committee (protocol number 0502A67586). Personnel practiced specific biosecurity protocols (13) to prevent PRRSV transmission between study groups throughout the experiment.
The 12 gilts were randomly allocated to 3 groups (4 gilts/group): A, B, and C. Gilts in group A (negative controls) were injected at 90 d of gestation with 3 mL of sterile minimum essential medium Eagle (Sigma-Aldrich, St. Louis, Missouri, USA). Gilts in group B received 3 mL of cell culture medium containing 101 50% tissue-culture infective dose (TCID50) of PRRSV isolate MN-30100 at cell culture passage four; and gilts in group C were inoculated with 102 TCID50 of the same isolate. The inoculation of gilts in group C with a higher dose was intended to increase the likelihood that PRRSV transplacental infection would occur in at least 4 litters. Inoculations were administered via the intramuscular route. Infection of fetuses following the transmission of the virus through the placenta has been reported to be more efficient during the last trimester of pregnancy (7,14). To confirm infection, RT-PCR and ELISA were performed on serum samples collected from the gilts at 5 and 14 d post-inoculation (DPI). The inoculated virus, PRRSV MN-30100, replicates at low levels in blood and tissues, induces only mild clinical signs in growing pigs (transient depression, lack of appetite for 24 to 48 h and mild fever of 40°C to 41°C) and is shed at significantly lower levels after experimental inoculation than a highly pathogenic isolate (15).
Farrowings were induced at 114 d of gestation. Serum and milk samples were collected from the sows within 2 h of farrowing, 4 d later, and at weaning (18 d). Piglets were weighed and individually identified using ear tags at birth. Serum samples collected at birth in sterile vacuum tubes (Becton-Dickinson Vacutainer, Franklin Lakes, New Jersey, USA) via jugular venipuncture were obtained before colostrum intake. Serum samples were collected again from the piglets at 4 d of age and at weaning. At weaning, piglets were weighed, humanely euthanized, and tissue samples were collected.
The PRRSV antibody response was evaluated by ELISA. The presence of PRRSV nucleic acid in serum, milk, and tissue samples was determined by RT-PCR. Tissue samples collected from the piglets at weaning (tonsil, sternal, and superficial inguinal lymph nodes) were pooled by individual animal and 0.5 g of this pool was placed in 7.5 mL of lysis buffer (BD Biosciences, Palo Alto, California, USA) in a sterile plastic tube (Falcon tube; Becton-Dickinson, Franklin Park, New Jersey, USA). After homogenization (Polytron PT 3100; Kinematica AG, Lucerne, Switzerland), samples were centrifuged at 3000 rpm for 15 min. Total RNA was extracted and purified from 200 μL of serum or 50 μL of the middle layer of the homogenized tissue supernatant using the Nucleospin II kit (BD Biosciences), according to the manufacturer’s protocol. The RNA was eluted in 50 μL of water. Every sample was assayed in duplicate using 2 μL of the rehydrated sample in a 20-μL RT-PCR reaction with primers and probe directed to the open reading frame (ORF) 7 region of the North American PRRSV (16). All reactions were conducted in a real-time PCR instrument (ABI 7500; Perkin-Elmer Applied Biosystems, Foster City, California, USA). A standard curve was developed for the quantitative RT-PCR procedure by preparing 10-fold dilutions of PRRSV MN-30100 stock. Results were reported as the number of RNA copies per mL of serum or milk (RNAc/mL) or number of RNA copies per gram of tissue (RNAc/g).
The RNAc/mL and the RNAc/g were log-transformed to stabilize the variance prior to analysis. The proportions of PRRSV PCR-positive piglets between groups and sampling days were compared using Fisher’s Exact Test. Group averages of log10 RNAc/mL, ELISA s/p ratios, and ADG in the piglets were compared, controlling for the litter effect, by one-way analysis of variance (ANOVA). Averages of total piglets born per litter, total piglets born alive per litter, log10 RNAc/mL, and ELISA s/p ratios in the sows were compared by Kruskal–Wallis one-way ANOVA (Statistix 8; Analytical Software, Tallahassee, Florida, USA).
The association between specific attributes including: piglet weight (kg), gender, presence of diarrhea, being visually sick, being a stillbirth piglet or a mummified fetus, presence of rough hair coat, farrowing 4 d earlier than the expected (date based on the herd average gestation length), presence of stillbirth piglets or mummified fetuses in 50% or more of a litter, and the detection of PRRSV PCR-positive piglets was screened by univariable logistic regression analysis (SAS/STAT 9.1, SAS Institute, Cary, North Carolina, USA). The multivariable logistic regression model, with PCR-result as the dependent variable, was determined with a backward variable selection procedure, including only those variables with P values ≤ 0.1 in the univariable analyses. Odds ratios (OR) and 95% confidence intervals (CI) were estimated by the logistic regression models.
Negative control females (group A) remained ELISA and PCR negative throughout the study and no clinical signs were observed. Mild fever and lack of appetite were detected in the inoculated gilts (groups B and C) for a period of 2 d following inoculation with PRRSV MN-30100. Four of the 8 inoculated gilts farrowed 4 to 5 d before the expected date, with 3 originating in group C. Piglets born to these females were undersized, weak, and died within a week after birth. No statistically significant differences between groups were detected between the proportion of early farrowings, the total piglets born per litter or total piglets born alive per litter (Table I). At 14 DPI all gilts in groups B and C were ELISA positive (s/p ratio greater than 0.4). Nucleic acid from PRRSV was detected in serum of all the gilts in groups B and C at 5 DPI. At farrowing, only 1 sow in group B and 1 in group C were viremic. However, the same day, PRRSV RNA was detected in milk samples in 3 sows of group B and in all 4 sows of group C. No statistically significant differences in mean log10 RNAc/mL of serum or milk or average ELISA s/p ratio were observed between groups B and C (P ≥ 0.05) (Table I).
Table I.
Effect of the inoculation dose on clinical signs, viral infection, and serologic response in the gilts
| Parameter | Group A (n = 4) | Group B (n = 4) | Group C (n = 4) |
|---|---|---|---|
| PRRSV inoculation dose | SHAM | 101 TCID50 | 102 TCID50 |
| Total piglets born per litter | 12 ± 2.7a | 11.8 ± 2.1a | 11.8 ± 2a |
| Piglets born alive per litter | 9.5 ± 2.2a | 7.3 ± 1.4a | 6.8 ± 1a |
| Proportion of early farrowings | 0/4 | 1/4 | 3/4 |
| log10 RNAc/mL serum 5 DPI | 0a | 1.1 ± 0.5b | 1.2 ± 0.2b |
| log10 RNAc/mL milk at farrowing | 0a | 1.5 ± 0.3b | 2.2 ± 0.6b |
| ELISA s/p ratio 14 DPI | 0a | 2.6 ± 0.4b | 2.2 ± 0.9b |
Total piglets born per litter, piglets born alive per litter, log10 RNAc/mL and ELISA s/p ratio are mean ± standard error.
Different superscripts on the same parameter (log10 RNAc/mL and ELISA s/p ratio) were statistically different (P < 0.05).
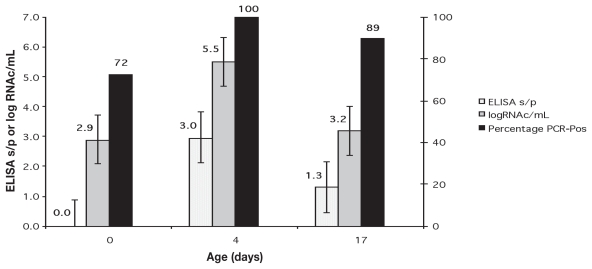
The proportion of PCR-positive piglets per litter at birth ranged from 55% to 100% in groups B and C. The proportion of infected piglets for the entire groups B (78%) and C (70%) were not statistically different (P = 0.817). The overall proportion at birth for both groups was 72%. Prevalence of PRRSV significantly increased from birth to 4 d of age (P = 0.001) when 100% of the piglets were viremic. At weaning, 89% of the piglets from the inoculated groups were still PCR-positive. No statistically significant difference was observed in the proportion of PCR-positive piglets at 4 d of age compared to weaning (P ≥ 0.05).
Viremia, measured as log10 RNAc/mL of serum, in piglets born alive from group B (2.9 ± 0.3), was not statistically different than the serum virus concentration in the piglets of group C (3.1 ± 0.4) at birth (P = 0.916). Litter had no significant effect on viremia levels at birth (P = 0.179). RNAc/mL and ELISA s/p ratio results of groups B and C were combined for comparisons at birth, 4 d of age and weaning. The viremia level of the suckling piglets was significantly higher at 4 d of age than at birth or at weaning (P ≥ 0.05) (Figure 1). Mean ELISA s/p ratio at 4 d of age and at weaning were statistically indistinguishable, but both were significantly higher that the mean at birth (P ≥ 0.05) (Figure 1). The mean viral load in the lymphoid tissues of the surviving piglets from inoculated groups at weaning was 4.6 ± 0.2 log10 RNAc/g of tissue.
Figure 1.
ELISA s/p ratio mean, log10 RNAc/mL and proportion of PRRSV PCR-positive piglets in groups B and C during lactation.
Note: Error bars are SE of the means and dots are percentage of PCR-positive piglets. Data from piglets in groups B and C were combined.
The only attribute at birth or at weaning with a P value ≤ 0.1 in the univariate logistic regression analysis was having at least 50% of a litter being stillbirth and/or mummified fetuses (OR, 0.31; 95% CI, 0.09–1.12; P = 0.075). There were no statistically significant associations between average litter weight, occurrence of early farrowing, weight, clinical condition, rough hair coat, or being a stillbirth piglet or a mummified fetus and the detection of PRRSV PCR-positive individuals (P ≥ 0.05). Neither the proportion of PCR-positive piglets at birth (P = 0.384) nor the serum virus concentration (P = 0.912) in piglets born from early farrowings were statistically different from piglets born from normal gestation length farrowings. Pigs defined as “very sick” (prostrate, not suckling, dyspnea) had a higher virus concentration in serum than the PCR-positive pigs that were apparently “healthy” at 4 d of age (P = 0.03). Serum virus concentration of stillbirth or mummified fetuses (4.95 ± 0.65 log10 RNAc/mL) tended to be higher than in PCR-positive born alive piglets (3.79 ± 0.19 log10 RNAc/mL) (P = 0.07). Birth weight of PCR positive pigs was not statistically different from birth-weight of PCR negative pigs (P ≥ 0.05).
The results of this study indicate that naïve gilts infected with a low dose of mildly virulent PRRSV strain late in gestation may become clinically ill and can efficiently transmit the virus in utero. The infection and consequent reproductive clinical signs following the inoculation of PRRSV at a dose of 101 TCID50 represents the lowest infectious dose that has been reported for this virus. Even though PRRSV was administered intramuscularly at 90 d of gestation, a considerable proportion of piglets (28%) were born as PCR-negative and up to 45% of the piglets within certain litters tested negative at birth. The prevalence of positive individuals reached 100% by 4 d of age and most of the piglets were still viremic at 17 d of age. Previous studies describing PRRSV vertical transmission following experimental inoculation during the last trimester of gestation detected between 19% and 94% of viremic piglets at birth using virus isolation (14,17–19), and reported that the efficiency of transmission was not affected by the virulence of the PRRSV strain (20). Viremia levels detected in suckling piglets were higher than those reported in a previous experimental inoculation with the same PRRSV strain in both 2- and 6-month-old pigs (15). A younger age of pig and/or a more sensitive RT-PCR technique may explain this difference.
The increase of serum virus concentration and prevalence of infected piglets after birth, as well as the prolonged viremia observed in our study, supports the sampling of piglets late during lactation as a tool to monitor PRRSV shedding from sow herds. Further evaluation of the dynamics of PRRSV transmission during lactation in endemically infected herds is required to improve monitoring protocols. This is because the prevalence of PCR-positive piglets at birth most likely will be lower than in our experiment due to previous PRRSV exposure that could provide immunological resistance and reduce the probability of vertical transmission.
None of the litter or individual attributes evaluated in the present study was significantly associated with a higher risk of being a PRRSV PCR-positive piglet at birth or at weaning. Although the limited number of observations included in the logistic regression model may have played a role in the results, it is more likely that the relatively high prevalence of PCR-positive piglets at birth (72%) and at weaning (89%) overwhelmed a possible association between specific attributes and infection. The application of a similar approach in an endemic infection scenario where the prevalence of piglet infection is lower, may offer a better estimate of the association.
In conclusion, under the conditions of this study, PRRSV transplacental transmission can occur following the inoculation of a small amount of virus, resulting in a high prevalence of viremic piglets at birth that remain infected until weaning. Littermates become infected horizontally from the dam or the other piglets. No specific attributes were associated with PRRSV infection in the piglets, indicating a need for more thorough studies to identify indicators of carrier piglets that could lead to the improvement of monitoring protocols for endemically infected sow herds.
Acknowledgments
The authors thank Colleen Finnegan and Martha Fuentes for expert technical support. Funding was provided by the US National Pork Board.
Footnotes
This study is part of the PhD thesis of Jean Paul Cano and it was funded by the PRRS Initiative of the USA National Pork Board (# 04-191).
References
- 1.Neumann EJ, Kliebenstein JB, Johnson CD, et al. Assessment of the economic impact of porcine reproductive and respiratory syndrome on swine production in the United States. J Am Vet Med Assoc. 2005;227:385–392. doi: 10.2460/javma.2005.227.385. [DOI] [PubMed] [Google Scholar]
- 2.Keffaber KK. Reproductive failure of unknown etiology. Am Assoc Swine Pract Newslett. 1989;1:1–10. [Google Scholar]
- 3.Loula T. Mystery pig disease. Agri-Practice. 1991;12:23–34. [Google Scholar]
- 4.Moore C. Clinical presentation of the mystery swine disease in the growing pig. Proceedings Mystery Swine Disease Committee Meeting; Denver, Colorado. 1990. pp. 41–49. [Google Scholar]
- 5.Wills RW, Zimmerman JJ, Yoon KJ, et al. Porcine reproductive and respiratory syndrome virus: Routes of excretion. Vet Microbiol. 1997;57:69–81. doi: 10.1016/S0378-1135(97)00079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Swenson SL, Hill HT, Zimmerman JJ, et al. Excretion of porcine reproductive and respiratory syndrome virus in semen after experimentally induced infection in boars. J Am Vet Med Assoc. 1994;204:1943–1948. [PubMed] [Google Scholar]
- 7.Christianson WT, Choi CS, Collins JE, Molitor TW, Morrison RB, Joo HS. Pathogenesis of porcine reproductive and respiratory syndrome virus infection in mid-gestation sows and fetuses. Can J Vet Res. 1993;57:262–268. [PMC free article] [PubMed] [Google Scholar]
- 8.Wagstrom EA, Chang CC, Yoon KJ, Zimmerman JJ. Shedding of porcine reproductive and respiratory syndrome virus (PRRSV) in mammary secretions of sows. Am J Vet Res. 2001;62:1876–1880. doi: 10.2460/ajvr.2001.62.1876. [DOI] [PubMed] [Google Scholar]
- 9.Christianson WT, Collins JE, Benfield DA, et al. Experimental reproduction of swine infertility and respiratory syndrome in pregnant sows. Am J Vet Res. 1992;53:485–488. [PubMed] [Google Scholar]
- 10.Dee SA, Joo HS. Recurrent reproductive failure associated with porcine reproductive and respiratory syndrome in a swine herd. J Am Vet Med Assoc. 1994;205:1017–1018. [PubMed] [Google Scholar]
- 11.Dee SA, Joo HS, Henry S, et al. Detecting subpopulations after PRRS virus infection in large breeding herds using multiple serologic tests. Swine Health and Prod. 1996;4:181–184. [Google Scholar]
- 12.Dee SA. Approaches to prevention, control, and eradication. PRRS Compendium. In: Zimmerman J, Yoon K-J, editors. The PRRS Compendium. 2nd ed. Des Moines, Iowa: National Pork Board; 2003. pp. 119–130. [Google Scholar]
- 13.Otake S, Dee SA, Rossow KD, et al. Transmission of porcine reproductive and respiratory syndrome virus by fomites (boots and coveralls) J Swine Health Prod. 2002;10:59–65. [Google Scholar]
- 14.Mengeling WL, Lager KM, Vorwald AC. Temporal characterization of transplacental infection of porcine fetuses with porcine reproductive and respiratory syndrome virus. Am J Vet Res. 1994;55:1391–1398. [PubMed] [Google Scholar]
- 15.Cho JG, Dee SA, Deen J, et al. Evaluation of the effects of animal age, concurrent bacterial infection, and pathogenicity of porcine reproductive and respiratory syndrome virus on virus concentration in pigs. Am J Vet Res. 2006;67:489–493. doi: 10.2460/ajvr.67.3.489. [DOI] [PubMed] [Google Scholar]
- 16.Schurrer JA, Dee SA, Moon RD, et al. Retention of ingested porcine reproductive and respiratory syndrome virus in houseflies. Am J Vet Res. 2005;66:1517–1525. doi: 10.2460/ajvr.2005.66.1517. [DOI] [PubMed] [Google Scholar]
- 17.Mengeling WL, Lager KM, Vorwald AC. Clinical effects of porcine reproductive and respiratory syndrome virus on pigs during the early postnatal interval. Am J Vet Res. 1998;59:52–55. [PubMed] [Google Scholar]
- 18.Kranker S, Nielsen J, Bille-Hansen V, Botner A. Experimental inoculation of swine at various stages of gestation with a Danish isolate of porcine reproductive and respiratory syndrome virus (PRRSV) Vet Microbiol. 1998;61:21–31. doi: 10.1016/s0378-1135(98)00176-x. [DOI] [PubMed] [Google Scholar]
- 19.Feng WH, Laster SM, Tompkins M, et al. In utero infection by porcine reproductive and respiratory syndrome virus is sufficient to increase susceptibility of piglets to challenge by Streptococcus suis Type II. J Virol. 2001;75:4889–4895. doi: 10.1128/JVI.75.10.4889-4895.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Park BK, Yoon IJ, Joo HS. Pathogenesis of plaque variants of porcine reproductive and respiratory syndrome virus in pregnant sows. Am J Vet Res. 1996;57:320–323. [PubMed] [Google Scholar]