Abstract
This study investigated the role of neutral, happy, fearful, and angry facial expressions in enhancing orienting to the direction of eye gaze. Photographs of faces with either direct or averted gaze were presented. A target letter (T or L) appeared unpredictably to the left or the right of the face, either 300 ms or 700 ms after gaze direction changed. Response times were faster in congruent conditions (i.e., when the eyes gazed toward the target) relative to incongruent conditions (when the eyes gazed away from the target letter). Facial expression did influence reaction times, but these effects were qualified by individual differences in self-reported anxiety. High trait-anxious participants showed an enhanced orienting to the eye gaze of faces with fearful expressions relative to all other expressions. In contrast, when the eyes stared straight ahead, trait anxiety was associated with slower responding when the facial expressions depicted anger. Thus, in anxiety-prone people attention is more likely to be held by an expression of anger, whereas attention is guided more potently by fearful facial expressions.
Keywords: emotion, facial expression, gaze direction, anxiety, attentional orienting
Facial expressions are potent social cues and are ideal stimuli to investigate how emotionally relevant information is prioritized over other information in attentional and perceptual processing (Vuilleumier, 2002). Strong individual differences have, however, been found in the processing of facial expressions of emotion. To illustrate, several studies have shown that individual differences in trait anxiety are associated with an increased propensity to orient attention toward facial expressions of threat (e.g., Bradley, Mogg, & Millar, 2000; Fox, 2002; Mogg & Bradley, 1999) and that amygdala response to threatening facial expressions is also modulated by the magnitude of self-reported anxiety (Bishop, Duncan, & Lawrence, 2004; Etkin et al., 2004). In a typical study, a dot-probe paradigm is used in which pairs of faces differing in expression are presented simultaneously on a computer screen for 500 ms and are immediately followed by a probe. Probes appearing in the location previously occupied by a threatening stimulus (e.g., threat word or facial expression) are detected faster by anxious individuals than probes appearing in the location of a neutral or positive stimulus (e.g., a happy face). Low-anxious people usually show no bias on this task, although sometimes they show an attentional vigilance for positive material (e.g., Fox, 1993; MacLeod & Mathews, 1988). This speeding to detect an object appearing near the location of threat has been well established in people with generalized anxiety disorder (e.g., MacLeod, Mathews, & Tata, 1986; Mogg, Bradley, & Williams, 1995) and social phobia (e.g., Mogg, Philippot, & Bradley, 2004), as well as in nonclinical groups reporting high levels of trait anxiety (e.g., Fox, 1993; Mathews & MacLeod, 1985). More recent research has focused more specifically on the various components of spatial attention to threat, which can be differentiated at both neural and cognitive levels. For instance, heightened anxiety may modulate the engagement of attention with threat (Fox, Russo, & Georgiou, 2005; Koster, Crombez, van Damme, Verschure, & De Houwer, 2004) and is also important in modulating the disengagement of spatial attention from threatening faces (Fox, Russo, Bowles, & Dutton, 2001; Fox, Russo, & Dutton, 2002), pictures (Yiend & Mathews, 2001), and locations associated with negative reinforcement (Derryberry & Reed, 2002). Thus, a substantial body of research has suggested that high-anxious individuals are more reactive to facial signals of threat at both cognitive and neural levels of analysis than are those with lower levels of anxiety.
A separate body of research has shown that eye gaze produces orienting of spatial attention in the direction signaled by the gaze. The ability to rapidly shift attention in the direction of a conspecific’s eye gaze may reflect a highly adaptive mechanism that is likely to be important for survival. Evidence for this notion comes from the finding that human infants are particularly sensitive to the direction of the eye gaze of human adults (Hood, Willen, & Driver, 1998). Moreover, studies with adult humans demonstrate that orienting attention in the direction of eye gaze appears to be automatic and occurs even when the gaze direction is counterpredictive (e.g., Driver et al., 1999; Friesen & Kingstone, 1998; Langton & Bruce, 1999). These studies used a derivative of the Posner (1980) cuing task to show that gaze direction is a powerful cue to orient attention. To illustrate, in the first study using this task, schematic faces with blanked-out eyes were displayed centrally for 670 ms, after which the pupils appeared, looking apparently straight ahead or to the left or the right. A target letter (F or T) then appeared unpredictably on either the left- or the right-hand side of the screen following a variable stimulus onset asynchrony (SOA) of 105 ms, 300 ms, 600 ms, or 1,005 ms. Strong gaze congruency effects were found such that response times were faster when the targets appeared in a location consistent with gaze direction. These findings occurred even though participants were explicitly told that gaze direction was not predictive of target location (Friesen & Kingstone, 1998). Broadly similar findings have subsequently been reported for photographs of human faces (Driver et al., 1999; Langton & Bruce, 1999).
Thus, one large body of research has indicated that high-anxious individuals show increased attentional processing of facial signals of threat, and another body of research has indicated that eye gaze produces orienting of spatial attention in the direction signaled by the gaze. Recently, these two areas of research have been combined to show that fearful faces with averted gaze produce increased orienting of spatial attention in high-anxious individuals. In the first demonstration of this interaction between gaze cuing, emotional expressions, and anxiety, we reported that those reporting high levels of trait anxiety showed a larger orienting effect with fearful expressions relative to neutral expressions, whereas the orienting effects for fearful and neutral expressions in those reporting low trait anxiety were equivalent (Mathews, Fox, Yiend, & Calder, 2003). We proposed that the effects of fearful gaze might be greater in a high trait-anxious group because the threshold of threat that triggers attentional orienting as a result of fearful expressions is lower in those with higher levels of anxiety (Mathews et al., 2003). Our results have now been replicated in two recent studies, both of which found that increased anxiety enhances the gaze cuing effect of fearful facial expressions (Putman, Hermans, & van Honk, 2006; Tipples, 2006), and another study found similar effects using peripheral rather than central presentation of faces (Holmes, Richards, & Green, 2006).
Although anxiety clearly seems to be an important modulator of the Gaze Direction × Emotional Expression interaction, some studies have also reported a general increase in gaze congruency effects for fearful expressions in an unselected sample of people (Holmes et al., 2006; Putman et al., 2006; Tipples, 2006; but see Hietanen & Leppänen, 2003, for contrasting results). These data are consistent with the idea that facial expressions of emotion and the direction of eye gaze can combine to facilitate social communication in humans. In the face-processing literature, it has been argued that many facial cues are processed independently (e.g., identity and expression; Bruce & Young, 1986). Hietanen and Leppänen (2003) have argued that facial expressions and gaze direction may also be processed by independent modules; however, a number of other studies, including those taking anxiety into account, have indicated that there may be a more integrated relationship between the analysis of facial expression and gaze (Adams & Kleck, 2003, 2005; Holmes et al., 2006; Mathews et al., 2003; Putman et al., 2006; Tipples, 2006). Moreover, neuropsychological research has shown that both of these facial cues are processed by the superior temporal sulcus and amygdala, and there is evidence that the latter may contribute to their integration (e.g., Adolphs, Tranel, Damasio, & Damasio, 1994; Calder & Jansen, 2005; George, Driver, & Dolan, 2001; Hoffman & Haxby, 2000; Young et al., 1995). Thus, it seems that the cognitive and neural processes involved in gaze and expressions analysis may overlap to a considerable degree.
Additional support for this notion comes from the findings of Adams and Kleck (2003). They required participants to simply categorize the emotional expression of a face and found that approach-related emotions (anger and joy) were detected more quickly when eye gaze was directed straight toward the observer. In contrast, the withdrawal-related emotions of fear and sadness were detected more quickly when the eye gaze was averted to the right or the left (Adams & Kleck, 2003). These findings suggest that the direction of eye gaze can play an important role in the perception of emotion from facial cues. More direct evidence for this hypothesis comes from a recent report that direct gaze enhances the perception of anger (but not fear), whereas averted gaze enhances the perception of fear and not anger (Adams & Kleck, 2005).
On the basis of this research, we reasoned that attentional orienting from gaze should produce different effects when the faces display fear and anger. In addition, consistent with previous research, we predicted that these effects would be most evident in high- relative to low-anxious individuals. One previous study did examine gaze cuing effects with both fearful and angry expressions, but individual differences in anxiety were not measured and no overall effect of emotion expression on gaze cuing was found (Hietanan & Leppänen, 2003). Investigations of individual differences in anxiety have contrasted just one negative facial expression with either neutral or happy expressions (Holmes et al., 2006; Mathews et al., 2003; Putman et al., 2006; Tipples, 2006). Thus, the current study is the first to examine the effect of a range of emotional expressions (angry, happy, fearful, and neutral) on gaze cuing in high- and low-anxious participants in the same experiment. The aim was twofold: (a) to investigate whether sensitivity to gaze direction as measured by orienting effects is modulated by the emotional expression (neutral, happy, fearful, or angry) of the face and (b) to determine whether level of trait anxiety can modulate the effects of emotional expression on gaze congruency effects. An additional aim was to replicate and extend previous reports by investigating the role of self-reported trait anxiety in influencing the ability of negative facial expressions with a direct gaze (fear and anger, relative to happy and neutral) to hold attention (Fox et al., 2001; Georgiou et al., 2005). The study by Georgiou et al. (2005) found that fearful faces with a direct gaze tended to slow the response times of high-anxious participants in categorizing peripheral targets to a much greater extent than either happy or neutral expressions (see also Mathews et al., 2003). The results of Adams and Kleck (2005), however, would predict that anxious individuals should be particularly sensitive to the direct gaze of angry faces relative to fearful faces and more sensitive to the averted gaze of fearful faces relative to angry faces.
Method
Participants
Participants were 40 undergraduate psychology students between 18 and 32 years old. Screening at the beginning of the academic year allowed us to preselect those reporting high levels of trait anxiety (scores of 45 or above; n = 20) and those reporting low levels of trait anxiety (scores of 35 or below; n = 20) on the trait scale of the State–Trait Anxiety Inventory (Spielberger, 1983). We selected on the basis of levels of trait anxiety rather than state anxiety because we wanted to investigate the more enduring effects of anxiety on emotion perception.
Materials and Apparatus
Sixteen photographs were selected from the Ekman and Friesen (1976) pictures of facial affect. The photographs were of four individuals (two men and two women), each depicting an angry, fearful, happy, and neutral expression, with the eyes looking straight ahead. The hair and nonfacial areas were removed from each photograph so that only the central face area was visible. Two new versions of each of these photographs were then produced in which the pupils were digitally moved to the far left or far right of both eyes to simulate a leftward or rightward gaze. Photographs were presented in the center of the computer screen and subtended a vertical visual angle of 7°. Targets were the uppercase letter T or L, subtending 3° of visual angle; these were positioned 5° to the left or the right of the midpoint of the screen.
The stimuli were presented on a 17-in. (43.18-cm) computer monitor, connected to an Apple Macintosh G4 computer. Stimulus presentation and data collection were controlled by PsyScope software in conjunction with a PsyScope response box (Cohen, MacWhinney, Flatt, & Provost, 1993).
Design and Procedure
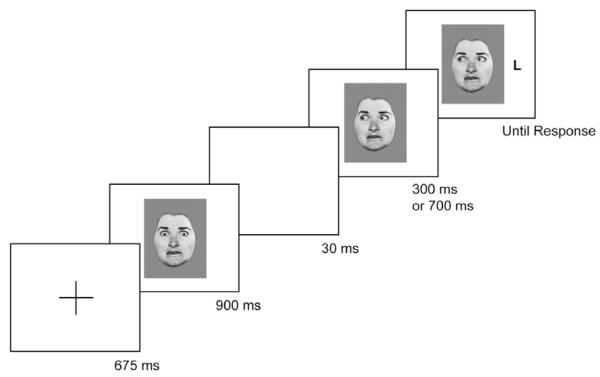
The participants completed a practice block of 10 trials, followed by two blocks of 192 experimental trials. The experimental trials consisted of equiprobable combinations of four individuals taken from the set provided by Ekman and Friesen (1976), four emotional expressions (neutral, happy, angry, or fearful), two target positions (left or right), two target types (T or L), three gaze directions (straight ahead, left, or right), and two SOAs (300 ms or 700 ms). Thus, there were 128 direct gaze trials and 256 averted gaze trials (128 congruent and 128 incongruent) across the entire experiment. Each trial began with the presentation of a fixation cross (+) at the center of the screen for 675 ms. This was followed by presentation of a face with eyes looking straight ahead for 900 ms. This display was then replaced by (a) a blank screen for 30 ms, followed by (b) the same photograph of the eyes looking straight ahead, (c) the same photograph with the eyes shifted left, or (d) the same photograph with the eyes shifted right. This gave the appearance of the eyes blinking slightly and then either maintaining a direct gaze or changing gaze toward the left or the right. The target letter then appeared unpredictably on the left- or the right-hand side of the screen either 300 ms or 700 ms after the second photograph. We used two SOAs so that target presentation time would not become predictable; these SOAs have been used in most previous research. The face and the target letter remained on the screen until the participant responded. An example of an incongruent trial with a fearful facial expression is illustrated in Figure 1. Responses were collected on a PsyScope response box with two buttons labeled T and L and arranged vertically to minimize any interference because of the left or right position of targets. Participants used the thumb and first finger of their dominant hand to make their responses.
Figure 1.
Example of a single trial in the experiment (not to scale). This example shows an incongruent averted gaze condition with a fearful expression.
Previous research has suggested that the appearance of a face looking directly at the participant tends to slow response times, especially if the face has a threatening expression (Georgiou et al., 2005; Mathews et al., 2003). Thus, as in our previous research (Mathews et al., 2003), we analyzed the central eye gaze condition separately from the averted gaze conditions. The averted gaze trials were analyzed by means of a 2 (anxiety group: high vs. low trait anxiety) × 4 (expression: neutral, happy, angry, or fearful) × 2 (congruency: congruent vs. incongruent) × 2 (SOA: 300 ms vs. 700 ms) analysis of variance (ANOVA) with mean correct reaction times as the dependent variable. The central gaze trials were analyzed by means of a 2 (anxiety group: high vs. low trait anxiety) × 4 (expression: neutral, happy, angry, or fearful) × 2 (SOA: 300 ms vs.700 ms) ANOVA with the mean correct reaction times as the dependent variable. The mean proportion of errors was low in this experiment (M = .025) and did not differ across conditions. Therefore, the statistical analysis focused on the mean reaction times.
Results
Averted Gaze Trials
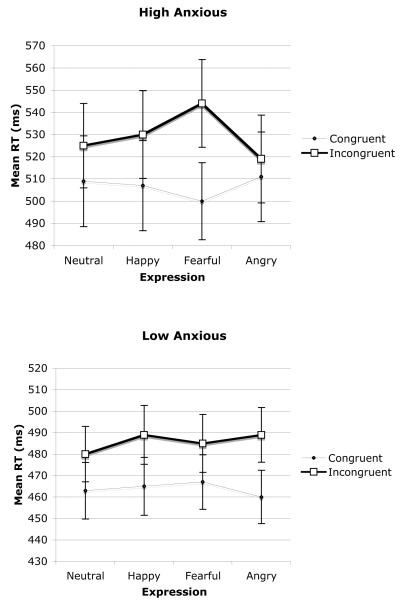
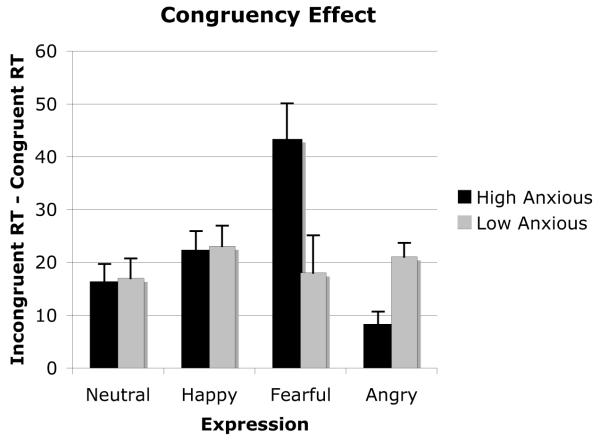
Errors and outlying latencies greater than 1,500 ms or less than 100 ms were removed (2.1% of all responses). The mean correct reaction times for the averted gaze trials (congruent and incongruent) were entered into an Anxiety Group × Expression × Congruency × SOA ANOVA with repeated measures on the last three variables. There was a main effect of SOA, F(1, 38) = 214.8, p < .001, such that reaction times were faster on the 700-ms SOA (M = 477 ms) relative to the 300-ms SOA (M = 517 ms), which is typical in attention research and probably reflects a buildup of alertness from the onset of the cue to the onset of the target (Posner, 1980). Of more theoretical interest, there was a main effect for congruency, F(1, 38) = 65.9, p < .001, such that reaction times were faster on congruent (M = 485 ms) relative to incongruent (M = 506 ms) trials. The highest level interaction was between anxiety group, expression, and congruency, F(3, 114) = 12.2, p < .001, which was not qualified by SOA. The mean reaction times collapsed over SOA are shown in Figure 2. Further analysis for each anxiety group separately confirmed that the Expression × Congruency interaction was significant only for the high trait-anxious group, F(3, 57) = 17.7, p < .001; for the low trait-anxious group, F(3, 57) < 1. Reaction times differed across expression for both the congruent, Pillai’s F(3, 17) = 3.52, p < .038, and the incongruent trials, Pillai’s F(3, 17) = 7.02, p < .003, for the high-anxious group. To further investigate the nature of this interaction, congruency effects were computed by subtracting the mean reaction times on congruent trials from the mean reaction time on incongruent trials; the resulting data are shown in Figure 3. Pairwise comparisons (t tests) were performed for all possible combinations of expression (collapsed across SOA) for each anxiety group separately. The Bonferroni correction (two-tailed) was applied to tests of statistical significance, and the significance level was set at .008. For the high trait-anxious group, the congruency effect for fearful expression was larger (M = 43.4 ms) than that observed for neutral (M = 15.8 ms), t(19) = 3.9, p < .001; happy (M = 22.4 ms), t(19) = 3.2, p < .005; or angry (M = 7.7 ms), t(19) = 4.9, p < .001, expressions. Moreover, the validity effect for angry expressions was also significantly less than that observed for neutral, t(19) = 3.6, p < .001, or happy, t(19) = 6.5, p < .001, expressions for this group.
Figure 2.
Mean reaction times in milliseconds for averted gaze trials as a function of trait anxiety, congruency, and facial expression.
Figure 3.
Mean congruency effect (i.e., difference between congruent and incongruent trials) as a function of trait anxiety and facial expressions.
A correlational analysis was conducted to examine the relationship between orienting to emotional expressions and the level of both trait and state anxiety. Pearson’s product–moment coefficients (Pearson’s r) were calculated for the relationship between trait and state anxiety and the mean gaze congruency effects for neutral, happy, fearful, and angry expressions. The analyses revealed significant positive correlations between the gaze congruency effect for fearful expressions and anxiety measures and negative correlations between gaze congruency for angry expressions and both trait and state anxiety measures. As shown in Table 1, these correlations remained even after controlling for the mean gaze congruency effect for neutral facial expressions. Thus, higher levels of trait and state anxiety were associated with increased orienting to eye gaze for fearful facial expressions and reduced orienting to eye gaze for angry facial expressions.
Table 1.
Correlation Coefficients and Partial Correlation Coefficients Between Trait and State Anxiety Levels and Mean Gaze Congruency Effects
| Anxiety scale | Expression Neutral (r) |
Happy |
Fearful |
Angry |
|||
|---|---|---|---|---|---|---|---|
| r | pr | r | pr | r | pr | ||
| Trait anxiety | −.031 | −.021 | .000 | .456* | .520** | −.453* | −.639** |
| State anxiety | −.109 | −.085 | −.015 | .450* | .554** | −.423* | −.512** |
Note. Partial correlation coefficients reflect the relationship between the anxiety measures and the mean gaze congruency effects for happy, fearful, and angry expressions, after controlling for the relationship between the anxiety measures and the mean gaze congruency effect for neutral facial expressions.
p < .01.
p < .001.
Central Gaze Trials
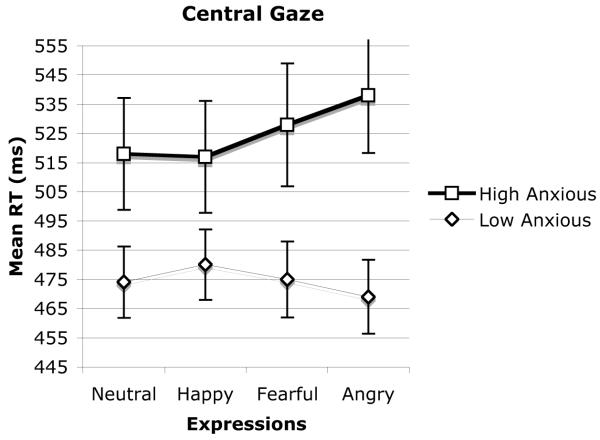
The mean correct reaction times for the central gaze trial conditions were entered into an Anxiety Group × Expression × SOA ANOVA. There was a main effect for expression, F(3, 114) = 4.7, p < .004, which interacted with anxiety group, F(3, 114) = 24.2, p < .001. There was also a main effect for SOA, F(1, 38) = 90.7, p < .001, such that reaction times were faster on the 700-ms trials (M = 482 ms) relative to the 300-ms trials (M = 518 ms), and for anxiety group, F(1, 38) = 4.7, p < .05, such that high-anxious participants were slower than low-anxious participants (Ms = 525 ms and 474 ms, respectively). The Anxiety Group × Expression interaction was not qualified by SOA, and the mean reaction times collapsed across SOA are shown in Figure 4. There was a main effect of expression for both the high, F(3, 57) = 18.4, p < .001, and low, F(3, 57) = 7.3, p < .001, trait anxiety groups. Pairwise comparisons (t tests) were performed for all possible combinations of expression collapsed across SOA for each anxiety group separately. The Bonferroni correction (two-tailed) was applied to tests of statistical significance, and the significance level was set at .008. For the high-anxious group, reaction times on the trials with an angry expression were slower relative to neutral, t(19) = 8.7, p < .001, and happy, t(19) = 9.6, p < .001, trials. No other comparisons reached significance on this strict criterion (all ts < 2.75, p < .013). We should note, however, that the difference between the angry and fearful expressions was significant by conventional standards (p < .05), but not by the strict criterion. In contrast, for the low-anxious group, reaction times on trials with angry expressions were faster relative to neutral, t(19) = 3.4, p < .003, and happy, t(19) = 6.4, p < .001, trials. In addition, reaction times on neutral trials were faster than reaction times on happy trials, t(19) = 5.2, p < .001. No other comparisons reached significance (all ts < 2.0, p < .06). To test the a priori hypothesis that angry expressions with direct gaze would produce slower response times relative to fearful expressions with direct gaze, a 2 (expression) × 2 (anxiety group) ANOVA was conducted examining only angry and fearful expressions. This analysis revealed an Expression × Anxiety Group interaction, F(1, 38) = 11.8, p < .001. Paired t tests using the Bonferroni correction (p < .025) revealed that response times were indeed slower with an angry face with a direct gaze relative to a fearful face with a direct gaze for high-anxious individuals (539 ms vs. 528 ms, respectively), t(19) = 2.75, p < .013, whereas this difference was marginally reversed for the low-anxious group (489 ms vs. 475 ms, respectively), t(19) = 2.04, p < .055.
Figure 4.
Mean reaction times in milliseconds for central gaze trials as a function of trait anxiety and facial expressions.
A correlational analysis was conducted to examine the relationship between speed of response when different emotional expressions with direct gaze were attended and the level of both trait and state anxiety. Partial correlation coefficients were calculated for the relationship between trait and state anxiety and the mean reaction times for happy, fearful, and angry expressions, using the mean reaction time to neutral expressions as a control. As shown in Table 2, the analyses revealed significant negative correlations between the anxiety measures and reaction time to happy facial expressions, but a positive correlation between the reaction time to angry expressions. Thus, higher levels of trait and state anxiety were associated with faster responses when happy expressions were fixated but slower responses when angry expressions were fixated.
Table 2.
Partial Correlation Coefficients Between Trait and State Anxiety Measures and Mean Reaction Time to Happy, Fearful, and Angry Expressions With Direct Gaze, Controlling for Mean Reaction Time to Neutral Expressions With Direct Gaze
| Anxiety Scale | Expression |
||
|---|---|---|---|
| Happy | Fearful | Angry | |
| Trait anxiety | −.528** | .238 | .722** |
| State anxiety | −.415* | .317 | .500** |
p < .01.
p < .001.
Discussion
The findings of this experiment are straightforward. In agreement with previous research, the level of trait anxiety enhanced the orienting response triggered by averted gaze in fearful relative to neutral facial expressions (Mathews et al., 2003; Putman et al., 2006; Tipples, 2006). An important finding was that this enhanced orienting response was specific to fearful expressions and that the gaze cuing effect was not enhanced for angry or happy relative to neutral expressions. Indeed, the gaze congruency effect triggered by angry expressions was actually reduced for high trait-anxious people relative to other facial expressions. This result conflicts with a recent report that angry expressions enhanced gaze cuing effects relative to happy and neutral expressions (Holmes et al., 2006, Experiment 3). However, this pattern only emerged in a post hoc analysis and the critical Anxiety × Congruency × Expression interaction was not significant in the Holmes et al. (2006) report. Our results show a very different pattern, and the interaction was significant. Further research is required to clarify whether averted fearful expressions enhance attentional orienting effects relative to angry facial expressions in anxious individuals, as found in the current study. A primary aim of the current study was to determine whether there was a general interaction between the gaze cuing effect and facial emotional expression. This interaction was found, but it was modified to a significant extent by the level of self-reported trait anxiety. Thus, as in previous research, observing a fearful facial expression with an averted gaze resulted in an enhanced cuing effect for those with high (but not low) levels of trait anxiety (Mathews et al., 2003; Putman et al., 2006; Tipples, 2006). Hietanen and Leppänen (2003) concluded after conducting six experiments that facial expressions do not affect the orientation of attention triggered by gaze direction. Although other studies have also failed to find general effects of facial expression on gaze cuing effects in unselected samples (e.g., Mathews et al., 2003), the current research emphasizes the importance of considering individual differences in anxiety as a determinant of the ability of emotional expressions to trigger enhanced orienting effects. Other research supporting this conclusion presented only fearful and neutral expressions in a gaze cuing task (Mathews et al., 2003; Putman et al., 2006; Tipples, 2006). The current results clearly demonstrate that fearful facial expressions are especially important in affecting the orientation of attention in anxious people, as fearful, neutral, angry, and happy expressions were all presented to the same participants in a random order. Thus, the present results show that it is not just negative expressions in general (e.g., fearful and angry) that are important; rather, the enhanced orienting appears to be specific for fearful expressions. This finding makes ecological sense in that the congruence of eye gaze and emotion can provide an important social cue to the source of threat. An angry face looking directly at you suggests clearly where the threat is coming from, as does a fearful face looking at another location. In contrast, an angry face looking away or a fearful face looking directly at you is a more ambiguous combination (Adams & Kleck, 2002).
Support for this assumption was also found in trials with direct gaze, in which individuals with high trait anxiety were slower to respond when the face showed anger relative to happy or neutral expressions. A planned analysis revealed that this slowing on trials with angry direct gaze expressions did indeed differ from fearful faces with a direct gaze. These results are consistent with the report of Adams and Kleck (2005), which argues that direct gaze enhances the perception of angry expressions and has no influence on the perception of fearful expressions. However, our results indicate that this is particularly the case for those reporting high levels of trait anxiety. The current results do not fully replicate previous reports of slower responses in anxious individuals to centrally located fearful faces relative to neutral faces with a direct gaze (Georgiou et al., 2005; Mathews et al., 2003). Nonetheless, although the current results did not survive the Bonferroni correction, the trend for slower response times with fearful relative to neutral expressions for high-anxious individuals was in the right direction (528 ms vs. 518 ms, respectively), t(19) = 2.03, p < .028. One difference between the current experiment and previous reports is the fact that in the present study faces with direct and averted gaze expressing a range of emotions (neutral, happy, fearful, and angry) were randomly presented to participants. In contrast, previous research presented either faces with fearful or neutral expressions with direct gaze only (Georgiou et al., 2005) or faces with both direct and averted gaze but only neutral and fearful expressions (Mathews et al., 2003). On the basis of the work of Adams and Kleck (2003, 2005), we can propose that direct gaze enhances the perception of anger, and averted gaze enhances the perception of fear (Adams & Kleck, 2005). This pattern is consistent with the hypothesis that anxiety is associated with a tendency for threat-related stimuli (i.e., averted fearful gaze and direct angry gaze) to hold the attention of anxious individuals for a disproportionate amount of time (Fox et al., 2001, 2002). It should also be noted, however, that faster response times for congruent trials with fearful expressions indicate that, in addition, there was also an enhanced orienting effect of fearful expressions as well as a greater tendency for these expressions to hold attention. It was not the case, for example, that averted congruent trials with fearful expressions were slower for anxious individuals than fearful expressions with a direct gaze (500 ms vs. 526 ms, respectively). A possible explanation of enhanced cuing effects in high-anxious participants for fearful facial expressions might be due to the possibility that these faces possess less ambiguity than happy or angry faces with averted gaze. Similarly, slower response times for angry relative to happy or fearful expressions with direct gaze may be because angry expressions possess less ambiguity under direct gaze conditions. This speculation would suggest that the effects of perceived gaze are enhanced as the ambiguity of a face stimulus increases.
An important finding in the current experiment is the apparent dissociation between the effects of fearful and angry facial expressions. Both of these expressions are generally rated as negatively valenced and high-arousal emotions (e.g., Adolphs, Russell, & Tranel, 1999). We found that high levels of self-reported trait anxiety led to an enhanced cuing effect for fearful facial expressions but a reduced cuing effect for angry facial expressions. In contrast, direct gaze resulted in slower reaction times for angry expressions relative to fearful expressions for the high-anxiety group. This emotion-specific pattern of responding does not support the view that sensitivity to eye gaze may be linked to a more general dimension of negative emotional arousal (Adolphs et al., 1999). Dimensional models of emotion perception (e.g., Russell, 1980) suggest that emotion perception is based on general dimensions of valence (negative vs. positive) and arousal (high vs. low) rather than specific discrete emotions. Because anger and fear are both negative, high-arousal emotions, this account would predict that gaze congruency effects should be relatively equivalent for expressions of both anger and fear. There is some evidence for this view in that event-related potential (ERP) analysis shows that fearful and angry expressions convey a processing advantage as they are processed within about 120 ms, and this advantage does not differ between the two expressions (Eimer, Holmes, & McGlone, 2003; Holmes, Vuilleumier, & Eimer, 2003). However, ERPs can only provide effective correlates of cortical systems, and a meta-analysis of functional MRI and positron emission tomography studies have identified relatively separate neural circuits underlying the perception of angry and fearful expressions, respectively (see Murphy, Nimmo-Smith, & Lawrence, 2003, for review). Likewise, studies of people with focal brain lesions have shown disproportionate deficits in the recognition of anger and fear expressions (Adolphs et al., 1994; Calder, Keane, Lawrence, & Manes, 2004; Calder et al., 1996). The current experiment is more in line with this discrete emotions account as opposite effects were found for fearful and angry expressions. These results are more supportive of a functional evolutionary view of emotions. If this is correct, our results would suggest that discrete emotions can influence attentional processing over and above effects on general dimensions of valence (negative vs. positive) and arousal.
The current results indicate that the processing of gaze direction is tightly integrated with facial expression analysis (cf. Adams & Kleck, 2003) rather than processed by independent modules (cf. Hietanen & Leppänen, 2003). Moreover, in combination with other research in this area, the current study provides compelling evidence that the integration of gaze direction and emotion expression analysis is modulated by the level of self-reported trait or state anxiety. Although most people are likely to attend to cues that signal danger (e.g., direct angry gaze and averted fearful gaze), this tendency is likely to occur at lower levels of threat in those individuals who are prone to experience anxiety. Presenting photographs of fearful and angry expressions in a laboratory when no real threat is apparent is a fairly innocuous situation. In real-life dynamic threat-related situations, it is likely that there would be some integration of emotion perception and gaze analysis in most people. However, the heightened sensitivity of anxious individuals to stimuli such as negative words, faces, or pictures (e.g., Fox et al., 2001; Mathews & MacLeod, 1985; Yiend & Mathews, 2001) provides an excellent experimental model to study emotion perception under laboratory conditions. In selective attention experiments using emotional facial expressions, it has also been demonstrated that the neural circuits underlying processing of emotional faces can be modulated to a significant extent by individual differences in traits such as anxiety (Bishop et al., 2004; Etkin et al., 2004) and extraversion (Canli, Sivers, Whitfield, Gotlib, & Gabrieli, 2002). The current results suggest that the neural systems involved in decoding eye gaze direction as well as emotional facial expressions should also be modulated by level of self-reported anxiety. In conclusion, the current results highlight the importance of taking individual differences into account when examining fundamental aspects of social perception at both cognitive and neural levels.
Acknowledgments
This work was partially supported by a project grant from the Wellcome Trust awarded to Elaine Fox (ref.: 076701/Z/05/Z). We are grateful to Konstantina Zougou for help with data collection.
Contributor Information
Elaine Fox, University of Essex.
Andrew J. Calder, Medical Research Council Cognition and Brain Sciences Unit
Andrew Mathews, University of California, Davis.
Jenny Yiend, University of Oxford.
References
- Adams RB, Kleck RE. Effects of gaze on amygdala sensitivity to anger and fear faces. Science. 2002 Jun 6;300:1536. doi: 10.1126/science.1082244. [DOI] [PubMed] [Google Scholar]
- Adams RB, Kleck RE. Perceived gaze direction and the processing of facial displays of emotion. Psychological Science. 2003;14:644–647. doi: 10.1046/j.0956-7976.2003.psci_1479.x. [DOI] [PubMed] [Google Scholar]
- Adams RB, Kleck RE. Effects of direct and averted gaze on the perception of facially communicated emotion. Emotion. 2005;5:3–11. doi: 10.1037/1528-3542.5.1.3. [DOI] [PubMed] [Google Scholar]
- Adolphs R, Russell JA, Tranel D. A role for the human amygdala in recognizing emotional arousal from unpleasant stimuli. Psychological Science. 1999;10:167–171. [Google Scholar]
- Adolphs R, Tranel D, Damasio H, Damasio A. Impaired recognition of emotion in facial expressions following bilateral damage to the human amygdala. Nature. 1994 Dec 15;372:669–672. doi: 10.1038/372669a0. [DOI] [PubMed] [Google Scholar]
- Bishop S, Duncan J, Lawrence AD. State anxiety modulation of the amygdala response to unattended threat-related stimuli. Journal of Neuroscience. 2004;24:10364––10368. doi: 10.1523/JNEUROSCI.2550-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley BP, Mogg K, Millar NH. Covert and overt orienting of attention to emotional faces in anxiety. Cognition & Emotion. 2000;14:789–808. [Google Scholar]
- Bruce V, Young AW. Understanding face recognition. British Journal of Psychology. 1986;77:305–327. doi: 10.1111/j.2044-8295.1986.tb02199.x. [DOI] [PubMed] [Google Scholar]
- Calder AJ, Jansen J. Configural coding of facial expressions: The impact of inversion and photographic negative. Visual Cognition. 2005;12:495–518. [Google Scholar]
- Calder AJ, Keane J, Lawrence AD, Manes F. Impaired recognition of anger following damage to the ventral striatum. Brain. 2004;127:1958–1969. doi: 10.1093/brain/awh214. [DOI] [PubMed] [Google Scholar]
- Calder AJ, Young AW, Rowland D, Perrett DI, Hodges JR, Etcoff NL. Facial emotion recognition after bilateral amygdala damage: Differentially severe impairment of fear. Cognitive Neuropsychology. 1996;13:699–745. [Google Scholar]
- Canli T, Sivers H, Whitfield SL, Gotlib IH, Gabrieli JD. Amygdala response to happy faces as a function of extraversion. Science. 2002 May 17;296:1291. doi: 10.1126/science.1068749. [DOI] [PubMed] [Google Scholar]
- Cohen JD, MacWhinney B, Flatt M, Provost J. PsyScope: A new graphic interactive environment for designing psychology experiments. Behavioural Research Methods, Instruments, and Computers. 1993;25:257–271. [Google Scholar]
- Derryberry D, Reed MA. Anxiety-related attentional biases and their regulation by attentional control. Journal of Abnormal Psychology. 2002;111:225–236. doi: 10.1037//0021-843x.111.2.225. [DOI] [PubMed] [Google Scholar]
- Driver J, Davis G, Ricciardelli P, Kidd P, Maxwell E, Baron-Cohen S. Gaze perception triggers reflexive visuospatial orienting. Visual Cognition. 1999;6:509–540. [Google Scholar]
- Eimer M, Holmes A, McGlone F. The role of spatial attention in the processing of facial expressions: An ERP study of rapid brain responses to six basic emotions. Cognitive, Affective, and Behavioral Neuroscience. 2003;3:97–110. doi: 10.3758/cabn.3.2.97. [DOI] [PubMed] [Google Scholar]
- Ekman P, Friesen WV. Pictures of facial affect. Consulting Psychologists Press; Palo Alto, CA: 1976. [Google Scholar]
- Etkin A, Klemenhagen KC, Dudman JT, Rogan MT, Hen R, Kandel ER, Hirsh J. Individual differences in trait anxiety predict the response of the basolateral amygdala to unconsciously processed fearful faces. Neuron. 2004;44:1043–1055. doi: 10.1016/j.neuron.2004.12.006. [DOI] [PubMed] [Google Scholar]
- Fox E. Allocation of visual attention and anxiety. Cognition & Emotion. 1993;7:207–215. doi: 10.1080/02699939308409185. [DOI] [PubMed] [Google Scholar]
- Fox E. Processing emotional facial expressions: The role of anxiety and awareness. Cognitive, Affective, & Behavioral Neuroscience. 2002;2:52–63. doi: 10.3758/cabn.2.1.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox E, Russo R, Bowles RJ, Dutton K. Do threatening stimuli draw or hold attention in subclinical anxiety? Journal of Experimental Psychology: General. 2001;130:681–700. [PMC free article] [PubMed] [Google Scholar]
- Fox E, Russo R, Dutton K. Attentional bias for threat: Evidence for delayed disengagement from emotional faces. Cognition & Emotion. 2002;16:355–379. doi: 10.1080/02699930143000527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox E, Russo R, Georgiou G. Anxiety modulates the degree of attentive resources required to process emotional faces. Cognitive, Affective, & Behavioral Neuroscience. 2005;5:396–404. doi: 10.3758/cabn.5.4.396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friesen CK, Kingstone A. The eyes have it! Reflexive orienting is triggered by nonpredictive gaze. Psychonomic Bulletin & Review. 1998;5:490–495. [Google Scholar]
- George N, Driver J, Dolan RJ. Seen gaze-direction modulates fusiform activity and its coupling with other brain areas during face processing. Neuroimage. 2001;13:1102–1112. doi: 10.1006/nimg.2001.0769. [DOI] [PubMed] [Google Scholar]
- Georgiou GA, Bleakley C, Hayward J, Russo R, Dutton K, Eltiti S, Fox E. Focusing on fear: Attentional disengagement from emotional faces. Visual Cognition. 2005;12:145–158. doi: 10.1080/13506280444000076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hietanen JK, Leppänen M. Does facial expression affect attention orienting by gaze direction cues? Journal of Experimental Psychology: Human Perception and Performance. 2003;29:1228–1243. doi: 10.1037/0096-1523.29.6.1228. [DOI] [PubMed] [Google Scholar]
- Hoffman EA, Haxby JV. Distinct representations of eye gaze and identity in the distributed human neural system for face perception. Nature Neuroscience. 2000;3:80–84. doi: 10.1038/71152. [DOI] [PubMed] [Google Scholar]
- Holmes A, Richards A, Green S. Anxiety and sensitivity to eye gaze in emotional faces. Brain and Cognition. 2006;60:282–294. doi: 10.1016/j.bandc.2005.05.002. [DOI] [PubMed] [Google Scholar]
- Holmes A, Vuilleumier P, Eimer M. The processing of emotional facial expression is gated by spatial attention: Evidence from event-related brain potentials. Cognitive Brain Research. 2003;16:174–184. doi: 10.1016/s0926-6410(02)00268-9. [DOI] [PubMed] [Google Scholar]
- Hood BM, Willen JD, Driver J. Adult’s eyes trigger shifts of visual attention in human infants. Psychological Science. 1998;9:131–134. [Google Scholar]
- Koster EHW, Crombez G, Van Damme S, Verschure B, De Houwer J. Does imminent threat capture and hold attention? Emotion. 2004;4:312–317. doi: 10.1037/1528-3542.4.3.312. [DOI] [PubMed] [Google Scholar]
- Langton SRH, Bruce V. Reflexive visual orienting in response to the social attention of others. Visual Cognition. 1999;6:541–567. [Google Scholar]
- Langton SRH, Watt RJ, Bruce V. Do the eyes have it? Cues to the direction of social attention. Trends in Cognitive Sciences. 2000;4:50–59. doi: 10.1016/s1364-6613(99)01436-9. [DOI] [PubMed] [Google Scholar]
- MacLeod C, Mathews AM. Anxiety and the allocation of attention to threat. Quarterly Journal of Experimental Psychology: Human Experimental Psychology. 1988;40A:653– 670. doi: 10.1080/14640748808402292. [DOI] [PubMed] [Google Scholar]
- MacLeod C, Mathews AM, Tata P. Attentional bias in emotional disorders. Journal of Abnormal Psychology. 1986;95:15–20. doi: 10.1037//0021-843x.95.1.15. [DOI] [PubMed] [Google Scholar]
- Mathews AM, Fox E, Yiend J, Calder A. The face of fear: Effects of eye-gaze and emotion on visual attention. Visual Cognition. 2003;10:823–835. doi: 10.1080/13506280344000095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathews AM, MacLeod C. Selective processing of threat cues in anxiety states. Behaviour Research & Therapy. 1985;23:563–569. doi: 10.1016/0005-7967(85)90104-4. [DOI] [PubMed] [Google Scholar]
- Mogg K, Bradley BP. Orienting of attention to threatening facial expressions presented under conditions of restricted awareness. Cognition & Emotion. 1999;13:713–740. [Google Scholar]
- Mogg K, Bradley BP, Williams R. Attentional bias in anxiety and depression: The role of awareness. British Journal of Clinical Psychology. 1995;34:17–36. doi: 10.1111/j.2044-8260.1995.tb01434.x. [DOI] [PubMed] [Google Scholar]
- Mogg K, Philippot P, Bradley BP. Selective attention to angry faces in clinical social phobia. Journal of Abnormal Psychology. 2004;113:160–165. doi: 10.1037/0021-843X.113.1.160. [DOI] [PubMed] [Google Scholar]
- Murphy FC, Nimmo-Smith I, Lawrence AD. Functional neuroanatomy of emotions: A meta-analysis. Cognitive, Affective and Behavioral Neuroscience. 2003;3:207–233. doi: 10.3758/cabn.3.3.207. [DOI] [PubMed] [Google Scholar]
- Posner MI. Orienting of attention. Quarterly Journal of Experimental Psychology: Human Experimental Psychology. 1980;32A:3–25. doi: 10.1080/00335558008248231. [DOI] [PubMed] [Google Scholar]
- Putman P, Hermans E, Van Honk J. Anxiety meets fear in perception of dynamic expressive gaze. Emotion. 2006;6:94–102. doi: 10.1037/1528-3542.6.1.94. [DOI] [PubMed] [Google Scholar]
- Russell JA. A circumplex model of affect. Journal of Personality and Social Psychology. 1980;39:1161–1178. [Google Scholar]
- Spielberger CD. Manual for the State-Trait Anxiety Inventory. Consulting Psychologists Press; Palo Alto, CA: 1983. [Google Scholar]
- Tipples J. Fear and fearfulness potentiate automatic orienting to eye gaze. Cognition & Emotion. 2006;20:309–320. [Google Scholar]
- Vuilleumier P. Facial expression and selective attention. Current Opinion in Psychiatry. 2002;15:291–300. [Google Scholar]
- Yiend J, Mathews AM. Anxiety and attention to threatening pictures. Quarterly Journal of Experimental Psychology: Human Experimental Psychology. 2001;54A:665–681. doi: 10.1080/713755991. [DOI] [PubMed] [Google Scholar]
- Young AW, Aggleton JP, Hellawell DJ, Johnson DJ, Broks P, Hanley JR. Face processing impairments after amygdalotomy. Brain. 1995;118:15–24. doi: 10.1093/brain/118.1.15. [DOI] [PubMed] [Google Scholar]