Abstract
T helper 1 cell fate is induced by overlapping signaling pathways, whose kinetic principles and regulatory motifs are largely unknown. We identified a simple positive feedback loop in the STAT4 signaling pathway, whereby activation by IL-12 leads to the increased expression in IL-12 receptor. A computational analysis shows that this feedback loop synergizes with the one mediated by the IFN-γ secreted by differentiating cells, when the induction of Th1 cell fate is weak. Positive feedback loops are often utilized to enhance phenotypic differentiation. This effect was confirmed by experiments showing that stochastic fluctuations in the expression of IL-12 receptor gene were amplified, leading to two discrete levels of expression in a cell population.
Keywords: positive feedback, stochastic, Interleukin-27, T-bet, computational modeling
1. Introduction
Activation of T helper (Th) lymphocytes by antigen presenting cells leads to their differentiation into Th1 or Th2 cell lineages depending on the cytokines present during the activation process [1]. Th2 cells produce IL-4, which leads to the inhibition of the Th1 cell lineage. Similarly, IFN-γ produced by Th1 cells acts to inhibit the development of Th2 cells [2].
Positive feedback has been shown to be a driving force in Th2 polarization. GATA3, a major transcriptional regulator of Th2 responses, was shown to induce its own expression [3]. Regulation of expression of a transcriptional activator through positive feedback has the capacity to create two states of expression level that can correspond to expression levels in undifferentiated and differentiated cells. Furthermore, the mutual inhibition of Th1 and Th2 cell types can lead to a bistable behavior in the form of a toggle switch [4–6] that can efficiently induce the polarization of T helper cell fates.
The differentiation of Th1 cells results from the activation of multiple interconnected signaling pathways. During the early stages of cell differentiation, IFN-γ and IL-27 direct commitment to the Th1 cell lineage [7–9]. IFN-γ, upon binding to its receptor, activates signal transducer and activator of transcription (STAT)1, while IL-27 activates both STAT1 and STAT4 [10]. STAT1 activation leads to the expression of the transcription factor T-bet, and the production of additional IFN-γ [11]. Given the presence of IFN-γ receptors on Th1 cells, IFN-γ has, in principle, the potential to contribute to the autoactivation of Th1 lymphocytes. STAT1 activation also leads to the transcription of the β2 subunit of the IL-12 receptor. This enables IL-12, upon binding to its receptor, to activate STAT4 which leads to a further enhancement of Th1 cells in the later stages of the differentiation [12]. Activation through the T-cell receptor may also initiate the expression of IL-12Rβ2 [13].
Despite an appreciation that multiple cytokines contribute to Th1 cell differentiation, what has remained unclear is the kinetic relationships of the above signaling pathways that enhance commitment to the Th1 cell fate. Given that positive feedback loops represent a robust, frequently utilized motif for induction of cell differentiation [14], we focused on distinguishing such loops in the STAT4 signaling pathway. For this purpose, we followed a three-step strategy. First, knockout cells were utilized in a way that signal propagation was confined to a small module of the differentiation network, which enables the detection of autoacatalytic processes. Next, we examined under what combination of cytokine concentrations the positive feedback displayed a discernible effect on the expression levels of IFN-γ and IL-12R in wild-type cells and how it interacted with other regulatory motifs. In the third step, we examined whether the IL-12 receptor mediated positive feedback, which was identified in the first step, amplified fluctuations in gene expression to create two cell phenotypes with distinct IL-12 receptor expression levels in wild-type cells.
2. Materials and methods
2. 1 Th1/Th2 cell differentiation assay
Naïve CD4+ T cells (1×106 cells/ml) were primed with plate coated anti-CD3 (2 μg/ml) and anti-CD28 (1 μg/ml) in the presence of IL-2 (2 ng/ml) in 48-well plates. To induce Th1 cell differentiation IL4 antibody (10 μg/ml) was added. Concentrations of IL-27, IL-12 and IFN-γ were varied in individual experiments. Th2 cell differentiation was induced in the presence of anti-IFN-γ (10 μg/ml) and IL-4 (10 ng/ml). On day 3, PMA (50 ng/ml), ionomycin (500 ng/ml) and monensin (2 μM) were added and incubated for 4 hours. After washing, cells were stained for intracellular IFN-γ using APC-conjugated anti-IFN-γ.
2. 2 IFNγ-capture assay and selection of IFN-γ+ cells
On the second day of priming of CD4+ T cells, PMA (50 ng/ml) and ionomycin (500 ng/ml) were added for 90 minutes. Cells were washed in cold RPMI medium 1640 and 20 μl IFN-γ-catch reagent was added (Miltenyi Biotech). After addition of 80 μl cold medium, cells were incubated on ice for 5 minutes. Subsequently, 10 ml 37°C medium was added to each sample and incubated at 37°C for 60 minutes. After an incubation of 1 minute on ice the cells were washed and stained with IFN-γ-detection antibody – PE conjugate (Miltenyi Biotech) and IFN-γ positive cells were selected by FACS (FACSAria)[15].
2.3 Computational model
A two-variable system of differential equation describes the changes in the concentration of IL-12Rβ2 mRNA denoted by x, and IFN-γ denoted by y.
Where,
The activation state of STAT1 and STAT4 were assumed to be in steady-state relative to the changes in the transcriptional responses of IL-12Rβ2 and IFN-γ. Therefore, the activity levels of STAT1 and STAT4 were denoted by f1(x) and f4(x), respectively. The mRNA and protein levels for IL-12Rβ2 and IFN-γ were considered to be linearly proportional. bx and by are basal transcription rates that determines the pre-induction level of IFN-γ receptor and the non-specific stimulation of IL-12Rβ2 mRNA expression during activation of the T-cell receptor. vx4, vx1, vy1 and vy4 are maximal transcription rates induced by STAT4 and STAT1 at the IL-12Rβ2 and IFN-γ promoters, respectively. mx4, mx1, my4 and my1 denote the corresponding dissociation equilibrium constants. γy is the decay rate of IFN-γ. IFN-γ is secreted into the extra-cellular space and a fraction of IFN-γ remains bound to the cell membrane[15]. my and m27 are apparent dissociation equilibrium constants of receptor-ligand interactions for IFN-γ and IL-27, respectively. ry-1 and r27-4 are the relative contributions of IFN-γ receptor and the IL-27 receptor to the STAT1 and STAT4 signaling, respectively.
3. Results & Discussion
3.1 Synergy between the IL-27 and IL-12 signaling pathways
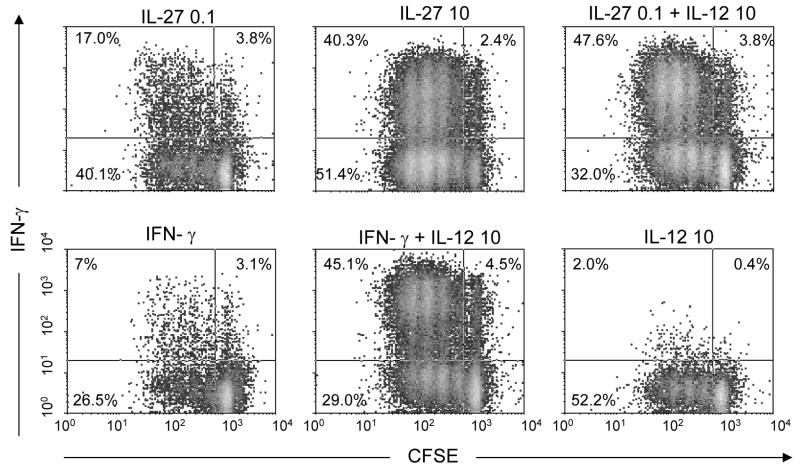
We activated Th cells in vitro to eliminate the interaction of unidentified cytokines when Th1 cells are activated by antigen-presenting cells. IL-27 elicited a dose dependent increase in the percentage of IFN-γ producing (Th1) cells (Fig. 1). The induction of Th1 cell fate by saturating concentrations of cytokines (10 ng/ml) revealed that IL-27 elicited a higher proportion of IFN-γ positive cells (40%) than did IFN-γ (7%), while IL-12 alone had only a modest but detectable effect (2%), three days after activation. The response to saturating concentrations of IL-27 was nearly identical to that seen with limiting concentrations of IL-27 (0.1 ng/ml) when combined with IL-12 at 10 ng/ml. IL-12 acted synergistically with either IL-27 or IFN-γ to promote Th1 cell differentiation (Fig. 1), consistent with the findings that the expression of IL-12Rβ2 is induced by STAT1, which is itself activated by IL-27 or IFN-γ.
Fig. 1.
IFN-γ production by wild-type lymphocytes in response to different cytokines. Cells were labeled with CFSE to detect the number of cell divisions and were stained with APC labeled anti-IFN-γ following activation. All concentration units are given in ng/ml. Under neutral conditions (in the absence of IL-12 and IL-27), 1% of the cells are IFN-γ positive, which corresponds to a background production.
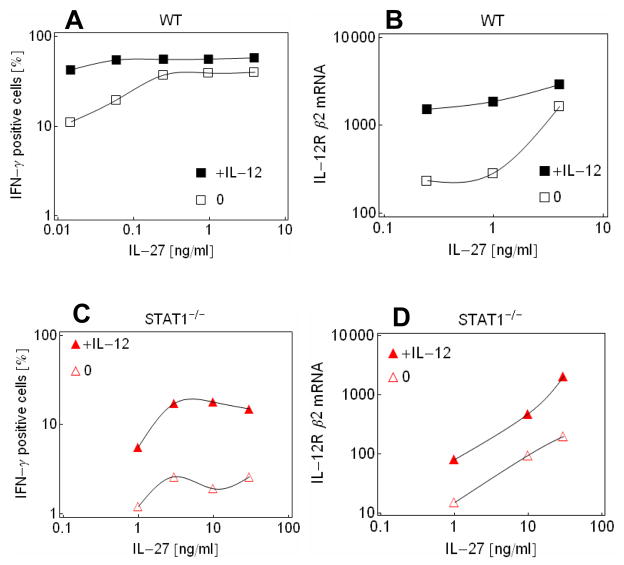
To further explore the synergistic action of IL-27 and IL-12, IFN-γ production was measured over a broad range of IL-27 concentrations in the presence or absence of IL-12. Nearly maximal IFN-γ production was seen, even at very low concentrations of IL-27, when IL-12 was present (Fig. 2A). At these same concentrations, IL-27 alone stimulated IFN-γ production only weakly. In these experiments, the response of IL-12Rβ2 expression was similar to that of IFN-γ production (Fig. 2B). The above findings can be explained by two different mechanisms. First, it is possible that the STAT1 activated by IL-27 induces the expression of IL-12Rβ2 mRNA at lower concentrations than what is required for the induction of IFN-γ and thus IL-12 bind to the IL-12R even when IFN-γ production is stimulated weakly by IL-27. Alternatively, it is possible that STAT1 activation alone is insufficient for inducing the expression of 12Rβ2 mRNA under these conditions and that some other amplification mechanism within the STAT4 pathway might contribute to the induction of IL-12Rβ2 mRNA expression. In order to distinguish between the two possibilities, we examined the effect of signaling through STAT4 when the STAT1 signaling pathway is eliminated.
Fig. 2.
Interaction of IL-27 and IL-12 on IFN-γ and IL-12Rβ2 mRNA production in wild-type and Stat1−/− cells (A) Proportion of IFN-γ positive wild-type cells as a function of IL-27 in the absence (black empty squares) and presence of 10 ng/ml IL-12 (black filled squares). The proportion of IFN-γ positive cells corresponds to the sum of relative cell counts in the upper two quadrants as shown in Fig. 1. (B) IL-12Rβ2 mRNA levels in wild-type cells as a function of IL-27 in the absence (black empty squares) and presence of 10 ng/ml IL-12 (black filled squares). (C) Proportion of IFN-γ positive Stat1−/− cells as a function of IL-27 concentration 5 days after induction with IL-27 in the absence (red empty triangles) or presence (red filled triangles) of 10 ng/ml IL-12. (D) IL-12Rβ2 mRNA levels in Stat1−/− cells as a function of IL-27 concentration 5 days after induction in the absence (red empty triangles) or presence (red filled triangles) of 10 ng/ml IL-12
3.2 Positive feedback in IL-12R expression
It has been shown that IL-27 promotes STAT4 phosphorylation [10]. In order, to study the contribution of STAT4 to Th1 cell commitment and to exclude the potential autoactivation effect of IFN-γ, signal propagation elicited by IL-27 and IL-12 was analyzed in STAT1-deficient cells. IL-27 increased IFN-γ production in a dose dependent manner (Fig. 2C). Next, we tested the effect of IL-12, which is mediated by STAT4 [12]. Interestingly, addition of IL-12 multiplied the effect of IL-27 on IFN-γ production over the entire range of applied IL-27 concentrations. IL-12Rβ2 expression responds similarly to the respective conditions but the dynamic range of expression levels was broader than that of the IFN-γ production (Fig. 2D). Thus, IL-12 induces the expression of IL-12Rβ2 proportionally to the IL-12Rβ2 levels induced by IL-27. Given that activation by IL-12 leads to enhanced expression of its receptor by increasing the expression of IL-12Rβ2, a positive feedback loop may be established in the presence of this cytokine.
IL-27 activates IL-12Rβ2 expression in wild-type cells at much lower concentrations than in STAT1-deficient cells (Fig. s 2B and 2D), which suggests that the STAT1 pathway responds more sensitively to IL-27 than the STAT4 pathway. The synergistic effect of IL-27 and IL-12 on wild-type cells can be then explained by the following scenario. The IL-12 receptor is not present on naïve T-cells; its expression is first weakly induced by IL-27 through STAT1. Subsequently, IL-12 activates the STAT4 signaling pathway leading to the further increase in the expression of IL-12Rβ2, creating a positive feedback loop.
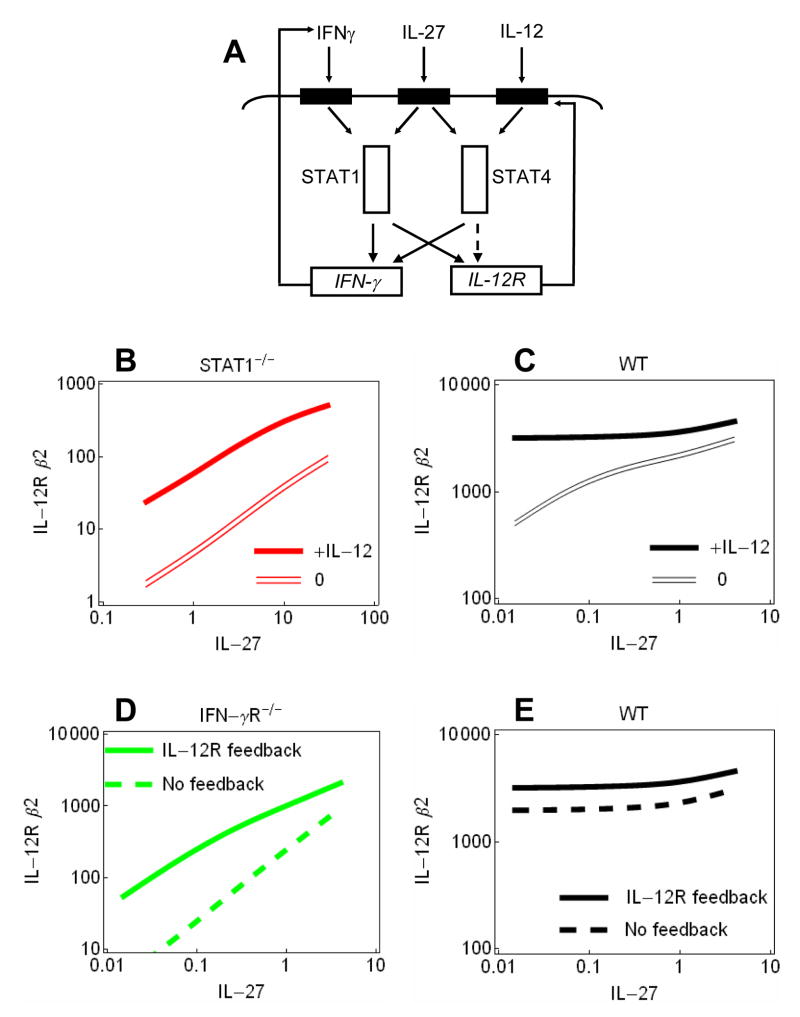
Next, we examined if the above experimental observations are consistent with a kinetic model including a positive feedback loop through the IL-12 receptor. The model comprised all four possible pair-wise interactions between STAT1 and STAT4, and their target genes, IFN-γ and IL-12Rβ2 (Fig. 3A). In order, to simulate the behavior of the positive feedback with realistic parameters, the kinetic model was fit to experimental data on IL-12Rβ2 expression using all described experimental conditions and the genotypes including wild-type, STAT1−/− and IFN-γR1−/− (see Methods). The simulation shows that the response of IL-12Rβ2 mRNA production to varying the IL-27 concentration shifts linearly upon addition IL-12 over a broad range of IL-27 concentration when only the STAT4 pathway is stimulated (i.e., in STAT1-deficient cells) (Fig. 3B). This indicates the presence of a positive feedback loop and that induction by IL-27 and IL-12 does not lead to a saturation of the STAT4 pathway activity in STAT1-deficient cells. Conversely, in wild-type cells this synergistic effect of IL-12 diminishes at higher concentrations of IL-27 (Fig. 3C). The fitted computational model also suggests that IL-27 elicits a stronger signal through STAT1 than STAT4. Indeed, STAT4 deficiency did not alter the induction of IFN-γ production by IL-27 (Fig. 4D).
Fig. 3.
Modeling the Th1 cell fate induction pathways (A) Schematic representation of the signaling network. The dashed line stands for the predicted interaction to close the IL-12R feedback loop. (B, C) Simulation of IL-12Rβ2 mRNA levels in Stat1−/− (B, red lines) and wild-type (C, black lines) background using fitted parameters (see Methods). Empty and filled lines denote were obtained using zero and saturating IL-12 concentrations, respectively. (D, E) The parameter vx4 was set to zero to eliminate the activation of IL-12Rβ2 by STAT4 in and thereby the positive feedback loop. IL-12Rβ2 mRNA levels were simulated in the presence (continuous line) and absence of the feedback loop (dashed line) in Ifngr1−/− (D, green lines) and wild-type networks (E, black lines).
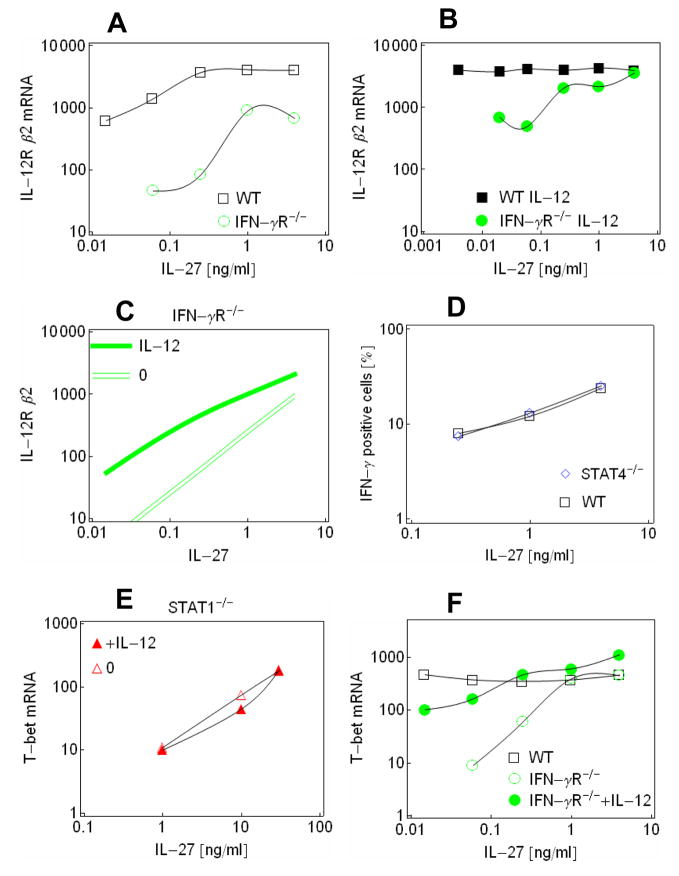
Fig. 4.
Self-activation of Th1 cells through IFN-γ (A) IL-12Rβ2 mRNA levels in wild-type (black empty squares) and Ifngr1−/− (green empty circles) cells as a function of IL-27 concentrations. (B) IL-12Rβ2 mRNA levels in wild-type (black filled squares) and Ifngr1−/− (green filled circles) cells as a function of IL-27 concentrations when IL-12 is present at 10 ng/ml concentration. (C) Simulation of IL-12Rβ2 mRNA levels in Ifngr1−/− cells using fitted parameters (see Methods). Empty and filled green lines denote were obtained using zero and saturating IL-12 concentrations, respectively. (D) Proportion of IFN-γ positive wild-type cells as a function of IL-27 in wild-type (BALB background, (black empty squares) and Stat4−/− cells (BALB background, blue empty diamonds). (E) T-bet mRNA levels in Stat1−/− cells, 5 days after induction with IL-27 in the presence (red filled triangles) or absence (red empty triangles) of IL-12 (10 ng/ml). (F) T-bet mRNA levels in wild-type (black empty squares), and Ifngr1−/− cells in the absence (green empty cicles) or presence of 10 ng/ml IL-12 (green filled circle) after induction with different concentrations of IL-27.
3.3 Autoactivation through secretion of IFN-γ
Next, we explored whether a mechanism other than the above positive feedback could contribute to an amplified induction of Th1 cell fate in wild-type cells. IFN-γ secreted from the differentiating Th1 cells may in principle enhance Th1 cell differentiation by activation of the STAT1-signaling pathway. To test this interaction, we compared wild-type and IFN-γR1-deficient cells. At higher IL-27 concentrations, IL-12Rβ2 expression was somewhat lower in IFN-γR1-deficient cells in comparison to wild-type cells (Fig. 4A). This difference became large at low IL-27 concentrations. Addition of IL-12 enhanced Th1 cell differentiation, but at low concentration of IL-27 IFN-γ-R-deficient cells had lower IL-12Rβ2 mRNA expression than wild-type cells (Fig. 4B).
The above experimental data are consistent with a model of positive feedback mediated by paracrine or autocrine effects IFN-γ when initiated by IL-27 (Fig. 4C). It remains to be determined at what cellular stage of IFN-γ secretion the maximal binding of IFN-γ to its receptor occurs if autoactivation is autocrine. The maximal activation potential of IFN-γR is smaller than that of IL-27R but the computational simulations show that secreted IFN-γ quickly reaches a level that contributes significantly to the STAT1 signaling in the early stages of Th1 cell differentiation. In comparison, the period to reach half-maximal expression of IL-12Rβ is twice as long as that for IFN-γ when these genes are induced constitutively.
T-bet may link STAT1 signaling to IFN-γ production to close the feedback loop. Unlike IFN-γ and IL-12Rβ2, T-bet, a STAT1 target gene [16], was shown to reach maximal expression a few days following activation, whereafter its levels decline [17]. Thus, T-bet is involved in the early steps of Th1 cell differentiation [18]. We compared the expression of the above genes in STAT1-deficient mice.
IL-27 induced expression of T-bet in STAT1-deficient mice in a dose-dependent way (Fig. 4E), suggesting that the STAT4 pathway can induce the expression of T-bet. While IL-12 synergized with IL-27 to induce the expression of IL-12Rβ2 (Fig. 2D), it had essentially no effect on T-bet expression (Fig. 4E). This can be explained by the responsiveness of T-bet expression in the early stages of Th1 differentiation. During the early stages of induction of STAT1-defficient cells by IL-12 and IL-27, only IL-27 contributes significantly to STAT-4 signaling and T-bet expression, given the slow induction of IL-12Rβ2 expression in these cells. In the later stages, when IL-12Rβ2 is expressed at sufficient levels in STAT1 deficient cells, T-bet expression is not responsive to inductive stimuli, and IL-12 does not contribute to expression of T-bet.
T-bet expression was greatly reduced in IFN-γR1−/− cells in comparison to wild-type cells when exposed to IL-27 (Fig. 4F), which is consistent with the involvement of a positive feedback loop through IFN-γ at the early stages of Th1 cell differentiation. IL-12 synergized with IL-27 to increase the expression of T-bet in IFN-γR1-deficient cells (Fig. 4F), unlike in STAT1-deficient cells. This can be explained by the stronger induction of IL-12Rβ2 expression by IL-12 and IL-27 in IFN-γR1-deficient cells in comparison with STAT1-deficient cells (compare Figures 2D and 4A). This suggests that the STAT4 pathway can also contribute to the control of T-bet expression, when sufficient levels of IL-12Rβ2 are expressed during the early stages of Th1 differentiation.
3.4 Contribution of the IL12R feedback loop to signaling in the Th1 network
The IL-12R and IFN-γ mediated feedback loops are integrated in the Th1 signaling network. The contribution of the IL12R feedback loop to the signaling network was calculated by removing the STAT4 activated expression of IL-12R in the model network. Consequently, IL-27 did not induce IL-12Rβ2 expression in STAT1-deficient model network (data not shown). The simulation shows a considerable, approximately 10fold reduction of IL-12Rβ2 expression in IFN-γR1-deficient network, at low concentrations of IL-27, when compared with expression levels predicted with model incorporating the IL12R feedback loop (Fig. 3D). In comparison, in wild-type network IL-12Rβ2 expression was reduced only 1.6 time (Fig. 3E). This suggests that the IL-12Rβ2 mediated feedback loop is particularly important when the functioning of other autocatalytic cycles is weakened. The above and similar compensation mechanisms may have led to the conclusion that fundamentally different pathways orchestrate Th1 differentiation in mouse and human cells; in particular, that STAT4 activates expression of IL-12Rβ2 expression in human cells, while IFN-γ induces expression of IL-12Rβ2 in mouse cells [19]. Our analysis indicates that both pathways are active in mice but that the STAT4 pathway, and consequently the IL12R mediated feedback loop exerts its effect in particular concentrations and combinations of cytokines.
3.5 Stochastic expression of IL-12Rβ2 mRNA
The experimental data and kinetic modeling described thus far suggests that the positive feedback loop speeds up the kinetics of IL-12Rβ2 and IFN-γ expression.
Positive feedback also has the capability to amplify slow and small fluctuations in gene expression and to promote differentiation of a cell population by creating two expression levels [14,20–23]. Fluctuations in eukaryotic gene expression may arise for example due to slow restructuring of chromatin in a chromosomal region, or due to the sensitivity of regulation of promoter activity in response to transcription factors activity [24–27]. For example single cell analysis of gene expression in immune cells has revealed that fluctuations are uncorrelated between the two alleles of the IL-4 gene within a single cell [28–30], while expression of the Pax5 gene switches from uncorrelated to correlated allelic expression during lymphoid development [31].
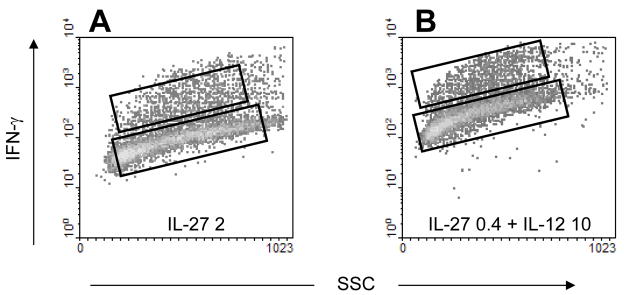
We examined this effect of positive feedback by analyzing the stochastic behavior of lymphocytes in two different conditions. We observed that induction of Th1 cell fate by higher concentration of IL-27 (2 ng/ml) (Fig. 5A) resulted in a percentage of IFN-γ positive cells comparable to that when Th1 cell fate was induced by IL-12 in combination with IL-27 at lower concentrations (0.4 ng/ml) (Fig. 5B). In both experiments, the proportion of IFN-γ positive cells was similar but the generation of a positive feedback loop was initiated by IL-12 only in one of the experiments. Cells expressing IFN-γ (on-cells) were separated from cells that do not contain detectable amounts of IFN-γ (off-cells). This was achieved by capturing the secreted IFN-γ on the surface of the cells, which was subsequently detected by fluorescent antibody (see Methods). The IL-12Rβ2 mRNA level was measured in both cell populations and in the ratio of mRNA level in on cells to off cells was calculated. Having similar proportions of on-cells in the two experimental conditions enables the examination to what extent fluctuations in the expression level of IL-12Rβ2 are amplified by the positive feedback.
Fig. 5.
Stochastic differentiation of Th1 cells. Lymphocytes from 129S6 mice were activated in the presence of indicated cytokines and 2 days later they were stained using the IFN-γ capture assay. Cells expressing low (off-cells) and high (on-cells) levels of IFN-γ were separated, and RNA was isolated from the sorted cells and the relative IL-12Rβ2 mRNA levels were measured. (A) 2 ng/ml IL-27 was used for induction of Th1 cell fate. The IL-12Rβ2 mRNA content of off and on-cells (77.5% and 13.2% of total population) was 121 and 393, respectively (B) 0.4 ng/ml IL-27 and 10 ng/ml IL-12 were used for induction of Th1 cell fate. The IL-12Rβ2 mRNA content of on and off-cells (84.5% and 11.7% of total population) was 49 and 455, respectively.
The ratio of IL-12Rβ2 mRNA in ON to OFF cells was around 3 when the cells were induced by high concentration of IL-27 while this ratio increased to 9 when both IL-27 and IL-12 were present. These results confirm that fluctuations in the expression of IL-12Rβ2 are amplified when the positive feedback is triggered by IL-12. The amplifying effect of the positive feedback on small initial fluctuations may also explain that stimulation by IL-12 alone induces IFN-γ production later than IL-27 and IFN-γ (see Supplementary Data).
Description of the regulatory motifs in the signaling network and stochastic gene expression in Th cells is important for understanding the mechanisms that amplify weak signals to generate responses of sufficient magnitude to deal with rapidly reproducing pathogens and, at the same time, prevent the overproduction of potentially dangerous cells [32,33]. It is necessary to maintain a delicate balance between intensity of Th1 and Th2 responses, because tipping this balance is associated with the appearance of overreacting cells giving rise to autoimmunity and allergic disorders. It has been hypothesized that interlinked slow and fast feedback loops increase the reliability of cellular fate decisions [14]. The interlinked slower IL12R- and the stronger IFN-γ-mediated feedback loops may provide efficient means to polarize Th in an adaptive way.
Supplementary Material
Acknowledgments
We thank A. Suto, M. Michaud, M. Soriano and members of the Glimcher lab for helpful discussions. Supported in part by grants from the National Institute of Health (AI40171 to MJG), the Swiss National Foundation (AB), Human Frontier Science Program (AB) and by a gift from the G. Harold and Leila Y. Mathers Charitable Foundation (MJG).
Abbreviations
- Th1
T helper 1
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Szabo SJ, Sullivan BM, Peng SL, Glimcher LH. Molecular mechanisms regulating Th1 immune responses. Annu Rev Immunol. 2003;21:713–58. doi: 10.1146/annurev.immunol.21.120601.140942. [DOI] [PubMed] [Google Scholar]
- 2.Zhang Y, Apilado R, Coleman J, Ben-Sasson S, Tsang S, Hu-Li J, Paul WE, Huang H. Interferon gamma stabilizes the T helper cell type 1 phenotype. J Exp Med. 2001;194:165–72. doi: 10.1084/jem.194.2.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ouyang W, Lohning M, Gao Z, Assenmacher M, Ranganath S, Radbruch A, Murphy KM. Stat6-independent GATA-3 autoactivation directs IL-4-independent Th2 development and commitment. Immunity. 2000;12:27–37. doi: 10.1016/s1074-7613(00)80156-9. [DOI] [PubMed] [Google Scholar]
- 4.Gardner TS, Cantor CR, Collins JJ. Construction of a genetic toggle switch in Escherichia coli. Nature. 2000;403:339–42. doi: 10.1038/35002131. [DOI] [PubMed] [Google Scholar]
- 5.Mariani L, Lohning M, Radbruch A, Hofer T. Transcriptional control networks of cell differentiation: insights from helper T lymphocytes. Prog Biophys Mol Biol. 2004;86:45–76. doi: 10.1016/j.pbiomolbio.2004.02.007. [DOI] [PubMed] [Google Scholar]
- 6.Roeder I, Glauche I. Towards an understanding of lineage specification in hematopoietic stem cells: a mathematical model for the interaction of transcription factors GATA-1 and PU.1. J Theor Biol. 2006;241:852–65. doi: 10.1016/j.jtbi.2006.01.021. [DOI] [PubMed] [Google Scholar]
- 7.Martin-Fontecha A, Thomsen LL, Brett S, Gerard C, Lipp M, Lanzavecchia A, Sallusto F. Induced recruitment of NK cells to lymph nodes provides IFN-gamma for T(H)1 priming. Nat Immunol. 2004;5:1260–5. doi: 10.1038/ni1138. [DOI] [PubMed] [Google Scholar]
- 8.Owaki T, Asakawa M, Morishima N, Hata K, Fukai F, Matsui M, Mizuguchi J, Yoshimoto T. A role for IL-27 in early regulation of Th1 differentiation. J Immunol. 2005;175:2191–200. doi: 10.4049/jimmunol.175.4.2191. [DOI] [PubMed] [Google Scholar]
- 9.Pflanz S, et al. IL-27, a heterodimeric cytokine composed of EBI3 and p28 protein, induces proliferation of naive CD4(+) T cells. Immunity. 2002;16:779–90. doi: 10.1016/s1074-7613(02)00324-2. [DOI] [PubMed] [Google Scholar]
- 10.Lucas S, Ghilardi N, Li J, de Sauvage FJ. IL-27 regulates IL-12 responsiveness of naive CD4+ T cells through Stat1-dependent and -independent mechanisms. Proc Natl Acad Sci U S A. 2003;100:15047–52. doi: 10.1073/pnas.2536517100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Afkarian M, Sedy JR, Yang J, Jacobson NG, Cereb N, Yang SY, Murphy TL, Murphy KM. T-bet is a STAT1-induced regulator of IL-12R expression in naive CD4+ T cells. Nat Immunol. 2002;3:549–57. doi: 10.1038/ni794. [DOI] [PubMed] [Google Scholar]
- 12.Kaplan MH, Sun YL, Hoey T, Grusby MJ. Impaired IL-12 responses and enhanced development of Th2 cells in Stat4-deficient mice. Nature. 1996;382:174–7. doi: 10.1038/382174a0. [DOI] [PubMed] [Google Scholar]
- 13.Usui T, Preiss JC, Kanno Y, Yao ZJ, Bream JH, O’Shea JJ, Strober W. T-bet regulates Th1 responses through essential effects on GATA-3 function rather than on IFNG gene acetylation and transcription. J Exp Med. 2006;203:755–66. doi: 10.1084/jem.20052165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brandman O, Ferrell JE, Jr, Li R, Meyer T. Interlinked fast and slow positive feedback loops drive reliable cell decisions. Science. 2005;310:496–8. doi: 10.1126/science.1113834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Assenmacher M, Scheffold A, Schmitz J, Segura Checa JA, Miltenyi S, Radbruch A. Specific expression of surface interferon-gamma on interferon-gamma producing T cells from mouse and man. Eur J Immunol. 1996;26:263–7. doi: 10.1002/eji.1830260141. [DOI] [PubMed] [Google Scholar]
- 16.Kamiya S, Owaki T, Morishima N, Fukai F, Mizuguchi J, Yoshimoto T. An indispensable role for STAT1 in IL-27-induced T-bet expression but not proliferation of naive CD4+ T cells. J Immunol. 2004;173:3871–7. doi: 10.4049/jimmunol.173.6.3871. [DOI] [PubMed] [Google Scholar]
- 17.Szabo SJ, Kim ST, Costa GL, Zhang X, Fathman CG, Glimcher LH. A novel transcription factor, T-bet, directs Th1 lineage commitment. Cell. 2000;100:655–69. doi: 10.1016/s0092-8674(00)80702-3. [DOI] [PubMed] [Google Scholar]
- 18.Mullen AC, et al. Role of T-bet in commitment of TH1 cells before IL-12-dependent selection. Science. 2001;292:1907–10. doi: 10.1126/science.1059835. [DOI] [PubMed] [Google Scholar]
- 19.Letimier FA, Passini N, Gasparian S, Bianchi E, Rogge L. Chromatin remodeling by the SWI/SNF-like BAF complex and STAT4 activation synergistically induce IL-12Rbeta2 expression during human Th1 cell differentiation. Embo J. 2007;26:1292–302. doi: 10.1038/sj.emboj.7601586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Acar M, Becskei A, van Oudenaarden A. Enhancement of cellular memory by reducing stochastic transitions. Nature. 2005;435:228–32. doi: 10.1038/nature03524. [DOI] [PubMed] [Google Scholar]
- 21.Austin DW, et al. Gene network shaping of inherent noise spectra. Nature. 2006;439:608–11. doi: 10.1038/nature04194. [DOI] [PubMed] [Google Scholar]
- 22.Becskei A, Seraphin B, Serrano L. Positive feedback in eukaryotic gene networks: cell differentiation by graded to binary response conversion. Embo J. 2001;20:2528–35. doi: 10.1093/emboj/20.10.2528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Guido NJ, Wang X, Adalsteinsson D, McMillen D, Hasty J, Cantor CR, Elston TC, Collins JJ. A bottom-up approach to gene regulation. Nature. 2006;439:856–60. doi: 10.1038/nature04473. [DOI] [PubMed] [Google Scholar]
- 24.Ahmad K, Henikoff S. Modulation of a transcription factor counteracts heterochromatic gene silencing in Drosophila. Cell. 2001;104:839–47. doi: 10.1016/s0092-8674(01)00281-1. [DOI] [PubMed] [Google Scholar]
- 25.Becskei A, Kaufmann BB, van Oudenaarden A. Contributions of low molecule number and chromosomal positioning to stochastic gene expression. Nat Genet. 2005;37:937–44. doi: 10.1038/ng1616. [DOI] [PubMed] [Google Scholar]
- 26.Lee GR, Kim ST, Spilianakis CG, Fields PE, Flavell RA. T helper cell differentiation: regulation by cis elements and epigenetics. Immunity. 2006;24:369–79. doi: 10.1016/j.immuni.2006.03.007. [DOI] [PubMed] [Google Scholar]
- 27.Cai S, Lee CC, Kohwi-Shigematsu T. SATB1 packages densely looped, transcriptionally active chromatin for coordinated expression of cytokine genes. Nat Genet. 2006;38:1278–88. doi: 10.1038/ng1913. [DOI] [PubMed] [Google Scholar]
- 28.Bix M, Locksley RM. Independent and epigenetic regulation of the interleukin-4 alleles in CD4+ T cells. Science. 1998;281:1352–4. doi: 10.1126/science.281.5381.1352. [DOI] [PubMed] [Google Scholar]
- 29.Guo L, Hu-Li J, Paul WE. Probabilistic regulation in TH2 cells accounts for monoallelic expression of IL-4 and IL-13. Immunity. 2005;23:89–99. doi: 10.1016/j.immuni.2005.05.008. [DOI] [PubMed] [Google Scholar]
- 30.Riviere I, Sunshine MJ, Littman DR. Regulation of IL-4 expression by activation of individual alleles. Immunity. 1998;9:217–28. doi: 10.1016/s1074-7613(00)80604-4. [DOI] [PubMed] [Google Scholar]
- 31.Nutt SL, Vambrie S, Steinlein P, Kozmik Z, Rolink A, Weith A, Busslinger M. Independent regulation of the two Pax5 alleles during B-cell development. Nat Genet. 1999;21:390–5. doi: 10.1038/7720. [DOI] [PubMed] [Google Scholar]
- 32.Bauch A, Superti-Furga G. Charting protein complexes, signaling pathways, and networks in the immune system. Immunol Rev. 2006;210:187–207. doi: 10.1111/j.0105-2896.2006.00369.x. [DOI] [PubMed] [Google Scholar]
- 33.Germain RN. The art of the probable: system control in the adaptive immune system. Science. 2001;293:240–5. doi: 10.1126/science.1062946. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.