Abstract
Exposure to uncontrollable stressors produces a variety of behavioral consequences (e.g. exaggerated fear, reduced social exploration) that do not occur if the stressor is controllable. In addition, an initial experience with a controllable stressor can block the behavioral and neural responses to a later uncontrollable stressor. The serotonergic (5-HT) dorsal raphe nucleus (DRN) has come to be viewed as a critical structure in mediating the behavioral effects of uncontrollable stress. Recent work suggests that the buffering effects of behavioral control on the DRN-dependent behavioral outcomes of uncontrollable stress require ventral medial prefrontal cortex (mPFCv) activation at the time of behavioral control. The present studies were conducted to directly determine whether or not controllable stress selectively activates DRN-projecting neurons within the mPFCv. To examine this possibility in the rat, we combined retrograde tracing (fluorogold iontophoresed into the DRN) with Fos immunohistochemistry, a marker for neural activation. Exposure to controllable, relative to uncontrollable, stress increased Fos expression in fluorogold-labeled neurons in the prelimbic region (PL) of the mPFCv. Furthermore, in a separate experiment, a prior experience with controllable stress led to potentiation of Fos expression in retrogradely labeled PL neurons in response to an uncontrollable stressor one week later. These results suggest that the PL selectively responds to behavioral control and utilizes such information to regulate the brainstem response to ongoing and subsequent stressors.
Keywords: stressor controllability, medial prefrontal cortex, prelimbic, fluorogold, rat
Introduction
Traumatic life events play a critical role in the etiology of a number of clinical conditions such as anxiety and depression, yet the outcome of a traumatic event can vary widely between individuals (Shalev et al., 1998; Charney, 2004). Research aimed at identifying psychosocial factors that determine vulnerability and resilience to trauma have focused on coping mechanisms, of which actual or perceived behavioral control is a central component (Yehuda et al., 2006). It is generally recognized that the degree of behavioral control that an organism (rat to human) has over an aversive experience is a potent variable in determining the behavioral and physiological impact of that aversive event (Weiss, 1968; Maier & Seligman, 1976). For example, rats exposed to a series of controllable escapable tail shocks (ES) later fail to show the typical behavioral outcomes of exposure to exactly equal yoked uncontrollable inescapable tail shocks (IS). Behavioral differences that are dependent solely on the controllability of the shock have been extensively reviewed (Maier & Watkins, 2005) and termed “stressor controllability effects”. In addition, prior ES can block the behavioral and neurochemical consequences of later exposure to IS, a process labeled “behavioral immunization” (Williams & Maier, 1977; Amat et al., 2006).
The difference in behavioral outcomes following uncontrollable and controllable stressors can be attributed, at least in part, to the fact that IS activates dorsal raphe nucleus (DRN) serotonergic (5-HT) neurons to a greater degree than does ES (Maswood et al., 1998; Grahn et al., 1999), thereby leading to a period of sensitization during which inputs to the DRN produce an exaggerated release of 5-HT in DRN projection regions (Amat et al., 1998). It has been argued that the DRN does not process information concerning the degree of controllability; rather the presence of control is detected by regions in the ventral medial prefrontal cortex (mPFCv) which, in turn, actively inhibits shock-induced activation of the DRN (Amat et al., 2005).
In support, inhibition of mPFCv output by microinjection of the GABAA agonist, muscimol, during IS and ES exposure selectively interferes with the buffering effects of ES (Amat et al., 2005; Baratta et al., 2007; Rozeske et al., 2009). That is, intra-mPFCv muscimol during ES leads ES to produce a comparable DRN 5-HT and behavioral responses to that of IS. Furthermore, mPFCv inactivation during an initial exposure to ES prevents the behavioral and neurochemical immunizing effects produced by ES to later IS exposure (Amat et al., 2006).
The foregoing suggests, but does not directly demonstrate, that a subpopulation of cells in the mPFCv that projects to the DRN selectively responds to the presence of behavioral control. The present experiments were conducted to investigate the possibility that control selectively activates infralimbic (IL) and/or prelimbic (PL) regions of the mPFCv 1) at the time of initial ES and 2) during subsequent IS exposure. In order to assess activation in IL and PL neurons that project to the DRN, retrograde tracing (fluorogold (FG)) was combined with double-labeling immunohistochemistry (IHC) for Fos, a marker for neuronal activation.
Materials and methods
Subjects
Male Sprague-Dawley rats (300-325 g; Harlan, Indianapolis) were housed in pairs on a 12-h light/dark cycle (lights on at 7:00 A.M.). Standard lab chow and water were available ad libitum. Rats were allowed to acclimate to colony conditions for 7-10 days prior to surgery. All animal procedures were approved by the Institutional Animal Care and Use Committee of University of Colorado at Boulder.
Iontophoresis of retrograde tracer
Rats were anesthetized with halothane anesthesia (Halocarbon Laboratories, River Edge, NJ). A small window (2 × 2 mm) was drilled in the skull bone to allow penetration of glass capillaries (20-25 μm external tip diameter) filled with a 2% solution of FG (Fluorochrome, Denver) dissolved in 0.9% sodium chloride buffer. Discrete FG deposits were iontophoretically applied (1.5 μA, 7 s on/off, 10 min) in the DRN (-8.3 mm caudal to bregma, -5.6 mm ventral from the dura mater, 2.5 mm relative to the midline, with an angle of 22.5° to avoid puncturing the sinus). Following iontophoresis, the capillaries were left in place for 10 min before removal to minimize FG leakage up the capillary tract. Rats were allowed 10-14 days recovery before experimentation.
Experiment 1: Effect of controllability on Fos expression in DRN-projecting mPFCv neurons
Rats were exposed to a single session of either ES or IS in Plexiglas boxes (14 × 11 × 17 cm) with a wheel mounted in the front and a Plexiglas rod protruding from the rear. The rat's tail was secured to the rod with tape and affixed with copper electrodes. Rats were run in yoked pairs (ES and IS) and each session consisted of 80 trials of tail shock (27 × 1.0 mA, 27 × 1.3 mA, and 26 × 1.6 mA) with an average 60 s intertrial interval. Tail shock was terminated for both rats when the ES rat met the escape requirement according to a protocol previously described (Baratta et al., 2007). Thus, the duration and the intensity of the tail shocks were identical for each rat in the pair. For the ES subject, the initial tail shock was terminated by a quarter turn of the wheel. The response requirement was increased by one quarter turn when each of three consecutive trials was completed in less than 5 s. Subsequent latencies under 5 s increased the requirement by 50% up to a maximum of four full turns. If the escape requirement was not reached by the end of 30 s, the shock was automatically terminated and the escape requirement was reduced to a single quarter turn. This procedure was used to insure that the ES subject maintained consistent operant escape responding throughout the 80 trials. Subjects were returned to the colony immediately following the tail shock procedure. Non-shocked home cage (HC) rats remained undisturbed in the colony.
Experiment 2: Effect of prior controllability on Fos expression in DRN-projecting mPFCv neurons in response to a subsequent exposure of IS
Rats were first subjected to the same wheel-turn ES/yoked-IS procedure as described in Experiment 1. Seven days later, rats were subjected to a second experimental treatment in which all rats were exposed to IS in Plexiglas tubes (17.5 cm long, 7.0 cm in diameter), rather than wheel-turn boxes, with the rat's tail extended from the rear of the tube and secured to a Plexiglas rod. The session consisted of 100 IS tail shocks (1.0 mA, 5 s duration each) with a variable intertrial interval ranging from 30 to 90 s (average intertrial interval of 60 s). Following the IS session, all subjects were returned to their colony until sacrifice (see below). Rather than repeating control groups, HC data is used for both experiments.
Tissue preparation
In both experiments, rats were anesthetized with sodium pentobarbital (65 mg/kg) two hours following the last tail shock. Rats were then perfused with 100 ml of ice-cold physiological saline followed by 250 ml of 4% paraformaldehyde in 0.1 M phosphate buffer (PB; pH 7.4). Brains were postfixed in the same fixative overnight. Brains were transferred to 30% sucrose and stored at 4°C until sectioning. Prior to sectioning, brains were flash frozen in -40°C isopentane. MPFCv (35 μm) sections were obtained in a -20°C cryostat while DRN sections (35 μm) were mounted onto SuperFrost Plus slides (Fisher Scientific, Pittsburgh), and coverslipped with VectaShield mounting media with DAPI (Vector Laboratories, Burlingame, CA).
IHC
IHC staining for Fos and FG were conducted sequentially in mPFCv sections. Staining for Fos was conducted first using the avidin-biotin-horseradish (ABC) method. Following a series of washes in phosphate buffer saline (PBS), sections were incubated in a 0.9% hydrogen peroxide solution in order to quench endogenous peroxidases. Then, sections were incubated for 24 h at room temperature (RT) with Fos primary antibody (1:15,000; Santa Cruz Biotechnology, Santa Cruz, CA) in a blocking solution containing 1% normal goat serum (NGS), 0.25% Triton-X, and 0.1% sodium azide. Following the primary antibody incubation, sections were incubated for 2 h at RT in biotinylated goat anti-rabbit secondary antibody (1:200, Jackson ImmunoResearch Laboratories, West Grove, PA) in blocking solution. After a series of PBS washes, slices were then incubated in ABC for 1 h at RT. Next, sections were washed in 0.1 M PB and then exposed to a solution containing 3, 3′-diaminobenzidine (DAB), cobalt chloride, nickel ammonium sulfate, ammonium chloride, and glucose oxidase in PB. The peroxidase reaction was initiated by the addition of a glucose solution that reacted with the tissue for approximately 7-10 min. The reaction was terminated by washing sections with PBS.
MPFCv sections were further processed for FG. Sections were incubated in rabbit polyclonal antisera directed against FG (1:50,000; Fluorochrome) for 48 h at 4°C. Slices were then incubated with a non-biotinylated goat anti-rabbit IgG (1:200; Jackson ImmunoResearch Laboratories) in blocking solution for 2 h at RT. This was followed by an incubation step with peroxidase-anti-peroxidase (PAP; 1:500) in PBS for 2 h and then into a series of .1 M PB washes before the chromagen was developed with a NovaRED substrate kit for peroxidase (Vector Laboratories). This yields a red reaction product as opposed to the dark reaction product obtained for the Fos nuclear stain. Following IHC, sections were mounted on gelatin treated slides and allowed to dry overnight. Slide mounted sections were defatted with Histoclear and coverslipped with Permount.
Image analysis
Tissue was examined on a microscope by an observer blind to animal treatment on an Olympus BX-61 microscope (Olympus America, Center Valley, Pennsylvania) and analyzed using Olympus Suite software. The number of Fos-stained nuclei, FG-positive cells, and the number of cells double-labeled for both Fos and FG were assessed in the IL and PL regions of the mPFCv. Fos-stained nuclei were identified by dark-brown or black ovoid particles. Neurons displaying red cell bodies were identified as immunopositive for FG. MPFCv sections were comparable to an anterior-posterior coordinate of +2.7 mm from bregma according to Paxinos and Watson (1986).
The localization of FG deposits in the DRN was verified with the same microscope using epifluorescence illumination with a mercury lamp coupled to a DAPI filter. Iontophoretic injections tended to produce a small necrotic area in the mid to caudal regions of the DRN.
Statistical analysis
All statistical comparisons were computed using StatView for windows (Version 5.0.1; SAS Institute, Cary, NC). Data were analyzed by between-subjects analysis of variance (ANOVA) followed by Fisher's protected least significant difference (PLSD) test post hoc comparison. To control for variability in the number of FG-immunoreacitve (ir) cells created by variations in FG deposit size and degree of retrograde transport, the number of FG-positive cells was added as a covariate in the analysis of the percentage of FG-labeled cells expressing Fos. The significance level was set at p < 0.05.
Results
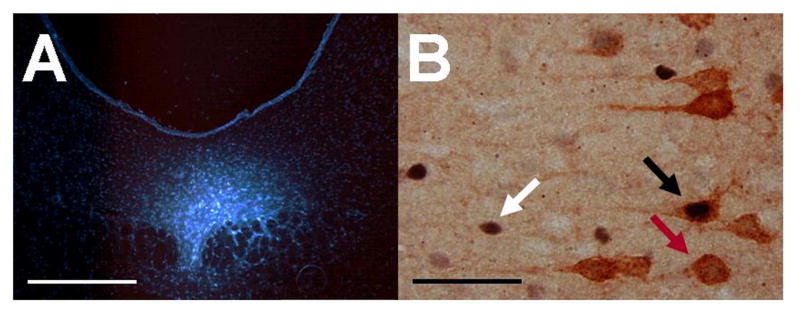
Figure 1A shows a representative FG deposit in the DRN, and Figure 1B shows representative Fos and FG labeling in the mPFCv. FG deposits tended to be restricted to the middle and caudal divisions of the DRN. Only subjects with minimal or no FG deposit beyond the DRN were included in the final analyses (n = 6-7/group in each experiment). FG-ir in the mPFCv was largely restricted to the deep layers of the PL and IL, with only occasional sparse labeling in the more dorsal anterior cingulate region, similar to previous observations following retrograde deposit into DRN (Gabbott et al., 2005; Goncalves et al., 2009).
Figure 1.
(A) Representative fluorescent photomicrograph showing a representative iontophoretic fluorogold deposit in the dorsal raphe nucleus, scale bar = 500 μm. (B) Representative brightfield photomicrograph (100× objective, oil) showing a Fos-immunoreactive (ir) nucleus (white arrow), a fluorogold-ir soma (yellow arrow), and a double-labeled (Fos- and fluorogold-ir) neuron (black arrow) in the ventral medial prefrontal cortex following tail shock, scale bar = 50 μm.
Experiment 1: Behavioral control increases Fos expression in DRN-projecting PL neurons
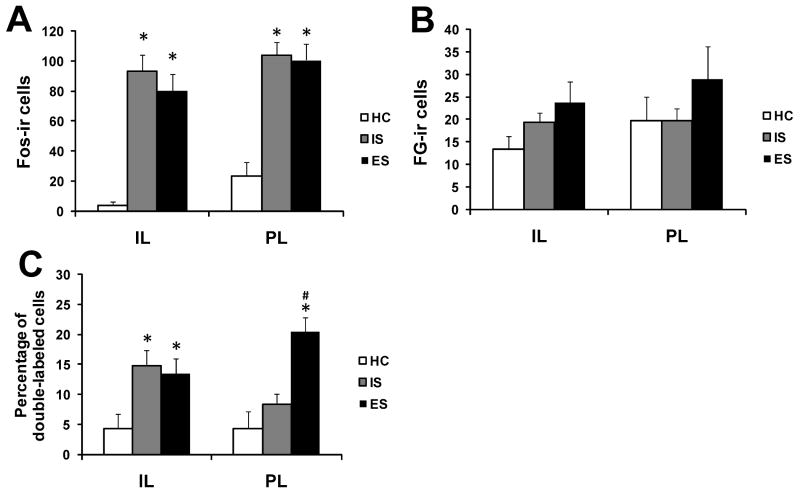
Fos- and FG-ir was examined in ES, IS, and HC subjects sacrificed 2 h following the last tail shock. HC subjects showed almost no Fos expression in the IL and little overall expression in the PL. In contrast, IS and ES led to a robust Fos enhancement in both the IL (F(2, 14) = 23.81, p < 0.001) and the PL (F(2, 15) = 19.16, p < 0.001). Post hoc analyses showed that IS (p < 0.001) and ES (p < 0.001) significantly increased Fos expression relative to HC in the IL and PL, although the two stress groups did not differ in amount (Fig. 2A). The volume of FG deposit is a critical variable in retrograde tracing as the number of retrogradely labeled cells is sensitive to the amount of tracer deposited. There were no significant differences in the number of FG-ir cells between groups in the IL or PL (Fig. 2B).
Figure 2.
Activation of neurons in the infralimbic (IL) and prelimbic (PL) regions of animals exposed to escapable shock (ES), inescapable shock (IS), and home cage (HC). (A) Mean and standard error of the mean (SEM) of the number of neurons expressing Fos in the IL and PL. (B) Mean and SEM of the number of neurons expressing fluorogold (FG) in the IL and PL. (C) Percentage of FG-immunoreactive (ir) neurons expressing Fos in the IL and PL. *P < 0.05 compared to HC; #P < 0.05 compared to IS.
The percent of DRN-projecting IL neurons expressing Fos was analyzed using an ANOVA that revealed a significant effect of group (F(2, 12) = 8.06, p < 0.01), no effect of the covariate (number of FG-positive cells), and a significant interaction between group and covariate (F(2, 12) = 4.14, p < 0.05). Post hoc analysis showed that IS (p < 0.01) and ES (p < 0.05) significantly increased double-labeled cells in the IL compared to HC, but there was no effect of controllability. The pattern for Fos expression in DRN-projecting PL neurons was different. An ANOVA indicated a significant effect of group (F(2, 13) = 5.46, p < 0.05), but no significant effect of the covariate (number of FG-positive cells) and interaction between group and covariate on the percent of DRN-projecting PL neurons expressing Fos. Post hoc analysis revealed that ES subjects significantly differed from IS and HC subjects (ps < 0.01), with ES treatment significantly increasing the percentage of double-labeled cells in the PL. IS did not differ from HC. Thus, behavioral control selectively induced activation in the PL neurons that project to the DRN (Fig. 2C).
Experiment 2: Behavioral control increases Fos expression in DRN-projecting PL neurons in response to a subsequent exposure of IS
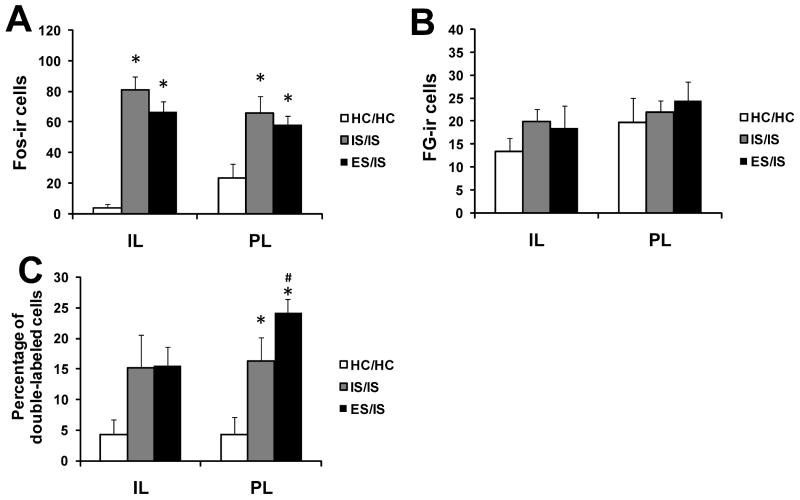
Here the question was whether or not prior behavioral control (treatment 1) facilitates the activation of the mPFCv-DRN pathway to subsequent IS given 7 days later (treatment 2). Exposure to IS (treatment 2) induced Fos expression in both the IL (F(2, 15) = 31.41, p < 0.001) and the PL (F(2, 15) = 5.19, p < 0.05). Post hoc analyses showed that prior ES (ES/IS) and prior IS (IS/IS) subjects had a significantly greater amount of Fos expression relative to HC/HC in the IL (ps < 0.001) and the PL (respectively, p < 0.05; p < 0.01), although the two prior stress groups did not differ in amount (Fig. 3A). In addition, there were no significant group differences in the number of FG-ir cells between groups in the IL or PL (Fig. 3B).
Figure 3.
Activation of neurons in the infralimbic (IL) and prelimbic (PL) regions following exposure to inescapable shock (IS). Subjects received either escapable shock (ES/IS), inescapable (IS/IS), or home cage (HC/HC) 7 days earlier. (A) Mean and standard error of the mean (SEM) of the number of neurons expressing Fos in the IL and PL. (B) Mean and SEM of the number of neurons expressing fluorogold (FG) in the IL and PL. (C) Percentage of FG-immunoreactive (ir) neurons expressing Fos in the IL and PL. *P < 0.05 compared to HC/HC; #P < 0.05 compared to IS/IS.
The percent of DRN-projecting IL neurons expressing Fos was analyzed using an ANOVA that revealed no significant effect of group, covariate (number of FG-positive cells), or interaction between group and covariate. Similar to Experiment 1, prior ES (ES/IS) led to a greater activation of DRN-projecting PL neurons. An ANOVA indicated a significant effect of group (F(2, 13) = 7.58, p < 0.01) and covariate (F(2, 13) = 5.53, p < 0.05), but no significant interaction between group and covariate. Post hoc analysis showed that both IS/IS (p < 0.01) and ES/IS (p < 0.001) significantly increased double-labeled cells in the PL compared to HC/HC. Additionally, ES/IS significantly increased Fos expression in DRN-projecting PL neurons compared to IS/IS (p < 0.05; Fig. 3C).
Discussion
The present results demonstrate that the PL–DRN pathway responds selectively to controllable, relative to exactly equal uncontrollable, stressors. Although total Fos expression in the PL was equivalent between ES and IS, ES led to a greater activation in DRN-projecting PL neurons. It is notable that the behavioral control-induced activation of PL neurons that project to the DRN was found 2 h after stressor exposure. At the same time point, Grahn et al. (1999) demonstrated that Fos expression in caudal DRN 5-HT neurons is preferentially induced by IS, relative to ES. Thus, the selective activation of DRN-projecting PL neurons by ES represents a potential mechanism by which behavioral control prevents DRN activation.
There are several lines of evidence that support this interpretation. Hajos and colleagues (1998) showed that electrical stimulation of the mPFCv potently reduces DRN 5-HT activity. The mPFCv-induced inhibition seemed counterintuitive since the mPFCv-DRN projections use the excitatory amino acid glutamate and a large number of cells in the DRN use 5-HT as their neurotransmitter. The possible mechanisms of this inhibition were clarified by several subsequent anatomical studies. For instance, the majority of DRN-projecting mPFCv neurons synapse onto GABA interneurons, not 5-HT cells (Jankowski & Sesack, 2004). These local inhibitory cells form synaptic contact with 5-HT dendrites and soma (Wang et al., 1992) and can inhibit 5-HT activity through activation of the GABAA receptor. Thus, the putative mechanism by which the mPFCv inhibits the DRN is through preferential targeting of local GABAergic circuits that switch an excitatory input to the DRN into an inhibitory signal onto DRN 5-HT neurons. Indeed, several groups have shown that pharmacological blockade of GABAergic transmission within the DRN prevents mPFCv-induced 5-HT inhibition (Celada et al., 2001; Varga et al., 2001).
Activation of the mPFCv during tail shock is critical for producing the buffering effects of behavioral control (Amat et al., 2005). Inactivation of the mPFCv during ES produces the typical behavioral outcomes of IS, while activation of the mPFCv during IS prevents the typical behaviors that follow IS (Amat et al., 2005; Amat et al., 2008). The data in Experiment 1 provide direct evidence that the PL neurons that project to the DRN selectively respond to controllable stressors and that their activation may provide a mechanism in which behavioral control engages the mPFCv in order to reduce DRN activation.
The second experiment explored the effect of prior ES on the activation of DRN-projecting mPFCv neurons in the behavioral immunization paradigm. The experiments reported by Amat et al. (2006) indicate that activation of the mPFCv is necessary for the resistance-inducing effects of prior ES on DRN and behavioral responses to later IS. That is, mPFCv inactivation at both the time of the initial experience of control and the time of the later uncontrollable stressor prevents the previous ES experience from exerting its protective effects. Furthermore, using the same behavioral immunization paradigm, Amat et al. (2008) activated the mPFCv by microinjecting the GABAA antagonist, picrotoxin, during exposure to controllable and uncontrollable stress. Picrotoxin did not affect the ability of ES to protect against later IS. Instead, picrotoxin-treated IS rats were resistant to the neural and behavioral impact of IS given in a different environment 7 days later. This suggests that activation of the mPFCv during controllable shock becomes ‘tied’ to some aspect of tail shock such that later uncontrollable stressors that normally do not activate mPFCv output to the DRN now do so.
Here, an initial experience with ES led to greater activation in the PL-DRN pathway during later IS than did intial IS. This occurred without prior ES modulating the number of Fos-ir neurons in the PL, highlighting the fact that total Fos expression alone may be an insufficient measure when interpreting data at a systems level. Given the extent to which Fos has been used to study the prefrontal cortex in response to a variety of stressors, it is surprising that relatively few studies have assessed the phenotype of prefrontal cells expressing Fos following stressor exposure.
The present results fit well with the emerging framework of functional differences among mPFCv subregions. Vertes (2004) and others have noted that the PL projects much more heavily to the DRN than does the IL (Vertes, 2004; Gabbott et al., 2005), especially to the caudal DRN (Goncalves et al., 2009), and it's the caudal DRN where controllability differences occur (Grahn et al., 1999; Amat et al., 2005). The DRN is the largest of the raphe nuclei and provides dense ascending 5-HT innervation to limbic and forebrain regions involved in the integration of emotional and cognitive aspects of behavior (Jacobs & Azmitia, 1992). As previously discussed (see Introduction), exaggerated 5-HT release into projection regions of the DRN is considered the proximate cause of the anxiety-like behavioral outcomes of IS. Thus, the DRN is viewed as a key integrative site at the efferent end of the stressor controllability circuit, which in turn can be modulated in the presence of behavioral control by “upstream” inputs such as the PL. This is consistent with the idea that the PL exerts control over brainstem and limbic areas that are known to influence cognition and goal-directed behavior, while the IL primarily regulates a network of structures that subserve autonomic/visceral functions (Vertes, 2004).
The focus on the DRN is not meant to suggest that other structures implicated in mediating stressor controllability effects are not similarly regulated by the PL. Analogous to the DRN, the noradrenergic (NE) locus coeruleus (LC) is able to influence a wide range of behavioral functions through broad efferent projections to limbic and cortical structures and activation of the LC is viewed as a critical requirement for the behavioral effects of IS to occur (Weiss & Simson, 1986; Simson & Weiss, 1988; but see McDevitt et al., in press). The rat LC receives glutamatergic afferents from the PL that terminate in the dendrite-rich peri-LC region that is populated by GABAergic interneurons, as opposed to the LC proper which is composed almost entirely of NE neuronal cell bodies (Sesack et al., 1989; Aston-Jones et al., 1995; Lee et al., 2005). Thus, the PL could potentially silence LC activity, in addition to the DRN, to produce the buffering effects of controllable stress. However, unlike the PL-DRN relationship, both electrical and chemical stimulation of the rat PL potently excites, rather than inhibits, LC neuronal activity (Jodo & Aston-Jones, 1997; Jodo et al., 1998). It may prove to be the case that the PL regulates the LC differently than the DRN during controllable stressors.
Overall, these data indicate that the PL is sensitive to the presence of stressor-controlling escape response and utilizes the information to regulate the DRN response to ongoing and subsequent stressors. More generally, the present results suggest that coping with traumatic life events may selectively engage human prefrontal regions functionally homologous to the rat PL in order to mitigate brainstem processes responding to aversive events.
Acknowledgments
This research was supported by grants MH 50479 (SFM), MH 75213 (MVB), and GM066728 (MVB) from the National Institutes of Health.
Abbreviations
- 5-HT
serotonin
- DRN
dorsal raphe nucleus
- ES
escapable tail shock
- FG
fluorogold
- HC
home cage
- IHC
immunohistochemistry
- IL
infralimbic
- ir
immunoreactive
- IS
inescapable tail shock
- LC
locus coeruleus
- mPFCv
ventral medial prefrontal cortex
- NE
noradrenergic
- PL
prelimbic
References
- Amat J, Baratta MV, Paul E, Bland ST, Watkins LR, Maier SF. Medial prefrontal cortex determines how stressor controllability affects behavior and dorsal raphe nucleus. Nat Neurosci. 2005;8:365–371. doi: 10.1038/nn1399. [DOI] [PubMed] [Google Scholar]
- Amat J, Matus-Amat P, Watkins LR, Maier SF. Escapable and inescapable stress differentially alter extracellular levels of 5-HT in the basolateral amygdala of the rat. Brain Res. 1998;812:113–120. doi: 10.1016/s0006-8993(98)00960-3. [DOI] [PubMed] [Google Scholar]
- Amat J, Paul E, Watkins LR, Maier SF. Activation of the ventral medial prefrontal cortex during an uncontrollable stressor reproduces both the immediate and long-term protective effects of behavioral control. Neuroscience. 2008;154:1178–1186. doi: 10.1016/j.neuroscience.2008.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amat J, Paul E, Zarza C, Watkins LR, Maier SF. Previous experience with behavioral control over stress blocks the behavioral and dorsal raphe nucleus activating effects of later uncontrollable stress: role of the ventral medial prefrontal cortex. J Neurosci. 2006;26:13264–13272. doi: 10.1523/JNEUROSCI.3630-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aston-Jones G, Shipley MT, Grzanna R. The locus coeruleus, A5 and A7 noradrenergic cell groups. In: Paxinos G, editor. The rat nervous system. Academic; New York: 1995. pp. 183–214. [Google Scholar]
- Baratta MV, Christianson JP, Gomez DM, Zarza CM, Amat J, Masini CV, Watkins LR, Maier SF. Controllable versus uncontrollable stressors bi-directionally modulate conditioned but not innate fear. Neuroscience. 2007;146:1495–1503. doi: 10.1016/j.neuroscience.2007.03.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celada P, Puig MV, Casanovas JM, Guillazo G, Artigas F. Control of dorsal raphe serotonergic neurons by the medial prefrontal cortex: involvement of serotonin-1A, GABA(A), and glutamate receptors. J Neurosci. 2001;21:9917–9929. doi: 10.1523/JNEUROSCI.21-24-09917.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charney DS. Psychobiological mechanisms of resilience and vulnerability: implications for successful adaptation to extreme stress. Am J Psychiatry. 2004;161:195–216. doi: 10.1176/appi.ajp.161.2.195. [DOI] [PubMed] [Google Scholar]
- Gabbott PL, Warner TA, Jays PR, Salway P, Busby SJ. Prefrontal cortex in the rat: projections to subcortical autonomic, motor, and limbic centers. J Comp Neurol. 2005;492:145–177. doi: 10.1002/cne.20738. [DOI] [PubMed] [Google Scholar]
- Goncalves L, Nogueira MI, Shammah-Lagnado SJ, Metzger M. Prefrontal afferents to the dorsal raphe nucleus in the rat. Brain Res Bull. 2009;78:240–247. doi: 10.1016/j.brainresbull.2008.11.012. [DOI] [PubMed] [Google Scholar]
- Grahn RE, Will MJ, Hammack SE, Maswood S, McQueen MB, Watkins LR, Maier SF. Activation of serotonin-immunoreactive cells in the dorsal raphe nucleus in rats exposed to an uncontrollable stressor. Brain Res. 1999;826:35–43. doi: 10.1016/s0006-8993(99)01208-1. [DOI] [PubMed] [Google Scholar]
- Hajos M, Richards CD, Szekely AD, Sharp T. An electrophysiological and neuroanatomical study of the medial prefrontal cortical projection to the midbrain raphe nuclei in the rat. Neuroscience. 1998;87:95–108. doi: 10.1016/s0306-4522(98)00157-2. [DOI] [PubMed] [Google Scholar]
- Jacobs BL, Azmitia EC. Structure and function of the brain serotonin system. Physiol Rev. 1992;72:165–229. doi: 10.1152/physrev.1992.72.1.165. [DOI] [PubMed] [Google Scholar]
- Jankowski MP, Sesack SR. Prefrontal cortical projections to the rat dorsal raphe nucleus: ultrastructural features and associations with serotonin and gamma-aminobutyric acid neurons. J Comp Neurol. 2004;468:518–529. doi: 10.1002/cne.10976. [DOI] [PubMed] [Google Scholar]
- Jodo E, Aston-Jones G. Activation of locus coeruleus by prefrontal cortex is mediated by excitatory amino acid inputs. Brain Res. 1997;768:327–332. doi: 10.1016/s0006-8993(97)00703-8. [DOI] [PubMed] [Google Scholar]
- Jodo E, Chiang C, Aston-Jones G. Potent excitatory influence of prefrontal cortex activity on noradrenergic locus coeruleus neurons. Neuroscience. 1998;83:63–79. doi: 10.1016/s0306-4522(97)00372-2. [DOI] [PubMed] [Google Scholar]
- Lee HS, Kim MA, Waterhouse BD. Retrograde double-labeling study of common afferent projections to the dorsal raphe and the nuclear core of the locus coeruleus in the rat. J Comp Neurol. 2005;481:179–193. doi: 10.1002/cne.20365. [DOI] [PubMed] [Google Scholar]
- Maier SF, Seligman ME. Learned helplessness: theory and evidence. J Exp Psychol Gen. 1976;105:3–46. [Google Scholar]
- Maier SF, Watkins LR. Stressor controllability and learned helplessness: the roles of the dorsal raphe nucleus, serotonin, and corticotropin-releasing factor. Neurosci Biobehav Rev. 2005;29:829–841. doi: 10.1016/j.neubiorev.2005.03.021. [DOI] [PubMed] [Google Scholar]
- Maswood S, Barter JE, Watkins LR, Maier SF. Exposure to inescapable but not escapable shock increases extracellular levels of 5-HT in the dorsal raphe nucleus of the rat. Brain Res. 1998;783:115–120. doi: 10.1016/s0006-8993(97)01313-9. [DOI] [PubMed] [Google Scholar]
- McDevitt RA, Szot P, Baratta MV, Bland ST, White SS, Maier SF, Neumaier JF. Stress-induced activity in the locus coeruleus is not sensitive to stressor controllability. Brain Res. doi: 10.1016/j.brainres.2009.06.017. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. Academic Press; San Diego: 1986. [DOI] [PubMed] [Google Scholar]
- Rozeske RR, Der-Avakian A, Bland ST, Beckley JT, Watkins LR, Maier SF. The medial prefrontal cortex regulates the differential expression of morphine-conditioned place preference following a single exposure to controllable or uncontrollable stress. Neuropsychopharmacology. 2009;34:834–843. doi: 10.1038/npp.2008.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sesack SR, Deutch AY, Roth RH, Bunney BS. Topographical organization of the efferent projections of the medial prefrontal cortex in the rat: an anterograde tract-tracing study with Phaseolus vulgaris leucoagglutinin. J Comp Neurol. 1989;290:213–242. doi: 10.1002/cne.902900205. [DOI] [PubMed] [Google Scholar]
- Shalev AY, Freedman S, Peri T, Brandes D, Sahar T, Orr SP, Pitman RK. Prospective study of posttraumatic stress disorder and depression following trauma. Am J Psychiatry. 1998;155:630–637. doi: 10.1176/ajp.155.5.630. [DOI] [PubMed] [Google Scholar]
- Simson PE, Weiss JM. Altered activity of the locus coeruleus in an animal model of depression. Neuropsychopharmacology. 1988;1:287–295. [PubMed] [Google Scholar]
- Varga V, Szekely AD, Csillag A, Sharp T, Hajos M. Evidence for a role of GABA interneurones in the cortical modulation of midbrain 5-hydroxytryptamine neurones. Neuroscience. 2001;106:783–792. doi: 10.1016/s0306-4522(01)00294-9. [DOI] [PubMed] [Google Scholar]
- Vertes RP. Differential projections of the infralimbic and prelimbic cortex in the rat. Synapse. 2004;51:32–58. doi: 10.1002/syn.10279. [DOI] [PubMed] [Google Scholar]
- Wang QP, Ochiai H, Nakai Y. GABAergic innervation of serotonergic neurons in the dorsal raphe nucleus of the rat studied by electron microscopy double immunostaining. Brain Res Bull. 1992;29:943–948. doi: 10.1016/0361-9230(92)90169-x. [DOI] [PubMed] [Google Scholar]
- Weiss JM. Effects of coping responses on stress. J Comp Physiol Psychol. 1968;65:251–260. doi: 10.1037/h0025562. [DOI] [PubMed] [Google Scholar]
- Weiss JM, Simson PG. Depression in an animal model: focus on the locus ceruleus. Ciba Found Symp. 1986;123:191–215. doi: 10.1002/9780470513361.ch11. [DOI] [PubMed] [Google Scholar]
- Williams JL, Maier SF. Transituational immunization and therapy of learned helplessness in the rat. J Exp Psychol Anim Behav Process. 1977;3:240–252. [Google Scholar]
- Yehuda R, Flory JD, Southwick S, Charney DS. Developing an agenda for translational studies of resilience and vulnerability following trauma exposure. Ann N Y Acad Sci. 2006;1071:379–396. doi: 10.1196/annals.1364.028. [DOI] [PubMed] [Google Scholar]