Abstract
The present study assessed food cravings in a cohort of 229 women who differed in smoking history (i.e., never smoker, former smoker, and current smoker) and body weight (i.e., normal weight, overweight, and obese). Each subject completed the Food Craving Inventory (FCI), which measures cravings for sweets, high fats, carbohydrates/starches, and fast-food fats, and the Profile of Mood States (POMS), which measures psychological distress. Smoking and obesity were independently associated with specific food cravings and mood states. Current smokers craved high fats more frequently than former and never smokers. They also craved starches more frequently and felt more depressed and angry than never smokers, but not former smokers. Whereas cravings for starchy foods and some mood states may be characteristic of women who are likely to smoke, more frequent cravings for fat among smokers is related to smoking per se. Similarly, obese women craved high fats more frequently than nonobese women and depression symptoms were intensified with increasing body weights. We hypothesize that the overlapping neuroendocrine alterations associated with obesity and smoking and the remarkable similarities in food cravings and mood states between women who smoke and women who are obese suggest that common biological mechanisms modulate cravings for fat in these women.
Introduction
Cigarette smoking is intimately related with body weight (see ref (1) for a review). Although the relationship is somewhat obscure in individuals who are currently smoking (some studies report smokers weigh less than nonsmokers (2), whereas others find they weigh the same (3)), men and women typically gain weight after they quit smoking (4). Especially for women, concern about gaining weight is a powerful deterrent to quitting and an incitement for relapse to smoking (5). The specific mechanisms underlying weight gain after smoking cessation remain unknown. Although changes in energy expenditure may account for some weight gain (6), most is due to greater caloric intake (7).
One reason why some women eat more after they quit smoking is because they confuse cravings for cigarettes with cravings for foods, and hunger (7,8). When abstaining from smoking, not only do women give in to these food cravings (7), but as cravings for cigarettes become more intensified, so do cravings for starchy carbohydrates and fats (9).
Another reason they eat more, especially highly palatable foods when abstaining from smoking, is to improve their dysphoric moods. Anxiety, depression and irritability are common symptoms of nicotine withdrawal and are manifested within 2–12 h after smoking the last cigarette (10). Thus, smokers experience daily periods of stress and symptoms of abstinence between cigarettes (11). Smokers may prefer to eat fatty and starchy foods to relieve withdrawal symptoms (12), which may be due to decreased serotoninergic and dopaminergic functioning observed during nicotine withdrawal (11,13). Eating diets poor in protein but rich in carbohydrates or fat increases brain serotonin (13,14) and inhibits corticotrophin-releasing factor (15). Such neurochemical and neurohormonal changes reduce stress and produce feelings of gratification and pleasure (15). Of interest is the finding that smokers living in many cultures throughout the world typically eat diets high in fat and energy, despite the extreme variability in food availability and cultural food habits (3,16,17).
The present study was crafted from the perspective that cravings for cigarettes and foods are closely linked in smokers and may reflect the efficacy of both stimuli in altering moods (18). The main objective of the present study was to determine whether the more frequent cravings for starchy foods and high fat foods by female smokers (9) were related to smoking per se, or a characteristic of women who were likely to smoke. To this end, we tested women who were current smokers as well as two control groups of women, one of which never smoked in their lifetime and the other which were former smokers and had not smoked for at least the last 6 months. Because obese individuals, like smokers, crave high fat foods more often than lean individuals (19), and because obesity, at least for women, is associated with depressive symptoms (20), we explored whether the associations between specific food cravings, smoking status, and mood were uniform among body weight categories.
Methods and Procedures
Subjects
The study population consisted of 229 women (55.7% black, 35.7% white, 8.6% mixed or other races) who participated in research studies on flavor perception and preferences at the Monell Chemical Senses Center from 2002 to 2004. Some of the data from these women have been reported elsewhere (21,22). All the women reported being healthy and were not on any medications with the exception of birth control pills at the time of testing. Twenty-two additional women participated in these studies but were excluded because of incomplete data (N = 8), or because they were underweight (N = 8), or pregnant (N = 6).
Smoking history was obtained by asking subjects the following questions: Do you smoke cigarettes? How old were you when you smoked your first cigarette? How old were you when you first started regular daily cigarette smoking? How many cigarettes are you currently smoking per day? When smoking the heaviest, how many cigarettes did you smoke per day? (2). Whenever subjects answered negatively to the first question, they were then asked if they had ever smoked, and if so, how much they had smoked and when they quit. From this data, three groups were formed: current smoker (N = 61), former smoker (N = 55), and never smoker (N = 113). Former smokers quit smoking at least 6 months before the study; whereas, never smokers had not smoked >20 cigarettes in their lifetime. All testing procedures were approved by the Office of Regulatory Affairs at the University of Pennsylvania and each woman gave written informed consent prior to testing.
Procedures
Each subject was tested at the Monell Center in a private testing room. They completed the Food Craving Inventory (FCI), a 28-item validated questionnaire designed to measure the frequency of overall food cravings as well as cravings for specific types of foods (19,23,24). Cravings for specific types of foods (i.e., an intense desire for a specific food that is difficult to resist) were measured by four independent subscales, each consisting of 4–8 items within the food category: high fats (i.e., fried chicken, gravy, sausage, hot dogs, fried fish, corn bread, bacon, steaks); sweets (i.e., cakes, cinnamon rolls, ice cream, cookies, chocolate, donuts, candy, brownies); carbohydrates/starches (i.e., sandwich bread, rice, biscuits, pasta, pancakes/waffles, rolls, cereal, baked potato); and fast-food fats (i.e., pizza, french fries, hamburger, chips). Participants rated how often they experienced a craving for each of the foods over the past month using a 5-point Likert scale (1 = never, 5 = always/almost every day). In addition to the four independent subscales, an average of the four was calculated and represents the general food craving score.
Subjects completed the 65-item Profile of Mood States Questionnaire (POMS) (25), which measures six independent, transient mood states: tension (range 0–36), depression (range 0–60), anger (range 0–48), vigor (range 0–32), fatigue (range 0–28), and confusion (range 0–28); higher scores reflect higher intensity of the mood states. They also completed the 21-item Beck Depression Inventory (BDI), which measures characteristic attitudes and symptoms of depression (26). Scores could range from 0 to 63 and a score of ≥10 are usually consistent with mild to moderate depression.
Height and weight were measured with women wearing light clothing and no shoes (Detecto Model 439, Physician Scale; Webb City, MO). BMI (the weight in kilograms divided by the square of the height in meters) was then computed and categorized as follows: 18.5–24.9 kg/m2 (normal weight), 25.0–29.9 kg/m2 (overweight), and ≥30.0 kg/m2 (obese) (27).
Data analyses
The dependent measures included the general food craving score as well as the craving scores for the four types of foods (high fats, carbohydrates/starches, sweets and fast-food fats), the six subscales of the POMS (tension, depression, anger, vigor, fatigue, and confusion) and BDI scores. For general food cravings and BDI scores, separate two-way ANOVAs were conducted with group (never smoker, former smoker, and current smoker) and BMI category (normal weight, overweight, obese) as the between subjects factors. For FCI and POMS data, separate repeated measure ANOVAs were conducted. In these analyses, group and BMI category were modeled as between subject factors and the cravings scores for the four types of foods or the six subscales of the POMS were modeled as repeated measure factors. Age was covaried in all analyses containing POMS data, as recommended (25). When ANOVAs revealed significant effects, post hoc Fisher Least Significant Difference analyses were conducted. Pearson correlation coefficients were used to determine whether relationships existed between food craving scores, BMI and mood states among the groups. All analyses were performed with STATISTICA 8.0 (StatSoft, Tulsa, OK), and the criterion for statistical significance was P < 0.05. The magnitude of group differences was further determined by calculating effect sizes using Cohen's d (28). Effect sizes make it possible to judge the importance of a group difference and was judged against standard criteria proposed by Cohen: trivial (<0.2), small (0.2 < 0.5), medium (0.5 < 0.8), and large (≥0.8).
Results
Subject characteristics
Sixty-one (26.6%) of the 229 women currently smoked cigarettes. These women reported that they had been smoking for 19 ± 7 (range 1–35) years and were currently smoking 8 ± 7 (range 1–30) cigarettes daily. They first experimented with a cigarette when they were 16 ± 4 years of age but did not begin smoking regularly until they were 18 ± 4 (range 5–31) years of age. Fifty-five women were former smokers. They quit smoking 7 ± 4 (range 0.5–12) years ago. At the time of quitting, they were smoking on average, 11 ± 10 (range 1–40) cigarettes daily. The never smoker group consisted of 113 women who either never smoked a cigarette (N = 95) or smoked <20 cigarettes (N = 18) in their lifetime. As shown in Table 1, there were no significant group differences in age, BMI, or parity. However, current smokers had lower income levels (χ2 (4 degrees of freedom (df) = 18.60; P = 0.001), fewer years of education (F (2, 226) = 7.62; P = 0.001), and scored higher on the BDI (F (2, 226) = 4.91; P = 0.008) than never and former smokers.
Table 1. Subjects Characteristics.
| Never smoker (N = 113) | Former smoker (N = 55) | Current smoker (N = 61) | Statistical analyses (P value) | |
|---|---|---|---|---|
| Age mean (s.d.) | 34.4 (6.5) | 36.5 (6.3) | 35.3 (5.5) | 0.11 |
| BMI mean (s.d.) | 25.9 (8.1) | 29.5 (6.5) | 28.1 (6.2) | 0.43 |
| BMI category (percentage of group) | 0.58 | |||
| Normal weight | 33.6% | 41.0% | 30.9% | |
| Overweight | 33.6% | 26.2% | 27.3% | |
| Obese | 32.7% | 32.8% | 41.8% | |
| Yearly income (percentage of group) | 0.001 | |||
| <$20,000 | 26.6%a | 18.9%a | 53.3%b | |
| $20,000 to $50,000 | 45.1%a | 43.4%a | 28.3%b | |
| >$50,000 | 28.3%a | 37.7%a | 18.3%b | |
| Years of education mean (s.d.) | 14.2 (2.1)a | 13.8 (1.8)a | 12.9 (2.4)b | 0.001 |
| Parity mean (s.d.) | 2.8 (1.5) | 2.8 (1.3) | 2.9 (1.4) | 0.96 |
| BDI score mean (s.d.) | 4.9 (4.9)a | 5.8 (5.8)a | 8.3 (9.7)b | 0.01 |
| Depressed, as determined by BDI (percentage of group) | 16.8% | 20.0% | 26.2% | 0.33 |
Significant P values are in bold.
Values in a row with different superscript letters are significantly different from each other.
Values in a row with different superscript letters are significantly different from each other.
BMI, body mass index; BDI, Beck Depression Inventory.
Food craving patterns
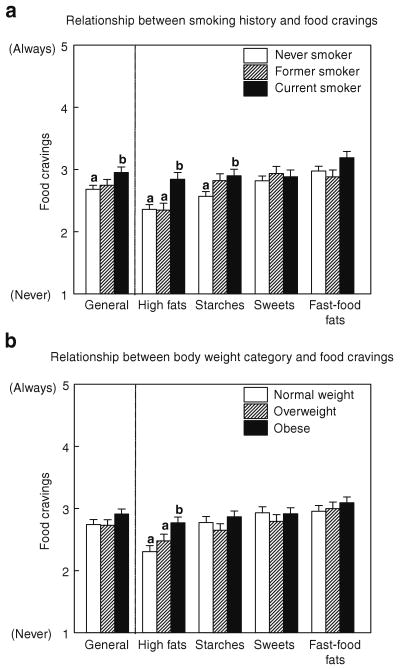
Although there were no interactions between group and body weight category, we found that food craving patterns were significantly and independently affected by each (group effect: (F (6, 660) = 4.42; P < 0.001); body weight category effect: (F (6, 660) = 3.18; P < 0.005)). This ANOVA yielded similar findings when income levels and years of education were used as covariates. As shown in Figure 1a, current smokers craved high fat foods more frequently than never and former smokers (Cohen's d = 0.5) and starches more frequently than never smokers (Cohen's d = 0.4). Former smokers craved starches at intermediate frequencies between current and never smokers. There was no significant relationship between the number of years since they quit smoking and how often they craved high fats or starches (P > 0.25). Nor were there significant differences among the three groups of women in the reported frequency of cravings for sweets, carbohydrates or fast-food fats. However, current smokers craved foods in general more often than never smokers (F (2, 220) = 3.14; P < 0.05; Cohen's d = 0.4).
Figure 1.
Frequency of general food cravings and cravings for high fat, starches, sweets, and fast-food fats during past month. (a) Relationship between smoking history and food cravings. (b) Relationship between body weight category and food cravings. Panel a presents the effects of smoking history: never smoker (white solid bars), former smoker (hatched bars) and current smoker (black solid bars) and panel b presents the effects of body weight category: Normal weight (white solid bars), Overweight (hatched bars) and Obese (black solid bars) on food cravings. Data are presented as Mean (±s.e.m.). Different superscript letters (i.e., a,b) are significantly different from each other at P < 0.05 level (LSD test).
Figure 1b shows that obese women craved high fat foods more frequently than normal-weight and overweight women (Cohen's d = 0.6). Cravings for specific types of foods (e.g., high fat, starches) as well as cravings for foods in general were significantly correlated with BMI in women who never smoked in their lifetimes (r (113 df) = 0.32; P = 0.001; r (113 df) = 0.20; P = 0.036; and r (113 df) = 0.21; P = 0.03, respectively). No such correlations were observed in the other two groups (former or current smokers; all P's > 0.14).
Mood states
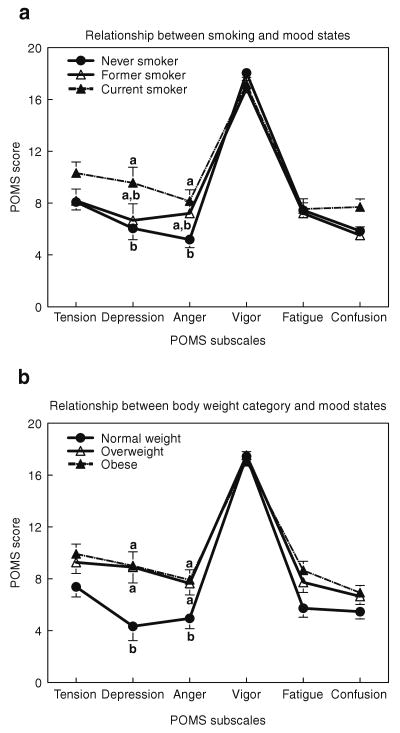
Although there were no interactions between group and body weight category, we found that mood states were significantly and independently affected by smoking group (POMS: F (10, 1095) = 2.26, P = 0.01; BDI: F (2, 220) = 5.71, P = 0.004) and BMI categories (POMS: F (10, 1095) = 2.17, P = 0.02; BDI: F (2, 220) = 5.71, P = 0.004). The results of these analyses remained the same when income levels and years of education were used as covariates. Current smokers felt more depressed and angry (Cohen's d = 0.4 for both), and tended to be more tense (P = 0.06) and confused (P = 0.09) than never smokers. Former smokers tended to score higher than never smokers on anger (P = 0.06) and lower than current smokers on depression (P = 0.07) (Figure 2a). As shown in Figure 2b, obese and overweight women felt more depressed (Cohen's d = 0.5) and angry (Cohen's d = 0.4) than normal-weight women.
Figure 2.
Mean response scores for tension, depression, anger, vigor, fatigue, and confusion, as determined by the Profile of Mood States. (a) Relationship between smoking and mood states. (b) Relationship between body weight category and mood states. Panel a presents the effects of smoking history: never smoker (closed circles), former smoker (open triangles) and current smoker (closed triangles) and panel b presents the effects of body weight category: Normal weight (closed circles), Overweight (open triangles) and Obese (closed triangles) on mood states. Data are presented as Mean (±s.e.m.). Different superscript letters are significantly different from each other at P < 0.05 level (LSD test).
Only among current smokers did we find significant correlations between BDI and BMI (r (61 df) = 0.33; P = 0.008) and a tendency for BDI to correlate with cravings for high fat foods (r (61 df) = 0.24; P = 0.06). The more depressed the women, the higher the BMI and the more frequent the cravings for high fat foods.
Discussion
Cravings for specific foods were related to the consequences of smoking per se and were not simply a characteristic of women who were likely to smoke. Consistent with previous findings (9), smokers craved foods, particularly those that are high in fat and starches, more often than women who never smoked in their lifetime. Former smokers craved high fat foods as infrequently as never smokers, suggesting that frequent cravings for high fat foods among smokers is related to effects of smoking per se. Among former smokers, we found no relationship between cravings for high fat (or starchy) foods and number of years since they quit smoking cigarettes, which was no <0.5 years prior to testing. This suggests that reductions in cravings for high fat foods occur within the immediate months after quitting smoking. That former smokers and current smokers craved starchy foods, like pasta and rice, more often and were different in their POMS scores than never smokers, suggests that some food cravings may be a characteristic of women who are likely to smoke, perhaps because they eat these foods to improve their moods (13,14).
In the present study, the FCI was used because it is one of a few psychometrically validated measures of food cravings (19), a subjective state that can motivate behavior (29). Although patterns of nutrient intake were not assessed, previous research revealed that the more frequent the cravings for specific foods, the more likely the person would “give in” to those cravings and consume that type of food (19,24). Further, data collected from 19 countries (i.e., United States, Bangladesh, China, Turkey, Australia, Finland, United Kingdom, Israel, Norway, Italy, Scotland, Sweden, Switzerland, Netherlands, Canada, Northern Ireland, France, New Zealand, China) revealed that despite different cultures, lifestyles and food habits, smokers consume more fat than nonsmokers (3,16,17). Based on these data, we would expect that the more frequent cravings for foods high in fat and starches reported by women in the present study will likely lead to increased consumption of these foods.
Several explanations, not mutually exclusive, are presented to explain the association between smoking and food cravings. First, the types of foods women who smoke crave and presumably eat, may be a consequence of tobacco damage to the sensory systems underlying flavor perception. Over time, smokers exhibited deficits in sweet (9) and bitter (30) taste perception as well as odor sensitivity (31). Tobacco use can damage the surface epithelium of the oral cavity (e.g., irritate salivary glands), and increase the risk for periodontal disease (32) and bacterial and viral infections in the oral and nasal cavities (33). Furthermore, chronic infections in the oral and nasal cavities increase the risk of developing ear infections (i.e., otitis media) and lead to damage peripheral nerves carrying taste information to the brain. Of particular interest are the recent findings by Bartoshuk and colleagues that adults with histories of severe otitis media had significantly higher BMIs and increased preferences for high-fat foods (34). Thus, heightened fat preferences may be the link between smoking and obesity.
Second, women who smoked were less educated and had lower income levels than those who did not smoke, a finding that is in agreement with worldwide trends in smoking (35). Because fats as well as sugars, constitute one of the most palatable and low-priced nutrients, smokers may consume and crave these foods because of affordability (36). However, the greater fat intake among smokers persisted after controlling for socio-demographic factors such as cultural environments (16) and education levels (17). Likewise, the more frequent cravings for high fat and starches among smokers in the present study persisted after adjustment for income level and years of education.
Third, we found that women who smoke felt more depressed than never smokers and tended to feel more depressed than former smokers. Because eating foods high in fat and carbohydrates produce feelings of gratification and pleasure (15), smokers may eat these foods to improve their dysphoric moods. Of interest was the tendency that the more depressed the woman was, the more frequently she craved high fat foods.
Independent of smoking history, obese women living in Philadelphia craved high fat foods more frequently than normal-weight or overweight women when assessed with the FCI. Similar findings have been previously reported for men and women living in the Southern United States (19). This suggests that the FCI is a tool that can measure food craving patterns in different geographic areas, at least within the United States (23). Also independent of smoking history, women in the obese and overweight groups felt more depressed and angrier than the women in the normal weight group, findings that are consistent with a recent study reporting that BMI was significantly related to depressive symptomatology in women (20). Such findings suggest that public health approaches aimed at reducing the burden of obesity or depression should consider the strong comorbidity between these two conditions (20).
Smoking and obesity were independently associated with certain mood states such as depression and anger as well as more frequent cravings for high fat foods. We hypothesize that these shared features are manifestations of similar alterations in the brain chemistry and metabolism of smokers and obese individuals. Several lines of evidence support this hypothesis. At the central level, obesity, like drug addiction, is associated with a reduction of dopamine in certain areas of the brain underlying reward, leading some to postulate that these disorders reflect a “reward deficiency syndrome” (37). Further, some of the same brain areas (e.g., striatum, orbitofrontal cortex) that are activated during drug cravings in addicted individuals are exaggeratedly activated during visual exposure to palatable foods as well as during sensations of stomach fullness in obese individuals (38,39). At the metabolic level, both smoking and obesity activate neuroendocrine and inflammatory responses that over time increase the risk of developing diabetes (1,40), a disorder known to be associated with depression (41). More knowledge of how smoking, obesity and mood states affect the perception of food-related cues may assist in designing programs to help women, a population especially concerned with weight gain, to stop smoking as well as to lose weight.
Acknowledgments
This project was funded by NIH Grant AA09523 and a grant from the Pennsylvania Department of Health. The Pennsylvania Department of Health specifically disclaims responsibility for any analyses, interpretations, or conclusions. Dr. Pepino is currently a fellow of a NIDA T32 DA07313 grant at the Department of Psychiatry and Center for Human Nutrition at Washington University School of Medicine, St Louis, MO 63108.
Footnotes
Disclosure: The authors declared no conflict of interest.
References
- 1.Chiolero A, Faeh D, Paccaud F, Cornuz J. Consequences of smoking for body weight, body fat distribution, and insulin resistance. Am J Clin Nutr. 2008;87:801–809. doi: 10.1093/ajcn/87.4.801. [DOI] [PubMed] [Google Scholar]
- 2.Albanes D, Jones DY, Micozzi MS, Mattson ME. Associations between smoking and body weight in the US population: analysis of NHANES II. Am J Public Health. 1987;77:439–444. doi: 10.2105/ajph.77.4.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Palaniappan U, Jacobs Starkey L, O'Loughlin J, Gray-Donald K. Fruit and vegetable consumption is lower and saturated fat intake is higher among Canadians reporting smoking. J Nutr. 2001;131:1952–1958. doi: 10.1093/jn/131.7.1952. [DOI] [PubMed] [Google Scholar]
- 4.Williamson DF, Madans J, Anda RF, et al. Smoking cessation and severity of weight gain in a national cohort. N Engl J Med. 1991;324:739–745. doi: 10.1056/NEJM199103143241106. [DOI] [PubMed] [Google Scholar]
- 5.Perkins KA. Smoking cessation in women. Special considerations. CNS Drugs. 2001;15:391–411. doi: 10.2165/00023210-200115050-00005. [DOI] [PubMed] [Google Scholar]
- 6.Hellerstein MK, Benowitz NL, Neese RA, et al. Effects of cigarette smoking and its cessation on lipid metabolism and energy expenditure in heavy smokers. J Clin Invest. 1994;93:265–272. doi: 10.1172/JCI116955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kos J, Hasenfratz M, Battig K. Effects of a 2-day abstinence from smoking on dietary, cognitive, subjective, and physiologic parameters among younger and older female smokers. Physiol Behav. 1997;61:671–678. doi: 10.1016/s0031-9384(96)00518-5. [DOI] [PubMed] [Google Scholar]
- 8.West R. Glucose for smoking cessation: does it have a role? CNS Drugs. 2001;15:261–265. doi: 10.2165/00023210-200115040-00001. [DOI] [PubMed] [Google Scholar]
- 9.Pepino MY, Mennella JA. Effects of cigarette smoking and family history of alcoholism on sweet taste perception and food cravings in women. Alcohol Clin Exp Res. 2007;31:1891–1899. doi: 10.1111/j.1530-0277.2007.00519.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hughes JR, Higgins ST, Bickel WK. Nicotine withdrawal versus other drug withdrawal syndromes: similarities and dissimilarities. Addiction. 1994;89:1461–1470. doi: 10.1111/j.1360-0443.1994.tb03744.x. [DOI] [PubMed] [Google Scholar]
- 11.Benowitz NL. Clinical pharmacology of nicotine: implications for understanding, preventing, and treating tobacco addiction. Clin Pharma Ther. 2008;83:531–541. doi: 10.1038/clpt.2008.3. [DOI] [PubMed] [Google Scholar]
- 12.Spring B, Wurtman J, Wurtman R, et al. Efficacies of dexfenfluramine and fluoxetine in preventing weight gain after smoking cessation. Am J Clin Nutr. 1995;62:1181–1187. doi: 10.1093/ajcn/62.6.1181. [DOI] [PubMed] [Google Scholar]
- 13.Berlin I, Vorspan F, Warot D, Maneglier B, Spreux-Varoquaux O. Effect of glucose on tobacco craving. Is it mediated by tryptophan and serotonin? Psychopharmacology (Berl) 2005;178:27–34. doi: 10.1007/s00213-004-1980-x. [DOI] [PubMed] [Google Scholar]
- 14.Fernstrom JD, Wurtman RJ. Brain serotonin content: increase following ingestion of carbohydrate diet. Science. 1971;174:1023–1025. doi: 10.1126/science.174.4013.1023. [DOI] [PubMed] [Google Scholar]
- 15.Dallman MF, Pecoraro N, Akana SF, et al. Chronic stress and obesity: a new view of “comfort food”. Proc Natl Acad Sci USA. 2003;100:11696–11701. doi: 10.1073/pnas.1934666100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dallongeville J, Marecaux N, Fruchart JC, Amouyel P. Cigarette smoking is associated with unhealthy patterns of nutrient intake: a meta-analysis. J Nutr. 1998;128:1450–1457. doi: 10.1093/jn/128.9.1450. [DOI] [PubMed] [Google Scholar]
- 17.Koh WP, Yuan JM, Sun CL, Lee HP, Yu MC. Middle-aged and older Chinese men and women in Singapore who smoke have less healthy diets and lifestyles than nonsmokers. J Nutr. 2005;135:2473–2477. doi: 10.1093/jn/135.10.2473. [DOI] [PubMed] [Google Scholar]
- 18.Hill AJ, Weaver CF, Blundell JE. Food craving, dietary restraint and mood. Appetite. 1991;17:187–197. doi: 10.1016/0195-6663(91)90021-j. [DOI] [PubMed] [Google Scholar]
- 19.White MA, Whisenhunt BL, Williamson DA, Greenway FL, Netemeyer RG. Development and validation of the food-craving inventory. Obes Res. 2002;10:107–114. doi: 10.1038/oby.2002.17. [DOI] [PubMed] [Google Scholar]
- 20.Simon GE, Ludman EJ, Linde JA, et al. Association between obesity and depression in middle-aged women. Gen Hosp Psychiatry. 2008;30:32–39. doi: 10.1016/j.genhosppsych.2007.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mennella JA, Pepino MY, Reed DR. Genetic and environmental determinants of bitter perception and sweet preferences. Pediatrics. 2005;115:e216–e222. doi: 10.1542/peds.2004-1582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pepino MY, Mennella JA. Sucrose-induced analgesia is related to sweet preferences in children but not adults. Pain. 2005;119:210–218. doi: 10.1016/j.pain.2005.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.White MA, Grilo CM. Psychometric properties of the Food Craving Inventory among obese patients with binge eating disorder. Eat Behav. 2005;6:239–245. doi: 10.1016/j.eatbeh.2005.01.001. [DOI] [PubMed] [Google Scholar]
- 24.Martin CK, O'Neil PM, Tollefson G, Greenway FL, White MA. The association between food cravings and consumption of specific foods in a laboratory taste test. Appetite. 2008;51:324–326. doi: 10.1016/j.appet.2008.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McNair DM, Lorr M, Droppleman LF. Revised Manual for the Profile of Mood States. Educational and Industrial Testing Services; San Diego, CA: 1992. [Google Scholar]
- 26.Beck AT, Steer RA, Garbin MG. Psychometric properties of the Beck Depression Inventory: Twenty-five years of evaluation. Clin Psychol Rev. 1988;8:77–100. [Google Scholar]
- 27.World Health Organization. Physical status: the use and interpretation of anthropometry. Report of a WHO Expert Committee. World Health Organization; Geneva: 1995. [PubMed] [Google Scholar]
- 28.Cohen J. Statistical Power Analysis for the Behavioral Sciences. Second. Lawrence Erlbaum; Hillsdale, NJ: 1988. [Google Scholar]
- 29.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. Fourth. American Psychiatric Association; Washington, DC: 1994. [Google Scholar]
- 30.Kaplan AR, Glanville EV, Fischer R. Taste thresholds for bitterness and cigarette smoking. Nature. 1964;202:1366. doi: 10.1038/2021366a0. [DOI] [PubMed] [Google Scholar]
- 31.Frye RE, Schwartz BS, Doty RL. Dose-related effects of cigarette smoking on olfactory function. JAMA. 1990;263:1233–1236. [PubMed] [Google Scholar]
- 32.Taybos G. Oral changes associated with tobacco use. Am J Med Sci. 2003;326:179–182. doi: 10.1097/00000441-200310000-00005. [DOI] [PubMed] [Google Scholar]
- 33.Arcavi L, Benowitz NL. Cigarette smoking and infection. Arch Intern Med. 2004;164:2206–2216. doi: 10.1001/archinte.164.20.2206. [DOI] [PubMed] [Google Scholar]
- 34.Bartoshuk LM, Duffy VB, Hayes JE, Moskowitz HR, Snyder DJ. Psychophysics of sweet and fat perception in obesity: problems, solutions and new perspectives. Philos Trans R Soc Lond B Biol Sci. 2006;361:1137–1148. doi: 10.1098/rstb.2006.1853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.World Health Organization. WHO Report on the Global Tobacco Epidemic: The MPower package. World Health Organization; Geneva: 2008. [Google Scholar]
- 36.Darmon N, Drewnowski A. Does social class predict diet quality? Am J Clin Nutr. 2008;87:1107–1117. doi: 10.1093/ajcn/87.5.1107. [DOI] [PubMed] [Google Scholar]
- 37.Wang GJ, Volkow ND, Logan J, et al. Brain dopamine and obesity. Lancet. 2001;357:354–357. doi: 10.1016/s0140-6736(00)03643-6. [DOI] [PubMed] [Google Scholar]
- 38.Stoeckel LE, Weller RE, Cook EW, 3rd, et al. Widespread reward-system activation in obese women in response to pictures of high-calorie foods. Neuroimage. 2008;41:636–647. doi: 10.1016/j.neuroimage.2008.02.031. [DOI] [PubMed] [Google Scholar]
- 39.Wang GJ, Yang J, Volkow ND, et al. Gastric stimulation in obese subjects activates the hippocampus and other regions involved in brain reward circuitry. Proc Natl Acad Sci USA. 2006;103:15641–15645. doi: 10.1073/pnas.0601977103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ozcan U, Cao Q, Yilmaz E, et al. Endoplasmic reticulum stress links obesity, insulin action, and type 2 diabetes. Science. 2004;306:457–461. doi: 10.1126/science.1103160. [DOI] [PubMed] [Google Scholar]
- 41.Golden SH, Lazo M, Carnethon M, et al. Examining a bidirectional association between depressive symptoms and diabetes. JAMA. 2008;299:2751–2759. doi: 10.1001/jama.299.23.2751. [DOI] [PMC free article] [PubMed] [Google Scholar]