Abstract
This study examined the role of dopamine D1-like receptor transmission in the lateral hypothalamus (LH) in flavor preference learning induced by intragastric (IG) infusions of glucose. Rats fitted with gastric catheters were injected daily in the LH with either saline or SCH23390 (12 nmol/brain), 10 min prior to training sessions with a flavor (CS+) paired with IG infusions of 8% glucose and a different flavor (CS−) paired with IG water infusions. In a post-training two-bottle test, SCH-treated rats preferred the CS+ to the CS− although their preference was weaker than that of the Control rats (61% vs. 87%). The same dose of SCH23390 reduced CS+ intake of the Control rats in a subsequent test but did not suppress their CS+ preference (90%). These results show that D1-like receptor activation in the lateral hypothalamus modulates the acquisition, but not the expression of flavor preference learning induced by the post-oral reinforcing properties of glucose.
Keywords: Carbohydrate, Conditioning, Forebrain, Learning, Mesolimbic, SCH23390
Learning plays an important role in the development of food and fluid preferences. If animals consume a food with an unfamiliar flavor (taste, odor, texture) and experience visceral malaise, they readily learn to avoid that flavor at subsequent exposures. This form of learning is well documented (Bures, Bermúdez-Rattoni, & Yamamoto, 1998), and is referred to as conditioned taste or conditioned flavor aversion when pure gustatory or complex flavor cues are used, respectively. Animals also learn to prefer the flavor of foods and fluids that provide positive nutritional consequences. This is documented by numerous studies showing that rodents acquire strong and long-lasting preferences for flavored foods and fluids that either contain a nutrient or are paired with intragastric (IG) infusions of nutrients (Capaldi, 1996; Sclafani, 1999).
Learned flavor preferences, like conditioned flavor aversions, are forms of classical conditioning in which associations are formed between a flavor cue (conditioned stimulus, CS) and the oral and/or post-oral reinforcing properties of nutrients (unconditioned stimulus, US). The neural substrates of taste or flavor aversion learning have been extensively investigated (Bures et al., 1998; Scalera, 2002), but much less is known about the brain mechanisms underlying learned flavor preferences. We recently investigated the role of the lateral hypothalamus (LH) in favor preferences induced by the postingestive action of nutrients, referred to here as flavor-nutrient conditioning, given that the LH has converging orosensory and viscerosensory inputs and its important role in the control of food intake (Bernardis & Bellinger, 1993; Berthoud, 2002; Norgren, 1976). Excitotoxic LH lesions attenuated flavor preference learning induced by concurrent intragastric (IG) carbohydrate infusions and totally blocked learning when the flavor was paired with delayed nutrient infusions. The same lesions also attenuated flavor avoidance learning induced by IG lithium chloride (Touzani & Sclafani, 2001; Touzani & Sclafani, 2002). The cellular mechanisms by which the LH modulates flavor learning are not certain but dopamine transmission is implicated. That is, dopamine D1-like but not D2-like receptor antagonism in the LH attenuated taste avoidance learning (Caulliez, Meile, & Nicolaidis, 1996; Fenu, Bassareo, & Di Chiara, 2001). We previously reported that systemic administration of a D1-like receptor antagonist impaired flavor-nutrient preference learning (Azzara, Bodnar, Delamater, & Sclafani, 2001), and in the present study we determined if this learning deficit involves D1-like receptors in the LH. To this end, the D1-like receptor antagonist, SCH23390, was infused in the LH during the acquisition or expression phases of flavor preferences conditioned by IG infusions of glucose. In the light of our previous findings with LH lesions (Touzani and Sclafani, 2001; Touzani and Sclafani, 2002) and systemic D1-like receptor antagonism (Azzara et al., 2001), we predicted that antagonism of LH D1-like receptors would retard the acquisition of a glucose-conditioned flavor preference but would have only a marginal effect on the expression of a previously learned flavor preference.
Twenty five adult male Sprague-Dawley rats (Charles River Laboratories, Wilmington, MA) were used. They weighed 408–522 g at the time of the surgery. The rats were individually housed in plastic tub cages in a vivarium maintained at 21°C and under a 12:12 h light:dark cycle (lights on at 0800h). They were given chow (Laboratory Rodent Diet 5001, PMI Nutrition International, Brentwood, MO) and tap water. Experimental protocols were approved by Brooklyn College Animal Care and Use Committee and were performed in accordance with the NIH Guidelines for the Care and Use of Laboratory Animals. All materials, procedures and testing apparatus are described in detail elsewhere (Touzani and Sclafani, 2001; Touzani, Bodnar, & Sclafani, 2008).
Under anesthesia, the rats underwent stereotaxic bilateral implantation of guide-cannulae (26-gauge, Plastics One Inc. Roanoke, VA) aimed above the LH (AP, −2.6 mm from bregma; ML, ±1.8 mm from midline, and DV, −6.8 mm from the skull surface) using a flat-skull position. The guide-cannulae were secured on the skull with stainless steel screws and dental cement. During the same surgery session, the rats were fitted with a gastric catheter as described by Touzani et al. (2008). Intramuscular penicillin (30,000 U) was given following the surgery.
The dopamine D1-like receptor antagonist, SCH23390 (Sigma Chemical Company, St. Louis, MO) was dissolved in sterile isotonic saline (vehicle) and administered at a volume of 0.5 μl/side. Infusions of the drug or the vehicle into the LH were performed bilaterally using an infusion pump and a 33-gauge stainless steel internal cannula (Plastics one, Roanoke, VA) connected to a 2-μl Hamilton microsyringe by polyethylene tubing. The tip of the internal cannulae protruded 2.0 mm beyond that of the guide. The injections were made at the rate of 0.5 μl/min and the cannulae were left in place for one more minute before their removal.
Two to three weeks after the surgery, the rats were placed on a food restriction schedule and maintained at 85% of their ad libitum body weights. They were adapted to drink an unflavored 0.2% sodium saccharin (Sigma, St. Louis, MO) solution in the test cages during 8–10 daily 30-min sessions. During the last four sessions, the rats were adapted to a mock injection, connected to the infusion system and given IG water infusions as they drank the saccharin solution. The rats were then trained to drink flavored saccharin solutions (0.05% grape or cherry Kool-Aide) with one flavor (the CS+) paired with IG infusions of 8% glucose and the other flavor (CS−) paired with IG water infusions during eight alternating one-bottle training sessions; infusion volumes were 8 ml/session. Rats in the SCH group received injections of 12 nmol SCH23390 (6 nmol/0.5 μl/side) in the LH, 10 min prior to each of the CS+ and CS− training sessions. The Control rats were injected with saline during training and their CS intakes were limited each session to the mean intake of the SCH rats, which had unrestricted access to the solutions. Following training, three two-bottle preference tests were conducted, each involved two 30 min/day sessions with the left-right position of the CS+ and CS− solutions counterbalanced; intakes were unlimited and there were no IG infusions. In Test 1, the SCH and Control groups received no LH injections. The SCH rats were then given another two pairs of identical tests without any brain injection, whereas the Control rats received LH injections of saline (Test 2) and then SCH23390 (6 nmol/0.5 μl/side, Test 3) 10 min prior to testing. At the completion of the experiment, the rats were deeply anesthetized and perfused transcardially with physiological saline followed by a 10% formalin solution. The 40-μm brain sections were prepared with a freezing microtome, stained with thionin, and cannula tracks were identified under a light microscope.
CS intakes during training and preference testing were measured to the neared 0.1 g and averaged over 2- or 4-day blocks. The data were analyzed using analyses of variance (ANOVA) procedures and individual comparisons were evaluated using simple main effects tests or t-test when appropriate. Two-bottle preference data were also expressed as percent CS+ intake [(CS+ intake/total intake) ×100]. The data were analyzed with ANOVA or t-test after an arcsine transformation as recommended by Kirk (1995).
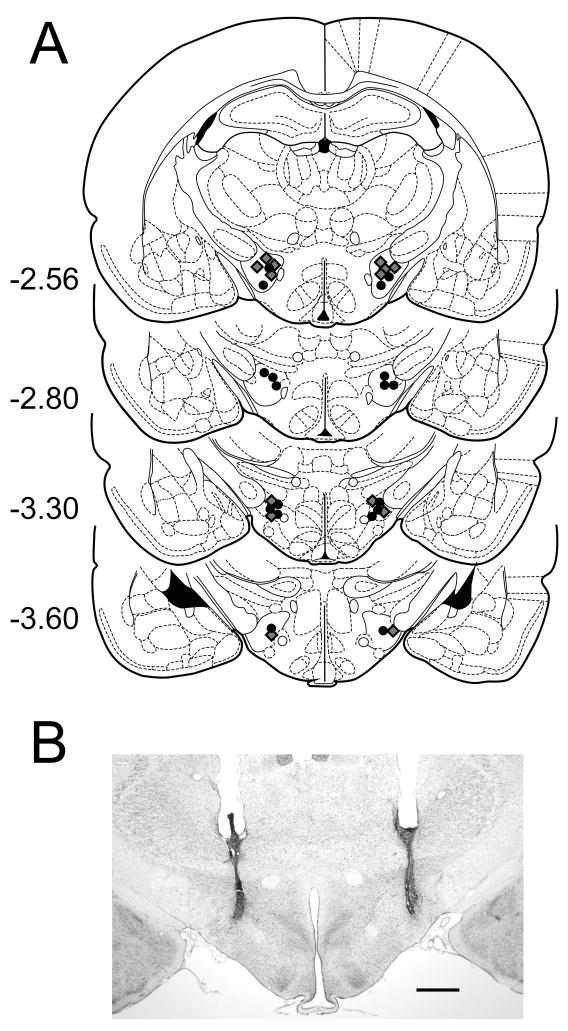
Cannula tip placements for all rats used are shown in Figure 1A. Placements were deemed appropriate for seven SCH rats and nine Control rats and were primarily restricted between the frontal planes −2.56 and −3.60 mm of the Paxinos & Watson (1998) atlas. The remaining nine rats had either very rostral cannula placements at the frontal plane −1.8 mm (four cases) or very low intakes during training and testing (five cases), and consequently their data were discarded. A photomicrograph of a representative bilateral microinjection site is shown in Figure 1B.
Figure 1.
A: Schematic representation of bilateral cannula tip placements in the lateral hypothalamus. Coronal sections were adapted from Paxinos and Watson (1998) with permission. Cannula tips are indicated by grey diamonds in the SCH group and black circles in the Control group. Numbers denote distance (in mm) posterior to bregma. B: Representative photomicrograph of a coronal section indicating bilateral cannula tracts terminating in the lateral hypothalamus. Scale bar: 1 mm.
The SCH rats consumed, on average 5.6 g/session of the CS+ and CS− during training and the intakes of the Control rats were limited to about this amount (4.8 g/session). The results of the first post-training preference test are summarized in Figure 2A. Overall, the rats consumed more CS+ than CS−, F(1,14) = 35.04; p< 0.001 and the two groups did not significantly differ in their total CS intakes. There was a significant Group × CS interaction [F(1,14) = 11.70, p < 0.01] and individual analyses revealed that the Control group consumed more CS+ than CS− (p < 0.001) whereas the CS intakes of the SCH group did not differ. The control rats also consumed more (p < 0.05) CS+ and marginally less (p = 0.07) CS− than did the SCH rats. Consequently, the percent CS+ intake of the Control group exceeded that of the SCH group in Test 1 (87% vs. 61%, p < 0.01). Whereas the CS intake of the SCH rats did not differ in Test 1, overall, they consumed more CS+ than CS− in Tests 2 and 3 conducted under identical conditions [Figure 2B, F(1,6) = 8.35, p < 0.05].
Figure 2.
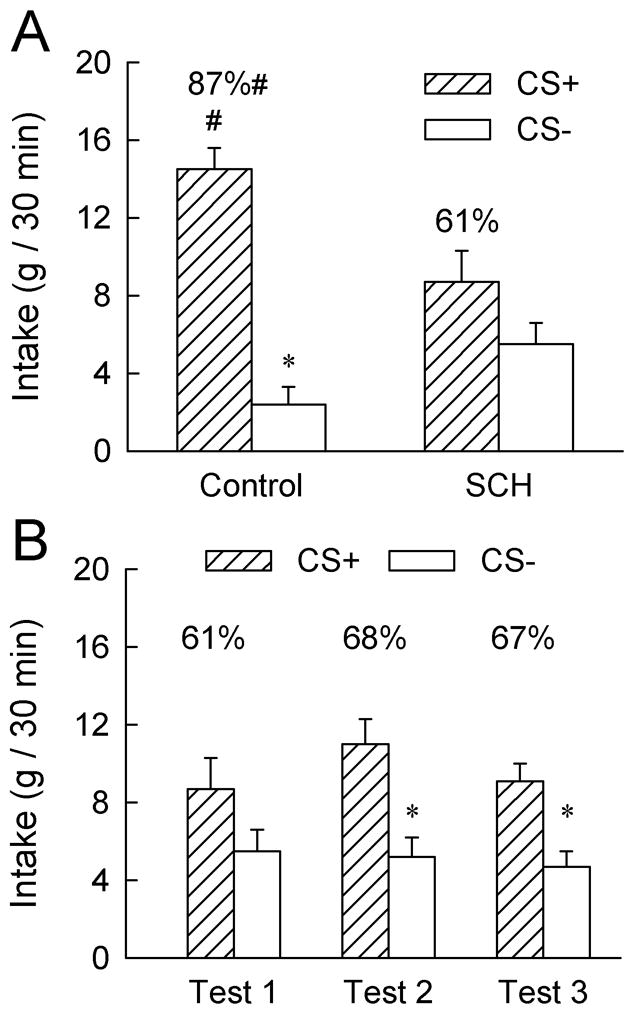
A: Intakes (+S.E.M.) of the CS+ and CS− during the first two-bottle choice test (Test 1); data represent the mean of two 30-min sessions. The CS+ and CS− were paired with intragastric infusions of 8 ml glucose and water, respectively, during training. No gastric infusions were given during testing. The SCH group was given injections of 12 nmol (6 nmol/0.5 μl/side) of SCH23390 into the lateral hypothalamus, ten minutes prior to the daily training sessions while the Control group was given vehicle injections. No brain injections were given prior to the two-bottle choice tests. The numbers atop the bars represent the mean of the individual rat’s percent CS+ intakes. The asterisk (*) denotes a significant (p < 0.05) difference between CS+ and CS− intakes within the Control group. The pound sign (#) denotes a significant difference in the CS+ and percent CS+ intakes between the Control and SCH groups. B: Intakes (+S.E.M.) of the CS+ and CS− during two-bottle choice Tests 1, 2 and 3 in the SCH group. No brain injections were given prior to these tests.
Following their strong CS+ preference in Test 1, the Control rats were given LH injections of saline and SCH23390 in Tests 2 and 3, respectively (Figure 3). The saline injection in Test 2 failed to reduce their CS+ preference compared to that observed in Test 1 with no injections (88% vs. 87%). Treatment with 12 nmol SCH23390 in Test 3 reduced the rats’ total CS intakes compared to the saline test (9.4 vs. 15.3 g/session, F(1,8) = 49.32, p < 0.001). This was due to a reduction in CS+ intake (p < 0.001); the already low CS− intake declined only slightly (p < 0.09) [CS × Test interaction [F(1,8) = 19.81, p < 0.01]. Nevertheless, the Control rats consumed significantly more CS+ than CS− in Test 3 and their percent CS+ intake was similar to that in the saline test (90% vs. 88%).
Figure 3.
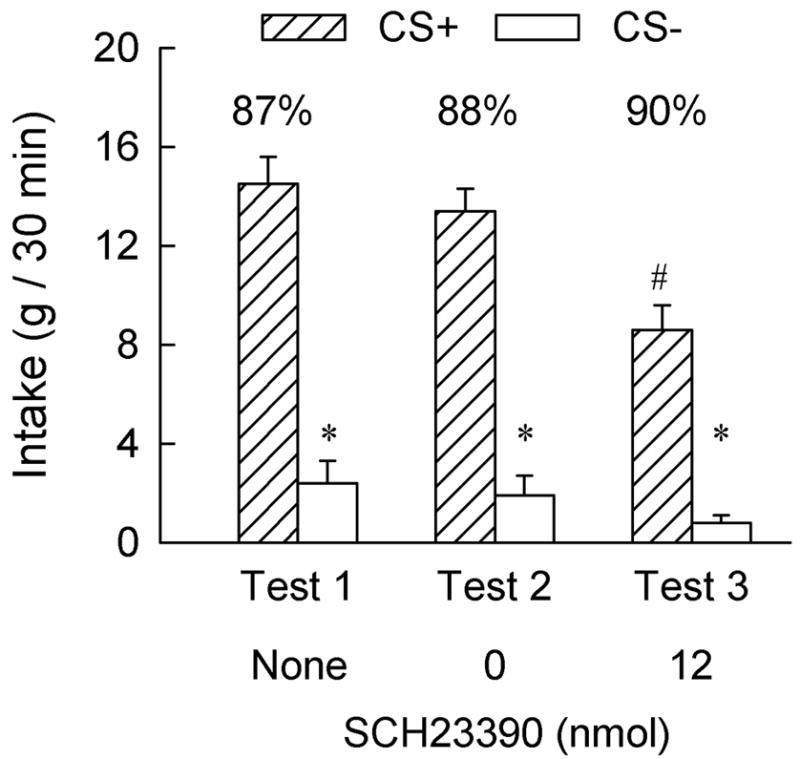
Intakes (+S.E.M.) of the CS+ and CS− during two-bottle choice Tests 1, 2 and 3 in the Control group; each test represents the mean of two 30-min sessions. In Test 1, no brain injections were given. In Tests 2 and 3, the rats were injected with 0 (saline) and 12 nmol of SCH23390 (6 nmol/0.5 μl/side), respectively, into the lateral hypothalamus, ten minutes prior to testing. The numbers atop the bars represent the mean of the individual rat’s percent CS+ intakes. The asterisk (*) denotes a significant (p < 0.05) difference between CS+ and CS− intakes. The pound sign (#) denotes a significant difference between the CS+ intakes following saline (Test 2) and SCH23390 (Test 3) injections.
The present study investigated the role of LH dopamine D1-like receptor activation in flavor preference learning induced by the post-oral reinforcing actions of glucose. The results revealed that antagonism of LH dopamine D1-like receptors during conditioning trials attenuated, but did not block, the acquisition of the glucose-conditioned flavor preference. In contrast, D1-like receptor antagonism during testing did not attenuate the expression of the previously learned glucose-based flavor preference in the Control rats although it did reduce the intake of the CS solutions. These findings demonstrate that activation of dopamine D1-like receptors in the LH is involved in the establishment of flavor-nutrient preference learning.
During training, the rats received bilateral administration of either saline or SCH23390 (12 nmol total dose) in the LH prior to each of eight training sessions. In the subsequent drug-free two-bottle choice test, the SCH rats displayed a weaker CS+ preference than did the Control group. The CS intakes of the Control rats were matched to those of the SCH rats during training and the rats were all infused with the same amount of glucose. Therefore, the attenuated CS+ preference of the SCH group cannot be attributed to reduced exposure to the CS+ or US (IG glucose). It is possible that the SCH rats would have displayed a stronger CS+ preference if they were tested following SCH23390 treatment (state-dependent learning effect). We previously observed, however, that rats given systemic injections of SCH23390 during training did not increase their CS+ preference when injected with the drug prior to the CS+ vs. CS− choice test (Azzara et al., 2001).
The present results are similar to those obtained with LH ibotenic acid lesions (Touzani and Sclafani, 2001; Touzani and Sclafani, 2002). When trained with CS+ and CS− solutions paired with concurrent infusions of maltodextrin (a glucose polymer) and water, respectively, the LH-lesioned group displayed an attenuated but still significant CS+ preference compared to the Control group (67% vs. 84%, Experiment 1 (Touzani and Sclafani, 2001). Because the lesions were made prior to training, it is not clear whether the LH is required for the acquisition and/or the expression of nutrient-conditioned flavor preference. In the present study, treating the Control rats, that had acquired a robust CS+ preference (88%), with SCH23390 in Test 3 did not reduce their CS+ preference (90%) although their absolute intake of the CS+ solution declined. This result clearly indicated that dopaminergic transmission in the LH is required for the normal acquisition, but not the expression, of a flavor preference conditioned by IG glucose infusions. It also confirmed that well learned behaviors become resistant to dopamine manipulations (Ikemoto & Panksepp, 1999).
There is extensive evidence linking dopamine, especially the mesocorticolimbic dopamine system, to reward processes and reward-related learning (Berridge, 2007; Wise, 2004). In this system, dopamine neurons located in the ventral tegmental area (VTA) project heavily to cortical and limbic structures including the nucleus accumbens (NAc), amygdala (AMY) and the prefrontal cortex (Swanson, 1982). We recently investigated the role of dopamine transmission in the NAc and AMY in flavor-nutrient preference learning and found that infusions of SCH23390 into the NAc or AMY completely blocked the acquisition but not the expression of glucose-conditioned flavor preferences (Touzani, Bodnar, & Sclafani, 2007, 2009). Interestingly, VTA dopamine neurons do not project to the LH (Swanson, 1982). Instead, the LH receives innervation from the neighboring zona incerta that harbors A13 dopamine-containing neurons (Wagner, Eaton, Moore, & Lookingland, 1995) although the distribution and density of D1-like receptors in the LH are not clear (Wamsley, Alburges, McQuade, & Hunt, 1992; Mansour, Meador-Wooruff, Zhou, Civelli, Akil, & Watson, 1992; Bubser, Fadel, Jackson, Meador-Wooruff, Jing, & Deutch, 2005). Our findings that D1-LIKE receptor antagonism in the LH attenuated flavor-nutrient preference learning implicate this second dopamine system in incentive learning. This extends prior evidence that LH dopamine D1-receptors are involved in taste aversion learning (Caulliez et al., 1996; Fenu et al., 2001).
The mechanisms by which dopamine transmission in the LH modulates flavor-nutrient preference learning remain to be established. Recent findings indicate that orexin-containing LH neurons innervate dopamine cells in the VTA that, in turn, project to corticolimbic areas involved in reward and reward-related learning (Fadel & Deutch, 2002; Balcita-Pedicino & Sesack, 2007). Furthermore, orexin receptors are expressed in VTA neurons (Korotkova, Sergeeva, Eriksson, Haas, & Brown, 2003) and these receptors are involved in behavioral effects associated with reward-paired stimuli (Aston-Jones, Smit, Moorman, & Richardson, 2009; Harris, Wimmer, & Aston-Jones, 2005). Collectively, these findings indicate that LH orexin neurons are involved in reward processing and learning. Interestingly, orexin cells in the LH are regulated by dopamine (Korotkova et al., 2003; Bubser et al., 2005) indicating that neurons of the LH that modulate the VTA dopamine cells are in turn influenced by dopamine. Thus, it is possible that dopamine release in the LH during flavor-nutrient preference learning modulates the LH-VTA orexin projection and thereby potentiates the activation of the mesolimbic dopamine system by nutrients and nutrient-associated flavor cues. Alternatively, dopamine transmission in the LH may modulate a population of neurons projecting to other brain areas involved in reward and incentive learning such as the mPFC, AMY and NAc. Further experiments are needed to elucidate the mechanisms by which dopamine transmission in the LH influences flavor-nutrient conditioning.
Acknowledgments
This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases grant DK071761.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- Aston-Jones G, Smit RJ, Moorman DE, Richardson KA. Role of lateral hypothalamic orexin neurons in reward processing and addiction. Neuropharmacology. 2009;56:112–121. doi: 10.1016/j.neuropharm.2008.06.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azzara AV, Bodnar RJ, Delamater AR, Sclafani A. D1 but not D2 dopamine receptor antagonism blocks the acquisition of a flavor preference conditioned by intragastric carbohydrate infusions. Pharmacology Biochemistry and Behavior. 2001;68:709–720. doi: 10.1016/s0091-3057(01)00484-1. [DOI] [PubMed] [Google Scholar]
- Balcita-Pedicino JJ, Sesack SR. Orexin axons in the rat ventral tegmental area synapse infrequently onto dopamine and gamma-aminobutyric acid neurons. Journal of Comparative Neurology. 2007;503:668–684. doi: 10.1002/cne.21420. [DOI] [PubMed] [Google Scholar]
- Bernardis LL, Bellinger LL. The lateral hypothalamic area revisited: Neuroanatomy, body weight regulation, neuroendocrinology and metabolism. Neuroscience and Biobehavioral Reviews. 1993;17:141–193. doi: 10.1016/s0149-7634(05)80149-6. [DOI] [PubMed] [Google Scholar]
- Berridge KC. The debate over dopamine’s role in reward: the case for incentive salience. Psychopharmacology (Berl) 2007;191:391–431. doi: 10.1007/s00213-006-0578-x. [DOI] [PubMed] [Google Scholar]
- Berthoud HR. Multiple neural systems controlling food intake and body weight. Neuroscience and Biobehavioral Reviews. 2002;26:393–428. doi: 10.1016/s0149-7634(02)00014-3. [DOI] [PubMed] [Google Scholar]
- Bubser M, Fadel JR, Jackson LL, Meador-Wooruff JH, Jing D, Deutch AY. Dopaminergic regulation of orexin neurons. European Journal of Neuroscience. 2005;21:2993–3001. doi: 10.1111/j.1460-9568.2005.04121.x. [DOI] [PubMed] [Google Scholar]
- Bures J, Bermúdez-Rattoni F, Yamamoto T. Conditioned Taste Aversion: Memory of a Special Kind. Oxford: Oxford University Press; 1998. [Google Scholar]
- Capaldi ED. Conditioned food preferences. In: Capaldi ED, editor. Why We Eat What We Eat: The Psychology of Eating. Washington, DC: American Psychological Association; 1996. pp. 53–80. [Google Scholar]
- Caulliez R, Meile MJ, Nicolaidis S. A lateral hypothalamic D1 dopaminergic mechanism in conditioned taste aversion. Brain Research. 1996;729:234–245. [PubMed] [Google Scholar]
- Fadel J, Deutch AY. Anatomical substrates of orexin-dopamine interactions: lateral hypothalamic projections to the ventral tegmental area. Neuroscience. 2002;111:379–387. doi: 10.1016/s0306-4522(02)00017-9. [DOI] [PubMed] [Google Scholar]
- Fenu S, Bassareo V, Di Chiara G. A role for dopamine D1 receptors of the nucleus accumbens shell in conditioned taste aversion learning. Journal of Neuroscience. 2001;21:6897–6904. doi: 10.1523/JNEUROSCI.21-17-06897.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris GC, Wimmer M, Aston-Jones G. A role for lateral hypothalamic orexin neurons in reward seeking. Nature. 2005;437:556–559. doi: 10.1038/nature04071. [DOI] [PubMed] [Google Scholar]
- Ikemoto S, Panksepp J. The role of nucleus accumbens dopamine in motivated behavior: a unifying interpretation with special reference to reward-seeking. Brain Research Reviews. 1999;31:6–41. doi: 10.1016/s0165-0173(99)00023-5. [DOI] [PubMed] [Google Scholar]
- Kirk RE. Experimental Design: Procedures for the Behavioral Sciences. Pacific Grove, CA: Brooks/Cole; 1995. [Google Scholar]
- Korotkova TM, Sergeeva OA, Eriksson KS, Haas HL, Brown RE. Excitation of ventral tegmental area dopaminergic and nondopaminergic neurons by orexins/hypocretins. Journal of Neuroscience. 2003;23:7–11. doi: 10.1523/JNEUROSCI.23-01-00007.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansour A, Meador-Wooruff JH, Zhou Q, Civelli O, Akil H, Watson SJ. A comparison of D1 receptor binding and mRNA in rat brain using receptor autoradiographic and in situ hybridization techniques. Neuroscience. 1992;46:959–971. doi: 10.1016/0306-4522(92)90197-a. [DOI] [PubMed] [Google Scholar]
- Norgren R. Taste pathways to hypothalamus and amygdala. Journal of Comparative Neurology. 1976;166:17–30. doi: 10.1002/cne.901660103. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. San Diego, CA: Academic Press; 1998. [Google Scholar]
- Scalera G. Effects of conditioned food aversions on nutritional behavior in humans. Nutritional Neuroscience. 2002;5:159–188. doi: 10.1080/10284150290013059. [DOI] [PubMed] [Google Scholar]
- Sclafani A. Macronutrient-conditioned flavor preferences. In: Berthoud H-R, Seeley R, editors. Neural and metabolic control of macronutrient intake. Boca Raton, FL: CRC Press; 1999. pp. 93–107. [Google Scholar]
- Swanson LW. The projections of the ventral tegmental area and adjacent regions: A combined fluorescent retrograde tracer and immunofluorescence study in the rat. Brain Research Bulletin. 1982;9:321–353. doi: 10.1016/0361-9230(82)90145-9. [DOI] [PubMed] [Google Scholar]
- Touzani K, Bodnar RJ, Sclafani A. Activation of dopamine D1 receptors in the nucleus accumbens is critical for the acquisition, but not the expression, of flavor preference conditioned by intragastric glucose in rats. European Journal of Neuroscience. 2008;27:1525–1533. doi: 10.1111/j.1460-9568.2008.06127.x. [DOI] [PubMed] [Google Scholar]
- Touzani K, Bodnar RJ, Sclafani A. Dopamine D1-like receptor antagonism in amygdala impairs the acquisition of glucose-conditioned flavor preference in rats. European Journal of Neuroscience. 2009 doi: 10.1111/j.1460-9568.2009.06829.x. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Touzani K, Sclafani A. Conditioned flavor preference and aversion: Role of the lateral hypothalamus. Behavioral Neuroscience. 2001;115:84–93. doi: 10.1037/0735-7044.115.1.84. [DOI] [PubMed] [Google Scholar]
- Touzani K, Sclafani A. Lateral hypothalamic lesions impair flavor-nutrient and flavor-toxin trace learning in rats. European Journal of Neuroscience. 2002;16:2425–2433. doi: 10.1046/j.1460-9568.2002.02404.x. [DOI] [PubMed] [Google Scholar]
- Wagner CK, Eaton MJ, Moore KE, Lookingland KJ. Efferent projections from the region of the medial zona incerta containing A13 dopaminergic neurons: a PHA-L anterograde tract-tracing study in the rat. Brain Research. 1995;677:229–237. doi: 10.1016/0006-8993(95)00128-d. [DOI] [PubMed] [Google Scholar]
- Wamsley JK, Alburges ME, McQuade RD, Hunt M. CNS distribution of D1 receptors: use of a new specific D1 receptor antagonist, [3H] SCH39166. Neurochemistry International. 1992;20:123S–128S. doi: 10.1016/0197-0186(92)90224-f. [DOI] [PubMed] [Google Scholar]
- Wise RA. Dopamine, learning and motivation. Nature Reviews Neuroscience. 2004;5:483–494. doi: 10.1038/nrn1406. [DOI] [PubMed] [Google Scholar]