Abstract
Small conductance calcium-activated potassium (SK) channels respond to intracellular Ca2+ via constitutively associated calmodulin (CaM). Previous studies have proposed a modular design for the interaction between CaM and SK channels. The C-lobe and the linker of CaM are thought to regulate the constitutive binding, whereas the N-lobe binds Ca2+ and gates SK channels. However, we found that coexpression of mutant CaM (E/Q) where the N-lobe has only one functional EF hand leads to rapid rundown of SK channel activity, which can be recovered with exogenously applied wild-type (WT), but not mutant, CaM. Our results suggest that the mutation at the N-lobe EF hand disrupts the stable interaction between CaM and SK channel subunits, such that mutant CaM dissociates from the channel complex when the inside of the membrane is exposed to CaM-free solution. The disruption of the stable interaction does not directly result from the loss of Ca2+-binding capacity because SK channels and WT CaM can stably interact in the absence of Ca2+. These findings question a previous conclusion that CaM where the N-lobe has only one functional EF hand can stably support the gating of SK channels. They cannot be explained by the current model of modular interaction between CaM and SK channels, and they imply a role for N-lobe EF hand residues in binding to the channel subunits. Additionally, we found that a potent enhancer for SK channels, 3-oxime-6,7-dichloro-1H-indole-2,3-dione (NS309), enables the recovery of channel activity with CaM (E/Q), suggesting that NS309 stabilizes the interaction between CaM and SK channels. CaM (E/Q) can regulate Ca2+-dependent gating of SK channels in the presence of NS309, but with a lower apparent Ca2+ affinity than WT CaM.
INTRODUCTION
By responding to elevated intracellular Ca2+ concentration, small conductance calcium-activated potassium (SK; KCa2, KCNN) channels play critical roles in regulating neuronal excitability (Stocker et al., 1999), hormone release (Tse and Hille, 1992), and smooth muscle tone (Doughty et al., 1999; Herrera et al., 2000). SK channel subunits do not directly bind Ca2+ but detect Ca2+ concentration via associated calmodulin (CaM) (Xia et al., 1998). Different from many other CaM-interacting proteins that only bind with Ca2+-loaded CaM (Yamniuk and Vogel, 2004), SK channels are constitutively associated with CaM, even in the absence of Ca2+ (Xia et al., 1998). This intimate association likely enables the fast activation of SK channels in response to Ca2+ as essential for their physiological functions (Xia et al., 1998). CaM consists of two globular domains (the C- and N-lobe) connected by a flexible linker. Each lobe contains two EF hand structures that serve as the Ca2+-binding sites. Structural, functional, and biochemical studies on SK channel–CaM complexes have suggested a modular mechanism for the molecular interaction between SK channels and CaM. The C-lobe and the linker of CaM are thought to be responsible for the constitutive association between CaM and SK channels, but C-lobe EF hands do not bind Ca2+. On the other hand, the N-lobe of CaM is considered not involved in the constitutive binding, but EF hands in the N-lobe bind Ca2+ and gate SK channels (Xia et al., 1998; Keen et al., 1999; Schumacher et al., 2001; Li and Aldrich, 2009).
A thorough understanding of the activation mechanism for SK channels is not yet available, partly resulting from the fact that each SK channel complex has up to four CaM molecules, each with at least two Ca2+-binding sites (EF hands) that likely bind Ca2+ cooperatively (Xia et al., 1998; Keen et al., 1999). The contribution of individual EF hands to the opening of SK channels has been difficult to evaluate. To establish a quantitative framework for the characterization of the coupling between Ca2+ binding and channel activation, it is useful to study SK channels associated with CaMs in which only one of the N-lobe EF hands binds Ca2+. A previous study suggested that when one of the EF hands at the N-lobe of CaM loses its Ca2+-binding capacity by mutation, CaM can still stably support SK channel gating, albeit with a reduced apparent affinity for Ca2+ (Keen et al., 1999). This mutation in CaM would greatly reduce the complexity of the SK channel gating scheme; therefore, we decided to study its effects more thoroughly to estimate the contribution of individual EF hands. We found unexpectedly that this mutation disrupts the constitutive interaction between CaM and SK channels. As such, mutant CaM dissociates rapidly from the SK channel complex and can be replaced by exogenously applied wild-type (WT) CaM. Our results suggest an involvement of the N-lobe of CaM in the constitutive binding, in contrast to the modular model of interaction, and call into question the previous conclusion that CaM with a single calcium-binding N-lobe EF hand is sufficient to stably gate the channel (Keen et al., 1999). Interestingly, mutant CaM can interact with SK channels and support gating in the presence of 3-oxime-6,7-dichloro-1H-indole-2,3-dione (NS309), a frequently used enhancer for SK and intermediate conductance calcium-activated potassium channels that increases the apparent affinity for Ca2+ activation (Strøbaek et al., 2004). The molecular mechanisms for the function of NS309 have remained unknown despite their apparent significance. Our results suggest that NS309 may enhance channel activity by stabilizing the interaction between channel subunits and CaM.
MATERIALS AND METHODS
Channel expression and molecular biology
For all electrophysiological recordings, rSK2 (KCa2.2) channels were expressed in Xenopus oocytes. WT or mutant CaM was coexpressed with SK channels as needed. The rSK2 clone was provided by J. Adelman (The Vollum Institute, Portland, OR). The clone for WT mammalian CaM was provided by S. Hamilton (Baylor College of Medicine, Houston, TX). Both clones were introduced into the pOX vector, from which RNA was transcribed in vitro. Approximately 10 ng rSK2 RNA and 10–20 ng CaM (WT or mutant) RNA (as needed) were injected into each oocyte (significantly higher doses than this were found to lead to unhealthy oocytes). Recordings were typically performed 4–10 d after injection. Mutations of CaM were generated using the Quickchange mutagenesis kit (Agilent Technologies) and verified by sequencing.
Electrophysiology
Patch clamp recordings of SK currents were performed in the inside-out configuration. Recording electrodes were fabricated with borosilicate glass pipets (VWR Scientific), coated with wax (Sticky Wax; Kerr Corporation), and fire-polished before use. Open electrode resistance was 1–2 MΩ in the bath solution. An Axopatch 200A amplifier (MDS Analytical Technologies) coupled with an ITC-16 interface (HEKA) was used to record data. Acquisition software (PULSE; HEKA) was used to sample data at 20-µs intervals, which were low-pass filtered at 5 KHz using the built-in filter of the amplifier. For long continuous recordings, data were sampled at 50- or 100-µs intervals. Unless otherwise noted, SK currents were elicited by a voltage ramp from −80 to 60 mV. Relative current levels at −80 mV were measured to estimate the open probability. During the course of some long experiments, there were small (usually <10 mV) changes in offset voltage. In these cases, driving force changes were corrected for based on the changes in reversal potential (which should be 0 mV in symmetrical K+ solutions). In some experiments where SK current ran down or up slightly (<15%) during the measurement of dose–response relationships, the maximal current level for normalization was corrected for based on an estimated linear time course of change. All experiments were performed at 22°C. Data analysis, fitting, and plotting were performed with IGOR Pro software (WaveMetrics). Average results were presented as mean ± SD.
Extracellular (pipette) solution contained (in mM): 140 KMeSO3, 20 HEPES, 2 KCl, and 2 MgCl2, pH 7.20. The standard internal (bath) solution contained (in mM): 136 KMeSO3, 20 HEPES, and 6 KCl, pH 7.20. Ca2+ chelators were used to control free Ca2+ concentration in internal solutions. For this purpose, 5 mM chelators (EGTA for Ca2+ concentrations of 0.5 µM and below, HEDTA for 0.77–15.4 µM, and NTA for 85 µM) were added to the standard internal solution. Different amounts of CaCl2 were added based on calculations with the WEBMAXC program. For most solutions with higher Ca2+ levels, the actual free Ca2+ concentrations were determined by measurements with a Ca2+-sensitive electrode (Orion Research, Inc.) and reported. We then deduced the affinities of chelators from these measurements and used them to calculate the free Ca2+ concentrations for other solutions, particularly those with <0.5 µM expected Ca2+, which cannot be accurately measured with Ca2+ electrode. Solution with 5 mM EGTA and no added Ca2+ was considered as nominally Ca2+ free. In this solution, the open probability of SK channels is extremely low as estimated with single-channel recordings; therefore, it was used to estimate leak current level for subtraction from currents recorded in various Ca2+ concentrations.
For solution changes, the perfusion chamber was washed with new solution of >10 times the chamber volume. In experiments to compare the activation time course of SK channels, the recording electrode was moved to a larger chamber containing Ca2+-free solution and positioned next to a quartz sewer pipe (100 µm in diameter) connected with a syringe, used to deliver high Ca2+ solution. To measure activation, the electrode was manually moved from the Ca2+-free bath into a laminar flow of high Ca2+ solution outside the sewer pipe during continuous current recording at −80 mV. Using junction potential as an indicator, we estimated that it routinely takes only a few milliseconds to move the electrode across the solution interface. However, it likely takes a much longer time to completely change the solution around the inside-out patch. Therefore, the measured activation time course is inevitably slower than the actual process. However, this does not affect our conclusions based on the comparison of activation time course under different conditions.
Preparation of CaM
WT and mutant mammalian CaM constructs in pET20b plasmid were expressed in a BL21 (DE3) strain of Escherichia coli (EMD). WT CaM was purified using phenyl sepharose chromatography with a slightly modified protocol developed by Gopalakrishna and Anderson (1982). Mutant CaMs with Ca2+-binding deficiency at individual EF hands were purified using ion exchange chromatography as described by Mukherjea et al. (1996). WT and mutant CaMs were dialyzed into water, concentrated to 400–1,000 µM, and stored in aliquots at −80°C. The concentration of WT CaM was determined by its extinction coefficient at 277 nm (Em = 3,300 cm2/M) (Richman and Klee, 1979). The concentrations of mutant CaMs were determined by the Lowry method using WT CaM as a standard (Lowry et al., 1951). Aliquots of stock CaM were thawed and added into solution immediately before use.
Chemicals
All chemicals were obtained from Sigma-Aldrich. NS309 stock solutions were prepared with DMSO at a concentration of 50 mM and stored at −20°C. Stocks were thawed and added to the solution immediately before use.
RESULTS
SK channels run down when coexpressed with N-lobe mutant CaM (E/Q)
Although it has been established that the gating of SK channels absolutely requires associated CaM (Xia et al., 1998; Keen et al., 1999), injection of rSK2 RNA alone into Xenopus oocytes results in functional SK channels due to the presence of endogenous CaM (Köhler et al., 1996). To study the functional effects of mutant CaM deficient in the ability to bind Ca2+ at one of the two N-lobe EF hands (EF1 and EF2), we overexpressed mutant CaM carrying a glutamate to glutamine mutation at the conserved position 12 in either N-lobe EF hand (E1Q or E2Q; “1” or “2” refers to EF hand 1 or 2. The mutation is at residue E32 or E68, respectively; Maune et al., 1992). In all aspects investigated in this study, we have performed comparable numbers of experiments with the E1Q and the E2Q mutant. As the effects of E1Q and E2Q mutations are essentially indistinguishable from each other, we have pooled the data together and refer to them as the E/Q mutant.
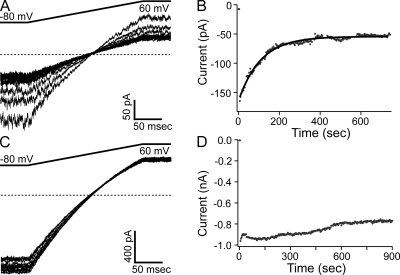
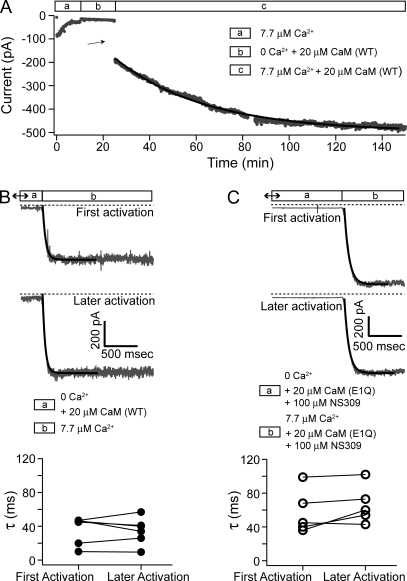
Although we were able to record SK currents in inside-out patches from oocytes coexpressing CaM (E/Q), we found that coinjection of mutant CaM (E/Q) RNA led to significantly lower SK current levels than control oocytes where the same amount of SK channel RNA was injected alone (SK alone) or coinjected with RNA for WT CaM (SK + CaM (WT)). The initial current level upon patch excision varied widely among experiments, but the average level for CaM (E/Q)–coinjected oocytes (160 ± 120 pA; n = 83) is only approximately one third of the average value for the control groups (SK alone, 465 ± 350 pA; n = 10; SK + CaM (WT), 470 ± 340 pA; n = 8). Additionally, currents from the CaM (E/Q)–coinjected oocytes ran down rapidly after patch excision, even in the presence of high Ca2+ (Fig. 1, A and B). As a result, after 10 min in solution, only a small amount of current remained, with 67 ± 15% (n = 83) of the initial current lost. Elevating the Ca2+ concentration from 7.7 to 85 µM in the bath solution failed to slow down the current rundown. Approximating the time course of current rundown with an exponential function yields a time constant of 161 ± 120 s (n = 66). It is known that SK channels associated with CaM (WT) also undergo rundown (Keen et al., 1999); however, the degree of rundown is much less pronounced. In our control experiments where SK channels were expressed alone or with CaM (WT), SK currents ran down only by a small fraction at 10 min after patch excision (SK alone, 10 ± 15%; n = 10; SK + CaM (WT), 17 ± 14%; n = 8) (Fig. 1, C and D).
Figure 1.
Coexpression of CaM (E/Q) leads to quick rundown of SK current. (A) Representative current traces recorded under voltage clamp with an inside-out patch excised from a Xenopus oocyte injected with RNA for SK channels and CaM (E1Q). Voltage protocol is shown on top. For clarity, only one trace recorded at the beginning of each minute after patch excision is shown. Horizontal dashed lines in this and other figures represent zero current level. (B) Average current level at −80 mV measured every 3 s from the same patch in A is plotted as a function of time. The jump in current at the beginning of the time course in this and later figures indicates patch excision. Data points during solution change were noisy and removed from the plot in this and other figures. Solid line represents a single-exponential fit of the time course with τ = 108 s. (C) Representative current traces recorded and shown as in A with a patch excised from an oocyte injected with RNA for CaM (WT) and SK channels. Note a small shift in the reversible potential during the course of recording. (D) Average current level at −80 mV from the patch shown in C measured and plotted as in B.
WT CaM can recover SK channels after rundown
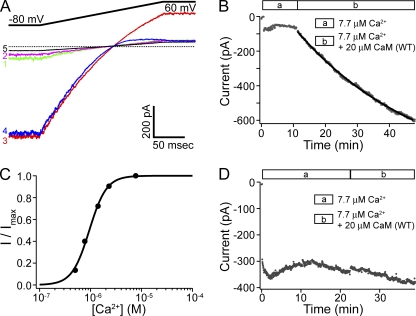
The fast rundown of SK current is reminiscent of a previous finding that disruption of the tight association between SK channel and CaM by mutations in either CaM or SK channel subunits causes rapid and extensive rundown of SK current (Lee et al., 2003). We therefore explored the possibility that the E/Q mutation also destabilizes the interaction between SK channels and CaM, and that the loss of CaM from the SK channel complex underlies current rundown in inside-out patches. Using the approach from the previous study, we tested whether the application of purified CaM protein can recover the SK channel activity after rundown in a patch from an oocyte coexpressing CaM (E/Q). As shown in Fig. 2 (A and B), after the current rundown stabilized, the application of 20 µM CaM (WT) protein led to a slow but robust recovery of currents. The kinetics of recovery varied considerably among experiments. Approximating the recovery time course with a single-exponential fit yields a time constant of 51 ± 20 min (n = 20), making it obvious that it was not always practical to achieve a complete recovery in our experiments. Nevertheless, exogenously applied CaM (WT) was able to recover the current to an average of ∼20-fold higher than the residual current level after rundown, and more than fivefold higher than the initial current level upon patch excision (n = 30).
Figure 2.
CaM (WT) can recover SK channel activity. (A) Representative current traces with a patch from a CaM (E2Q)–coinjected oocyte at the following time points: (1) immediately after patch excision; (2) after rundown stabilized; (3) 50 min after the application of 20 µM CaM (WT); (4) the application of solution containing 16 µM Ba2+; and (5) finally in Ca2+-free solution. (B) Average current level at −80 mV measured every 3 s with the patch shown in A during the course of rundown and recovery. Legends in this and later figures show the different solutions that the patch is subjected to. Solid line represents fit with a single-exponential time course (τ = 39.7 min). (C) With the same patch shown in A and B, after recovery of SK channel activity with CaM (WT), solutions containing different free Ca2+ concentrations were applied to the patch. Average current level at −80 mV was measured at each concentration, normalized to the maximal value, and plotted as a function of Ca2+ concentration. The dose–response relationship is fitted with the Hill equation: I/Imax = 1/(1+(EC50/[ Ca2+])h), yielding EC50 = 0.94 µM and h = 2.6. (D) A representative time course of the average current level at −80 mV with a patch excised from an oocyte coinjected with RNA for SK channels and CaM (WT).
Several lines of evidence demonstrate conclusively that the recovered currents are carried by SK channels. (1) The inward rectification of the recovered currents (Fig. 2 A, trace 3) is typical of SK currents recorded in control experiments (Fig. 1 C) and in previous studies (Soh and Park, 2001). (2) The recovered currents were inhibited by Ca2+-free solution (Fig. 2 A, trace 5). (3) The recovered currents were blocked by 16 µM of internal Ba2+ (Fig. 2 A, trace 4) with similar voltage dependence as previously reported for Ba2+ block of SK channels (Soh and Park, 2001, 2002). (4) The recovered currents were stable in CaM-free solution, consistent with the known stable interaction between SK channels and CaM (WT) (Xia et al., 1998). (5) The Ca2+ dose–response relationship for the recovered currents (Fig. 2 C) is similar when compared with control SK channels that were originally associated with endogenous or coexpressed CaM (WT) (see Fig. 3 C for comparison). (6) The application of CaM (WT) to SK channels expressed alone or with CaM (WT) only led to a slight increase in the current (16 ± 4%; n = 3) in 10 min (Fig. 2 D). By comparison, for SK channels coexpressed with CaM (E/Q), the application of CaM (WT) protein led to an average of a 6.8-fold increase in the current level in 10 min (n = 30).
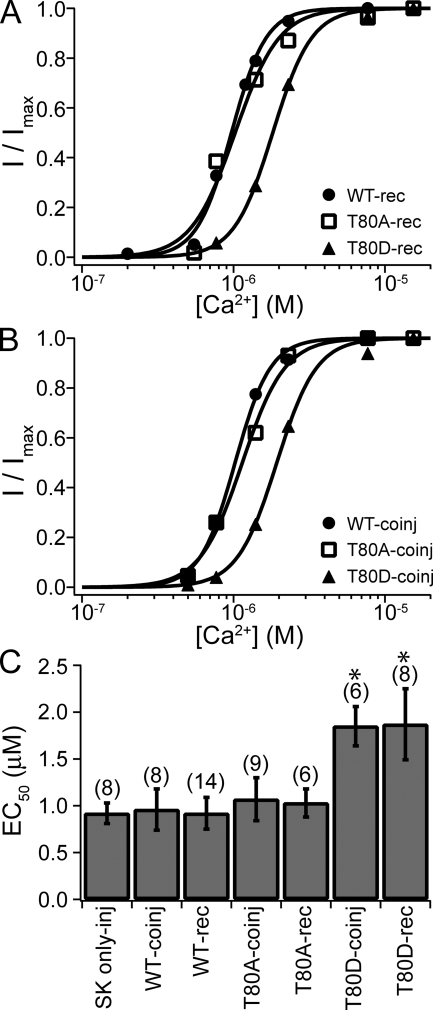
Figure 3.
SK channels recovered with CaM (T80D) have reduced apparent Ca2+ affinity. (A) Representative dose–response relationships for SK channels recovered with CaM (WT) (filled circles), CaM (T80A) (open squares), and CaM (T80D) (filled triangles). In all cases, patches were excised from oocytes coinjected with RNA for SK channels and CaM (E/Q). Currents were allowed to run down before the application of 20 µM of individual types of CaM. Dose–response relationships were measured in CaM-free solutions. Solid lines are fits with the Hill equation (WT, EC50 = 0.97 µM and h = 3.8; T80A, EC50 = 1.03 µM and h = 3.0; T80D, EC50 = 1.82 µM and h = 3.4). (B) Representative dose–response relationships for SK channels coexpressed with CaM (WT) (filled circles), CaM (T80A) (open squares), or CaM (T80D) (filled triangles). Solid lines are fits with the Hill equation (WT, EC50 = 1.03 µM and h = 3.7; T80A, EC50 = 1.14 µM and h = 3.1; T80D, EC50 = 1.93 µM and h = 3.3). (C) Average values for EC50 from experiments as shown in A and B. Dose–response relationships were individually fitted for each experiment. Error bars represent standard deviation. Trial numbers are shown in the brackets on the top. Labeling is interpreted as the following: SK only-inj, oocytes were injected with SK RNA alone; WT-coinj, CaM (WT) RNA was coinjected with SK RNA; WT-rec, SK channels recovered by CaM (WT) in patches from oocytes coinjected with RNA for the SK channel and CaM (E/Q). The same applies for CaM (T80A) and (T80D). EC50 values for T80D-rec and T80D-coinj are not significantly different from each other (P > 0.05; Student's t test), but significantly different from all other columns (P < 0.01), and are therefore labeled with stars. All other columns are not significantly different from each other (P > 0.05).
Recovery results from coassembly of SK channels with exogenously applied CaM
To confirm that the recovered activity is due to reassembly of SK channels with the exogenously applied CaM rather than an indirect effect of CaM, we applied other types of CaM to the patch. Previously, it has been shown that the phosphorylation status of the threonine at position 80 (T80) of the associated CaM (mediated by endogenous CK2) has a dramatic effect on the Ca2+ dependence of SK channel activation. A point mutation T80D in CaM, which presumably mimics the phosphorylated state of T80, results in lower apparent Ca2+ affinity compared with T80A, a surrogate for the dephosphorylated state of T80 (Bildl et al., 2004). If exogenously applied CaM became directly associated with SK channels in the patch, we would expect that SK channels should also have a different apparent Ca2+ affinity when the channel activity is recovered with purified CaM (T80A) compared with recovery with CaM (T80D).
Both mutant CaMs, (T80A) and (T80D), were able to recover SK channel activity in patches from oocytes coexpressing SK channels with CaM (E/Q) with comparable levels and kinetics of recovery compared with CaM (WT) (fold recovery in 10 min: CaM (T80D), 4.3 ± 3.8; n = 15; (T80A), 8.0 ± 5.8; n = 8). Fig. 3 A shows representative dose–response relationships for SK channels recovered with CaM (WT), (T80A), and (T80D). CaM (T80A) had a similar effect as CaM (WT) on the Ca2+-dependent activation of SK channels, but CaM (T80D) led to a reduction in the apparent Ca2+ affinity by about twofold (Fig. 3 C). The reduction in apparent affinity with T80D is qualitatively consistent with the earlier study (Bildl et al., 2004), but the effect was smaller in our study (two- vs. fivefold), which will be discussed later. In complementary experiments, we coexpressed mutant CaM (T80A) or (T80D) with SK channels. The coexpression of CaM (T80A) or (T80D) led to SK channels with stable activity (no rapid rundown); thus, the dose–response relationship could be directly measured without application of exogenous CaM. Fig. 3 B shows the representative dose–response relationships for SK channels coexpressed with CaM (WT), (T80A), and (T80D). Coexpression of CaM (T80D) also led to a two-fold increase in EC50 for Ca2+ activation of SK channels. The average EC50 values for coinjection experiments compare favorably with recovery experiments with the corresponding CaMs (Fig. 3 C). The similarity between the effect of CaM (T80A) and CaM (WT) suggests that residue T80 in both the coexpressed CaM (WT) and the exogenously applied CaM (WT) is mostly dephosphorylated. These results confirm that the applied CaM indeed assembled with SK channels in patches from oocytes expressing CaM (E/Q). CaM (WT) is known to bind tightly with SK channels and rarely dissociates (Xia et al., 1998). However, our results indicate that CaM (E/Q) must dissociate more readily, leaving empty CaM-binding sites available for the exogenously applied CaM (WT).
N-lobe mutant CaM (E/Q) cannot recover SK channels
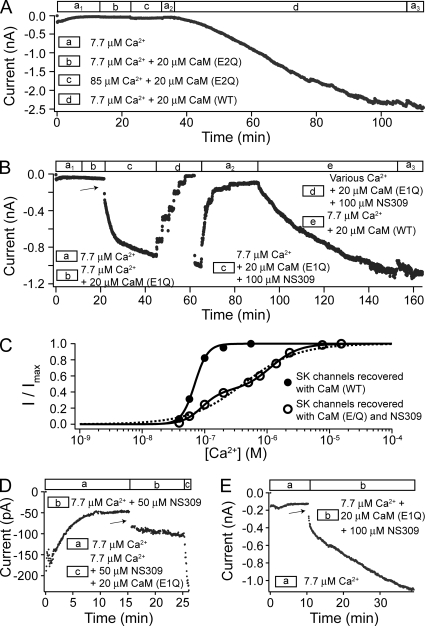
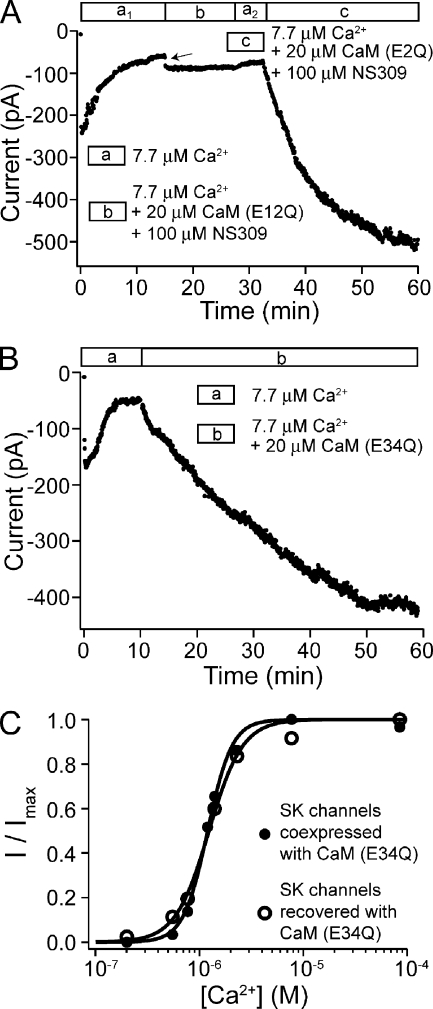
Due to small currents and rapid rundown (Fig. 1, A and B), the contribution of CaM (E/Q) to SK channel activation could not be accurately measured from the initial currents. Knowing that CaM (E/Q) dissociates rapidly from SK channels, we applied purified CaM (E/Q) protein to the patch after current rundown, testing whether the dissociation of CaM can be compensated by rebinding. However, we found that the application of CaM (E/Q) protein failed to recover SK channel activity (Fig. 4 A, segment b). Increasing Ca2+ concentration (from 7.7 to 85 µM, and up to 1 mM in additional experiments) led to an immediate but small increase in the residual current (Fig. 4 A, segment c), suggesting that 7.7 µM Ca2+ is not sufficient to saturate the activation of residual channels that remain activatable (indicating a reduced apparent Ca2+ affinity). However, there was no further recovery of SK channel activity after the immediate current increase. In the same patch that was unresponsive to CaM (E/Q), subsequent application of CaM (WT) successfully recovered SK channel activity (Fig. 4 A, segment d). On average, the application of 20 µM CaM (E/Q) led to an only 15 ± 22% increase in current in 10 min (n = 14), compared with an average 6.8-fold increase in 10 min with CaM (WT) (n = 30). This result suggests that either CaM (E/Q) cannot assemble with SK channels in excised patches, or they assemble but cannot gate the channel. A simple reduction in apparent Ca2+ affinity is not likely to account for the lack of recovery because up to 1 mM Ca2+ had no further effect.
Figure 4.
CaM (E/Q) can only recover SK channel activity in the presence of NS309. (A) Time course of the average SK current level at −80 mV with a patch excised from an oocyte coexpressing SK channels and CaM (E2Q). Patch was subjected to solutions containing different amount of Ca2+ and 20 µM WT or mutant CaM as shown by the legend. (B) Time course of the average SK current level at −80 mV with a patch from an oocyte coexpressing SK channels and CaM (E1Q). (C) A representative dose–response relationship for SK channels recovered with CaM (E2Q) and NS309 in a patch from an oocyte coexpressing SK channels and CaM (E2Q) (open circles). Initial currents were allowed to run down before the application of 20 µM CaM (E2Q) and 100 µM NS309. After recovery, the dose–response relationship was measured in the presence of 20 µM CaM (E2Q) and 100 µM NS309. Dashed line represents a fit of the data with a single Hill equation (EC50 = 0.41 µM and h = 1.0). Solid line represents a fit of the data with a two-component Hill equation, I/Imax = c/(1+(EC50a/[ Ca2+])ha) + (1−c)/(1+(EC50b/[Ca2+])hb), in which c is the fraction of the first component (c = 0.41, EC50a = 90 nM, and ha = 2.9; EC50b = 1.14 µM and hb = 1.9). Filled circles are a representative dose–response relationship in the presence of 100 µM NS309 for CaM (WT)–recovered SK channels. Solid line is a fit with a single Hill equation (EC50 = 69 nM and h = 4.2). (D) A representative experiment where NS309 was applied in the absence of CaM to a patch from an oocyte coexpressing SK channels with CaM (E/Q). (E) An example of recovery using CaM (E1Q) and NS309 without pretreatment with CaM (E1Q) alone.
NS309 enables N-lobe mutant CaM (E/Q) to recover channel activity
In an attempt to facilitate the recovery of SK channels with CaM (E/Q), we resorted to a potent SK channel enhancer, NS309 (Strøbaek et al., 2004), to test whether it could enable the assembly of CaM (E/Q) with SK channels and Ca2+-dependent gating. In fact, CaM (E/Q) was able to recover SK channel activity in the presence of 50–100 µM (>>saturation) NS309 (Fig. 4 B, segment c), despite CaM (E/Q) alone having no effect in the same patch (Fig. 4 B, segment b). On average, the application of CaM (E/Q) with NS309 led to a 6.9 ± 6.6–fold increase from the residual current level in 10 min (n = 19). The time course of recovery was variable, and approximation with a single-exponential function (not always well-fit) yields an average time constant of 14 ± 11 min (n = 17), faster than the recovery with CaM (WT) (τ = 51 ± 20 min; n = 20). In control experiments such as the one shown in Fig. 4 D, application of NS309 alone led to an immediate increase (arrow) in the residual current level by 1.1 ± 0.9–fold (n = 5), again suggesting that the 7.7 µM Ca2+ alone is insufficient to saturate the activation of residual SK current. However, in the 10 min after the application of NS309, currents only increased by 31 ± 12% (n = 5), suggesting that NS309 alone cannot efficiently recover SK channel activity.
After recovery, when CaM and NS309 were removed from the solution by washing, the current once again ran down rapidly (Fig. 4 B, segment a2). This is clearly different from the recovered SK currents with CaM (WT), which no longer ran down even in CaM-free solution. As an example, in the same patch after the current ran down again, CaM (WT) could recover SK channel activity that was stable even after CaM (WT) was removed from solution (Fig. 4 B, segments e and a3). In separate experiments, the activity of SK channels recovered with CaM (WT) remained stable for hours in CaM-free solution. This difference confirms that the interaction between CaM (E/Q) and SK channels is unstable, such that CaM (E/Q) dissociates rapidly from the SK channel complex.
SK channels recovered with NS309 and CaM (E/Q) have lower apparent Ca2+affinity
In light of the unstable association between SK channels and CaM (E/Q), we measured the Ca2+-dependent activation of the recovered SK channels with solutions containing both CaM (E/Q) and NS309. A representative dose–response relationship for such recovered SK channels is shown in Fig. 4 C (open circles). Fitting of the dose–response relationships from similar experiments with the Hill equation (Fig. 4 C, dashed line) yielded an average EC50 = 0.41 ± 0.18 µM and h = 1.1 ± 0.2 (n = 8). These values reflect a significantly reduced apparent Ca2+ affinity and cooperativity when compared with the activation of CaM (WT)–recovered SK channels in the presence of NS309, with an average EC50 = 66 ± 12 nM and h = 4.3 ± 0.8 (n = 5) (Fig. 4 C, filled circles and solid line). However, as discussed later, this reduced slope for the dose–response relationship can also result from subgroups of recovered SK channels with different apparent Ca2+ affinity. A two-component Hill equation clearly improved the quality of fit (Fig. 4 C, solid line), with one EC50 at ∼100 nM and the other at ∼1 µM. The two-component Hill equation consistently provided a better fit of the dose–response relationship with similar parameters in all eight experiments where SK channels were recovered with NS309 and CaM (E/Q). On average, 46% of the recovered SK channels had a higher affinity, with EC50 = 116 ± 53 nM and h = 2.9 ± 0.8, whereas the other 54% had EC50 = 1.20 ± 0.52 µM and h = 2.2 ± 0.9 (n = 8). Comparison between channels recovered with CaM (WT) and CaM (E/Q) suggests that in the presence of saturating NS309, a fraction of the channels recovered with CaM(E/Q) behave like those associated with CaM (WT), whereas the remaining have much reduced apparent Ca2+ affinity. The possible explanations for these two groups will be discussed later.
N-lobe mutant CaM (E/Q) cannot assemble with SK channels without NS309
Is it possible that CaM (E/Q) does assemble with SK channels in the patch but cannot gate them unless NS309 is present? The data in Fig. 4 B may seem to support this at first glance. The immediate stepwise increase in the current level after the application of CaM (E/Q) plus NS309 (marked by the arrow) could suggest that pretreatment of the patch with CaM (E/Q) alone had already led to the assembly of some channels with CaM (E/Q), which was quickly activated when NS309 was applied. However, additional experiments revealed that this immediate current increase did not depend on pretreatment of the patch with CaM (E/Q). When CaM (E/Q) and NS309 were coapplied without pretreatment of CaM (E/Q), we saw qualitatively similar time courses of recovery, including the initial immediate increase of current level and the fast recovery phase (Fig. 4 E, arrow). Rather, this immediate current increase is likely due to the effect of NS309 because the application of NS309 without CaM (E/Q) also led to an immediate increase in the residual current (Fig. 4 D, arrow). If during the 10 min of pretreatment CaM (E/Q) could assemble with SK channels as effectively without NS309, we would expect to see a much larger immediate current increase, as extrapolated from the fast recovery phase when CaM (E/Q) and NS309 were coapplied (Fig. 4 B, segment c). Therefore, it seems that CaM (E/Q) cannot assemble with the SK channels in the patch without NS309 present at the same time. However, together with NS309, CaM (E/Q) does appear to gate the channel. Additionally, this result suggests that diffusion of CaM into the recording pipette is not likely a limiting factor for the slow recovery of SK channels; otherwise, we would expect to see a more pronounced speeding of the recovery process by the pretreatment of patch with CaM (E/Q) alone before adding NS309. This argument is based on the assumption that the action of NS309 on SK channels already assembled with CaM is immediate on the slow time scale for recovery. This was verified to be the case with SK channels associated with CaM (WT) in additional experiments (not depicted), and it is consistent with the immediate increase of residual current level when NS309 was applied alone (Fig. 4 D, arrow).
Recovery of SK channels does not require Ca2+ binding to CaM
Why is CaM (E/Q) without NS309 unable to assemble with SK channels in the patch? As the primary known effect of the E/Q mutation at the EF hand is the loss of Ca2+ binding, the most obvious possibility is that assembly of CaM with SK channels in the patch requires Ca2+ binding at both EF hands in the N-lobe of CaM. Although CaM (WT) is known to constitutively bind with SK channels even in the absence of Ca2+ (Xia et al., 1998), it is possible that new assembly of CaM onto SK channels already in the membrane has different requirements. A previous study showed that CaM (WT) can assemble with mutant SK channels (SK2:R464E/K467E) in a patch in the absence of Ca2+, although with a reduced affinity (Lee et al., 2003). We therefore also tested whether the recovery of SK channels (WT channels in our case) requires the presence of Ca2+.
As shown in Fig. 5 A, after the rundown of SK channels coexpressed with CaM (E/Q), we pretreated the patch with CaM (WT) in Ca2+-free solution for 15 min (segment b), followed by CaM (WT) in high Ca2+ (segment c). The immediate increase in the current level (arrow) after the application of high Ca2+ solution suggests that a significant amount of SK channels were already assembled with CaM (WT) in the absence of Ca2+ and ready to be activated by Ca2+. In contrast to the earlier discussion on the effect of CaM (E/Q) and NS309 in Fig. 4 B, in this case the immediate increase in the current level can only be explained by channel recovery during the Ca2+-free pretreatment. Given the slow time course of later recovery in CaM (WT) and high Ca2+ (Fig. 5 A, segment c), the immediate increase is unlikely due to an instantaneous recovery of some SK channels, but rather to an accumulated effect during the pretreatment. The degree of recovery in Ca2+-free solutions varied considerably among experiments, but on average, 15 min of pretreatment with CaM (WT) in the absence of Ca2+ led to an immediate increase of current by approximately eightfold of the residual current when Ca2+ was applied afterward (n = 6). This number is not far from the average fold of recovery in 10 min of CaM (WT) with high Ca2+ (approximately sevenfold). However, a detailed comparison in the levels and kinetics of recovery between high Ca2+ and Ca2+-free condition was not attempted in light of the large patch-to-patch variation.
Figure 5.
Recovery of SK channels in the absence of Ca2+. (A) Time course of the average current level at −80 mV with a patch from an oocyte coexpressing SK channels with CaM (E1Q). Solid line is a single-exponential fit with a τ = 39.1 min. (B) After patch was excised from an oocyte expressing SK channel and CaM (E1Q), current ran down to 60 pA before the patch was subjected to 20 µM CaM (WT) in Ca2+-free solution for 60 min. Then, recording pipette was quickly moved into a laminar flow of solution containing 7.7 µM Ca2+ to measure the time course of activation. Current was continuously recorded at −80 mV during the movement and shown in the top panel. Later in the experiment, pipette was moved back to Ca2+-free solution and then to 7.7 µM Ca2+ again (middle). Solid lines are fits of data with a single-exponential time course (top, τ = 46 ms; middle, τ = 38 ms). The bottom panel compares the time constants for the first activation after recovery to the average values for later activations. Results were from six similar experiments. (C) Similar experiment as in B, except that CaM (WT) was replaced by 20 µM CaM (E1Q) plus 100 µM NS309, which were also present during the measurement of the activation time course. Before the application of Ca2+-free solution containing CaM (E1Q) and NS309, there was 18 pA of residual current after rundown. Solid lines are fits of data with a single-exponential time course (top, τ = 70 ms; middle, τ = 66 ms). Data from five similar experiments are shown in the bottom panel as in B.
To formally exclude the possibility that a fast component of SK channel recovery at the beginning of the application of high Ca2+ and CaM (WT) underlies the immediate increase of current, we measured the time course of SK channel activation with a higher temporal resolution after pretreatment with CaM (WT) in Ca2+-free solution. For this purpose, the recording pipette was first bathed in Ca2+-free solution containing CaM (WT) for an extended time (>1 h), and then quickly moved into a laminar flow of solution containing high Ca2+ while SK current was continuously recorded. If SK channels needed to be recovered in high Ca2+ before being activated by Ca2+ for the first time, we would expect the time course of activation to be slower compared with channels that are already recovered, as long as the recovery process is not significantly faster than the activation time course. As shown in Fig. 5 B, the time course of activation for the first time after pretreatment with CaM (WT) in Ca2+-free solution is not significantly different from later activations, in which the channels were known to be already assembled with CaM (WT). The time constants for activation (approximated with a single-exponential time course) of recovered SK channels were variable from patch to patch, likely reflecting the variable time it takes to change the solution around the inside-out patches, depending on factors such as patch geometry and endogenous Ca2+ buffer. As a result, the measured time constants are inevitably an overestimate of the true values. However, within each experiment, the time constants for the first activation are always similar compared with the average values for later activations (Fig. 5 B, bottom). On average, these numbers only differ by 18 ± 10% (in either direction; n = 6). Within the variation in the measurements, these results show that activation for the first time after pretreatment with CaM (WT) in Ca2+-free solution is not significantly slower than later activations, confirming that CaM (WT) indeed can assemble with SK channels in the patch in the absence of Ca2+, making them ready for activation. We have also performed similar experiments to estimate the recovery of SK channels with CaM (E/Q) and NS309 in the absence of Ca2+. In this case, CaM (E/Q) and NS309 were present at all times to avoid current rundown after recovery. As shown in Fig. 5 C, the time course of activation for the first time after pretreatment with CaM (E/Q) and NS309 in the absence of Ca2+ is also comparable to the time course for later activations. On average, the time course differs by 16 ± 16% (n = 5) between the first and later activations within the same patches (Fig. 5 C, bottom), indicating that CaM (E/Q) can also assemble with SK channels in the absence of Ca2+, as long as NS309 is present.
At least one functional N-lobe EF hand is required for recovery with NS309
In light of our finding that CaM with E/Q mutation at either EF1 or EF2 can only assemble with SK channels and regulate gating in the presence of NS309, we asked whether at least one functional N-lobe EF hand is required for this effect. As shown in Fig. 6 A, CaM (E12Q), in which both EF1 and EF2 carry the E/Q mutation, failed to recover SK channel activity, even in the presence of NS309. The application of CaM (E12Q) with NS309 led to an immediate increase of residual current (arrow), likely due to the effect of NS309 as explained earlier. However, there was no further increase in current. In the same patch, after CaM (E12Q) was washed away from the solution, SK channel activity could be recovered by CaM (E/Q) in the presence of NS309 as usual (Fig. 6 A, segment c). This result demonstrates again that NS309 alone is not sufficient to recover SK channels. However, in this case it is impossible to distinguish whether CaM (E12Q) cannot assemble with SK channels in the patch, or it assembles but cannot gate the channels even in the presence of NS309.
Figure 6.
CaM (E34Q), but not CaM (E12Q), can recover SK channel activity. (A) Time course of the average current level at −80 mV with a patch from an oocyte coexpressing SK channels with CaM (E2Q) while the patch was subjected to different solutions as indicated by the legend. (B) Time course of the average current level at −80 mV with a patch from an oocyte coexpressing SK channels with CaM (E2Q). (C) Dose–response relationship for the patch shown in B was measured after recovery of SK channels with CaM (E34Q) (open circles). Solid line is a fit with the Hill equation (EC50 = 1.24 µM and h = 2.7). Filled circles represent the dose–response relationship measured with a patch from an oocyte coexpressing SK channels with CaM (E34Q) fitted with the Hill equation (EC50 = 1.20 µM and h = 3.7).
C-lobe mutant CaMs can recover channel activity
Previously by coexpressing different EF hand mutant CaMs, it was concluded that intact EF hands at the C-lobe of CaM (EF3 and EF4) are dispensable for Ca2+-dependent gating of SK channels, whereas EF hands at the N-lobe are critical (Keen et al., 1999). However, the interpretation of such results is inevitably complicated by the presence of high levels of endogenous CaM (WT) in the expression system. Our findings with CaM (E/Q) have provided a way of verifying these results by applying different mutant CaMs directly onto WT SK channels from which CaM (E/Q) has dissociated. A mutant CaM in which Ca2+ binding is ablated at both the C-lobe EF hands with the E/Q mutations (E34Q, mutations at residues E105 and E141) could recover SK channel activity in a similar fashion as CaM (WT) (Fig. 6 B). In other experiments, CaM (E3Q) and (E4Q) with mutations to single C-lobe Ca2+-binding sites were both found able to recover SK channel activity likewise. The levels and kinetics of recovery by these mutant CaMs were comparable to those by CaM (WT). In complementary experiments, coexpression of CaM (E34Q, E3Q, or E4Q) led to SK channels that are functionally indistinguishable from control SK channels either expressed alone or coexpressed with CaM (WT). Additionally, the Ca2+-dependent activation of the SK channels recovered by CaM (E34Q, E3Q, or E4Q) is similar to SK channels coexpressed with the corresponding CaMs (e.g., Fig. 6 C), and not far from the control SK channels associated with CaM (WT). With data pooled together, the SK channels recovered by CaM (E34Q, E3Q, or E4Q) are activated by Ca2+ with EC50 = 1.16 ± 0.10 µM and h = 3.0 ± 0.4 (n = 7), only slightly different from SK channels recovered with CaM (WT) (EC50 = 0.92 ± 0.17 µM and h = 4.1 ± 1.8; n = 14). These results indicate that, consistent with the previous study with coexpression of mutant CaMs (Keen et al., 1999), intact C-lobe EF hands are neither important for the stable interaction between SK channels and CaM, nor for Ca2+-dependent activation.
DISCUSSION
Involvement of N-lobe EF hands in CaM–SK channel association
Previous studies on the interaction between SK channels and CaM have led to a modular functional assignment, with the C-lobe and the linker region of CaM underlying the constitutive association, and the N-lobe responsible for the Ca2+-dependent gating (Xia et al., 1998; Keen et al., 1999; Schumacher et al., 2001; Li and Aldrich, 2009). The contribution of either N-lobe EF hand to the gating of SK channels is not clear. By coexpression of mutant CaM with SK channels, a previous study concluded that when one of two N-lobe EF hands loses Ca2+-binding capacity, CaM can still stably gate the channel, albeit with reduced apparent affinity (Keen et al., 1999). However, we have found that when one N-lobe EF hand is mutated in such a way to lose its Ca2+-binding capacity, CaM can no longer stably associate with SK channels, and can only gate the SK channels in the presence of NS309. How do we reconcile our results with previous findings? One potential explanation would be that we have used a different mutation to remove the Ca2+-binding capacity of the EF hands. We have mutated the conserved glutamate (E) at position 12 to a glutamine (Q) (Maune et al., 1992) in contrast to the aspartate (D) to alanine (A) mutation at position 1 used in the previous study (Keen et al., 1999). For comparison, we made the same D to A mutation and conducted parallel experiments with the E to Q mutants. In all aspects that have been tested, we found that the D to A mutation produced very similar results as the E to Q mutation. Therefore, the difference in the mutations cannot explain our different findings. The functional effects of both E/Q and D/A mutations suggest that N-lobe EF hands are involved in the association between CaM and SK channel subunits.
Effects of endogenous WT CaM may explain discrepancies with previous work
We next considered the possible contribution by the endogenous CaM (WT), which is highly expressed in Xenopus oocytes, the expression system used in both studies. When mutant CaMs are coexpressed with SK channels, they have to compete with the endogenous CaM (WT) to assemble with SK channels. If, as our results suggest, the interaction between CaM (E/Q) and SK channels is destabilized, CaM (E/Q) is needed in great excess compared with the endogenous CaM (WT) to ensure that channels are only associated with mutant CaM. Given the high level of endogenous CaM (WT), it is possible that SK channels can be assembled with a mixture of CaM (WT) and mutant CaM. With a significant number of CaM (WT) (e.g., 3) in the complex, SK channels may maintain stable activity but with reduced apparent Ca2+ affinity, accounting for the stable activity of SK channels coexpressed with mutant CaM in the previous study (Keen et al., 1999), and the variable amounts of residual current after rundown in our study. With a lower number (or none) of CaM (WT) in the complex, SK channels may gate briefly after patch excision and then lose their activity when CaM (E/Q) dissociates from the complex, explaining the current rundown after patch excision. Some patches in our study had more stable residual current after rundown, allowing estimates of the dose–response relationship. The apparent Ca2+ affinity for these channels is variable (EC50 = 1.5–4 µM), but always considerably lower than control SK channels associated with CaM (WT) (EC50 = ∼1 µM), suggesting that SK channels conducting the residual current have variable, and often fewer than four, CaMs (WT) in the channel complex. Also in support of the idea that coassembly of mutant and WT CaM underlies the SK channels in the previous study and the residual channel activity in ours, we found that reducing the amount of RNA injection for CaM (E/Q) led to higher levels of stable SK channels with reduced apparent affinity for Ca2+.
If CaM (WT) and CaM (E/Q) are indeed coassembled into the same SK channel complex, different numbers of CaM(WT) and CaM(E/Q) can also help explain the two components in the fit of the dose–response relationship for SK channels recovered with CaM (E/Q) and NS309 (Fig. 4 C). More CaMs (WT) could allow the channels to gate in the presence of NS309 with an apparent affinity close to that of control SK channels associated with only CaM (WT) (∼100 nM), whereas more CaMs (E/Q), and therefore fewer CaMs (WT), in the complex would allow the channels to gate with a reduced apparent affinity (∼1 µM). Additionally, the contribution of endogenous CaM (WT) could also help explain the observed smaller difference between the effects of T80A and T80D mutant CaMs (Fig. 3) compared with an earlier study (Bildl et al., 2004), if in our study there were more endogenous CaMs (WT) coassembled with CaM (T80D) or CaM (T80A), diminishing the difference.
Additional evidence supporting the coassembly of mutant and WT CaM with SK channels came from the experiments with oocytes coexpressing SK channels and CaM (E12Q). We were able to record some initial SK currents (although very little) upon patch excision, which quickly ran down, and channel activity could be recovered with CaM (WT). CaM (E12Q) cannot bind Ca2+ at either of the two N-lobe EF hands and should not be able to gate the channel (Keen et al., 1999; Li and Aldrich, 2009). Therefore, the initial channel activity that quickly ran down must be due to channels coassembled with both CaM (WT) and CaM (E12Q). Although CaM (E12Q) cannot bind Ca2+, it may somehow help support the gating of SK channels by the coassembled WT CaMs in the complex, such that channel activity ran down when CaM (E12Q) dissociated. One possibility would be that a certain number (e.g., three) of CaMs (even if some cannot bind Ca2+) are always required for stable gating of SK channels. Interestingly, an earlier study using a tandem dimer SK construct also suggests that at least three CaMs must be bound for SK channel function (Keen et al., 1999).
Disruption of stable SK channel–CaM interaction by N-lobe EF hand mutation
Surface expression of SK channels is known to require association with CaM (Lee et al., 2003). The initial rundown of channel activity, the reduced apparent affinity of the residual current, and the ability of exogenous CaM to recover channel activity all demonstrate that CaM (E/Q) is able to interact with SK channels, preventing them from assembling with four endogenous CaMs (WT). However, our recovery experiments (Fig. 4) suggest that CaM (E/Q) in solution does not effectively bind with SK channels in the patch after rundown. This suggests that CaM (E/Q) can more readily interact with SK channels during protein synthesis and the initial assembly of the channel complex before delivery to the membrane. Alternatively, assembly of CaM (E/Q) with SK channels could require intracellular factors that are lost during patch excision, or perhaps CaM (E/Q) is present at a much higher local concentration during the initial assembly of channel complex than we could achieve in our recovery experiments.
Regardless of the exact reasons underlying the differences between the previous study (Keen et al., 1999) and ours, our results clearly demonstrate that CaM (E/Q), with only one N-lobe EF hand losing its ability to bind Ca2+, cannot stably bind with SK channels in inside-out patches. This finding is unexpected from the current modular hypothesis for the interaction between CaM and SK channels, according to which the EF hands at the N-lobe should be unimportant for the constitutive binding. Then how does the mutation at N-lobe EF hands affect the stable interaction? CaM can assemble with SK channels in the absence of Ca2+ (Fig. 5), consistent with similar findings with a mutant SK channel in an earlier study (Lee et al., 2003). Therefore, the disrupted interaction cannot be accounted for by a simple loss of Ca2+ binding at one EF hand. It seems that E to Q (or D to A) mutations at the EF hand not only result in the loss of its ability to bind Ca2+, but also lead to additional changes in the part of CaM that regulates its stable interaction with SK channels independent of Ca2+. In other words, even in the Apo (Ca2+-free) form, CaM (E/Q) is functionally different from CaM (WT). Of note, E to Q mutations at EF hands of drosophila CaM were previously found to result in abnormal conformations in their complexes with model target peptides (Mukherjea and Beckingham, 1993). To this end, it is interesting that the same mutation at the EF hands of the C-lobe of CaM (EF3 and 4), supposedly regulating the constitutive binding with SK channels, had no such effects. Further studies are needed to elucidate the exact mechanism for the mutational effect, but our results imply a role of the N-lobe of CaM in the constitutive association between the CaM and SK channels. The interaction between the two is likely more complicated than explained by the modular hypothesis.
Potential molecular mechanism of NS309
Interestingly, a potent SK channel enhancer, NS309, facilitated the assembly of CaM (E/Q) with SK channel and recovery of channel activity (Fig. 4). Since its identification (Strøbaek et al., 2004), NS309 has been frequently used as a positive modulator for SK and intermediate conductance calcium-activated potassium channels, but the molecular mechanisms for its modulation remain unclear. However, another less potent but functionally similar modulator, EBIO, was thought to modulate the Ca2+ gating of SK channels by stabilizing the interaction between SK channels and CaM (Pedarzani et al., 2001). Our results are consistent with NS309 having a similar effect of stabilizing this interaction, but not consistent with NS309 simply increasing Ca2+ affinity of CaM, as higher Ca2+ concentrations up to 1mM failed to substitute for the effect of NS309 in channel recovery with CaM (E/Q). This stabilizing effect seems independent of Ca2+, as suggested by our finding that CaM (E/Q) can assemble with channels in the absence of Ca2+ (Fig. 5 C). On the other hand, the enhanced interaction between CaM and SK channels by NS309 is not sufficient to open SK channels. In the absence of Ca2+, the open probability of SK channels remains very low, even in the presence of saturating concentrations of NS309 (Fig. 4 C), consistent with earlier studies with both NS309 and EBIO (Pedarzani et al., 2001; Strøbaek et al., 2004). It is possible that NS309, by stabilizing the interaction between CaM and SK channels, enhances the coupling between Ca2+ binding and the gating of the SK channel, such that less Ca2+ needs to bind for the channel to open. This could explain the fact that NS309 increases apparent Ca2+ affinity for channels associated with CaM (WT), as well as the fact that CaM (E/Q) and NS309 could recover significantly higher channel activity than the initial level upon patch excision, when the channels may still have CaM (E/Q) assembled but gate with low open probability (Fig. 4 B).
Conclusions
We have confirmed some aspects of the current understanding of the gating mechanisms for SK channels in that EF hands in the N-lobe, but not the C-lobe, bind Ca2+ and gate the channels. However, in contrast to the current modular hypothesis, our results suggest that EF hands in the N-lobe of CaM are also involved in the Ca2+-independent stable interaction between CaM and SK channel subunits. Calling into question a previous conclusion that a single functional N-lobe EF hand in CaM is sufficient to stably gate SK channels, we found that two intact N-lobe EF hands are required for the stable interaction between CaM and SK channels. Additionally, our findings suggest that NS309 likely enhances SK channels by stabilizing this interaction.
Acknowledgments
The authors wish to thank Dr. John Adelman for providing the rSK2 construct and Dr. Susan Hamilton for the CaM construct. We are grateful for Dr. Adron Harris for providing Xenopus oocytes.
Christopher Miller served as editor.
Footnotes
Abbreviations used in this paper:
- CaM
- calmodulin
- NS309
- 3-oxime-6,7-dichloro-1H-indole-2,3-dione
- SK
- small conductance calcium-activated potassium
- WT
- wild-type
References
- Bildl W., Strassmaier T., Thurm H., Andersen J., Eble S., Oliver D., Knipper M., Mann M., Schulte U., Adelman J.P., Fakler B. 2004. Protein kinase CK2 is coassembled with small conductance Ca2+-activated K+ channels and regulates channel gating.Neuron. 43:847–858 doi:10.1016/j.neuron.2004.08.033 [DOI] [PubMed] [Google Scholar]
- Doughty J.M., Plane F., Langton P.D. 1999. Charybdotoxin and apamin block EDHF in rat mesenteric artery if selectively applied to the endothelium.Am. J. Physiol. 276:H1107–H1112 [DOI] [PubMed] [Google Scholar]
- Gopalakrishna R., Anderson W.B. 1982. Ca2+-induced hydrophobic site on calmodulin: application for purification of calmodulin by phenyl-Sepharose affinity chromatography.Biochem. Biophys. Res. Commun. 104:830–836 doi:10.1016/0006-291X(82)90712-4 [DOI] [PubMed] [Google Scholar]
- Herrera G.M., Heppner T.J., Nelson M.T. 2000. Regulation of urinary bladder smooth muscle contractions by ryanodine receptors and BK and SK channels.Am. J. Physiol. Regul. Integr. Comp. Physiol. 279:R60–R68 [DOI] [PubMed] [Google Scholar]
- Keen J.E., Khawaled R., Farrens D.L., Neelands T., Rivard A., Bond C.T., Janowsky A., Fakler B., Adelman J.P., Maylie J. 1999. Domains responsible for constitutive and Ca2+-dependent interactions between calmodulin and small conductance Ca2+-activated potassium channels.J. Neurosci. 19:8830–8838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Köhler M., Hirschberg B., Bond C.T., Kinzie J.M., Marrion N.V., Maylie J., Adelman J.P. 1996. Small-conductance, calcium-activated potassium channels from mammalian brain.Science. 273:1709–1714 doi:10.1126/science.273.5282.1709 [DOI] [PubMed] [Google Scholar]
- Lee W.S., Ngo-Anh T.J., Bruening-Wright A., Maylie J., Adelman J.P. 2003. Small conductance Ca2+-activated K+ channels and calmodulin: cell surface expression and gating.J. Biol. Chem. 278:25940–25946 doi:10.1074/jbc.M302091200 [DOI] [PubMed] [Google Scholar]
- Li W., Aldrich R.W. 2009. Activation of the SK potassium channel-calmodulin complex by nanomolar concentrations of terbium.Proc. Natl. Acad. Sci. USA. 106:1075–1080 doi:10.1073/pnas.0812008106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowry O.H., Rosebrough N.J., Farr A.L., Randall R.J. 1951. Protein measurement with the Folin phenol reagent.J. Biol. Chem. 193:265–275 [PubMed] [Google Scholar]
- Maune J.F., Klee C.B., Beckingham K. 1992. Ca2+ binding and conformational change in two series of point mutations to the individual Ca2+-binding sites of calmodulin.J. Biol. Chem. 267:5286–5295 [PubMed] [Google Scholar]
- Mukherjea P., Beckingham K. 1993. Calcium binding site mutants of calmodulin adopt abnormal conformations in complexes with model target peptides.Biochem. Mol. Biol. Int. 29:555–563 [PubMed] [Google Scholar]
- Mukherjea P., Maune J.F., Beckingham K. 1996. Interlobe communication in multiple calcium-binding site mutants of Drosophila calmodulin.Protein Sci. 5:468–477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedarzani P., Mosbacher J., Rivard A., Cingolani L.A., Oliver D., Stocker M., Adelman J.P., Fakler B. 2001. Control of electrical activity in central neurons by modulating the gating of small conductance Ca2+-activated K+ channels.J. Biol. Chem. 276:9762–9769 doi:10.1074/jbc.M010001200 [DOI] [PubMed] [Google Scholar]
- Richman P.G., Klee C.B. 1979. Specific perturbation by Ca2+ of tyrosyl residue 138 of calmodulin.J. Biol. Chem. 254:5372–5376 [PubMed] [Google Scholar]
- Schumacher M.A., Rivard A.F., Bächinger H.P., Adelman J.P. 2001. Structure of the gating domain of a Ca2+-activated K+ channel complexed with Ca2+/calmodulin.Nature. 410:1120–1124 doi:10.1038/35074145 [DOI] [PubMed] [Google Scholar]
- Soh H., Park C.S. 2001. Inwardly rectifying current-voltage relationship of small-conductance Ca2+-activated K+ channels rendered by intracellular divalent cation blockade.Biophys. J. 80:2207–2215 doi:10.1016/S0006-3495(01)76193-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soh H., Park C.S. 2002. Localization of divalent cation-binding site in the pore of a small conductance Ca2+-activated K+ channel and its role in determining current-voltage relationship.Biophys. J. 83:2528–2538 doi:10.1016/S0006-3495(02)75264-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stocker M., Krause M., Pedarzani P. 1999. An apamin-sensitive Ca2+-activated K+ current in hippocampal pyramidal neurons.Proc. Natl. Acad. Sci. USA. 96:4662–4667 doi:10.1073/pnas.96.8.4662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strøbaek D., Teuber L., Jørgensen T.D., Ahring P.K., Kjaer K., Hansen R.S., Olesen S.P., Christophersen P., Skaaning-Jensen B. 2004. Activation of human IK and SK Ca2+-activated K+ channels by NS309 (6,7-dichloro-1H-indole-2,3-dione 3-oxime).Biochim. Biophys. Acta. 1665:1–5 doi:10.1016/j.bbamem.2004.07.006 [DOI] [PubMed] [Google Scholar]
- Tse A., Hille B. 1992. GnRH-induced Ca2+ oscillations and rhythmic hyperpolarizations of pituitary gonadotropes.Science. 255:462–464 doi:10.1126/science.1734523 [DOI] [PubMed] [Google Scholar]
- Xia X.M., Fakler B., Rivard A., Wayman G., Johnson-Pais T., Keen J.E., Ishii T., Hirschberg B., Bond C.T., Lutsenko S., et al. 1998. Mechanism of calcium gating in small-conductance calcium-activated potassium channels.Nature. 395:503–507 doi:10.1038/26758 [DOI] [PubMed] [Google Scholar]
- Yamniuk A.P., Vogel H.J. 2004. Calmodulin's flexibility allows for promiscuity in its interactions with target proteins and peptides.Mol. Biotechnol. 27:33–57 doi:10.1385/MB:27:1:33 [DOI] [PubMed] [Google Scholar]