Summary
Background
Obesity is a significant risk factor for psoriasis and body mass index (BMI) correlates with disease severity.
Objectives
To investigate the relationship between obesity and psoriasis, focusing on the role of adipokines such as leptin and resistin.
Patients/Methods
Psoriasis patients (n=30) were recruited and their BMI, waist circumference and disease severity (PASI) were recorded. Fasting serum samples were obtained on enrollment and after a course of UVB treatment. Age, sex and BMI-matched healthy controls were also recruited.
Results
On enrollment, serum leptin and soluble leptin receptor levels were not raised compared with the controls. However, resistin, IL-1β, IL-6, CCL2, CXCL8 and CXCL9 were all significantly elevated in the patient group and serum resistin correlated with disease severity (r=0.372, p=0.043). Improvement after UVB treatment was accompanied by decreased serum CXCL8. In vitro, both leptin and resistin could induce CXCL8 and TNF-α production by blood monocytes, and leptin could additionally induce IL-1β and IL-1ra production. Leptin also dose dependently increased secretion of the growth factor amphiregulin by ex vivo-cultured lesional psoriasis skin.
Conclusions
These data support the view that leptin and resistin may be involved in the pathogenesis of psoriasis in overweight individuals, possibly by augmenting the cytokine expression by the inflammatory infiltrate.
Keywords: Psoriasis, Obesity, Leptin, Resistin, UVB, Cytokines
Introduction
Psoriasis is a common inflammatory T cell-mediated skin disorder, affecting 2-3% of the population 1, in which the most prominent microscopic abnormality is hyperproliferation and altered differentiation of keratinocytes. While the disease has several distinct yet overlapping phenotypes 2 by far the most common is chronic plaque psoriasis, which affects about 90% of patients. The etiology of psoriasis is unknown but the disease is believed to have an autoimmune basis and a strong genetic component 3. Several HLA alleles are associated with psoriasis, in particular HLA-Cw*0602 which is probably the major genetic determinant of the disease 4. Despite strong hereditary factors exogenous stimuli such as infection, trauma, and stress play an important role in disease manifestation 5-8.
Obesity has long been associated with and considered detrimental for psoriasis. Henseler and Christophers reported in 1995 that a substantial proportion of psoriasis patients hospitalized for treatment were obese 9. Patients over ideal bodyweight also tend to have worse psoriasis in terms of the proportion of involved skin 10, and the extent of their psoriasis lesions correlates with body mass index (BMI) 11. In a recent case-control study, Naldi and colleagues 8 found that a moderately increased BMI (26 to 29), was associated with slightly increased risk of psoriasis and clinical obesity (BMI>29) more than doubled the risk of psoriasis. Further support for a link between these two conditions comes from the observation that obesity is more prevalent in patients with severe as opposed to mild psoriasis 12 and an increased prevalence of the metabolic syndrome in psoriasis patients has recently been reported 13. Reports also exist of a favorable outcome after 4 weeks on a low-energy (855 kcal day-1) diet 14 or resolution of psoriasis after gastric bypass surgery 15, but such treatment modalities require closer examination and controlled trials. Thus, a causal relationship between obesity and psoriasis has not been fully established as obesity may occur as a consequence of developing psoriasis 16, although the obese state may well exacerbate the severity of the disease or derive from a common underlying pathophysiology 17.
White adipose tissue is composed of mature triglyceride-filled adipocytes, along with pre-adipocytes, endothelial cells, fibroblasts and leukocytes 18. Expansion of adipose tissue during weight gain leads to the recruitment of macrophages into the adipose tissue 19 and this is probably mediated by adipocyte-derived chemokines such as CCL2 (monocyte chemoattractant protein-1) 20. Macrophages are the chief source of adipose tissue-derived tumor necrosis factor (TNF)-α 21 and are an important component of the non-adipocyte fraction of this tissue which is also the main source of IL-6 and CXCL8 22. These cytokines are abundant in psoriasis skin 23, their levels in suction blister fluids of involved psoriasis skin correlate with disease severity 24 and both have established roles in psoriasis pathogenesis 25.
Leptin is one of the main adipose-derived cytokines and has been investigated primarily for its role in controlling energy homeostasis by regulating appetite 26,27. Leptin is also important for cell-mediated immunity and CD4+ T cells are hyporeactive in leptin deficient mice 28. Congenital leptin deficiency in humans results in lower frequency of blood CD4+ T cells as well as impaired T cell proliferation and production of cytokines such as IFN-γ 29, and this hyporesponsiveness can be restored by exogenous leptin 30. Patients suffering from common variable immunodeficiency have lower serum leptin levels than healthy individuals; however, administration of exogenous leptin could not reverse this deficiency 31,32. Leptin appears to contribute to Th1 and suppresses Th2 immune responses. In vitro, leptin acts on naive T cells, increasing their IL-2 secretion and proliferation, as well as increasing IFN-γ production by memory T cells 28. Leptin is required for the induction and progression of autoimmune encephalomyelitis in mice 33, and it was recently shown that leptin inhibits the proliferation of CD4+FoxP3+ T regulatory cells 34. Thus, elevated leptin levels may lead to enhanced Th1 type immune responses due to diminished T regulatory activity. Leptin also increases macrophage activity and their production of IL-1β, IL-6, TNF-α and IL-12 35,36. Additionally, leptin can alter the morphology of monocyte-derived dendritic cells and increase their production of IL-1β, IL-6, TNF-α and IL-12p70, and priming of naïve T cells by leptin-treated dendritic cells resulted in increased Th1 polarization 37.
Resistin was originally discovered in mouse adipocytes and assigned a key role in the induction of murine insulin resistance 38. Human adipocytes however do not produce resistin 39, but resistin is expressed by cells in the stromal compartment of adipose tissue 21, particularly macrophages 19. Resistin mRNA is increased in the subcutaneous adipose tissue of obese compared with lean individuals 40, but there is only a very weak correlation between BMI and serum resistin levels 41 although the proportion of mononuclear leukocytes within adipose tissue correlates well with BMI 19. Resistin is also expressed by peripheral blood mononuclear cells (PBMC) 42,43 particularly by monocytes 44,45 and is upregulated during their differentiation into macrophages 44,45. Lipopolysaccharide (LPS) and the inflammatory cytokines IL-1β, IL-6 and TNF-α have all been demonstrated to induce resistin mRNA expression by human PBMCs 42, and elevated levels of resistin can be induced in the blood of healthy individuals in response to exogenous LPS 45. Resistin dose-dependently stimulates its own production (autocrine effect) and stimulates TNF-α, IL-1β, IL-6, and CXCL8 in PBMCs 43 as well as IL-12 in macrophages 46. The autocrine effect of resistin and its ability to induce other pro-inflammatory cytokines that in turn can stimulate more resistin synthesis, suggests a vicious cycle type pathogenic role for resistin.
Although numerous clinical studies have suggested that obesity has an adverse effect on psoriasis 8-13,16 information is lacking about potential pathophysiological pathways that may be responsible for this association. Here we examine serum adipokine and cytokine levels of patients before and after a course of narrow-band 310 nm ultraviolet B (NB-UVB) treatment and compare with BMI-matched non-psoriatic controls. Further, we investigate how either local or systemic increases in leptin or resistin might trigger or exacerbate psoriasis.
Materials & Methods
Recruitment and clinical evaluation of patients and controls
Thirty chronic plaque psoriasis patients and 29 age, sex and body mass index (BMI)-matched controls were recruited to the study. None of the patients were on systemic treatment. On recruitment, weight, height and waist circumference of all individuals in the study were recorded. Disease severity was assessed before and after treatment with the Psoriasis Area and Severity Index (PASI) 47 by the same physician (JTS). All patients completed a questionnaire involving past treatment (medication or visits to the Blue Lagoon) and whether they had noticed a change in their condition after losing or gaining weight. Patients underwent treatment in the Blue Lagoon Dermatological Clinic, which involves regular bathing in the lagoon water combined with NB-UVB irradiation. On completion of treatment, the PASI score, weight and waist measurements were again recorded and a second fasting serum sample taken. All participants gave their informed consent before enrolment. The National Bioethics Committee of Iceland and the Icelandic Data Protection Authority approved the study. A further 16 chronic plaque psoriasis patients and 3 healthy control volunteers were recruited for skin biopsy for ex-vivo skin culture and imunohistochemistry. Informed consent was obtained from all subjects, under protocols approved by the Institutional Review Board of the University of Michigan.
Measurement of cytokines, adipokines and leptin receptor in serum
Blood was collected from patients and controls after overnight fast. Serum was isolated after clotting and stored in aliquots at -70°C until used. Leptin, soluble leptin receptor, adiponectin, resistin, CXCL8, IL-22 were determined by enzyme-linked immunosorbent assay (ELISA) (R&D Systems, Oxford, UK). The cytokines IL-1β, IL-6, IL-10, IL-12p70, CCL2 and CXCL9 were measured using a microsphere-based multiplexed immunoassay (Bio-Plex, Bio-Rad, Sundbyberg, Sweden).
Monocyte cytokine production in stimulated whole blood
Sodium heparin-treated whole blood was collected from healthy volunteers and incubated for 16 hours with recombinant human resistin (SCBT, Heidelberg, Germany) or recombinant human leptin (SCBT) in the presence of 10 μg mL-1 brefeldin A (Sigma). Cells were first stained for surface CD14 expression (PerCP-CD14, clone MφP9, BD Biosciences), then erythrocytes were lysed (FACS lysing solution, BD Biosciences), lymphocytes fixed and permeabilised (FACS permeabilising solution, BD Biosciences), and stained intracellularly with FITC, PE or APC-labeled monoclonal antibodies against IL-1ra (clone AS17), IL-1β (AS10), CXCL8 (AS14) and TNF-α (6401.1111, BD Biosciences). After washing, cells were analyzed using a FACScalibur flow cytometer and Cell Quest Pro software (BD Biosciences).
Ex vivo skin culture
Three psoriatic and three control donors each gave eight 2mm punch skin biopsies. The biopsies were treated with different concentrations of recombinant leptin (R&D Systems, Minneapolis, MN, USA) for a total of 5 days in M154 medium (Cascade Biologics, Portland, OR, USA) when the tissue supernatants were harvested and stored at -70°C. Amphiregulin was quantified using an ELISA (R&D Systems) according to the manufacturer’s instructions. Recombinant human amphiregulin (R&D Systems) was used as the standard, and the blank was unexposed culture medium.
Immunohistochemical staining and automated analysis
Immunohistochemistry was performed with anti-leptin receptor antibody (R&D Systems) according to the manufacturer’s instructions and visualized with 3-amino-9-ethyl-carbazole (BioGenex, San Ramon, CA), followed by hematoxylin counterstain (Biocare Medical, Concord, CA). Stained sections were examined by light microscopy, and each stained tissue section was subjected to image capture in its entirety via 5 digital images taken through the 20X objective. All images were then analyzed using Image Pro software (Image-Pro Plus, Media Cybernetics, Silver Spring, MD, USA), to capture the area of positive staining for leptin receptor in epidermis versus dermis. Statistical analysis was performed using a two-tailed unpaired t test assuming equal variances, p values ≤ 0.05 were considered to be significant.
Real-time quantitative PCR
Leptin and leptin receptor expression were validated by quantitative real-time PCR (QRT-PCR). Primers for leptin (Cat. No PPH00581E), leptin receptor (Cat No. PPH00028B) and the housekeeping gene RPLP0 (36B4, Cat No. PPH21138E) were obtained from Superarray Biosciences (Frederick, MD, USA). The reverse transcription reaction was performed on 0.5μg of RNA template and cDNA was synthesized using anchored-oligo (dT)18 primers as instructed by the manufacturer (Roche Diagnostics, Mannheim, Germany). QRT-PCR was carried out on the LightCycler 2.0 system (Roche Diagnostics, Mannheim, Germany). LightCycler FastStart DNA MasterPLUS SYBR Green I was used for all PCR reactions as instructed by the manufacturer. The reaction profile consisted of an initial denaturation at 95°C for 15 minutes followed by 40 cycles of PCR at 95°C for 10 seconds (denaturation), 58°C for 10 seconds (annealing) and 72°C for 10 seconds (extension). The fluorescence emitted was captured at the end of the extension step of each cycle at 530nm. Results were normalized to the expression of the housekeeping gene 18S ribosomal RNA.
Statistical analyses
Datasets were tested for normality using the Kolmogorov-Smirnov test, and statistical significance determined by Student’s t-test, paired t-test (pre- and post-treatment) or Mann-Whitney Rank Sum tests where appropriate using Sigma Plot version 10 for windows (Systat, Erkrath, Germany.) Data series were also tested for significance with a one-way ANOVA with Dunnett’s post test using GraphPad Prism version 4 for Windows (GraphPad Software, San Diego, CA, USA.)
Results
The study groups
The treatment group comprised 30 chronic plaque psoriasis patients (14 women and 16 men) and 29 age, sex and body mass index (BMI)-matched volunteers (16 women and 13 men) with no skin disease. As shown in table 1, the patients and controls were very similar regarding age, weight, waist circumference, BMI, fasting serum triglycerides, cholesterol or high-density lipoproteins. The weight and waist circumference of the patients did not change during the treatment. The patients responded to a questionnaire. While the majority of the patients had not noticed any marked changes in their body weight after the onset of psoriasis, six (20%, 2 women and 4 men) reported improvement in their disease following weight loss. Four reported that scaling and erythema lessened after weight loss and worsened following weight gain and the remaining 2 patients reported that scaling and erythema was decreased following weight loss.
Table 1. Characteristics of the patient and control study groups.
Mean values for each measurement are shown together with minimum and maximum values.
| Controls | Patients | |
|---|---|---|
| Age (y) | 47.14 | 52.87 |
| 22 - 83 | 24 - 77 | |
| Height (m) | 1.67 | 1.73 |
| 1.52 - 1.83 | 1.54 - 1.88 | |
| Weight (kg) | 86.05 | 90.54 |
| 59.6 - 143 | 58.7 - 125 | |
|
Waist circumference (cm) |
105.61 | 105.00 |
| 73.6 - 149 | 77 - 129 | |
| BMI (kg m-2) | 30.81 | 30.51 |
| 22.0 - 49 .5 | 18.8 - 39.4 | |
|
Triglycerides (mmol L-1) |
1.32 | 1.57 |
| 0.6 - 4.6 | 0.5 - 4.8 | |
|
Cholesterol (mmol L-1) |
5.69 | 6.01 |
| 3.9 - 7.8 | 3.7 - 8.7 | |
|
High density lipoproteins (mmol L-1) |
1.61 | 1.51 |
| 1.0 - 2.6 | 0.8 - 2.8 |
Disease severity
The patients’ disease severity of was evaluated before and after the treatment using the PASI score. The mean score was 15.33 on enrollment (range 4.9 – 34.8). As expected, a dramatic decrease in disease severity (PASI) was recorded after the UVB and bathing treatment (mean PASI 3.84, p < 0.001).
BMI and waist circumference correlate with serum leptin
The patients and matched controls enrolled in this study had a mean BMI of 30.5 and 30.8 kg m-2 respectively. Amongst men in the control group, there was a strong positive correlation between serum leptin and BMI (r = 0.87, p < 0.001), but this was not the case for the female controls (r = 0.26, not significant). Interestingly, both male and female psoriasis patients demonstrated a correlation between BMI and leptin (r = 0.65, p = 0.012 for women and r = 0.59, p = 0.016 for men). A very similar relationship existed between waist circumference and serum leptin in both groups. There was a weak correlation between BMI and disease severity amongst the men (r = 0.32) but this was not statistically significant and no correlation between the two variables was seen amongst the women.
Leptin is not elevated in the blood of psoriasis patients
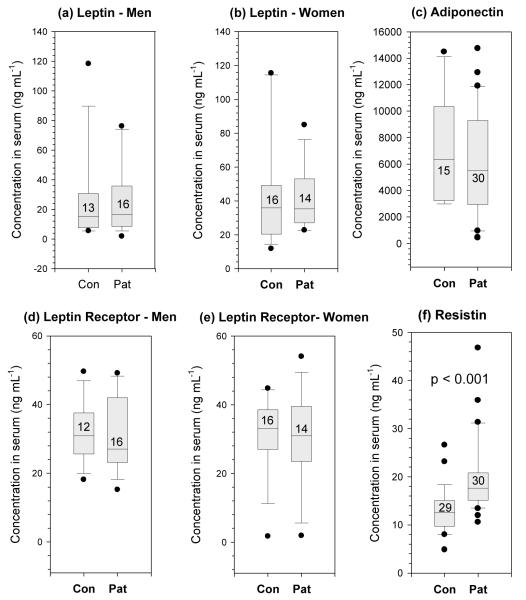
There were no significant differences in the serum concentrations of leptin, soluble leptin receptor or adiponectin between patients and controls (Figure 1a-e). As expected, women in both the patient and control groups had significantly higher serum leptin levels than the men (p = 0.017). However, patients’ leptin levels were not significantly different from those of the BMI-matched non-psoriasis controls for either sex (Figure 1a and b). Serum leptin levels did not correlate with disease severity in neither male nor female patients.
Figure 1. Adipokine levels in serum from patients and matched controls.
Adipokines were measured by ELISA in the serum of psoriasis patients and healthy BMI, age and sex matched controls after overnight fast. Leptin, soluble leptin receptor and adiponectin were found not to be elevated in patients compared with matched controls. Resistin, however, was significantly increased. Boxes represent median and interquartile range, whiskers indicate the 10th and 90th percentiles, with values outside this range plotted individually; numbers within boxes indicate the number of individuals tested.
Serum resistin is elevated in psoriasis and correlated with disease severity
The patients had significantly more resistin in their serum than did the controls (p < 0.001, Figure 1f) and this correlated with their disease severity (r = 0.372, p = 0.043 Figure 2).
Figure 2.
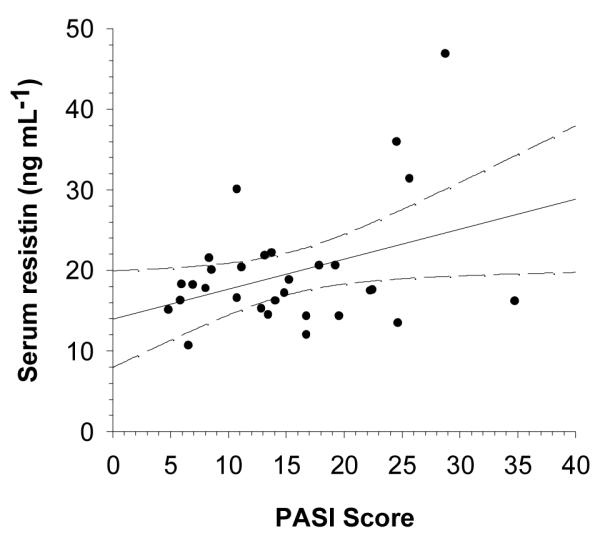
Disease severity (PASI) correlates with patients’ serum resistin concentration (n = 30, r = 0.372, p = 0.043). Dashed lines indicate 95% confidence intervals.
Serum cytokine levels are altered in psoriasis patients, and responded to NB-UVB therapy
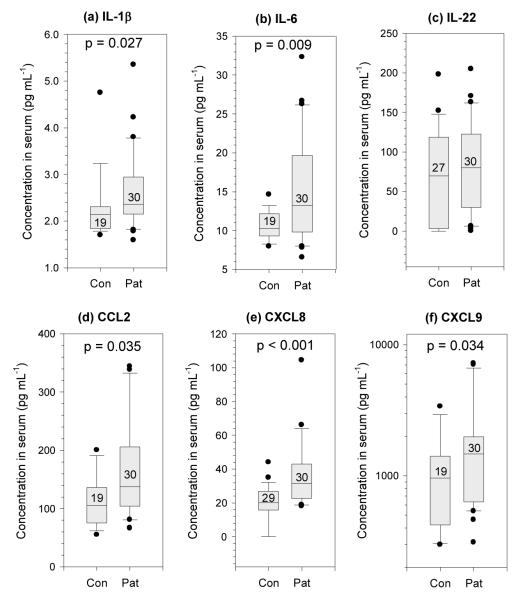
IL-1β, IL-6, CCL2, CXCL8, and CXCL9 were all significantly elevated in the pre-treatment serum of the patients compared with the controls (Figure 3); however no difference was seen in levels of IL-22 (Figure 3c). Serum IL-10 and IL-12p70 were also increased in the patients (data not shown). Following the treatment, a significant decrease was seen in the serum concentration of both CXCL8 (p = 0.001) and IL-22 (p < 0.001), but no significant changes were observed in serum for IL-1β or IL-6 (data not shown). Although some decrease was observed in resistin levels after treatment this was not significant (p = 0.1).
Figure 3. Cytokine levels in serum from patients and controls.
Cytokines were measured in fasting serum by multiplex assay or ELISA. IL-1β, IL-6, CCL2 (MCP-1), CXCL8 (IL-8) and CXCL9 (MIG) were all significantly elevated in patients compared with matched healthy controls. Boxes and whiskers are described in Figure 1.
Leptin and resistin induce pro-inflammatory cytokine production by monocytes
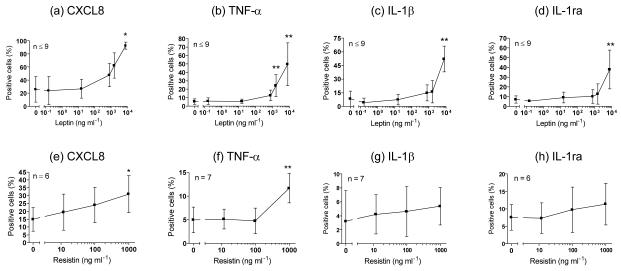
As serum leptin correlated with the BMI of psoriasis patients and serum resistin correlated with their disease severity, we chose to examine the effects of leptin and resistin on cytokine production by blood monocytes ex vivo using intracellular cytokine staining and flow cytometry. The cytokine content of monocytes after 16 hours of incubation with recombinant leptin or resistin is shown in Figure 4. Both leptin and resistin could induce significant amounts of CXCL8 and TNF-α production by the monocytes. In addition, leptin was able to induce the accumulation of IL-1β and IL-1ra in monocytes (Figure 4c and d).
Figure 4. Exogenous leptin (a-d) and resistin (e-h) induce blood monocytes from healthy volunteers to produce pro-inflammatory cytokines ex vivo in a dose-dependent manner.
Plots show proportion of cytokine+ CD14+ monocytes detected by intracellular cytokine staining and flow cytometry (mean ± SD). Statistical significance indicated as * p < 0.05, ** p < 0.01, by 1-way ANOVA with Dunnett’s test.
Leptin induced amphiregulin expression by in vitro cultured skin
As the direct effects of leptin on skin are currently unknown, we exposed biopsies from healthy and plaque psoriasis skin to leptin in vitro. Addition of exogenous leptin led to a dose-dependent accumulation of the EGF-like growth factor amphiregulin in the medium from cultured psoriasis skin, but an increase in amphiregulin was not observed when non-psoriatic control skin was cultured (Figure 5).
Figure 5. Exogenous leptin induces in vitro cultured psoriasis skin to express the growth factor amphiregulin in a dose-dependent manner.
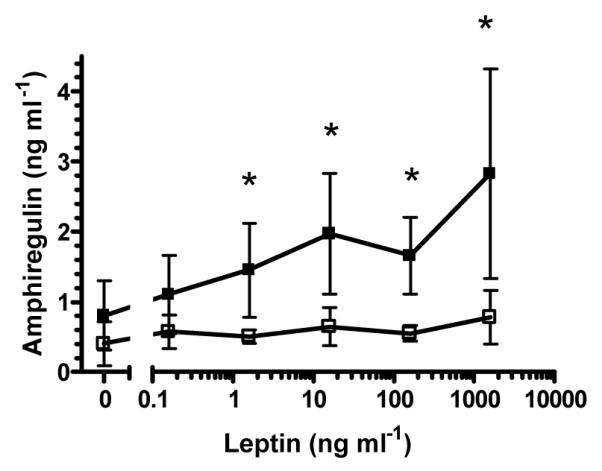
Control skin- open squares, psoriasis skin — filled squares, mean (n = 3) ± S.D. * p < 0.05 1-tailed t-test.
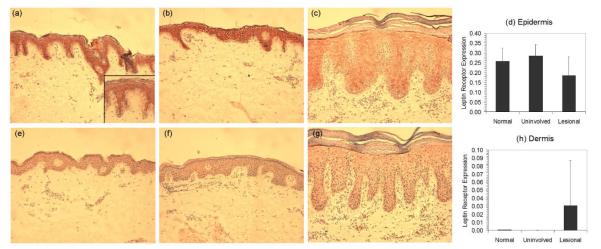
Enhanced expression of leptin receptor was seen in uninvolved psoriasis skin, but was downregulated in lesional epidermis
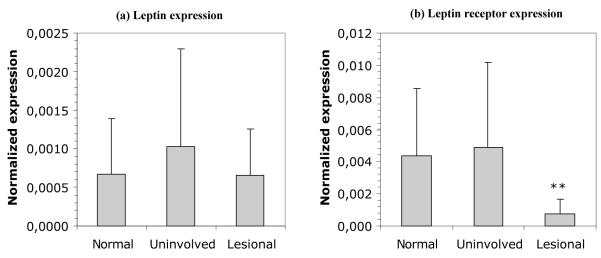
Because psoriasis skin, but not healthy skin, responded to the addition of exogenous leptin by producing the growth factor amphiregulin, we investigated whether this difference was due to variations in leptin receptor expression. First we examined the leptin and leptin receptor mRNA expression in healthy as well as symptom-free and lesional psoriasis skin using quantitative RT-PCR (Figure 6). All three skin phenotypes contained similar amounts of leptin mRNA (Figure 6a). Lesional psoriasis skin, however, contained significantly (p < 0.005) less mRNA for leptin receptor than either healthy or symptom-free psoriasis skin (Figure 6b) and this observation was supported immunohistochemically (Figure 7). Thus, lesional psoriasis epidermis (Figure 7c) expressed less leptin receptor than either healthy (Figure 7a) or uninvolved psoriatic epidermis (Figure 7b) and this finding was confirmed using computer-assisted image quantification (Figure 7d). Interestingly, the converse was seen in the dermal compartment, where very little leptin receptor staining was seen in healthy or clinically uninvolved dermis, but some cells within the lesional dermis stained strongly for leptin receptors (Figure 7c and h).
Figure 6. Evalution of (a) leptin and (b) leptin receptor mRNA expression levels in skin by QRT-PCR.
Leptin mRNA levels are of similar magnitude in healthy, symptomless psoriasis and lesional psoriasis skin. However, leptin receptor mRNA expression is significantly diminished in lesional psoriasis skin compared with both healthy and symptomless skin. Mean (n = 13) ± SD ** p < 0.005, 2-tailed t-test.
Figure 7. Immunohistochemical evaluation of leptin receptor expression in (a) healthy skin, (b) uninvolved and (c) lesional psoriasis skin.
Following immunohistochemical detection of leptin receptor in skin biopsies from 3 healthy and 3 psoriatic donors the strength and extent of receptor expression (red staining) was evaluated in the (d) epidermal and (h) dermal compartments using automated analysis with Image Pro software. For comparison isotype control staining is also shown (e-g).
Discussion
In this study we show that serum resistin is elevated in psoriasis patients compared with age-, sex- and BMI-matched healthy controls and that resistin correlates with disease severity. This finding confirms a small prospective study assessing the effects of treatment with the oral retinoid acitretin 48 where increased resistin levels were measured in the blood of psoriasis patients and compared with age, sex and BMI-matched controls, however that study did not report any correlation between disease severity and serum resistin, probably because only 10 patients were enrolled. However, we and recently others 49 now show a positive correlation between psoriasis disease severity and serum resistin levels. There was some decrease in serum resistin after the treatment but this was not significant (p=0.1).
We also demonstrate that exogenous resistin can induce monocytes to produce the inflammatory cytokines CXCL8 and TNF-α in vitro Figure 4e and f) which is in agreement with Bokarewa et al., who showed increases in IL-6 and TNF-α but not IL-1β in the culture medium of PBMC cultures stimulated with 1000 ng ml-1 resistin 43. Resistin has also been shown to increase the expression by human endothelial cells of the vascular cell adhesion molecule-1, CCL2 and endothelin-1 50. Resistin has furthermore been reported to promote proliferation and migration of cultured endothelial cells and to increase the expression of vascular endothelial growth factor receptors-1 and -2, and matrix metalloproteinases-1 and -2, culminating in in vitro angiogenesis 51. Collectively these various activities of resistin make it an attractive effector molecule in psoriasis.
Like resistin, leptin also induces the production of inflammatory cytokines by monocytes, and in addition to CXCL8 and TNF-α it also markedly induces the production of IL-1β and, IL-1ra (Figure 4 a-d). Further, using an ex-vivo organotypic culture system, we show that exogenously added leptin induces psoriasis skin to produce amphiregulin, an ErbB1-binding member of the EGF family that is known to drive autocrine keratinocyte proliferation in culture 52, and to promote marked inflammatory hyperplasia when overexpressed in the epidermis of transgenic mice. We also show that leptin receptors are downregulated in lesional psoriasis skin while they are constitutively expressed in healthy and uninvolved psoriatic skin, thus leptin, like IL-23 53, may induce pro-inflammatory cytokine production from infiltrating lymphocytes rather than acting directly on the keratinocytes themselves. It is interesting in this context that leptin receptors have been shown to be downregulated during early wound healing, and then strongly expressed by mitotic keratinocytes at the wound edge later in the healing process 54. Such differential regulation of leptin-driven epidermal proliferation is impaired in Lepob/Lepob mice 55.
CXCL8, a strong neutrophil chemoattractant, is also known to stimulate the proliferation of keratinocytes 56. We report elevated CXCL8 levels in the serum of psoriasis patients and that both resistin and leptin can induce the production of CXCL8 by blood monocytes. Given that keratinocytes may both respond to and secrete CXCL8, this chemokine is likely to contribute to the keratinocyte hyperproliferation in psoriasis.
We can now perhaps begin to envision some links between increases in the volume of adipose tissue and severity of psoriasis. Thus, increased adiposity is associated with raised levels of circulating cytokines, including leptin and resistin, which may promote activation of T cells and monocytes, driving both Th1 and Th17 immune responses and at the same time impairing the function of regulatory T cells. High concentrations of leptin may furthermore induce local production of amphiregulin which, together with leptin- and resistin-stimulated production of CXCL8, could help to drive the keratinocyte proliferation which is characteristic for psoriasis.
Acknowledgments
The authors would like to thank Esther Hjálmarsdóttir at the Blue Lagoon Dermatological Clinic for her assistance with collecting serum samples and patient information.
Abbreviations used
- BMI
body mass index
- CCL
CC chemokine ligand
- CXCL
CXC chemokine ligand
- EGF
Epidermal growth factor
- IL-1β
interleukin-1β
- IL-1ra
interleukin-1 receptor antagonist
- LPS
lipopolysaccharide
- mRNA
messenger RNA
- NB-UVB
narrow-band ultra violet B radiation
- PASI
psoriasis area and severity index
- PBMC
peripheral blood mononuclear cells
- QRT-PCR
quantitative real time reverse transcriptase PCR
- TNF-□
tumor necrosis factor-□
Footnotes
Conflict of Interest None.
References
- 1.Christophers E. Psoriasis - epidemiology and clinical spectrum. Clin Exp Dermatol. 2001;26:314–20. doi: 10.1046/j.1365-2230.2001.00832.x. [DOI] [PubMed] [Google Scholar]
- 2.Griffiths CE, Christophers E, Barker JN, et al. A classification of psoriasis vulgaris according to phenotype. Br J Dermatol. 2007;156:258–62. doi: 10.1111/j.1365-2133.2006.07675.x. [DOI] [PubMed] [Google Scholar]
- 3.Valdimarsson H. The genetic basis of psoriasis. Clinics in Dermatology. 2007;23:563–67. doi: 10.1016/j.clindermatol.2007.08.010. [DOI] [PubMed] [Google Scholar]
- 4.Nair RP, Stuart PE, Nistor I, et al. Sequence and haplotype analysis supports HLA-C as the psoriasis susceptibility 1 gene. Am J Hum Genet. 2006;78:827–51. doi: 10.1086/503821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Valdimarsson H, Baker BS, Jonsdottir I, et al. Psoriasis: a T-cell-mediated autoimmune disease induced by streptococcal superantigens?*1. Immunology Today. 1995;16:145–9. doi: 10.1016/0167-5699(95)80132-4. [DOI] [PubMed] [Google Scholar]
- 6.Telfer NR, Chalmers RJ, Whale K, et al. The role of streptococcal infection in the initiation of guttate psoriasis. Arch Dermatol. 1992;128:39–42. [PubMed] [Google Scholar]
- 7.Gudjonsson JE, Thorarinsson AM, Sigurgeirsson B, et al. Streptococcal throat infections and exacerbation of chronic plaque psoriasis: a prospective study. Br J Dermatol. 2003;149:530–4. doi: 10.1046/j.1365-2133.2003.05552.x. [DOI] [PubMed] [Google Scholar]
- 8.Naldi L, Chatenoud L, Linder D, et al. Cigarette smoking, body mass index, and stressful life events as risk factors for psoriasis: results from an Italian case-control study. J Invest Dermatol. 2005;125:61–7. doi: 10.1111/j.0022-202X.2005.23681.x. [DOI] [PubMed] [Google Scholar]
- 9.Henseler T, Christophers E. Disease concomitance in psoriasis. J Am Acad Dermatol. 1995;32:982–6. doi: 10.1016/0190-9622(95)91336-x. [DOI] [PubMed] [Google Scholar]
- 10.Raychaudhuri SP, Gross J. Psoriasis risk factors: role of lifestyle practices. Cutis. 2000;66:348–52. [PubMed] [Google Scholar]
- 11.Marino MG, Carboni I, De Felice C, et al. Risk factors for psoriasis: a retrospective study on 501 outpatients clinical records. Ann Ig. 2004;16:753–8. [PubMed] [Google Scholar]
- 12.Neimann AL, Shin DB, Wang X, et al. Prevalence of cardiovascular risk factors in patients with psoriasis. J Am Acad Dermatol. 2006;55:829–35. doi: 10.1016/j.jaad.2006.08.040. [DOI] [PubMed] [Google Scholar]
- 13.Sommer DM, Jenisch S, Suchan M, et al. Increased prevalence of the metabolic syndrome in patients with moderate to severe psoriasis. Arch Dermatol Res. 2006;298:321–8. doi: 10.1007/s00403-006-0703-z. [DOI] [PubMed] [Google Scholar]
- 14.Rucevic I, Perl A, Barisic-Drusko V, et al. The role of the low energy diet in psoriasis vulgaris treatment. Coll Antropol. 2003;27:41–8. [PubMed] [Google Scholar]
- 15.Higa-Sansone G, Szomstein S, Soto F, et al. Psoriasis remission after laparoscopic Roux-en-Y gastric bypass for morbid obesity. Obes Surg. 2004;14:1132–4. doi: 10.1381/0960892041975569. [DOI] [PubMed] [Google Scholar]
- 16.Herron MD, Hinckley M, Hoffman MS, et al. Impact of obesity and smoking on psoriasis presentation and management. Arch Dermatol. 2005;141:1527–34. doi: 10.1001/archderm.141.12.1527. [DOI] [PubMed] [Google Scholar]
- 17.Sterry W, Strober BE, Menter A. Obesity in psoriasis: the metabolic, clinical and therapeutic implications. Report of an interdisciplinary conference and review. Br J Dermatol. 2007;157:649–55. doi: 10.1111/j.1365-2133.2007.08068.x. [DOI] [PubMed] [Google Scholar]
- 18.Ahima RS, Flier JS. Adipose Tissue as an Endocrine Organ. Trends in Endocrinology and Metabolism. 2000;11:327–32. doi: 10.1016/s1043-2760(00)00301-5. [DOI] [PubMed] [Google Scholar]
- 19.Curat CA, Wegner V, Sengenes C, et al. Macrophages in human visceral adipose tissue: increased accumulation in obesity and a source of resistin and visfatin. Diabetologia. 2006;49:744–7. doi: 10.1007/s00125-006-0173-z. [DOI] [PubMed] [Google Scholar]
- 20.Kanda H, Tateya S, Tamori Y, et al. MCP-1 contributes to macrophage infiltration into adipose tissue, insulin resistance, and hepatic steatosis in obesity. J Clin Invest. 2006;116:1494–505. doi: 10.1172/JCI26498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fain JN, Madan AK, Hiler ML, et al. Comparison of the release of adipokines by adipose tissue, adipose tissue matrix, and adipocytes from visceral and subcutaneous abdominal adipose tissues of obese humans. Endocrinology. 2004;145:2273–82. doi: 10.1210/en.2003-1336. [DOI] [PubMed] [Google Scholar]
- 22.Bruun JM, Lihn AS, Madan AK, et al. Higher production of IL-8 in visceral vs. subcutaneous adipose tissue. Implication of nonadipose cells in adipose tissue. Am J Physiol Endocrinol Metab. 2004;286:E8–13. doi: 10.1152/ajpendo.00269.2003. [DOI] [PubMed] [Google Scholar]
- 23.Gearing AJ, Fincham NJ, Bird CR, et al. Cytokines in skin lesions of psoriasis. Cytokine. 1990;2:68–75. doi: 10.1016/1043-4666(90)90045-u. [DOI] [PubMed] [Google Scholar]
- 24.Bonifati C, Carducci M, Fei P Cordiali, et al. Correlated increases of tumour necrosis factor-alpha, interleukin-6 and granulocyte monocyte-colony stimulating factor levels in suction blister fluids and sera of psoriatic patients--relationships with disease severity. Clin Exp Dermatol. 1994;19:383–7. doi: 10.1111/j.1365-2230.1994.tb02687.x. [DOI] [PubMed] [Google Scholar]
- 25.Gudjonsson JE, Johnston A, Sigmundsdottir H, et al. Immunopathogenic mechanisms in psoriasis. Clin Exp Immunol. 2004;135:1–8. doi: 10.1111/j.1365-2249.2004.02310.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Muoio DM, Dohm G Lynis. Peripheral metabolic actions of leptin. Best Pract Res Clin Endocrinol Metab. 2002;16:653–66. doi: 10.1053/beem.2002.0223. [DOI] [PubMed] [Google Scholar]
- 27.Pelleymounter MA, Cullen MJ, Baker MB, et al. Effects of the obese gene product on body weight regulation in ob/ob mice. Science. 1995;269:540–3. doi: 10.1126/science.7624776. [DOI] [PubMed] [Google Scholar]
- 28.Lord GM, Matarese G, Howard JK, et al. Leptin modulates the T-cell immune response and reverses starvation-induced immunosuppression. Nature. 1998;394:897–901. doi: 10.1038/29795. [DOI] [PubMed] [Google Scholar]
- 29.Palacio A, Lopez M, Perez-Bravo F, et al. Leptin Levels Are Associated with Immune Response in Malnourished Infants. J Clin Endocrinol Metab. 2002;87:3040–6. doi: 10.1210/jcem.87.7.8636. [DOI] [PubMed] [Google Scholar]
- 30.Farooqi IS, Matarese G, Lord GM, et al. Beneficial effects of leptin on obesity, T cell hyporesponsiveness, and neuroendocrine/metabolic dysfunction of human congenital leptin deficiency. J Clin Invest. 2002;110:1093–103. doi: 10.1172/JCI15693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ferraroni NR, Geloneze B, Mansour E, et al. Severe hypoleptinaemia associated with insulin resistance in patients with common variable immunodeficiency. Clin Endocrinol (Oxf) 2005;63:63–5. doi: 10.1111/j.1365-2265.2005.02300.x. [DOI] [PubMed] [Google Scholar]
- 32.Goldberg AC, Eliaschewitz FG, Montor WR, et al. Exogenous leptin restores in vitro T cell proliferation and cytokine synthesis in patients with common variable immunodeficiency syndrome. Clinical Immunology. 2005;114:147–53. doi: 10.1016/j.clim.2004.09.002. [DOI] [PubMed] [Google Scholar]
- 33.Matarese G, Di Giacomo A, Sanna V, et al. Requirement for leptin in the induction and progression of autoimmune encephalomyelitis. J Immunol. 2001;166:5909–16. doi: 10.4049/jimmunol.166.10.5909. [DOI] [PubMed] [Google Scholar]
- 34.De Rosa V, Procaccini C, Cali G, et al. A key role of leptin in the control of regulatory T cell proliferation. Immunity. 2007;26:241–55. doi: 10.1016/j.immuni.2007.01.011. [DOI] [PubMed] [Google Scholar]
- 35.Loffreda S, Yang SQ, Lin HZ, et al. Leptin regulates proinflammatory immune responses. FASEB J. 1998;12:57–65. [PubMed] [Google Scholar]
- 36.Zarkesh-Esfahani H, Pockley G, Metcalfe RA, et al. High-Dose Leptin Activates Human Leukocytes Via Receptor Expression on Monocytes. J Immunol. 2001;167:4593–9. doi: 10.4049/jimmunol.167.8.4593. [DOI] [PubMed] [Google Scholar]
- 37.Mattioli B, Straface E, Quaranta MG, et al. Leptin promotes differentiation and survival of human dendritic cells and licenses them for Th1 priming. J Immunol. 2005;174:6820–8. doi: 10.4049/jimmunol.174.11.6820. [DOI] [PubMed] [Google Scholar]
- 38.Steppan CM, Bailey ST, Bhat S, et al. The hormone resistin links obesity to diabetes. Nature. 2001;409:307–12. doi: 10.1038/35053000. [DOI] [PubMed] [Google Scholar]
- 39.Nagaev I, Smith U. Insulin resistance and type 2 diabetes are not related to resistin expression in human fat cells or skeletal muscle. Biochem Biophys Res Commun. 2001;285:561–4. doi: 10.1006/bbrc.2001.5173. [DOI] [PubMed] [Google Scholar]
- 40.Savage DB, Sewter CP, Klenk ES, et al. Resistin / Fizz3 expression in relation to obesity and peroxisome proliferator-activated receptor-gamma action in humans. Diabetes. 2001;50:2199–202. doi: 10.2337/diabetes.50.10.2199. [DOI] [PubMed] [Google Scholar]
- 41.Utzschneider KM, Carr DB, Tong J, et al. Resistin is not associated with insulin sensitivity or the metabolic syndrome in humans. Diabetologia. 2005;48:2330–3. doi: 10.1007/s00125-005-1932-y. [DOI] [PubMed] [Google Scholar]
- 42.Kaser S, Kaser A, Sandhofer A, et al. Resistin messenger-RNA expression is increased by proinflammatory cytokines in vitro. Biochem Biophys Res Commun. 2003;309:286–90. doi: 10.1016/j.bbrc.2003.07.003. [DOI] [PubMed] [Google Scholar]
- 43.Bokarewa M, Nagaev I, Dahlberg L, et al. Resistin, an adipokine with potent proinflammatory properties. J Immunol. 2005;174:5789–95. doi: 10.4049/jimmunol.174.9.5789. [DOI] [PubMed] [Google Scholar]
- 44.Patel L, Buckels AC, Kinghorn IJ, et al. Resistin is expressed in human macrophages and directly regulated by PPAR gamma activators. Biochem Biophys Res Commun. 2003;300:472–6. doi: 10.1016/s0006-291x(02)02841-3. [DOI] [PubMed] [Google Scholar]
- 45.Lehrke M, Reilly MP, Millington SC, et al. An inflammatory cascade leading to hyperresistinemia in humans. PLoS Med. 2004;1:e45. doi: 10.1371/journal.pmed.0010045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Silswal N, Singh AK, Aruna B, et al. Human resistin stimulates the pro-inflammatory cytokines TNF-alpha and IL-12 in macrophages by NF-kappaB-dependent pathway. Biochem Biophys Res Commun. 2005;334:1092–101. doi: 10.1016/j.bbrc.2005.06.202. [DOI] [PubMed] [Google Scholar]
- 47.Fredriksson T, Pettersson U. Severe psoriasis--oral therapy with a new retinoid. Dermatologica. 1978;157:238–44. doi: 10.1159/000250839. [DOI] [PubMed] [Google Scholar]
- 48.Corbetta S, Angioni R, Cattaneo A, et al. Effects of retinoid therapy on insulin sensitivity, lipid profile and circulating adipocytokines. Eur J Endocrinol. 2006;154:83–6. doi: 10.1530/eje.1.02057. [DOI] [PubMed] [Google Scholar]
- 49.Boehncke S, Thaci D, Beschmann H, et al. Psoriasis patients show signs of insulin resistance. Br J Dermatol. 2007;157:1249–51. doi: 10.1111/j.1365-2133.2007.08190.x. [DOI] [PubMed] [Google Scholar]
- 50.Verma S, Li SH, Wang CH, et al. Resistin promotes endothelial cell activation: further evidence of adipokine-endothelial interaction. Circulation. 2003;108:736–40. doi: 10.1161/01.CIR.0000084503.91330.49. [DOI] [PubMed] [Google Scholar]
- 51.Mu H, Ohashi R, Yan S, et al. Adipokine resistin promotes in vitro angiogenesis of human endothelial cells. Cardiovasc Res. 2006;70:146–57. doi: 10.1016/j.cardiores.2006.01.015. [DOI] [PubMed] [Google Scholar]
- 52.Cook PW, Mattox PA, Keeble WW, et al. A heparin sulfate-regulated human keratinocyte autocrine factor is similar or identical to amphiregulin. Mol. Cell. Biol. 1991;11:2547–57. doi: 10.1128/mcb.11.5.2547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zheng Y, Danilenko DM, Valdez P, et al. Interleukin-22, a T(H)17 cytokine, mediates IL-23-induced dermal inflammation and acanthosis. Nature. 2007;445:648–51. doi: 10.1038/nature05505. [DOI] [PubMed] [Google Scholar]
- 54.Stallmeyer B, Kampfer H, Podda M, et al. A Novel Keratinocyte Mitogen: Regulation of Leptin and its Functional Receptor in Skin Repair. J Invest Dermatol. 2001;117:98–105. doi: 10.1046/j.0022-202x.2001.01387.x. [DOI] [PubMed] [Google Scholar]
- 55.Frank S, Stallmeyer B, Kampfer H, et al. Leptin enhances wound re-epithelialization and constitutes a direct function of leptin in skin repair. J. Clin. Invest. 2000;106:501–9. doi: 10.1172/JCI9148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rennekampff HO, Hansbrough JF, Kiessig V, et al. Bioactive interleukin-8 is expressed in wounds and enhances wound healing. J Surg Res. 2000;93:41–54. doi: 10.1006/jsre.2000.5892. [DOI] [PubMed] [Google Scholar]