Abstract
Adult human bone marrow-derived mesenchymal stem cells (hMSCs) are under study as therapeutic delivery agents that assist in the repair of damaged tissues. To achieve the desired clinical outcomes for this strategy requires a better understanding of the mechanisms that drive the recruitment, migration and engraftment of hMSCs to the targeted tissues. It is known that hMSCs are recruited to sites of stress or inflammation to fulfill their repair function. It is recognized that toll-like receptors (TLRs) mediate stress responses of other bone marrow-derived cells. This study explored the role of TLRs in mediating stress responses of hMSCs. Accordingly, the presence of TLRs in hMSCs was established initially by RT-PCR assays. Flow cytometry and fluorescence immunocytochemical analyses confirmed these findings. The stimulation of hMSCs with TLR agonists led to the activation of downstream signaling pathways, including NF-κB, AKT and MAPK. Consequently, activation of these pathways triggered the induction and secretion of cytokines, chemokines and related TLR gene products as established from cDNA array, immunoassay and cytokine antibody array analyses. Interestingly, the unique patterns of affected genes, cytokines and chemokines measured, identify these receptors as critical players in the clinically established immunomodulation, observed for hMSCs. Lastly, hMSCs migration was promoted by TLR ligand exposure as demonstrated by transwell migration assays. Conversely, disruption of TLRs by neutralizing TLR antibodies compromised hMSCs migration. This study defines a novel TLR-driven stress and immune modulating response for hMSCs that is critical to consider in the design of stem cell-based therapies.
Keywords: toll-like receptors, danger signals, stress responses, migration, immune modulation, human mesenchymal stem cells
Introduction
Toll-like receptors (TLRs) are a conserved family of receptors that recognize pathogen-associated molecular patterns and promote the activation of immune cells [1-5]. To date, several TLR (numbered 1−11) have been identified in humans. Agonists for TLRs include exogenous microbial components such as lipopolysaccharide, LPS (TLR2 and 4), lipoproteins and peptidoglycans (TLR1, 2, 6), viral RNA (TLR3), bacterial and viral unmethylated CpG-DNA (TLR9), and endogenous molecules including heat shock proteins (HSP, TLR4) and extracellular matrix molecules (fibronectin, TLR4) [2, 3, 5, 6]. TLR agonist stimulation leads to the expression of inflammatory cytokines or co-stimulatory molecules by a MyD88 (a TLR adapter protein)-dependent or MyD88-independent signaling pathway and can promote chemotaxis of the stimulated cell. TLRs are differentially expressed on leukocyte subsets and non-immune cells and appear to regulate important aspects of innate and adaptive immune responses [2, 7-10].
The ability of TLRs to recognize seemingly unrelated molecules shed from both pathogens (e.g. LPS) and injured tissues (e.g. HSP70) served as the premise for the proposed “danger model” of immune response [11]. This model is based on the idea that the immune system responds to signals that represent potential harm to the host rather than to signals that are foreign to the host. In doing so, this model addresses the shortcomings of other immune recognition models that rely on the notion that host immune cells recognize only non-self molecules [3, 11, 12]. These latter models are limited since they fail to explain certain observed immune responses: mothers not rejecting fetuses that contain foreign proteins or tumor cells being tolerated despite producing non-self proteins. The “danger model” that relies upon TLRs and their ability to respond to a multitude of endogenously and exogenously derived and aberrantly shed molecules has spawned a great deal of interest from researchers in diverse fields including but not limited to tumor immunology, inflammation and vaccine development.
Initially, research on TLRs focused on their expression and signaling consequences in immune cells. However, recent reports indicate that other bone marrow-derived cells including mesenchymal stem/progenitor cells (MSCs) are among the cells that express TLR proteins [7-9]. MSCs are separated from other cells in the bone marrow by their tendency to adhere to plastic. MSCs are typically known to differentiate into osteoblasts, chondrocytes, and adipocytes in culture [13, 14]. These cells specifically home to damaged and inflammed tissues and contribute to their repair in part by secretion of immunomodulating cytokines, chemokines and extracellular matrix proteins. Critically, these cells are immunosuppressive to the host and can be easily expanded to large numbers in culture [13]. As a result of these and other qualities, human MSCs (hMSCs) are very attractive candidates in stem cell-based strategies for tissue repair and gene therapy. Numerous investigators have now demonstrated the successful recruitment and multi-organ engraftment capability of hMSCs in various animal models and human clinical trials [15, 16]. However, the success of this strategy has been limited since the net engraftment measured for the infused hMSCs in pre-clinical and clinical trials is relatively poor [16]. Therefore, a better understanding of the precise molecular mechanisms governing hMSC's stem cell fate, mobilization, and recruitment to the sites of engraftment is warranted to improve treatment efficacy.
To this end, our study sought to determine if like other bone marrow-derived cells, hMSCs migration and/or recruitment was also driven by TLRs. Recent reports concerning adult adipose tissue-derived, mesenchymal, and hematopoetic stem cells suggest that TLRs play a role in stem cell biology [7-9]. However, these reports mainly focused on the role of TLRs in stem cell proliferation and their potential role in disrupting the differentiation capabilities of the stem cells. These reports did not focus on the role of TLRs in critical stress responses of stem cells as analyzed here. Note that stress or danger signal responses of hMSCs are defined here as one of the potential mesenchymal stem cell fates that is different from self-renewal, differentiation or apoptosis and that drives their migration, invasion and engraftment into damaged and inflamed tissues sites. This hMSC fate is initiated once the host encounters various conditions of tissue pathology including mechanical injury (wounds), inflammation, infection or cancer that then drives the egress of the hMSCs from their niche and allows their migration into the circulation, their invasion across vessels and their engraftment to the injured site to fulfill their repair function.
We report here that stimulation of TLRs within hMSCs leads to the activation of established TLR signaling pathways. Interestingly, these activated pathways mediate the secretion of discrete patterns of cytokines and chemokines depending on the TLR-ligand employed. This observation implicates these receptors in the immune modulating function established for hMSCs [13]. We also describe that TLR stimulation particularly promotes their migration capabilities. Thus, TLR-stimulation may be one mechanism that specifically drives the recruitment, migration and immune modulating function of the hMSCs at injured or stressed sites. Apart from establishing a new aspect of their biology, the identification of TLRs as critical mediators of stress responses within hMSCs also provides a novel target to exploit in the improvement of stem cell-based therapeutic strategies.
Materials and Methods
Cell Culture
Human MSCs were obtained from our collaborators at the Tulane University Center for Gene Therapy led by Darwin J. Prockop, M.D., Ph.D. Additionally, MSCs were obtained from two commercial suppliers Cambrex BioScience (Walkersville, MD) and Allcells (Emeryville, CA) to ensure variability of the starting cell population and to make certain that findings are universal and not unique to single donor pools derived from a unique source. These suppliers test the hMSCs for their homogeneity and provide test results for their differential potential to chondro-, osteo-, and adipo- genic lineages. Once obtained, expanded and established in our lab the hMSCs are also verified for positive staining of CD90, CD105, CD106, CD164, CD56, CD166, CD29, and CD44, and negative antibody staining for CD45, CD14, CD31, CD34, HLA DR and CD117. Consistent fibroblast-like morphology was monitored by microscopy. MSC cultivation consisted of growth in tissue culture treated flasks or dishes in minimum essential medium alpha with GlutaMax I (MEM-α Invitrogen Carlsbad, CA) supplemented with 16.5% fetal bovine serum (Atlanta Biologicals, Lawrenceville, GA). For serum-free cultivation, growth medium without serum supplementation was added to the MSC cultures for at least 18 hr prior to the beginning of the experiment. MSCs of a passage number no greater than six were routinely used in all the experiments to maintain consistency.
TLR Ligands
In this study, endotoxin or LPS, synthetic CpG-oligodeoxynucleotides (CpG-ODN), flagellin and polyriboinosinic polyribocytidylic acid, (poly(I:C)) represented exogenous TLR ligands. Fibronectin-derived fragments (III1c, 45kDa) and the human secreted antimicrobial peptide LL-37 alone or in combination with LPS served as the endogenous danger signals. Typically, TLR ligands were used in these concentrations: 1μM poly(I:C) (Sigma-Aldrich, St. Louis, MO); 10ng/mL LPS (Sigma-Aldrich, St. Louis, MO); 5μ flagellin (Sigma-Aldrich, St. Louis, MO); 1μM CpG-ODN (IDTDNA, Coralville, IA); 1μg/mL fibronectin III1c or 45kDa (Sigma-Aldrich, St. Louis, MO); 5μg/mL LL-37 (Innovagen, Lund, Sweden).
RT-PCR
The hMSCs (70% confluency) were washed and total RNA was isolated with 1 mL TriReagent as standard (Invitrogen, Carlsbad, CA). Potential DNA contamination in the RNA sample was removed by Turbo DNA-free treatment (Ambion, Austin, TX). CDNA was elaborated from RNA using SuperScript II (Invitrogen Carlsbad, CA). TLR transcripts were amplified using the primers published [10] (IDTDNA, Coralville, IA). Human peripheral blood mononuclear cells (PBMCs) RNA was used as a positive control. PCR assay cycles: 94oC for 2 min, 35 cycles of 94 oC for 20s, 56 oC for 30s, 72 oC for 30s [10].
Flow Cytometry
Human MSCs were harvested and analyzed by flow cytometry with a BD-FACSCalibur flow cytometer (BD Biosciences, San Jose, CA) as described previously [17]. Intracellular antibody staining was achieved after fixation and permeabilization of the cells as indicated by the manufacturer (cytofix/cytoperm buffers, BD Biosciences, San Jose, CA).
Primary antibodies
Isotype-control FITC mouse IgG1K (BD#556649); isotype-control PE mouse IgG1K (BD#551436); isotype-control mouse IgG1K (BD#557224); CD105 (BD#555690); CD166 (BD#559263); CD90 (BD#555597); CD44 (BD#555478); CD34 (BD#5507610); CD31 (BD#550761); CD106 (BD#551148); TLR1 (all anti-human TLR from Abcam, Cambridge, MA, #ab11209); TLR2 (#ab9100); TLR3 (#ab12085); TLR4 (#ab30667); TLR5 (#ab13875); TLR6 (#ab22046); TLR7 (#ab13722); TLR8 (#ab12120); TLR9 (#ab17236); MyD88 (#ab2068); IKK α/β (Cell Signaling Beverly, MA #2697S); pMAPK 42/44 (Cell Signaling Beverly, MA #9101); MAPK 42/44 (Cell Signaling Beverly, MA #9102); pAKT Ser473 (Cell Signaling Beverly, MA #4058S); AKT (Cell Signaling Beverly, MA #272); β Actin (Sigma-Aldrich, MO, #A-2066).
Flurescence Immunocytochemical Analysis
Fluorescence immunocytochemistry was performed on cells grown to near confluence (70%) on chamber slides, fixed and permeabilized with BD cytofix/cytoperm™ buffer (BD Pharmigen, San Jose, CA). The primary antibodies diluted in stain buffer in the appropriate concentration (ratio of 0.5 μg Ab/1×106 cells) were added for 1 h at 4°C in the dark. Next, unbound primary antibody was washed twice with BD cytoperm wash buffer. The secondary antibody (Alexa488-conjugated, Molecular Probes, Invitrogen, CA) was diluted in stain buffer in the appropriate concentrations and added for 1 h at 4°C in the dark. Slides were washed again twice prior to DAPI staining and mounting with ProLong Gold antifade reagent (Molecular Probes, Invitrogen, CA). Micrographs were taken on a Zeiss Axioplan 2 Fluorescence Microscope with Intelligent Imaging Innovations Deconvolution Hardware and Software (SlideBook ver. 4).
Western Blot Analysis
Cells were plated on 10 cm plates to 70% confluence at 37°C before treatments. The cells were washed twice with cold PBS and protein was isolated as standard (mammalian protein extraction reagent, M-PER (Pierce Biotechnology, Rockford, IL) containing a protease inhibitor cocktail (Roche Applied Science, Indianapolis, IN), a phosphatase inhibitor cocktail (Sigma-Aldrich, St. Louis, MO) and 1 mM PMSF (Sigma-Aldrich, St. Louis, MO). Lysates were clarified by centrifugation at 14,000 rpm at 4°C. Protein concentration was measured by the Micro BCA™ Protein Assay Kit (Pierce Biotechnology, Rockford, IL). Lysates (50 μg) were resolved on 4−12% SDS-polyacrylamide gels and transferred to nitrocellulose membranes (Invitrogen, Carlsbad, CA). Membranes were blocked with 5% nonfat dried milk in PBS containing 0.2% Tween-20 (PBST) for 1 h at room temperature and blots were incubated at 4°C overnight with the primary antibodies. The blots were then washed in PBST and incubated with species-specific IgG conjugated to horseradish peroxidase (1:5000; Amersham Biosciences Corp., Piscataway, NJ) for 1 h at room temperature. Antigen-antibody complexes were visualized after exposure to X-ray film by enhanced chemiluminescence (Amersham Biosciences Corp. Piscataway, NJ).
TLR Pathway cDNA Array Assay. hMSCs were treated with TLR ligands for four hours. Total RNA was isolated with RNAzol (Invitrogen, Carlsbad, CA) and analyzed as per manufacturer's instructions (human TLR pathway-specific gene expression profiling system, SuperArray Bioscience, Frederick, MD) on an iCycler iQ5 Real-Time PCR Detection System (Bio-Rad, Hercules, CA). The raw data from both the control and the treated groups were obtained and uploaded onto GEarray Analyzer software (SuperArray Inc., Bethesda, MD) for analysis and verification of appropriate experimental procedures.
Cytokine Antibody Array Assay. Conditioned medium from TLR-ligand treated or untreated hMSCs cultures were tested for cytokine, chemokine and matrix metalloprotease inhibitor secretion by cytokine antibody arrays following the manufacturer's instructions (human cytokine array 3, MA6150; Panomics, Redwood City, CA).
Fluorescence Bead Assay
Conditioned medium from similarly treated hMSCs cultures were tested for cytokine or chemokine secretion by fluorescence bead immunoassay as per manufacturer's instruction (Bender MedSystems, Inc, Burlingame, CA). Primary hMSCs cultures were treated with various TLR ligands, for 48 h prior to harvesting and concentrating the spent culture medium with centricon-10 (5-fold) and loading 100 μL per sample in triplicate. Following flow cytometry the data were analyzed as indicated by manufacturer (FlowCytomixPro ver. 2.2, Bender MedSystems, Inc, Burlingame, CA).
Transwell Migration Assay
Migration assays were performed in transwell inserts with 8-μm pore membrane filters (Falcon, BD Biosciences, San Jose, CA). Human MSCs were grown to subconfluence, (70%) prior to harvesting by trypsinization and labeling with CellTrackerTM green (1 μM, Molecular Probes, Eugene, OR) for 1 h at 37°C. Fluorescently labeled hMSCs (2.5 to 5×105 cells/well in 300 μL) were loaded onto the upper chamber, and 500 μL hMSCs growth medium with chemotactic factors or TLR ligands, as indicated was loaded onto the bottom chamber. After overnight incubation the upper side of the filters was carefully washed with cold PBS and non-migrating cells remaining were removed with a cotton tip applicator. Fluorescence images of the migrating cells were collected using a Nikon TE300 inverted epifluorescence microscope (DP Controller v1.2.1.108, Olympus Optical Company, LTD; Nikon USA, Lewisville, TX). Each experiment was performed in triplicate with two separate hMSCs donors. Data are expressed as numbers of counted, migrated cells per 200X field micrograph for each sample well and normalized to those cell counts obtained for the untreated control.
Statistical analysis
Data are shown as average +/− standard error of the mean (S.E.M.). Multiple group comparison was performed by one-way analysis of variance (ANOVA) followed by the Bonferroni procedure for comparison of means. Comparison between any two groups was analyzed by the two-tailed Student's t-test or one-way ANOVA (Prism4, GraphPad Software Inc. CA). Values of p < 0.05 were considered statistically significant.
Results
Toll-like receptors (TLRs) are expressed in human mesenchymal stem cells (hMSCs)
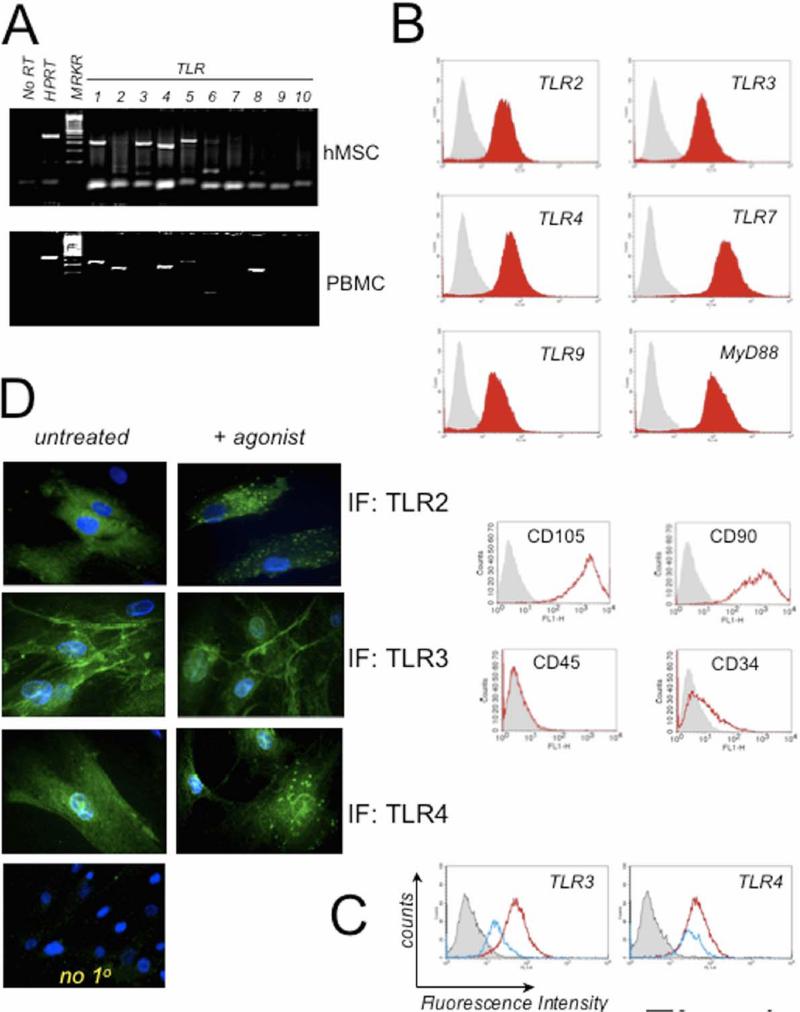
Initially a set of primers specific for human TLR1−10 cDNA was used in RT-PCR analyses to establish their expression in hMSCs [10]. As a positive control, RNA from peripheral blood mononuclear cells (PBMC) was tested with the same primer sets in the assay. In agreement with recent reports, this strategy convincingly revealed the presence of TLR1, 2, 3, 4, 5, and 6 (Fig. 1A [7]). TLR1, 2, 4, 5 and 8 RNA expression were confirmed for PBMC. Most striking was the expression of TLR3 RNA in hMSCs but not PBMC and lack of TLR8 expression in hMSCs in contrast to PBMC expression.
Figure 1. Human Mesenchymal Stem Cells (hMSCs) Express Toll-like Receptors (TLRs) and downstream signaling molecule, MyD88.
A. RNA was isolated from hMSCs and PBMC and analyzed by RT-PCR for expression of TLR 1−10. HPRT was used as loading control. hMSC expressed TLR 1, 2, 3, 4, 5, 6, and 9 whereas PBMC expressed TLR 1, 2, 4, 5, and 8 (n>3). B. Human MSC donor pools were routinely examined by flow cytometry for expression of discriminating MSC cell surface markers as described in Materials and Methods. Shown in bottom panels are representative findings for hMSCs: positive expression of CD90 and CD105 and mostly negative expression of CD45 and CD34 (n>12). HuMSCs were examined also by flow cytometry for expression of TLR2, 3, 4, 7, 9 and MyD88 as indicated by red filled-in curves on the top panels (n>7). The grey filled-in line represents hMSCs incubated with corresponding isotype antibody controls. C. Representative flow cytometry analysis of hMSCs for TLR3 and TLR4 after ligand stimulation for 30 min (Blue line) or without stimulation (constitutive expression, Red line). D. Antibody staining of TLRs was performed following fixation and membrane permeabilization of the ligand-treated hMSCs seeded on chamber slides. Samples were pre-treated for 1 h with ligands: 1 μM poly(I:C) (TLR3) or 10 ng/mL LPS (TLR2 & 4) prior to harvest and immunofluorescence (IF). As a control, the primary antibody was omitted from staining procedure (no 1o, n>3).
Measurement of TLR protein expression in hMSCs was achieved by flow cytometry and immunofluorescence assays with specific antibodies to human TLR proteins. Flow cytometry analyses indicated that primary cultures of hMSCs contain ample amounts of MyD88, TLR2, 3, 4 and 7 (Fig.1B). TLR9 protein levels appeared to be the smallest measured (Fig. 1B). Verification of hMSC populations was also achieved by flow cytometry with established surface markers as described in the Methods (Fig. 1B). Treatment of the hMSCs with the established TLR ligands generally resulted in diminished receptor levels in flow cytometry analysis most likely due to receptor activation, internalization and degradation as evident from the leftward shift of the plot measured for the ligand treated samples (blue line, Fig. 1C). Treatment of the hMSCs with the TLR9 agonist CpG-ODN was an exception and did not follow this pattern (data not shown). Consistent findings were noted in immunofluorescence assays (Fig. 1D). Note that TLR agonist treatment leads to endosomal-like partitioning for both TLR2 and 4. TLR3 expression was diffuse in the cytoplasm and along the cell's edge. TLR3 agonist treatment led to a more focused expression of TLR3 along the cell's edge as well as to endosomal-like compartments.
TLR stimulation of hMSCs leads to activation of expected downstream signaling molecules
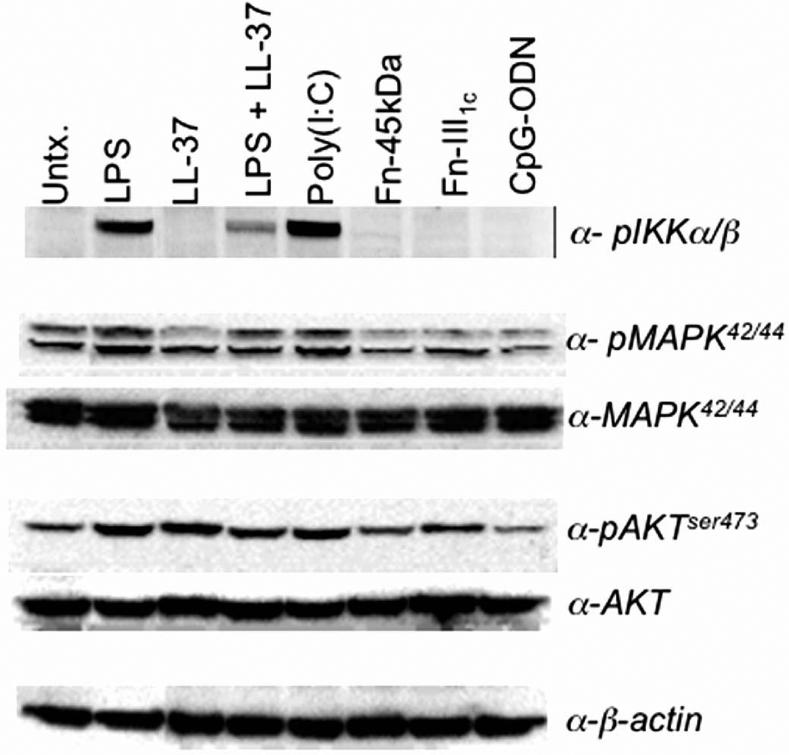
TLRs within hMSCs were stimulated for 1h by various ligands and assessed by Western blot analysis to examine their downstream signaling capabilities (Fig. 2). Interestingly, treatment of the hMSCs with poly(I:C) the ligand for TLR3 resulted in the greatest activation of the NF-κB pathway. LPS treatments also led to increased phospho-IKKα/β expression and this expression was dampened by combined LL-37 treatment as previously reported [18]. CpG-ODN (TLR9) treatment of hMSCs appeared not to affect these pathways. Analysis of PI3K pathway stimulation upstream of both the MAPK and NF-κB pathways revealed that LPS, LL-37, poly(I:C) and fibronectin fragment (III1c) stimulation also activated this pathway.
Figure 2.
hMSC stimulation by discrete TLR-ligands affects established TLR-downstream signaling components. Downstream signaling potential by the stimulated TLRs within the hMSCs was assessed by Western blot analysis. LPS, CpG-ODN, and poly(I:C) represented exogenous ligands. Fibronectin-derived fragments (Fn-45kDa, -III1c) and the human secreted antimicrobial peptide LL-37 served as the endogenous danger signals (n>3).
TLR stimulation in hMSCs triggers the induction of cytokines, chemokines and other established TLR-regulated genes
To identify the genomic consequences of TLR stimulation within hMSCs, focused microarray analysis was performed. The hMSCs were treated with various TLR ligands for 4 hr prior to RNA isolation and array analysis of 84 TLR-related genes. The most dramatic fold changes observed for several genes in the treated over untreated controls are recorded in Table 1. The results are arranged by molecule type: TLR, then cytokines and chemokines followed by other downstream signaling genes. TNFα gene expression was enhanced for all the TLR-ligands tested confirming TLR-stimulation within the hMSCs. Similar to the results presented above unique expression patterns resulted for each TLR agonist employed. Strikingly, regardless of the agonist used to treat the hMSCs there was uniform vast induction of TLR3. Smaller induction for TLR6 and 9 were also noted following LPS treatment of hMSCs. Poly(I:C) treatment of hMSCs led to almost exclusive TLR3 induction compared to other TLRs whereas LL-37 treatment led to modest induction of TLR3, TLR4 and 6. Fibronectin III1C treatment followed the LPS profile but in a more dampened way. LPS treatment caused increased expression of many cDNAs including CXCL10 (IP10), IL6, IL8, IFN1β and NF-κB. Poly(I:C) exposure caused the highest induction of the chemokine ligand, CCL2 or monocyte chemotactic protein 1 (MCP1). This treatment also caused induction of IRAK2, CXCL10 (IP10), IL6, IL8, IFN1β and NF-κB.
Table 1. TLR stimulation in hMSCs triggers the induction of cytokines, chemokines and other established TLR-regulated genes.
The effect of TLR stimulation on gene expression within hMSCs was analyzed by TLR-pathway focused cDNA array (see “http://www.superarray.com/genetable.php?pcatn=APHS-018A” for details on the arrayed genes). Results are presented as fold changes in gene expression of TLR-stimulated relative to unstimulated hMSCs. Genes included in the table are those that were most dramatically induced in comparison to the untreated control. The expected TLR-mediated response of increased expression of TNFα was observed for all ligands tested (shaded). Those genes whose induction was consistent in both the cDNA array and cytokine antibody array are denoted in bold and italicized.
| cDNA | LPS | LL-37 | LL37+LPS | Poly(I:C) | FN-III1C |
|---|---|---|---|---|---|
| Toll-like receptors | |||||
| TLR3 | 164.2 | 30.0 | 3.6 | 10.8 | 7.0 |
| TLR4 | 5.3 | 4.7 | 4.4 | 2 | 0.6 |
| TLR6 | 11.0 | 7.8 | 1.6 | 1.5 | 3.1 |
| TLR9 | 20.8 | 2.9 | 1.0 | 1.0 | 4.5 |
| Cytokines and Chemokines | |||||
| CCL2 | 259.0 | 17.6 | 190.0 | 3191.5 | 4.3 |
| CXCL10 | 29853.2 | 1.0 | 1.0 | 1009.9 | 1.0 |
| IFN1β | 806.8 | 3.2 | 12.4 | 37.0 | 3.3 |
| IL10 | 1.0 | 1.0 | 2.0 | 1.0 | 1.0 |
| IL1α | 200.0 | 8.9 | 30.1 | 35.3 | 3.2 |
| IL1β | 904.1 | 66.3 | 83.9 | 99.0 | 6.6 |
| IL6 | 829.9 | 173.6 | 17.8 | 20.1 | 8.9 |
| IL8 | 5066.6 | 3.2 | 96.3 | 69.6 | 3.7 |
| TNFα | 42.8 | 2.8 | 10.6 | 9.1 | 3.2 |
| TLR-signaling related proteins | |||||
| BTK | 1.5 | 20.0 | 962.1 | 3.3 | 4 |
| E1F2AK2 | 6.7 | 68.6 | 54.2 | 313.0 | 30.9 |
| HSPA1A | 1338.5 | 1.0 | 1.9 | 1.8 | 278.2 |
| HSPD1 | 19.9 | 29.4 | 1.2 | 0.9 | 4.8 |
| IRAK2 | 1.0 | 1.0 | 13.0 | 12.2 | 1.0 |
| IRF1 | 539.4 | 32 | 22.3 | 72.5 | 2.6 |
| MYD88 | 1.0 | 1.2 | 3.2 | 4.3 | 1.0 |
| NFκB1 | 78.8 | 32.2 | 3.2 | 3.8 | 5.5 |
| NFκB2 | 7.7 | 1.6 | 6.9 | 5.9 | 1.0 |
| RIPK2 | 84.0 | 37.5 | 22.6 | 28.4 | 5.9 |
| TIRAP | 16.7 | 5.7 | 0.8 | 0.6 | 2.3 |
| TRAF6 | 48.8 | 23.9 | 20.3 | 7.1 | 4.3 |
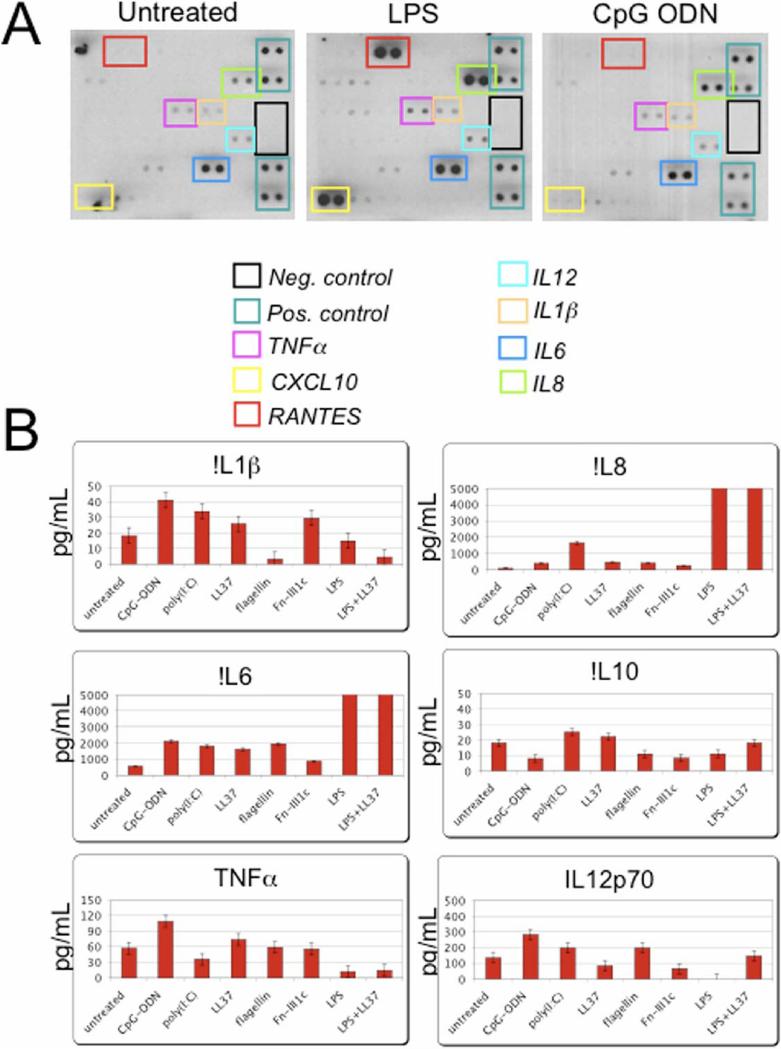
Enhanced chemokine and cytokine secretion by the hMSCs was demonstrated by both cytokine antibody arrays (Fig. 3A) and fluorescence bead array (Fig. 3B) assays. Conditioned medium from treated hMSCs cultures tested by these methods also resulted in unique cytokine and chemokine secretion patterns depending on the ligand used. For comparison, those genes found in both the cDNA array and the cytokine antibody array are shown in bold in Table 1. Cytokine antibody arrays confirmed that LPS-treatment resulted in the increased secretion of CXCL10 (IP10), IL6, IL8 and TNFα. The increased secretion of IL12 was also noted following both poly(I:C) and CpG-ODN exposure of the hMSCs. IL4 secretion was uniquely noted following LL-37 treatment whereas MMP3 was secreted following fibronectin-fragment treatment of the hMSCs (data not shown). Similar secretion patterns were observed in fluorescence bead array (Fig. 3B). Overall, the secretion pattern of LPS-treated cells appears to favor pro-inflammatory mediators such as IL1β, IL6 and TNFα whereas poly(I:C)-mediated secretion patterns appear to favor anti-inflammatory mediators like IL10 and IL12.
Figure 3.
The hMSCs secrete various cytokines and chemokines following stimulation with different TLR ligands. Primary hMSC cultures were treated with various TLR-ligands: 1μM poly(I:C); 10ng/mL LPS; 5μM flagellin; 1μM CpG-ODN; 1μg/mL fibronectin III1c; 5μg/mL LL-37; or LPS and LL-37 combined; as noted, for 24 h (A.) or 48 h (B.), prior to harvesting and concentrating the spent culture medium as indicated in Materials and Methods. A. Cytokine antibody array assay (n>3) and B. Fluorescence bead immunoassay (n>4) analysis revealed that TLR-stimulation induced unique cytokine and chemokine secretion profiles dependent on ligand used to treat hMSCs. Error bars indicate +/− standard error of the mean (SEM). Values exceeding the bar graph scale are enumerated above the corresponding bars.
TLR stimulation within hMSCs triggers their enhanced migration
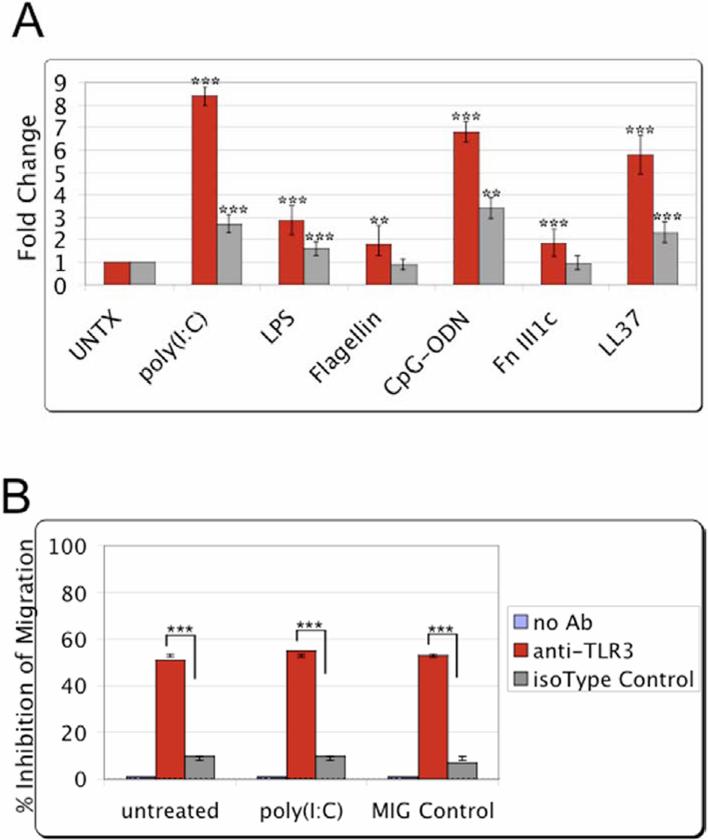
The effect of TLR stimulation on hMSCs migration was examined by transwell migration assay. Several representative TLR ligands (as indicated) were added as chemoattractant on the bottom wells and single-cell suspensions of hMSCs were loaded on top inserts. Consistently, poly(I:C) treatment enhanced the migration response from exposed hMSCs (Fig. 4A). LPS, CpG-ODN, and LL-37 resulted in moderate migration by the exposed hMSCs. Fibronectin (fragments III1C) and flagellin resulted in limited migration. Addition of the TLR ligands prior to migration assay resulted in improved migration (data not shown). As expected, minimal hMSC migration was measured in serum-free medium [17].
Figure 4.
TLR stimulation promotes the migration of the treated hMSCs. MSC migration towards TLR-specific ligands was examined by transwell migration assay. After overnight incubation, migration towards the various TLR-ligands was visualized and recorded by fluorescence microscopy. Migration was quantified from the obtained micrographs by counting the number of fluorescently labeled cells remaining after removal of non-migrating cells. A. Bar graph of the obtained results normalized to untreated (without TLR-ligand or chemotactic factor) control samples for two donors (red and gray). Error bars indicate SEM. Statistical significance analyzed for samples compared with the untreated control samples (n=6). ***p>0.001, **p>0.01
B. hMSC migration was examined following pre-incubation of the cells for 1 h with a human TLR3 specific monoclonal antibody or an isotype IgG control in the transwell migration assay as indicated. The equilibrated chemoattractant wells were loaded with growth medium in combination with poly(I:C), the TLR3 ligand, or a routinely used chemoattractant as a migration control (MIG control). After overnight incubation, migration was quantified as in A. Error bars indicate SEM. Statistical significance analyzed for samples compared with the isotype IgG treated control samples (n=3). ***p>0.001, **p>0.01.
TLR3 is critical to the migration responses of the stimulated hMSCs
The collective results indicated that TLR3 and its downstream signaling consequences are critical to hMSCs stress responses. Consequently, hMSCs were treated in the transwell migration assay with a neutralizing TLR3 antibody to test this notion. hMSCs were pre-incubated for 1hr with a human TLR3 specific monoclonal antibody or an isotype IgG control prior to loading in transwell migration assay inserts. After overnight incubation, migration was quantified as indicated in the Methods. Regardless of chemotactic factor used, pre-incubation of the hMSCs with anti-TLR3 antibody for 1 h prior to migration assay inhibited their migration by at least 55% (Fig. 4B). By contrast, pre-treatment of the hMSCs with an isotype IgG control led to minimal inhibition of hMSCs migration (10%).
Discussion
To understand the molecular details of how hMSCs sense stress, mobilize and engraft at inflamed, injured or pathological sites (tumors) to fulfill their repair function, we investigated TLRs and their downstream signaling consequences in these cells. Concurrent to our study, hematopoetic and other adult marrow-derived stem cells were reported to express TLRs. However, these reports mostly focused on the role of TLRs in stem cell proliferation and their potential role in disrupting the differentiation capability of these stem cells. These reports did not focus on the role of TLRs in critical stress responses of stem cells as described here. Specifically, since hMSCs are increasingly being employed in cell-based therapies the molecular details that drive their migration, recruitment and engraftment is critical to improving controlled and desired clinical outcomes. We report here that stimulation of TLRs within hMSCs leads to activation of established TLR signaling pathways, increased secretion of cytokines and chemokines, and promotes their migration. From these observations we propose that TLR stimulation within hMSCs may be one critical mechanism that specifically drives their stress responses. Notably, it appears that of all the expressed TLRs in this cell population TLR3 stimulation specifically plays a significant role in hMSCs stress responses. This observation is unique to this bone marrow-derived cell and is different from immune cells where TLR4 is the established LPS sensor responsible for affecting innate and adaptive immune responses.
In agreement with recent reports, hMSCs were found to express TLR1, 2, 3, 4, 5, 6 and 9 RNA by RT-PCR assays (Fig 1A). Protein expression of the corresponding gene products was confirmed by flow cytometry and immunofluorescence assays [7, 9]. Additionally, it was similarly found that hMSCs express reduced levels of TLR9 [9]. Typically, overall TLR expression in hMSCs was high with mean fluorescence intensities (MFI) comparable to established hMSC markers (e.g. CD90). The trend of TLR expression was the same for all the donors tested (n>7) and was from highest to lowest MFI: TLR5>>TLR2=3=4=6=7>>TLR9. Stimulation of the TLRs was found to lead to their transient internalization and degradation indicating that TLRs are activated after ligand treatment of the hMSCs (Fig 1B, C and D). TLR9 appeared to be the only exception with little degradation noted after ligand treatment of the hMSCs (data not shown).
We report for the first time that downstream signaling consequences of the stimulated TLRs within the hMSCs yielded specific activation patterns of the NF-κB, MAPK and AKT pathways consistent with the expected activation of established TLR-downstream pathways (Fig 2). Any one of these signaling pathways is known to affect cell migration and invasion properties of the stimulated cell [19-21]. Notably, treatment of the hMSCs with poly(I:C), the ligand for TLR3, resulted in the greatest activation of the NF-κB pathway. Similar effects for this ligand were reported for murine MSC [9].
Exposure of the hMSCs to TLR ligands led to unique cytokine and chemokine secretion as established by cytokine antibody arrays and confirmed with bead arrays (Fig 3A, B). LPS treatment of hMSCs distinctively led to the induced secretion of CCL5 (RANTES) and CXCL10 (IP10). Poly(I:C) treatment resulted in dramatically induced expression of CCL2 (MCP-1). In our hands, the hMSCs secreted high levels of IL6 constitutively and indiscriminately after treatment of the cells with growth factors, hypoxia, erythropoietin or various TLR ligands (unpublished observations, [17] and [22]). By contrast, IL6 secretion was used previously as the criteria to primarily follow TLR2 related pathways in murine MSC [9].
These observations support the notion that the different TLRs expressed within the hMSCs are competent in relaying distinct stress signals. Critically, many of the signaling consequences of TLR stimulation, like secretion of CCL5 (RANTES), IL8, and TNFα imply that TLRs may partially mediate the immune modulating responses established for hMSCs [13]. Further, these observations suggest that by stimulating specific TLR molecules different immune responses might be elicited by the hMSCs at the site of engraftment. In fact, the prediction from our studies is that LPS pre-treatment of hMSCs would create a pro-inflammatory milieu due to elevated IL6, TNF-α and CCL5 (RANTES) secretion. Conversely, poly(I:C) pre-treatment of hMSCs is expected to yield an anti-inflammatory environment due to increased IL10 and IL12 secretion. Intriguingly, a recent report dealing with the use of LPS moieties as vaccine adjuvants supports this notion [18]. The improved adjuvant effect of the monophosphoryl lipid A (MPLA) moiety was attributed to the fact that this adjuvant, unlike lipid A (LPS), activates only one-arm of the TLR signaling pathway. Instead of activating both the MyD88-dependent pathway that leads to inflammation and the TRIF-dependent pathway that leads to T-cell activation and promotes effective immune responses, this moiety only activates TRIF signaling and potentially avoids the harmful side effects caused by inflammation. Essentially, MPLA acts like a TLR3 ligand (poly(I:C)) since TLR3 signaling is unique and generally mediated exclusively through TRIF- and not MyD88-signaling pathways.
It should be noted that although there was consistency in the ability of TLR-ligands to induce discrete or unique cytokine and chemokine secretion patterns dependent on ligand employed we did observe donor variability in these patterns. For example, the secretion of CCL5 (RANTES) following LPS treatment in one donor pool was by far the greatest measured chemokine whereas IL8 levels were higher than CCL5 (RANTES) secretion following LPS stimulation of another donor pool. These findings might be explained by subtle differences in TLR-signaling for each donor hMSC. Additionally, our study confirms previous reports demonstrating that when LL-37 is combined with LPS treatment the overall effect is an observed dampening of the LPS effect (Table 1, Fig 2 and 3) [23]. Similarly, stimulation of hMSCs with fibronectin components yielded limited TLR-specific results most likely reflecting the fact that endogenously-derived agonists affect not only TLRs but several other receptor classes on the cells.
Importantly, our study demonstrated that specific TLR stimulation drives the migration of the hMSCs (Fig. 4A). While there was cursory mention of this potential effect by TLRs in a recent study of murine MSCs, this concept was not the focus of that report [9]. In their study, the authors concluded from a rudimentary wound-healing assay that the TLR2 ligand impaired murine MSC migration. It is not surprising that there are differences in migration responses mediated by specific TLR ligands on MSC from different species. In particular, in light of the fact that it was recently reported that TLR-mediated responses are both species-specific and cell-type specific [24]. Admittedly, the effect on hMSC migration by TLR stimulation could be improved from our original strategy. For instance, there is the possibility that the order of exposure to the hMSCs of the TLR ligands prior to or along with other stress signal molecules can be further explored to achieve more dramatic manipulation of hMSCs migration and invasion capabilities. This point is particularly important since a recent report stated that CCL5 (RANTES) driven hMSC migration was highly induced by pre-treatment of the hMSCs with TNFα [25].
Several lines of evidence point uniquely to TLR3 as primarily mediating the stress migration responses within hMSCs. First, TLR3 appears to be highly expressed in this cell population as demonstrated from RT-PCR, flow cytometry and immunofluorescence assay results presented (Fig. 1). Next, the dramatic effects of TLR3 ligand or poly(I:C) treatment of hMSCs that led to the specific induction of NF-κB, MAPK and AKT pathways, as well as cytokine, chemokine and other TLR pathway genes supports this notion (Fig. 2, 3, Table 1). Migration assays also demonstrated that poly(I:C) exposure of the hMSCs led to one of the most enhanced effects on hMSC migration when compared to other TLR-ligands (Fig 4A). This was confirmed by transwell migration (Fig 4A) and Boyden chamber migration (data not shown) assays with several of the hMSCs donors. This observation prompted further investigation. Thus, transwell migration assays performed with anti-TLR3 neutralizing antibodies resulted in consistent inhibition of hMSC migration (Fig. 4B). Surprisingly, this inhibition does not depend on specific TLR3 ligand exposure suggesting that hMSC migration is dependent on TLR3 regardless of its activation. A detailed investigation is currently underway in the laboratory to strengthen support for these observations, as well as to study the possibility of manipulating this TLR pathway for the in vivo guided manipulation of infused hMSCs to improve their migration, invasion and immune modulating responses at targeted sites. In summary, this study defines a novel TLR-driven stress and immune modulating response for hMSCs that is critical to consider in the design of stem cell-based therapies.
Conclusion
We demonstrated that stimulation of TLRs within hMSCs leads to the activation of established TLR signaling pathways. These activated pathways mediate the secretion of discrete patterns of cytokines and chemokines depending on the TLR-ligand employed. This observation critically implicates these receptors in the immune modulating function established for hMSCs. We describe also that TLR stimulation particularly promotes their migration capabilities. Thus, TLR-stimulation may be one mechanism that specifically drives the recruitment, migration and immune modulating function of the hMSCs at injured or stressed sites. Along with establishing a new aspect of their biology, the identification of TLRs as critical mediators of stress responses within hMSCs also provides a novel target to exploit in the improvement of stem cell-based therapeutic strategies.
Acknowledgments
The authors would like to thank the laboratory of Donald Phinney for their generous assistance with initially acquiring and cultivating the human mesenchymal stem cells. We would also like to thank Joanna DeSalvo for her assistance in the preparation of figures and Elizabeth Norton for insightful discussions of our work.
This work was supported by the following grants, NIH AI056229, NIH 1P20RR20152-01 and a research grant award from Cancer Association of Greater New Orleans (CAGNO).
References
- 1.Wright SD. Toll, a new piece in the puzzle of innate immunity. J Exp Med. 1999 Feb 15;189(4):605–609. doi: 10.1084/jem.189.4.605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.West AP, Koblansky AA, Ghosh S. Recognition and signaling by toll-like receptors. Annu Rev Cell Dev Biol. 2006;22:409–437. doi: 10.1146/annurev.cellbio.21.122303.115827. [DOI] [PubMed] [Google Scholar]
- 3.Sabroe I, Read RC, Whyte MK, Dockrell DH, Vogel SN, Dower SK. Toll-like receptors in health and disease: complex questions remain. J Immunol. 2003 Aug 15;171(4):1630–1635. doi: 10.4049/jimmunol.171.4.1630. [DOI] [PubMed] [Google Scholar]
- 4.Miggin SM, O'Neill LA. New insights into the regulation of TLR signaling. J Leukoc Biol. 2006 Aug;80(2):220–226. doi: 10.1189/jlb.1105672. [DOI] [PubMed] [Google Scholar]
- 5.Anders HJ, Banas B, Schlondorff D. Signaling danger: toll-like receptors and their potential roles in kidney disease. J Am Soc Nephrol. 2004 Apr;15(4):854–867. doi: 10.1097/01.asn.0000121781.89599.16. [DOI] [PubMed] [Google Scholar]
- 6.Triantafilou K, Triantafilou M, Dedrick RL. A CD14-independent LPS receptor cluster. Nat Immunol. 2001 Apr;2(4):338–345. doi: 10.1038/86342. [DOI] [PubMed] [Google Scholar]
- 7.Hwa Cho H, Bae YC, Jung JS. Role of Toll-Like Receptors on Human Adipose-Derived Stromal Cells. Stem Cells. 2006 December 1;24(12):2744–2752. doi: 10.1634/stemcells.2006-0189. %R 10.1634/stemcells.2006-0189. 2006. [DOI] [PubMed] [Google Scholar]
- 8.Nagai Y, Garrett KP, Ohta S, et al. Toll-like receptors on hematopoietic progenitor cells stimulate innate immune system replenishment. Immunity. 2006 Jun;24(6):801–812. doi: 10.1016/j.immuni.2006.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pevsner-Fischer M, Morad V, Cohen-Sfady M, et al. Toll-like receptors and their ligands control mesenchymal stem cell functions. Blood. 2006 October 12; doi: 10.1182/blood-2006-06-028704. %R 10.1182/blood-2006-06-028704. 2006:blood-2006-2006-028704. [DOI] [PubMed] [Google Scholar]
- 10.Mempel M, Voelcker V, Kollisch G, et al. Toll-like receptor expression in human keratinocytes: nuclear factor kappaB controlled gene activation by Staphylococcus aureus is toll-like receptor 2 but not toll-like receptor 4 or platelet activating factor receptor dependent. J Invest Dermatol. 2003 Dec;121(6):1389–1396. doi: 10.1111/j.1523-1747.2003.12630.x. [DOI] [PubMed] [Google Scholar]
- 11.Matzinger P. The danger model: a renewed sense of self. Science. 2002 Apr 12;296(5566):301–305. doi: 10.1126/science.1071059. [DOI] [PubMed] [Google Scholar]
- 12.Kim YM, Romero R, Oh SY, et al. Toll-like receptor 4: a potential link between “danger signals,” the innate immune system, and preeclampsia? Am J Obstet Gynecol. 2005 Sep;193(3 Pt 2):921–927. doi: 10.1016/j.ajog.2005.07.076. [DOI] [PubMed] [Google Scholar]
- 13.Aggarwal S, Pittenger MF. Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood. 2005 Feb 15;105(4):1815–1822. doi: 10.1182/blood-2004-04-1559. [DOI] [PubMed] [Google Scholar]
- 14.Phinney DG, Kopen G, Isaacson RL, Prockop DJ. Plastic adherent stromal cells from the bone marrow of commonly used strains of inbred mice: variations in yield, growth, and differentiation. J Cell Biochem. 1999 Mar 15;72(4):570–585. [PubMed] [Google Scholar]
- 15.Hall B, Dembinski J, Sasser AK, Studeny M, Andreeff M, Marini F. Mesenchymal stem cells in cancer: tumor-associated fibroblasts and cell-based delivery vehicles. Int J Hematol. 2007 Jul;86(1):8–16. doi: 10.1532/IJH97.06230. [DOI] [PubMed] [Google Scholar]
- 16.Aicher A, Brenner W, Zuhayra M, et al. Assessment of the tissue distribution of transplanted human endothelial progenitor cells by radioactive labeling. Circulation. 2003 Apr 29;107(16):2134–2139. doi: 10.1161/01.CIR.0000062649.63838.C9. [DOI] [PubMed] [Google Scholar]
- 17.Zwezdaryk KJ, Coffelt SB, Figueroa YG, et al. Erythropoietin, a hypoxia-regulated factor, elicits a pro-angiogenic program in human mesenchymal stem cells. Exp Hematol. 2007 Apr;35(4):640–652. doi: 10.1016/j.exphem.2007.01.044. [DOI] [PubMed] [Google Scholar]
- 18.Mata-Haro V, Cekic C, Martin M, Chilton PM, Casella CR, Mitchell TC. The vaccine adjuvant monophosphoryl lipid A as a TRIF-biased agonist of TLR4. Science. 2007 Jun 15;316(5831):1628–1632. doi: 10.1126/science.1138963. [DOI] [PubMed] [Google Scholar]
- 19.Primo L, di Blasio L, Roca C, et al. Essential role of PDK1 in regulating endothelial cell migration. J Cell Biol. 2007 Mar 26;176(7):1035–1047. doi: 10.1083/jcb.200607053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Helbig G, Christopherson KW, 2nd, Bhat-Nakshatri P, et al. NF-kappaB promotes breast cancer cell migration and metastasis by inducing the expression of the chemokine receptor CXCR4. J Biol Chem. 2003 Jun 13;278(24):21631–21638. doi: 10.1074/jbc.M300609200. [DOI] [PubMed] [Google Scholar]
- 21.Jeong HJ, Na HJ, Hong SH, Kim HM. Inhibition of the stem cell factor-induced migration of mast cells by dexamethasone. Endocrinology. 2003 Sep;144(9):4080–4086. doi: 10.1210/en.2003-0115. [DOI] [PubMed] [Google Scholar]
- 22.Haynesworth SE, Baber MA, Caplan AI. Cytokine expression by human marrow-derived mesenchymal progenitor cells in vitro: effects of dexamethasone and IL-1 alpha. J Cell Physiol. 1996 Mar;166(3):585–592. doi: 10.1002/(SICI)1097-4652(199603)166:3<585::AID-JCP13>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 23.Kandler K, Shaykhiev R, Kleemann P, et al. The antimicrobial peptide LL-37 inhibits the activation of dendritic cells by TLR ligands. Int Immunol. 2006 Dec;18(12):1729–1736. doi: 10.1093/intimm/dxl107. [DOI] [PubMed] [Google Scholar]
- 24.Lundberg AM, Drexler SK, Monaco C, et al. Key differences in TLR3/POLY I:C signaling and cytokine induction by human primary cells: a phenomenon absent from murine cell systems. Blood. 2007 Jul 27; doi: 10.1182/blood-2007-02-072934. [DOI] [PubMed] [Google Scholar]
- 25.Lopez Ponte A, Marais E, Gallay N, et al. The in vitro migration capacity of human bone marrow mesenchymal stem cells: Comparison of chemokine and growth factor chemotactic activities. Stem Cells. 2007 Mar 29; doi: 10.1634/stemcells.2007-0054. [DOI] [PubMed] [Google Scholar]