Abstract
The contribution of peripheral expression of tissue-specific CNS Ags to the generation of tolerance is uncertain. To study this question, we examined mice transgenic (Tg) for expression of beta-galactosidase (βgal) on the retinal photoreceptor cell arrestin promoter, in conjunction with TCR Tg mice producing CD4+ T cells specific for βgal (βgalTCR). Several strategies were used to test the hypothesis that βgal expressed in the retina supported thymus-independent tolerance and regulatory T cell development. Retinal expression generated an immunoregulatory response that depressed development of immune responses to βgal following systemic immunization with βgal. This regulation was transferable to naive mice by CD3+4+25+ T cells from naive retinal βgal+ donors. Experiments that removed the βgal+ retina by enucleation showed that subsequent development of a regulatory response was lost. Adoptive transfer of CD25- βgalTCR T cells into retinal βgal Tg mice on the Rag(-/-) background led to regulatory activity that limited lymphopenia-induced proliferation of βgalTCR T cells in mice with retinal expression of βgal, and inhibited the ear swelling assay for delayed type hypersensitivity. These results show that retinal expression of very small amounts of a tissue-specific Ag can generate tolerance that includes regulatory T cells.
Keywords: Neuroimmunology, Tolerance/Suppression/Anergy, Autoimmunity, Transgenic/Knockout mice, T cells
Introduction
Immunologic self-tolerance is required for an effective immune system, and is provided by the concerted activities of central and peripheral tolerance. A growing body of evidence describes mechanisms underlying the generation and activity of regulatory T cells (Tregs) that bear the CD4+25+Foxp3+ phenotype (1, 2). These Ag-specific Tregs play an important role in tolerance to tissue-specific self-Ags (TSA) (3). Expression of TSA in thymus, including aire gene-directed expression of TSA in medullary thymic epithelial cells, leads to negative selection of T cells specific for TSA (4, 5), and to positive selection of Tregs specific for TSA (6), providing protection from autoimmune disease. While the development of Tregs in young mice is largely thymus-dependent (7), CD4+25+ Tregs re-develop spontaneously several months following thymectomy (8, 9). It has also been demonstrated that CD4+25+ Tregs can develop from mature, peripheral CD4+ T cells in vivo in response to exogenous Ag administered by i.v. or oral routes (10, 11).
Experimental autoimmune uveoretinitis (EAU) is a retinal autoimmune disease mediated by CD4 (12) or CD8 (13) T cells directed to retinal Ags, including interphotoreceptor retinoid binding protein (IRBP). Through use of IRBP-deficient and wild-type mice, thymic expression of IRBP was shown to provide central tolerance to IRBP, through negative selection (14), and generation of CD25+ Tregs (15). Aire-deficient mice developed autoimmune retinitis that was dependent on retinal IRBP expression; mice deficient in aire and IRBP did not develop retinal inflammation, but other organs remained targets of autoimmune disease (16). Since thymic expression of IRBP was not required to generate Tregs that protected from retinal inflammation (15), it is possible that Tregs with specificity for other retinal TSAs could contribute to protection from retinal autoimmunity.
We propose that Tregs result from contact with retinal Ags in the periphery, contributing to the generation of tolerance, separate from the contribution of thymic expression. Using E. coli beta-galactosidase (βgal) transgenic (Tg) mice to achieve Ag expression from the arrestin promoter in retinal photoreceptor cells, we found spontaneous immunoregulation that altered the immune response to βgal (17). Although analysis of retinal βgal Tg mice has not revealed detectable levels of βgal in thymus, whether by X-gal staining, RT-PCR, or evidence of thymic selection, very low levels could contribute to thymic generation of Tregs. The present results show that intracellular expression of Ag in neurons (photoreceptor cells) in normal, quiescent retina led to peripheral generation of Tregs that could be attributed to retinal-derived Ag.
Materials and Methods
Mice
βgal-expressing Tg mice have been described elsewhere (17). βgal expression in rod photoreceptor cells of arrβgal mice produces 150 ng of βgal/retina and < 0.5 ng/pineal gland. GFAPβgal mice express βgal in CNS astrocytes (175 ng/brain). βgal expression in adult ROSA26 mice was low, but widespread. T cell receptor (TCR) Tg mice carrying an α/β TCR conferring specificity for a class II MHC-restricted response to a βgal peptide were described (βgalTCR mice, (18)). All βgal Tg mice and Rag(-/-) mice were backcrossed onto the B10.A background (Charles River, Wilmington, MA). Mice were housed under SPF conditions on lactose-free chow, and handled in accordance with the ARVO Statement for Use of Animals in Ophthalmic and Vision Research, and University of Minnesota animal use and care guidelines.
Ags and immunization
βgal was purchased from Prozyme (San Leandro, CA). A class II-restricted immunodominant epitope of βgal (YVVDEANIETHGMV) was prepared by the Microchemical Facility at the University of Minnesota. Some mice received a s.c. injection on a hind thigh with 50-200 μg of βgal in CFA containing 1 mg/mL of killed M. tb, H37Ra (Sigma, St. Louis, MO). Others were inoculated i.p. with 1.25 - 5 × 106 pfu/mouse of live, recombinant vaccinia virus (VSC 56) expressing βgal under control of the synthetic early-late promoter (19). VSC 56 was obtained from Dr. J. Yewdell (NIAID/NIH, Bethesda, MD).
T cells and T cell culture
Naive CD4+ T cells specific for βgal were isolated from βgalTCR Tg mice. Cultures were done with or without βgal peptide (1 – 10 μM), APCs and βgalTCR T cells as indicated, in RPMI 1640 with 10 % FCS. Cytokines in culture supernatants were determined by cytometric bead array. Abs and cytokine standards were purchased from BD Biosciences and eBiosciences (San Diego, CA), R&D Systems (Minneapolis, MN), and Millipore (St. Charles, MO).
Positive and negative selection of cells
Isolation of CD25+ (clone 7D4), CD4+, CD8+ or Thy1.2+ cells, or depletion of these populations, was done using the MACS system (Miltenyi Biotec, Auburn, CA). Cell suspensions were incubated with Abs conjugated with paramagnetic beads and applied to MS+, LS+ or LD columns according manufacturer's protocols. For transfer, cells were washed and resuspended to 5 × 107 cells/mL in saline. Recipient mice received up to 5 × 106 cells in 0.1 mL by i.v. injection. Larger numbers or volumes of cells were given by i.p. injection as indicated.
Ear test measurement of delayed-type hypersensitivity (DTH)
The difference in ear thickness due to a DTH response to Ag was measured with a micrometer (Mitutoyo, Kawasaki, Japan), before and after Ag inoculation. A 30 g needle was used to give an intrapinna injection of 50 - 100 μg of βgal in 10 μL saline.
Enucleations
To perform bilateral enucleations to remove all retina-associated Ag, mice were anesthetized with xylazine/ketamine HCl. The lids were retracted to proptose the eye. The globes were grasped with a curved forceps and scissors were used to do a blunt dissection, followed by cutting the optic nerve to free the eye. Pressure was applied with a cotton swab to control bleeding. The lids were closed with a single gut suture, and antibiotic ointment was applied. Buprenorphine was used post-operatively.
RT-PCR Method
mRNA was prepared using μMACS mRNA isolation kit (Miltenyi) and DNase I (Ambion) per manufacturer's protocol. 1-5 μL of mRNA was reverse transcribed in a 20 μL reaction containing 100 ng of reverse primer for the indicated gene (see below) and 10 U RNase inhibitor (Ambion) with dNTPs (0.5mM final), reaction buffer (1× final), and reverse transcriptase (4 U final) from Omniscript RT kit (Qiagen). The RT reaction was incubated at 37°C for 60 min and terminated at 93°C for 7 min. 1 μL of the RT reaction was then amplified by PCR using 100 ng of the forward and reverse primers, 1 mM MgCl2, 0.2 mM dNTPs, and 1× reaction buffer plus 1.25 U HotStarTaq DNA polymerase (Qiagen) in a final volume of 50 μL. The PCR reactions were subjected to an initial denaturation at 95°C for 15 min followed by 40 cycles of 92°C for 30 sec, 60°C for 45 sec, 72°C for 60 sec, and then a final extension at 72°C for 7 min. The RT-PCR products were analyzed by Gel Star (Lonza)-stained agarose gels. The forward and reverse primers are as follows: rod photoreceptor cell arrestin 5′-TCTCAGTCTCCCTCAGCAAAGAG and 5′-TCACTCATCCTGGCCAGCATCCTC; βgal 5′-GATGATTTCAGCCGCGCTGTAC and 5′-CTCACTCGGGTGATTACGATCG; acidic ribosomal binding protein (ARBP) 5′-AAACAGTAGTACAGCCATTGGG and 5′-CTGCAACTGCCATTCCAACA; β-actin 5′-GTGGGCCGCTCTAGGCACCAA and 5′-CTCTTTGATGTCACGCACGATTTC.
Results
Evidence for tolerance in retinal Ag Tg mice
We previously reported that expression of βgal in retinal rod photoreceptor cells led to spontaneous tolerance to βgal, but the tolerance was overwhelmed by immunization with βgal in CFA containing a high dose of M. tb. H37Ra and concurrent i.v. pertussis toxin, as required to induce EAU (17). Given this sensitivity to a highly aggressive immunization protocol, we asked if the regulatory activity was relevant to physiological challenges. Mice vaccinated with live VSC 56 virus showed that DTH to βgal in convalescent arrβgal mice was inhibited (Fig. 1A). The GFAPβgal and βgal+ROSA26 control mice also demonstrated reduced DTH following viral challenge. Control virus lacking βgal expression induced no evidence of immunity or tolerance to βgal (data not shown). No autoimmune disease was seen in βgal+ tissues of βgal Tg mice vaccinated with virus. Ag-specific inhibition in arrβgal mice was shown by use of bovine serum albumin (BSA) in CFA-immunizations and ear swelling assays to BSA in control and arrβgal mice (Fig. 1B).
FIGURE 1.
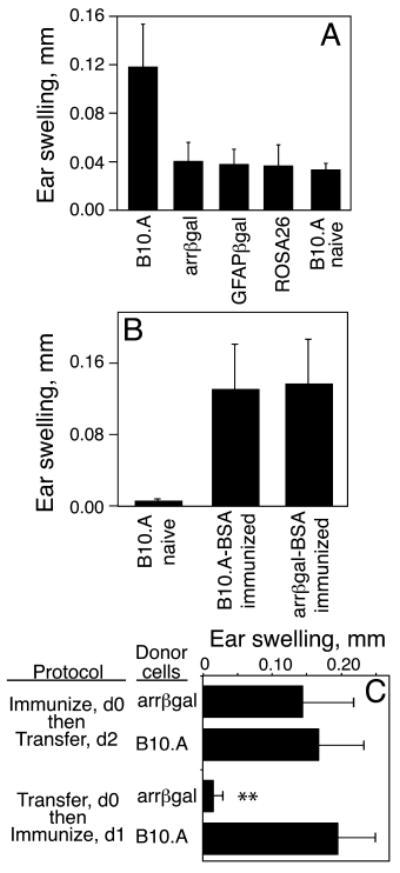
Transgenic expression of βgal inhibited the immune response to βgal elicited by (A) infection with VSC 56, or by (B-C) immunization with Ag in CFA. (A) Analysis of the DTH response to βgal. Mice were ear tested with purified βgal 21 days post-infection. (B) Analysis of the specificity of tolerance in arrβgal mice. Mice were immunized with BSA/CFA and the DTH response to BSA was assayed 21 days later. (C) The regulatory activity of arrβgal mice acted on the afferent response in vivo. Recipient mice were immunized with βgal, and 30 × 106 spleen cells from control B10.A or arrβgal donor mice were transferred i.p. as indicated. DTH was analyzed by ear test 7 days post-immunization. All results are given as the mean ± SD. N = 5 - 7 mice/group. **P < 0.01 (t test) for arrβgal vs. B10.A mice.
Tolerance was transferred by SPL, and acted on the afferent response to Ag
Adoptive transfer of immunoregulation induced by peripheral, intraocular inoculation of exogenous Ag has been reported to inhibit established immune responses to retinal Ags in immunized recipients (20). To test naive arrβgal mice for transferable activity, SPL from naïve, B10.A and arrβgal mice were transferred into βgal-negative recipients. After 1 day, the recipients were immunized with βgal in CFA and ear tested 7 days later. SPL from arrβgal donors, but not wt donors, depressed the DTH response (Fig. 1C). However, the regulatory activity was only effective if transferred before Ag priming.
The tolerogenic activity of arrβgal mice was transferred by CD3+4+25+ T cells
If inhibition of DTH in arrβgal mice was mediated by Tregs, depletion of CD3+ cells should abrogate the transfer of inhibition of βgal DTH. CD3-depleted or unseparated SPL (Fig. 2A) were transferred, followed by infection with VSC 56, and ear testing (Fig. 2B). B10.A mice that received CD3-depleted arrβgal SPL had full-scale ear swelling, relative to controls, showing that splenic CD3 cells from arrβgal mice transferred inhibition of DTH. To determine whether the inhibitory activity was carried by CD4+ or CD8+ T cells, these T cell populations from arrβgal mice were prepared as described in the Methods (Fig. 2C). The CD4+ population from arrβgal mice clearly inhibited the expression of DTH to βgal after VSC56 vaccination, but the CD8+ population did not (Fig. 2D). The association of inhibitory activity with CD3+4+ cells suggested the activity of CD4+25+ Tregs. SPL and LN cells from arrβgal mice were enriched for CD3+4+ cells as above, and separated into CD25+ and CD25- fractions (Fig. 2E). Recipient B10.A mice were vaccinated and ear tested after 26 days. While unseparated cells transferred inhibition of ear swelling, CD25-depleted cells did not (Fig. 2F).
FIGURE 2.
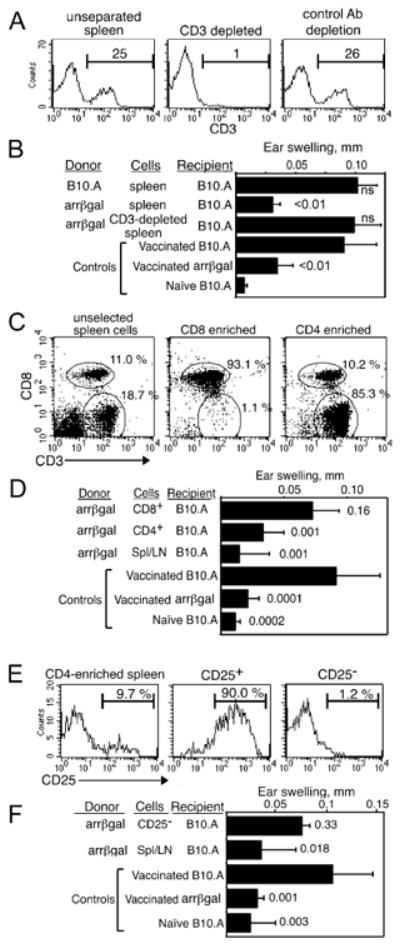
Regulatory cells from arrβgal mice are CD3+4+25+. (A, B) Analysis of CD3+ cells from arrβgal mice for Treg activity. (A) FACS analysis of CD3-depleted (center), and unfractionated spleen/LN cells (left). Percent of cells is indicated. (B) Depletion of CD3+ cells eliminates the ability to inhibit DTH upon transfer to B10.A mice. 30 × 106 cells were transferred. (C, D). Analysis of CD4+ cells from arrβgal spleen/LN for Treg activity. (C) FACS analysis of fractions selected for CD4+ cells (right), CD8+ cells (center), and unfractionated spleen/LN (left). (D) CD4+ cells transferred inhibition of DTH to B10.A mice. 7 × 106 CD4+ or CD8+ T cells, or 30 × 106 unseparated cells were transferred to normal B10.A mice. (E, F). Transfer of CD25-depleted cells failed to inhibit the βgal ear swelling response. (E) FACS analysis of spleen/LN cells depleted of CD25+. Different clones of anti-CD25 were used for depletion (PC-61) or detection (7D4). 50 × 106 CD25- cells or 30 × 106 unseparated cells were transferred. (F) CD25- cells did not inhibit ear swelling. For all assays, the cells were transferred to B10.A mice on day 0, mice were immunized on day 1 by vaccination with VSC 56, and ear tested on day 26 - 28 with βgal. P values (t test) for comparisons with vaccinated B10.A control groups are given. Error bars indicate SD.
Removal of the retina alters tolerance in arrβgal mice
The thymus produces CD4+25+Foxp3+ Tregs to many self-Ags, as shown by day 3 thymectomy (8), Foxp3 deficiency (21), and aire deficiency (5, 6). Thymic expression of βgal was not detected in arrβgal mice by RT-PCR (Fig. 3A). Conversely, arrestin mRNA was detected in retina and thymus (Fig. 3B). The control ROSA26 mice expressed readily detectable βgal mRNA in thymus and retina. The results suggested that the recombinant arrestin promoter construct in the arrβgal Tg mice had reduced activity in the thymus, raising the possibility that retinal βgal expression could contribute to the observed tolerance.
FIGURE 3.
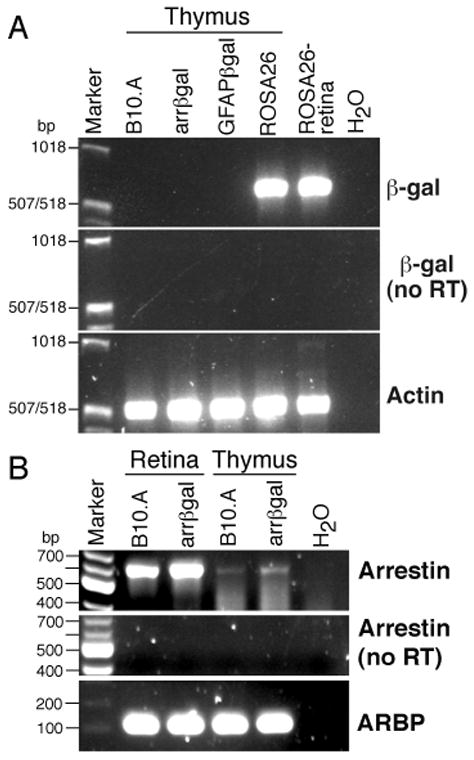
Examination of thymus and retina for expression of βgal and arrestin mRNA in βgal Tg and control B10.A mice. (A) RT-PCR for βgal mRNA in samples from thymus and retina. (B) Detection of endogenous arrestin mRNA in thymus and retina from B10.A and arrβgal mice.
Approximately 99.9 % of βgal in arrβgal mice is expressed in the retina; the remainder is expressed in the pineal gland. Therefore, enucleation may test the role of retinal Ag expression in generation of tolerance. B10.A and arrβgal mice were enucleated, and irradiated with 800 R two days later to ablate existing T cells. Control mice were either irradiated only, or unmanipulated. Five months later, the mice were immunized with βgal in CFA, and ear tested. Enucleated arrβgal mice had reduced ability to inhibit DTH elicited by ear testing βgal-immunized mice (Fig. 4A). Since the thymus was not manipulated, the outcome reflects retina-dependent processes. DTH in control ROSA26 mice, which have thymic and extra-ocular expression of βgal, was inhibited after recovery from enucleation and irradiation (Fig. 4B).
FIGURE 4.
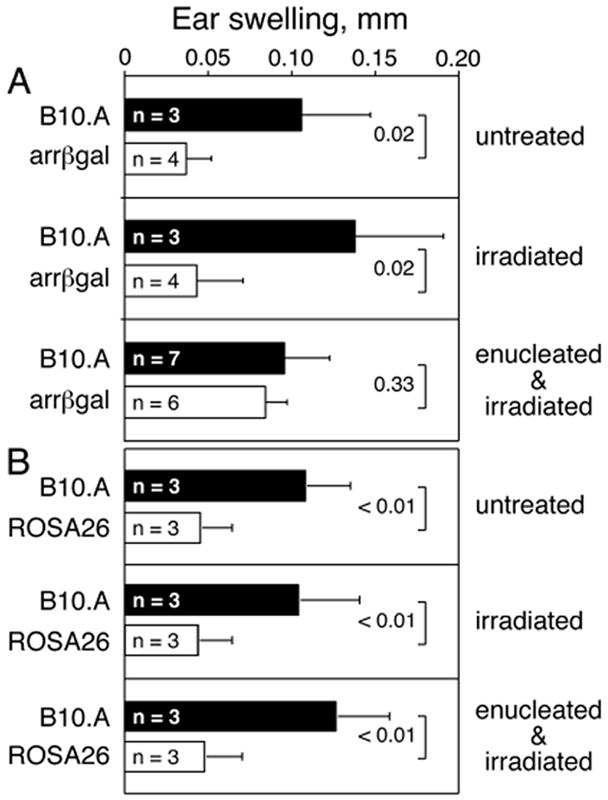
Retinal βgal expression contributed to the production of tolerance. (A) Arrβgal and control mice were enucleated and/or irradiated to eliminate the retina as a source of Ag, and to deplete existing Tregs. Five months later, all mice were immunized with βgal and the DTH response was analyzed by ear testing with βgal 7 days post-immunization. (B) ROSA26 and control mice were similarly treated and then ear tested. Results are given as the mean ± SD and P values (t test) are indicated.
The tolerogenic activity of arrβgal SPL acts on naive βgalTCR T cells in vitro
Since the regulatory activity acted on afferent responses in vivo, it was tested on naive βgalTCR T cells in vitro. Approximately 60 % of mature T cells, and 80-90 % of CD4+ T cells in these mice on the normal background, carry the α/β TCR conferring specificity for a class II MHC-restricted epitope of βgal (18). The effect of arrβgal SPL on the T cell response to Ag was assessed by secretion of IFNγ in vitro. Enriched, naive βgalTCR T cells were obtained by positive selection for CD4+ SPL and LN cells from βgalTCR mice, and cultured with and without Ag in the presence of non-irradiated arrβgal or normal B10.A SPL. Non-irradiated arrβgal SPL inhibited Ag-induced IFNγ production by naive βgalTCR T cells (Fig. 5), but had less effect on production of IL-2, IL-4, IL-10, or TNFα (data not shown).
FIGURE 5.
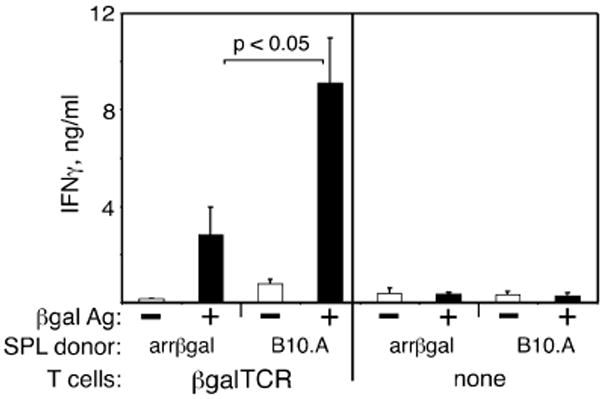
Spleen cells from arrβgal mice inhibited the in vitro Ag response of naive βgalTCR T cells. (A) 5 × 106 non-irradiated splenocytes from arrβgal mice or control B10.A mice were cultured with/without 1 × 105 βgalTCR CD4+ T cells and 50 μg/mLβgal as indicated. Supernatants were collected at 72 h after Ag stimulation and assayed by ELISA. The results are given as the mean ± SD, P values (t test) are indicated.
Functional Tregs can be peripherally induced in βgalTCR T cells
Oral, i.v., and i.p. administration of Ags has been reported to induce CD4+25+ Tregs in the periphery (10, 22, 23). As proof of principle that peripheral βgal can generate Tregs, the i.v. route to generation of βgal-specific CD4+25+ Tregs in normal B10.A mice was tested (Fig. 6). Ten days after i.v. inoculation of 200 μg of soluble βgal, SPL and LN cells were pooled, and CD4+25+ T cells were isolated. Regulatory activity was assessed by testing co-cultures for effects on Ag-dependent cytokine production by purified CD4+25- naive βgalTCR T cells. IL-2 and IFNγ production by βgalTCR T cells was inhibited by addition of CD4+25+ cells from mice pre-treated with βgal, but there was little effect of adding CD4+25+ cells from untreated B10.A mice (Fig. 6D). While elevated levels of TGFβ1 were found in co-cultures of βgal-induced Tregs and βgalTCR T cells, Tregs from either donor did not make significant amounts when cultured separately, and made no detectable IL-2, IL-10 or IFNγ.
FIGURE 6.
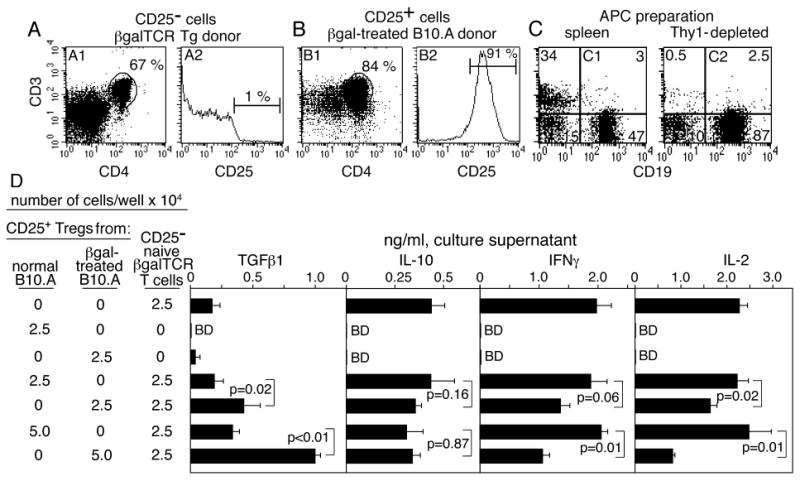
Induction and analysis of CD4+25+ Tregs induced by peripheral administration of βgal in B10.A mice. (A-C) FACS analysis of CD4+25- (A), CD4+25+ (B), and Thy-1 depleted cells (C) for use in the functional assays. The cells in A and B were depleted with anti-CD19, -CD11b, -CD11c, and -CD8 followed by selection for CD4+25- or CD4+25+ cells. (D) Ag-stimulated cytokine production by naive CD4+25- βgalTCR T cells in co-culture with populations of CD4+CD25+ T cells from normal B10.A mice or βgal-inoculated B10.A mice. Irradiated, Thy1-depleted splenocytes (3 × 105/well) were added as APCs and βgal Ag was added at 100 μg/mL. Results are given as the mean ± SD of duplicate assays (3 wells/sample in each assay) of 72 h supernatants. BD = below detection. Cytokine production in unstimulated cultures was at background levels, and is not shown.
Tolerance is found in arrβgal × βgalTCR double Tg mice on the Rag(-/-) background
We previously showed that arrβgal × βgalTCR double Tg mice spontaneously developed CD25+ Tregs that inhibited DTH to βgal (24). These mice were backcrossed onto the Rag(-/-) background, so that both βgal-specific effector T cells capable of mediating DTH, and Tregs specific for βgal, would be generated from the same precursors. Inhibition of DTH was found in these mice with retinal βgal expression on the Rag(-/-) background (Fig. 7A), consistent with peripheral generation of tolerance due to retinal Ag expression. The βgalTCR T cells gave rise to populations of effector and regulatory T cells depending on whether they were generated in wt or arrβgal mice, respectively.
FIGURE 7.
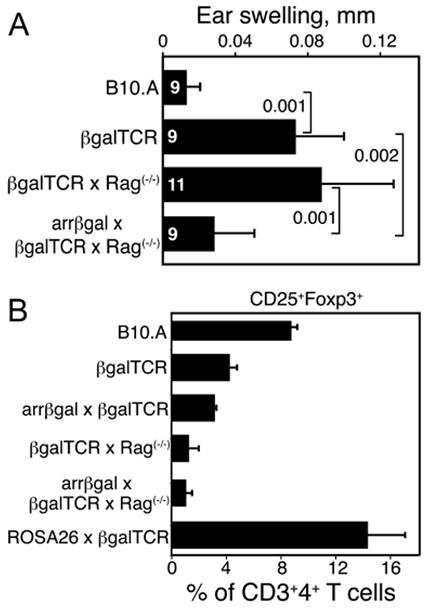
CD25+ Tregs were generated endogenously in arrβgal × βgalTCR double Tg mice. (A) Tolerance developed spontaneously in the arrβgal × βgalTCR mice on the Rag(-/-) background. Ear testing was done with βgal without prior immunization. Number of mice (N) as indicated. Results are given as the mean ± SD, P values (t test) are indicated. (B) A reduced frequency of T cells with a regulatory phenotype developed in arrβgal × βgalTCR mice. FACS analysis for CD25+Foxp3+ T cells as a percentage of the total CD3+4+ splenic T cells, N ≥ 3 for all groups. The results are given as the mean ± SD.
CD25+Foxp3+ T cells are found in wt and Tg mice
It has been reported that generation of CD4+25+Foxp3+ Tregs in TCR Tg T cells on the Rag(-/-) background requires positive thymic selection by cognate Ag (25). In the case of TSAs, this is thought to be dependent on the aire gene (26, 27). CD4+25+Foxp3+ T cells were poorly selected, at least numerically, in arrβgal × βgalTCR double Tg mice on the Rag(-/-) background (Fig. 7B). The results suggest an Ag source other than thymus, since there was no evidence of enhanced positive selection of CD25+Foxp3+ cells in the arrβgal mice, unlike the βgalTCR × ROSA26 double Tg mice, which exhibit thymic expression and produced an elevated number of Tregs. No discrete population of CD25+Foxp3+ cells was found in thymus from either the βgalTCR × Rag(-/-) mice or the arrβgal × βgalTCR × Rag(-/-) mice (data not shown).
Rag(-/-) mice reveal a peripheral contribution of retinal Ag to tolerance in mature T cells
The peripheral induction of a regulatory response was further tested by adoptive transfer of purified, mature, peripheral CD25- βgalTCR T cells into Rag(-/-) recipients that can not thymically generate mature CD4+25+ Tregs (28, 29). Backcrossing the arrβgal mice onto the Rag(-/-) background provided βgal+ retina as the peripheral source of specific Ag for peripheral generation and/or maintenance of Tregs from the transferred T cell populations. Analysis of CD25- βgalTCR donor cells prior to inoculation showed that contamination by Foxp3+CD25+ double positive cells was reduced to 0.5 % from a starting point of 10 – 15 % (Fig. 8A). Several populations of CD25+ T cells were also prepared for transfer, and contained approximately 75 % Foxp3+CD25+ double positive cells (Fig. 8A).
FIGURE 8.
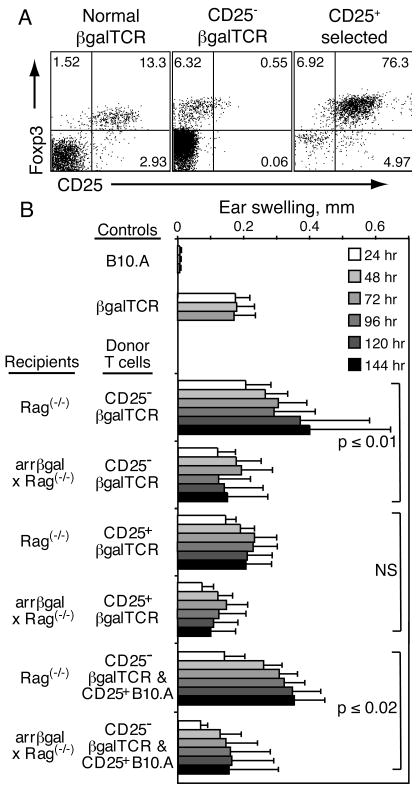
Transfer of CD25- βgalTCR T cells into lymphopenic recipients, whether arrβgal × Rag(-/-) or Rag(-/-), demonstrated the development of a systemic regulatory response in βgal+ recipients. (A) CD25+Foxp3+ status of the CD4+ donor cells prior to transfer. (B) Ear swelling of control and recipient mice 10 – 11 weeks post-transfer of the indicated T cell populations. CD25-depleted naive βgalTCR T cells, or CD25-enriched T cells from βgalTCR mice, were given at 2.5 × 105/mouse. CD25-enriched T cells from B10.A mice were administered at 5 × 104/mouse, where indicated, with the CD25-depleted cells.
Rag(-/-) and arrβgal × Rag(-/-) mice were inoculated with these T cells, alone or in combination. Ten - 11 weeks after adoptive transfer, recipient mice were ear tested with βgal. Transfer of CD25- T cells into Rag(-/-) recipients resulted in unusually strong and progressive DTH responses (Fig. 8B). In contrast, arrβgal × Rag(-/-) recipients exhibited a self-limiting DTH response that was significantly smaller. By comparison, transfer of CD25+ T cells from βgalTCR donors gave smaller, but still robust, self-limited ear swelling responses in Rag(-/-) recipients, while arrβgal × Rag(-/-) recipients showed nominally less swelling over the 7 day course. Mice receiving combinations of CD25- cells from βgalTCR mice with CD25+ cells from normal B10.A mice showed that inclusion of the CD25+ cells had little effect on the ear swelling assays, whether the recipients were Rag(-/-) or arrβgal × Rag(-/-) mice.
CD25+ cells inhibited EAU in arrβgal × Rag(-/-) mice
Although inclusion of CD25+ cells from wt mice in transfers to arrβgal recipients did not inhibit DTH, they were not without effect. We recently found that adoptive transfer of naive, CD25-depleted βgalTCR T cells to arrβgal × Rag(-/-) mice led to a high incidence of severe EAU (18). If 5 × 104 CD25+ cells from B10.A mice were added to 2.5 × 105 naive, CD25-depleted βgalTCR T cells in the inoculum, pathogenesis of EAU was substantially inhibited in arrβgal × Rag(-/-) recipients (Table I). Similarly, isolation and transfer of the CD4+ population from βgalTCR mice, which also contains approximately 6% CD25+ T cells, led to a substantial reduction in incidence and severity of EAU relative to transfer of the CD25- population only.
Manipulation of the retina alters tolerance in arrβgal mice
If development of Tregs from circulating, mature CD4 T cells required recognition of retinal Ag, preventing this process should reduce tolerance. The enucleation strategy shown in Fig. 4 supported the hypothesis that retinal Ag contributed to the regulatory activity, but the irradiation used to lymphoablate the mice could damage the retina of control mice, and promote Ag trafficking. To avoid radiation damage, the Tg T cells and arrβgal mice on the Rag(-/-) background were used. The mice were enucleated and given naive CD25- T cells from βgalTCR mice on the Rag(-/-)background. After 8 weeks, the mice were tested for evidence of Treg development from the donor cells (Fig. 9A). The recipients with intact, βgal+ retinas gave inhibited DTH responses, while the enucleated recipients developed full scale responses similar to Rag(-/-) controls, showing that Ag in the retina elicited the regulatory response. Since there was no irradiation of the mice, or manipulation of the thymus, the outcome was imposed on mature peripheral T cells by retinal Ag. This result was followed by the demonstration that the cells responsible for the inhibited DTH in the control recipients that were not enucleated were sensitive to anti-CD25 depletion, which restored their DTH to full scale levels (Fig. 9B).
FIGURE 9.
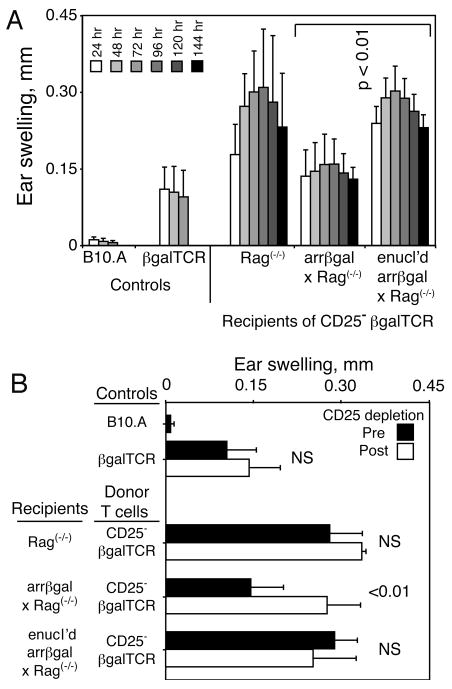
Enucleation prevents induction of tolerance in CD25- βgalTCR T cells transferred into arrβgal × Rag(-/-) mice. (A) Resting CD25- T cells (2.5 × 105/mouse) from βgalTCR × Rag(-/-) mice were inoculated into the indicated recipients. Mice were ear tested after 56 days. (B) After ear testing, the mice were rested for 9 days, and treated with anti-CD25. Three days later, the mice were again ear tested with βgal; measurements at 48 h are shown. Results are given as the mean ± SD and P values (t test) are indicated. Enucleated = enucl'd.
Discussion
Our goal was to determine if retinal expression of Ag in arrβgal mice was a factor in generating tolerance. Attempts to detect thymic expression of βgal by Xgal staining for enzymatic activity, and RT-PCR, yielded no evidence for expression. Functional assays for thymic selection have also failed to implicate thymic βgal. Grafting bone marrow from the βgalTCR mice into irradiated arrβgal versus B10.A recipients gave no evidence of activation in peripheral populations from LN, spleen, or blood, nor differences in maturation of cells from thymus (18). Conversely, βgalTCR T cells were substantially affected when βgalTCR bone marrow was grafted into ROSA26 mice, consistent with their thymic and systemic βgal expression.
We show here the presence of an Ag-specific activity from arrβgal mice that transfers with CD3+4+25+ T cells, and inhibits the afferent response to βgal immunization. The generation of the tolerance was significantly dependent on the presence of a βgal+ retina. Although the Ag is localized to the retina, its presence in normal and injured retina altered the systemic DTH response in naive, arrβgal × βgalTCR double Tg mice, showing that the effect was not limited to local (retinal) mediators or mechanisms. Elsewhere, we showed that systemic tolerance exhibited by the double Tg mice was reversed by treatment with anti-CD25 in vivo (24). Double Tg mice on the Rag(-/-) background also developed tolerance. Foxp3+ T cells were generated in vivo from βgalTCR T cells during LIP, and βgal-specific Tregs could be generated in adult βgalTCR mice by i.v. inoculation of βgal, confirming the ability to induce regulatory activity in mature βgalTCR T cells. In addition to inhibiting CD4 T cell-mediated DTH, as described here, the regulatory activity generated in arrβgal mice was also able to inhibit the ear swelling mediated by βgal-specific CD8 T cells raised by the VSC 56 vaccination (30).
Ear swelling assays for DTH in lymphopenic recipients of CD25- βgalTCR T cells were inhibited if βgal was present in the retina, but βgal+ retina was also susceptible to autoimmune destruction, showing a dissociation between the mechanisms of DTH and the pathogenesis of retinal autoimmune disease. Inclusion of exogenous CD25+ T cells from βgal- donors in the lymphopenia experiments showed no effect on βgal induction of ear swelling, but they inhibited the EAU, again indicating the dissociation between EAU susceptibility, and the systemic readout of tolerance. Exogenous Tregs limited the LIP expansion of the βgalTCR T cells, similar to findings in other models (31), but no more so than having βgal in the retina.
A similar fraction of CD25+Foxp3+ T cells was recovered from βgalTCR × Rag(-/-) mice, with or without retinal βgal expression, suggesting that positive selection did not contribute significantly to the size of the Treg population. Our results are consistent with peripheral tolerance and induction of Tregs to βgal in naive arrβgal mice, supporting our previous observation that elicitation of DTH is inhibited in βgal-immunized arrβgal mice (17). The findings provide evidence that extends the mechanisms of retinal immune privilege to include the extrathymic development of CD4+25+ Tregs that are dependent on local production of an endogenous retinal Ag. We do not disregard the possibility that thymic expression of βgal may occur at some point in development, and contribute to the overall production of Tregs. But, we found that in adult mice, significant generation of the regulatory response to βgal could be accomplished by Ag from the retina.
There is substantial strain and species heterogeneity in the repertoire of TSAs expressed in thymus, including retinal TSAs (32). Curiously, thymic expression of a single retinal protein, IRBP, not only negatively selects the repertoire for IRBP reactivity (33), but also serves to protect the retina from spontaneous autoimmune attack against a variety of retina-specific self-Ags (16). It was also shown that thymic expression of IRBP may not be required to generate Tregs that protect retina (15). One interpretation is that tolerance to other retinal Ags is not required. Alternatively, it raises the possibility that other retinal Ags contribute to induction of peripheral tolerance. We have not found evidence for arrestin-promoted βgal expression in thymus from arrβgal mice, even though there is evidence for arrestin expression in thymus (32). We can only speculate about βgal expression, due to uncertainty in the mechanism by which aire gives expression of TSAs. The arrestin promoter:βgal construct used to make the arrβgal mice uses only a portion of the relatively uncharacterized promoter region of arrestin, and it serves to give a low level of expression of βgal, even in retinal photoreceptor cells. Further, the location effects on the activity of the transgenic arrestin promoter are unknown, and may strongly affect its activity. In any case, the possible role of thymus is not being disputed; we have asked if Ag of retinal origin can generate regulatory T cells, and found evidence for it. The mechanisms that maintain tolerance in the periphery, including the extrathymic expression of aire (34), and the extrathymic generation of new regulatory cells specific for peripheral self-Ags, are increasingly important topics.
The literature on the generation of Foxp3+ Tregs in Rag(-/-) mice is unclear, with evidence both for (35) and against (36) their production. The role of cognate Ag is an obvious variable. Our results suggest that very little Ag in a privileged site altered the response of Ag-specific donor CD25- T cells in lymphopenic mice. Clearly, oral or i.v. administration of large doses of Ags can give rise to Tregs (37), but these conditions are not physiologically relevant to the arrβgal Tg mice used in this study where nanograms of intracellular Ag are expressed in neurons behind the blood-retina barrier. Models for peripheral induction of Ag-specific Tregs that are potentially relevant to our observations are based on the presentation of very low doses of Ag by DC under sub-immunogenic conditions (29). Using antibody to target delivery of subnanogram doses of Ag to DC (28), or release of submicrogram doses of Ag via an osmotic pump (38), induced Tregs indistinguishable from Tregs developed in thymus.
How retinal Ag is gathered by APCs in normal retina, and where it is delivered so that it can induce tolerance are fundamental questions. There has been no demonstration that naive T cells enter retina and encounter retinal Ag on APCs that could lead to development of a regulatory response. Analysis using labeled T cells has found them undetectable except in inflamed retina, or if the T cells were activated (39). Regardless of the mechanism, the retinal origin of the Ag, rather than thymic, was the issue examined by our experiments. Although there is no direct evidence that Ag-laden APCs leave the retina, we propose that trace amounts of retinal Ag are gathered by cells with APC potential. Parenchymal microglia have a very low rate of turnover (40), and may not perform this function. Other candidates include retinal perivascular cells, which may turn over with kinetics similar to that in brain (approximately 50 % in 2-3 months (41, 42)), possibly providing slow, on-going delivery of retinal Ag to peripheral lymphoid tissues. Previously, we used the optic nerve crush (ONC) to induce apoptosis in retinal ganglion cells (24). ONC is a well-known model for glaucoma that leads to retinal microglial migration and activation in the retina (43). Although the βgal+ photoreceptor cells are not directly affected by the ONC, we found that two weeks after an ONC, the inhibition of DTH was lost in arrβgal × βgalTCR double Tg mice. The ONC experiment suggests that changing the interaction of innate immune cells with the neural retina can alter the balance of effector/regulatory activity to a local Ag.
Another candidate population for delivery of retinal Ag to lymphoid tissue is circulating monocytes that have transiently exited the circulation. The possibility for monocyte “surveillance” is based on observations that activated T cells, without specificity for Ag in the tissue, migrated into retina (44). Their entry into retina was accompanied by ED1+MHC class II+ monocytes. Local, minor breakdown of the blood/retinal barrier was found, together with local activation of retinal microglia. The migration and reverse transmigration of human monocyte-derived DC has been described (45). Randolph et al found that CD16+ monocytes underwent ablumenal migration. If the cells encountered activation factors, CD80/86 and class II were upregulated. In the presence of TGFβ, their differentiation into DC was completed, and reverse migration back into the circulation was found. Dendritic cells are now well-known to possess the ability to promote differentiation of Tregs (46, 47). The differentiation resulting from TGFβ exposure has been shown to induce some DC to be regulatory or tolerizing (48, 49), and TGFβ is known to promote the generation of CD4+25+Foxp3+ Tregs (50). Based on these observations, it is possible that generation of activated T cells to on-going environmental challenges provides opportunities for small numbers of activated T cells to enter retina, bringing monocytes with them. These cells have the opportunity to gather Ag, and in the TGFβ-rich retinal environment, may develop the regulatory phenotype, exit in the circulation and travel to spleen. Over time, even a low level of this migration may produce a regulatory response to Ags of retinal origin. Treatment of immature bone marrow DC with TGFβ and IRBP, and transfer to naive mice, made them resistant to EAU induction by IRBP (51). Previous studies from our lab suggest that the APCs that support pathogenesis of retinal autoimmunity can be recruited (52). Where the retinal Ag-bearing cells interact with T cells to produce the regulatory response is unknown at this time. Recent results show that very low, systemic cell-associated Ag levels can produce functional changes in the response to an Ag, even when the Ag is already present in the eye (53-55).
Acknowledgments
The authors thank Chunzhi Dou, Kathleen Lew, Thien Sam and Katie Pierson for technical assistance. We thank Dr. Alex Khoruts for a critique of the manuscript.
Abbreviations
- EAU
experimental autoimmune uveoretinitis
- SPL
splenocytes
- βgal
beta-galactosidase
- VSC 56
recombinant vaccinia virus expressing βgal
- Tregs
regulatory T cells
- GFAP
glial fibrillary acidic protein
- Tg
transgenic
- IRBP
interphotoreceptor retinoid binding protein
Footnotes
Supported by NIH grants R01 EY011542dsg, R01 EY016376dsg, T32 EY007133ul; Research to Prevent Blindness, Inc.; and the Minnesota Lions and Lionesses Clubs.
“This is an author-produced version of a manuscript accepted for publication in The Journal of Immunology (The JI). The American Association of Immunologists, Inc. (AAI), publisher of The JI, holds the copyright to this manuscript. This version of the manuscript has not yet been copyedited or subjected to editorial proofreading by The JI; hence, it may differ from the final version published in The JI (online and in print). AAI (The JI) is not liable for errors or omissions in this author-produced version of the manuscript or in any version derived from it by the U.S. National Institutes of Health or any other third party. The final, citable version of record can be found at www.jimmunol.org.”
References
- 1.Fehervari Z, Sakaguchi S. Development and function of CD25+CD4+ regulatory T cells. Curr Opin Immunol. 2004;16:203–208. doi: 10.1016/j.coi.2004.01.004. [DOI] [PubMed] [Google Scholar]
- 2.Sakaguchi S, Yamaguchi T, Nomura T, Ono M. Regulatory T cells and immune tolerance. Cell. 2008;133:775–787. doi: 10.1016/j.cell.2008.05.009. [DOI] [PubMed] [Google Scholar]
- 3.Sakaguchi S, Sakaguchi N, Shimizu J, Yamazaki S, Sakihama T, Itoh M, Kuniyasu Y, Nomura T, Toda M, Takahashi T. Immunologic tolerance maintained by CD25+ CD4+ regulatory T cells: their common role in controlling autoimmunity, tumor immunity, and transplantation tolerance. Immunol Rev. 2001;182:18–32. doi: 10.1034/j.1600-065x.2001.1820102.x. [DOI] [PubMed] [Google Scholar]
- 4.Anderson MS, Venanzi ES, Klein L, Chen Z, Berzins S, Turley SJ, Von Boehmer H, Bronson R, Dierich A, Benoist C, Mathis D. Projection of an immunological self-shadow within the thymus by the aire protein. Science. 2002;10:10. doi: 10.1126/science.1075958. [DOI] [PubMed] [Google Scholar]
- 5.Liston A, Lesage S, Wilson J, Peltonen L, Goodnow CC. Aire regulates negative selection of organ-specific T cells. Nat Immunol. 2003;4:350–354. doi: 10.1038/ni906. [DOI] [PubMed] [Google Scholar]
- 6.Aschenbrenner K, D'Cruz LM, Vollmann EH, Hinterberger M, Emmerich J, Swee LK, Rolink A, Klein L. Selection of Foxp3+ regulatory T cells specific for self antigen expressed and presented by Aire+ medullary thymic epithelial cells. Nat Immunol. 2007;8:351–358. doi: 10.1038/ni1444. [DOI] [PubMed] [Google Scholar]
- 7.Itoh M, Takahashi T, Sakaguchi N, Kuniyasu Y, Shimizu J, Otsuka F, Sakaguchi S. Thymus and autoimmunity: production of CD25+CD4+ naturally anergic and suppressive T cells as a key function of the thymus in maintaining immunologic self-tolerance. J Immunol. 1999;162:5317–5326. [PubMed] [Google Scholar]
- 8.Asano M, Toda M, Sakaguchi N, Sakaguchi S. Autoimmune disease as a consequence of developmental abnormality of a T cell subpopulation. J Exp Med. 1996;184:387–396. doi: 10.1084/jem.184.2.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Taguchi O, Kontani K, Ikeda H, Kezuka T, Takeuchi M, Takahashi T. Tissue-specific suppressor T cells involved in self-tolerance are activated extrathymically by self-antigens. Immunology. 1994;82:365–369. [PMC free article] [PubMed] [Google Scholar]
- 10.Thorstenson KM, Khoruts A. Generation of anergic and potentially immunoregulatory CD25+CD4 T cells in vivo after induction of peripheral tolerance with intravenous or oral antigen. J Immunol. 2001;167:188–195. doi: 10.4049/jimmunol.167.1.188. [DOI] [PubMed] [Google Scholar]
- 11.Zhang X, Izikson L, Liu L, Weiner HL. Activation of CD25(+)CD4(+) regulatory T cells by oral antigen administration. J Immunol. 2001;167:4245–4253. doi: 10.4049/jimmunol.167.8.4245. [DOI] [PubMed] [Google Scholar]
- 12.Caspi RR, Grubbs BG, Chan CC, Chader GJ, Wiggert B. Genetic control of susceptibility to experimental autoimmune uveoretinitis in the mouse model. Concomitant regulation by MHC and non-MHC genes. J Immunol. 1992;148:2384–2389. [PubMed] [Google Scholar]
- 13.McPherson SW, Heuss ND, Roehrich H, Gregerson DS. Bystander killing of neurons by cytotoxic T cells specific for a glial antigen. Glia. 2006;53:457–466. doi: 10.1002/glia.20298. [DOI] [PubMed] [Google Scholar]
- 14.Avichezer D, Grajewski RS, Chan CC, Mattapallil MJ, Silver PB, Raber JA, Liou GI, Wiggert B, Lewis GM, Donoso LA, Caspi RR. An immunologically privileged retinal antigen elicits tolerance: major role for central selection mechanisms. J Exp Med. 2003;198:1665–1676. doi: 10.1084/jem.20030413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Grajewski RS, Silver PB, Agarwal RK, Su SB, Chan CC, Liou GI, Caspi RR. Endogenous IRBP can be dispensable for generation of natural CD4+CD25+ regulatory T cells that protect from IRBP-induced retinal autoimmunity. J Exp Med. 2006;203:851–856. doi: 10.1084/jem.20050429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.DeVoss J, Hou Y, Johannes K, Lu W, Liou GI, Rinn J, Chang H, Caspi R, Fong L, Anderson MS. Spontaneous autoimmunity prevented by thymic expression of a single self-antigen. J Exp Med. 2006;203:2727–2735. doi: 10.1084/jem.20061864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gregerson DS, Dou C. Spontaneous induction of immunoregulation by an endogenous retinal antigen. Invest Ophthalmol Vis Sci. 2002;43:2984–2991. [PubMed] [Google Scholar]
- 18.McPherson SW, Heuss ND, Gregerson DS. Lymphopenia-induced proliferation is a potent activator for CD4+ T cell mediated autoimmune disease in the retina. J Immunol. 2009;182:969–979. doi: 10.4049/jimmunol.182.2.969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bennink JR, Yewdell JW. Recombinant vaccinia viruses as vectors for studying T lymphocyte specificity and function. Curr Top Microbiol Immunol. 1990;163:153–184. doi: 10.1007/978-3-642-75605-4_6. [DOI] [PubMed] [Google Scholar]
- 20.Hara Y, Caspi RR, Wiggert B, Chan CC, Wilbanks GA, Streilein JW. Suppression of experimental autoimmune uveitis in mice by induction of anterior chamber-associated immune deviation with interphotoreceptor retinoid-binding protein. J Immunol. 1992;148:1685–1692. [PubMed] [Google Scholar]
- 21.Lin W, Haribhai D, Relland LM, Truong N, Carlson MR, Williams CB, Chatila TA. Regulatory T cell development in the absence of functional Foxp3. Nat Immunol. 2007;8:359–368. doi: 10.1038/ni1445. [DOI] [PubMed] [Google Scholar]
- 22.Akbar AN, Taams LS, Salmon M, Vukmanovic-Stejic M. The peripheral generation of CD4+ CD25+ regulatory T cells. Immunology. 2003;109:319–325. doi: 10.1046/j.1365-2567.2003.01678.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen TC, Cobbold SP, Fairchild PJ, Waldmann H. Generation of anergic and regulatory T cells following prolonged exposure to a harmless antigen. J Immunol. 2004;172:5900–5907. doi: 10.4049/jimmunol.172.10.5900. [DOI] [PubMed] [Google Scholar]
- 24.Gregerson DS, Heuss ND, Lehmann U, McPherson SW. Evidence for extrathymic generation of regulatory T cells specific for a retinal antigen. Ophthalmic Res. 2008;40:154–159. doi: 10.1159/000119868. [DOI] [PubMed] [Google Scholar]
- 25.Jordan MS, Boesteanu A, Reed AJ, Petrone AL, Holenbeck AE, Lerman MA, Naji A, Caton AJ. Thymic selection of CD4+CD25+ regulatory T cells induced by an agonist self-peptide. Nat Immunol. 2001;2:301–306. doi: 10.1038/86302. [DOI] [PubMed] [Google Scholar]
- 26.Cabarrocas J, Cassan C, Magnusson F, Piaggio E, Mars L, Derbinski J, Kyewski B, Gross DA, Salomon BL, Khazaie K, Saoudi A, Liblau RS. Foxp3+ CD25+ regulatory T cells specific for a neo-self-antigen develop at the double-positive thymic stage. Proc Natl Acad Sci U S A. 2006;103:8453–8458. doi: 10.1073/pnas.0603086103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Apostolou I, Sarukhan A, Klein L, von Boehmer H. Origin of regulatory T cells with known specificity for antigen. Nat Immunol. 2002;3:756–763. doi: 10.1038/ni816. [DOI] [PubMed] [Google Scholar]
- 28.Kretschmer K, Apostolou I, Hawiger D, Khazaie K, Nussenzweig MC, von Boehmer H. Inducing and expanding regulatory T cell populations by foreign antigen. Nat Immunol. 2005;6:1219–1227. doi: 10.1038/ni1265. [DOI] [PubMed] [Google Scholar]
- 29.Jaeckel E, Kretschmer K, Apostolou I, von Boehmer H. Instruction of Treg commitment in peripheral T cells is suited to reverse autoimmunity. Semin Immunol. 2006;18:89–92. doi: 10.1016/j.smim.2006.01.011. [DOI] [PubMed] [Google Scholar]
- 30.McPherson SW, Yang J, Chan CC, Dou C, Gregerson DS. Resting CD8 T cells recognize beta-galactosidase expressed in the immune-privileged retina and mediate autoimmune disease when activated. Immunology. 2003;110:386–396. doi: 10.1046/j.1365-2567.2003.01750.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hagen KA, Moses CT, Drasler EF, Podetz-Pedersen KM, Jameson SC, Khoruts A. A role for CD28 in lymphopenia-induced proliferation of CD4 T cells. J Immunol. 2004;173:3909–3915. doi: 10.4049/jimmunol.173.6.3909. [DOI] [PubMed] [Google Scholar]
- 32.Egwuagu CE, Charukamnoetkanok P, Gery I. Thymic expression of autoantigens correlates with resistance to autoimmune disease. J Immunol. 1997;159:3109–3112. [PubMed] [Google Scholar]
- 33.Avichezer D, Grajewski RS, Chan CC, Mattapallil MJ, Silver PB, Raber JA, Liou GI, Wiggert B, Lewis GM, Donoso LA, Caspi RR. An immunologically privileged retinal antigen elicits tolerance: major role for central selection mechanisms. J Exp Med. 2003;198:1665–1676. doi: 10.1084/jem.20030413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gardner JM, Devoss JJ, Friedman RS, Wong DJ, Tan YX, Zhou X, Johannes KP, Su MA, Chang HY, Krummel MF, Anderson MS. Deletional tolerance mediated by extrathymic Aire-expressing cells. Science. 2008;321:843–847. doi: 10.1126/science.1159407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Curotto de Lafaille MA, Lino AC, Kutchukhidze N, Lafaille JJ. CD25- T cells generate CD25+Foxp3+ regulatory T cells by peripheral expansion. J Immunol. 2004;173:7259–7268. doi: 10.4049/jimmunol.173.12.7259. [DOI] [PubMed] [Google Scholar]
- 36.Wan YY, Flavell RA. Identifying Foxp3-expressing suppressor T cells with a bicistronic reporter. Proc Natl Acad Sci U S A. 2005;102:5126–5131. doi: 10.1073/pnas.0501701102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.O'Garra A, Vieira P. Regulatory T cells and mechanisms of immune system control. Nat Med. 2004;10:801–805. doi: 10.1038/nm0804-801. [DOI] [PubMed] [Google Scholar]
- 38.Apostolou I, von Boehmer H. In vivo instruction of suppressor commitment in naive T cells. J Exp Med. 2004;199:1401–1408. doi: 10.1084/jem.20040249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Prendergast RA, Iliff CE, Coskuncan NM, Caspi RR, Sartani G, Tarrant TK, Lutty GA, McLeod DS. T cell traffic and the inflammatory response in experimental autoimmune uveoretinitis. Invest Ophthalmol Vis Sci. 1998;39:754–762. [PubMed] [Google Scholar]
- 40.Matsumoto Y, Fujiwara M. Absence of donor-type major histocompatibility complex class I antigen-bearing microglia in the rat central nervous system of radiation bone marrow chimeras. J Neuroimmunol. 1987;17:71–82. doi: 10.1016/0165-5728(87)90032-4. [DOI] [PubMed] [Google Scholar]
- 41.Xu H, Chen M, Mayer EJ, Forrester JV, Dick AD. Turnover of resident retinal microglia in the normal adult mouse. Glia. 2007;55:1189–1198. doi: 10.1002/glia.20535. [DOI] [PubMed] [Google Scholar]
- 42.Hickey W, Vass K, Lassmann H. Bone marrow-derived elements in the central nervous system: an immunohistochemical and ultrastuctural survey of rat chimeras. J Neuropathol Exp Neurol. 1992;51:246–256. doi: 10.1097/00005072-199205000-00002. [DOI] [PubMed] [Google Scholar]
- 43.Panagis L, Thanos S, Fischer D, Dermon CR. Unilateral optic nerve crush induces bilateral retinal glial cell proliferation. Eur J Neurosci. 2005;21:2305–2309. doi: 10.1111/j.1460-9568.2005.04046.x. [DOI] [PubMed] [Google Scholar]
- 44.Hu P, Pollard JD, Chan-Ling T. Breakdown of the blood-retinal barrier induced by activated T cells of nonneural specificity. Am J Pathol. 2000;156:1139–1149. doi: 10.1016/S0002-9440(10)64982-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Randolph GJ, Sanchez-Schmitz G, Liebman RM, Schakel K. The CD16(+) (FcgammaRIII(+)) subset of human monocytes preferentially becomes migratory dendritic cells in a model tissue setting. J Exp Med. 2002;196:517–527. doi: 10.1084/jem.20011608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–252. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 47.Steinman RM, Nussenzweig MC. Avoiding horror autotoxicus: the importance of dendritic cells in peripheral T cell tolerance. Proc Natl Acad Sci U S A. 2002;99:351–358. doi: 10.1073/pnas.231606698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kobie JJ, Wu RS, Kurt RA, Lou S, Adelman MK, Whitesell LJ, Ramanathapuram LV, Arteaga CL, Akporiaye ET. Transforming growth factor beta inhibits the antigen-presenting functions and antitumor activity of dendritic cell vaccines. Cancer Res. 2003;63:1860–1864. [PubMed] [Google Scholar]
- 49.Yarilin D, Duan R, Huang YM, Xiao BG. Dendritic cells exposed in vitro to TGF-beta1 ameliorate experimental autoimmune myasthenia gravis. Clin Exp Immunol. 2002;127:214–219. doi: 10.1046/j.1365-2249.2002.01748.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chen W, Jin W, Hardegen N, Lei KJ, Li L, Marinos N, McGrady G, Wahl SM. Conversion of peripheral CD4+CD25- naive T cells to CD4+CD25+ regulatory T cells by TGF-beta induction of transcription factor Foxp3. J Exp Med. 2003;198:1875–1886. doi: 10.1084/jem.20030152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jiang HR, Muckersie E, Robertson M, Forrester JV. Antigen-specific inhibition of experimental autoimmune uveoretinitis by bone marrow-derived immature dendritic cells. Invest Ophthalmol Vis Sci. 2003;44:1598–1607. doi: 10.1167/iovs.02-0427. [DOI] [PubMed] [Google Scholar]
- 52.Gregerson DS, Kawashima H. APC derived from donor splenocytes support retinal autoimmune disease in allogeneic recipients. J Leukoc Biol. 2004;76:383–387. doi: 10.1189/jlb.0404249. [DOI] [PubMed] [Google Scholar]
- 53.McPherson SW, Roberts JP, Gregerson DS. Systemic expression of rat soluble retinal antigen induces resistance to experimental autoimmune uveoretinitis. J Immunol. 1999;163:4269–4276. [PubMed] [Google Scholar]
- 54.McPherson SW, Roberts JP, Gregerson DS. Peripheral expression of rod photoreceptor arrestin induces an epitope-specific, protective response against experimental autoimmune uveoretinitis. Curr Eye Res. 2005;30:491–502. doi: 10.1080/02713680590956270. [DOI] [PubMed] [Google Scholar]
- 55.Agarwal RK, Kang Y, Zambidis E, Scott DW, Chan CC, Caspi RR. Retroviral gene therapy with an immunoglobulin-antigen fusion construct protects from experimental autoimmune uveitis. J Clin Invest. 2000;106:245–252. doi: 10.1172/JCI9168. see comments. [DOI] [PMC free article] [PubMed] [Google Scholar]


