Abstract
Zebrafish is gaining popularity as a vertebrate model for screening small molecules that affect specific phenotypes or genetic pathways. In this study, we present a targeted drug screen to identify drug modifiers of the melanocyte migration defect of a temperature-sensitive allele of the Kit receptor tyrosine kinase, kitts. We first test two candidate drugs, the phosphatidylinositol-3-kinase kinase inhibitor (LY294002) and the Erk/MAP kinase inhibitor (PD98059), for their effect on melanocyte migration and survival. We find that LY294002 enhances the migration defect of kitts, implicating the phosphatidylinositol-3-kinase kinase pathway in promoting kit-dependent melanocyte migration, but not survival. We then used the kitts-sensitized genetic background to screen a panel of 1280 pharmacologically active drugs to identify drug enhancers and suppressors of the kitts melanocyte migration defect. We identified three drug enhancers of migration, two of which, Papaverine and Isoliquiritigenin, specifically enhance the kitts migration defect, while 8-DPAT affected both melanocyte migration and survival. These drugs now provide additional experimental tools for investigating the mechanisms of kit-promoted melanocyte migration and survival in the zebrafish embryo.
Introduction
The zebrafish has long been recognized as a powerful vertebrate model for large-scale forward genetic screens.1,2 Mutations that affect pigment pattern in the embryo and adult were among the largest classes of mutants first isolated, and much has been learned about cell–cell signaling, cell fate specification, pigment evolution, and cell migration from the study of these mutants.3–7 Characteristics that make zebrafish amenable to forward genetics, such as small size, external fertilization, optical clarity, and ease of producing many embryos, have also been recognized as useful for large-scale screening of small molecules.8–14 Drug discovery in the zebrafish can be aided by taking advantage of mutants to identify specific chemical–genetic interactions and pathways.15
The Kit receptor tyrosine kinase is required for several developmental functions in the zebrafish melanocyte lineage, including differentiation, migration, survival, and regeneration.16–18 The migration and survival functions are separable molecularly and temporally, suggesting that they may be independent functions of the Kit receptor.19 Evidence from other model systems is consistent with multiple signaling pathways working downstream of the Kit receptor (see reviews by Linnekin20 and Kent et al.21), including the phosphatidylinositol-3-kinase (PI3K) and the Ras-Raf-MAP kinase (MAPK) signaling pathways.22 Although well studied in murine models, it is not yet known which signaling pathway is involved in the zebrafish melanocyte functions, or indeed, whether zebrafish kit signaling uses different pathways for these processes. Here, we use a temperature-sensitive allele of kit, kitj1e99 (kitts), which, at an intermediate temperature, provides a sensitized genetic background to test whether the PI3 kinase inhibitor (LY294002) or the MAP kinase inhibitor (PD98059) enhances kit defects in migration and survival. Our results suggest that kit signals through the PI3K pathway to promote migration, but not survival. We find no evidence that kit promotes either migration or survival through the MAPK pathway.
We then conducted a small molecule screen of 1280 pharmacologically active compounds to identify drugs that either enhanced or suppressed the sensitized migration defect provided by the kitts mutant. This identified three additional drug enhancers of kitts migration defect: Papaverine, Isoliquiritigenin, and 8-DPAT. Each drug was further tested for its interaction with kitts in concentration–response curves, and for their effect on the survival function of kit signaling in both kitts and wild type (WT). The drug enhancers identified in this screen will be useful tools in modulating kit-dependent cell signaling in zebrafish embryos. The annotated targets of these drugs also suggest two additional pathways that need to be explored in kit signaling, including one that may increase cyclic nucleotides and a second that involves serotonin levels.
Materials and Methods
Role of PI3 kinase pathway, MAP kinase pathway, and Kit kinase function in migration and survival
We tested two candidate downstream pathways of kit, the PI3K pathway using the inhibitor LY294002 and the MAP kinase pathway using an inhibitor to the Map Kinase, Mek, PD98059, for their effect on WT and kitts melanocyte migration and survival.
Pharmacological screen for drug modifiers of kit-promoted migration
We screened the Library of Pharmacologically Active Compounds (LOPAC; Sigma, St. Louis, MO) for drugs that modified a sensitized allele of kit. This panel contains 1280 compounds from all major drug target classes. Drugs were diluted to 10 μM in 96-well plates to a final volume of 200 μL. We used the kitj1e99 (kitts) allele of kit described previously as temperature sensitive for its defects in migration and survival.19 Three kitts embryos were placed in each well using a Pasteur pipette while adding as little extra embryo medium as possible (∼20 μL).
To find drug enhancers of the kitts migration defect, kitts animals were placed in drug at 10–12 hpf and reared at 28°C (just above the permissive temperatures for kitts). Animals were assayed for extent of melanocyte migration using an inverted microscope at 2 and 3 dpf. Conversely, we screened for drug suppressors of kitts, by rearing animals at 30°C (just below the restrictive temperature for kitts) and assayed for WT-like pigment pattern at 2 and 3 dpf. Embryonic lethality, in which all three embryos in the drug died before 2 dpf, was ∼4% for the migration screen. We initially selected 43 possible enhancers and 34 possible suppressors of migration. Drugs that reliably replicated their effects are shown in Table 1. To identify drugs that would affect melanocyte survival, we followed the procedure above, except that embryos were added to the drug panel at 2 dpf and screened at 6 dpf. Embryos were again reared at 28°C to identify enhancers and 30°C for suppressors. The intermediate survival phenotype of kitts was too variable for the screen to be effective, and no melanocyte survival modifying drugs were identified from the screen.
Table 1.
Drug Enhancers of Kit Melanocyte Defects
| Name | Source | Annotated target | Migration | Survival |
|---|---|---|---|---|
| Candidate drug enhancers of kit defects | ||||
| LY294002 | Sigma L9908 | PI3K inhibitor | Enhance | No effect |
| PD98059 | Sigma P215 | Mek inhibitor | No effect | No effect |
| Gleevec | LCLaboratories I5508 | RTK type III (Kit) kinase inhibitor | No effect | No effect |
| Drug enhancers of kit defects identified in screen | ||||
| Papaverine | Sigma P3510 | Phosphodiesterase inhibitor | Enhance | No effect |
| Isoliquiritigenin | Sigma I3766 | Guanylyl cyclase activator | Enhance | No effect |
| 8-DPAT (R-(+)-8-hydroxy-DPAT) | Sigma H140 | Serotonin agonist | Enhance | Enhance |
PI3K, phosphatidylinositol-3-kinase.
Lethality concentration response
To further investigate the three enhancers of the kitts migration defect identified in the screen, we first identified the maximal sublethal concentration for each drug. We placed 10 embryos/well at 10 hpf in various concentrations of drug ranging from 1 to 1000 μM. We selected the drug concentration for which greater than 50% embryos survived for 6 days postfertilization for further experiments.
Melanocyte migration
Drugs that were identified in the screen were retested to quantify the enhancement effect on kitts, and whether they could affect WT embryos at higher concentrations. WT and kitts embryos were retested at 10 μM drug as in the original screen, as well as the maximal sublethal concentration. All embryos were placed in drug at 10–12 hpf, reared at either 28°C for enhancers or 30°C for suppressors, and fixed at 72 hpf using 4% paraformaldehyde in phosphate-buffered saline solution. Ten embryos were used for each treatment. Quantitation of migrated and nonmigrated melanocytes was performed by counting a subpopulation of melanocytes in the embryo, referred to as the migratory subpopulation. The migratory subpopulation includes melanocytes on the head, yolk, near the ear, and in the dorsal, lateral, and abdominal stripes above the hind yolk as previously described,23 and highlighted in Figure 1B. Melanocytes in the head, yolk, and abdominal stripes (highlighted by hash-marked regions) were considered as migrated. Cells near the ear, on the dorsum, and in the lateral stripe (highlighted by clear regions) were categorized as nonmigrated. To test whether each drug had a significant effect on the number of migrated cells, a Student's t-test was conducted on the ratio of migrated to nonmigrated melanocytes compared to that of control.
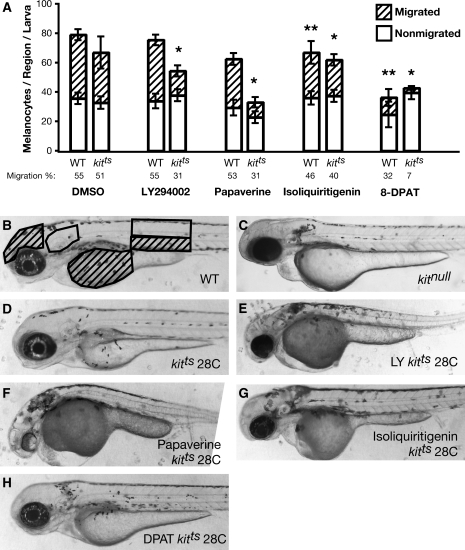
FIG. 1.
Drug enhancers of kitts migration defect. (A) Quantification of melanocyte migration after each drug treatment at 3 dpf in wild-type (WT) and kitts genetic backgrounds. Regions that were counted are highlighted in (B). Melanocytes near the ear and in the dorsal and lateral stripe above the hind yolk were categorized as nonmigrated and are highlighted by clear boxes. Melanocytes in the head, on the yolk, and the ventral stripe were considered migrated and are highlighted by hash-marked boxes. Bars represent the mean, with error bars indicating standard deviation. Statistically significant differences in the ratio of migrated melanocytes between drug treatment and the DMSO control are indicated for kitts (*) and WT (**). (B) Untreated WT embryos at 3 dpf have melanocytes that have almost completed migration, with melanocytes present on the yolk and head, and few near the ear. With our metric, WT embryos have a total of 80 melanocytes with 55% migration. Embryos with null mutations in kit (C) show a migration defect with few melanocytes on yolk or head and a cluster of melanocytes near the ear, and have 25% migration (data not shown). Embryos with a temperature-sensitive mutation in kit, kitts, at 28°C (D) have slightly fewer total melanocytes, but have insignificantly fewer migrated cells (51%) than WT (p = 0.410). Embryos treated with LY294002 (10 μM) in WT have a developmental delay but are nearly identical to untreated embryos with regard to melanocyte migration (p = 0.811), but in kitts show a significant defect (E) in the number of migrated melanocytes when compared to untreated kitts (p = 4.4E–6). Papaverine (20 μM) also does not significantly affect WT embryos (p = 0.570) but shows a large deficit of migration (31%) in kitts (F, p = 3.8E–5) and also shows a developmental delay and pericardial edema. Isoliquiritigenin (20 μM) has a significant effect on both WT (46%, p = 0.002) and kitts (40%, p = 0.008) migration and in kitts (G) appears much like a kit null mutant (C). Treatment of WT embryos with 10 μM 8-DPAT results in a large deficit in the number of total melanocytes (36.7 compared with 79.0 in untreated, p = 8.9E–8) as well as migrated melanocytes (32%, p = 1.710–7). In kitts (H), 8-DPAT appears like kitnull animals, and has only 7% migration (p = 7.1E–14), which is less than that observed in kitnull. The effect of 8-DPAT on total melanocytes in kitts is similar to that observed in WT-treated animals (p = 0.247). For all treatments, sample size was >8 animals.
Drug enhancer concentration–response curves
Concentration–response experiments were conducted on drug enhancers that reliably replicated kitts migration effect using various sublethal concentrations of drug on both WT and kitts embryos. Embryos were added to drug at 10 hpf, reared at 28°C, and fixed at 72 hpf. For these experiments (Fig. 2), we chose to count the regions where migration defects are most apparent in kitnull embryos: the yolk and near the ear. Migration factor is defined as number of melanocytes on the yolk divided by the total of melanocytes on the yolk plus near the ear. We normalized each of the WT drug treatments to the untreated WT control, and the kitts drug treatments to the kitts untreated control. Thus, both kitts and WT have a normalized migration factor of 1 when no drug is added. Drugs enhance the kitts migration defect when the slope of the kitts concentration curve is steeper than that of WT. We then tested whether each point in the concentration–response curves was significantly less in kitts than in WT using Student's t-test.
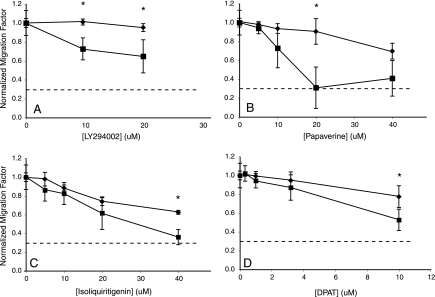
FIG. 2.
Concentration response of drug enhancers on kitts and wild-type (WT) melanocyte migration. (A–D) Drug enhancers of kitts migration were retested at various concentrations in both kitts and WT embryos. Migration factor is the ratio of yolk melanocytes to the number of melanocytes near the ear plus the number on the yolk. We normalized each migration factor in drug treatments of each kitts and WT to its respective untreated control. This method emphasizes the migration phenotype as a function of drug concentration. The dotted line in each graph represents the kitnull migration value (0.3) using this metric. *p-Values from t-tests between kitts and WT less than 0.05 are indicated. (A) LY294002 shows a sharp decline in kitts but a fairly flat response in WT embryos with a significant difference at 10 μM (p = 0.001) and at 20 μM (p = 0.002). (B) Papaverine has a large effect in kitts from 5 to 20 μM. At 20 μM, the migration effect in kitts is significantly different from that of WT (p < 0.001) and is identical to the kitnull defect. In WT embryos, the slope of the concentration–response curve is quite shallow with the 40 μM effect identical to the effect of 10 μM in kitts animals, indicating a shift in the concentration response of 30 μM from WT to kitts. (C) Isoliquiritigenin concentration response reveals that the effect of Isoliquiritigenin on kitts defect is greater than that of WT. From 0 to 20 μM the two curves appear to have the same slope, but from 20 to 40 μM the slope of kitts is greater, with a significant difference between kitts and WT at 40 μM (p = 0.01). (D) Concentration response of 8-DPAT also reveals a similarity between the responses of WT and kitts between 0 and 3.2 μM, but between 3.2 and 10 μM displays a greater effect on kitts with a significant difference in migration factor at 10 μM (p = 0.001).
Melanocyte survival
Drug enhancers of the kitts migration defect were tested for their effect on melanocyte survival. WT and kitts were added to drug at 2 dpf, reared at 28°C, and fixed at 6 dpf. Quantitation of melanocyte survival was accomplished by counting the total number of dorsal stripe melanocytes at 6 dpf. Student's t-tests were performed to test for statistical significance on difference of total dorsal melanocytes with that of untreated controls.
Results
Role of PI3K or MAPK in kit-promoted processes
We first tested two candidate drugs, LY294002 and PD98059, for their effect on enhancing the migration and survival defects of kitj1e99 (kitts), a temperature-sensitive mutation at the kit receptor tyrosine kinase. At the restrictive temperature (33°C) melanocytes fail to migrate to the periphery, whereas at the permissive temperature (25°C) they migrate normally. We reasoned that at an intermediate temperature (28°C), where kitts have approximately 80% of the WT complement of migrated melanocytes, this mutation provides a sensitized background, whereby slight perturbation of the kit pathway will exhibit large changes in the migration phenotype of kitts. Thus, the enhancement of the kitts defect might reveal drugs that interact with the kit pathway that would not be revealed in a WT genetic background. We previously used kitts as a sensitized allele to confirm that a putative kit ligand, kitla, is the functional ligand to kit.23 As in that study, we used a subthreshold concentration that did not show an effect in WT animals to ask whether either LY294002 or PD98059 could interact with the kitts phenotype.
LY294002 enhances the migration defect of kitts (Fig. 1A–E). When kitts animals are treated with 20 μM LY294002 (Fig. 1E), only approximately 16.6 melanocytes, in the migratory subpopulation counted, migrate to the periphery, or 31%, significantly less than in untreated controls (54%, p = 4.4E–6). Slightly more melanocytes remain localized to the dorsum (37.8 compared to 32.8 of control), and there are fewer total melanocytes in the LY294002-treated animals (54.4 compared to 66.9 melanocytes, p = 0.031). The defect in migration is most obvious when considering the cluster of melanocytes near the ear and the absence on the yolk in drug-treated animals (Fig. 1E). In contrast, when WT fish are treated with 20 μM LY, there is no difference in melanocyte migration compared to untreated controls (55% compared to 54%, p = 0.811). LY294002-treated fish also appear developmentally delayed and have pericardial edema at concentrations above 10 μM.
To further investigate the chemical–genetic interaction of LY294002 with kitts, we assayed for migration as a function of drug concentration to create concentration–response curves for both WT and kitts animals (Fig. 2A). In these graphs, we represent migration based on the number of melanocytes on the yolk compared to the number near the ear. Each series of drug treatments is normalized with the untreated control group. Thus, if the LY294002 effects on migration are independent of its interaction with kit, the slopes of the concentration response will be equal. If, however, LY294002 affects migration through the kit pathway, the slope of the concentration response will be steeper in the kitts mutants. The effect on migration in WT embryos treated with LY294002 is negligible at concentrations up to 20 μM. However, Figure 2A shows that the curve for kitts is markedly steeper between 5 and 10 μM. At 10 μM LY294002, kitts animals have a migration defect that is approximately halfway between untreated kitts and the kitnull allele (represented by the dashed line at a migration factor of 0.3) and is significantly different from that of WT at 10 μM LY294002 (p = 0.001). This effect levels off at 20 μM, but is still significantly different than WT (p = 0.002).
We tested whether LY294002 could also enhance the kitts melanocyte survival defect. We found that treatment with LY294002 after 2 dpf did not affect melanocyte survival in either WT or kitts (Table 2).WT larvae have approximately 104.3 dorsal melanocytes at 6 dpf. When treated with the maximal sublethal concentration of 20 μM LY294002, WT animals have nearly identical number of melanocytes (97.5). Untreated kitts animals have only about 70.9 dorsal melanocytes at 6 dpf when reared at 28°C, due to partial melanocyte cell death beginning at 4 dpf in kitts. LY294002-treated kitts have approximately 67.4 dorsal melanocytes, which is insignificantly different from the untreated kitts (p = 0.150).
Table 2.
Effect of Drug Enhancers on Kitts Melanocyte Survival Defect
| Wild type | p-Value | kitts | p-Value | |
|---|---|---|---|---|
| No drug | 104.3 ± 5.6 | — | 70.9 ± 5.7 | — |
| LY294002 (20 μM) | 97.5 ± 13.6 | 0.811 | 67.4 ± 5.5 | 0.151 |
| Papaverine (10 μM) | ND | 66.9 ± 6.4 | 0.124 | |
| Isoliquiritigenin (20 μM) | ND | 67.5 ± 5.4 | 0.131 | |
| 8-DPAT (10 μM) | 44.3 ± 13.5 | 2.0E–10 | 26.3 ± 3.5 | 1.2E–16 |
Embryos were treated with drug from 2 dpf, when kit requirement for survival begins, until 6 dpf. Average number of dorsal melanocytes at 6 dpf with standard deviation is presented. p-Value of Student's t-test comparing untreated wild type with drug treatment is listed for each drug. Number of sample size was >8 for each treatment.
ND, not determined.
We next tested whether PD98059 affected melanocyte migration or survival in kitts animals. PD98059 showed no effect on WT melanocyte migration or survival nor showed enhancement of the kitts migration or survival defect (data not shown).
Pharmacological screen for enhancers of kit-promoted melanocyte migration
We next sought an unbiased approach to finding drug modifiers of the kitts-sensitized allele to identify potential signaling pathways involved in kit-dependent migration or survival. We reasoned that this sensitized genetic background could reveal drugs that enhance or suppress the kitts phenotype that may not have a specific or obvious effect in a WT genetic background when kit functioning is robust, and thus would be missed in a screen using WT fish. We screened a panel of 1280 drugs (LOPAC; Sigma) that are biologically active in mammals for both enhancers and suppressors of the kitts migration defect.
Through this screen, we first identified 43 possible drug enhancers and 34 possible drug suppressors of melanocyte migration. These drugs were retested, and three enhancers, Papaverine, Isoliquiritigenin, and 8-DPAT, consistently replicated the initial migration phenotype. None of the 34 possible drug suppressors replicated their effect on the kitts migration defect consistently.
Papaverine and Isoliquiritigenin enhance the kitts migration defect, but not the survival defect
We identified two drugs, Papaverine and Isoliquiritigenin, that specifically enhance the migration defect of kitts (Fig. 1F, G). When kitts embryos are treated with 20 μM Papaverine, only approximately 10.2 melanocytes, or 31% of the melanocytes in the subpopulation counted, migrate to the periphery. This is significantly lower than the 34.1 migrated melanocytes seen in untreated kitts controls (p = 3.8E–5). In contrast, when 20 μM Papaverine is given to WT embryos, an insignificant decrease in migrated melanocytes is observed (33.2 melanocytes or 53% of the migratory subpopulation in Papaverine WT, compared to 43 melanocytes or 55% in untreated embryos, p = 0.571).
To further investigate whether Papaverine can affect WT embryos and to examine whether Papaverine acts synergistically with kitts, we conducted a concentration–response assay of melanocyte migration (Fig. 2B). WT embryos showed a migration defect of 0.70 with 40 μM Papaverine. This effect is comparable to that observed with only 10 μM of Papaverine in kitts (0.73). In fact, 20 μM Papaverine in kitts animals phenocopies the kitnull mutants, which have a migration factor of 0.30 (dashed line). Thus, slightly reducing the activity of kit signaling from WT to kitts at 28°C results in a large shift in the concentration–response curve of Papaverine, indicating a strong interaction between Papaverine and kitts.
Isoliquiritigenin also affects kitts migration. When kitts embryos are treated with 20 μM Isoliquiritigenin (Fig. 1G), melanocytes are clustered near the ear and very few appear on the head or yolk. Quantification reveals that only 24.6%, or 40%, of melanocytes migrate to the periphery, which is significantly fewer than migrate in untreated kitts (p = 0.008). WT embryos treated with 20 μM Isoliquiritigenin also show a significant defect in the amount of melanocyte migration (30.9% or 46% migrated melanocytes compared with 55% in untreated controls, p = 0.002). To test whether Isoliquiritigenin enhances the kitts defect at other concentrations, we examined the concentration–response curve for Isoliquiritigenin between 5 and 40 μM. The response curve reveals a gradual increase in the migration defect response in kitts (Fig. 2C) from 5 to 40 μM. The slope of the curve is slightly steeper than that observed in WT embryos. At 40 μM, Isoliquiritigenin-treated kitts animals are significantly different from WT (p = 0.01). The effect of 40 μM Isoliquiritigenin is 0.63, similar to that of 20 μM Isoliquiritigenin in kitts, indicating a shift in the concentration–response curve that is less dramatic than observed with Papaverine.
We wanted to test whether drug modifiers of the migration defect of kitts could also affect melanocyte survival. Melanocytes in kitnull mutants begin to die from the embryo at 4 dpf. We have shown previously that the requirement of kit activity for embryonic melanocyte survival begins after 2 dpf. We therefore can test whether the drugs modify kit-dependent melanocyte survival by treating kitts animals with the maximal sublethal concentration of drug after 2 dpf and assaying for melanocyte death at 6 dpf. Embryos treated with Papaverine or Isoliquiritigenin at concentrations above 10 μM resulted in embryonic death by 4 dpf. However, kitts embryos treated with 10 μM Papaverine did survive to 6 dpf, and they show no significant difference in the amount of melanocyte survival (66.9 melanocytes for Papaverine vs. 70.9 melanocytes for control, p = 0.124; see Table 2).Similarly, 20 μM Isoliquiritigenin also shows no enhancement of the kitts survival defect (67.5 melanocytes, p = 0.131). Because there was no effect in the kitts-sensitized background, we did not test for an effect on WT survival.
8-DPAT enhances both migration and survival defects of kitts
In addition to the migration-specific drug enhancers, we identified 8-DPAT as a drug that enhanced the migration defect and also enhanced the survival defect of kitts in the secondary screen. When kitts embryos are treated with 10 μM 8-DPAT, they have only 3.1 migrated melanocytes (Fig. 1H), or about 7% of melanocytes in the subpopulation counted. Untreated kitts have significantly more (34.1 melanocytes, p = 7.19E–14). Additionally, when WT embryos are treated with 10 μM 8-DPAT, they also show a substantial defect in melanocyte migration and have fewer total melanocytes at 3 dpf. Untreated WT embryos have 43.3 migrated melanocytes, or about 55% of the migratory subpopulation (Fig. 1A, B). 8-DPAT–treated animals have an average of 11.7 migrated melanocytes, only 32% of the migratory subpopulation. This is significantly different from the expected number of migrated melanocytes (19.6 expected migrated melanocytes, p = 1.74E–7). In addition to the migration defect, these animals also have very small melanocytes much like null mutants for kit (Fig. 1C).
8-DPAT also enhances the survival defect of kitts. Untreated kitts embryos have 70.9 dorsal melanocytes at 6 dpf when reared at 28°C. When kitts are treated with 10 μM 8-DPAT after 2 dpf, animals have only approximately 13.5 dorsal melanocytes (p = 1.2E–16). In addition, 8-DPAT–treated WT have 43.3 dorsal melanocytes at 6 dpf, significantly less than the untreated WT, which have 112.1 (p = 2.0E–10). This reduction of melanocytes from 2 dpf is most likely due to melanocyte-specific cell death rather than improper ontogenetic development, as embryos develop a nearly full complement of melanocytes before the drug is added by 2 dpf. Further, the melanocytes appear to fragment and congregate in small groups, much like that observed in MoTP-induced melanocyte death.18
Discussion
The role of Kit signaling in melanocyte migration, proliferation, and survival has been extensively studied in mice in many developmental and oncogenic contexts. Several signaling pathways are known to act downstream of Kit or be required for particular Kit-dependent processes. Among the more widely studied pathways downstream of Kit include the PI3K pathway and the MAPK pathway. However, both pathways have been implicated for promoting migration and survival functions. For example, PI3K activity is required for Kit-activated survival of gastrointestinal stromal tumor cell lines24 and in survival of mast cells.25 The MAPK pathway has also been shown to promote survival of many cell types during zebrafish embryogenesis.26 With regard to migration, both MAPK and PI3K pathways have been shown to be required for cell migration in murine peripheral blood cells.27
To better understand the roles of the PI3K and MAPK pathways in zebrafish melanocyte migration and survival, we tested two candidate drugs, LY294002 and PD98059, for their effect in enhancing the migration and survival defects of kitts. Both these drugs have been used effectively in zebrafish to knock down the function of their annotated targets. The PI3K inhibitor LY294002 inhibits activation of PKB/Akt by PI3K,28,29 and PD98059 has been shown to inhibit Erk activation in the MAPK pathway28,30 in zebrafish cells.31
Our data are consistent with previous studies that kit interacts with the PI3K signaling pathway, specifically that PI3K signaling promotes kit-dependent melanocyte migration in zebrafish embryos. LY294002 strongly enhances the migration defect of kitts. Our failure to find an effect of LY294002 on melanocyte survival through our sensitized assay suggests that PI3K signaling may not have a necessary role in kit-promoted survival.
In contrast, the Mek/MAPK inhibitor, PD98059, did not have an effect on enhancement of kitts migration or survival. This could be due to the MAPK pathway not being involved in melanocyte survival. However, because we did not test directly whether Erk activation was inhibited with PD98059, we cannot rule out the possibility that our drug did not inhibit Mek. The lack of effect in enhancing the kitts defect might also be explained by the fact that the presumed target of PD98059, Mek, is too far downstream of kit to see an enhancement of the defect. Moreover, it remains possible that the MAPK pathway is promoting other kit-dependent processes that we did not assay for, such as proliferation, differentiation, or regeneration of the melanocyte.
In addition to using drugs that are known to affect likely downstream targets of Kit signaling, we used the kitts-sensitized background to screen for additional drugs that would affect unsuspected pathways involved in Kit signaling. This study took advantage of a sensitized genetic background to find drugs that interacted with the Kit receptor tyrosine kinase. As above, we reasoned that using a sensitized allele might amplify the effects of perturbations in interacting pathways. Because many pathways are required for development and survival of the embryo, it is likely that inhibiting them completely using high concentrations of drug would not result in a specific melanocyte phenotype in WT embryos. However, it may be possible to find drugs that at subthreshold concentrations may reveal a specific melanocyte phenotype in the kitts genetic background. Thus, using a sensitized allele, we were able to identify two drugs that specifically enhanced the kitts migration defect and one drug that enhanced both the migration and survival defects of kitts. Stern et al.15 used a similar strategy to find drug suppressors of a bymb allele that has increased mitotic cells in the embryo. Because of this phenotype, they were able to find several suppressors that inhibit the cell cycle. We designed our screen to identify both enhancers and suppressors of the kit temperature-sensitive migration defect; however, we failed to find suppressors of migration that consistently replicated in kitts. Several drugs selected as suppressors enlarged melanocytes in kitts (data not shown), but did not alter migration quantitatively. The study presented here is the first demonstration of identifying drug enhancers for a specific phenotypic defect in zebrafish. Further, our result that two of our drugs, Papaverine and Isoliquiritigenin, would not have shown a phenotype in WT animals at the concentration used in the screen (10 μM) indicates that these drugs would have been missed in a screen using WT animals. This demonstrates the attraction for finding temperature sensitive, or hypomorphic, alleles in other pathways in zebrafish.
Although many novel drugs have been identified using larger and more diverse synthetic libraries with the zebrafish, we chose a smaller panel of known, pharmacologically active drugs for our screen. One of the more challenging problems in drug development is identifying the target of a novel compound.32 The compounds in the LOPAC panel have been well characterized and have proposed mechanisms of action in mammals. Although these mechanisms may not always be conserved between mammals and fish, we can consider the annotated target in generating models of possible modes of action in zebrafish. We discuss each of our identified drug enhancers, their known target in mammals, and their potential role in zebrafish melanocyte development below.
Papaverine is a strong enhancer of the kitts migration phenotype. The concentration–response curve of Papaverine's effect on kitts and WT reveals a large shift in the effective concentration on migration. In fact, it is possible to achieve a migration defect identical to the kitnull allele in kitts with 20 μM Papaverine, a concentration that in WT animals causes no defect in migration. This chemical–genetic interaction suggests that Papaverine inhibits processes that interact with the kit-signaling pathway involved in promoting migration or possibly interacts with the part of the kit receptor that is responsible for cell migration. Embryos treated with higher concentrations of Papaverine are also developmentally delayed, indicating that it is blocking an essential pathway in development. Although we find that Papaverine-treated animals have slightly fewer ontogenetic melanocytes, it does not appear to affect melanocyte survival in either WT or kitts animals. Papaverine-treated animals also display a change in melanocyte morphology, resulting in slightly larger melanocytes. In mammals, Papaverine acts as a nonselective phosphodiesterase inhibitor, increasing the amount of the secondary messengers cyclic AMP (cAMP) and cyclic GMP (cGMP) available for signaling. Consistent with this notion, the retinal pigmented epithelium, which is a melanocyte population independent of the neural crest, has been shown to have increased cAMP levels after treatment with Papaverine.33 This mechanism predicts that increased cAMP or cGMP levels should inhibit melanocyte migration. An inhibitory role for cell migration has previously been proposed for both cAMP34,35 and cGMP.36,37
Isoliquiritigenin also appears to have a migration-specific enhancement of kitts. Its migration defect is not as strong as Papaverine in kitts, and its effect appears synergistic, as the slope of the concentration response is steeper in kitts than in WT. However, this slope is not as severe as Papaverine in kitts. The concentration response suggests that Isoliquiritigenin interacts with kitts, but that this interaction is weak. Isoliquiritigenin activates guanylyl cyclase in mammals, but has been shown to increase both cGMP and cAMP levels.38,39 Thus, its mode of action may be similar to that of Papaverine, in that it increases the amount of cGMP and cAMP and that they both inhibit the kit pathway in promoting migration specifically. This finding suggests that increases in these secondary messengers inhibit kit-dependent melanocyte migration. The reports that Papaverine more effectively raises cAMP levels39 and our finding that it is more potent in enhancing kitts migration defects than Isoliquiritigenin raise the possibility that cAMP levels may be more important to inhibiting melanocyte migration than cGMP.
To further test whether increased levels of cAMP inhibit migration, we asked if Forskolin, which increases cAMP levels through adenylyl cyclase activation,40,41 also can inhibit melanocyte migration. Forskolin is in the LOPAC panel, and we noted during the primary screen that it caused large melanocytes in kitts embryos consistent with previous reports.42 However, we scored Forskolin as having no effect on migration in kitts. Indeed, when Forskolin was retested at 10 and 100 μM in kitts animals, we noted normal amounts of migration on the yolk and few melanocytes near the ear (data not shown), tending to rule out that increased cAMP levels are sufficient to inhibit melanocyte migration. We have not tested agents that specifically increase cGMP. Thus, we suggest that the drugs Papaverine and Isoliquiritigenin may act to inhibit migration by increasing cGMP levels, or instead by an unidentified pathway.
8-DPAT appears to have several roles in zebrafish melanocyte development, including migration and survival. Our data show that 8-DPAT affects migration in kitts and WT embryos, and that, like Isoliquiritigenin, the effect on kitts is slightly more than an additive effect, because the slope of the concentration–response curve is steeper in kitts animals than in WT animals. This suggests that there is a specific interaction with kit signaling, but that this interaction is weak. Additionally, when animals are treated with 8-DPAT after melanocytes have finished developing at 2 dpf, they have fewer melanocytes at 6 dpf, indicating that 8-DPAT also blocks melanocyte survival.
To address where in the kit genetic pathway 8-DPAT was acting, we asked whether activating kit could suppress the effect of 8-DPAT. Activating kit through the overexpression of the kit ligand, kitla, in cmv:kitla transgenic animals results in 50% more melanocytes than WT at 6 dpf.23 To test whether the cmv:kitla phenotype is epistatic to the 8-DPAT phenotype, we administered 8-DPAT to cmv:kitla animals. 8-DPAT–treated cmv:kitla animals are categorically identical to WT fish treated with 8-DPAT, indicating that 8-DPAT is epistatic to cmv:kitla. This result raises the possibility that the inhibition of kit signaling of 8-DPAT is downstream of kitla activity. None of the other drugs in this manuscript had an effect on melanocyte number or migration in cmv:kitla animals.
8-DPAT acts in mammals specifically as an agonist to 5-HT1A serotonin receptors to decrease the levels of cAMP in the cell. Thus, if 8-DPAT mode of action is conserved in zebrafish, it might have had the opposite effect of Papaverine and Isoliquiritigenin. However, we observe that all three are acting to inhibit melanocyte migration in zebrafish. Our findings are consistent, however, with in vitro evidence that serotonin may promote migration of neural crest cells.43 However, no other serotonergic antagonists or agonists were identified in the primary screen or in a follow-up screen with 15 other serotonergic drugs that were retested from the LOPAC panel (not shown). It is possible that 8-DPAT is acting either as an antagonist to serotonin receptors or as an agonist to other G-protein–coupled receptors that may increase cAMP in the cell, or function through cAMP-independent means. Previous work has shown that the G-protein–coupled receptor, CXCR4, and its ligand, Stromal cell–derived factor 1 (sdf1), are required in zebrafish for neuromast migration in the posterior lateral line,44 as well as melanocyte migration to the lateral stripe.6 Our results may suggest a larger role for G-protein–coupled receptors in melanocyte migration and suggest a new role in melanocyte survival.
This study reveals that care must be taken while drawing inferences about the targets of small molecules even when drugs are identified from panels of well-studied pharmaceuticals. In mammals, each of the drugs we identified from our LOPAC screen has targets that together suggest roles for cyclic nucleotides in regulating melanocyte migration. Yet, our efforts to substantiate the mechanism of drug action, by more directly modifying cAMP levels with Forskolin, or blocking serotonin receptors with other serotonergic antagonists, failed to support the predicted mode of action. In zebrafish, which also allows for the use of genetic screens, additional clues to drug targets may come from reversing the logic of the screen performed in this study, and instead screening for mutations that specifically enhance the efficacy of the drug. Such screens seem particularly attractive when the phenotype involves the melanocyte, a cell that is not essential for viability of zebrafish. Indeed, proof of this principle is provided by screens that found hypomorphic and viable alleles of the cuproenzyme ATP7a among enhancers of the copper chelator neocuproine.45
Acknowledgments
We thank Jonathan Gitlin for generously supplying the LOPAC drug panel. This work was funded in part by NIH RO1-GM56988.
Disclosure Statement
No competing financial interests exist.
References
- 1.Solnica-Krezel L. Schier AF. Driever W. Efficient recovery of ENU-induced mutations from the zebrafish germline. Genetics. 1994;136:1401–1420. doi: 10.1093/genetics/136.4.1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mullins MC. Hammerschmidt M. Haffter P. Nusslein-Volhard C. Large-scale mutagenesis in the zebrafish: in search of genes controlling development in a vertebrate. Curr Biol. 1994;4:189–202. doi: 10.1016/s0960-9822(00)00048-8. [DOI] [PubMed] [Google Scholar]
- 3.Johnson SL. Africa D. Walker C. Weston JA. Genetic control of adult pigment stripe development in zebrafish. Dev Biol. 1995;167:27–33. doi: 10.1006/dbio.1995.1004. [DOI] [PubMed] [Google Scholar]
- 4.Kelsh RN. Brand M. Jiang YJ. Heisenberg CP. Lin S. Haffter P, et al. Zebrafish pigmentation mutations and the processes of neural crest development. Development. 1996;123:369–389. doi: 10.1242/dev.123.1.369. [DOI] [PubMed] [Google Scholar]
- 5.Lamason RL. Mohideen MA. Mest JR. Wong AC. Norton HL. Aros MC, et al. SLC24A5, a putative cation exchanger, affects pigmentation in zebrafish and humans. Science. 2005;310:1782–1786. doi: 10.1126/science.1116238. [DOI] [PubMed] [Google Scholar]
- 6.Svetic V. Hollway GE. Elworthy S. Chipperfield TR. Davison C. Adams RJ, et al. Sdf1a patterns zebrafish melanophores and links the somite and melanophore pattern defects in choker mutants. Development. 2007;134:1011–1022. doi: 10.1242/dev.02789. [DOI] [PubMed] [Google Scholar]
- 7.Lister JA. Robertson CP. Lepage T. Johnson SL. Raible DW. nacre Encodes a zebrafish microphthalmia-related protein that regulates neural-crest-derived pigment cell fate. Development. 1999;126:3757–3767. doi: 10.1242/dev.126.17.3757. [DOI] [PubMed] [Google Scholar]
- 8.Khersonsky SM. Jung DW. Kang TW. Walsh DP. Moon HS. Jo H, et al. Facilitated forward chemical genetics using a tagged triazine library and zebrafish embryo screening. J Am Chem Soc. 2003;125:11804–11805. doi: 10.1021/ja035334d. [DOI] [PubMed] [Google Scholar]
- 9.Peterson RT. Shaw SY. Peterson TA. Milan DJ. Zhong TP. Schreiber SL, et al. Chemical suppression of a genetic mutation in a zebrafish model of aortic coarctation. Nat Biotechnol. 2004;22:595–599. doi: 10.1038/nbt963. [DOI] [PubMed] [Google Scholar]
- 10.Fleming A. Sato M. Goldsmith P. High-throughput in vivo screening for bone anabolic compounds with zebrafish. J Biomol Screen. 2005;10:823–831. doi: 10.1177/1087057105279952. [DOI] [PubMed] [Google Scholar]
- 11.Mendelsohn BA. Yin C. Johnson SL. Wilm TP. Solnica-Krezel L. Gitlin JD. Atp7a determines a hierarchy of copper metabolism essential for notochord development. Cell Metab. 2006;4:155–162. doi: 10.1016/j.cmet.2006.05.001. [DOI] [PubMed] [Google Scholar]
- 12.Murphey RD. Stern HM. Straub CT. Zon LI. A chemical genetic screen for cell cycle inhibitors in zebrafish embryos. Chem Biol Drug Des. 2006;68:213–219. doi: 10.1111/j.1747-0285.2006.00439.x. [DOI] [PubMed] [Google Scholar]
- 13.Anderson C. Bartlett SJ. Gansner JM. Wilson D. He L. Gitlin JD, et al. Chemical genetics suggests a critical role for lysyl oxidase in zebrafish notochord morphogenesis. Mol Biosyst. 2007;3:51–59. doi: 10.1039/b613673g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Choi TY. Kim JH. Ko DH. Kim CH. Hwang JS. Ahn S, et al. Zebrafish as a new model for phenotype-based screening of melanogenic regulatory compounds. Pigment Cell Res. 2007;20:120–127. doi: 10.1111/j.1600-0749.2007.00365.x. [DOI] [PubMed] [Google Scholar]
- 15.Stern HM. Murphey RD. Shepard JL. Amatruda JF. Straub CT. Pfaff KL, et al. Small molecules that delay S phase suppress a zebrafish bmyb mutant. Nat Chem Biol. 2005;1:366–370. doi: 10.1038/nchembio749. [DOI] [PubMed] [Google Scholar]
- 16.Parichy DM. Rawls JF. Pratt SJ. Whitfield TT. Johnson SL. Zebrafish sparse corresponds to an orthologue of c-kit and is required for the morphogenesis of a subpopulation of melanocytes, but is not essential for hematopoiesis or primordial germ cell development. Development. 1999;126:3425–3436. doi: 10.1242/dev.126.15.3425. [DOI] [PubMed] [Google Scholar]
- 17.Mellgren EM. Johnson SL. A requirement for kit in embryonic zebrafish melanocyte differentiation is revealed by melanoblast delay. Dev Genes Evol. 2004;214:493–502. doi: 10.1007/s00427-004-0428-y. [DOI] [PubMed] [Google Scholar]
- 18.Yang CT. Johnson SL. Small molecule-induced ablation and subsequent regeneration of larval zebrafish melanocytes. Development. 2006;133:3563–3573. doi: 10.1242/dev.02533. [DOI] [PubMed] [Google Scholar]
- 19.Rawls JF. Johnson SL. Temporal and molecular separation of the kit receptor tyrosine kinase's roles in zebrafish melanocyte migration and survival. Dev Biol. 2003;262:152–161. doi: 10.1016/s0012-1606(03)00386-5. [DOI] [PubMed] [Google Scholar]
- 20.Linnekin D. Early signaling pathways activated by c-Kit in hematopoietic cells. Int J Biochem Cell Biol. 1999;31:1053–1074. doi: 10.1016/s1357-2725(99)00078-3. [DOI] [PubMed] [Google Scholar]
- 21.Kent D. Copley M. Benz C. Dykstra B. Bowie M. Eaves C. Regulation of hematopoietic stem cells by the steel factor/KIT signaling pathway. Clin Cancer Res. 2008;14:1926–1930. doi: 10.1158/1078-0432.CCR-07-5134. [DOI] [PubMed] [Google Scholar]
- 22.Timokhina I. Kissel H. Stella G. Besmer P. Kit signaling through PI 3-kinase and Src kinase pathways: an essential role for Rac1 and JNK activation in mast cell proliferation. EMBO J. 1998;17:6250–6262. doi: 10.1093/emboj/17.21.6250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hultman KA. Bahary N. Zon LI. Johnson SL. Gene duplication of the zebrafish kit ligand and partitioning of melanocyte development functions to kit ligand a. PLoS Genet. 2007;3:e17. doi: 10.1371/journal.pgen.0030017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bauer S. Duensing A. Demetri GD. Fletcher JA. KIT oncogenic signaling mechanisms in imatinib-resistant gastrointestinal stromal tumor: PI3-kinase/AKT is a crucial survival pathway. Oncogene. 2007;26:7560–7568. doi: 10.1038/sj.onc.1210558. [DOI] [PubMed] [Google Scholar]
- 25.Serve H. Yee NS. Stella G. Sepp-Lorenzino L. Tan JC. Besmer P. Differential roles of PI3-kinase and Kit tyrosine 821 in Kit receptor-mediated proliferation, survival and cell adhesion in mast cells. EMBO J. 1995;14:473–483. doi: 10.1002/j.1460-2075.1995.tb07023.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhu S. Korzh V. Gong Z. Low BC. RhoA prevents apoptosis during zebrafish embryogenesis through activation of Mek/Erk pathway. Oncogene. 2008;27:1580–1589. doi: 10.1038/sj.onc.1210790. [DOI] [PubMed] [Google Scholar]
- 27.Ueda S. Mizuki M. Ikeda H. Tsujimura T. Matsumura I. Nakano K, et al. Critical roles of c-Kit tyrosine residues 567 and 719 in stem cell factor-induced chemotaxis: contribution of src family kinase and PI3-kinase on calcium mobilization and cell migration. Blood. 2002;99:3342–3349. doi: 10.1182/blood.v99.9.3342. [DOI] [PubMed] [Google Scholar]
- 28.Pozios KC. Ding J. Degger B. Upton Z. Duan C. IGFs stimulate zebrafish cell proliferation by activating MAP kinase and PI3-kinase-signaling pathways. Am J Physiol. 2001;280:R1230–R1239. doi: 10.1152/ajpregu.2001.280.4.R1230. [DOI] [PubMed] [Google Scholar]
- 29.Hong CC. Peterson QP. Hong JY. Peterson RT. Artery/vein specification is governed by opposing phosphatidylinositol-3 kinase and MAP kinase/ERK signaling. Curr Biol. 2006;16:1366–1372. doi: 10.1016/j.cub.2006.05.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Le HY. Zhang Y. Liu H. Ma LH. Jin Y. Huang QH, et al. eena Promotes myeloid proliferation through stimulating ERK1/2 phosphorylation in zebrafish. J Biol Chem. 2008;283:17652–17661. doi: 10.1074/jbc.M710517200. [DOI] [PubMed] [Google Scholar]
- 31.Keller JM. Escara-Wilke JF. Keller ET. Heat stress-induced heat shock protein 70 expression is dependent on ERK activation in zebrafish (Danio rerio) cells. Comp Biochem Physiol. 2008;150:307–314. doi: 10.1016/j.cbpa.2008.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lindsay MA. Target discovery. Nat Rev. 2003;2:831–838. doi: 10.1038/nrd1202. [DOI] [PubMed] [Google Scholar]
- 33.Friedman Z. Hackett SF. Linden J. Campochiaro PA. Human retinal pigment epithelial cells in culture possess A2-adenosine receptors. Brain Res. 1989;492:29–35. doi: 10.1016/0006-8993(89)90885-8. [DOI] [PubMed] [Google Scholar]
- 34.Fuse S. Esemuede N. Yamaguchi M. Maier KG. Nesselroth SM. Sumpio BE, et al. The role of G proteins in thromospondin-1-induced vascular smooth muscle cell migration. Surgery. 2008;144:86–92. doi: 10.1016/j.surg.2008.03.028. [DOI] [PubMed] [Google Scholar]
- 35.Chen L. Zhang JJ. Huang XY. cAMP inhibits cell migration by interfering with Rac-induced lamellipodium formation. J Biol Chem. 2008;283:13799–13805. doi: 10.1074/jbc.M800555200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jacob A. Molkentin JD. Smolenski A. Lohmann SM. Begum N. Insulin inhibits PDGF-directed VSMC migration via NO/cGMP increase of MKP-1 and its inactivation of MAPKs. Am J Physiol Cell Physiol. 2002;283:C704–C713. doi: 10.1152/ajpcell.00110.2002. [DOI] [PubMed] [Google Scholar]
- 37.Zhang S. Yang Y. Kone BC. Allen JC. Kahn AM. Insulin-stimulated cyclic guanosine monophosphate inhibits vascular smooth muscle cell migration by inhibiting Ca/calmodulin-dependent protein kinase II. Circulation. 2003;107:1539–1544. doi: 10.1161/01.cir.0000056766.45109.c1. [DOI] [PubMed] [Google Scholar]
- 38.Yu SM. Kuo SC. Vasorelaxant effect of isoliquiritigenin, a novel soluble guanylate cyclase activator, in rat aorta. Br J Pharmacol. 1995;114:1587–1594. doi: 10.1111/j.1476-5381.1995.tb14943.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Abdollahi M. Chan TS. Subrahmanyam V. O'Brien PJ. Effects of phosphodiesterase 3,4,5 inhibitors on hepatocyte cAMP levels, glycogenolysis, gluconeogenesis and susceptibility to a mitochondrial toxin. Mol Cell Biochem. 2003;252:205–211. doi: 10.1023/a:1025568714217. [DOI] [PubMed] [Google Scholar]
- 40.Seamon KB. Padgett W. Daly JW. Forskolin: unique diterpene activator of adenylate cyclase in membranes and in intact cells. Proc Natl Acad Sci USA. 1981;78:3363–3367. doi: 10.1073/pnas.78.6.3363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Barresi MJ. Stickney HL. Devoto SH. The zebrafish slow-muscle-omitted gene product is required for Hedgehog signal transduction and the development of slow muscle identity. Development. 2000;127:2189–2199. doi: 10.1242/dev.127.10.2189. [DOI] [PubMed] [Google Scholar]
- 42.Jin EJ. Thibaudeau G. Effects of lithium on pigmentation in the embryonic zebrafish (Brachydanio rerio) Biochim Biophys Acta. 1999;1449:93–99. doi: 10.1016/s0167-4889(98)00176-1. [DOI] [PubMed] [Google Scholar]
- 43.Moiseiwitsch JR. Lauder JM. Serotonin regulates mouse cranial neural crest migration. Proc Natl Acad Sci USA. 1995;92:7182–7186. doi: 10.1073/pnas.92.16.7182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.David NB. Sapede D. Saint-Etienne L. Thisse C. Thisse B. Dambly-Chaudiere C, et al. Molecular basis of cell migration in the fish lateral line: role of the chemokine receptor CXCR4 and of its ligand, SDF1. Proc Natl Acad Sci USA. 2002;99:16297–16302. doi: 10.1073/pnas.252339399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Madsen EC. Gitlin JD. Zebrafish mutants calamity and catastrophe define critical pathways of gene-nutrient interactions in developmental copper metabolism. PLoS Genet. 2008;4:e1000261. doi: 10.1371/journal.pgen.1000261. [DOI] [PMC free article] [PubMed] [Google Scholar]