Abstract
Previous studies have demonstrated that rats exposed to methamphetamine (MA) during the neonatal period display deficits in spatial learning and memory. The underlying correlates are unknown; therefore, this study was devised to determine whether neuronal changes occur in the dentate gyrus (DG), nucleus accumbens (NAcc) and cortex of adult rats exposed to 10 mg/kg MA administered four times daily from P11−20 using Golgi–Cox staining [Gibb, R. & Kolb, B. (1998) J. Neurosci. Meth., 79, 1−4]. The DG and NAcc demonstrated a decrease in the number of spines per neuron and the NAcc showed an associated decrease in dendritic length. Selective changes in cortex were observed because increased dendritic length in the parietal cortex occurred with no change in the number of spines, and no differences were noted for either dendritic length or spines in the medial frontal cortex. The data suggest a potential cause for the learning and memory deficits induced by neonatal MA exposure; however, the underlying mechanism that produces these neuronal changes is unknown.
Keywords: cortex, dentate gyrus, development, Golgi–Cox staining
Introduction
The use of methamphetamine (MA) among women of child-bearing age increases the probability that fetuses will be exposed to this drug. Nonetheless, the long-term effects of gestational exposure are unknown. Several short-term effects of MA have been documented in humans and these include intrauterine growth retardation, reduced head circumference, increased morbidity and reduced performance in a visual recognition test during the first year of life (Oro & Dixon, 1987; Little et al., 1988; Dixon & Bejar, 1989; Struthers & Hansen, 1992; Hansen et al., 1993). Newer data demonstrate, using MRI spectroscopy, that children exposed to MA during gestation have an increase in creatine and in the ratio of glutamate : glutamine concentrations, suggesting increased metabolic activity, but no changes in neuronal or glial markers (Smith et al., 2001). While limited, these data suggest that MA causes at least short-term central nervous system changes. It remains to be seen whether such effects result in long-term deleterious effects.
Rodent models are valuable for understanding the potential long-term effects of developmental drug exposures. However, because the brain matures on a different time scale in rats than in humans, in order to model human third-trimester drug exposure, treatment corresponding to the same events in the rat has to be administered during the neonatal period (Bayer et al., 1993). Rat hippocampal granule cell proliferation continues until postnatal day (P)19 (Bayer et al., 1993) and we have demonstrated that MA administration from P11 to P20 produces deficits in spatial learning and memory but does not affect working memory, cued learning or swimming capabilities (Vorhees et al., 1994a, 1998, 1999; Vorhees et al., 2000; Williams et al., 2002). Unlike in adult animals, neonatal exposure to MA in rats does not produce neurotoxicity as characterised by astrogliosis nor does it produce immediate reductions in dopaminergic and serotonergic markers (Cappon et al., 1997), although neonatal exposure to MA in gerbils appears to induce long-lasting changes in dopaminergic and serotonergic systems (Busche et al., 2002; Neddens et al., 2002; 2003). Perturbations of the hypothalamic–pituitary–adrenal (HPA) axis do occur in neonatal rats exposed to MA, as demonstrated by increases in corticosterone (CORT) and adrenocorticotropin hormone (Williams et al., 2000). CORT is known to alter neuronal development within the hippocampus (Gould et al., 1991a, b), an area important for spatial learning and memory (Morris et al., 1982).
This study was designed to analyse the effects of neonatal MA administration on dendritic length and spine density in adult rats and thus provide further insight into possible neural correlates that may help to identify regions of the brain altered by developmental MA exposure. This is important relative to behavioural function, as our laboratory has demonstrated that neonatal MA treatment in rats produces spatial learning deficits in adulthood. The underlying mechanism or affected brain regions for these effects has been elusive. Accordingly we investigated, using the Golgi–Cox technique, whether MA administration from P11 to P20 produces changes in neuronal morphology in several regions of the brain important for cognitive function.
Materials and methods
Subjects
Nulliparous female and male Sprague-Dawley CD IGS rats (Charles River Laboratories, Raleigh, USA) were mated in hanging wire cages following an acclimatisation period to the vivarium of at least 2 weeks. The day a sperm plug was detected was considered embryonic day zero (E0). Fourteen days after being placed with the males, the females were transferred to polycarbonate cages (45.7 × 23.8 × 20.3 cm). The offspring were the test subjects for these experiments. The vivarium was maintained on a 14-h light : 10-h dark cycle (lights on at 06.00 h) and food and water were freely available. All procedures were approved by the Cincinnati Children's Research Foundation's Laboratory Animal Care and Use Committee and the vivarium was fully accredited by the Association for the Assessment and Accreditation of Laboratory Animal Care (AAALAC).
Beginning on E21 females were checked twice daily for the presence of a litter. The day of birth was considered P0 and on P1 litters were standardized to eight pups with at least four males and two females present in each litter. Offspring were weighed on P7 and weekly thereafter and uniquely identified by an ear punch on P11.
Methamphetamine treatment
d-Methamphetamine HCl (MA; Sigma) was dissolved in the sterile saline (SAL) vehicle at a concentration of 10 mg/3 mL (expressed as the freebase). MA was administered to animals at a dose of 10 mg/kg through a subcutaneous route in the dorsum. Beginning on P11 and continuing through P20 pups received MA four times per day with 2 h between injections. In order to minimise possible irritation to the dermis, injection sites were varied. Prior to each injection, animals were weighed. Animals were allowed to be weaned naturally by their dams (Redman & Sweney, 1976; Blass & Teicher, 1980) and separated on P28 and housed in pairs. A total of six litters were produced for this study and one male of each condition was used from each litter. The other animals in the litter were given to collaborators for pilot work.
Golgi–Cox analysis
On approximately P60 (a day that is encompassed by our behavioural testing period), the animals were given an overdose of sodium pentobarbital, perfused intracardially with 0.9% saline, and whole brains harvested. Tissue was collected from all animals on the same day within a 2-h time period and littermates (i.e. one MA and one SAL animal) were perfused simultaneously. Brains were placed in Golgi–Cox solution for 14 days and subsequently in a Golgi–Cox–30% sucrose solution for 3 days. The tissue was cut in 200-μm sections using a vibratome, mounted onto gelatinized slides and stained using the method described previously (Gibb & Kolb, 1998). Neurons were first identified at a lower magnification (100×) in each hemisphere and then a total of five neurons per hemisphere were traced at a higher magnification (250×) using the camera lucida method. An experimenter who was blind to treatment membership performed the cell selection and tracing. In order for a neuron to be selected it had to meet certain criteria: the dendritic tree had to be well visualized and not obscured by stain precipitate, blood vessels or astrocytes. If more than five neurons were identified in each hemisphere as reaching these criteria, then the cells included in the study were randomly selected from the total number of cells identified as analysable. Four brain areas were traced and quantified; these were Zilles’ areas P1 (Layer III, parietal cortex), Cg3 (Layer V, medial frontal cortex), nucleus accumbens (NAcc) and the dentate gyrus (DG). Pyramidal cells were traced in P1, Cg3, and NAcc, and granule cells located in the granular cell layer were traced in the DG. For the NAcc, only pyramidal cells in the shell were traced.
Dendritic length was quantified using a Sholl analysis of ring intersections (Sholl, 1981). For example, to obtain a measure of length the total number of ring intersections was summed and then averaged across cells. Spine density was quantified by counting spines on one fourth-order terminal tip from basilar dendrites and one third-order terminal tip from apical dendrites in the cortex. These regions of the dendrites were used for analysis, firstly because this is typically where differences have been observed in past studies following stimulant administration in adult rats (Robinson & Kolb, 1999; Brown & Kolb, 2001). Secondly, when there are increases in dendritic length they must occur in the distal end, thus increasing the likelihood of changes in dendritic spine densities at this location. For both the NAcc and DG, neurons do not have an apical and basilar dendritic tree so spine density was quantified using a third-order terminal tip. Spine density was consistently performed on the same branch order and figured as the number of spines per cm of the dendritic branch, even though the branch length may have varied minimally.
Statistical analyses were performed by averaging dendritic length and spine density across cells per hemisphere, thus representing one mean per animal per brain region of analysis. Cells harvested in different hemispheres were not treated as independent samples. Group differences were assessed using an independent t-test on dendritic length and dendritic spine density in each brain area. For both the frontal and parietal cortices, the basilar and apical dendritic trees were analysed separately.
Results
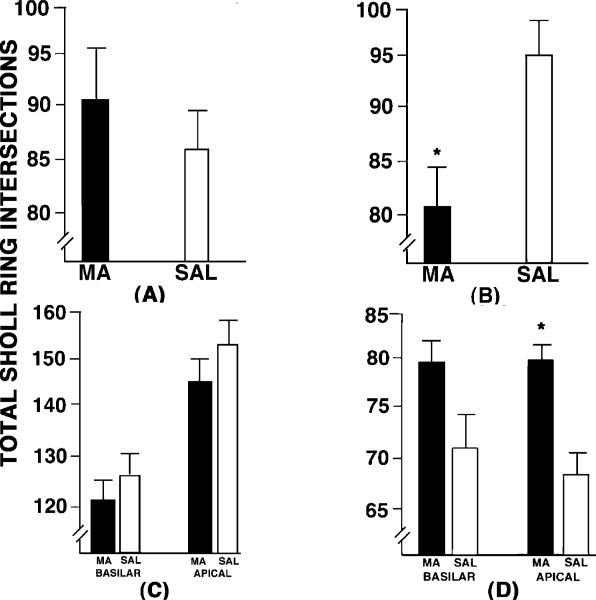
Representative photomicrographs of dendritic length and spine densities of the DG and NAcc are shown in Fig. 1. Dendritic length (Fig. 2A–D) and dendritic spine density (Fig. 3A–D) are shown for each of the four brain areas analysed. Camera lucida drawings of representative spiny neurons in the NAcc and DG are shown in Figs 4 and 5, respectively. The arrow indicates the terminal tip from which spines were traced. An independent t-test revealed a significant group effect in dendritic length in the NAcc (t10 = 5.00, P < 0.05; Fig. 2B) and the apical tree of the parietal cortex (t11 = 8.15, P < 0.01; Fig. 2D). In the NAcc, the effect was a 14.5% decrease in dendritic length in animals given MA compared to SAL controls. In the parietal cortex, the effect was a 12.8% increase in dendritic length in MA-treated animals compared to SAL-treated controls. The t-test on the basilar parietal tree was not significant (P = 0.12), although there was a comparable tendency for the MA animals to have an increase in dendritic length (10.3%) vs. SAL controls. No other comparisons were statistically significant for dendritic length.
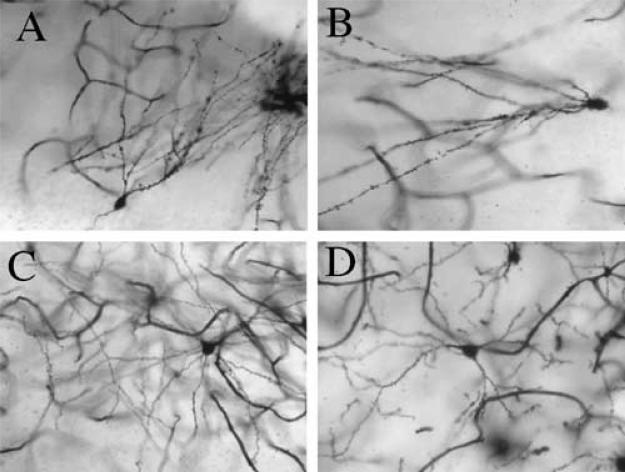
Fig. 1.
Displayed are representative photomicrographs of (A and B) dentate gyrus (DG) granular cells and of (C and D) nucleus accumbens (NAcc) pyramidal cells from animals treated neonatally with SAL (A and C) or MA (B and D). For the DG, spine density was decreased in MA animals (B) relative to SAL (A) animals; in the NAcc, dendritic branch length as well as spine density was decreased in MA animals (D) compared to SAL animals (C). It is important to note that these pictures do not represent the entire cell, as dendrites that course through tissue are not all on the same plane; however, the pictures depicted here are within the same plane of tissue. Pictures were taken with a Sony digital camera equipped with a Zeiss lens, and all pictures were taken at the drawing power for dendritic length (250×).
Fig. 2.
The dendritic lengths in (A) the DG, (B) NAcc, (C) frontal cortex and (D) parietal cortex in animals treated with either methamphetamine (MA) or the saline vehicle (SAL) from P11 to P20. Decreased dendritic lengths were observed in the NAcc and increased lengths were observed in the parietal cortex. *P < 0.05.
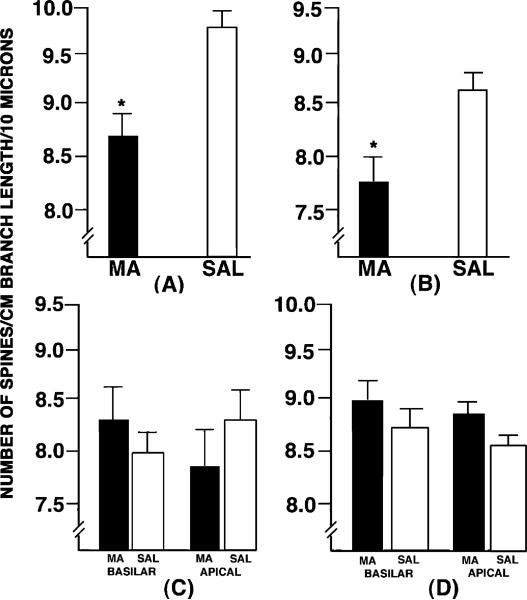
Fig. 3.
The dendritic spine densities in (A) the DG, (B) NAcc, (C) frontal cortex and (D) parietal cortex in animals treated with either methamphetamine (MA) or the saline vehicle (SAL) from P11 to P20. Decreased dendritic spine denisites were observed in both the dentate gyrus and the nucleus accumbens. *P < 0.05.
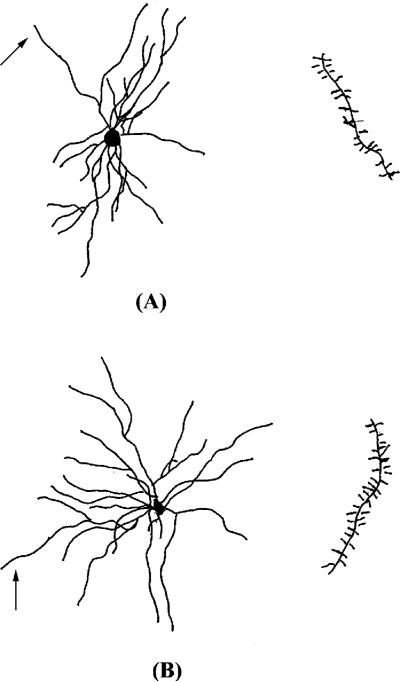
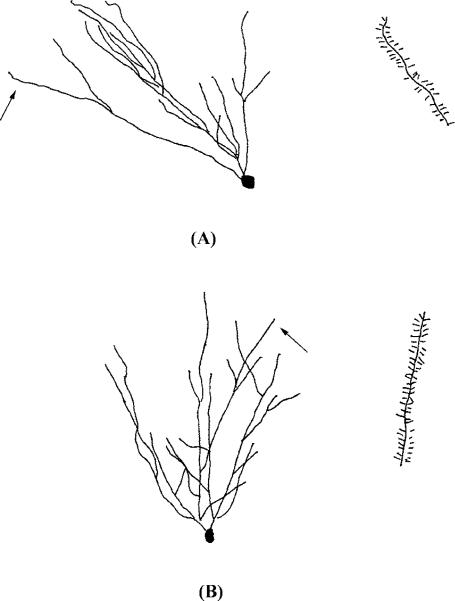
Fig. 4.
Representative camera lucida drawings of spiny neurons in the shell of the NAcc from animals administered (A) SAL or (B) methamphetamine from P11 to P20. The arrow indicates the terminal tip where the spines were traced.
Fig. 5.
Representative camera lucida drawings of spiny neurons in the DG from animals administered (A) SAL or (B) methamphetamine from P11 to P20. The arrow indicates the terminal tip where the spines were traced.
Regarding dendritic spine density, there was a significant effect of group in the NAcc (t11 = 5.21, P < 0.03) and DG (t10 = 8.69, P < 0.001). In the NAcc, there was a 9.2% decrease in spine density in MA animals as compared to SAL controls. In the DG, there was a slightly more robust decrease of 11.3% in spine density in MA animals compared to controls. There were no other comparisons that were statistically significant for spine density. An inspection of the same region in Figs 1, 4 and 5 illustrate the lower spine density seen in the NAcc and DG.
Discussion
In this study we demonstrated that injections of MA from P11 to P20 produced region-specific changes in the dendritic morphology of the DG, NAcc and parietal cortex when these regions were examined ≈40 days after the cessation of drug. This is the first demonstration that administration of a psychostimulant, and MA in particular, reduces dendritric length in the NAcc as well as the number of spines in both the NAcc and DG. We also demonstrated that neonatal MA produced increases in dendritic length in the parietal cortex but did not alter spine density in this region. Interestingly, the effect of MA on parietal cortex appears to be specific because no differences in either the dendritic length or the number of spines was observed in the medial frontal cortex. Similar to the changes induced in the parietal cortex, others have demonstrated that a single dose of MA (50 mg/kg) administered on P14 to gerbils produces longer dendritic lengths as well as increased numbers of spines in the prefrontal cortex, although the parietal cortex was not examined in that study (Blaesing et al., 2001), and there is a reduction in dopaminergic fibers in the NAcc in these animals that appears to be influenced if the animals are isolated at weaning (Neddens et al., 2002). Others have also demonstrated that housing conditions influence neuronal morphology and that these changes are region-specific, such that no changes were observed, changes in spine density were observed, or changes in both spine density and length were reported (Kolb et al., 2003).
Unique to the present study is that we have consistently observed spatial learning and memory deficits in the Morris water maze in animals treated with MA from P11 to P20 (Vorhees et al., 1994a, 1998, 1999, 2000; Williams et al., 2002, 2003a, 2003b) and have also shown spatial deficits in the Barnes maze (Williams et al., 2003). The spatial deficits are observed in animals of the same age as those showing morphological changes here. It should be noted that increased errors in delayed alternation and locomotor activity has been noted in a previous study following a P14 administration of MA to gerbils (Dawirs et al., 1996); however, the locomotor effect observed in gerbils is opposite to that observed following P11−20 MA administration in rats (Vorhees et al., 1994b). Therefore, taken together with the Golgi findings following neonatal MA in gerbils, direct comparison of data from gerbils and rats must be interpreted with caution especially because the dosing paradigms are much different and it is known that substituted amphetamines can induce different neurotransmitter responses among different species (Logan et al., 1988).
The morphological changes in Golgi-stained dendrites are considered evidence of altered synaptic connectivity. For example, rats reared in complex environments or trained in learning tasks demonstrate increases in the surface of cortical and subcortical dendrites as well as the number of synapses measured per neuron (Greenough & Bailey, 1988; Greenough et al., 1988; Faherty et al., 2003; Kolb et al., 2003). Therefore, it suggests that neural changes that accompany changes in behaviour can be expressed utilizing Golgi-stained tissue. This is relevant to the present study because neonatal MA exposure produces spatial learning impairments, and this may be reflected by the decrease in dendritic length and spine density in two areas that have been implicated in spatial memory and goal-directed behaviour, namely the DG and NAcc (Floresco et al., 1997; Roullet et al., 1997; Gilbert et al., 2001; Jeltsch et al., 2001). No differences were noted in the medial frontal cortex in this study, a region that is thought to be important in sequential learning and memory (Kolb, 1990; Etienne, 1992). Consistent with this lack of effect in the frontal cortex, we find no differences in a test of sequential learning and memory, the Cincinnati water maze, when rats are treated with MA from P11 to P20 (Vorhees et al., 1994a; Williams et al., 2003b).
The administration of drugs of abuse such as cocaine, d-amphetamine or nicotine in adult animals produce changes in patterns of synaptic connectivity in the frontal cortex and NAcc (Robinson & Kolb, 1997, 1999; Brown & Kolb, 2001). These changes generally are exhibited by increases in dendritic length or spine density and have been shown to persist for as long as 3.5 months after drug administration ceased. A single dose of MA in gerbils has also been shown to produce increases in dendritic length and spine density in the prefrontal cortex; however, Golgi-impregnated neurons within the NAcc were not examined in these studies (Dawirs et al., 1991; Blaesing et al., 2001). Unlike the adult effects of psychostimulants, neonatal MA exposure in rats either directly produced, or influenced secondary pathways to induce, significant decreases or suppression in dendritic length and dendritic spine density in the NAcc compared to SAL-treated controls. Because tissue was only collected in adulthood in this study, it is uncertain whether synaptic connectivity was immediately altered during the period of drug administration and the effects persisted into adulthood or whether neurons in the various regions were predisposed by the early MA administration and the effect occurred later following a precipitating event such as puberty. A time-course study to determine the exact effect of MA would be informative.
The differences in results between the adult administration and the present study may involve several factors. For example, repeated amphetamine or nicotine treatment in adult rats have been shown to produce increases in several neurotrophic factors, including basic fibroblast growth factor (bFGF), nerve growth factor (NGF) and brain-derived neurotrophic factor (BDNF) (Flores & Stewart, 2000; Kenny et al., 2000; Jonnala & Buccafusco, 2001). In addition, adult mice given a single administration of MA demonstrated increased transcripts for BDNF (Thomas et al., 2004). Although there is less information regarding the effect of neonatal MA administration on neurotrophic factors, it has been suggested that these factors may be involved in the detrimental effects following early MA exposure (Frost & Cadet, 2000). Furthermore, it is known that several neurotrophic factors (i.e. BDNF, NGF) are at peak concentrations developmentally between P7 and P21 in various regions of the rat brain, including the hippocampus and cortex (Das et al., 2001). Therefore, alterations in these factors during development may be more critical for neuronal morphology, more so than in adult animals.
Probably the most important difference between adult administration of psychostimulants and the results of the present study is the period of development when MA was administered. For instance, neonatal amphetamine exposure has been shown to produce a reduction in synapses in the hippocampus that appears to be related to decreased cell proliferation (Bendek & Hahn, 1981). Neonatal drug administration, especially during the time period that MA was administered in the present study, may affect synaptogenesis and result in long-term changes in brain function. Other than the neurotrophic factors already mentioned, CORT also plays an important role in cell birth and death and synaptic connectivity (Woolley et al., 1990; Gould et al., 1991a, b). Of interest here is that CORT alters neurotrophic factor expression and protein content (Smith et al., 1995; Schaaf et al., 1998, 2000; Scaccianoce et al., 2000). Furthermore, neonatal MA administration has been shown to produce a protracted release of CORT induced on P11 as well as transient increases on P15 and P20 (Williams et al., 2000). CORT is especially appealing as a mediator or permissive factor in the morphological changes, because others have demonstrated that high levels of this steroid administered during the neonatal period reduces the number of neurons within the hippocampus (Gould et al., 1991b) and it is known to induce atrophy of CA3 dendrites in adults (Magarinños & McEwen, 1995a, b). Taken together, it is likely that MA influences the release or suppression of factors important for normal neuronal development to occur.
In conclusion, the results show that neonatal MA exposure produces specific changes within the brain, and the major differences are in brain areas that play a role in cognition and drug reinforcement. It is recognized that Golgi histological staining is only suggestive for a loss of synaptic connectivity, and that the underlying mechanism for these changes still remains in question. Nonetheless, the lower spine densities in both the DG and NAcc and shorter dendritic lengths in the NAcc certainly suggest that there is long-term synaptic reorganization as the result of neonatal MA exposure and this may have important implications regarding MA exposure during pregnancy in humans.
Acknowledgements
Portions of these data were presented at the 33rd annual meeting of the Society for Neuroscience. Supported by US National Institutes of Health research grants DA06733 (C.V.V.) and DA14269 and ES07051 (M.T.W.)
Footnotes
M.T.W. and R.W.B. contributed equally to this work.
Abbreviations
BDNF, brain-derived neurotrophic factor; CORT, corticosterone; DG, dentate gyrus; E, embryonic day; HPA, hypothalamic–pituitary–adrenal; MA, methamphetamine; NAcc, nucleus accumbens; P, postnatal day; SAL, saline.
References
- Bayer SA, Altman J, Russo RJ, Zhang X. Timetables of neurogenesis in the human brain based on experimentally determined patterns in the rat. Neurotoxicology. 1993;14:83–144. [PubMed] [Google Scholar]
- Bendek G, Hahn Z. Effect of amphetamine on the metabolism and incorporation of [3H]-thymidine into DNA of developing rat brain. Dev. Neurosci. 1981;4:55–65. doi: 10.1159/000112741. [DOI] [PubMed] [Google Scholar]
- Blaesing B, Nossoll M, Teuchert-Noodt G, Dawirs RR. Postnatal maturation of prefrontal pyramidal neurones is sensitive to a single early dose of methamphetamine in gerbils (Meriones unguiculatus). J. Neural Transm. 2001;108:101–113. doi: 10.1007/s007020170101. [DOI] [PubMed] [Google Scholar]
- Blass EM, Teicher MH. Suckling. Science. 1980;210:15–22. doi: 10.1126/science.6997992. [DOI] [PubMed] [Google Scholar]
- Brown RW, Kolb B. Nicotine sensitization increases dendritic length and spine density in the nucleus accumbens and cingulate cortex. Brain Res. 2001;899:94–100. doi: 10.1016/s0006-8993(01)02201-6. [DOI] [PubMed] [Google Scholar]
- Busche A, Neddens J, Dinter C, Dawirs RR, Teuchert-Noodt G. Differential influence of rearing conditions and methamphetamine on serotonin fibre maturation in the dentate gyrus of gerbils (Meriones unguiculatus). Dev. Neurosci. 2002;24:512–521. doi: 10.1159/000069362. [DOI] [PubMed] [Google Scholar]
- Cappon G, Morford LL, Vorhees CV. Ontogeny of methamphetamine-induced neurotoxicity and associated hyperthermic response. Dev. Brain Res. 1997;103:155–162. doi: 10.1016/s0165-3806(97)81791-9. [DOI] [PubMed] [Google Scholar]
- Das KP, Chao SL, White LD, Haines WT, Harry GJ, Tilson HA, Barone S., Jr Differential patterns of nerve growth factor, brain-derived neurotrophic factor and neurotrophin-3 mRNA and protein levels in developing regions of rat brain. Neuroscience. 2001;103:739–761. doi: 10.1016/s0306-4522(01)00011-2. [DOI] [PubMed] [Google Scholar]
- Dawirs RR, Teuchert-Noodt G, Busse M. Single doses of methamphetamine cause changes in the density of dendritic spines in the prefrontal cortex of gerbils (Meriones unguiculatus). Neuropharmacology. 1991;30:275–282. doi: 10.1016/0028-3908(91)90155-5. [DOI] [PubMed] [Google Scholar]
- Dawirs RR, Teuchert-Noodt G, Czaniera R. Ontogeny of PFC-related behaviours is sensitive to a single non-invasive dose of methamphetamine in neonatal gerbils (Meriones unguiculatus). J. Neural Transm. 1996;103:1235–1245. doi: 10.1007/BF01271184. [DOI] [PubMed] [Google Scholar]
- Dixon SD, Bejar R. Echoencephalographic findings in neonates associated with maternal cocaine and methamphetamine use: incidence and clinical correlates. J. Pediatr. 1989;115:770–778. doi: 10.1016/s0022-3476(89)80661-4. [DOI] [PubMed] [Google Scholar]
- Etienne AS. Navigation of a small mammal by dead reckoning and local cues. Curr. Dir. Psychol. Sci. 1992;1:48–52. [Google Scholar]
- Faherty CJ, Kerley D, Smeyne RJ. A Golgi-Cox morphological analysis of neuronal changes induced by environmental enrichment. Brain Res. Dev. Brain Res. 2003;141:55–61. doi: 10.1016/s0165-3806(02)00642-9. [DOI] [PubMed] [Google Scholar]
- Flores C, Stewart J. Basic fibroblast growth factor as a mediator of the effects of glutamate in the development of long-lasting sensitization to stimulant drugs: studies in the rat. Psychopharmacology (Berl) 2000;151:152–165. doi: 10.1007/s002130000417. [DOI] [PubMed] [Google Scholar]
- Floresco SB, Seamans JK, Phillips AG. Selective roles for hippocampal, prefrontal cortical, and ventral striatal circuits in radial-arm maze tasks with or without a delay. J. Neurosci. 1997;17:1880–1890. doi: 10.1523/JNEUROSCI.17-05-01880.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frost DO, Cadet JL. Effects of methamphetamine-induced neurotoxicity on the development of neural circuitry: a hypothesis. Brain Res. Rev. 2000;34:103–118. doi: 10.1016/s0165-0173(00)00042-4. [DOI] [PubMed] [Google Scholar]
- Gibb R, Kolb B. A method for vibratome sectioning of Golgi-Cox stained whole rat brain. J. Neurosci. Meth. 1998;79:1–4. doi: 10.1016/s0165-0270(97)00163-5. [DOI] [PubMed] [Google Scholar]
- Gilbert PE, Kesner RP, Lee I. Dissociating hippocampal sub-regions: double dissociation between dentate gyrus and CA1. Hippocampus. 2001;11:626–636. doi: 10.1002/hipo.1077. [DOI] [PubMed] [Google Scholar]
- Gould E, Woolley CS, Cameron HA, Daniels DC, McEwen BS. Adrenal steroids regulate postnatal development of the rat dentate gyrus. II. Effects of glucocorticoids and mineralocorticoids on cell birth. J. Comp. Neurol. 1991a;313:486–493. doi: 10.1002/cne.903130309. [DOI] [PubMed] [Google Scholar]
- Gould E, Woolley CS, McEwen BS. Adrenal steroids regulate postnatal development of the rat dentate gyrus. I. Effects of glucocorticoids on cell death. J. Comp. Neurol. 1991b;313:479–485. doi: 10.1002/cne.903130308. [DOI] [PubMed] [Google Scholar]
- Greenough WT, Bailey CH. The anatomy of a memory: convergence of results across a diversity of tests. Trends Neurosci. 1988;11:142–147. [Google Scholar]
- Greenough WT, Withers GS, Wallace CS. Morphological changes in the nervous system arising from behavioral experience: What is the evidence that they are involved in learning and memory. In: Squire LR, Lindenlaub E, editors. The Biology of Memory, Symposia Medica Hoechst. Wiley; New York: 1988. pp. 159–185. [Google Scholar]
- Hansen RL, Struthers JM, Gospe SM., Jr Visual evoked potentials and visual processing in stimulant drug-exposed infants. Dev. Med. Child Neurol. 1993;35:798–805. doi: 10.1111/j.1469-8749.1993.tb11731.x. [DOI] [PubMed] [Google Scholar]
- Jeltsch H, Bertrand F, Lazarus C, Cassel JC. Cognitive performances and locomotor activity following dentate granule cell damage in rats: role of lesion extent and type of memory tested. Neurobiol. Learn. Mem. 2001;76:81–105. doi: 10.1006/nlme.2000.3986. [DOI] [PubMed] [Google Scholar]
- Jonnala RR, Buccafusco JJ. Relationship between the increased cell surface alpha7 nicotinic receptor expression and neuroprotection induced by several nicotinic receptor agonists. J. Neurosci. Res. 2001;66:565–572. doi: 10.1002/jnr.10022. [DOI] [PubMed] [Google Scholar]
- Kenny PJ, File SE, Rattray M. Acute nicotine decreases, and chronic nicotine increases the expression of brain-derived neurotrophic factor mRNA in rat hippocampus. Brain Res. Mol. Brain Res. 2000;85:234–238. doi: 10.1016/s0169-328x(00)00246-1. [DOI] [PubMed] [Google Scholar]
- Kolb B. Prefrontal cortex. In: Kolb B, Tees RC, editors. The Cerebral Cortex of the Rat. MIT Press; Cambridge: 1990. pp. 437–458. [Google Scholar]
- Kolb B, Gorny G, Soderpalm AH, Robinson TE. Environmental complexity has different effects on the structure of neurons in the prefrontal cortex versus the parietal cortex or nucleus accumbens. Synapse. 2003;48:149–153. doi: 10.1002/syn.10196. [DOI] [PubMed] [Google Scholar]
- Little BB, Snell LM, Gilstrap LC., III Methamphetamine abuse during pregnancy: outcome and fetal effects. Obstet. Gynecol. 1988;72:541–544. [PubMed] [Google Scholar]
- Logan BJ, Laverty R, Sanderson WD, Yee YB. Differences between rats and mice in MDMA (methylenedioxymethylamphetamine) neurotoxicity. Eur. J. Pharmacol. 1988;152:227–234. doi: 10.1016/0014-2999(88)90717-0. [DOI] [PubMed] [Google Scholar]
- Magariños AM, McEwen BS. Stress-induced atrophy of apical dendrites of hippocampal CA3c neurons: Comparison of stressors. Neuroscience. 1995a;69:83–88. doi: 10.1016/0306-4522(95)00256-i. [DOI] [PubMed] [Google Scholar]
- Magariños AM, McEwen BS. Stress-induced atrophy of apical dendrites of hippocampal CA3c neurons: Involvement of glucocorticoid secretion and excitatory amino acid receptors. Neuroscience. 1995b;69:89–98. doi: 10.1016/0306-4522(95)00259-l. [DOI] [PubMed] [Google Scholar]
- Morris RGM, Garrud P, Rawlins JNP, O'Keefe J. Place navigation impaired in rats with hippocampal lesions. Nature. 1982;297:681–683. doi: 10.1038/297681a0. [DOI] [PubMed] [Google Scholar]
- Neddens J, Bagorda F, Busche A, Horstmann S, Moll GH, Dawirs RR, Teuchert-Noodt G. Epigenetic factors differentially influence postnatal maturation of serotonin (5-HT) innervation in cerebral cortex of gerbils: interaction of rearing conditions and early methamphetamine challenge. Brain Res. Dev. Brain Res. 2003;146:119–130. doi: 10.1016/j.devbrainres.2003.09.016. [DOI] [PubMed] [Google Scholar]
- Neddens J, Lesting J, Dawirs RR, Teuchert-Noodt G. An early methamphetamine challenge suppresses the maturation of dopamine fibres in the nucleus accumbens of gerbils: on the significance of rearing conditions. J. Neural Transm. 2002;109:141–155. doi: 10.1007/s007020200010. [DOI] [PubMed] [Google Scholar]
- Oro AS, Dixon SD. Perinatal cocaine and methamphetamine exposure: maternal and neonatal correlates. J. Pediatr. 1987;111:571–578. doi: 10.1016/s0022-3476(87)80125-7. [DOI] [PubMed] [Google Scholar]
- Redman RS, Sweney LR. Changes in diet and patterns of feeding activity in developing rats. J. Nutrit. 1976;106:615–626. doi: 10.1093/jn/106.5.615. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Kolb B. Persistent structural modifications in nucleus accumbens and prefrontal cortex neurons produced by previous experience with amphetamine. J. Neurosci. 1997;17:8491–8497. doi: 10.1523/JNEUROSCI.17-21-08491.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson TE, Kolb B. Alterations in the morphology of dendrites and dendritic spines in the nucleus accumbens and prefrontal cortex following repeated treatment with amphetamine or cocaine. Eur. J. Neurosci. 1999;11:1598–1604. doi: 10.1046/j.1460-9568.1999.00576.x. [DOI] [PubMed] [Google Scholar]
- Roullet P, Mele A, Ammassari-Teule M. Ibotenic lesions of the nucleus accumbens promote reactivity to spatial novelty in nonreactive DBA mice: implications for neural mechanisms subserving spatial information encoding. Behav. Neurosci. 1997;111:976–984. doi: 10.1037//0735-7044.111.5.976. [DOI] [PubMed] [Google Scholar]
- Scaccianoce S, Lombardo K, Angelucci L. Nerve growth factor brain concentration and stress: changes depend on type of stressor and age. Intern. J. Dev. Neurosci. 2000;18:469–479. doi: 10.1016/s0736-5748(00)00014-9. [DOI] [PubMed] [Google Scholar]
- Schaaf MJM, de Jong J, de Kloet ER, Vreugdenhil E. Down-regulation of BDNF mRNA and protein in the rat hippocampus by corticosterone. Brain Res. 1998;813:112–120. doi: 10.1016/s0006-8993(98)01010-5. [DOI] [PubMed] [Google Scholar]
- Schaaf MJM, de Kloet ER, Vreugdenhil E. Corticosterone effects on BDNF expression in the hippocampus: Implications for memory formation. Stress. 2000;3:201–208. doi: 10.3109/10253890009001124. [DOI] [PubMed] [Google Scholar]
- Sholl DA. The Organization of the Cerebral Cortex. Methunen; London: 1981. [Google Scholar]
- Smith LM, Chang L, Yonekura ML, Grob C, Osborn D, Ernst T. Brain proton magnetic resonance spectroscopy in children exposed to methamphetamine in utero. Neurology. 2001;57:255–260. doi: 10.1212/wnl.57.2.255. [DOI] [PubMed] [Google Scholar]
- Smith MA, Makino S, Kvetnansky R, Post RM. Stress and glucocorticoids affect the expression of brain-derived neurotrophic factor and neurotrophin-3 mRNAs in the hippocampus. J. Neurosci. 1995;15:1768–1777. doi: 10.1523/JNEUROSCI.15-03-01768.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Struthers JM, Hansen RL. Visual recognition memory in drug-exposed infants. J. Dev. Behav. Pediatr. 1992;13:108–111. doi: 10.1097/00004703-199204000-00005. [DOI] [PubMed] [Google Scholar]
- Thomas DM, Francescutti-Verbeem DM, Liu X, Kuhn DM. Identification of differentially regulated transcripts in mouse striatum following methamphetamine treatment – an oligonucleotide microarray approach. J. Neurochem. 2004;88:380–393. doi: 10.1046/j.1471-4159.2003.02182.x. [DOI] [PubMed] [Google Scholar]
- Vorhees CV, Ahrens KG, Acuff-Smith KD, Schilling MA, Fisher JE. Methamphetamine exposure during early postnatal development in rats. I. Acoustic startle augmentaion and spatial learning deficits. Psychopharmacology. 1994a;114:392–401. doi: 10.1007/BF02249328. [DOI] [PubMed] [Google Scholar]
- Vorhees CV, Ahrens KG, Acuff-Smith KD, Schilling MA, Fisher JE. Methamphetamine exposure during early postnatal development in rats. II. Hypoactivity and altered responses to pharmacological challenge. Psychopharmacology. 1994b;114:402–408. doi: 10.1007/BF02249329. [DOI] [PubMed] [Google Scholar]
- Vorhees CV, Inman-Wood SL, Morford LL, Broening HW, Fukumura M, Moran MS. Adult learning deficits after neonatal exposure to d-methamphetamine: selective effects on spatial navigation and memory. J. Neurosci. 2000;20:4732–4739. doi: 10.1523/JNEUROSCI.20-12-04732.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vorhees CV, Morford LL, Inman SL, Reed TM, Schilling MA, Cappon GD, Moran MS, Nebert DW. Genetic differences in spatial learning between Dark Agouti and Sprague-Dawley strains: possible correlation with the CYP2D2 polymorphism in rats treated neonatally with methamphetamine. Pharmacogenetics. 1999;9:171–181. [PubMed] [Google Scholar]
- Vorhees CV, Reed TM, Schilling MA, Fisher JE, Moran MS, Cappon GD, Nebert DW. CYP2D1 polymorphism in methamphetamine-treated rats: genetic differences in neonatal mortality and effects on spatial learning and acoustic startle. Neurotoxicol. Teratol. 1998;20:265–273. doi: 10.1016/s0892-0362(97)00129-3. [DOI] [PubMed] [Google Scholar]
- Williams MT, Blankenmeyer TL, Schaefer TL, Brown CA, Gudelsky GA, Vorhees CV. Long-term effects of neonatal methamphetamine exposure in rats on spatial learning in the Barnes maze and on cliff avoidance, corticosterone release, and neurotoxicity in adulthood. Dev. Brain Res. 2003;147:163–175. doi: 10.1016/j.devbrainres.2003.11.001. [DOI] [PubMed] [Google Scholar]
- Williams MT, Inman-Wood SL, Morford LL, McCrea AE, Ruttle AM, Moran MS, Rock SL, Vorhees CV. Preweaning treatment with methamphetamine induces increases in both corticosterone and ACTH in rats. Neurotoxicol. Teratol. 2000;22:751–759. doi: 10.1016/s0892-0362(00)00091-x. [DOI] [PubMed] [Google Scholar]
- Williams MT, Moran MS, Vorhees CV. Refining the critical period for methamphetamine-induced spatial deficits in the Morris water maze. Psychopharmacology. 2003a;168:329–338. doi: 10.1007/s00213-003-1433-y. [DOI] [PubMed] [Google Scholar]
- Williams MT, Morford LL, Wood SL, Wallace TL, Fukumura M, Broening HW, Vorhees CV. Developmental d-methamphetamine treatment selectively induces spatial navigation impairments in reference memory in the Morris water maze while sparing working memory. Synapse. 2003b;48:138–148. doi: 10.1002/syn.10159. [DOI] [PubMed] [Google Scholar]
- Williams MT, Vorhees CV, Boon F, Saber AJ, Cain DP. Methamphetamine exposure from postnatal day 11−20 causes impairments in both behavioral strategies and spatial learning in adult rats. Brain Res. 2002;958:312–321. doi: 10.1016/s0006-8993(02)03620-x. [DOI] [PubMed] [Google Scholar]
- Woolley CS, Gould E, McEwen BS. Exposure to excess glucocorticoids alters dendritic morphology of adult hippocampal pyramidal neurons. Brain Res. 1990;531:225–231. doi: 10.1016/0006-8993(90)90778-a. [DOI] [PubMed] [Google Scholar]