Abstract
Examining visual word recognition memory (WRM) with nose-referenced EEGs, we reported a preserved ERP ‘old-new effect’ (enhanced parietal positivity 300–800 ms to correctly-recognized repeated items) in schizophrenia (Kayser et al., 1999). However, patients showed reduced early negative potentials (N1, N2) and poorer WRM. Because group differences in neuronal generator patterns (i.e., sink-source orientation) may be masked by choice of EEG recording reference, the current study combined surface Laplacians and principal components analysis (PCA) to clarify ERP component topography and polarity and to disentangle stimulus- and response-related contributions. To investigate the impact of stimulus modality, 31-channel ERPs were recorded from 20 schizophrenic patients (15 male) and 20 age-, gender-, and handedness-matched healthy adults during parallel visual and auditory continuous WRM tasks. Stimulus- and response-locked reference-free current source densities (spherical splines) were submitted to unrestricted Varimax-PCA to identify and measure neuronal generator patterns underlying ERPs. Poorer (78.2±18.7% vs. 87.8±11.3% correct) and slower (958±226 vs. 773±206 ms) performance in patients was accompanied by reduced stimulus-related left parietal P3 sources (150 ms pre-response) and vertex N2 sinks (both overall and old/new effects) but modality-specific N1 sinks were not significantly reduced. A distinct mid-frontal sink 50-ms post-response was markedly attenuated in patients. Reductions were more robust for auditory stimuli. However, patients showed increased lateral-frontotemporal sinks (T7 maximum) concurrent with auditory P3 sources. Electrophysiologic correlates of WRM deficits in schizophrenia suggest functional impairments of posterior cortex (stimulus representation) and anterior cingulate (stimulus categorization, response monitoring), primarily affecting memory for spoken words.
Keywords: event-related potentials (ERP), recognition memory, old/new effect, auditory/visual modality, schizophrenia, current source density (CSD), principal components analysis (PCA), response monitoring
1. Introduction
Abnormalities of cognitive function are a key component of schizophrenia, compromising working memory and episodic memory processes (e.g., Barch, 2005; Pelletier et al., 2005). It has been hypothesized that receptive language dysfunction in schizophrenia represents a learning disorder that is caused by a core deficit in the temporal dynamics of brain function (Condray, 2005), which may be linked to abnormalities of left hemisphere structure and morphology within the supratemporal plane (i.e., planum temporale; e.g., Barta et al., 1990; Falkai et al., 1995; Kawasaki et al., 2008) and associated left-lateralized processes typically mediating language-related functions (e.g., Flor-Henry, 1969, 1976; Crow, 1990, 1997, 2004b; DeLisi et al., 1997). Moderate impairments of verbal episodic memory and learning have repeatedly been reported in schizophrenia (e.g., Goldberg et al., 1993; Saykin et al., 1991), and these verbal memory deficits appear to be unrelated to medication status or chronicity (e.g., Albus et al., 2006; Hill et al., 2004; Saykin et al., 1994). Although it may be difficult to differentiate verbal memory from a more generalized cognitive dysfunction (Blanchard and Neale, 1994), there is growing evidence that a subgroup of patients with schizophrenia has a selective deficit in verbal working memory (e.g., Bruder et al., 2004; Wexler et al., 1998).
1.1. Temporal lobe abnormalities in schizophrenia
Several studies have reported structural temporal lobe abnormalities in schizophrenia, including superior temporal gyrus and hippocampus (e.g., Arnold et al., 1991; Shenton et al., 1992; Bogerts et al., 1990, 1993; Menon et al., 1995; Pearlson et al., 1997), brain structures critical involved in the formation and retrieval of memory representations (e.g., Damasio, 1989; Smith and Halgren, 1989). Neuroimaging studies have provided evidence linking the verbal memory deficits in schizophrenia to left medial temporal lobe (MTL) structures (e.g., Gur et al., 1994; Mozley et al., 1996; Nestor et al., 2007). It has been suggested that structural abnormalities of the temporal lobe are directly linked to the frequently-reported reductions of event-related brain potentials (ERPs) in schizophrenia, most notably of the late cognitive P3 component (e.g., McCarley et al., 1991, 1993, 2002, 2008; O’Donnell et al., 1993, 1999; Egan et al., 1994; Kawasaki et al., 1997). Although several studies supported the concept that both structural (e.g., Barta et al., 1990; Rossi et al., 1992; Bilder et al., 1994; Faux et al., 1993; Falkai et al., 1995; Vita et al., 1995) and functional abnormalities in schizophrenia as measured by P3 amplitude during target detection (‘oddball’) tasks involve primarily the left side of the brain (e.g., McCarley et al., 1993, 2002; Salisbury et al., 1998; Strik et al., 1994; van der Stelt et al., 2004), other studies failed to find abnormal structural (e.g., Flaum et al., 1995; Kulynych et al., 1996; Weinberger et al., 1991) or electrophysiological asymmetries (e.g., Pfefferbaum et al., 1989; Ford et al., 2000; Kayser et al., 2001; for a review, see Ford, 1999). This inconsistency may be related to the clinical heterogeneity of schizophrenic samples and other methodological issues, which, with regard to the functional abnormalities, include ERP paradigm, stimulus characteristics and modality, response requirements and component definition (e.g., Strik et al., 1994; Ford et al., 2000; Salisbury et al., 2001; Kayser et al., 2001, 2006).
1.2. Electrophysiological correlates of recognition memory
ERP research in schizophrenia relying on simple oddball tasks has generally not used paradigms specifically designed to probe left or right hemispheric functions (but see Kayser et al., 2001; Bruder et al., 2001), or to target linguistic and mnemonic processes. Semantic context and recognition memory have been widely studied in healthy populations with a variety of experimental paradigms (e.g., N400), which are increasingly applied to psychiatric populations (e.g., Kumar and Debruille, 2004). One of the most robust findings in ERP memory research is the old/new effect, a more positive-going potential for previously-studied and correctly-recognized old than new items that begins at about 300 ms post stimulus onset, lasts several hundred milliseconds, and has a left parietal maximum (e.g., reviewed by Johnson, 1995; Allan et al., 1998; Friedman, 2000; Mecklinger, 2000). Whereas this late ERP old/new effect is considered an electrophysiological correlate of explicit memory-retrieval processes (conscious recollection), an earlier mid-frontal old/new effect that peaks around 400 ms is regarded as an index of implicit knowledge that a stimulus event has been previously experienced (item familiarity), suggesting different neural generators of two distinct retrieval processes postulated in dual-process models of recognition memory (e.g., Rugg and Curran, 2007; Yonelinas, 2001). Whereas the early mid-frontal old/new effect appears to primarily originate from lateral regions of the prefrontal cortex, the lateral posterior parietal cortex seems to be the main contributor to the late parietal old/new effect (e.g., Yonelinas et al., 2005; Wagner et al., 2005). However, both old/new effects receive contributions from frontal and parietal regions (Iidaka et al., 2006) and MTL structures (Rugg et al., 1991; Guillem et al., 1995; Wegesin and Nelson, 2000) indicative of a more complex recognition memory network. While these positive-going old/new effects overlap either a positive (parietal P3 or P600) or a negative component (mid-frontal FN400, N400, N2) in visual recognition memory tasks, with both ERP topography and polarity affected by the choice of EEG recording reference (cf. Kayser et al., 2003), the different scalp distributions of N2, P3 and the overlying old/new effects strongly suggest that separable cognitive processes with distinct neuronal generators are superimposed, though volume-conducted, to produce these scalp-recorded ERPs (cf. Johnson et al., 1998; Friedman, 2000; Kayser et al., 2003).
1.3. ERP old/new effects in schizophrenia
A few studies have investigated ERP old/new effects in schizophrenia, the typical behavioral finding being poorer task performance (recognition accuracy and response latency) in patients. In the context of a word detection task (button press response to animal names embedded in a series of unrelated words), Matsumoto et al. (2001) found a markedly reduced nose-referenced positivity in 20 schizophrenic patients to immediately repeated nontarget words (zero lag), peaking around 450 ms, whereas a 5-lag repetition delay revealed a smaller old/new effect and no group difference to targets. These results largely matched the reduced word ERP repetition effects in schizophrenia observed by Matsuoka et al. (1999). This research group also reported that reduced ERP repetition effects for words were more pronounced for schizophrenic patients with more severe formal thought disorder (Matsumoto et al., 2005). Similar reduced immediate ERP word repetition effects were described by Kim et al. (2004) for 14 schizophrenic patients in an explicit continuous recognition task using 21-channel, linked-mastoids recordings, and, in contrast to Matsumoto et al. (2001), a 5-lag repetition delay revealed essentially comparable group differences (i.e., reduced ERP old/new effects). One common problem across these studies concerns the observed ERP waveforms, which revealed a substantially weaker component structure (i.e., N1, N2, and particularly P3) in patients compared to controls, raising questions of comparable signal-to-noise ratios in the two study groups and the need for appropriate component measures that take into account ERP topography as a defining characteristic. Moreover, given the ambiguity as to whether the ERP repetition effect represents an electrophysiologic correlate of non-conscious memory retrieval processes (Friedman, 2000), and evidence that repetition priming effects – unlike recognition memory old/new effects – do not require intact MTL structures (Rugg et al., 1991), the findings for immediate item repetition, while interesting, provide limited electrophysiological evidence of impaired episodic memory in schizophrenia.
Recording 9-channel ERPs with a linked-mastoids reference during a study-test paired-associate word learning task, Baving et al. (2000) found reduced old/new effects for 16 schizophrenic patients compared with 16 healthy controls, which overlapped a P2 followed by a positive slow wave (SW), and larger P2 old/new effects correlated with greater recognition accuracy in controls. Although patients performed more poorly, there were no group differences in overall amplitudes of P2 and positive SW, and old/new effects in controls were rather small. Using a study-test word recognition task combined with a Remember/Know procedure to disentangle recollection- and familiarity-based retrieval processes, Tendolkar et al. (2002) reported left-parietal and right-frontal recollection (Remember) old/new effects for both 14 schizophrenic patients and 14 healthy controls, but these effects extended over a longer time interval in controls. Although more robust Remember old/new effects were evident for both groups at mid-centroparietal sites, ERP analysis was restricted to four medial-lateral electrode pairs despite the use of 28-channel EEG montage with an average reference. In contrast, familiarity (Know) old/new effects were observed for patients over frontal sites beyond 500 ms, but for controls over temporoparietal sites between 500 and 800 ms, leaving it unclear whether these condition differences indeed reflect early mid-frontal old/new effects (FN400) linked to item familiarity (e.g., Curran, 1999; Curran and Cleary, 2003).
Guillem et al. (2001) studied ERP old/new effects in 15 schizophrenia patients and 15 healthy controls during a continuous recognition memory paradigm with unfamiliar faces under implicit (indicate the gender) and explicit (item previously presented) task instructions. Using a 13-channel EEG montage referenced to the right ear lobe, reduced old/new effects were reported in patients over medial parietal sites for the implicit task at about 300 ms, overlapping a relative negative ERP deflection (N300), but not at about 500 ms, overlapping a late positive complex (P500). During the explicit task, patients performed substantially poorer than controls (but nevertheless clearly above chance) and showed reduced old/new effects over posterior sites during a prolonged late positive complex at about 500 ms (N500), but not at 700 ms (P700). In the same task, however, patients had an enhanced late old/new effect over frontal sites, rendering a complex and difficult to interpret pattern of old/new effects.
In a prior study, we recorded nose-referenced 30-channel ERPs during a visual continuous word recognition paradigm in 24 schizophrenic patients and 19 healthy controls (Kayser et al., 1999). Although patients showed poorer accuracy of word recognition, they had the same ERP old-new effect as controls (i.e., greater late positivity between 400–700 ms at medial and parietal sites) overlapping a late positive complex of comparable amplitude in both groups. Patients did, however, show a striking reduction of earlier negative potentials (N1, N2), and the amplitude of N2 and the N2-P3 complex to words was greater over left than right inferior temporal-parietal sites in controls but not patients. Notably, these ERP measures correlated with performance accuracy in either group, except for the N2-P3 asymmetry in patients, suggesting that the poorer performance of schizophrenic patients in the word recognition memory task stems at least in part from a deficit in a left-lateralized system involved in phonological processing, comparable to that seen in dyslexia (Salmelin et al., 1996).
In summary, the findings of these recognition memory studies in schizophrenia suggest largely preserved late ERP old/effects overlapping the late positive complex, presumably associated with explicit retrieval or conscious recollection of items, whereas earlier old/new effects may be compromised. Likewise, the late cognitive components observed during these recognition memory tasks, notably those showing high topographic similarity to a classical P3b with a mid-parietal maximum, appear unimpaired in schizophrenia, whereas earlier potentials (N1, P2, N2) are reduced in amplitude. However, profound differences between these studies in almost all methodological aspects, particularly ERP component identification, definition and analysis, as well as choice of EEG recording reference, severely limits the ability to draw general conclusions from these findings.
1.4. Reference-free ERP components by means of CSD/PCA methodology
Two persistent issues in ERP research are the dependency of surface potentials on a reference location (e.g., linked-mastoids, ear lobe, nose, average, sternum) and the definition and measurement of appropriate ERP components (e.g., specific time windows for peak or integral amplitudes), which crucially affect component interpretation (e.g., polarity, topography, generator) and statistical analysis (e.g., Kayser and Tenke, 2003, 2005; Nunez and Srinivasan, 2006). Although these issues are generally known and disseminated through textbooks (e.g., Nunez, 1981; Luck, 2005) and other seminal publications (e.g., Picton et al., 2000), the implications for applied electrophysiologic research have as of yet not been fully appreciated by the field at large. For example, the visual appearance of a limited set of surface potential waveforms may change dramatically if referenced to mastoid (ear lobe) or nose (e.g., see visual condition in Fig. 8 of Kayser et al., 2007, depicting the shift and polarity inversion of a nose-referenced N1/N2 complex from inferior-lateral sites to mid-centroparietal sites when using a linked-mastoids reference), which may also influence which electrode sites are selected for statistical analysis. A particular bias is introduced with an asymmetric (unilateral) reference, which has the inherent capacity to modulate (i.e., amplify or decrease) existing hemisphere asymmetries. Whereas some researchers are aware of these problems (e.g., Baving et al., 2000, note the absence of negative ERP deflections in their data as a consequence of activity at the averaged-mastoids reference), others seem to erroneously assume that ERP condition or group effects observed at a particular recording sites reflect neuronal activity of the underlying brain regions. However, depending on the orientation of the underlying equivalent current generator responsible for a particular ERP deflection, which may differ between experimental groups or conditions, the (arbitrary) reference choice may mask existing effects if the reference region is itself differentially affected through volume-conducted activity by the experimental manipulations (e.g., Nunez and Westdorp, 1994). This indeterminancy is not resolved by using an average reference, which has the additional caveat of being different for different EEG montages and, due to practical limits of sampling from the ventral side of the brain, is biased toward the montage center or pole, which is typically the vertex (e.g., Dien, 1998; Junghöfer et al., 1999).
Related problems concern: 1) how to best quantify ERP effects (i.e., group and/or condition differences) in multichannel surface potentials with or without “obvious” and “meaningful” waveform peaks, some of which may easily go unnoticed in grand mean averages given the overwhelming temporal and spatial complexity; 2) how to avoid experimenter bias is selecting time intervals and recording sites for statistical analysis; and 3) how to ensure statistical independency of the analyzed effects (cf. Kayser and Tenke, 2003, 2005). All studies reviewed in the previous section differ in this regard, for instance, ranging from baseline-to-peak amplitude measures within certain time intervals (Baving et al., 2000), including individual peak identification used to determine individual time windows for amplitude integrals (Guillem et al., 2001) and fixed, sequential time window amplitudes at a priori determined sites covering regions-of-interest (Tendolkar et al., 2002), to data-driven ERP components measures derived from temporal principal components analysis (PCA; Kayser et al., 1999). It is not unreasonable to suspect that these different analytic approaches may themselves have led to different results and conclusions when applied to the same data.
We have proposed a generic analytic strategy for multi-channel ERP recordings that can overcome these limitations: first, convert reference-dependent surface potentials into reference-free current source density (CSD; surface Laplacian) waveforms representing the radial current flow into (sources) and out of (sinks) the scalp (any EEG reference will yield the same, unique CSD waveforms, with reduced volume-conducted contributions and sharper topographies than ERPs), and second, identify unique and orthogonal variance patterns in these reference-free data by means of temporal, unrestricted Varimax-PCA using the covariance matrix (Kayser and Tenke, 2006a, 2006b;for details on surface Laplacian estimates, see Tenke and Kayser, 2005; for detailed arguments regarding unrestricted factor extraction/rotation and preferability for covariance- over correlation-based factor loadings, see Kayser and Tenke, 2005, 2006c). Apart from the theoretical advantages of this CSD-PCA approach over traditional ERP analytic methods, a systematic comparison between ERP-PCA and CSD-PCA solutions has provided empirical evidence that no ERP information is distorted or lost after eliminating ambiguities stemming from the recording reference; instead, experimental effects were clarified and additional insights emerged when utilizing CSD as a bridge between montage-dependent scalp potentials and their underlying current generators (Kayser and Tenke, 2006a). CSD compared to ERP analysis offers better spatial and temporal resolution without the need for prior assumptions about generator sources (e.g., tissue conductivity and geometry, laminar orientation, number and independence of generators), which is in striking contrast to EEG source localization methods (Michel et al., 2004).
Despite a common belief that a high-density EEG montage (i.e., more than 100 channels) is required to reliably compute CSD estimates, given previous findings highlighting problems with spatial aliasing for the interpretation of undersampled CSD estimates (Junghöfer et al., 1997; Srinivasan et al., 1998), low-density CSD estimates (i.e., 31 channels) can not only improve ERP data analysis and interpretation, but are also surprisingly accurate and reliable for a group of subjects. Because averaging individual CSD topographies across subjects effectively applies a spatial low pass filter to the data, the resulting spatially-smoothed CSD group topographies are sufficiently represented by fewer recording sites, revealing effectively identical estimates at these sites when compared with CSDs obtained from high-density (i.e., 129-channel) ERPs (Kayser and Tenke, 2006b). Therefore, when group findings are the primary research objective, low-resolution CSD topographies can be as efficient as their high-density counterparts, and have been useful in studying long-lasting ERP components during working memory in schizophrenia (Kayser et al., 2006), disentangling overlapping P3 generators in depression (Tenke et al., 2008), and clarifying stimulus- and response-related neuronal generator contributions to ERP old/new effects during auditory and visual word recognition memory (Kayser et al., 2007).
1.5. Performance monitoring in schizophrenia
It has been suggested that “willed” intentions are not correctly monitored in schizophrenia patients, that the discrepancy between will and action gives rise to positive symptoms, and that the effectiveness of dopamine receptor antagonists in reducing positive symptoms is directly linked to the induced Parkinsonism (Frith, 1987). There is growing evidence of impaired self- or performance-monitoring in schizophrenia (e.g., Ullsperger, 2006), including electrophysiologic findings of reduced error negativity (Ne; e.g., Falkenstein et al., 2000) or error-related negativity (ERN; e.g., Gehring et al., 1993), a mid-frontal negativity peaking about 100 ms after initiating an erroneous response. Several studies have reported markedly smaller ERN amplitudes in schizophrenia (e.g., Kopp and Rist, 1999; Mathalon et al., 2002; Alain et al., 2002b; Bates et al., 2002; Morris et al., 2006), with paranoid patients and those with more positive symptoms having the most prominent reductions (Kopp and Rist, 1999; Mathalon et al., 2002; Bates et al., 2002). There is some evidence that reduced ERN is modulated by clinical state in schizophrenia, because ERN amplitude increased after successful treatment with atypical antipsychotics (Bates et al., 2004). However, the observed ERN impairments were nonetheless rather robust and suggestive of a trait deficit in schizophrenia. At the same time, other response-related ERP components, such as the correct response negativity (CRN) or the ensuing positivity to errors (Pe) and correct responses (Pc), appear to be unaffected in schizophrenia or yielded inconsistent results (Alain et al., 2002b; Mathalon et al., 2002; Bates et al., 2002, 2004; Kim et al., 2006).
Given converging evidence stemming from studies using functional magnetic resonance (fMRI; e.g., Kiehl et al., 2000), ERP source localization techniques (e.g., Dehaene et al., 1994), combined electrophysiologic, magnetoencephalographic and MRI data (e.g., Miltner et al., 2003), concurrent EEG and fMRI data (e.g., Debener et al., 2005) and intracranial EEG recordings in monkeys (e.g., Emeric et al., 2008), it is widely assumed that the ERN is generated within the anterior cingulate cortex (ACC; for reviews, e.g., van Veen and Carter, 2002, 2006). Because imaging data have documented structural (i.e., reduced volume; Baiano et al., 2007) and functional ACC abnormalities in schizophrenia patients (e.g., Carter et al., 2001; Polli et al., 2008), their reduced ERN amplitudes may originate from an underlying ACC dysfunction (e.g., Mathalon et al., 2002).
The ERN belongs to a family of ERP components that are characterized by a distinct medial-frontal negativity (FCz maximum), including CRN (e.g., Vidal et al., 2000) and the feedback-related negativity (FRN; e.g., Donkers et al., 2005; Hajcak et al., 2006), which is not directly linked to a response. Interestingly, Morris et al. (2008) reported reduced amplitudes of both ERN and feedback negativity (FBN) in schizophrenia. Using temporal CSD-PCA methodology, we have repeatedly observed a CSD component exhibiting high topographic similarity with this group of ERP components. Its underlying neuronal generator pattern consisted of a focal mid-frontal sink (Fz−) accompanied by bilateral centroparietal sources (CP+) and was evident in healthy adults during stimulus-locked auditory oddball ERPs, partially overlapping but distinct from a classical late P3b, and peaking at or around the time when subjects pressed a response button or silently updated a target count (Kayser and Tenke, 2006a, 2006b; Tenke et al., 1998, 2008). This highly-distinct sink-source pattern was also present for response-locked ERPs of correct trials recorded during auditory and visual recognition memory tasks (Kayser et al., 2007), revealing inverse old/new effects consistent with bilateral activation of anterior cingulate and supplementary motor area (orthogonal to the medial cortical surface within the longitudinal fissure, with opposite orientations in the two hemispheres). Given that these overlapping old/new effects imply motivational and/or self-monitoring processes (e.g., Luu et al., 2000), this distinct response-related, mid-frontal negativity in schizophrenic patients is also of interest in the context of a word recognition memory paradigm.
1.6. The present study
A repeated notion is that P3 abnormalities in schizophrenia are more robust and common with tasks using auditory than visual stimuli (e.g., Pfefferbaum et al., 1989; Egan et al., 1994; Ford et al., 1994; Jeon and Polich, 2003), which may be linked to a higher incidence of auditory than visual hallucinations (Ford, 1999), the close association between language and phonological representations (Crow, 2004a), or to systemic differences in processing visual or auditory information, particularly considering the neurophysiological aspects of temporal integration for sounds. When discussing the absence of a reduced old/new effect in schizophrenia during a visual word recognition memory task, we proposed to investigate whether the old/new effect is impaired in schizophrenia during an auditory word recognition memory task (Kayser et al., 1999). As ERP old/new effects have rarely been studied in the auditory modality, we developed and compared closely-matched auditory and visual continuous word recognition memory tasks in healthy adults in a within-subjects paradigm (Kayser et al., 2003, 2007). These studies revealed highly comparable old/new effects for both modalities despite prominent differences in scalp topography and peak latency of auditory and visual N2 and P3 amplitudes, supporting the view that word retrieval is a common, high-level cognitive process associated with old/new effects (cf. Johansson and Mecklinger, 2003) that are largely independent from, but superimposed on, the modality-specific ERP component structure. Taking full advantage of the CSD-PCA approach, we described left parietal old/new source effects accompanied by lateral frontocentral old/new sink effects in both modalities, which overlapped modality-specific P3 sources at about 160 ms before the response (Kayser et al., 2007).
The main purpose of the present study was to compare these reference-independent visual and auditory old/new effects in schizophrenia patients with those for a carefully-matched sample of healthy adults. A second aim was to clarify polarity, topography, and underlying current generators of distinct ERP components (N1, N2, P3) previously observed during the visual version of this continuous recognition memory task, and examine whether the marked amplitude and asymmetry reductions of early negativities (N1, N2) in schizophrenia would be reproducible in the auditory modality. The final goal was to draw on our previous findings of a distinct mid-frontal sink 45 ms postresponse of presumably anterior cingulate origin, and use this CSD component to explore self-monitoring deficits in schizophrenia during correct responses in a word recognition memory paradigm.
2. Material and Methods
2.1. Participants
Twenty-three inpatients and two outpatients (20 male, 5 female) at New York State Psychiatric Institute were recruited for the study, excluding left-handed individuals and those with a history of neurological illness or substance abuse. Five male patients did not provide sufficient correct, artifact-free trials for stable ERP waveforms (more than 15 for new or old items) and were removed from the study. The patients included in the final patient sample met DSM-IV (American Psychiatric Association, 1994) criteria for schizophrenia (paranoid, n = 11; undifferentiated, n = 4), schizoaffective disorder (bipolar type, n = 3), or psychosis not otherwise specified (n = 2).1 Diagnoses were based on clinical interviews by psychiatrists and a semistructured interview (Nurnberger et al., 1994) including items from commonly-used instruments (e.g., SCID-P, Spitzer et al., 1990; SANS, SAPS, Andreasen 1983, 1984). Symptom ratings were obtained using the Positive and Negative Syndrome Scale (PANSS; Kay et al., 1992). The total BPRS score indicated that patients were mildly-to-moderately disturbed (Table 1). Most patients (n = 11) did not receive antipsychotic medications for at least 14 days before testing. Nine patients were treated with risperidone (n = 3), clozapine (n = 2), olanzapine (n = 2), aripriprazole (n = 1), or fluphenazine (n = 1).
Table 1.
Means, Standard Deviations (SD), and Ranges for Demographic and Clinical Variables
| Patients (n = 20, 15 male) | Healthy Controls (n = 20, 15 male) | |||||
|---|---|---|---|---|---|---|
| Variable | Mean | SD | Range | Mean | SD | Range |
| Age (years) | 29 | 7.7 | 19 – 44 | 28.9 | 6.4 | 20 – 42 |
| Education (years) | 13.4a | 2.2 | 9 – 16 | 16.1 | 2.1 | 12 – 20 |
| Handedness (LQ) b | 79.8 | 30.8 | 0 – 100 | 74.9 | 24.8 | 0 – 100 |
| Verbal IQ (WAIS) | 102.4c | 10.9 | 87 – 123 | |||
| Onset age (years) | 21.1 | 6.1 | 7 – 35 | |||
| Illness duration (years) | 6.2d | 9.7 | 0 – 37 | |||
| Total BPRS | 32.8d | 7.9 | 22 – 48 | |||
| PANSS general | 28.4d | 7.2 | 16 – 38 | |||
| PANSS positive | 14.3d | 5.9 | 7 – 26 | |||
| PANSS negative | 12.3d | 5.5 | 7 – 27 | |||
Patients differ significantly from healthy controls (F[1,38] = 16.7, p < .001).
Laterality quotient (Oldfield, 1971) can vary between −100.0 (completely left-handed) and +100.0 (completely right-handed).
n = 7.
n = 19.
Patients were compared to 20 healthy volunteers (15 men, 5 women) selected from a larger sample (N = 40) included in our previous report (Kayser et al., 2007). Control participants, who had been recruited from the New York metropolitan area and paid US$15/hr, were without a history of neurological illness or substance abuse and without current or past psychopathology based on a standard screening interview (SCID-NP; First, Spitzer et al., 1996). Importantly, patient and control participants had been tested under the same protocol and during the same time period. Without knowledge about their behavioral performance or ERP data, healthy adults were carefully matched to individual patients with regard to gender, age, and handedness (all participants were right-handed except for one ambidextral individual in each group; Table 1). Whereas patients had significantly less education than control participants, the available verbal IQ data (WAIS) suggested that the patients’ verbal skills were well within normal range.
All participants had normal or corrected-to-normal vision. Hearing acuity was assessed using standard audiometric procedures, requiring all participants to have an ear difference of less than 10 dB and a hearing loss no greater than 25 dB at 500, 1000, or 2000 Hz. The ethnic composition in both groups was representative for the New York region, with an approximately equal number of participants in each racial category (patients vs. controls: White 8/9, Black 6/5, Asian 1/1, Native-American 1/1, more than one race 1/2, unknown 3/2). Five participants in each group had also been included in a different working memory ERP study (Kayser et al., 2006). The experimental protocol had been approved by the institutional review board and was undertaken with the understanding and written consent of each participant.
2.2. Stimuli and procedure
As the study’s procedural protocol has already been described in detail (Kayser et al., 2007), largely repeating the auditory and visual continuous word recognition memory paradigm used previously (Kayser et al., 2003), a brief summary will suffice for this report. During the serial presentation of words, participants indicated for each word whether it was new (never presented in the series) or old (presented previously) by pressing one of two buttons on a response pad. Words were 320 English nouns selected from the MRC Psycholinguistic database (Coltheart, 1981), which were presented as auditory or visual stimuli and arranged in four separate block sequences (114 trials each, 456 trials total, two auditory and two visual blocks or sequences). Ratings for word frequency (Kucera and Francis, 1967) and concreteness (Paivio et al., 1968) were balanced across blocks. There were no item repetitions either within or between modalities, except for the new-old item pairs. Item sequence and modality assignments were counterbalanced across participants.
For each block, the item sequence consisted of 34 words that repeated once after either a short or a long lag (8 or 24 intervening items; n = 17 each; pseudo-randomized order), and 46 filler words that did not repeat. Items that were to be repeated were considered new items at the first presentation, and old items at the second presentation, and these repeated items formed the basis for the subsequent data analysis to compare “true” memory effects that are largely independent of the physical and connotational differences between stimuli. In contrast, never-repeated words were considered filler items and not included in the data analysis.
Auditory word items (484 ms median duration; range 311 to 830 ms) synthesized for a male voice (Lucent Technologies, 2001) were presented binaurally through headphones at a comfortable listening level of about 72 dB SPL. Visual word items (500 ms duration) were foveally presented in black on a light gray background on a CRT computer monitor (0.95° vertical angle; 3.3 – 8.7° horizontal angle). A constant 2.5 s stimulus onset asynchrony and a fixation cross to minimize eye movements was used for both modalities. Participants were instructed to respond to every stimulus as quickly and accurately as possible and that there would be no overlap between blocks for word repetitions. Responses were accepted from 200 ms post-stimulus onset until the next stimulus onset (2500 ms). Response hand assignment (i.e., left/right button press for old/new responses) was systematically alternated across blocks but balanced within participants across modalities.
2.3. Data acquisition, recording, and artifact procedures
Nose-referenced scalp EEG (AFz ground) was continuously recorded at 200 samples/s from 30 extended 10–20-system locations (4 midline and 13 lateral pairs of tin electrodes embedded in a Lycra stretch cap) within .1–30 Hz (−6dB/octave), along with bipolar recordings of vertical and horizontal eye movements (for complete montage and recording details, see Kayser et al., 2007). Volume-conducted blink artifacts were effectively removed from the raw EEG by means of spatial PCA generated from identified blinks and artifact-free EEG periods (NeuroScan, 2003).
Recording epochs of 2,000 ms (including a 300 ms pre-stimulus baseline) were extracted off-line from the blink-corrected continuous data, tagged for A/D saturation, and low-pass filtered at 20 Hz (−24 dB/octave). To maximize the number of artifact-free epochs, volume-conducted horizontal eye movements, which were systematically prompted in the visual condition (i.e., reading the word stimuli), were reduced by computing the linear regressions between the horizontal EOG and the EEG differences of homologous lateral recording sites (i.e., Fp2 - Fp1, F8 - F7, etc.) for each epoch, and the correlated eye activity was then removed by applying ±beta weight/2 to each lateral EEG signal (cf. Kayser et al., 2006). Residual artifacts due to amplifier drift, muscle or movement-related activity, or residual eye activity were identified on a channel-by-channel and trial-by-trial basis by employing a reference-free electrical distance measure (Kayser and Tenke, 2006d). Artifactual surface potentials were replaced by spherical spline interpolation (Perrin et al., 1989) using the data from artifact-free channels if eight or less channels were affected; otherwise, a trial was rejected. Artifact detection and electrode replacement was verified by visual inspection.
Stimulus-locked ERP waveforms were averaged from correct, artifact-free trials using the entire 2-s epoch. The mean number of trials used to compute new and old ERP averages after pooling across lag (M ± SD, min-max range, controls vs. patients) were 56.1 ± 7.3 (37 – 67) vs. 49.0 ± 11.5 (29 – 67) for auditory, and 54.3 ± 8.4 (33 – 65) vs. 50.5 ± 13.4 (19 – 68) for visual stimuli. Whereas about the same number of trials entered into new ERP averages for controls and patients (56.9 ± 7.0 vs. 56.9 ± 7.5), owing to their better performance, controls had more old trials than patients (53.5 ± 8.4 vs. 42.8 ± 12.3; group × condition interaction, F[1,38] = 12.9, p = .0009), but the number of old trials were nonetheless sufficient in each group. Furthermore, a satisfactory signal-to-noise ratio for each condition was confirmed by visual inspections of the individual ERP waveforms of each participant. ERP waveforms were screened for electrolyte bridges (Tenke and Kayser, 2001), low-pass filtered at 12.5 Hz (−24 dB/octave), and baseline-corrected using the 100 ms preceding stimulus onset.
Response-locked ERP waveforms were averaged from 1-s sub-epochs including 700 ms before and 300 ms after the recorded response and applying the stimulus-locked baseline correction. Although trials with responses faster than 400 ms or slower than 1,400 ms had to be excluded when computing response-locked ERPs, because of insufficient sample points at the beginning or end of the sub-epochs, the number of remaining trials was still sufficient in each group (92.3% for controls and 85.7% for patients of stimulus-locked trials).
2.4. Current Source Density (CSD) and Principal Components Analysis (PCA)
Averaged ERP waveforms were transformed into current source density (CSD) estimates (μV/cm2 units) using a spherical spline surface Laplacian (Perrin et al., 1989) as detailed elsewhere (e.g., Kayser and Tenke, 2006a; Kayser et al., 2007). To determine common sources of variance in these reference-free transformations of the original ERP data, CSD waveforms were submitted to temporal principal components analysis (PCA) derived from the covariance matrix, followed by unrestricted Varimax rotation of the covariance loadings. However, only a limited number of meaningful, high-variance CSD factors are retained for further statistical analysis (for complete rationale, see Kayser and Tenke, 2003, 2005 for complete rationale, see Kayser and Tenke, 2006a, 2006c). By virtue of the reference-independent Laplacian transform, CSD factors have an unambiguous component polarity and topography.
Auditory and visual stimulus-locked CSD waveforms (400 sample points spanning the time interval from −300 to 1,695 ms around stimulus-onset) and response-locked CSD waveforms (201 sample points spanning the time interval from −700 to 300 ms around response-onset) were submitted to four separate temporal PCA to better account for the modality-dependent ERP/CSD component structure and to isolate their stimulus and response contributions (MatLab emulation of BMDP-4M algorithms; cf. appendix of Kayser and Tenke, 2003). Hence, input data matrices consisted of either 400 or 201 variables and 2,480 observations stemming from 40 participants, 2 conditions (new/old pooled across short and long lags) and 31 electrode sites, including the nose.
Additional temporal PCAs were computed for combined stimulus- and response-locked CSD waveforms (cf. Kayser et al., 2007). The extracted factors and the ensuing statistical analysis were entirely consistent with the extracted factors and statistical results stemming from the separated CSD waveforms. However, because one primary objective was to contrast stimulus- and response-locked old/new effects, the findings for the combined analysis are not detailed in this report.
2.5. Statistical analysis
Factor scores of meaningful PCA factors for each modality were submitted to repeated measures ANOVA with condition (old, new) as a within-subjects factor and group (controls, patients) as a between-subjects factor. Because lag emerged as an insufficient manipulation of task difficulty and did not differ between groups, it was not included as an independent variable for electrophysiologic data. Gender was also omitted as a design factor given the imbalance of men and women in each group (3:1 ratio), rendering very small cell sizes for females (n = 5). The ANOVA designs included one or more recording sites at which PCA factor scores were largest and most representative of the associated CSD components (cf. Kayser et al., 2006; Kayser and Tenke, 2006a). Subsets of recording sites consisted of either midline sites or lateral, homologous recording sites over both hemispheres, in which case either site, or site and hemisphere were added as within-subjects factors to the design. However, because recording sites were selected on the premise that they collectively represent sink or source activity targeted in these statistical analyses, site effects were of secondary interest.
Greenhouse-Geisser epsilon (ε) correction was used to compensate for violations of sphericity when appropriate (e.g., Keselman 1998). Simple effects (BMDP-4V; Dixon 1992) provided means to systematically examine interaction sources, or to further explore group effects even in the absence of superordinate interactions. A conventional significance level (p < .05) was applied for all effects.
For analyses of the behavioral data, response latency (mean response time of correct responses) and percentages of correct responses were submitted to repeated measures ANOVA with condition (old, new), lag (short, long), and modality (visual, auditory) as within-subjects factors and group (controls, patients) as the between-subjects factor. As in our previous word recognition memory studies, the d′-like sensitivity measure dL (logistic distribution; cf. footnote 5 in Kayser et al., 1999) was calculated from hit and false alarm rates (Snodgrass and Corwin, 1988) and submitted to a similar ANOVA without the condition factor.
3. Results
3.1. Behavioral data
Table 2 summarizes the behavioral performance for both groups and modalities. Both patients and controls distinguished old from new items well above chance for both modalities, as can be seen from both the correct responses for old items and the sensitivity measure (dL). Still, patients’ accuracy was significantly poorer compared to controls, and this performance impairment was not affected by modality. Patients had also about 200-ms longer response latencies than controls, but this overall group difference did not interact with modality or condition. However, as expected, mean response latency was about 200–300 ms longer for auditory than visual stimuli, and this modality effect interacted with condition.
Table 2.
Behavioral Data Summary: Grand Means (SD) and ANOVA F Ratios
| Correct Responses [%] |
Sensitivity [dL] |
Latency [ms] |
|||||
|---|---|---|---|---|---|---|---|
| Modality | Group | New | Old | New | Old | ||
| Auditory | Controls | 90.3 (9.3) | 86.2 (12.4) | 4.53 (1.26) | 920 (180) | 883 (177) | |
| Patients | 89.1 (9.0) | 66.4 (15.1) | 3.13 (1.41) | 1106 (178) | 1111 (142) | ||
| Visual | Controls | 91.5 (8.9) | 83.3 (12.4) | 4.41 (1.27) | 619 (136) | 668 (141) | |
| Patients | 93.5 (6.2) | 63.7 (19.1) | 3.49 (1.35) | 743 (166) | 873 (166) | ||
| Effect a | F | p | F | p | F | p | |
| Group | 21.8 | < .0001 | 15.5 | .001 | 17.4 | .002 | |
| Condition b | 47.1 | < .0001 | - | - | 4.11 | < .05 | |
| Condition × Group b | 18.3 | .0001 | - | - | |||
| Modality | 366.5 | < .0001 | |||||
| Modality × Condition b | 4.86 | .03 | - | - | 76.8 | < .0001 | |
For all effects, df = 1, 38. Only F ratios with p < .10 are reported.
Not applicable to dL sensitivity measure.
Lag showed only small and inconsistent effects on behavioral measures of accuracy, sensitivity and response latency, and did not appear to corroborate the intended manipulation of task difficulty. Thus, these effects will not be further detailed for sake of brevity. Most importantly, lag failed to interact with group in any performance measure. As a consequence, electrophysiologic measures were pooled across lag to increase their signal-to-noise ratio and to reduce the complexity of the design (cf. Kayser et al., 2003, 2007).
3.2. Electrophysiologic data
3.2.1. Stimulus-locked ERP and CSD waveforms
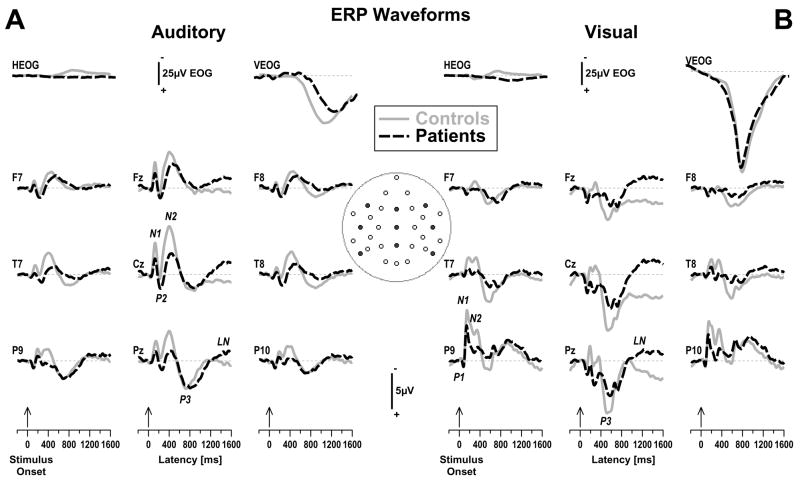
Auditory and visual nose-referenced, grand mean surface potentials locked to stimulus onset (averaged across condition) are shown in Figure 1 for patients and controls at nine selected sites, along with the bipolar eye activity traces. Whereas horizontal and vertical eye movements differed across modalities, these eye movements were largely comparable across groups. Furthermore, blink activity was effectively removed by the spatial blink filter applied to the continuous data and did not differentially affect the ERPs of either group. The overall ERP deflections were highly comparable to our prior studies (Kayser et al., 1999, 2003). For the auditory task, distinct ERP deflections were identified as N1, P2, and N2, particularly over central sites (e.g., see Cz in Figure 1A), and as P3 and a late negativity (LN) over posterior sites (e.g., see site P3 in Figure 1A). For the visual task, distinct ERP deflections were identified as P1, N1, and N2, particularly over inferior-parietal sites (e.g., see P9 in Figure 1B), and as P3 and LN over posterior sites (e.g., see Pz in Figure 1B). As can be seen, peak latencies and regional maxima of individual ERP deflections varied according to modality. They were, however, comparable across groups, despite patients showing notable reductions in most of these prominent peaks.2
Figure 1.
Nose-referenced, stimulus-locked (−200 to 1600 ms) grand average event-related surface potential (ERP) [μV] waveforms (100 ms pre-stimulus baseline) for auditory (A) and visual (B) stimuli (averaged across old and new items) comparing 20 patients (dashed black lines) and 20 healthy controls (solid gray lines) at selected lateral (F7/8, T7/8, P9/10) and midline (Fz, Cz, Pz) recording sites (indicated by black dots in inset). Horizontal and vertical electrooculograms (EOG) are shown at a smaller scale before blink correction. Distinct ERP components are labeled for auditory stimuli at Cz (N1, P2, N2), for visual stimuli at P9 (P1, N1, N2), and for both modalities at Pz (P3, LN).
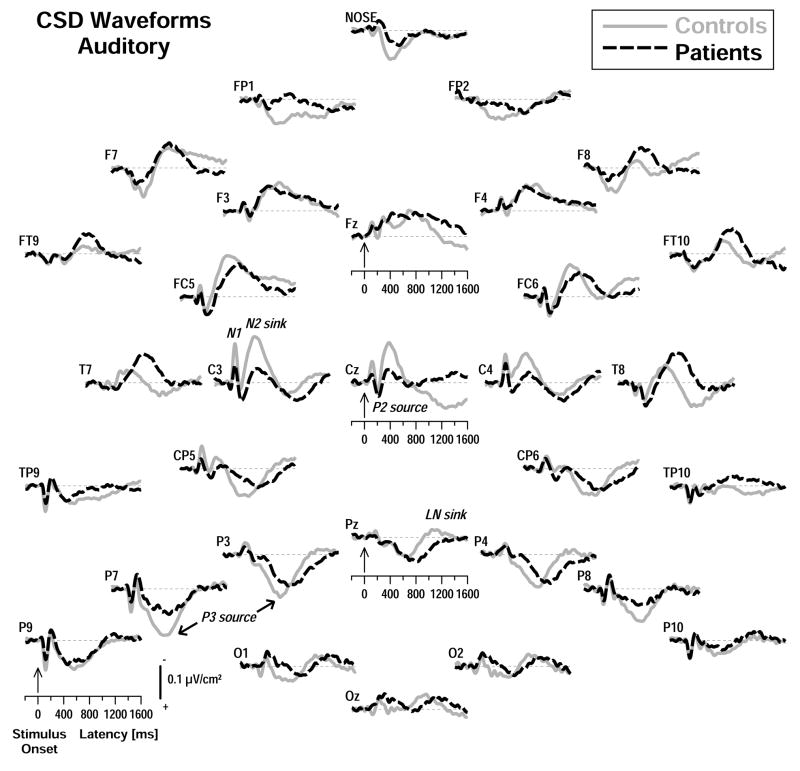
The CSD transformations of these ERP waveforms, which eliminate reference-dependent ambiguities, are given in Figures 2 and 3, showing sink (negative) and source (positive) activity collapsed across new and old stimuli for controls and patients at each recording site.3 Distinct stimulus-locked auditory CSD components (Figure 2) included central N1 and N2 sinks (approximate peak latencies 120 and 420 ms at C3 for controls) and a central P2 source (200 ms at Cz). Lateral-parietal P3 sources (685 ms at P3, P7) were accompanied by lateral-frontal sinks (700 ms at F7, F8). Whereas early central sink activity (N1, N2) and the late lateral-posterior P3 source appeared markedly reduced in patients, the lateral-frontal sink activity was not, or was even more robust at lateral-temporal sites (e.g., see T7, T8).
Figure 2.
Stimulus-locked, reference-free current source density (CSD) [μV/cm2] waveforms for auditory stimuli (averaged across old and new items) comparing 20 patients and 20 controls at all 31 sites (lines as in Figure 1). Distinct CSD components included central N1 and N2 sinks (approximate peak latencies 120 and 420 ms at C3 for controls), central P2 (200 ms at Cz) and lateral-posterior P3 sources (620 ms at P7).
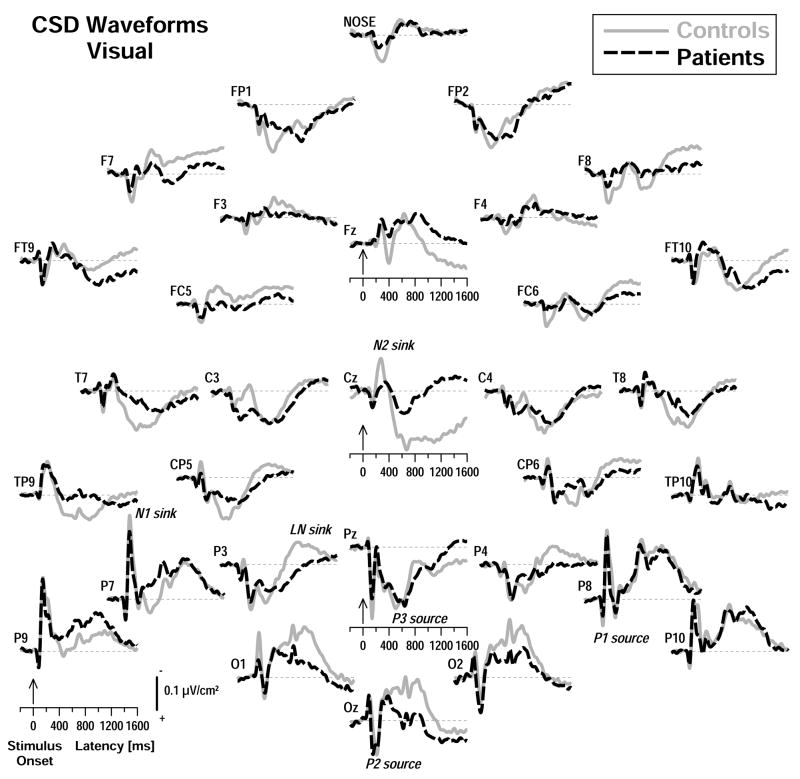
Figure 3.
Stimulus-locked CSD waveforms as in Figure 2 for visual stimuli. Distinct CSD components included inferior lateral-parietal N1 sinks (approximate peak latency 145 ms at P7 for controls), occipital P2 sources (210 ms at Oz), a central N2 sink (270 ms at Cz), and mid-parietal P3 sources (500 ms at Pz).
Prominent stimulus-locked visual CSD components (Figure 3) included inferior lateral-parietal P1 sources (approximate peak latency 80 ms at P8 in controls) and N1 sinks (145 ms at P7), followed by an occipital P2 source (210 ms at Oz), a central N2 sink (270 ms at Cz), and mid-parietal P3 sources (500 ms at Pz). In contrast to nose-referenced ERPs, which showed a visual N2 maximum over the left inferior-parietal region, the reference-free CSDs revealed a vertex maximum for a visual N2 sink, indicating that the true origin of the underlying N2 generator is substantially masked by volume-conduction when using a nose reference. Like its ERP counterpart, however, the vertex N2 sink was severely diminished in patients compared with controls, and earlier N1 sink activity was also reduced in patients over left occipital-parietal sites (at O1, P7). Furthermore, an extended late source at Cz in controls that followed the N2 sink was almost absent in patients. In contrast, P3 source amplitude was comparable in controls and patients at site P3 where it was greatest.
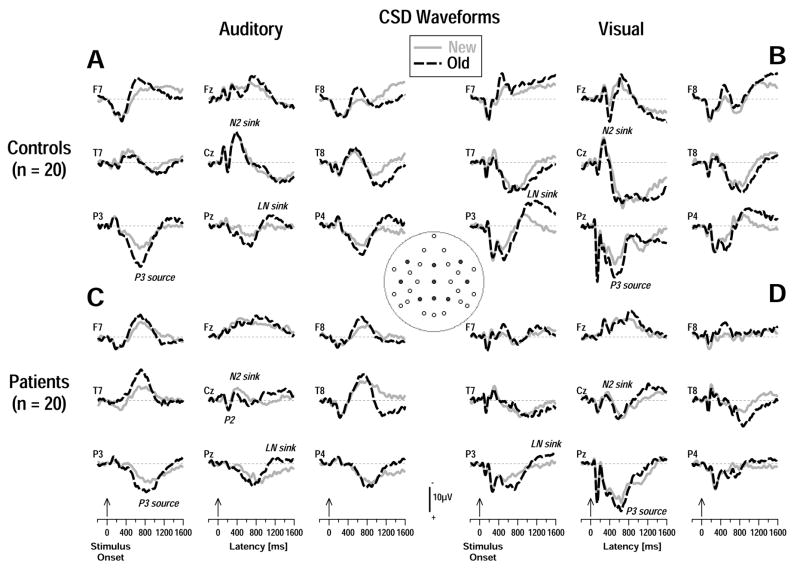
The corresponding auditory and visual old/new effects overlapping the stimulus-locked CSD component structure are depicted in Figure 4 at nine representative sites for controls and patients. Across groups and tasks, increased parietal P3 sources (at P3, Pz, P4) were accompanied by increased lateral-frontal sinks (at F7, F8) for old compared to new stimuli, although these effects were somewhat weaker in patients. Interestingly, for spoken words, patients showed increased lateral-temporal sinks (at T7, T8; Figure 4C) for old than new items that coincided with the frontal-parietal sink-source pattern associated with P3 amplitude. A posterior late negativity (LN) that peaked at or around response onset showed inverted old/new effects across modalities and groups.
Figure 4.
Stimulus-locked CSD waveforms from controls (A, B) and patients (C, D) for auditory (A, C) and visual (B, D) stimuli comparing old (dashed black lines) and new (solid gray lines) stimuli at selected lateral (F7/8, T7/8, P3/4) and midline (Fz, Cz, Pz) sites (indicated by black dots in inset). Increased medial- and mid-parietal P3 sources (P3/4, Pz) and lateral-frontal sinks (F7/8) were seen for old compared to new auditory and visual stimuli in both groups. These old/new effects, however, were generally smaller in patients.
3.2.2. Stimulus-locked PCA component waveforms and topographies
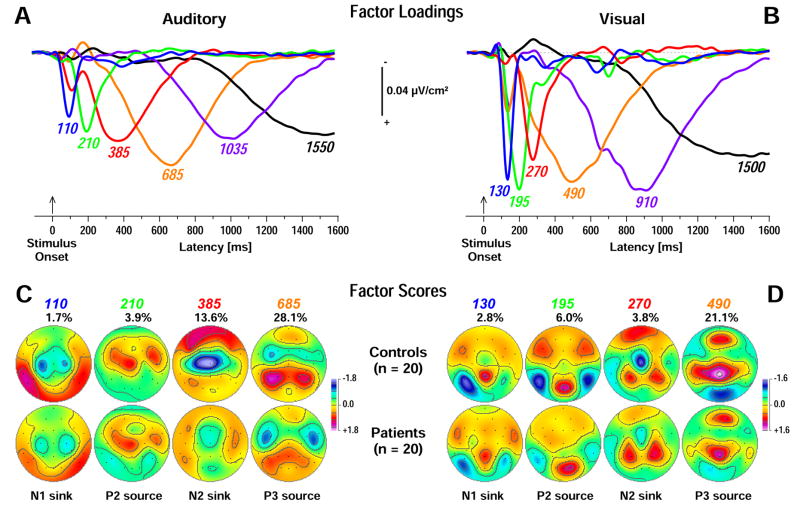
Figure 5 shows the time courses of factor loadings for the first six CSD factors extracted for each modality (both more than 85% explained variance after rotation) and the corresponding topographies for factors peaking before response onset. Labels were chosen to indicate the peak latency of the factor loadings relative to stimulus onset, along with brief functional interpretations of factors given a signature topography. However, these identifying labels refer nevertheless to an entire CSD factor, which consists of characteristic time courses and topographies.
Figure 5.
Unrestricted PCA solutions using auditory (A, C) or visual (B, D) stimulus-locked CSD waveforms. A, B: Time courses of Varimax-rotated covariance loadings for the first six CSD factors extracted for auditory (86.5% total variance explained) or visual (85.2%) stimuli. Labels indicate the peak latency of the factor loadings relative to stimulus onset. C, D: Corresponding factor score topographies (nose at top) with percentage of explained variance for the earliest four factors in each PCA solution (peak latency < 900 ms) corresponding to N1 and N2 sinks and P2 and P3 sources for each modality, separately plotted for controls (top row) and patients (bottom row). The same symmetric scale was used for all topographic maps within a modality.
As the present CSD-PCA solutions were entirely consistent with those of the combined stimulus-and response-locked analyses for the larger sample of healthy adults (Kayser et al., 2007), the stimulus-locked analysis focused on factors summarizing stimulus-driven, modality-specific CSD activity preceding the response.4 Auditory CSD factors corresponded to N1 sink (peak latency 110 ms; left mid-central maximum; 1.7% explained variance), P2 source (210 ms; frontocentral maximum; 3.9%), N2 sink (385 ms; vertex maximum; 13.6%), and P3 source (685 ms; lateral-parietal sources paired with lateral-frontal sinks; 28.1%; Figure 4C). Similarly, visual CSD factors corresponded to N1 sink (130 ms; left lateral inferior-parietal maximum; 2.8%), P2 source (195 ms; occipital maximum with lateral-parietal sinks; 6.0%), N2 sink (270 ms; mid-frontocentral maximum with medial-parietal sources; 3.8%), and P3 source (490 ms; mid-parietal and frontopolar sources paired with lateral-frontal sinks; 21.1%; Figure 5D).
3.2.3. Stimulus-locked repeated measures ANOVA
Table 3 summarizes the primary statistics obtained for stimulus-locked auditory and visual CSD-PCA factors at targeted regions (i.e., where sink or source activity was most prominent). There were no significant effects involving group or condition for either N1 sink (auditory or visual). The only significant N1 sink finding was a hemisphere main effect for visual stimuli confirming a left-greater-than-right N1 sink across groups (cf. factor 130 in Figure 5D). However, a simple hemisphere effect for the visual N1 sink was observed for controls, F(1, 38) = 7.12, p = .01, but not for patients, F(1, 38) < 1.0, ns.
Table 3.
Summary of F Ratios from Repeated Measures ANOVA Performed on Stimulus-locked CSD-PCA Factors at Selected Sites.
| A | Auditory Factor (Sites) |
|||||
|---|---|---|---|---|---|---|
|
110 N1 sink |
210 P2 source |
385 N2 sink |
685 P3 source |
|||
| Variable | (C3/4) | (FC5/6, C3/4) | (Cz) | (C3/4) | (P3/4, P7/8, CP5/6) | (FC5/6, F7/8, FT9/10, T7/8) |
| G | 9.79 ** | 27.0 **** | 19.5 **** | 6.25 * | ||
| C | 4.62 * | 26.7 **** | 42.0 **** | |||
| C × G | 13.2 *** | |||||
| H | 10.4 ** | - | 11.6 ** | 10.3 ** | ||
| H × G | - | 3.72 | ||||
| H × C × G | - | 3.13 | 5.41 * | |||
| B | Visual Factor (Sites) |
|||||
|
130 N1 sink |
195 P2 source |
270 N2 sink |
490 P3 source |
|||
| Variable | (P7/8, P9/10, O1/2) | (O1/2) | (Cz) | (P3/4, P7/8, CP5/6) | (Cz, Pz) | |
| G | 6.94 ** | 11.0 ** | 3.17 | |||
| C | 6.81 ** | 27.9 **** | ||||
| C × G | 5.22 * | |||||
| H | 4.90 * | 9.47 ** | - | 12.7 ** | - | |
| H × G | - | 3.49 | - | |||
| H × C × G | - | - | ||||
Note. G = Group (patients, controls); C = condition (new, old); H = hemisphere (left, right). Only F ratios with p < .10 are reported for effects pooled over site (subsets as indicated; for all tabled effects, df = 1, 38; see supplementary material for complete ANOVA summary).
Effect not applicable.
p ≤ .05.
p ≤ .01.
p ≤ .001,
p ≤ .0001.
Likewise, there were no significant effects involving group or condition for the auditory or visual P2 source, only significant hemisphere main effects for both modalities. An overall left-greater-than-right auditory P2 source at lateral frontocentral sites was comparable in both groups (cf. factor 210 in Figure 5C; simple hemisphere effects for controls, F(1, 38) = 6.68, p = .01; for patients, F(1, 38) = 3.91, p = .05). For visual stimuli, there was a significant right-greater-than-left P2 source at lateral occipital sites, which was largely due to a hemispheric asymmetry in patients, F(1, 38) = 9.86, p = .003, but not in controls, F(1, 38) = 1.47, p = .23 (cf. factor 195 in Figure 5D).5 Thus, the statistical analyses performed on early stimulus-locked CSD factors (N1 sink, P2 source) did not reveal prominent group or condition differences.
In contrast, robust group and condition differences were found for late stimulus-locked CSD factors (N2 sink, P3 source). Across modalities, the vertex N2 sink was significantly reduced in patients compared to controls (cf. factors 385 and 270 in Figures 5C and 5D; Table 3). The vertex N2 sink was also greater for old than new visual stimuli.6 Likewise, the analysis for the auditory N2 sink at medial central sites yielded a strong group difference, an old-greater-than-new condition main effect (M ± SD, old = −0.76 ± 1.04, new = −0.62 ± 0.98), and a left-greater-than-right hemisphere main effect (cf. factor 385 in Figure 5C; Table 3A). Although there was only a marginally significant group × hemisphere interaction, a significant simple hemisphere main effect was observed for controls, F(1, 38) = 14.2, p = .0006 (M ± SD, C3 = −1.57 ± 0.78, C4 = −0.90 ± 1.00), but not patients, F(1, 38) = 1.09, p = .30 (M ± SD, C3 = −0.24 ± 0.76, C4 = −0.05 ± 0.68).
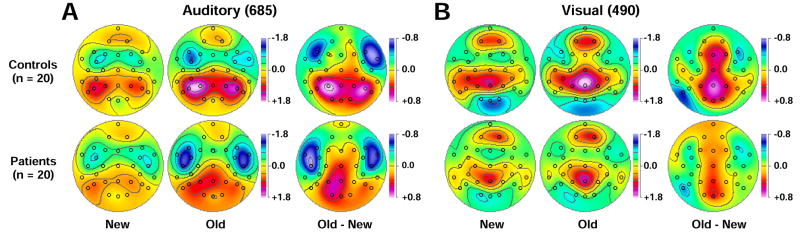
Robust overall auditory old/new effects were also observed for P3 source over lateral parietal sites (Figure 6A; Table 3A). However, a significant group × condition interaction revealed that the old-greater-than-new P3 source was only significant in controls, F(1, 38) = 38.8, p < .0001, but not in patients, F(1, 38) = 1.18, p = .28. Moreover, patients had overall reduced auditory P3 source compared to controls. Although auditory P3 source was overall strongly left-lateralized, a significant simple hemisphere effect was only observed for controls, F(1, 38) = 9.94, p = .003, but not patients, F(1, 38) = 1.92, p = .17. Thus, patients showed markedly reduced and less asymmetric old/new effects for auditory P3 source.
Figure 6.
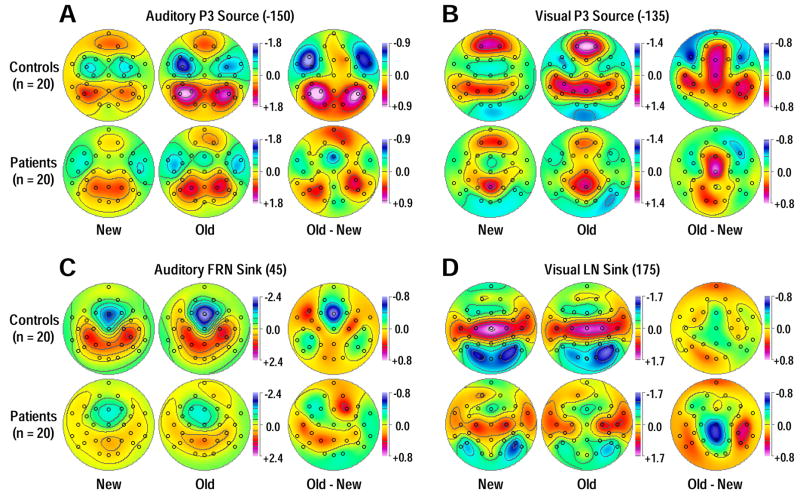
Mean topographies of CSD factor scores for PCA components corresponding to auditory (A; factor 685) and visual (B; factor 490) stimulus-locked P3 source. Topographies are shown for new and old stimuli and their respective old-minus-new difference for 20 controls (top) and 20 patients (bottom). Circles indicate the spherical positions of the 31-channel EEG montage (nose at top). All maps are 2D-representations of spherical spline surface interpolations (Perrin et al., 1989) derived from the mean factors scores available for each recording site.
Similarly, robust overall visual old/new effects for P3 source were observed over mid-centroparietal sites (Figure 6B; Table 3B). Although old-greater-than-new P3 source was again more robust in controls, F(1, 38) = 23.4, p < .0001, than patients, F(1, 38) = 6.96, p = .01, there was no significant group × condition interaction.7 Likewise, patients showed only marginal reductions in overall visual P3 source compared to controls at these midline sites. In contrast, the analysis for the lateral parietal extension (P3/4, P7/8, CP5/6) of visual P3 source revealed a marked amplitude reduction for patients compared with controls, along with a significant group × condition interaction stemming from an old-greater-than-new P3 source in controls, F(1, 38) = 5.70, p = .02, but not in patients, F(1, 38) < 1.0, ns. Analogous to the auditory modality, the visual P3 source was greater over the left than right hemisphere, and a significant simple hemisphere effect was again observed for controls only, F(1, 38) = 14.8, p = .0004, but not for patients, F(1, 38) = 1.45, p = .24, providing the basis for a marginally significant group × hemisphere interaction. An additional analysis for the frontopolar source of factor 490 using sites Fp1/2 (Figure 5D) revealed only a significant right-larger-than-left hemisphere main effect, F(1, 38) = 5.72, p = .02, but no group or condition effects. Thus, at off-midline parietal sites, patients showed also markedly reduced and less asymmetric old/new effects for visual P3 source.
Finally, the prominent inverted old/new effects for the associated sink activity at lateral-frontotemporal sites (FC5/6, F7/8, FT9/10, T7/8) for auditory P3 source were targeted in an additional analysis (Figure 6A; Table 3A), which confirmed the presence of this condition effect across groups. There was also a group main effect stemming from increased sinks in patients compared with controls, but a group × site interaction (see supplementary material) and simple group effects at each site revealed that this group difference was significant at lateral-temporal sites only (at T7/8, F(1, 38) = 14.3, p = .0005; at FT9/10, F7/8, FC5/6, all F(1, 38) ≤ 2.61, all p ≥ .11). A significant three-way group × condition × hemisphere interaction originated from greater inverted old/new effects over right than left sites for controls, F(1, 38) = 4.44, p = .04, whereas patients had no asymmetric condition effects, F(1, 38) = 1.40, p = .24 (cf. old-minus-new difference maps in Figure 6A). Thus, inverted lateral-frontotemporal old/new effects for stimulus-locked auditory P3 source were present in both groups but most robust in patients over the left lateral-temporal region.
3.2.4. Response-locked CSD waveforms and topographies
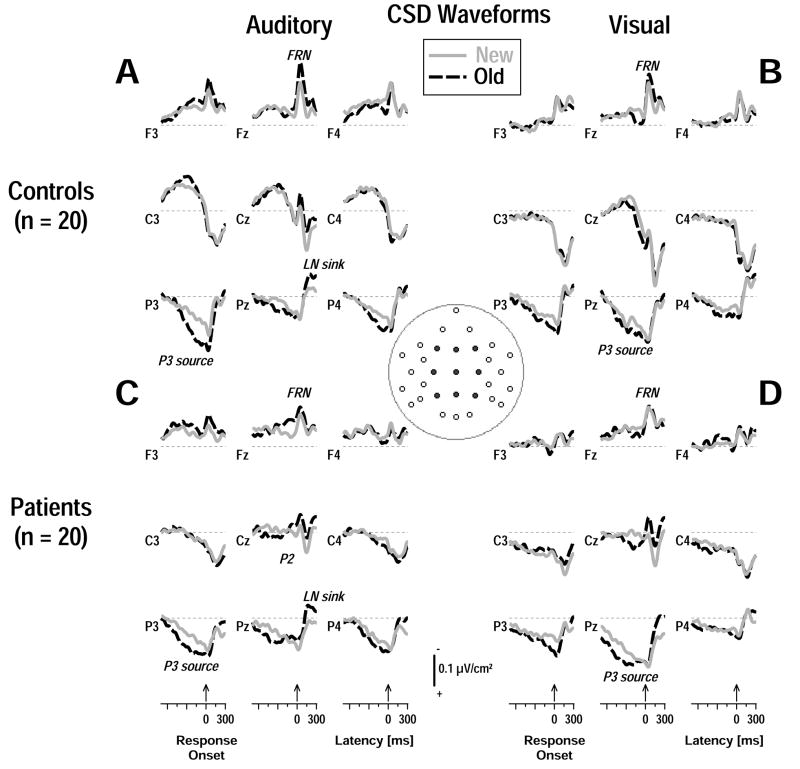
Figure 7 gives the reference-free CSD transformations of the response-locked auditory and visual ERP waveforms at a representative subset of recording sites for both groups and conditions. Across modality, a mid-frontal response-related negativity (FRN), peaking at approximately 50 ms after response onset, was prominent for controls (Figure 7AB) but markedly reduced in patients (Figure 7CD). The FRN was preceded in both groups by distinct old/new effects overlapping the P3 source at medial-parietal (auditory) and mid-parietal (visual) sites, which were larger over the left than right hemisphere, particularly for auditory stimuli (cf. sites P3 and P4 in Figure 7AC). The P3 source was evidently terminated by the response, giving rise to a late negativity (LN) sink over posterior sites, which appeared to be more robust for old than new words.
Figure 7.
Response-locked CSD waveforms (−700 to 300 ms, 100 ms baseline preceding stimulus onset) at selected midline (Fz, Cz, Pz) and adjacent medial (F3/4, C3/4, P3/4) sites (indicated by black dots in inset) comparing new and old items for each group and modality. A distinct mid-frontal response-related negativity (FRN) terminated the preceding P3 source in all conditions, giving rise to a late inverted old/new effect (LN sink) over mid-posterior sites. However, the focal Fz sink was markedly reduced in patients.
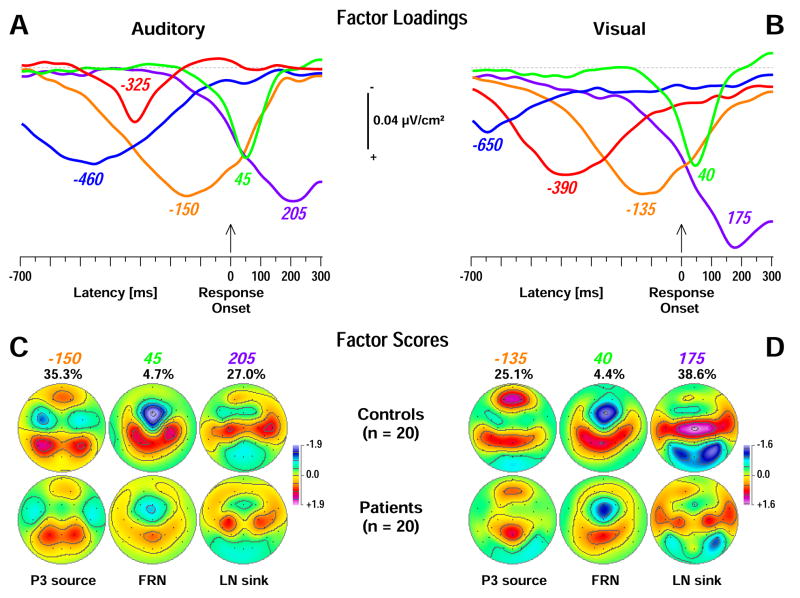
The time courses of factor loadings for the first five response-locked CSD factors extracted for each modality (both more than 90% explained variance after rotation) and corresponding factor score topographies of primary interest are given in Figure 8. Labels were chosen to indicate the peak latency of the factor loadings relative to response onset. In each modality, three CSD factors corresponded to the P3 source immediately preceding the response (auditory peak latency −150 ms; lateral-parietal sources paired with lateral-frontal sinks; 35.3% explained variance; visual −135 ms; mid-parietal and frontopolar sources; 25.1%), the FRN (auditory 45 ms; 4.7%; visual 40 ms; 4.4%; Fz sink maxima with centroparietal sources) and the LN sink (auditory 205 ms; 27.0%; visual 175 ms; 38.6%; occipital sinks with centrotemporal sources) following the response. In each modality, two earlier factors gathered variance evidently associated with stimulus-related activity.
Figure 8.
Unrestricted PCA solutions using auditory (A, C) or visual (B, D) response-locked CSD waveforms. A, B: Time courses of Varimax-rotated covariance loadings for the first five CSD factors extracted for auditory (90.7% total variance explained) or visual (92.6%) stimuli. Labels indicate the peak latency of the factor loadings relative to response onset. C, D: Corresponding factor score topographies (nose at top) with percentage of explained variance for the three factors in each PCA solution corresponding to P3 source (peak latencies −150 and −135 ms), FRN (45 and 40 ms), and LN sink (205 and 175 ms) for each modality, separately plotted for controls (top row) and patients (bottom row). The same symmetric scale was used for all topographic maps within a modality.
3.2.5. Response-locked repeated measures ANOVA
Table 4 summarizes the primary statistics obtained for response-locked auditory and visual CSD-PCA factors at targeted regions (i.e., where sink or source activity was most prominent). As for the stimulus-locked data, robust auditory old/new P3 source effects were observed over lateral parietal sites, and patients had overall reduced auditory P3 source compared to controls (Figure 9A; cf. factor −150 in Table 4A). There was only a marginal group × condition interaction, and significant old/new effects for auditory P3 source were present in controls, F(1, 38) = 32.2, p < .0001, and patients, F(1, 38) = 9.81, p = .003. Moreover, the left-greater-than-right hemisphere asymmetry of the response-locked auditory P3 source was weaker than its stimulus-locked counterpart, although a simple hemisphere main effect was still present for controls, F(1, 38) = 6.15, p = .02.
Table 4.
Summary of F Ratios from Repeated Measures ANOVA Performed on Response-locked CSD-PCA Factors at Selected Sites.
| A | Auditory Factor (Sites) |
||||||
|---|---|---|---|---|---|---|---|
| −150 P3 source |
45 FRN |
205 LN sink |
|||||
| Variable | (P3/4, P7/8, CP5/6) | (FC5/6, F7/8, FT9/10, T7/8) | (Fz) | (CP5/6, P3/4) | (O1/2) | (C3/4, T7/8) | |
| G | 4.34 * | 9.16 ** | 14.3 *** | ||||
| C | 38.8 **** | 16.1 *** | 3.58 | 3.72 | 21.2 **** | ||
| C × G | 3.22 | 6.84 ** | 7.26 ** | ||||
| H | 3.04 | - | |||||
| H × G | 3.11 | - | |||||
| H × C | 2.85 | - | 7.05 ** | ||||
| H × C × G | 3.41 | - | 5.37 * | ||||
| B | Visual Factor (Sites) |
||||||
| −135 P3 source |
40 FRN |
175 LN sink |
|||||
| Variable | (P3/4, P7/8, CP5/6) | (Cz, Pz) | (Fz) | (CP5/6, P3/4) | (O1/2) | (C3/4, T7/8) | (Cz, Pz) |
| G | 10.0 ** | 6.62 ** | 10.8 ** | 5.97 * | 13.4 *** | ||
| C | 4.70 * | 12.6 *** | 6.50 ** | 10.4 ** | 46.0 **** | ||
| C × G | 2.95 | 11.6 ** | |||||
| H | 15.4 *** | - | - | ||||
| H × C | - | - | 5.44 * | 5.82 * | |||
Note. G = Group (patients, controls); C = condition (new, old); H = hemisphere (left, right). Only F ratios with p < .10 are reported for effects pooled over site (subsets as indicated; for all tabled effects, df = 1, 38; see supplementary material for complete ANOVA summary).
Effect not applicable.
p ≤ .05.
p ≤ .01.
p ≤ .001,
p ≤ .0001.
Figure 9.
Mean topographies of CSD-PCA components corresponding to auditory (A; factor −150) and visual (B; factor −135) response-locked P3 source, auditory FRN (C; factor 45) and visual LN sink (D; factor 175). As in Figure 6, topographies are separately shown for controls and patients and for new and old stimuli with their respective old-minus-new difference.
Likewise, old/new effects for visual P3 source effects were observed over mid-centroparietal sites (Figure 9B; cf. factor −135 in Table 4B) for both controls, F(1, 38) = 8.66, p = .006, and patients, F(1, 38) = 4.28, p = .046, but there were no significant effects involving group. In contrast, and similar to the stimulus-locked analysis, visual P3 source was markedly reduced in patients compared with controls at lateral-parietal sites. The overall old-greater-than-new P3 source at these sites was modulated by a marginal group × condition interaction due to a significant simple condition effect in controls, F(1, 38) = 7.55, p = .009, but not patients, F(1, 38) < 1.0, ns. Visual P3 source was again left-lateralized but the hemispheric asymmetry did not interact with group. Thus, the response-locked findings for auditory and visual P3 source are in close agreement with their stimulus-locked counterparts.
As for the inverted auditory old/new sink effects, the analysis at lateral-frontotemporal sites (Figure 9A; cf. factor −150 in Table 4A) revealed an overall condition main effect and a group × condition interaction stemming from old-greater-than-new sinks in controls, F(1, 38) = 22.0, p < .0001, but not patients, F(1, 38) < 1.0, ns. In contrast to the stimulus-locked analysis, there were no increased sinks in patients compared with controls. Thus, the response-locked findings for inverted lateral-frontotemporal old/new effects for auditory P3 source differ from their stimulus-locked counterparts in that patients did not show increased lateral-frontotemporal sinks for old items seen in controls.
The auditory response-related midfrontal sink (FRN) was significantly reduced in patients compared with controls (Figure 9C; cf. factor 45 in Table 4A). A group × condition interaction confirmed that an old-greater-than-new sink was present in controls, F(1, 38) = 10.5, p = .003, but not patients, F(1, 38) < 1.0, ns. The corresponding centroparietal source was also smaller in patients compared to controls but there were no condition effects at these sites. In contrast, the visual FRN revealed only an overall significant old/new effect (cf. factor 40 in Table 4B) stemming from greater sinks to old than new stimuli. The corresponding centroparietal source, which also revealed an overall old/new effect originating from old-greater-than-new sources (M ± SD, old = 0.78 ± 0.85, new = 0.54 ± 0.70), was again reduced in patients (cf. factor 40 in Figure 8D). Thus, a response-related midfrontal sink and centroparietal source activity was markedly reduced in patients, particularly for auditory stimuli.
There were no significant group or condition effects for the auditory late occipital negativity (LN sink; cf. factor 205 in Figure 8C; Table 4A). However, the corresponding centrotemporal source was greater for old than new stimuli (M ± SD, old = 0.90 ± 1.04, new = 0.59 ± 0.89). In contrast, the visual LN sink (cf. factor 175 in Figure 8D; Table 4B) was reduced in patients compared with controls, and so was the corresponding centrotemporal source. Despite these reductions, inverted old/new effects at mid-centroparietal sites (Figure 9D) were more robust in patients, F(1, 38) = 51.9, p < .0001, than in controls, F(1, 38) = 5.70, p = .02, resulting in a significant group × condition interaction (cf. factor 175 in Table 4B). Thus, although their response-locked visual LN sink was markedly reduced, patients showed, unlike controls, a prominent old/new effect in this component.
4. Discussion
In agreement with prior evidence of impaired verbal learning and memory in schizophrenia (e.g., Saykin et al., 1991; Gur et al., 1994; Mozley et al., 1996; Baving et al., 2000; Tendolkar et al., 2002; Barch, 2005), patients having schizophrenia or schizophrenia spectrum disorders showed poorer word recognition memory and increased response latencies than healthy adults during visual and auditory tasks. The extent of this deficit was comparable to that previously seen for schizophrenic patients for a visual version of the continuous recognition memory task (Kayser et al., 1999), and equal in size for auditory stimuli (cf. Kayser et al., 2003). Still, patients’ performance was adequate and well above chance for both modalities, rendering mere task disengagement an unlikely source for the observed electrophysiologic abnormalities.
Benefitting from the improved spatial resolution and reference-independence of CSD-transformed ERPs, the present study confirmed a largely preserved visual old/new effect in schizophrenia over mid-parietal sites (cf. Kayser et al., 1999), but found marked old/new source reductions over lateral parietal regions for both visual and auditory tasks. Schizophrenic patients also lacked the left-greater-than-right hemisphere asymmetry observed for healthy adults, the typical topographical finding for the late old/new effect (e.g., Johnson, 1995; Allan et al., 1998; Friedman and Johnson, 2000). Most importantly, impairments of these electrophysiologic correlates of conscious memory retrieval were more robust for auditory word recognition, suggesting a particular deficit in encoding and/or retrieval of phonological information. A completely new finding is that the abnormalities of prominent CSD/ERP effects were followed by marked reductions in a distinct, response-related mid-frontal negativity, which is likely associated with performance monitoring (e.g., Luu et al., 2000; Ullsperger, 2006; Botvinick, 2007). These reductions were also most prominent for auditory stimuli.
4.1. Left parietal old/new effects and temporal lobe abnormalities in schizophrenia
Both healthy adults and schizophrenic patients showed late parietal old/new effects that overlapped distinct auditory and visual P3 sources, which may reflect conscious experience of item retrieval (e.g., Friedman and Johnson, 2000; Rugg and Curran, 2007). This is entirely consistent with other neuroimaging evidence implicating old/new effects for the lateral posterior parietal cortex (e.g., Wagner et al., 2005). However, there were several topographical abnormalities associated with this parietal old/new effect in schizophrenia. First, whereas old/new source effects were fairly preserved over posterior midline sites, particularly in the visual modality, more lateral old/new sources were more severely reduced in patients. This was more obvious for the lateral-parietal auditory source pattern but nevertheless also present for visual stimuli. Second, these findinge were bolstered by the failure of patients to show the typical left-lateralized asymmetry of this parietal old/new effect, matching our prior data (Kayser et al., 1999). These abnormalities of parietal old/new source effects in this continuous recognition memory paradigm, as well as reduced overall P3 source amplitude and asymmetry, are not merely the result of slower or more variable responses in schizophrenia because the response-locked data indicated comparable, but somewhat weaker, reductions over left lateral parietal sites.
These results are in striking agreement with the reduced left inferior parietotemporal P3 source found during visual word encoding in schizophrenia (Kayser et al., 2006). In their meta-analysis of P3 asymmetry in schizophrenia for auditory oddball data, Jeon and Polich (2001) reported no reliable effect sizes for lateral temporal (T7/8) scalp locations, but instead for homologous sites located more posteriorly, towards the medial-parietal regions (referred to as TCP1/2), which more closely matches the present results. It is generally well-known that multiple generators contribute to the scalp recorded P3 potential, and although task-specific requirements, including stimulus modality and response mode, critically affect and modulate its topography, temporal-parietal activity is considered a main source contributing to stimulus-driven P3b (e.g., Picton, 1992; Halgren et al., 1995a, 1995b; Molnar, 1994; Brazdil et al., 2003; Polich, 2007). Tenke et al. (2008) recently supplemented the current CSD-PCA approach with a hemispatial PCA to disentangle parietal and temporal neuronal generators as contributors to P3 source activity during a dichotic oddball task. Interestingly, by tackling the recording reference conundrum of auditory oddball data with CSD transformations and spatial PCA, Turetsky et al. (1998a, 1998b) identified a reduction of a left temporal P3 source subcomponent as a unifying ERP feature among various schizophrenic subgroups.
The current study adds to this more differentiated picture of P3 generator patterns by clearly identifying the contributions of lateral frontocentral sinks to the late parietal old/new source effects (cf. Kayser et al., 2007). These sinks were broader and asymmetrically enhanced in schizophrenic patients during the auditory task, predominantly affecting the left lateral temporal recording site (T7), reminiscent of left hemisphere overactivation in schizophrenia during verbal processing (cf. Gur 1978; Ragland et al., 2005). Although the implications of this unexpected finding remain unclear, it is obvious that the neuronal generator patterns underlying the (left) parietal old/new effect (i.e., the relative orientation of the lateral frontocentral sinks compared to the parietal sources) differed between patients and controls, a group difference with profound implications for ERP studies relying on referenced surface potentials. Given that the generator patterns of the old/new effects resembled the pattern for auditory P3 but differed from that for visual P3, one may contemplate whether this pattern reflects phonological processing common to both modalities (e.g., Price, 2000), and therefore indicates disturbed temporal integration in schizophrenia within a recognition memory network involving frontal and parietal regions (e.g., Kim et al., 2003; Iidaka et al. 2006).
The importance of the lateral parietal cortex for memory processes, particularly successful retrieval, has been rediscovered by functional neuroimaging findings, which more frequently implicate lateral parietal cortex in recognition memory than other brain areas generally recognized as key components for memory, including temporal lobe regions (Simons and Mayes, 2008). Researchers, however, are puzzled by the exact meaning of this contribution because lesion studies indicate that the lateral parietal cortex, while activated during conscious item recollection, is not necessary for successful retrieval (Cabeza, 2008; Simons et al., 2008). If parietal ERP old/new effects are the electrophysiologic correlate of the subjective experience of recollection (Ally et al., 2008), preserved old/new effects in schizophrenia for correct trials may come as less of a surprise. On the other hand, Cabeza (2008) has proposed that the contributions of dorsal and ventral regions of the parietal cortex can be dissociated on the basis of top-down (dorsal) and bottom-up (ventral) attentional processes. Following this distinction, our findings of largely intact late old/new source effects over mid-parietal sites but weakened old/new source effects over inferior lateral parietal sites in schizophrenia may be indicative of a largely preserved goal-directed effort to retrieve memory information as opposed to a compromised retrieval output.
We should note that a midfrontal extension of the parietal old/new effect was present in the visual but not auditory modality (cf. discussion in Kayser et al., 2007). To the extent that this positive-going old/new difference reflects the midfrontal FN400 effect linked to item familiarity (e.g., Rugg and Curran, 2007), the present data do not provide evidence of abnormal implicit knowledge of previous word presentations in schizophrenia (cf. Tendolkar et al., 2002). However, as a continuous recognition memory paradigm does not specifically probe predictions stemming from the dual-process model of episodic memory, no definitive conclusions are possible.
4.2. Modality-independent reductions of early negativities in schizophrenia
Although visual and auditory N1 sink amplitudes were nominally smaller in schizophrenic patients than controls, there was no statistical support for these group differences, which is in agreement with our previous studies using visual word stimuli in the same recognition memory paradigm with nose-referenced ERPs (Kayser et al., 1999, footnote 6) and in a working memory tasks with CSD waveforms (Kayser et al., 2006). This suggests that reductions of early, mostly stimulus-driven ERP components in schizophrenia, which are frequently observed during simple auditory or visual tasks (e.g., Ford et al., 1994; Bruder et al., 1998; Kayser et al., 2001; Doniger et al., 2002; Clunas and Ward, 2005; Butler et al., 2007) and likely indicative of a deficit of attention or early perceptual processing (e.g., P50, MMN, P1, N1; cf. Javitt et al., 2008), were of subordinate importance to the deficits in later components observed during the more cognitively-demanding memory tasks. Nevertheless, while both groups showed left-larger-than-right visual N1 sinks over inferior-parietal sites, an asymmetry linked to the recognition of linguistic visual material (e.g., Dehaene, 1995; Hauk et al., 2006), this effect was only significant for healthy adults. The reduced N1 sink asymmetry in schizophrenia closely matches recent N150 findings for a small sample of chronic schizophrenic outpatients, which have been taken as evidence for a deficit of automatically activating linguistic networks (Spironelli et al., 2008).
In contrast to N1, robust reductions of N2 sink amplitudes were found for patients across modalities (cf. Alain et al., 2001 cf. Alain et al., 2002a). Interestingly, the presence of a left-lateralized auditory N2 sink at medial central sites in controls but not patients parallels the visual N1 sink findings, which may also hint at a failure of automatically accessing phonological/semantical word representations in the left hemisphere (cf. Price et al., 2003). ERP studies reporting robust N2 reductions in schizophrenia have often used a nose reference (e.g., Bruder et al., 1998, 1999; Kayser et al., 1999, 2001) or target-minus-standard difference waveforms (e.g., O’Donnell et al., 1993; Umbricht et al., 2006), whereas a linked-mastoids or ear lobe reference tends to lessen its noticeability. While temporal characteristics of the visual N2 sink and its group difference in the current study were in close agreement with our previous findings (Kayser et al., 1999), the reference-free CSD transform revealed maximum current flow at vertex and not over the left inferior parietal region, as presumed when interpreting our nose-referenced ERP data. Therefore, we must conclude that early, left-lateralized inferior parietal ERP negativities (N1, N2) to visual stimuli observed with a nose reference are due to a volume-conducted fusion of two or more (cf. inferior parietal sinks associated with visual P2 source) partly-overlapping potentials of different cortical origin. In fact, a linked-mastoids reference will likely project an N2 towards the mid-frontocentral region, a location conveniently fitting dipole models suggesting an underlying ACC source (van Veen and Carter, 2002). More importantly, almost the same cortical region was implicated in both neuronal generator patterns underlying the visual and auditory N2 sink, which may suggest reduced, modality-independent ACC contribution to this frontocentral component in schizophrenia. This is also entirely consistent with recent combined fMRI and ERP evidence in healthy adults during auditory and visual oddball tasks, showing for both modalities similar overall ACC activation, the main contributor of N2b, but modality-specific connectivity to primary auditory (Heschl’s gyrus) and visual (striate) cortices (Crottaz-Herbette and Menon, 2006). These findings suggest top-down attentional modulation of sensory processing by ACC, which may be compromised in schizophrenia.
Nevertheless, patients and controls showed comparable condition-dependencies of these N2 sinks (i.e., inverted old/new effects), which fits well with the idea that N2 indexes stimulus classification (e.g., Simson et al., 1976). There appears also to be a striking analogy between the sequence of the current N2 sinks and P3 sources and the N2/P3 complex typically observed for target stimuli during oddball tasks, with both components showing condition sensitivity but with opposite polarity. However, exceeding caution is warranted when likening the present N2 generator patterns, which were derived by means of CSD-PCA in the context of a specific recognition memory task, to previous discourses on the meaning of N2 derived from surface ERPs using various reference schemes and other paradigms (e.g., Fabiani et al., 2000; Folstein and Van Petten, 2008). These data suggest that several functional processes can be associated with negative ERP deflections in the 200–400 ms time range after stimulus onset, with some processes, such as attention, tied in with modality-specific N2 topographies and generators. It is also plausible to conjecture that these vertex N2 sinks belong in the class of N400-like potentials indexing decision making during linguistic processes (cf. Silva-Pereyra et al., 2003). In any case, the marked N2 sink reductions across modality for words are similar to those seen for schizophrenic patients in visual or auditory discrimination tasks (e.g., O’Donnell et al., 1993; Egan et al., 1994; Strandburg et al., 1994; Bruder et al., 1998, 1999; Kayser et al., 2001; Umbricht et al., 2006). They are also consistent with our ERP findings involving phonetic discrimination of consonant-vowel syllables, in which smaller N2 amplitude in schizophrenic patients was associated with poorer verbal memory performance (Bruder et al., 2001). In context of these converging findings, we interpret the present N2 sink results as evidence of impaired stimulus categorization in schizophrenia at a cognitive stage beyond visual or auditory word form processing, which adversely affects word recognition memory.
4.3. Impaired medial frontal cortex function and performance monitoring in schizophrenia
Whereas anterior cingulate involvement in the generation of visual and auditory N2 sink activity may be debatable, there is little doubt that the response-related, midfrontal negativity (FRN) belongs to the class of ERN-like components attributed to generators within the ACC (e.g., van Veen and Carter, 2002, 2006). The present findings of markedly smaller auditory FRN in patients, combined with their reduced old/new sink effects across modalities, matches previous evidence of reduced error-related and feedback negativity in schizophrenia (e.g., Kopp and Rist, 1999; Mathalon et al., 2002; Alain et al., 2002b; Bates et al., 2002; Morris et al., 2006, 2008), which is likely linked to structural and/or functional ACC impairments (e.g., Carter et al., 2001; Laurens et al., 2003; Baiano et al., 2007; Polli et al., 2008). Still, only correct trials were analyzed in the present study, which appears to be at odds with prior reports of normal or even larger CRN amplitude in schizophrenic patients (e.g., Kopp and Rist 1999; Mathalon et al., 2002). However, a comparison of these findings may be difficult due to differences in experimental task and ERP methods, which apart from the CSD-PCA approach also include using a pre- rather than post-stimulus baseline for response-locked ERP activity to avoid the confound of subtracting stimulus-locked topographies (cf. Urbach and Kutas, 2006; Kayser et al., 2007). The presence of this component in healthy adults during these word recognition memory tasks is presumably an index of active performance monitoring to adaptively adjust response behavior in subsequent trials (Debener et al., 2005), and conversely, its reduction in schizophrenic patients reflects a dysfunction in effectively monitoring their ongoing response behavior (cf. Ullsperger, 2006), again impacting more severely on auditory information processing. These findings are consistent with evidence suggesting that a dysfunction of corollary discharge in schizophrenia, in which a failure to build internal representations of self-generated behavioral responses (efference copies) may be associated with, and specific to, auditory hallucinations (e.g., Ford et al., 2001, 2007; Ford and Mathalon, 2004; Mathalon and Ford, 2008).
The reduced visual centrotemporal source, which followed the FRN as part of the generator pattern associated with a late occipital negativity, underscores the failure of schizophrenic patients to show response-related brain activation. Topography (vertex maximum) and time course of this distinct response-locked source suggest that it belongs to the family of Pe-like components reflecting different monitoring processes than the ERN/Ne but also of cingulate origin (e.g., Falkenstein et al., 2000; Vocat et al., 2008). The late occipital negativity, which has frequently been observed in visual recognition memory tasks showing an inverted old/new effect (cf. Johansson & Mecklinger, 2003), was also reduced in the visual modality for schizophrenic patients. Despite this overall reduction in amplitude, no abnormalities were found for the overlapping inverted late episodic memory effect over centroparietal midline sites consistent with a generator pattern posteriorly along the cingulate sulcus (cf. Kayser et al., 2007). However, more research is clearly needed on how different response-related ERP components relate to and reflect impaired performance monitoring in schizophrenia.
4.4. Limitations and conclusions
Typical limitations of ERP studies in schizophrenia concern small and heterogeneous samples, the potential influence of antipsychotic medication on cognition and brain function, and the tendency of patients to perform more poorly than healthy controls (e.g., Barch, 2005). With regard to sample size, the current number of 20 individuals ranks among the largest patient groups tested during a recognition memory paradigm (only exceeded by Kayser et al., 1999). Furthermore, patients were particularly well-matched (yoked) to controls in core demographic variables (gender, age, ethnicity) as well as handedness, which is a crucial moderator of lateralized brain functions, including language (e.g., Annett, 1991; Knecht et al., 2000). Although the sample was heterogeneous, most of the patients in the present study met DSM-IV criteria for schizophrenia (n = 15), and all had schizophrenia spectrum disorders. The reductions of the response-related mid-frontal sink in the current patient sample, with a majority being of paranoid subtype (n = 11), are of interest in light of previous reports linking reduced ERN to paranoid schizophrenia patients (Kopp and Rist, 1999; Mathalon et al., 2002). However, subtyping with small samples is likely to be problematic because of unpredictable differences in other variables (e.g., gender, age, etc.), thereby severely limiting the ability to draw valid conclusions. Because most of the current patients were off medication during testing (n = 11), it is unlikely that the observed recognition memory deficits and associated electrophysiologic abnormalities were a mere byproduct of drug treatment, and this appears to be a widely-accepted notion (cf. Barch, 2005). The adequate task performance of patients and confining the ERP/CSD analysis to correct trials, combined with a distinct ERP/CSD component structure in patients indicative of modality-specific activation of auditory and visual processing streams, strongly suggest that our findings are indicative of impaired word recognition memory processing in schizophrenia.
While the present study focused on the influence of modality on verbal recognition memory in schizophrenia, it does not address whether abnormalities in patients are specific to linguistic or language-related aspects of episodic memory. One meta-analysis found that non-word stimuli yielded even larger effect sizes of compromised recognition memory performance in schizophrenia (Pelletier et al., 2005), and old/new ERP abnormalities in schizophrenia have been reported for unfamiliar faces that are difficult to verbalize (Guillem et al., 2001). To further address these issues of material specificity in a within-subjects design, we are now recording ERPs of larger samples of schizophrenia patients and healthy adults during continuous recognition memory tasks using both words and unfamiliar faces.
Whereas ERPs provide direct, real-time measures of brain activity associated with successive stages of information processing, they lack the spatial resolution supplied by other neuroimaging methods that indirectly quantify neuronal activity. However, the reference-free ERP/CSD approach addresses this limitation by eliminating many of the pitfalls of volume-conducted scalp potentials (e.g., redundancy, reference-dependence), thereby effectively bridging the gap between surface potentials and their underlying neuroanatomical generators (Kayser and Tenke, 2006a). Future approaches will likely include combined measurements of electrophysiologic and metabolic brain responses in schizophrenia during word recognition memory tasks to further narrow the gap between scalp current flow estimates and underlying brain activation.
Merging CSD and PCA methods into a generic ERP strategy and applying it to both stimulus- and response-locked activity has again been found to be a potent approach in advancing knowledge of cognitive dysfunction in schizophrenia. The present findings establish robust reductions of the ERP old/new effects over left lateral temporoparietal, but not mid-parietal, sites as an electrophysiologic correlate of word recognition memory deficits in schizophrenia, implicating impaired stimulus representation involving posterior regions. Marked reductions of stimulus-related vertex N2 sinks preceded and response-related mid-frontal sink activity followed these impairments, indicating additional deficits in word classification (i.e., stimulus categorization) and performance monitoring in schizophrenia involving anterior cingulate cortex. These impairments, while essentially present for both visual and auditory recognition memory tasks, were clearly more robust for spoken words, suggesting a specific impairment of temporal integration and retrieval of semantic information by means of a phonological code (cf. Baddeley, 1983; see also Kayser et al., 2003, p. 12) involving left parietal-temporal regions typically associated with language-related processing.
Supplementary Material
Acknowledgments
Supported by grants MH50715 and MH066597 from the National Institute of Mental Health (NIMH). Waveform plotting software developed and generously provided by Charles L. Brown, III. We thank Barbara Stuart, Paul Leite, Carlye Griggs, Mia Sage, Christopher Kroppmann, and Nathan Gates for help with data collection and artifact screening. Preliminary analyses of these data were presented at the 43rd Annual Meeting of the Society for Psychophysiological Research (SPR), Chicago, IL, October 29 – November 2, 2003, the 1st Joint Meeting of the EEG and Clinical Neuroscience Society (ECNS) and International Society for Neuroimaging in Psychiatry (ISNIP), Irvine, CA, September 28 - October 2, 2004, and the 63rd Annual Meeting of the Society of Biological Psychiatry (SoBP) in Washington, DC, May 1 – 3, 2008. We greatly appreciate helpful suggestions by Stuart Steinhauer.
Footnotes
One 44-year old male had a clinical diagnosis of schizoaffective disorder at the time of testing. His cardinal symptom consisted of auditory command hallucinations for many years. Similarly, the clinical diagnosis of one 27-year old female patient with auditory hallucinations and paranoid delusions had been schizoaffective disorder for most of her hospitalization. At discharge, however, the final consensus diagnosis of both patients was changed to psychosis not otherwise specified. As these individuals clearly fall in the range of schizophrenia spectrum disorders, and due to the small sample, they were not excluded from the study. Furthermore, CSD waveforms and all crucial ANOVA results were virtually unaffected by including these patients.
It should be obvious that a linked-mastoids reference (i.e., the mean of sites TP9 and TP10) would render a substantial and inverted potential at frontal sites, mostly affecting visual ERPs of healthy adults (i.e., a different reference will result in a different pattern of positive and negative ERP activity, and will differentially impact on visual and auditory ERPs of controls or patients; e.g., cf. Figure 8 in Kayser et al., 2007), thereby likely altering group and/or condition effects.
Animated topographies of stimulus- and response-locked CSD waveforms can be obtained at URL http://psychophysiology.cpmc.columbia.edu/cwrm2007csd.html.
Separate PCA solutions derived from the patients’ CSD data only (n = 20) revealed a highly comparable factor structure, thereby validating the use of a common extraction with both groups.
The analysis for the associated sink activity over inferior temporoparietal sites (TP9/10, P9/10, P7/8) for factor 195 (visual P2 source) revealed no significant effects (see supplementary material).
We note, however, that this condition effect is not apparent from the grand mean CSD waveforms or the corresponding animated topographies. This presumably indicates that higher-variance factors removed overlapping, condition-related variance associated with the time course of this N2 sink, thereby unmasking relevant differences, although it is still prudent to evaluate this finding cautiously.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alain C, Cortese F, Bernstein LJ, He Y, Zipursky RB. Auditory feature conjunction in patients with schizophrenia. Schizophr Res. 2001;49(1–2):179–191. doi: 10.1016/s0920-9964(00)00138-9. [DOI] [PubMed] [Google Scholar]
- Alain C, Bernstein LJ, He Y, Cortese F, Zipursky RB. Visual feature conjunction in patients with schizophrenia: an event-related brain potential study. Schizophr Res. 2002;57(1):69–79. doi: 10.1016/s0920-9964(01)00303-6. [DOI] [PubMed] [Google Scholar]
- Alain C, McNeely HE, He Y, Christensen BK, West R. Neurophysiological evidence of error-monitoring deficits in patients with schizophrenia. Cereb Cortex. 2002;12(8):840–846. doi: 10.1093/cercor/12.8.840. [DOI] [PubMed] [Google Scholar]
- Albus M, Hubmann W, Mohr F, Hecht S, Hinterberger Weber P, Seitz NN, Kuchenhoff H. Neurocognitive functioning in patients with first-episode schizophrenia: results of a prospective 5-year follow-up study. Eur Arch Psychiatry Clin Neurosci. 2006;256(7):442–451. doi: 10.1007/s00406-006-0667-1. [DOI] [PubMed] [Google Scholar]
- Allan K, Wilding EL, Rugg MD. Electrophysiological evidence for dissociable processes contributing to recollection. Acta Psychol (Amst) 1998;98(2–3):231–252. doi: 10.1016/s0001-6918(97)00044-9. [DOI] [PubMed] [Google Scholar]
- Ally BA, Simons JS, McKeever JD, Peers PV, Budson AE. Parietal contributions to recollection: Electrophysiological evidence from aging and patients with parietal lesions. Neuropsychologia. 2008;46(7):1800–1812. doi: 10.1016/j.neuropsychologia.2008.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and Statistical Manual of the Mental Disorders Fourth Edition (DSM-IV) American Psychiatric Association; Washington, DC: 1994. [Google Scholar]
- Andreasen NC. The Scale for the Assessment of Negative Symptoms (SANS) The University of Iowa; Iowa City, IA: 1983. [Google Scholar]
- Andreasen NC. The Scale for the Assessment of Positive Symptoms (SAPS) The University of Iowa; Iowa City, IA: 1984. [Google Scholar]
- Annett M. Speech lateralisation and phonological skill. Cortex. 1991;27(4):583–593. doi: 10.1016/s0010-9452(13)80007-x. [DOI] [PubMed] [Google Scholar]
- Arnold SE, Hyman BT, Van Hoesen GW, Damasio AR. Some cytoarchitectural abnormalities of the entorhinal cortex in schizophrenia. Arch Gen Psychiatry. 1991;48(7):625–632. doi: 10.1001/archpsyc.1991.01810310043008. [DOI] [PubMed] [Google Scholar]
- Baddeley AD. Working Memory. Philos Trans R Soc Lond, B, Biol Sci. 1983;302(1110):311–324. [Google Scholar]
- Baiano M, David A, Versace A, Churchill R, Balestrieri M, Brambilla P. Anterior cingulate volumes in schizophrenia: a systematic review and a meta-analysis of MRI studies. Schizophr Res. 2007;93(1–3):1–12. doi: 10.1016/j.schres.2007.02.012. [DOI] [PubMed] [Google Scholar]
- Barch DM. The cognitive neuroscience of schizophrenia. Annu Rev Clin Psychol. 2005;1:321–353. doi: 10.1146/annurev.clinpsy.1.102803.143959. [DOI] [PubMed] [Google Scholar]
- Barta PE, Pearlson GD, Powers RE, Richards SS, Tune LE. Auditory hallucinations and smaller superior temporal gyral volume in schizophrenia. Am J Psychiatry. 1990;147(11):1457–1462. doi: 10.1176/ajp.147.11.1457. [DOI] [PubMed] [Google Scholar]
- Bates AT, Kiehl KA, Laurens KR, Liddle PF. Error-related negativity and correct response negativity in schizophrenia. Clin Neurophysiol. 2002;113(9):1454–1463. doi: 10.1016/s1388-2457(02)00154-2. [DOI] [PubMed] [Google Scholar]
- Bates AT, Liddle PF, Kiehl KA, Ngan ET. State dependent changes in error monitoring in schizophrenia. J Psychiatr Res. 2004;38(3):347–356. doi: 10.1016/j.jpsychires.2003.11.002. [DOI] [PubMed] [Google Scholar]
- Baving L, Rockstroh B, Rossner P, Cohen R, Elbert T, Roth WT. Event-related potential correlates of acquisition and retrieval of verbal associations in schizophrenics and controls. J Psychophysiol. 2000;14(2):87–96. [Google Scholar]
- Bilder RM, Wu H, Bogerts B, Degreef G, Ashtari M, Alvir JM, Snyder PJ, Lieberman JA. Absence of regional hemispheric volume asymmetries in first-episode schizophrenia. Am J Psychiatry. 1994;151(10):1437–1447. doi: 10.1176/ajp.151.10.1437. [DOI] [PubMed] [Google Scholar]
- Blanchard JJ, Neale JM. The neuropsychological signature of schizophrenia: generalized or differential deficit? Am J Psychiatry. 1994;151(1):40–48. doi: 10.1176/ajp.151.1.40. [DOI] [PubMed] [Google Scholar]
- Bogerts B, Ashtari M, Degreef G, Alvir JM, Bilder RM, Lieberman JA. Reduced temporal limbic structure volumes on magnetic resonance images in first episode schizophrenia. Psychiatry Res. 1990;35(1):1–13. doi: 10.1016/0925-4927(90)90004-p. [DOI] [PubMed] [Google Scholar]
- Bogerts B, Lieberman JA, Ashtari M, Bilder RM, Degreef G, Lerner G, Johns C, Masiar S. Hippocampus-amygdala volumes and psychopathology in chronic schizophrenia. Biol Psychiatry. 1993;33(4):236–246. doi: 10.1016/0006-3223(93)90289-p. [DOI] [PubMed] [Google Scholar]
- Botvinick MM. Conflict monitoring and decision making: reconciling two perspectives on anterior cingulate function. Cogn Affect Behav Neurosci. 2007;7(4):356–366. doi: 10.3758/cabn.7.4.356. [DOI] [PubMed] [Google Scholar]
- Brazdil M, Roman R, Daniel P, Rektor I. Intracerebral somatosensory event-related potentials: effect of response type (button pressing versus mental counting) on P3-like potentials within the human brain. Clin Neurophysiol. 2003;114(8):1489–1496. doi: 10.1016/s1388-2457(03)00135-4. [DOI] [PubMed] [Google Scholar]
- Bruder G, Kayser J, Tenke C, Rabinowicz E, Friedman M, Amador X, Sharif Z, Gorman J. The time course of visuospatial processing deficits in schizophrenia: an event-related brain potential study. J Abnorm Psychol. 1998;107(3):399–411. doi: 10.1037//0021-843x.107.3.399. [DOI] [PubMed] [Google Scholar]
- Bruder G, Kayser J, Tenke C, Amador X, Friedman M, Sharif Z, Gorman J. Left temporal lobe dysfunction in schizophrenia: event-related potential and behavioral evidence from phonetic and tonal dichotic listening tasks. Arch Gen Psychiatry. 1999;56(3):267–276. doi: 10.1001/archpsyc.56.3.267. [DOI] [PubMed] [Google Scholar]
- Bruder GE, Kayser J, Tenke CE, Friedman M, Malaspina D, Gorman JM. Event-related potentials in schizophrenia during tonal and phonetic oddball tasks: relations to diagnostic subtype, symptom features and verbal memory. Biol Psychiatry. 2001;50(6):447–452. doi: 10.1016/s0006-3223(01)01168-4. [DOI] [PubMed] [Google Scholar]
- Bruder GE, Wexler BE, Sage MM, Gil RB, Gorman JM. Verbal memory in schizophrenia: additional evidence of subtypes having different cognitive deficits. Schizophr Res. 2004;68(2–3):137–147. doi: 10.1016/S0920-9964(03)00156-7. [DOI] [PubMed] [Google Scholar]
- Butler PD, Martinez A, Foxe JJ, Kim D, Zemon V, Silipo G, Mahoney J, Shpaner M, Jalbrzikowski M, Javitt DC. Subcortical visual dysfunction in schizophrenia drives secondary cortical impairments. Brain. 2007;130(2):417–430. doi: 10.1093/brain/awl233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabeza R. Role of parietal regions in episodic memory retrieval: The dual attentional processes hypothesis. Neuropsychologia. 2008;46(7):1813–1827. doi: 10.1016/j.neuropsychologia.2008.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter CS, MacDonald AW, 3rd, Ross LL, Stenger VA. Anterior cingulate cortex activity and impaired self-monitoring of performance in patients with schizophrenia: an event-related fMRI study. Am J Psychiatry. 2001;158(9):1423–1428. doi: 10.1176/appi.ajp.158.9.1423. [DOI] [PubMed] [Google Scholar]
- Clunas NJ, Ward PB. Auditory recovery cycle dysfunction in schizophrenia: a study using event-related potentials. Psychiatry Res. 2005;136(1):17–25. doi: 10.1016/j.psychres.2005.05.009. [DOI] [PubMed] [Google Scholar]
- Coltheart M. The MRC psycholinguistic database. Q J Exp Psychol A. 1981;33:497–505. [Google Scholar]
- Condray R. Language disorder in schizophrenia as a developmental learning disorder. Schizophr Res. 2005;73(1):5–20. doi: 10.1016/j.schres.2004.05.022. [DOI] [PubMed] [Google Scholar]
- Crottaz-Herbette S, Menon V. Where and when the anterior cingulate cortex modulates attentional response: combined fMRI and ERP evidence. J Cogn Neurosci. 2006;18(5):766–780. doi: 10.1162/jocn.2006.18.5.766. [DOI] [PubMed] [Google Scholar]
- Crow TJ. Temporal lobe asymmetries as the key to the etiology of schizophrenia. Schizophr Bull. 1990;16(3):433–443. doi: 10.1093/schbul/16.3.433. [DOI] [PubMed] [Google Scholar]
- Crow TJ. Schizophrenia as failure of hemispheric dominance for language. Trends Neurosci. 1997;20(8):339–343. doi: 10.1016/s0166-2236(97)01071-0. [DOI] [PubMed] [Google Scholar]
- Crow TJ. Auditory hallucinations as primary disorders of syntax: an evolutionary theory of the origins of language. Cognit Neuropsychiatry. 2004;9(1–2):125–145. doi: 10.1080/13546800344000192. [DOI] [PubMed] [Google Scholar]
- Crow TJ. Cerebral asymmetry and the lateralization of language: core deficits in schizophrenia as pointers to the gene. Curr Opin Psychiatry. 2004;17(2):97–106. [Google Scholar]
- Curran T. The electrophysiology of incidental and intentional retrieval: ERP old/new effects in lexical decision and recognition memory. Neuropsychologia. 1999;37(7):771–785. doi: 10.1016/s0028-3932(98)00133-x. [DOI] [PubMed] [Google Scholar]
- Curran T, Cleary AM. Using ERPs to dissociate recollection from familiarity in picture recognition. Cogn Brain Res. 2003;15(2):191–205. doi: 10.1016/s0926-6410(02)00192-1. [DOI] [PubMed] [Google Scholar]
- Damasio AR. Time-locked multiregional retroactivation: a systems-level proposal for the neural substrates of recall and recognition. Cognition. 1989;33(1–2):25–62. doi: 10.1016/0010-0277(89)90005-x. [DOI] [PubMed] [Google Scholar]
- Debener S, Ullsperger M, Siegel M, Fiehler K, von Cramon DY, Engel AK. Trial-by-trial coupling of concurrent electroencephalogram and functional magnetic resonance imaging identifies the dynamics of performance monitoring. J Neurosci. 2005;25(50):11730–11737. doi: 10.1523/JNEUROSCI.3286-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dehaene S. Electrophysiological evidence for category-specific word processing in the normal human brain. Neuroreport. 1995;6(16):2153–2157. doi: 10.1097/00001756-199511000-00014. [DOI] [PubMed] [Google Scholar]
- Dehaene S, Posner MI, Tucker DM. Localization of a neural system for error detection and compensation. Psychol Sci. 1994;5(5):303–305. [Google Scholar]
- DeLisi LE, Sakuma M, Kushner M, Finer DL, Hoff AL, Crow TJ. Anomalous cerebral asymmetry and language processing in schizophrenia. Schizophr Bull. 1997;23(2):255–271. doi: 10.1093/schbul/23.2.255. [DOI] [PubMed] [Google Scholar]
- Dien J. Issues in the application of the average reference: review, critiques, and recommendations. Behav Res Methods Instrum Comput. 1998;30(1):34–43. [Google Scholar]
- Dixon WJ, editor. BMDP Statistical Software Manual: To Accompany the 7.0 Software Release. University of California Press; Berkeley, CA: 1992. [Google Scholar]
- Doniger GM, Foxe JJ, Murray MM, Higgins BA, Javitt DC. Impaired visual object recognition and dorsal/ventral stream interaction in schizophrenia. Arch Gen Psychiatry. 2002;59(11):1011–1020. doi: 10.1001/archpsyc.59.11.1011. [DOI] [PubMed] [Google Scholar]
- Donkers FC, Nieuwenhuis S, van Boxtel GJ. Mediofrontal negativities in the absence of responding. Cogn Brain Res. 2005;25(3):777–787. doi: 10.1016/j.cogbrainres.2005.09.007. [DOI] [PubMed] [Google Scholar]
- Egan MF, Duncan CC, Suddath RL, Kirch DG, Mirsky AF, Wyatt RJ. Event-related potential abnormalities correlate with structural brain alterations and clinical features in patients with chronic schizophrenia. Schizophr Res. 1994;11(3):259–271. doi: 10.1016/0920-9964(94)90020-5. [DOI] [PubMed] [Google Scholar]
- Emeric EE, Brown JW, Leslie M, Pouget P, Stuphorn V, Schall JD. Performance monitoring local field potentials in the medial frontal cortex of primates: anterior cingulate cortex. J Neurophysiol. 2008;99(2):759–772. doi: 10.1152/jn.00896.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabiani M, Gratton G, Coles MGH. Event-related brain potentials: methods, theory, and applications. In: Cacioppo JT, Tassinary LG, Bernston GG, editors. Handbook of Psychophysiology. 2. Cambridge University Press; Cambridge, UK: 2000. pp. 53–84. [Google Scholar]
- Falkai P, Bogerts B, Schneider T, Greve B, Pfeiffer U, Pilz K, Gonsiorzcyk C, Majtenyi C, Ovary I. Disturbed planum temporale asymmetry in schizophrenia. A quantitative postmortem study. Schizophr Res. 1995;14(2):161–176. doi: 10.1016/0920-9964(94)00035-7. [DOI] [PubMed] [Google Scholar]
- Falkenstein M, Hoormann J, Christ S, Hohnsbein J. ERP components on reaction errors and their functional significance: a tutorial. Biol Psychol. 2000;51(2–3):87–107. doi: 10.1016/s0301-0511(99)00031-9. [DOI] [PubMed] [Google Scholar]
- Faux SF, McCarley RW, Nestor PG, Shenton ME, Pollak SD, Penhune V, Mondrow E, Marcy B, Peterson A, Horvath T, et al. P300 topographic asymmetries are present in unmedicated schizophrenics. Electroenceph Clin Neurophysiol. 1993;88(1):32–41. doi: 10.1016/0168-5597(93)90026-l. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV Axis-I Disorders - Non-patient Edition (SCID-NP) Biometrics Research Department, New York State Psychiatric Institute; New York, NY: 1996. [Google Scholar]
- Flaum M, Swayze VW, 2nd, O'Leary DS, Yuh WT, Ehrhardt JC, Arndt SV, Andreasen NC. Effects of diagnosis, laterality, and gender on brain morphology in schizophrenia. Am J Psychiatry. 1995;152(5):704–714. doi: 10.1176/ajp.152.5.704. [DOI] [PubMed] [Google Scholar]
- Flor-Henry P. Psychosis and temporal lobe epilepsy. A controlled investigation. Epilepsia. 1969;10(3):363–395. doi: 10.1111/j.1528-1157.1969.tb03853.x. [DOI] [PubMed] [Google Scholar]
- Flor-Henry P. Lateralized temporal-limbic dysfunction and psychopathology. Ann N Y Acad Sci. 1976;280:777–797. doi: 10.1111/j.1749-6632.1976.tb25541.x. [DOI] [PubMed] [Google Scholar]
- Folstein JR, Van Petten C. Influence of cognitive control and mismatch on the N2 component of the ERP: a review. Psychophysiology. 2008;45(1):152–170. doi: 10.1111/j.1469-8986.2007.00602.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford JM. Schizophrenia: the broken P300 and beyond. Psychophysiology. 1999;36(6):667–682. [PubMed] [Google Scholar]
- Ford JM, White PM, Csernansky JG, Faustman WO, Roth WT, Pfefferbaum A. ERPs in schizophrenia: effects of antipsychotic medication. Biol Psychiatry. 1994;36(3):153–170. doi: 10.1016/0006-3223(94)91221-1. [DOI] [PubMed] [Google Scholar]
- Ford JM, Mathalon DH, White PM, Pfefferbaum A. Left temporal deficit of P300 in patients with schizophrenia: effects of task. Int J Psychophysiol. 2000;38(1):71–79. doi: 10.1016/s0167-8760(00)00131-8. [DOI] [PubMed] [Google Scholar]
- Ford JM, Mathalon DH, Kalba S, Whitfield S, Faustman WO, Roth WT. Cortical responsiveness during talking and listening in schizophrenia: an event-related brain potential study. Biol Psychiatry. 2001;50(7):540–549. doi: 10.1016/s0006-3223(01)01166-0. [DOI] [PubMed] [Google Scholar]
- Ford JM, Gray M, Faustman WO, Roach BJ, Mathalon DH. Dissecting corollary discharge dysfunction in schizophrenia. Psychophysiology. 2007;44(4):522–529. doi: 10.1111/j.1469-8986.2007.00533.x. [DOI] [PubMed] [Google Scholar]
- Ford JM, Mathalon DH. Electrophysiological evidence of corollary discharge dysfunction in schizophrenia during talking and thinking. J Psychiatr Res. 2004;38(1):37–46. doi: 10.1016/s0022-3956(03)00095-5. [DOI] [PubMed] [Google Scholar]
- Friedman D. Event-related brain potential investigations of memory and aging. Biol Psychol. 2000;54(1–3):175–206. doi: 10.1016/s0301-0511(00)00056-9. [DOI] [PubMed] [Google Scholar]
- Friedman D, Johnson R., Jr Event-related potential (ERP) studies of memory encoding and retrieval: a selective review. Microsc Res Tech. 2000;51(1):6–28. doi: 10.1002/1097-0029(20001001)51:1<6::AID-JEMT2>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- Frith CD. The positive and negative symptoms of schizophrenia reflect impairments in the perception and initiation of action. Psychol Med. 1987;17(3):631–648. doi: 10.1017/s0033291700025873. [DOI] [PubMed] [Google Scholar]
- Gehring WJ, Goss B, Coles MGH, Meyer DE, Donchin E. A neural system for error-detection and compensation. Psychol Sci. 1993;4(6):385–390. [Google Scholar]
- Goldberg TE, Torrey EF, Gold JM, Ragland JD, Bigelow LB, Weinberger DR. Learning and memory in monozygotic twins discordant for schizophrenia. Psychol Med. 1993;23(1):71–85. doi: 10.1017/s0033291700038861. [DOI] [PubMed] [Google Scholar]
- Guillem F, N'Kaoua B, Rougier A, Claverie B. Intracranial topography of event-related potentials (N400/P600) elicited during a continuous recognition memory task. Psychophysiology. 1995;32(4):382–392. doi: 10.1111/j.1469-8986.1995.tb01221.x. [DOI] [PubMed] [Google Scholar]
- Guillem F, Bicu M, Hooper R, Bloom D, Wolf MA, Messier J, Desautels R, Debruille JB. Memory impairment in schizophrenia: a study using event-related potentials in implicit and explicit tasks. Psychiatry Res. 2001;104(2):157–173. doi: 10.1016/s0165-1781(01)00305-5. [DOI] [PubMed] [Google Scholar]
- Gur RE. Left hemisphere dysfunction and left hemisphere overactivation in schizophrenia. J Abnorm Psychol. 1978;87(2):226–238. doi: 10.1037//0021-843x.87.2.226. [DOI] [PubMed] [Google Scholar]
- Gur RE, Jaggi JL, Shtasel DL, Ragland JD, Gur RC. Cerebral blood flow in schizophrenia: effects of memory processing on regional activation. Biol Psychiatry. 1994;35(1):3–15. doi: 10.1016/0006-3223(94)91160-6. [DOI] [PubMed] [Google Scholar]
- Hajcak G, Moser JS, Holroyd CB, Simons RF. The feedback-related negativity reflects the binary evaluation of good versus bad outcomes. Biol Psychol. 2006;71(2):148–154. doi: 10.1016/j.biopsycho.2005.04.001. [DOI] [PubMed] [Google Scholar]
- Halgren E, Baudena P, Clarke JM, Heit G, Marinkovic K, Devaux B, Vignal JP, Biraben A. Intracerebral potentials to rare target and distractor auditory and visual stimuli. II. Medial, lateral and posterior temporal lobe. Electroenceph Clin Neurophysiol. 1995;94(4):229–250. doi: 10.1016/0013-4694(95)98475-n. [DOI] [PubMed] [Google Scholar]
- Halgren E, Baudena P, Clarke JM, Heit G, Liegeois C, Chauvel P, Musolino A. Intracerebral potentials to rare target and distractor auditory and visual stimuli. I. Superior temporal plane and parietal lobe. Electroenceph Clin Neurophysiol. 1995;94(3):191–220. doi: 10.1016/0013-4694(94)00259-n. [DOI] [PubMed] [Google Scholar]
- Hauk O, Davis MH, Ford M, Pulvermuller F, Marslen-Wilson WD. The time course of visual word recognition as revealed by linear regression analysis of ERP data. Neuroimage. 2006;30(4):1383–1400. doi: 10.1016/j.neuroimage.2005.11.048. [DOI] [PubMed] [Google Scholar]
- Hill SK, Beers SR, Kmiec JA, Keshavan MS, Sweeney JA. Impairment of verbal memory and learning in antipsychotic-naive patients with first-episode schizophrenia. Schizophr Res. 2004;68(2–3):127–136. doi: 10.1016/S0920-9964(03)00125-7. [DOI] [PubMed] [Google Scholar]
- Iidaka T, Matsumoto A, Nogawa J, Yamamoto Y, Sadato N. Frontoparietal network involved in successful retrieval from episodic memory. Spatial and temporal analyses using fMRI and ERP. Cereb Cortex. 2006;16(9):1349–1360. doi: 10.1093/cercor/bhl040. [DOI] [PubMed] [Google Scholar]
- Javitt DC, Spencer KM, Thaker GK, Winterer G, Hajos M. Neurophysiological biomarkers for drug development in schizophrenia. Nat Rev Drug Discov. 2008;7(1):68–83. doi: 10.1038/nrd2463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeon YW, Polich J. P300 asymmetry in schizophrenia: a meta-analysis. Psychiatry Res. 2001;104(1):61–74. doi: 10.1016/s0165-1781(01)00297-9. [DOI] [PubMed] [Google Scholar]
- Jeon YW, Polich J. Meta-analysis of P300 and schizophrenia: patients, paradigms, and practical implications. Psychophysiology. 2003;40(5):684–701. doi: 10.1111/1469-8986.00070. [DOI] [PubMed] [Google Scholar]
- Johansson M, Mecklinger A. The late posterior negativity in ERP studies of episodic memory: action monitoring and retrieval of attribute conjunctions. Biol Psychol. 2003;64(1–2):91–117. doi: 10.1016/s0301-0511(03)00104-2. [DOI] [PubMed] [Google Scholar]
- Johnson R., Jr . Event-related potential insights into the neurobiology of memory systems. In: Boller F, Grafman J, editors. Handbook of Neuropsychology. Vol. 10. Elsevier; Amsterdam: 1995. pp. 135–163. [Google Scholar]
- Johnson R, Jr, Kreiter K, Russo B, Zhu J. A spatio-temporal analysis of recognition-related event-related brain potentials. Int J Psychophysiol. 1998;29(1):83–104. doi: 10.1016/s0167-8760(98)00006-3. [DOI] [PubMed] [Google Scholar]
- Junghöfer M, Elbert T, Leiderer P, Berg P, Rockstroh B. Mapping EEG-potentials on the surface of the brain: a strategy for uncovering cortical sources. Brain Topogr. 1997;9(3):203–217. doi: 10.1007/BF01190389. [DOI] [PubMed] [Google Scholar]
- Junghöfer M, Elbert T, Tucker DM, Braun C. The polar average reference effect: a bias in estimating the head surface integral in EEG recording. Clin Neurophysiol. 1999;110(6):1149–1155. doi: 10.1016/s1388-2457(99)00044-9. [DOI] [PubMed] [Google Scholar]
- Kawasaki Y, Maeda Y, Higashima M, Nagasawa T, Koshino Y, Suzuki M, Ide Y. Reduced auditory P300 amplitude, medial temporal volume reduction and psychopathology in schizophrenia. Schizophr Res. 1997;26(2–3):107–115. doi: 10.1016/s0920-9964(97)00055-8. [DOI] [PubMed] [Google Scholar]
- Kawasaki Y, Suzuki M, Takahashi T, Nohara S, McGuire PK, Seto H, Kurachi M. Anomalous cerebral asymmetry in patients with schizophrenia demonstrated by voxel-based morphometry. Biol Psychiatry. 2008;63(8):793–800. doi: 10.1016/j.biopsych.2007.08.008. [DOI] [PubMed] [Google Scholar]
- Kay SR, Opler LA, Fishbein A. Positive and negative syndrome scale (PANSS) rating manual. Multihealth System Inc; Toronto, Canada: 1992. [Google Scholar]
- Kayser J, Bruder GE, Friedman D, Tenke CE, Amador XF, Clark SC, Malaspina D, Gorman JM. Brain event-related potentials (ERPs) in schizophrenia during a word recognition memory task. Int J Psychophysiol. 1999;34(3):249–265. doi: 10.1016/s0167-8760(99)00082-3. [DOI] [PubMed] [Google Scholar]
- Kayser J, Bruder GE, Tenke CE, Stuart BK, Amador XF, Gorman JM. Event-related brain potentials (ERPs) in schizophrenia for tonal and phonetic oddball tasks. Biol Psychiatry. 2001;49(10):832–847. doi: 10.1016/s0006-3223(00)01090-8. [DOI] [PubMed] [Google Scholar]
- Kayser J, Fong R, Tenke CE, Bruder GE. Event-related brain potentials during auditory and visual word recognition memory tasks. Brain Res Cogn Brain Res. 2003;16(1):11–25. doi: 10.1016/s0926-6410(02)00205-7. [DOI] [PubMed] [Google Scholar]
- Kayser J, Tenke CE, Gates NA, Kroppmann CJ, Gil RB, Bruder GE. ERP/CSD indices of impaired verbal working memory subprocesses in schizophrenia. Psychophysiology. 2006;43(3):237–252. doi: 10.1111/j.1469-8986.2006.00398.x. [DOI] [PubMed] [Google Scholar]
- Kayser J, Tenke CE, Gates NA, Bruder GE. Reference-independent ERP old/new effects of auditory and visual word recognition memory: joint extraction of stimulus- and response-locked neuronal generator patterns. Psychophysiology. 2007;44(6):949–967. doi: 10.1111/j.1469-8986.2007.00562.x. [DOI] [PubMed] [Google Scholar]
- Kayser J, Tenke CE. Optimizing PCA methodology for ERP component identification and measurement: theoretical rationale and empirical evaluation. Clin Neurophysiol. 2003;114(12):2307–2325. doi: 10.1016/s1388-2457(03)00241-4. [DOI] [PubMed] [Google Scholar]
- Kayser J, Tenke CE. Trusting in or breaking with convention: towards a renaissance of principal components analysis in electrophysiology. Clin Neurophysiol. 2005;116(8):1747–1753. doi: 10.1016/j.clinph.2005.03.020. [DOI] [PubMed] [Google Scholar]
- Kayser J, Tenke CE. Principal components analysis of Laplacian waveforms as a generic method for identifying ERP generator patterns: I. Evaluation with auditory oddball tasks. Clin Neurophysiol. 2006;117(2):348–368. doi: 10.1016/j.clinph.2005.08.034. [DOI] [PubMed] [Google Scholar]
- Kayser J, Tenke CE. Principal components analysis of Laplacian waveforms as a generic method for identifying ERP generator patterns: II. Adequacy of low-density estimates. Clin Neurophysiol. 2006;117(2):369–380. doi: 10.1016/j.clinph.2005.08.033. [DOI] [PubMed] [Google Scholar]
- Kayser J, Tenke CE. Consensus on PCA for ERP data, and sensibility of unrestricted solutions. Clin Neurophysiol. 2006;117(3):703–707. [Google Scholar]
- Kayser J, Tenke CE. Electrical distance as a reference-free measure for identifying artifacts in multichannel electroencephalogram (EEG) recordings. Psychophysiology. 2006;43:S51. [Google Scholar]
- Keselman HJ. Testing treatment effects in repeated measures designs: an update for psychophysiological researchers. Psychophysiology. 1998;35(4):470–478. [PubMed] [Google Scholar]
- Kiehl KA, Liddle PF, Hopfinger JB. Error processing and the rostral anterior cingulate: an event-related fMRI study. Psychophysiology. 2000;37(2):216–223. [PubMed] [Google Scholar]
- Kim JJ, Kwon JS, Park HJ, Youn T, Kang do H, Kim MS, Lee DS, Lee MC. Functional disconnection between the prefrontal and parietal cortices during working memory processing in schizophrenia: a[15(O)]H2O PET study. Am J Psychiatry. 2003;160(5):919–923. doi: 10.1176/appi.ajp.160.5.919. [DOI] [PubMed] [Google Scholar]
- Kim MS, Kwon JS, Kang SS, Youn T, Kang KW. Impairment of recognition memory in schizophrenia: event-related potential study using a continuous recognition task. Psychiatry Clin Neurosci. 2004;58(5):465–472. doi: 10.1111/j.1440-1819.2004.01287.x. [DOI] [PubMed] [Google Scholar]
- Kim MS, Kang SS, Shin KS, Yoo SY, Kim YY, Kwon JS. Neuropsychological correlates of error negativity and positivity in schizophrenia patients. Psychiatry Clin Neurosci. 2006;60(3):303–311. doi: 10.1111/j.1440-1819.2006.01506.x. [DOI] [PubMed] [Google Scholar]
- Knecht S, Drager B, Deppe M, Bobe L, Lohmann H, Floel A, Ringelstein EB, Henningsen H. Handedness and hemispheric language dominance in healthy humans. Brain. 2000;123(12):2512–2518. doi: 10.1093/brain/123.12.2512. [DOI] [PubMed] [Google Scholar]
- Kopp B, Rist F. An event-related brain potential substrate of disturbed response monitoring in paranoid schizophrenic patients. J Abnorm Psychol. 1999;108(2):337–346. doi: 10.1037//0021-843x.108.2.337. [DOI] [PubMed] [Google Scholar]
- Kucera N, Francis WN. Computational analysis of present-day American English. Brown University Press; Providence, RI: 1967. [Google Scholar]
- Kulynych JJ, Vladar K, Jones DW, Weinberger DR. Superior temporal gyrus volume in schizophrenia: A study using MRI morphometry assisted by surface rendering. Am J Psychiatry. 1996;153(1):50–56. doi: 10.1176/ajp.153.9.A50. [DOI] [PubMed] [Google Scholar]
- Kumar N, Debruille JB. Semantics and N400: insights for schizophrenia. J Psychiatry Neurosci. 2004;29(2):89–98. [PMC free article] [PubMed] [Google Scholar]
- Laurens KR, Ngan ET, Bates AT, Kiehl KA, Liddle PF. Rostral anterior cingulate cortex dysfunction during error processing in schizophrenia. Brain. 2003;126(3):610–622. doi: 10.1093/brain/awg056. [DOI] [PubMed] [Google Scholar]
- Luck SJ. An introduction to the event-related potential technique. MIT Press; Cambridge, MA: 2005. [Google Scholar]
- Luu P, Flaisch T, Tucker DM. Medial frontal cortex in action monitoring. J Neurosci. 2000;20(1):464–469. doi: 10.1523/JNEUROSCI.20-01-00464.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathalon DH, Fedor M, Faustman WO, Gray M, Askari N, Ford JM. Response-monitoring dysfunction in schizophrenia: an event-related brain potential study. J Abnorm Psychol. 2002;111(1):22–41. [PubMed] [Google Scholar]
- Mathalon DH, Ford JM. Corollary discharge dysfunction in schizophrenia: evidence for an elemental deficit. Clin EEG Neurosci. 2008;39(2):82–86. doi: 10.1177/155005940803900212. [DOI] [PubMed] [Google Scholar]
- Matsumoto K, Matsuoka H, Yamazaki H, Sakai H, Kato T, Miura N, Nakamura M, Osakabe K, Saito H, Ueno T, Sato M. Impairment of an event-related potential correlate of memory in schizophrenia: effects of immediate and delayed word repetition. Clin Neurophysiol. 2001;112(4):662–673. doi: 10.1016/s1388-2457(01)00475-8. [DOI] [PubMed] [Google Scholar]
- Matsumoto K, Yamazaki H, Nakamura M, Sakai H, Miura N, Kato T, Miwa S, Ueno T, Saito H, Matsuoka H. Reduced word-repetition effect in the event-related potentials of thought-disordered patients with schizophrenia. Psychiatry Res. 2005;134(3):225–231. doi: 10.1016/j.psychres.2004.03.013. [DOI] [PubMed] [Google Scholar]
- Matsuoka H, Matsumoto K, Yamazaki H, Sakai H, Miwa S, Yoshida S, Numachi Y, Saito H, Ueno T, Sato M. Lack of repetition priming effect on visual event-related potentials in schizophrenia. Biol Psychiatry. 1999;46(1):137–140. doi: 10.1016/s0006-3223(98)00330-8. [DOI] [PubMed] [Google Scholar]
- McCarley RW, Faux SF, Shenton ME, Nestor PG, Adams J. Event-related potentials in schizophrenia: their biological and clinical correlates and a new model of schizophrenic pathophysiology. Schizophr Res. 1991;4(2):209–231. doi: 10.1016/0920-9964(91)90034-o. [DOI] [PubMed] [Google Scholar]
- McCarley RW, Shenton ME, O'Donnell BF, Faux SF, Kikinis R, Nestor PG, Jolesz FA. Auditory P300 abnormalities and left posterior superior temporal gyrus volume reduction in schizophrenia. Arch Gen Psychiatry. 1993;50(3):190–197. doi: 10.1001/archpsyc.1993.01820150036003. [DOI] [PubMed] [Google Scholar]
- McCarley RW, Salisbury DF, Hirayasu Y, Yurgelun Todd DA, Tohen M, Zarate C, Kikinis R, Jolesz FA, Shenton ME. Association between smaller left posterior superior temporal gyrus volume on magnetic resonance imaging and smaller left temporal P300 amplitude in first-episode schizophrenia. Arch Gen Psychiatry. 2002;59(4):321–331. doi: 10.1001/archpsyc.59.4.321. [DOI] [PubMed] [Google Scholar]
- McCarley RW, Nakamura M, Shenton ME, Salisbury DF. Combining ERP and structural MRI information in first episode schizophrenia and bipolar disorder. Clin EEG Neurosci. 2008;39(2):57–60. doi: 10.1177/155005940803900206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mecklinger A. Interfacing mind and brain: a neurocognitive model of recognition memory. Psychophysiology. 2000;37(5):565–582. [PubMed] [Google Scholar]
- Menon RR, Barta PE, Aylward EH, Richards SS, Vaughn DD, Tien AY, Harris GJ, Pearlson GD. Posterior superior temporal gyrus in schizophrenia: Grey matter changes and clinical correlates. Schizophr Res. 1995;16(2):127–135. doi: 10.1016/0920-9964(94)00067-i. [DOI] [PubMed] [Google Scholar]
- Michel CM, Murray MM, Lantz G, Gonzalez S, Spinelli L, Grave de Peralta R. EEG source imaging. Clin Neurophysiol. 2004;115(10):2195–2222. doi: 10.1016/j.clinph.2004.06.001. [DOI] [PubMed] [Google Scholar]
- Miltner WH, Lemke U, Weiss T, Holroyd C, Scheffers MK, Coles MG. Implementation of error-processing in the human anterior cingulate cortex: a source analysis of the magnetic equivalent of the error-related negativity. Biol Psychol. 2003;64(1–2):157–166. doi: 10.1016/s0301-0511(03)00107-8. [DOI] [PubMed] [Google Scholar]
- Molnar M. On the origin of the P3 event-related potential component. Int J Psychophysiol. 1994;17:129–144. doi: 10.1016/0167-8760(94)90028-0. [DOI] [PubMed] [Google Scholar]
- Morris SE, Yee CM, Nuechterlein KH. Electrophysiological analysis of error monitoring in schizophrenia. J Abnorm Psychol. 2006;115(2):239–250. doi: 10.1037/0021-843X.115.2.239. [DOI] [PubMed] [Google Scholar]
- Morris SE, Heerey EA, Gold JM, Holroyd CB. Learning-related changes in brain activity following errors and performance feedback in schizophrenia. Schizophr Res. 2008;99(1–3):274–285. doi: 10.1016/j.schres.2007.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mozley LH, Gur RC, Gur RE, Mozley PD, Alavi A. Relationships between verbal memory performance and the cerebral distribution of fluorodeoxyglucose in patients with schizophrenia. Biol Psychiatry. 1996;40(6):443–451. doi: 10.1016/0006-3223(95)00421-1. [DOI] [PubMed] [Google Scholar]
- Nestor PG, Kubicki M, Kuroki N, Gurrera RJ, Niznikiewicz M, Shenton ME, McCarley RW. Episodic memory and neuroimaging of hippocampus and fornix in chronic schizophrenia. Psychiatry Res. 2007;155(1):21–28. doi: 10.1016/j.pscychresns.2006.12.020. [DOI] [PubMed] [Google Scholar]
- NeuroScan, Inc. Compumedics Neuroscan. El Paso, TX: 2003. SCAN 4.3 - Vol. II. EDIT 4.3 - Offline analysis of acquired data (Document number 2203, Revision D) [Google Scholar]
- Nunez P. Electric fields of the brain. Oxford University Press; New York: 1981. [Google Scholar]
- Nunez PL, Srinivasan R. Electric fields of the brain: the neurophysics of EEG. Oxford University Press; New York: 2006. [Google Scholar]
- Nunez PL, Westdorp AF. The surface Laplacian, high resolution EEG and controversies. Brain Topogr. 1994;6(3):221–226. doi: 10.1007/BF01187712. [DOI] [PubMed] [Google Scholar]
- Nurnberger JI, Jr, Blehar MC, Kaufmann CA, York Cooler C, Simpson SG, Harkavy Friedman J, Severe JB, Malaspina D, Reich T. Diagnostic interview for genetic studies. Rationale, unique features, and training. NIMH Genetics Initiative. Arch Gen Psychiatry. 1994;51(11):849–859. doi: 10.1001/archpsyc.1994.03950110009002. [DOI] [PubMed] [Google Scholar]
- O'Donnell BF, Shenton ME, McCarley RW, Faux SF, Smith RS, Salisbury DF, Nestor PG, Pollak SD, Kikinis R, Jolesz FA. The auditory N2 component in schizophrenia: relationship to MRI temporal lobe gray matter and to other ERP abnormalities. Biol Psychiatry. 1993;34(1–2):26–40. doi: 10.1016/0006-3223(93)90253-a. [DOI] [PubMed] [Google Scholar]
- O'Donnell BF, McCarley RW, Potts GF, Salisbury DF, Nestor PG, Hirayasu Y, Niznikiewicz MA, Barnard J, Shen ZJ, Weinstein DM, Bookstein FL, Shenton ME. Identification of neural circuits underlying P300 abnormalities in schizophrenia. Psychophysiology. 1999;36(3):388–398. doi: 10.1017/s0048577299971688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9(1):97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Paivio A, Yuille JC, Madigan SA. Concreteness, imagery, and meaningfulness values for 925 nouns. J Exp Psychol. 1968;76(1):1–25. doi: 10.1037/h0025327. [DOI] [PubMed] [Google Scholar]
- Pearlson GD, Barta PE, Powers RE, Menon RR, Richards SS, Aylward EH, Federman EB, Chase GA, Petty RG, Tien AY. Medial and superior temporal gyral volumes and cerebral asymmetry in schizophrenia versus bipolar disorder. Biol Psychiatry. 1997;41(1):1–14. doi: 10.1016/s0006-3223(96)00373-3. [DOI] [PubMed] [Google Scholar]
- Pelletier M, Achim AM, Montoya A, Lal S, Lepage M. Cognitive and clinical moderators of recognition memory in schizophrenia: a meta-analysis. Schizophr Res. 2005;74(2–3):233–252. doi: 10.1016/j.schres.2004.08.017. [DOI] [PubMed] [Google Scholar]
- Perrin F, Pernier J, Bertrand O, Echallier JF. Spherical splines for scalp potential and current density mapping [Corrigenda EEG 02274, EEG Clin. Neurophysiol, 1990, 76, 565] Electroenceph Clin Neurophysiol. 1989;72(2):184–187. doi: 10.1016/0013-4694(89)90180-6. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Ford JM, White PM, Roth WT. P3 in schizophrenia is affected by stimulus modality, response requirements, medication status, and negative symptoms. Arch Gen Psychiatry. 1989;46(11):1035–1044. doi: 10.1001/archpsyc.1989.01810110077011. [DOI] [PubMed] [Google Scholar]
- Picton TW. The P300 wave of the human event-related potential. J Clin Neuropsychol. 1992;9(4):456–479. doi: 10.1097/00004691-199210000-00002. [DOI] [PubMed] [Google Scholar]
- Picton TW, Bentin S, Berg P, Donchin E, Hillyard SA, Johnson R, Jr, Miller GA, Ritter W, Ruchkin DS, Rugg MD, Taylor MJ. Guidelines for using human event-related potentials to study cognition: recording standards and publication criteria. Psychophysiology. 2000;37(2):127–152. [PubMed] [Google Scholar]
- Polich J. Updating P300: an integrative theory of P3a and P3b. Clin Neurophysiol. 2007;118(10):2128–2148. doi: 10.1016/j.clinph.2007.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polli FE, Barton JJ, Thakkar KN, Greve DN, Goff DC, Rauch SL, Manoach DS. Reduced error-related activation in two anterior cingulate circuits is related to impaired performance in schizophrenia. Brain. 2008;131(4):971–986. doi: 10.1093/brain/awm307. [DOI] [PubMed] [Google Scholar]
- Price CJ. The anatomy of language: contributions from functional neuroimaging. J Anat. 2000;197(3):335–359. doi: 10.1046/j.1469-7580.2000.19730335.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price CJ, Winterburn D, Giraud AL, Moore CJ, Noppeney U. Cortical localisation of the visual and auditory word form areas: a reconsideration of the evidence. Brain Lang. 2003;86(2):272–286. doi: 10.1016/s0093-934x(02)00544-8. [DOI] [PubMed] [Google Scholar]
- Ragland JD, Gur RC, Valdez JN, Loughead J, Elliott M, Kohler C, Kanes S, Siegel SJ, Moelter ST, Gur RE. Levels-of-processing effect on frontotemporal function in schizophrenia during word encoding and recognition. Am J Psychiatry. 2005;162(10):1840–1848. doi: 10.1176/appi.ajp.162.10.1840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi A, Stratta P, Mattei P, Cupillari M, Bozzao A, Gallucci M, Casacchia M. Planum temporale in schizophrenia: a magnetic resonance study. Schizophr Res. 1992;7(1):19–22. doi: 10.1016/0920-9964(92)90069-h. [DOI] [PubMed] [Google Scholar]
- Rugg MD, Curran T. Event-related potentials and recognition memory. Trends Cogn Sci. 2007;11(6):251–257. doi: 10.1016/j.tics.2007.04.004. [DOI] [PubMed] [Google Scholar]
- Rugg MD, Roberts RC, Potter DD, Pickles CD, Nagy ME. Event-related potentials related to recognition memory. Effects of unilateral temporal lobectomy and temporal lobe epilepsy. Brain. 1991;114(5):2313–2332. doi: 10.1093/brain/114.5.2313. [DOI] [PubMed] [Google Scholar]
- Salisbury DF, Shenton ME, Sherwood AR, Fischer IA, Yurgelun-Todd DA, Tohen M, McCarley RW. First-episode schizophrenic psychosis differs from first-episode affective psychosis and controls in P300 amplitude over left temporal lobe. Arch Gen Psychiatry. 1998;55(2):173–180. doi: 10.1001/archpsyc.55.2.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salisbury DF, Rutherford B, Shenton ME, McCarley RW. Button-pressing affects P300 amplitude and scalp topography. Clin Neurophysiol. 2001;112(9):1676–1684. doi: 10.1016/s1388-2457(01)00607-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salmelin R, Service E, Kiesilä P, Uutela K, Salonen O. Impaired visual word processing in dyslexia revealed with magnetoencephalography. Ann Neurol. 1996;40(2):157–162. doi: 10.1002/ana.410400206. [DOI] [PubMed] [Google Scholar]
- Saykin AJ, Gur RC, Gur RE, Mozley PD, Mozley LH, Resnick SM, Kester DB, Stafiniak P. Neuropsychological function in schizophrenia. Selective impairment in memory and learning. Arch Gen Psychiatry. 1991;48(7):618–624. doi: 10.1001/archpsyc.1991.01810310036007. [DOI] [PubMed] [Google Scholar]
- Saykin AJ, Shtasel DL, Gur RE, Kester DB, Mozley LH, Stafiniak P, Gur RC. Neuropsychological deficits in neuroleptic naive patients with first-episode schizophrenia. Arch Gen Psychiatry. 1994;51(2):124–131. doi: 10.1001/archpsyc.1994.03950020048005. [DOI] [PubMed] [Google Scholar]
- Shenton ME, Kikinis R, Jolesz FA, Pollak SD, LeMay M, Wible CG, Hokama H, Martin J, Metcalf D, Coleman M, et al. Abnormalities of the left temporal lobe and thought disorder in schizophrenia: A quantitative magnetic resonance imaging study. N Engl J Med. 1992;327(9):604–612. doi: 10.1056/NEJM199208273270905. [DOI] [PubMed] [Google Scholar]
- Silva-Pereyra J, Rivera Gaxiola M, Aubert E, Bosch J, Galan L, Salazar A. N400 during lexical decision tasks: a current source localization study. Clin Neurophysiol. 2003;114(12):2469–2486. doi: 10.1016/s1388-2457(03)00248-7. [DOI] [PubMed] [Google Scholar]
- Simons JS, Mayes AR. What is the parietal lobe contribution to human memory? Neuropsychologia. 2008;46(7):1739–1742. doi: 10.1016/j.neuropsychologia.2008.05.001. [DOI] [PubMed] [Google Scholar]
- Simons JS, Peers PV, Hwang DY, Ally BA, Fletcher PC, Budson AE. Is the parietal lobe necessary for recollection in humans? Neuropsychologia. 2008;46(4):1185–1191. doi: 10.1016/j.neuropsychologia.2007.07.024. [DOI] [PubMed] [Google Scholar]
- Simson R, Vaughan HG, Ritter W. The scalp topography of potentials associated with missing visual or auditory stimuli. Electroenceph Clin Neurophysiol. 1976;40(1):33–42. doi: 10.1016/0013-4694(76)90177-2. [DOI] [PubMed] [Google Scholar]
- Smith ME, Halgren E. Dissociation of recognition memory components following temporal lobe lesions. J Exp Psychol Learn Mem Cogn. 1989;15(1):50–60. doi: 10.1037//0278-7393.15.1.50. [DOI] [PubMed] [Google Scholar]
- Snodgrass JG, Corwin J. Pragmatics of measuring recognition memory: applications to dementia and amnesia. J Exp Psychol Gen. 1988;117(1):34–50. doi: 10.1037//0096-3445.117.1.34. [DOI] [PubMed] [Google Scholar]
- Spironelli C, Angrilli A, Stegagno L. Failure of language lateralization in schizophrenia patients: an ERP study on early linguistic components. Journal of Psychiatry and Neuroscience. 2008;33(3):235–243. [PMC free article] [PubMed] [Google Scholar]
- Spitzer RL, Williams JBW, Gibbon M, First MB. Structured Clinical Interview for DSM-III-R: Patient Edition. American Psychiatric Press; Washington, DC: 1990. [Google Scholar]
- Srinivasan R, Tucker DM, Murias M. Estimating the spatial Nyquist of the human EEG. Behav Res Methods Instrum Comput. 1998;30(1):8–19. [Google Scholar]
- Strandburg RJ, Marsh JT, Brown WS, Asarnow RF, Guthrie D, Higa J, Yee Bradbury CM, Nuechterlein KH. Reduced attention-related negative potentials in schizophrenic adults. Psychophysiology. 1994;31(3):272–281. doi: 10.1111/j.1469-8986.1994.tb02216.x. [DOI] [PubMed] [Google Scholar]
- Strik WK, Dierks T, Franzek E, Stober G, Maurer K. P300 asymmetries in schizophrenia revisited with reference-independent methods. Psychiatry Res. 1994;55(3):153–166. doi: 10.1016/0925-4927(94)90023-x. [DOI] [PubMed] [Google Scholar]
- Tendolkar I, Ruhrmann S, Brockhaus A, Pukrop R, Klosterkotter J. Remembering or knowing: electrophysiological evidence for an episodic memory deficit in schizophrenia. Psychol Med. 2002;32(7):1261–1271. doi: 10.1017/s0033291702006335. [DOI] [PubMed] [Google Scholar]
- Tenke CE, Kayser J, Fong R, Leite P, Towey JP, Bruder GE. Response- and stimulus-related ERP asymmetries in a tonal oddball task: a Laplacian analysis. Brain Topogr. 1998;10(3):201–210. doi: 10.1023/a:1022261226370. [DOI] [PubMed] [Google Scholar]
- Tenke CE, Kayser J, Shankman SA, Griggs CB, Leite P, Stewart JW, Bruder GE. Hemispatial PCA dissociates temporal from parietal ERP generator patterns: CSD components in healthy adults and depressed patients during a dichotic oddball task. Int J Psychophysiol. 2008;67(1):1–16. doi: 10.1016/j.ijpsycho.2007.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tenke CE, Kayser J. A convenient method for detecting electrolyte bridges in multichannel electroencephalogram and event-related potential recordings. Clin Neurophysiol. 2001;112(3):545–550. doi: 10.1016/s1388-2457(00)00553-8. [DOI] [PubMed] [Google Scholar]
- Tenke CE, Kayser J. Reference-free quantification of EEG spectra: combining current source density (CSD) and frequency principal components analysis (fPCA) Clin Neurophysiol. 2005;116(12):2826–2846. doi: 10.1016/j.clinph.2005.08.007. [DOI] [PubMed] [Google Scholar]
- Turetsky BI, Colbath EA, Gur RE. P300 subcomponent abnormalities in schizophrenia: I. Physiological evidence for gender and subtype specific differences in regional pathology. Biol Psychiatry. 1998;43(2):84–96. doi: 10.1016/S0006-3223(97)00258-8. [DOI] [PubMed] [Google Scholar]
- Turetsky B, Colbath EA, Gur RE. P300 subcomponent abnormalities in schizophrenia: II. Longitudinal stability and relationship to symptom change. Biol Psychiatry. 1998;43(1):31–39. doi: 10.1016/s0006-3223(97)00261-8. [DOI] [PubMed] [Google Scholar]
- Ullsperger M. Performance monitoring in neurological and psychiatric patients. Int J Psychophysiol. 2006;59(1):59–69. doi: 10.1016/j.ijpsycho.2005.06.010. [DOI] [PubMed] [Google Scholar]
- Umbricht DS, Bates JA, Lieberman JA, Kane JM, Javitt DC. Electrophysiological indices of automatic and controlled auditory information processing in first-episode, recent-onset and chronic schizophrenia. Biol Psychiatry. 2006;59(8):762–772. doi: 10.1016/j.biopsych.2005.08.030. [DOI] [PubMed] [Google Scholar]
- Urbach TP, Kutas M. Interpreting event-related brain potential (ERP) distributions: implications of baseline potentials and variability with application to amplitude normalization by vector scaling. Biol Psychol. 2006;72(3):333–343. doi: 10.1016/j.biopsycho.2005.11.012. [DOI] [PubMed] [Google Scholar]
- van der Stelt O, Frye J, Lieberman JA, Belger A. Impaired P3 generation reflects high-level and progressive neurocognitive dysfunction in schizophrenia. Arch Gen Psychiatry. 2004;61(3):237–248. doi: 10.1001/archpsyc.61.3.237. [DOI] [PubMed] [Google Scholar]
- van Veen V, Carter CS. The anterior cingulate as a conflict monitor: fMRI and ERP studies. Physiol Behav. 2002;77(4–5):477–482. doi: 10.1016/s0031-9384(02)00930-7. [DOI] [PubMed] [Google Scholar]
- van Veen V, Carter CS. Error detection, correction, and prevention in the brain: a brief review of data and theories. Clin EEG Neurosci. 2006;37(4):330–335. doi: 10.1177/155005940603700411. [DOI] [PubMed] [Google Scholar]
- Vidal F, Hasbroucq T, Grapperon J, Bonnet M. Is the 'error negativity' specific to errors? Biol Psychol. 2000;51(2–3):109–128. doi: 10.1016/s0301-0511(99)00032-0. [DOI] [PubMed] [Google Scholar]
- Vita A, Dieci M, Giobbio GM, Caputo A, Ghiringhelli L, Comazzi M, Garbarini M, Mendini AP, Morganti C, Tenconi F, et al. Language and thought disorder in schizophrenia: brain morphological correlates. Schizophr Res. 1995;15(3):243–251. doi: 10.1016/0920-9964(94)00050-i. [DOI] [PubMed] [Google Scholar]
- Vocat R, Pourtois G, Vuilleumier P. Unavoidable errors: a spatio-temporal analysis of time-course and neural sources of evoked potentials associated with error processing in a speeded task. Neuropsychologia. 2008;46(10):2545–2555. doi: 10.1016/j.neuropsychologia.2008.04.006. [DOI] [PubMed] [Google Scholar]
- Wagner AD, Shannon BJ, Kahn I, Buckner RL. Parietal lobe contributions to episodic memory retrieval. Trends Cogn Sci. 2005;9(9):445–453. doi: 10.1016/j.tics.2005.07.001. [DOI] [PubMed] [Google Scholar]
- Wegesin DJ, Nelson CA. Effects of inter-item lag on recognition memory in seizure patients preceding temporal lobe resection: evidence from event-related potentials. Int J Psychophysiol. 2000;37(3):243–255. doi: 10.1016/s0167-8760(00)00101-x. [DOI] [PubMed] [Google Scholar]
- Weinberger DR, Suddath RL, Casanova MF, Torrey EF, Kleinman JE. Crow's 'lateralization hypothesis' for schizophrenia. Arch Gen Psychiatry. 1991;48(1):85–87. doi: 10.1001/archpsyc.1991.01810250087013. [DOI] [PubMed] [Google Scholar]
- Wexler BE, Stevens AA, Bowers AA, Sernyak MJ, Goldman-Rakic PS. Word and tone working memory deficits in schizophrenia. Arch Gen Psychiatry. 1998;55(12):1093–1096. doi: 10.1001/archpsyc.55.12.1093. [DOI] [PubMed] [Google Scholar]
- Yonelinas AP. Components of episodic memory: the contribution of recollection and familiarity. P. 2001;356(1413):1363–1374. doi: 10.1098/rstb.2001.0939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yonelinas AP, Otten LJ, Shaw KN, Rugg MD. Separating the brain regions involved in recollection and familiarity in recognition memory. J Neurosci. 2005;25(11):3002–3008. doi: 10.1523/JNEUROSCI.5295-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.