Abstract
Decades of intensive research of primary cardiac pacemaker, the sinoatrial node, have established potential roles of specific membrane channels in the generation of the diastolic depolarization, the major mechanism allowing sinoatrial node cells generate spontaneous beating. During the last three decades, multiple studies made either in the isolated sinoatrial node or sinoatrial node cells have demonstrated a pivotal role of Ca2+ and, specifically Ca2+-release from sarcoplasmic reticulum, for spontaneous beating of cardiac pacemaker. Recently, spontaneous, rhythmic local subsarcolemmal Ca2+ releases from ryanodine receptors during late half of the diastolic depolarization have been implicated as a vital factor in the generation of sinoatrial node cells spontaneous firing. Local Ca2+ releases are driven by a unique combination of high basal cAMP production by adenylyl cyclases, high basal cAMP degradation by phosphodiesterases and a high level of cAMP-mediated PKA-dependent phosphorylation. These local Ca2+ releases activate an inward Na+-Ca2+ exchange current which accelerates the terminal diastolic depolarization rate and, thus, controls the spontaneous pacemaker firing. Both the basal primary pacemaker beating rate and its modulation via β-adrenergic receptor stimulation appear to be critically dependent upon intact RyR function and local subsarcolemmal sarcoplasmic reticulum generated Ca2+ releases. This review aspires to integrate the traditional viewpoint that has emphasized the supremacy of the ensemble of surface membrane ion channels in spontaneous firing of the primary cardiac pacemaker, and these novel perspectives of cAMP-mediated PKA-dependent Ca2+ cycling in regulation of the heart pacemaker clock, both in the basal state and during β-adrenergic receptor stimulation.
Keywords: sinoatrial nodal cells, local Ca2+ release, ryanodine receptor, ionic channels, beta-adrenergic receptor stimulation, phosphodiesterase
I. Introduction
As the knowledge base within a given field of research expands, classical perspectives often clash with novel insights. A transient state of confusion prevails until older and newer concepts become integrated into new “dogma” (which usually has a finite life of its own, as new knowledge evolves). The field of cardiac pacemaker cell research is no exception. The sinoatrial (SA) node, the primary physiological pacemaker of the heart, initiates more than 3 billion heart beats during a life span. SA node cells (SANC) differ from primary contractile myocardial cells in their unique ability to generate spontaneous action potentials (APs), due to a spontaneous gradual change of the membrane potential called diastolic depolarization (DD, Fig.1B).
Figure 1.
A, Schematic illustration of the basal and reserve cardiac pacemaker regulation by cAMP-mediated, PKA-dependent Ca signaling. Intracellular Ca cycling (in red) operates in tight cooperation with the classic ensemble of membrane ion channels. This interaction, i.e. intracellular Ca-surface membrane ion channel coupling, produces robust heartbeats: submembrane Ca releases during late DD ignite membrane excitations; surface membrane depolarization, in turn, via the AP-induced Ca transient, resets the periodicity of Ca cycling during each spontaneous cycle. The elevation of Ca also inactivates ICa,L. Constitutive activation of the basal AC activity results in a high level of cAMP and cAMP/PKA dependent phosphorylation. Note that Ca, cAMP or PKA-dependent phosphorylation modulate not only the function of Ca cycling proteins (phospholamban, ryanodine receptors, L-type Ca channel, NCX current) but also sarcolemmal ion channels (If, Ist, IK). Note that L-type Ca channel and NCX current are both Ca cycling proteins and surface membrane current generators. The basal Ca/cAMP-PKA “feed-forward” regulation is kept in check by a high basal PDE activity, which prevents cAMP/PKA signaling to become excessive i.e., acts as negative feedback mechanism to prevent an excessive basal beating rate, and to insure reserve cAMP/PKA modulation of spontaneous beating via activation of β-ARs.
B, Schematic illustration of spontaneous SANC APs and major currents involved in generating the linear and nonlinear components of diastolic depolarization (DD). The deviation of the membrane potential from the dashed line (linear DD) occurs concomitantly with an LCR-induced increase in subsarcolemmal [Ca] and activation of inward NCX current. Thus, the interaction of SR Ca cycling and membrane ion channel proteins ignites the membrane to generate an AP.
Early theories of the initiation of the heartbeat included both intracellular metabolism and surface membrane events [1]. But later, as the cardiac pacemaker field adopted the Hodgkin-Huxley theory of membrane excitation that emphasized voltage- and time-dependent gating of various surface membrane channels, the ensemble of surface membrane ion channels in pacemaker cells was envisioned as the clock that regulates normal automaticity. An extensive research effort then attempted to target which of the surface membrane ion channels had the major role in controlling spontaneous DD and sinoatrial node pacemaker firing. Sequential ideas regarding the importance of ionic current candidates emerged, e.g. decay of an outward delay rectifier potassium current, Ik, or activation of inward currents, e.g. hyperpolarization activated current, If, and other ionic currents gated by membrane depolarization, i.e. L-type calcium (Ca) current, ICa,L, T-type Ca current, ICa,T, sustained current, Ist, Na-Ca exchange current, INCX, and others (Fig. 1). For some time now, the If channel has been considered to be the most important ion channel involved in the regulation of DD in cardiac pacemaker cells, and is often referred to as “the pacemaker channel.”
But, over time, it became apparent that in addition to voltage and time, surface membrane electrogenic molecules were strongly modulated by Ca and phosphorylation. The studies of a sub-group of pacemaker cell researchers focusing upon intracellular Ca movements spawned the idea that intracellular Ca is an important player in controlling pacemaker cell automaticity [2-7]. This elevated the status of INCX current as a major Ca-activated electrogenic mechanism. Like atrial, ventricular and other excitable cells, SANC rhythmically cycle Ca during each duty cycle (Fig. 1). Confocal Ca imaging beneath sarcolemma, coupled with simultaneous recordings of membrane potential via perforated patch-clamp technique, have provided convincing evidence that critically timed local Ca releases occur during late DD and activate NCX current, causing exponential increase in the DD rate to drive the membrane potential to the threshold of AP upstroke [8-10]. Such rhythmic, sarcoplasmic reticulum (SR) generated spontaneous local Ca releases (LCRs) beneath the cell membrane driven by a high basal cAMP and cAMP-mediated PKA-dependent phosphorylation has been referred to as an intracellular Ca clock, i.e. a component that interacts with the classic sarcolemmal membrane voltage clock to form the overall pacemaker clock [11-14].
Needless to say, there is presently some degree of uncertainty about the relative roles of If vs. that of intracellular Ca cycling in controlling normal SA node pacemaker automaticity. This review aspires to bridge the gap between traditional perspectives that have emphasized the ensemble of surface membrane ion channels in spontaneous firing of the primary cardiac pacemaker and novel perspectives of cAMP-mediated, PKA-dependent Ca cycling in regulation of the pacemaker cell clock, both in the basal state and in response to β-adrenergic receptor (β-AR) stimulation (Fig. 1).
2. The basal cAMP level is linked to spontaneous beating in cardiac pacemaker tissues/cells
a. cAMP effects on basal spontaneous pacemaker activity
Early studies had documented that exposure of the isolated rabbit SA node to adenylyl cyclase (AC) inhibitors suppressed spontaneous beating, and that this effect could be reversed when dibutyryl cAMP was added to the bath solution [15]. An ionophoretic injection of cAMP into Purkinje fibers, or superfusion of isolated rabbit SA node with a cAMP containing solution, produced a marked increase in the DD slope, and a concomitant increase in the spontaneous beating rate [16-18]. These results support the idea that intracellular cAMP accelerates the spontaneous pacemaker activity. One creative model proposed that a release of endogenous catecholamines, stored within pacemaker cells, activated adrenergic receptors in an autocrine manner, leading to activation of adenylyl cyclases (AC) and an increase in the intracellular cAMP level [19]. In this model, cAMP-mediated protein kinase activation increased the phosphorylation level of membrane proteins and Ca channels, allowing Ca to enter, depolarizing the cell. Catecholamine discharge from the pacemaker cell in this model was Ca-dependent, so that, the more Ca entered the cell, the higher was the rate of the catecholamine discharge; this positive feedback loop resulted in an exponential depolarization. Within a given cycle, the cell catecholamine pool became depleted, the cell was repolarized by Ca extrusion, the vesicles of catecholamine storage became replenished and began again to spontaneously discharge catecholamines to initiate the next cycle [19]. In spite of the appeal of this model, it did not work, at least in its existing form, because, the prolonged application of β-AR blockers did not alter the spontaneous activity of the rabbit SA node [20].
The original measurements of the basal cAMP level in SANC, made almost 3 decades later, showed that basal cAMP level is several fold higher in isolated rabbit SANC than in atrial or ventricular myocytes [11]. A pivotal role of constitutive AC activation in the generation of high basal cAMP production, and its crucial role for spontaneous beating of SANC, had been established by specific AC inhibition, which markedly decreased level of cAMP and suppressed SANC spontaneous beating [21; 22]. A link between Ca and cAMP soon followed with the discovery of Ca-activated type of ACs, i.e. AC1 and AC8, in rabbit and guinea-pig SANC [21-23], and localization of the basal Ca-activated AC activity in SANC within lipid raft microdomains [23].
b. A high level of basal phosphodiesterase (PDE) activity controls basal cAMP level in cardiac pacemaker cells
The cell cAMP level is a result of a balance between cAMP production by AC and its hydrolysis into 5′-AMP by cyclic nucleotide phosphodiesterases (PDE), the only known mechanism to degrade cAMP [24]. An earlier observation by Tsien et al. in Purkije fibers provided a clue that the level of cAMP in pacemaker cells was regulated by basal PDE activity, because various PDE inhibitors produced a marked increase in the DD slope and shortened AP plateau, mimicking effects of cAMP injection [25]. Later, several studies demonstrated that, similar to Purkinje fibers, suppression of PDE activity in isolated SA node [26-28] produced an increase in cAMP level [26] and an augmentation of the DD slope [27, 28] that resulted in a marked acceleration of the spontaneous beating rate. The extent of PDE-dependent control of the basal cAMP level, and of spontaneous firing of isolated rabbit SANC, has recently been demonstrated: inhibition of total PDE activity by a broad-spectrum PDE inhibitor, IBMX, produces a 9-fold increase in the cAMP level and ~50% increase in the SANC spontaneous beating rate (both effects being greater than those of the saturating concentration of β- AR agonist, isoproterenol [29]. The suppression of different PDE subtypes in isolated rabbit SANC revealed that PDE3 and PDE4 represent a major part of the basal PDE activity [30]. Thus, basal PDE-dependent control counters the effect of constitutive AC activation and restricts the level of basal cAMP and spontaneous firing rate of SANC.
c. Contribution of ion channels to spontaneous beating and their regulation by cAMP or PDE inhibition
Hyperpolarization activated current, If
In 1980 Brown and DiFrancesco using small strips of rabbit SA node identified If, “funny” current [31]. The current was called “funny” because it had unusual features: it was an inward current activated on hyperpolarization and carried by both Na+ and K+ ions with an activation threshold between −50 and −65 mV [32]. Since If current is activated within a voltage range typical for the pacemaker potential, this current had been proposed as the predominant ionic current underlying cardiac pacemaking [32, 33]. If current is widely expressed among species and present almost in all cardiac pacemaker cells. However, If is absent in: amphibian sinus venosus from the cane toad Bufo marinus [34]; some spontaneously beating SANC from monkey [35]; and ~50% of spontaneously beating rat SANC [36].
Controversial views regarding the actual role of If in the generation of the pacemaker potential and regulation of spontaneous SANC beating have appeared from nearly the time of If discovery and continue to be a matter of debate till nowadays [37-50]. On the basis of the negative activation threshold of If current and its slow time course of activation, concerns were raised regarding If current contribution in normal pacemaker activity [51]. Many laboratories using various If inhibitors tried to evaluate the contribution of If to cardiac pacemaking. The results of multiple studies in isolated SA nodes of different species consistently demonstrated a reduction of the spontaneous beating rate after If suppression, and the magnitude of this reduction ranged from 3% to 24%. Only a single study showed a dramatic ~53% suppression of guinea-pig SA node firing (Table 1A). The results acquired in SANC isolated from different species are consistent with the data in isolated SA node, and estimate the contribution of the If current to cardiac pacemaking to be between ~3% and 30% (Table 1B). Thus, although If current does indeed make a substantial contribution to the pacemaker beating rate, the data in table 1 strongly suggest, to us, that If current is not a unique determinant of the spontaneous SA node beating rate, and that the combined control of other mechanisms have at least a two fold greater impact on the pacemaker rate.
Table 1.
A. Changes in the spontaneous beating rate of the intact isolated sinoatrial (SA) node or isolated heart after suppression of the If current.B. Changes in the spontaneous beating rate of isolated sinoatrial node cells (SANC) after suppression of the If current.
| Isolated sinoatrial node, species, [reference number] |
Average beating rate before drug (beat/min) |
Drug and concentration (μM) |
Decrease in the beating rate produced by If suppression (%) |
|---|---|---|---|
| Small SAN preparations, rabbit, [37] | 138 | Cs+, 2 mM | 3 |
| Isolated SA node, rabbit, [38] | ~125 ~125 ~125 |
Cs+, 2 mM UL-FS-49, 1 μM ZD7288, 3 μM |
12 16 13 |
| Isolated SA node, rabbit, [39] | 162 | Cs+, 2 mM | 24 |
| Isolated SA node, guinea-pig, [39] | 176 | ZD7288, 0.64 μM | 53 |
| Isolated SA node, guinea-pig, [40] | ~126 ~126 |
Cs+, 1 mM Cs+, 2 mM |
3 7.3 |
| Isolated SA node, dog, [41] | 95 | ZD7288, 3 μM | 8 |
| Isolated SA node, mice, [42] | 339 | Cs+, 1 mM | 20 |
| Isolated SA node, mice [43] | 335 | ZD7288, 1 μM | 13 |
| Isolated rabbit heart, [39] | 171 | Cs+, 2 mM | 21 |
| Isolated sinoatrial node cells (SANC) or sinus venous cells (SVC), species, [reference number] |
Average beating rate before drug (beat/min) |
Drug and concentration (μM) |
Decrease in the beating rate produced by If suppression (%) |
|---|---|---|---|
| SANC, rabbit [44] | 166 | Cs+, 2 mM | 30 |
| SANC, rabbit, [45] | 205 | Cs+, 2 mM | 5 |
| SANC, rabbit, [46] | 190 | Ivabradine, 0.3μM | 23 |
| SANC, rabbit, [47] | 174 | Ivabradine, 3μM | 16 |
| SANC, porcine [48] | 80 | Cs+, 2 mM | 10 |
| SANC, human [49] | 72 | Cs+, 2 mM | 26 |
| SANC, guinea-pig [50] | 201 | ZD7288, 3 μM | 14 |
A direct effect of cAMP on the If current was shown by DiFrancesco in 1991, who discovered that cAMP activated If by shifting its activation curve to more positive voltages [52]. PDE inhibition by broad-spectrum PDE inhibitor, IBMX, or amrinone increased If by shifting the current activation to more positive potentials, an effect similar to an increase in the cAMP level [53, 54]. However, when If current in rabbit SANC was suppressed by pretreatment with 2 mmol/L Cs+, resulting in ~10% reduction of the spontaneous beating rate, IBMX produced a similar ~50% increase in the spontaneous firing rate, either in the absence or presence of Cs+, suggesting, to us, a minor role for If in the positive chronotropic effect of PDE inhibition (Fig. 8A) [29].
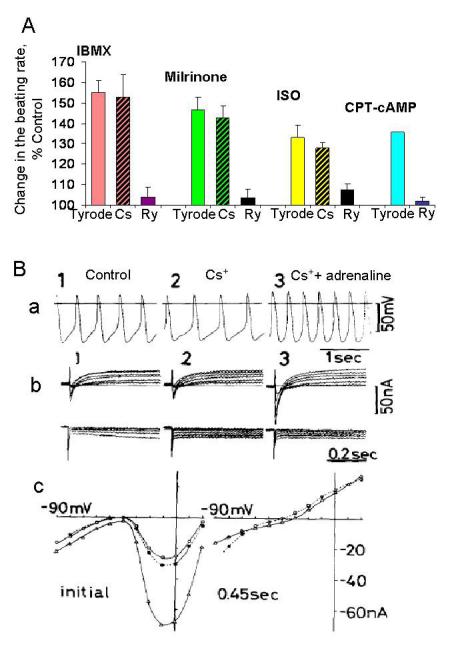
Figure 8.
A, Relative increase in the average firing rate of rabbit SANC by PDE inhibition (100 μmol/L IBMX, 50 μmol/L milrinone); β-AR stimulation (1 μmol/L ISO), before (Tyrode) and after either inhibition of If current with Cs+ (2 mmol/L, Cs) or suppression of LCRs with ryanodine (3 μmol/L, Ry). Last columns, the effect of CPT-cAMP before (Tyrode) and after suppression of LCRs with ryanodine. B (from [37]), Effects of adrenaline on the spontaneous beating rate of the small rabbit SA node preparation in the presence of If current blockade. Panel (a), APs recorded in the control (1), in presence of 5 mmol/L CsCl (2), and on addition of 0.5 μmol/L adrenaline (3). The spontaneous rate decreased from 138 bpm to 113 bpm in the CsCl-containing solution; addition of adrenaline enhanced the rate to 195 bpm. Membrane currents recorded in each solution are illustrated in the panel (b), with clamp pulses applied in 10 mV steps from a holding potential of −40mV in the depolarizing (upper panel) and hyperpolarizing direction (lower panel). Note, that in Cs+-containing solutions (panel b2, b3), adrenaline fails to generate the time-dependent current ih. Panel (c) shows current voltage relations in the control (filled circles), in the Cs+-containing Tyrode (open circles) and in the presence of adrenaline (triangle). isl is markedly enhanced by adrenaline in the presence of Cs+.
The channels underlying If current are encoded by four genes (HCN1-4). When heterologously expressed, HCN1-4 channels have distinct gating characteristics and cAMP sensitivities, i.e., HCN2, HCN3, and HCN4 channels are sensitive to cAMP, while HCN1 channels are almost insensitive. In spite of species variation, the dominant HCN isoform present in the adult SA node is HCN4, accounting for ~80% of the total HCN message [55; 56]. In both the rabbit and murine SA node ~80% of If current is carried by HCN4, while the residual 20% is carried by HCN1 and HCN2 in the rabbit and in murine SA node, respectively [57].
Homozygous HCN2 and HCN4 knockout mice provide an additional and unique opportunity to directly study the contribution of If current and specific HCN2 and HCN4 isoforms to cardiac pacemaking. A deletion of HCN2 reduced If by ~30% without changing current activation [58]. The average heart rate at rest and during exercises was not different between wild-type and HCN2-deficient mice (Figure 2C). Marked dysrhythmia, however, was present in mutant mice [58].
Figure 2. Neither HCN4 nor HCN2 are required for basal mouse heart beating or for flight or fight response.
A (modified from [60]), Mean heart rates of control and HCN4 knockout mice do not differ significantly during exercises (running on a treadmill) or stimulation of β-AR with isoproterenol (ISO, 0.5 mg/kg). B (modified from [61]), Mean heart rate of adult wild-type (open columns, n=8) and HCN4-KiT mice with controlled deletion of HCN4 only within the SA and AV nodes (filled columns, n=7) before (−) and after (+) tamoxifen injection. Heart rates under basal conditions and after application of isoproterenol (0.1 mg/kg) did not significantly differ between the genotypes. n.s., not significant. C (based on data in table 1, from: [58]), Mean heart rates of control and knockout HCN2-deficient mice do not differ significantly during: normal activity; exercises or stimulation by ISO. D (based on data in Fig. 4C from [62]), Heart beating rates in freely moving adult wild type and heterozygous HCN+/R669Q mice (in which cAMP binding to HCN4 is suppressed) during normal activity or swimming, and in the isolated beating hearts from wild type mice and heterozygous HCN+/R669Q mice. There are no significant differences among groups.
Homozygous HCN4 knockout mice, generated by deleting exon 4 of the HCN4 gene, are embryonic lethal [59], suggesting a crucial, but yet to be defined role of HCN4 isoform in the embryonic heart. It seems that the formation of the heart, itself, requires the presence of HCN4 [59]. Conditional knock out of HCN4 in adult mice reduced If by ~75-80%, and while this was accompanied by persistent sinus pauses, the average heart rate in knockout mice was similar to the wild type mice [60] (Figure 2A). Controlled deletion of HCN4 only within the SA and atrio-ventricular nodes of adult mice confirmed the results obtained in mice with conditional knock out of HCN4 [61] (Figure 2B). Mice with a homozygous mutation of a single amino-acid exchange (R669Q) that prevented binding of cAMP to HCN4 channels, HCN4R669Q/R669Q mice, are also embryonic lethal [62]. The heart rate of adult heterozygous HCN+/R669Q mice at rest was ~23% slower than in the wild type mice, but the intrinsic rate of isolated hearts was not different between HCN+/R669Q and wild type mice [62] (Figure 2D). Thus, the results of studies in which either HCN2 or HCN4 channel were genetically modified (Figure 2) are consistent with the previous studies (in which If current was blocked, Table 1), and suggest that the most important function of HCN2, HCN4 channels and If current may be to stabilize the pacemaker rhythm, the idea spawned by German groups [58-62].
Mutations discovered within human HCN4 gene are associated with cardiac arrhythmias. A heterozygous deletion (1631delC) in exon 5 of the human HCN4 gene has been observed in a single person, and results in truncated C-terminus lacking the cyclic nucleotide-binding domain [63]. The mutated If channel is insensitive to cAMP, and exhibits altered deactivation kinetics [63]. The patient with this mutation had idiopathic sinus bradycardia ~41 bpm [63]. However, during exercises the heart rate of this patient increased, similar to a healthy person, by ~150% (from 41 bpm at rest to 101 bpm during exercises) [63]. Another type of HCN4 mutation (a point mutation in CNBD, cAMP-binding domain) has been detected in an entire family. This mutation shifts If channel activation to more negative potentials (cAMP modulation remains intact) and the heart rate was reduced by ~29% [64]. These data indicate that If current is implicated in the generation of rhythmic basal beating rate in humans, and, suggest to us, that the magnitude of its contribution is consistent with that of If current in the basal spontaneous beating of the isolated SA node, or of isolated SANC of other species (Table 1).
Recently, artificial biological pacemakers, based upon overexpression of If current have been created [65, 66]. Neonatal ventricular myocytes infected with an adenovirus carrying murine HCN2 expressed a marked ~5-fold elevated If current, and a ~2-fold increase in the spontaneous beating rate. Injection of the adenoviral murine HCN2 construct into dog left atrium resulted in spontaneous rhythms during vagal stimulation, which was used to silence physiological SA node rhythm [65, 66]. Artificial pacemaker activity was also recorded when human mesenchymal stem cells, transfected with murine HCN2 gene, were injected in the canine left ventriclar myocardium [65, 66]. These data show that an inward current generator, in that case markedly elevated If due to overexpressed HCN2 channel, may be able to drive the heart. But, it is not clear whether this result is specific to HCN channel or whether overexpression of any inward current generator would produce a similar result.
T-type Ca current
Voltage clamp studies of single rabbit SANC have identified two types of Ca currents: a long lasting, L-type Ca current, ICa;L, activated at a holding potential of −40 mV, and a transient, rapidly decaying T-type Ca current, ICa;T, activated at a holding potential −80 mV [67]. ICa;T is activated within the voltage range of the pacemaker potential and, thus, might contribute to spontaneous depolarization. The contribution of ICa;T to spontaneous activity of rabbit SANC has been studied in the presence of 40 μmol/L Ni2+ (since this current is blocked by 40 μmol/L Ni2+, leaving L-type Ca current almost unaffected). Most studies have demonstrated that in the presence of Ni2+ there was ~10% decrease in the beating rate confirming that, indeed, ICa;T participates in the generation of cardiac pacemaking [45, 67, 68].
Three different types of α1 subunit for T-type Ca channel had been discovered [69], i.e. Cav3.1, Cav3.2 and Cav3.3. Both Cav3.1and Cav3.2 have been discovered in the mice heart and the contribution of ICa;T in cardiac pacemaking has been studied using mice lacking either Cav3.2 [70] or Cav3.1 [71] isoforms. Mice lacking Cav3.2 channels have no change in the beating rate, suggesting that this isoform is not vital for cardiac pacemaking [70, 71]. In contrast, ICa;T was completely abolished in SANC from mice lacking Cav3.1, suggesting that this isoform is the major one in adult mouse SANC. In line with this idea, the spontaneous beating rate of isolated SANC from mice lacking Cav3.1 is decreased by 30% [71, 72]. But when the amplitude of ICa;T is compared among various mammalian species, it is became clear that while ICa;T is substantial in mouse SANC, it is smaller in guinea-pig SANC, is further reduced in rabbit SANC, and is almost absent in porcine SANC, demonstrating a reverse dependence upon the animal size [73]. It is not clear whether ICa;T is expressed in human SANC, since there have been no reports either in human cardiomyocytes or SANC [73].
L-type Ca current
In primary SANC ICa,L is a main source of the inward current for the AP upstroke and, thus, is obligatory for the generation of rhythmic spontaneous APs [51]. L-type Ca channels are a multi-subunit complex, comprising a central pore subunit (α subunit) and regulatory/ auxiliary subunits (β, α2/δ, and γ) [69, 74]. Four genes encode L-type Ca channel α1-subunits in mammals (alpha S, C, D, and F); α1C represents the most abundant isoform in the cardiovascular system, but recently α1D has been detected in the mouse SA node [69, 75]. L-type Ca current with α1D as a central pore subunit is activated at a lower voltage range relative to α1C subunit, suggesting that this channel might be involved in the generation of DD. The density of ICa,L in Cav1.3 knockout mice compared with wild-type mice was reduced by 79%, demonstrating a marked contribution of Cav1.3 channel in total ICa,L in mouse SANC [69, 71]. Indeed, sinus bradycardia, both in isolated hearts and in intact animals, has been observed in α1D knockout mice [69, 75]. The presence of low-voltage-activated ICa,L has also been demonstrated in rabbit SANC, but the exact contribution of this current to the total ICa,L in rabbit is still unknown [76].
Flash photolisys of caged cAMP in rabbit SANC markedly increased both ICa,L and firing frequency [77]. Application of membrane-permeable cAMP in ventricular myocytes produced a several fold increase in ICa,L current [78]. Suppression of total PDE activity in rat ventricular myocytes with IBMX increased [cAMP]i by 70% and ICa,L by 120%; but specific PDE1, PDE2, PDE3 or PDE4 inhibitors applied separately had no stimulatory effects on ICa,L, and small effects on basal [cAMP]i [79]. In contrast to ventricular myocytes, a specific PDE3 inhibitor, amrinone, increased basal ICa,L amplitude by ~23% in guinea-pig SANC [54], and another PDE3 inhibitor, milrinone, increased basal ICa,L amplitude by ~45% in rabbit SANC [29]. These studies did not consider whether the effect of cAMP on ICa,L was, in fact, due to cAMP, or to cAMP-mediated protein kinase A (PKA)-dependent phosphorylation, which accompanied an increase in cAMP [29, 54, 79]. Other studies, however, suggested that the cAMP-induced increase in Ca current depends on activation of cAMP-mediated PKA-dependent phosphorylation which markedly increases the opening probability of the channel [80]. A membrane associated anchoring protein, AKAP, has been shown to target PKA to the L-type Ca channel [81; 82], by interacting directly with a1C-subunit of the channel via a leucine zipper motif [83]. The effect of release of caged cAMP in rabbit SANC to markedly enhance ICa,L is completely abolished after pretreatment with the selective PKA inhibitor, Rp-cAMPS [77]. While there is no functionally relevant basal PKA-dependent phosphorylaton of L-type Ca channel in ventricular myocytes, in rabbit SANC ICa,L declines by ~80% in the presence of specific PKA inhibitor peptide, PKI, suggesting the presence of functionally relevant basal PKA-dependent phosphorylation of L-type Ca channel [84].
Delayed rectifier potassium current (IK)
The essential role of potassium conductance for generation of pacemaker potential was initially proposed by Weidmann in 1951 [85]. Several decades of intensive studies in Purikinje fibers and SA node tissue identified numerous K currents [86]. However, data from the single SANC patch-clamp recordings recognized a delayed rectifier potassium current (IK) as the most important K current. This current regulates the repolarization of SANC action potential, and its deactivation plays a key role in the pacemaker depolarization [51]. IK conductance slowly declines following repolarization decreasing the hyperpolarizing effect of IK, and increasing the role of inward current to shift the membrane to more positive potentials [51]. IK has rapid (IKr) and slow (IKs) activating components [87]. While IK in guinea-pig and porcine SA node is mostly represented by IKs, in rat and rabbit SA node IKr is the major component [35].
Recently it has been shown in rabbit SANC that basal PDE activation controls the delayed rectifier K+ current, IK: PDE inhibition by IBMX substantially increases (by ~12%) the amplitude of IK,tail current and shifts (by ~5 mV) IK activation to more negative potentials [29]. In ventricular myocytes the slow component of the delayed rectifier K+ current, IKs, is also regulated by cAMP, which increases IKs, amplitude, slows its deactivation kinetics, and shifts its activation curve to more negative potentials [88,89]. These changes are mainly attributable to cAMP-mediated PKA-dependent phosphorylation [88,89]. The effects of cAMP stimulation are prevented by global disruption of PKA anchoring, suggesting that AKAPs are required for cAMP-mediated PKA-dependent regulation of the IKs channel [90].
NCX current
NCX current in its normal, forward, mode extrudes Ca by exchanging intracellular Ca for extracellular Na+, with coupling ratio per transport cycle of 1:3 resulting in inward NCX current. Early patch clamp studies suggested that NCX current may contribute to the last third of DD [91], but the contribution of NCX current to pacemaker depolarization and spontaneous beating of cardiac pacemaker cells had remained an area of debate for a long time (for detailed review see Noble, [86]). The role of NCX current in cardiac pacemaking and the contribution of this current into DD was later clarified in cat latent atrial pacemaker cells isolated from the Eustachian ridge [92]. This study clearly demonstrated that the slow inward current during DD was, indeed, NCX exchange current and was activated by SR Ca release [92].
The idea that the NCX exchanger could potentially be phosphorylated was suggested in 1990 by Philipson, who cloned NCX and discovered several phosphorylation sites which could be a substrate for either CaMKII or PKA-dependent phosphorylation [93]. Recently, it was shown that in rat heart a number of proteins are associated with NCX1 on its intracellular side, including catalytic and regulatory subunits of PKA and the catalytic subunit of PKC [94]. However, the functional significance of NCX phosphorylation remains controversial [94-96]. There are no reports that have been focused on NCX phosphorylation in SANC.
3. Ca signaling in pacemaker cells
a. Effects of [Ca] on ion channels in SANC
That an increase in extracellular [Ca] increases the rate of DD and spontaneous beating of the SA node had been known for over 30 years [97, 98]. But most of studies have failed to note or emphasize that intracellular Ca also increases when extracellular [Ca] increases. The gating of several time and voltage dependent ion currents, i.e. If, ICa,T, ICa,L, IK, Ist, chloride current, and NCX current are Ca dependent. Since these currents become activated during DD, it has been widely accepted that Ca-induced changes in ionic currents may be involved in changes of the pacemaker potential and spontaneous beating of SANC.
Effects of [Ca] on If
The Ca-dependent increase of If and shift of If activation curve in a positive direction was initially explained by a direct effect of [Ca]i on the If channel [99, 100]. However, experiments using inside-out macropatch techniques failed to show a direct effect of [Ca] on the If current in rabbit SANC [101]. The chelation of intracellular Ca with BAPTA in guinea-pig SANC significantly reduced the amplitude of If current and altered the voltage dependence of If activation. But in the presence of the calmodulin blocker W-7, BAPTA had no significant effect on If current, suggesting that effects of [Ca] on If involved a Ca calmodulin-sensitive mechanism without direct action of Ca on If channel [102]. Later, these results were explained on the basis of Ca-calmodulin activation of AC activity in SANC to increase cAMP which, in its turn, activates If [21]. The link between Ca activation of AC and spontaneous beating of intact SANC, however, requires signaling downstream of cAMP, i.e. cAMP-mediated PKA-dependent phosphorylation [11].
Effects of [Ca] on ICa,T and ICa,L
Both ICa,T and ICa,L are strongly regulated by [Ca]. An elevation of extracellular [Ca]o from 0.5 to 5 mmol/L causes a 6-fold increase in the amplitude of ICa,T and ICa,L in rabbit SANC [67]. Inactivation of ICa,L confers a negative feedback on L-type Ca channel to terminate Ca entry during AP [103], and the decay kinetics of ICa,L are highly dependent on the local Ca milieu [78, 104]. In contrast to rabbit SANC, an elevation of intracellular Ca from pCa 9 to 6.8 in canine Purkinje cells progressively suppressed ICa,L, but enhanced ICa,T [105].
Effects of [Ca] on delayed rectifier potassium current (IK)
The delayed rectifier potassium current (IK) is strongly regulated by [Ca]. In guinea-pig ventricular myocytes IK increases 3-fold when [Ca]i is elevated from 10 to 100 nmol/L, an effect probably due to the increased number and open probability of functional IK channels [106]. The amplitude of IKr, the rapid component of IK, is markedly suppressed by buffering [Ca]i with BAPTA-AM in guinea-pig ventricular myocytes [107].
Effects of [Ca] on sustained current, Ist
Ist is a nicardipine-sensitive inward Na+ current that becomes activated and exhibits very small inactivation over the membrane potential range encompassing DD [108, 109]. This current is considered, by some researchers, to be an important current that underlies the spontaneous DD of SANC [110]. In contrast to other ionic currents in SANC, Ist is increased by lowering extracellular [Ca]o [108]. Though Ist current has characteristics similar to those of ICa,L [110], it is distinct from ICa,L, being activated at more negative potentials, achieving peak amplitude at about −50 mV, and having reversal potential close to +37 mV [108]. Although 40 μmol/L Ni2+ blocks Ist by ~50% [108], neither specific blockers for Ist nor the molecular origin have been identified.
Effects of [Ca] on Ca-activated Cl-current (ICl(Ca))
This current, ICl(Ca), is present in only ~30% of spontaneously active rabbit SANC. Blockade of ICl(Ca) in rabbit SANC affects neither AP parameters, nor the spontaneous beating rate, suggesting that ICl(Ca) has a limited role in the generation of spontaneous pacemaker activity [111].
4. SANC pacemaker function requires basal phosphorylation by both protein kinase A (PKA) and calcium/calmodulin dependent protein kinase II (CaMKII)
Recent studies in isolated SANC have demonstrated that the high basal cAMP level mediates robust, basal cAMP-mediated PKA-dependent phosphorylation, which is required for cardiac pacemaking [11]. When PKA-dependent phosphorylation in SANC is inhibited by specific PKA-inhibitor peptide, PKI, spontaneous beating ceases and this effect is reversable [11]. Indirect evidence also suggest the presence of basal L-type Ca channel PKA-dependent phosphorylation in rabbit SANC [84] as there is direct evidence for phosphorylation of Ca cycling proteins, including phospholamban (PLB) [11], ryanodine receptors (RyRs) [11] and probably others, yet to be discovered. The relative level of basal PLB phosphorylation is ~10-fold higher in rabbit SANC than in ventricular myocytes. Gradations in basal PKA activity, indexed by gradations in phospholamban phosphorylation effected by a specific PKA inhibitory peptide, PKI, were highly correlated with concomitant gradations that occur in the spontaneous SANC beating rate [11].
Signals that increase intracellular [Ca]i activate CaMKII [112], a ubiquitous and multifunctional enzyme, widely involved in Ca-dependent cellular processes. CaMKII activation can persist independently of its initial Ca/CaM activators (autophosphorylated form) and retain enzymic activity even in the absence of Ca/CaM [112] enabling CaMKII to prolong the action of a transient Ca signal. In isolated rabbit SANC basal active CaMKII is localized beneath the cell membrane, whereas the total CaMKII is present uniformly in the cell (Fig. 3A) [113]. This restricted localization of active CaMKII to the surface membrane of SANC is consistent with the idea that CaMKII targets sarcolemmal and subsarcolemmal compartments, and that CaMKII activity is likely regulated by local Ca gradients in sub-membrane microdomains.
Figure 3. The intracellular distribution of CaMKII, RyR and NCX in SANC.
A (from [113]), The intracellular distribution of total and active CaMKII in SANC. A top panel shows a uniform distribution of the total CaMKII immunolabeling; middle panel shows the localization of active CaMKII beneath the sarcolemmal membrane; bottom panel shows the negative control, i.e., in the absence of the primary antibodies. B (from [123]), RyR in single cells isolated from the guinea-pig SA node. C, Confocal image of SANC doubly immunolabeled for NCX and RyR. D, pixel-by-pixel fluorescence intensities of labeling along an arbitrary (white) line in panel C. The horizontal dashed lines show the average pixel intensity. E, Topographical profiles of the pixel intensity levels of each antibody labeling and overlay in the small SANC in panel C. The maximum height represents the brightest possible pixel in the source image. C-E from [122].
In cardiac ventricular myocytes ICa,L is regulated by basal phosphorylation of CaMKII which facilitates channel opening in response to membrane depolarization or Ca entry, thereby modulating ICa,L amplitude and reactivation [114-116]. A basal level of CaMKII activity is required for spontaneous beating of rabbit SANC, since CaMKII inhibitors suppress SA node pacemaking [113]. In the heart, CaMKII regulates SR Ca cycling by phosphorylating RyRs, phospholamban, a SR Ca-ATPase modulator [117], and by SERCA2 activation directly [118]. Thus, the key role of CaMKII-dependent regulation of basal SANC beating rate has been explained by both the regulation of ICa;L inactivation and reactivation kinetics [113], and by augmentation of SERCA activity by PLB phosphorylation at CaMKII dependent Thr17 site [119].
5. Intracellular Ca in cardiac pacemaker automaticity
Several lines of evidence obtained in ventricular or atrial tissue/cells have suggested that spontaneous oscillations of myoplasmic calcium occur under physiological conditions and are generated by cycles of Ca uptake and release by the SR (for review see Lakatta [120]). Later, Ca cycling proteins, previously identified in atrial or ventricular mycytes, i.e., SERCA, RyRs (the SR Ca release channels) and Na/Ca exchanger (NCX), were also identified in SA nodal pacemaker cells. Several recent studies clearly demonstrated immunolabeling of SERCA [121; 122], RyR [122, 123] and NCX [121; 122] in SANC (Fig. 3C-E). Perspectives on the density of such labeling, however, varied among these studies and conclusions about expression of NCX, in particular, differed between studies by Musa et al. [121] and Lyashkov et al. [122]. The latter study revealed colocalized, submembrane immunolabling of NCX and RyR (Fig.3D-E) regardless of SANC size, and demonstrated NCX/RyR immunolabling within the center (connexin 43 null area) of the SA node [122]. In contrast, the former study showed that the density of labeling by anti-RYR2 and anti-NCX was significantly less in small SANC presumably isolated from the center of the SA node [121].
Ryanodine, a rhizome alkaloid extracted from Rynia speciosa Vahl has been an invaluable experimental tool to probe RyR Ca release. At low concentrations ryanodine binds directly to open RyRs, locking these channels in an open subconductance state, which leads to depletion of SR Ca content and suppression of the heart’s contraction [124]. SR Ca release was implicated in pacemaker activity of cat latent pacemaker area, the Eustachian ridge, located between inferior vena cava and right atrium [2]. In this a very small area, 1μmol/L ryanodine caused a marked (172%) increase in the spontaneous cycle length and decrease of the later DD slope, without changing the earlier part of DD [2]. These results were interpreted to indicate that Ca released from the SR is implicated into latent pacemaker activity [2].
Subsequent studies in isolated SA node tissue of different species confirmed that ryanodine produces a dose-dependent reduction of the spontaneous beating rate in the range from 12% to 70% (Table 2A) [3, 4, 11, 41, 125-129]. Some studies which employed gradually increasing ryanodine concentrations, demonstrated dose-dependent effect of ryanodine both in the isolated mouse SA node [129] and rabbit or mouse SANC [9, 130]. Table 2 shows that suppression of the pacemaker firing by ryanodine might be not just dose-dependent but also species-dependent. For example, in mouse SA node 42% suppression of spontaneous firing was achieved at ryanodine concentration of 0.2 μmol/L [129], while in rabbit SANC almost the same 50% suppression of spontaneous firing was achieved at 3 μmol/L ryanodine [9], the concentration 15-fold greater.
Table 2.
A. Suppression of SR Ca2+ release by ryanodine decreases spontaneous beating rate of intact isolated sinoatrial node tissue.B. Suppression of SR Ca2+ release by ryanodine decreases spontaneous beating rate of isolated single sinoatrial node cells.
| Sinoatrial node (SAN) (isolated or intact), species, [reference number] |
Pre Ry average beating rate (beat/min) |
Ry concentration |
Ry-induced decrease in the beating rate (%) |
|---|---|---|---|
| SAN tissue strips, rabbit [3] | 213 | 1 μM | 20 |
| SAN, guinea pig [4] | 230 | 2 μM | 29 |
| SAN, rabbit [125] | 125 | 30 μM | 19 |
| SAN, rabbit [126] | ~120 | 10 μM | up to100 |
| SAN, mice [127] | ~150 | 20 μM | 45 |
| SAN, mice [128] | 255 | 0.003 μM | 24 |
| SAN, mice [129] | 243 243 |
0.2 μM 2 μM |
42 64 |
| Intact, in situ SAN, dog [11] | 108 | 5 nmol/min | 12 |
| Isolated SAN, dog [41] | 95 | 3 μM | 16 |
| Isolated sinoatrial node cells (SANC) or sinus venous cells (SVC), species, [reference number] |
Pre Ry average beating rate (beat/min) |
Ry concentration (μM) |
Ry induced decrease in the beating rate (%) |
|---|---|---|---|
| SVC, cane toad, [131] | 31 | 2-10 | 100 |
| SANC guinea pig [123] | 145 | 2 | 75 |
| SANC, mice [132] | 135 | 10 | 22 |
| SANC, mice [130] | 312 | 1 | 60 |
| SANC in cultured cells, rabbit [5] | 93 | 10 | 33 |
| SANC, rabbit [6] | 181 | 1 | 25 |
| SANC rabbit [9] | 205 200 202 |
1 3 30 |
32 52 95 |
| Small SANC, rabbit [122] | 210 | 3 | 50 |
| Large SANC, rabbit [122] | 210 | 3 | 50 |
| SANC, rabbit [125] | 216 | 30 | 22 |
| Small SANC rabbit [133] Large SANC, rabbit [133] |
151 151 207 207 |
2 30 2 30 |
0 0 24 24 |
| SANC, rabbit [134] | 182 | 3 | 30 |
| SANC, rabbit [47] | 204 | 3 | 30 |
All studies that have employed ryanodine observed a reduction of the isolated SA node beating rate (Table 2A), although the extent of Ry-induced suppression of cardiac pacemaking varied among studies. For example, in one study 10 μmo/L ryanodine nearly stopped spontaneous beating of the isolated rabbit SA node [126], while in another study, also in the isolated rabbit SA node, there was a modest ~19% decrease in the beating rate by 30 μmo/L ryanodine [125]. A small suppression of the sinoatrial node beating rate by ryanodine may be due to an insufficient concentration or insufficient time of ryanodine treatment, the latter is especially important for SA node tissue, because several minutes of ryanodine treatment is required even for isolated SANC to reach a new stable level of spontaneous beating. One study has even found that different ryanodine concentrations (1 and 10 μmol/L) produce the same 60% suppression of the isolated mouse SANC beating rate, but, it takes 15 and 4 minutes to reach a stable level of the suppressant effect, respectively [130].
A substantial ryanodine-induced suppression of spontaneous SANC beating has also been observed in isolated SANC from different species exposed to ryanodine [5-6, 9, 47, 122-123, 125, 130-134] (Table 2B). The extent of beating rate suppression produced by ryanodine in both isolated SANC or isolated SA node is similar, and ranges between 12 and 100% [3-6, 9, 11, 41, 47, 122-123, 125-134] (Table 2). However, one study failed to find any changes in the spontaneous beating of small SANC, thought to be isolated from the central area of the SA node [133]. A large variation around a null average change in the beating rate of small SANC in this study indicated that half of cells increased beating rate, whilst the other half decreased the beating rate [133]. It is known that following a rapid application of ryanodine to isolated SANC, prior to SR Ca depletion, RyR Ca release initially increases, and the spontaneous beating rate concomitantly increases; as the SR becomes depleted with time, the spontaneous beating slows [2, 134]. Thus, it seems to us, that observations by Lancaster et al. in small SANC might reflect differences in the time-course of this biphasic effect of ryanodine, when small SANC have been studied at earlier and bigger cells at later time points [133]. The immunostaining data from the same group demonstrated less RyR and NCX in small SANC than in large SANC, [121], however, the location of small and large cells within SA node before isolation was not verified. It was suggested, that small SANC, thought to originate from SA node center, might be less dependent on Ca and, thus, less susceptible to ryanodine-induced suppression of spontaneous firing [133]. Subsequent extensive studies of a larger number of SANC demonstrated robust RyR and NCX immunolabeling in both small and large SANC, and a similar degree of ryanodine-induced suppression of spontaneous firing in SANC of all sizes [122] (Table 2B).
The mechanism of Ry-induced suppression of spontaneous beating was first studied in latent pacemaker cells isolated from the Eustachian ridge. In these cells the inhibition of SR Ca release by Ry abolished the NCX current during DD and increased spontaneous cycle length (fig. 4 A-D) [92]. Subsequently, inhibition of SR Ca release by Ry was also observed to suppress the NCX current and prolong spontaneous cycle length in the sinus venosus of the cane toad Bufo marinus [7]. However, since within a given cycle, there was a time gap between AP-induced Ca transient and activation of NCX current during late DD, the exact scenario of how Ca release can control the DD rate in pacemaker cells was not clear. But, the later discovery of another form of Ca release during late DD, appears to solve this conundrum (see below).
Figure 4. The role of SR Ca release and NCX current in cat latent atrial pacemaker cells.
(A-G; from [10]) and rabbit SANC (H-M; from [45]). A, Compared to control exposure to 1 μmol/L ryanodine (B) decreases the pacemaker firing rate and the slope of the pacemaker potential. In control (C), voltage clamp of the late pacemaker potential at −50 mV elicits a transient inward current that decays to a background net inward current. Ryanodine (D) abolishes the inward currents and decreases the slope of the pacemaker potential. Compared to control (E), 500 nmol/L isoproterenol (Iso; F) significantly increases inward currents and the slope of the pacemaker potential. Addition of ryanodine (Rya; G) inhibits the isoproterenol-stimulated inward currents and decreases the slope of the pacemaker potential. Effects of suppression of RyR Ca release by ryanodine (Ry) on the ISO produced increase of NCX current (H) and ICa,L (K) in SANCs: original current recordings for the NCX current (H) and ICa,L (K). Relative increase in mean NCX current (L) and ICa,L (M) amplitude by 1 μmol/L of ISO before and after block of RyR with 3 μmol/L of ryanodine. *P<0.05.
6. Spontaneous Ca releases in cardiac pacemaker cells
a. Internal Ca oscillators in overloaded pacemaker cells
By the late 70’s perspectives gleaned from voltage clamped and permeabilized atrial or ventricular myocytes led to the idea of the existence of an intracellular Ca oscillator. Numerous studies had observed current oscillations associated with spontaneous contractile fluctuations in Ca overloaded Purkinje fibers [135]. Tsien et al, based upon such oscillations, conceptualized two coexisting oscillators in cardiac pacemaker cells, i.e. membrane oscillator and internal Ca oscillator [135]. In their model a surface membrane oscillator was represented by a control loop in which membrane potential changes evoke delayed conductance changes. The internal Ca oscillator was an intracellular rhythm generator that was largely independent of the surface membrane. The critical link between the two oscillators, i.e., the internal Ca oscillator and membrane oscillator, might be executed, as Noble proposed earlier, by NCX current which is activated during Ca release from SR, since an extrusion of Ca via NCX exchange generates a functionally significant inward Na current. However, because this dual oscillator pacemaker model required unphysiological high intracellular Ca, it was concluded that internal Ca oscillator might be implicated in abnormal, but not in normal pacemaker function.
b. Rhythmic spontaneous local subsarcolemmal Ca releases (LCRs) under physiological conditions
Simultaneous confocal line-scan Ca imaging and membrane potential recordings using perforated-patch clamp technique have permitted detection of localized submembrane Ca releases during late DD in cat latent atrial pacemaker cells [8] and in rabbit SANC cells [9]. In both cell types local Ca releases appeared during late DD to activate inward NCX and, thus, control DD rate. In cat atrial pacemaker cells local subsarcolemmal Ca releases appeared to be triggered by voltage-dependent activation of ICa,T [8; 10]. In contrast, in rabbit SANC using acute voltage clamp (Fig. 5) or in permeabilized rabbit SANC bathed in physiological [Ca], it was demonstrated, that local subsarcolemmal Ca releases (LCRs) could occur spontaneously and did not require membrane depolarization [136]. Furthermore, blockade of T-type Ca current suppressed LCRs in cat latent atrial pacemaker cells [8], but it did not affect LCRs in rabbit primary SANC [136] (Fig 5A).
Figure 5. Local RyR Ca releases persist during voltage clamp to −70 mV, when T-type Ca current is blocked by Ni2+.
(from: [136]). A, recordings of APs (top), line-scan image (middle) and normalized subsarcolemmal fluorescence averaged spatially over the image width in a representative SANC during superfusion with 50 μmol/L Ni2+. B, control recordings of line-scan image and normalized subsarcolemmal fluorescence in the same cell before exposure to Ni2+.
When the fine structure of LCRs in rabbit SANC was assessed by 2D-images, it was observed that following the decay of the AP-induced Ca transient, multiple LCRs began to emerge throughout the cell as spark-like events, and that with time they converged to generate wavelets that propagate locally beneath the sarcolemma with a velocity of ~150 μm/s to a distance of up to ~12 μm [137]. Subsarcolemmal LCRs activate an inward NCX current which accelerates the rate of late DD, an effect which determines the time when the subsequent spontaneous AP occurs, i.e., the spontaneous SANC beating rate [9]. When, in either cat atrial pacemaker cells or rabbit SANC, RyR Ca release was inhibited by ryanodine, NCX current was suppressed, the late DD became flattened and spontaneous beating slowed (fig. 4A-D) [8-10]. A rapid application of solution in which Na+ was substituted for Li+ directly on SANC (to block NCX current) reduced the slope of the late DD and prevented the subsequent AP from firing without altering the diastolic RyR Ca release [9]. A longer exposure to Li+ abolished spontaneous SANC beating [9, 50, 122].
Numerical model simulations demonstrated that each LCR in SANC could activate an inward NCX current equal to ~0.27pA producing a membrane depolarization of ~0.17mV [138]. Thus, the estimated total NCX current generated by small (32 pF), primary SANC was about ~10pA, which was in a good agreement with experimental data: ryanodine-sensitive NCX current per 32 pF SANC was equal to ~9pA [29]. Due to the high membrane resistance of SANC, a net increase of ~3 pA in the ionic current during DD is enough for 30 pF cell to drive the membrane potential to the threshold of AP upstroke [139]. An increase in NCX current ~9pA, therefore, is sufficient to drive DD to the threshold to fire APs and, therefore, sufficient to control the spontaneous SANC beating rate.
7. Coupling between ‘membrane clock’ and ‘Ca clock’ drives cardiac pacemaker automaticity
Rhythmically occurring subsarcolemmal LCRs in SANC are a manifestation of ‘Ca clock’ that spontaneously cycles Ca under physiological conditions [1, 12-14, 136, 140]. When disconnected from the surface membrane, e.g. during voltage clamp at potentials that prevent SANC from Ca loss via NCX, the SR clock in SANC becomes ‘free running’ (Fig. 6A) and in physiologic intracellular [Ca], estimated to be ~160 nmol/L Ca in beating SANC [136], generates spontaneous, roughly periodic LCRs with frequency 2-4 Hz [11]. Similar, persistent LCRs occur in ‘skinned’ SANC, bathed in 100 nM [Ca] [136]. In voltage-clamped SANC, rhythmic LCRs generate rhythmic current fluctuations, and the frequency of current fluctuations is the same as the frequency of LCRs [11] (Fig. 6A, right); both periodic LCRs and current fluctuations are abolished by ryanodine [1, 12-14, 138, 140].
Figure 6. LCRs and current fluctuations in intact SANC during voltage clamp.
(from [11]). A, (left) Simultaneous recordings of membrane potential or current (top), confocal line-scan image (middle), and normalized fluo-3 fluorescence (bottom) averaged over the line-scan image, in a representative spontaneously beating SANC before and during voltage clamp to −10 mV; (right) Fast Fourier transform (FFT) of Ca (red) and membrane current fluctuations (green). B: PKA inhibition with PKI shifts FFT of both Ca and membrane current fluctuations to lower frequencies during voltage clamp. C: β-adrenergic receptor stimulation with isoproterenol shifts FFT of both Ca and membrane current fluctuations to higher frequencies during voltage clamp.
During spontaneous SANC firing the LCR period, the delay between the AP-induced cytosolic Ca transient and subsequent LCR is regulated by the speed at which SR Ca clock “ticks”, i.e., the kinetics of SR Ca cycling influenced by the SR Ca load, speed and efficiency of the SR Ca pump and activation of RyRs, the SR Ca release channels (Fig. 7 A, B). Similar to that in ventricular myocytes, Ca cycling in SANC depends upon restitution of the SR Ca load following the global SR Ca release and removal of RyR inactivation effected by the prior AP. The intracellular “Ca clock” dynamically interacts with the surface membrane ion channel clock in SANC during each spontaneous cycle. Both L-type Ca current and NCX current, via modulation of cell Ca balance, regulate the SR Ca load, the amount of Ca available for pumping into SR, a key determinant of LCR periodicity, and the periodicity of NCX. The AP upstroke in primary SANC is due to ICa,L which triggers synchronized global RyR Ca release from SR causing a global SR Ca depletion. This synchronized depletion resets the SR Ca clock. Ca influx via L-type Ca channels replenishes Ca lost via NCX during the prior cycle providing Ca to be pumped into SR. The magnitude of L-type channel Ca influx, itself, is finely tuned by the channel inactivation by the cytosolic Ca transient that it triggers.
Figure 7. cAMP-mediated PKA-dependent periodicity of LCRs during spontaneous beating in rabbit SANC.
A, Confocal linescan images of a representative SANC. Single-headed arrows indicate LCRs, double-headed arrows delineate the LCR period and spontaneous cycle length. B (modified from [136]), Relationship between LCR period and the cycle length during spontaneous beating (10 cells, 86 LCRs). C, The relationship between relative changes in the PLB phosphorylation in SANC suspensions produced by PDE inhibition or β-AR stimulation and LCR period produced by the same interventions (IBMX; milrinone; ISO). D, The effects of PDE inhibition or β-AR stimulation to alter the spontaneous cycle length are linked to their ability to change the LCR period. Dash line in B and C is the line of identity. C, D from [29].
It has been proposed that the mutual interaction of SR Ca cycling and surface membrane ion channels is the essence of an integrated pacemaker cell clock [12, 13]. A novel numerical model featuring the generation of spontaneous rhythmic Ca releases and their interaction with the surface membrane provides support for this concept [141]. It is now well recognized, that cAMP-mediated PKA-dependent and CaMKII-dependent phosphorylation modulates SR Ca cycling in SANC [11, 119], as in other excitable cells. Both basal PKA and CaMKII-activity also modulate the membrane component of SR Ca clock, in part at least, by increasing open probability of L-type Ca channels and accelerating their reactivation [84, 113].
Abnormalities in Ca cycling are related to human SA node disfunction
Recently, the essential role of RyR Ca release for human cardiac pacemaking, i.e., sinoatrial node and atrio-ventricular node functions, has been demonstrated [142]. Sixteen members from 2 separate families with genomic deletion of RYR2 exon-3 had sinoatrial and atrioventricular node disfunction, atrial fibrillation and atrial standstill. These data clearly show that mutations of human RyR might lead to extended abnormalities in cardiac pacemaker function.
A dysfunction in ankyrin-based pathways (ANK2 (ankyrin-B/AnkB) locus) has been recently linked to human SA node dysfunction and reported for two large families with severe SA node dysfunction [143]. Mice with reduced expression of AnkB, i.e. heterozygous AnkB, demonstrated SA node dysfunction similar to humans with severe bradycardia and heart rate variability associated with loss of normal cell Ca handling and loss of normal automaticity [143]. Specifically, reduction of AnkB resulted in the reduction of NCX and L-type Ca currents; T-type Ca and If currents, however, remained unchanged [143]. In our opinion, these data further indicate an essential role of Ca handling proteins and Ca cycling for normal SA node function in human.
8. cAMP and PDE effects to elevate the basal beating rate are mediated via local subsarcolemmal Ca releases
Elevation of cAMP by suppression of basal PDE activity markedly increases cAMP-mediated PKA-dependent phosphorylation of Ca cycling proteins, i.e., L-type Ca channel and PLB. Specifically, broad spectrum PDE inhibitor, IBMX, produces more than 2-fold increase in PLB phosphorylation at the PKA-dependent Ser16 site (Fig. 7C) [29]. The PDE-inhibition produces increase in ICa,L amplitude, amplifying Ca influx via these channels, and, thus, elevates the amount of Ca available for pumping into SR; a concomitant increase in PLB phosphorylation accelerates the SR Ca pump, increasing SR Ca load [29]. As a result, LCR’s amplitude and spatial width are markedly increased and inward current via NCX, activated by LCRs, is substantially elevated [29]. Suppression of PDE activity also markedly decreases the LCR period, shifting LCR occurrence to earlier times during DD (Fig. 7C); this shift in LCR period is highly correlated with a concomitant decrease in the spontaneous cycle length (Fig. 7D) [29].
When RyR Ca release is inhibited by a low concentration of Ry, the positive chronotropic effect of PDE inhibition is markedly blunted (Fig. 8A), indicating that modulation of Ca release via RyR is obligatory for the PDE inhibition -induced acceleration of the SANC firing rate [29]. Ry pretreatment, per se, has no effect on the average ICa,L or IK amplitude, and PDE inhibition increases ICa,L and IK amplitude to a similar extent in the absence or presence of Ry [29]. Thus, although suppression of PDE activity markedly increases Ca influx via an increase of ICa,L amplitude, the positive chronotropic effect of PDE inhibition is blunted if RyR are disabled and LCRs are inhibited by Ry. Note, in fig. 8A that, in contrast to the marked inhibitory effect of ryanodine on the PDE-inhibition induced increase in the spontaneous SANC beating rate, suppression of If current by Cs+ has only a very minor effect.
The membrane permeable cAMP analog, CPT-cAMP, increases the spontaneous beating rate of rabbit SANC by ~36% (Fig. 8A) [11]. But in the presence of Ry the ability of maximal CPT-cAMP concentration to increase the spontaneous SANC beating rate is markedly limited and reached only ~13% (Fig. 8A), confirming a vital role of normal RyR Ca release in the modulation of spontaneous SANC firing by cAMP [11]. The demonstration of this Ry effect appears to require a robust effect of cAMP prior to Ry, because, in another study [134], when the CPT-cAMP induced response is relatively small (~18% increase in the beating rate) a significant difference in the beating rate acceleration by CPT-cAMP in the presence or absence of Ry was not observed. Specifically, when CPT-cAMP was reapplied after exposure to ryanodine, the acceleration of SANC beating rate was the same, as in the absence of ryanodine ~17% [134].
9. Effects of β-adrenergic stimulation on ion channels in SANC: role of cAMP-mediated, PKA-dependent phosphorylation and Ca
The activation of the β-AR and their coupling to Gs proteins leads to: activation of AC; conversion of ATP to cAMP; PKA activation and PKA-dependent protein phosphorylation and enhancement of Ca signaling. It is well known that β-AR stimulation amplifies several membrane currents, e.g. ICa,L; If; Ist; IK. The positive chronotropic effect of β-AR stimulation had originally been exclusively ascribed to effects on one or more of surface membrane currents. Both earlier and recent experimental evidence from many different laboratories have demonstrated crucial importance of RyR Ca release in the positive chronotropic effect of β-AR stimulation. However, it would appear, that a common view remains that the positive chronotropic effect of β-AR stimulation is due exclusively to its effects on surface membrane currents, with no consideration of any role of the well documented β-AR stimulation effects to augment internal Ca cycling [47, 125, 133, 144, 145].
Effects of β-AR stimulation on If
When a positive chronotropic effect of epinephrine in Purkinje fibers was first discovered, a possible explanation for this effect on spontaneous beating rate was attributed to its effect on IK2 (which later was discovered to be If) [146, 147]. Since epinephrine (adrenaline) augmented If current in the rabbit SA node, it was concluded that β-AR stimulation of the SA node, similar to that in Purkinje fibers, increased spontaneous beating rate through amplification of If current [148]. Later, it was discovered that β-AR stimulation increases cAMP, and hence the effect of β-AR stimulation to increase SANC beating rate was ascribed to direct cAMP-dependent facilitation of If channel opening, via a positive shift of the voltage dependence of the activation curve [149]. But, the main contribution of If current to the positive chronotropic effect of β-AR stimulation was immediately and subsequently challenged in the isolated SA node [37, 41-42, 150] (Table 3), and later in isolated SANC (Table 3) [45]. Several groups have demonstrated that when If current is suppressed, the major part of the positive chronotropic response to β-AR stimulation remains intact (Table 3A), suggesting that mechanisms other than If amplification, contribute substantially to the positive chronotropic effect of β-AR stimulation (Table 3; Fig. 8). This idea was recently further tested in: adult HCN4 deficient mice ([60], Fig. 2A); adult HCN4-KiT mice with controlled deletion of HCN4 in SA and atrio-ventricular nodes ([61], Fig. 2B); HCN2 knockout mice ([58], Fig. 2C); and adult heterozygous HCN+/R669Q mice ([62], Fig. 2D). All of aforementioned mice showed no defect in heart-rate regulation during exercises or during sympathetic stimulation (Fig. 2). Moreover, in adult HCN4-deficient mice in the absence of enhancement in the amplitude of the small residual If by β-AR agonist, isoproterenol (ISO), a full dose-response of heart rate acceleration to ISO was preserved, and ISO was still able to accelerate the heart rate when the residual If current was suppressed with the If blocker, cilobradine [60]. The data in table 3 and fig. 2 suggest to us, that β-AR induced amplification of If current is not the major player in β-AR stimulation induced increase in the SANC beating rate or increase of the heart rate in vivo. A key role of If current in pacemaker cells, however, may not be to drive the beating rate [151] but to stabilize the pacemaker rhythm [58, 60-62, 141, 151].
Table 3.
Effect of β-AR stimulation to increase the spontaneous SANC or isolated SA node beating rate in the absence or presence of the If current blockade
| Preparation, species, [reference number] |
If blocker concentrati on |
Concentration of β-AR agonist |
Control beating rate, beat/min |
Acceleratio n by β-AR stimulation |
Acceleration by β-AR stimulation in the presence of If blockade |
|---|---|---|---|---|---|
| SANC, rabbit [45] | Cs+, 2 mM | ISO, 1 μM | 170 | 32% | 22% |
| Small SA node preparations, rabbit, [37] |
Cs+, 5 mM | Adrenaline, 0.5 μM |
113 | 73% | |
| Isolated SA node, dog, [41] |
ZD7288, 3 μM |
ISO, 1 μM | 95 | 83% | 40% |
| Isolated SA node, mice [42] |
Cs+, 1 mM Cs+, 1 mM |
Sympathetic nerve stimu- lation, 10 Hz Norepinephrine 0.1 μM |
339 339 |
41% 52% |
35% 44% |
| Isolated SA node, rat [150] |
Cs+, 2 mM | ISO, 0.05 μM | 166 | 208% | 158% |
Effects of β-AR stimulation on delayed rectifier potassium current (IK)
An increase of IK in the SA node in response to a β-AR agonist was first observed in small multicellular rabbit SA node preparations [148]. These results were later confirmed in isolated SANC: the β-AR agonist, ISO, increased IK in rabbit SANC [152] and a slow component of IK, IKs, in guinea-pig SANC [153, 154] which was attributable to the increase in cAMP mediated, PKA-dependent phosphorylation of this current [152, 153]. The cAMP-mediated PKA-dependent regulation of the IKs channels is mediated by a macromolecular signaling complex including the targeting protein AKAP9 (Yotiao) that regulates channel activity by phosphorylation of Ser27 in the N-T of KCNQ1, and of Ser43 in the N-T of Yotiao [155]. Additionally, a study by Lei et al demonstrates that although IKs does not contribute to the basal rabbit SANC beating rate, it contributes significantly to the increase in the spontaneous pacemaker rate during β-AR stimulation [156].
Effects of β-AR stimulation on ICa,T and ICa,L
A catecholamine-induced increase in Ca influx through Ca channels in Purkinje fibers was first discovered by Reuter in 1967 who put forward the hypothesis that the availability of Ca channels could be regulated by catecholamines through a metabolic intermediate, like cyclic AMP, which may increase the open probability of Ca channels and therefore the Ca influx [157-159]. Later, the amplification of L-type Ca current by the β-AR agonist, ISO, was also demonstrated in rabbit SANC [45, 67], guinea-pig SANC [160], latent atrial pacemaker cells [92] and the sinus venosus of the cane toad [131]. Surprisingly, T-type Ca current was not amplified by ISO [67].
Intensive studies in ventricular myocytes have demonstrated that β-AR stimulation increases ICa,L by augmenting the mean channel open time as well as the probability of channel opening [161]. The increase in ICa,L by -AR stimulation is linked to PKA-dependent phosphorylation of serine 1928 residue of the L-type Ca channel located at the C-terminal of α1C [82, 162]. When PKA-dependent phosphorylation is suppressed by dialisys with Rp-CAMPs, or is inhibited with PKA inhibitor peptide, PKI, the stimulation of ICa,L by either ISO or forskolin is markedly suppressed [78, 163]. AKAP plays a crucial role for L-type Ca channel phosphorylation: PKA is anchored to the membrane through AKAP15/18 facilitating PKA-dependent phosphorylation of L-type Ca channel [164]. An amplification of L-type Ca current in response to PKA-dependent activation is lost on expression of the inactive AKAP15/18 mutant [164].
Effects of β-AR stimulation on sustained current, Ist
Ist is increased by ~2-fold by by the β-AR agonist, ISO [108, 109, 165], suggesting that part of the positive chronotropic effect of the β-AR stimulation might be explained by increase of Ist. However, application of 50 μmol/L Ni2+, which blocks Ist by 50% produces only ~10% decrease in the ISO-induced acceleration [45], suggesting to us that Ist current likely has a minor role in β-AR acceleration of rabbit SANC firing.
10. The positive chronotropic effects of β-AR stimulation in SANC are critically dependent upon intact RyR function and NCX current
Confocal imaging of local Ca releases have identified an important contribution of LCRs not only to the positive chronotropic response of SANC to PDE inhibition but also to β-AR stimulation [29, 45, 166]. In rabbit SANC β-AR stimulation increases the number, amplitude and size of LCRs during late DD, reflecting the recruitment of additional RyRs and their partial synchronization due, in part at least, to the increase in PKA-dependent PLB phosphorylation (Fig. 7C), increase in ICa,L (Fig. 4M) and an elevated SR Ca load [45]. β-AR stimulation might also increase local Ca releases via direct effects on RyR Ca release due to RyR phosphorylation, as in ventricular myocytes [167; 168]. The spatiotemporal synchronization of RyR Ca releases in response to β-AR stimulation is accompanied by a concomitant decrease in LCR period, indicating that SR ‘Ca clock’ “operates at a higher speed” to increase the frequency of spontaneous Ca oscillations (Fig 6C, 7D). These augmented and earlier occurring Ca releases produce an amplified and earlier activation of inward NCX current, leading to earlier increase of DD rate and the spontaneous SANC beating rate (Fig. 9) [45].
Figure 9. Disabling RyRs dramatically reduces the effects of β-AR stimulation to increase either the isolated rabbit SANC or intact canine SA node spontaneous beating rate.
A, Representative linescan images of LCRs and AP-induced Ca transients in SANC, before (top) and during (bottom) application β-AR stimulation (0.1 μM ISO). B (from [45]), Effects of RyR inhibition on the concentration response of SANC firing rate to β-AR stimulation. ISO causes a dose-dependent increase in the firing rate, and this effect is markedly suppressed in the presence of ryanodine. C, Schematic of intranodal SA nodal microdialysis of an anesthetized open-chest dog in vivo. D (from [11]), The dose-responses of the canine heart rate to two sequential ISO infusions delivered by microdialysis into the SA node. ISO produced a brisk, reproducible tachycardia, and sequential dose responses in controls were superimposable. Disabling of RyRs with ryanodine (5nmol/min) dramatically reduces the effects of β-AR stimulation with ISO to increase the heart rate in vivo. Local nodal dialysis with ryanodine reduced resting heart rate by 12% (from 108 ± 5 to 96 ± 6 bpm; P < 0.05) and suppressed the response to subsequently added ISO by 75%. *P < 0.05.
In cat latent atrial pacemaker cells ISO induces a significant increase in inward NCX current, resulting in a concomitant increase of the DD slope (Fig. 4E,F) [92], suggesting that NCX current is an important factor mediating β-AR regulation of latent pacemaker automaticity [92]. An increase in NCX current during β-AR stimulation has also been observed in the sinus venosus of the cane toad and was attributed to elevated [Ca]i, rather than to changes in the intrinsic properties of Na-Ca exchanger [7, 131]. Studies in cat latent atrial pacemaker cells (Fig. 4), SANC and isolated SA nodes of different species (Table 4), supported the discovery that inhibition of RyR Ca release by low Ry concentrations markedly blunted, but not always abolished, the effect of ISO to increase the spontaneous SANC beating rate [11, 41, 45, 123, 125, 130-131, 134]. PKA-dependent phosphorylation of NCX1 during β-AR stimulation remains controversial [94-96].
Table 4.
A. Suppression of SR Ca2+ release by ryanodine suppresses effect of β-AR stimulation to accelerate the spontaneous beating rate of isolated single SANC.B. Suppression of SR Ca2+ release by ryanodine suppresses effect of β-AR stimulation to increase the spontaneous beating rate of the isolated or intact sinoatrial node.
| Isolated SVC or SANC, species, [reference number] |
Concentr ation of Ry |
Concentration of β-AR agonist |
Control beating rate, beat/min |
β-AR stimulation- induced acceleration |
β-AR stimulation induced acceleration (Ry present) |
|---|---|---|---|---|---|
| SVC, cane toad [131] | 1 μM 20 μM |
ISO, 2 μM adrenaline, 2 μM |
22 29 |
41% 66% |
0 32% |
| SANC, guinea- pig [123] | 2 μM | ISO, 0.1 μM | 132 | 138% | 0 |
| SANC, rabbit [45] | 3 μM | ISO, 1 μM | 157 | 31% | 5% |
| SANC, rabbit [134] | 3 μM | ISO, 1 μM | 197 | 24% | 8% |
| SANC, mouse [130] | 1 μM | ISO, 1 μM | ~312 | 40% |
55% rate reduction |
| Isolated or intact SA node, species, [reference number] |
Concentra tion of Ry |
Concentration of β-AR agonist |
Control beating rate, beat/min |
β-AR stimulation induced acceleration |
β-AR stimulation induced acceleration (Ry present) |
|---|---|---|---|---|---|
| Isolated SA node, guinea- pig [123] |
2 μM | ISO, 0.1 μM | 215 | 35% | 23% |
| Isolated SA node, rabbit [125]* |
30 μM | ISO, 0.2 μM | 125 | No data | 53% |
| Intact in vivo SA node, dog [11] |
5 nmol/min |
ISO, 1-150 nmol/min |
118 | 42% | 8% |
| Isolated SA node, dog, [41] |
3 μM | ISO, 1 μM | 95 | 83% | 23% |
no report of ISO-induced increase in the beating rate in the absence of ryanodine
It has recently been demonstrated that acceleration of the heart rate during β-AR stimulation in mice requires CaMKII activation [130]. Intact mice in which CaMKII was genetically disabled via conditional expression of CaMKII peptide inhibitor, AC3-I, had similar basal heart beating rates as wild type mice; but AC3-I mice showed significantly slower acceleration of the beating rate during stress than control mice. It was discovered that during β-AR stimulation of mouse SANC, CaMKII was implicated in: the increase of SR Ca load via CaMKII-dependent phosphorylation of PLB at Thr17 site; increase in diastolic RyR Ca release; increase in the DD rate and increase in SA node beating rate [130]. Ryanodine significantly and equivalently reduced the beating rate in both AC3-I and control SANC. Strikingly, ryanodine treated mouse SANC of both genotypes showed a reduction rather than an increase in the beating rate after ISO, suggesting that RyR Ca release is required for the positive chronotropic effect of the β-AR stimulation [130] (Table 4). Moreover, results of this study strongly suggest that the reduced response to β-AR stimulation in AC3-I mice was independent of If, since this current, as well as its response to ISO, was fully preserved.
When the SR Ca load in rabbit SANC is depleted by ryanodine, there is an approximate three-fold decrease in the ability of ISO to accelerate rabbit SANC firing rate, and about a six-fold increase in the ISO EC50 (Fig 9B) [45]. When RyR function is inhibited by ryanodine, β-AR stimulation fails to amplify LCRs, fails to augment NCX current and DD rate, and fails to increase the spontaneous SANC firing rate, even though the β-AR effect to increase ICa,L amplitude remains intact (Fig 4K,M). Thus, in our opinion, the RyR Ca release flux acts as a “switchboard” that links β-AR stimulation to an increase in SANC firing rate. These data are also consistent with other studies in isolated SANC of different species. Table 4A shows that in the presence of ryanodine the ability of β-AR stimulation to increase the spontaneous SANC beating rate is strikingly, up to 100%, suppressed [45, 123, 130-131, 134]. The requirement of intact RyR function for β-AR stimulation induced positive chronotropic effect has also been tested in the isolated or intact SA node (Table 4B). In the isolated canine SA node there was 3.5-fold decrease in the ability of β-AR stimulation to increase spontaneous firing rate in the presence of low Ry concentration [41] (Table 4B). In open chest dogs, a robust, dose-dependent increase in the spontaneous beating rate effected by microdialysis of the SA node artery with ISO was virtually abolished when RyR were inhibited by ryanodine (Fig. 9C, D) (Table 4B). In contrast, Honjo et al. showed that the effect of β-AR stimulation to increase the SANC firing rate did not require intact RyR function: ISO could still augment the spontaneous beating rate in the presence of 30 μmol/L Ry (Table 4B)[125]. However, an interpretation of whether ryanodine had a suppressant effect on the ISO-induced acceleration of the beating rate in this study is precluded by the fact that the ISO-induced acceleration of the beating rate in the absence of ryanodine was not reported [125]. This study also used a very high concentration of ryanodine (30 μmol/L), which, in contrast to a lower ryanodine concentration used in the most studies of this sort (see Table 4), locks RyRs in a closed state, preventing SR Ca depletion. Indeed, when SR store is still loaded with Ca in the presence of high ryanodine concentration, β-AR stimulation effect to increase SANC firing rate persists [131] (Table 4A).
11. β-AR stimulation induced shift of the primary pacemaker site in the intact SA node
The SA node is a complex heterogeneous structure and the intrinsic spontaneous cycle length and AP characteristics are the result of cooperative efforts of multiple individual rabbit SANC. It is well known that different interventions such as autonomic stimulation, change in temperature or ion concentrations produce shift of the primary pacemaker site from the center of the SA node to the periphery, suggesting that, under different experimental conditions, different subsets of SANC could become the initiation sites of spontaneous SA node firing. This suggests that results obtained in the isolated SANC may not be extrapolated to SA node tissue. However, a suppression of the RyR Ca release by ryanodine decreases the spontaneous beating rate and positive chronotropic effect of β-AR stimulation to a similar extent, regardless of whether experiments were performed in the isolated SANC or in SA node tissue of different species (Tables 2 and 4). Thus, in our opinion, the important contribution of Ca cycling in the basal pacemaker function as well as in the acceleration produced by β-AR stimulation reflects properties acquired by all SANC, which is reflected in the response of the entire SA node. Nevertheless, the different “neighborhoods” within SA node may harbor cells with varying robustness of PKA or β-AR dependent Ca cycling.
Until recently, LCRs have been reported only in isolated SANC, and there were no direct measurements of LCR in SA node tissue. But recently, Joung et al., using simultaneous mapping of intracellular Ca and membrane potential, reported the occurrence of LCRs in the canine isolated SA node [41]. This was a challenging task, under basal conditions LCRs were hardly visible and were observed only in few preparations. During β-AR stimulation with isoproterenol, which shifted a primary pacemaker site to the superior part of the SA node, LCRs were markedly amplified and were recorded in all canine SA nodes studied. Consistent with previous observations (Tables 2, 4), depletion of SR Ca with either ryanodine or a combination of ryanodine and thapsigargin (to inhibit SERCA function) prevented the isoproterenol-induced LCRs and blunted β-AR stimulation induced canine SA node acceleration [41].
12. Summary
Although our understanding of cardiac pacemaker mechanisms has greatly advanced over the past two decades, many questions on how the sinoatrial node rhythm is generated and controlled remain an enigma. Even if it is still a matter of debate which ion channels are required for generation of DD, it is clear that cardiac pacemaker cannot relay upon a single channel to drive SA node automaticity, and many different mechanisms including ionic currents and transporters are involved in the generation of spontaneous SA node beating.
Multiple studies have demonstrated an essential role of RyR Ca release and NCX current for spontaneous beating of both SANC and intact SA node. While the ensemble of SANC ion channel membrane clocks is required to generate APs, it is not sufficient to generate rhythmic APs at normal and stable rates. The more recent discovery of rhythmic local subsarcolemmal Ca releases from RyR in rabbit SANC has provided the direct evidence that LCRs deliver their “pay load” at a time most appropriate to activate NCX during late DD to drive membrane potential to the threshold to fire AP. Thus, the robust cardiac pacemaker clock includes a balanced integration of both intracellular and surface membrane processes, i.e., an internal ‘Ca clock’ interacts with the membrane ‘ion channel clock’, both forming a unitary pacemaker clock that insures normal pacemaker cell automaticity. The basal spontaneous beating rate is controlled by high level of cAMP production by Ca-activated ACs that is kept in check by high level of PDE activity. There is substantial evidence to indicate that modulation of pacemaker automaticity via β-AR stimulation acts via an augmentation of the basal PKA and CaMKII-dependent phosphorylation regulating Ca cycling which drives the surface membrane channels to ignite APs at a forth rate. When augmentation of Ca cycling is precluded, the ‘fight or flight’ response provided by β-AR stimulation cannot effectively accelerate the spontaneous SANC beating rate.
Acknowledgments
This work is supported, in part, by Intramural Research Program of the National Institute on Aging, National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Maltsev VA, Vinogradova TM, Lakatta EG. The emergence of a general theory of the initiation and strength of the heartbeat. J Pharmacol Sci. 2006;100:338–69. doi: 10.1254/jphs.cr0060018. [DOI] [PubMed] [Google Scholar]
- [2].Rubenstein DS, Lipsius SL. Mechanisms of automaticity in subsidiary pacemakers from cat right atrium. Circ Res. 1989;64:648–657. doi: 10.1161/01.res.64.4.648. [DOI] [PubMed] [Google Scholar]
- [3].Hata T, Noda T, Nishimura M, Watanabe Y. The role of Ca2+ release from sarcoplasmic reticulum in the regulation of sinoatrial node automaticity. Heart Vessels. 1996;11:234–41. doi: 10.1007/BF01746203. [DOI] [PubMed] [Google Scholar]
- [4].Rigg L, Terrar DA. Possible role of calcium release from the sarcoplasmic reticulum in pacemaking in guinea-pig sino-atrial node. Exp Physiol. 1996;81:877–80. doi: 10.1113/expphysiol.1996.sp003983. [DOI] [PubMed] [Google Scholar]
- [5].Li J, Qu J, Nathan RD. Ionic basis of ryanodine’s negative chronotropic effect on pacemaker cells isolated from the sinoatrial node. Am. J. Physiol. 1997;273:H2481–H2489. doi: 10.1152/ajpheart.1997.273.5.H2481. [DOI] [PubMed] [Google Scholar]
- [6].Satoh H. Electrophysiological actions of ryanodine on single rabbit sinoatrial nodal cells. Gen. Pharmac. 1997;28:31–38. doi: 10.1016/s0306-3623(96)00182-6. [DOI] [PubMed] [Google Scholar]
- [7].Ju YK, Allen DG. Intracellular calcium and Na+Ca2+ exchange current in isolated toad pacemaker cells. J. Physiol. 1998;508:153–166. doi: 10.1111/j.1469-7793.1998.153br.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Hüser J, Blatter LA, Lipsius SL. Intracellular Ca2+ release contributes to automaticity in cat atrial pacemaker cells. J Physiol (Lond) 2000;524:415–422. doi: 10.1111/j.1469-7793.2000.00415.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Bogdanov KY, Vinogradova TM, Lakatta EG. Sinoatrial nodal cell ryanodine receptor and Na— Ca2+ exchanger: molecular partners in pacemaker regulation. Circ Res. 2001;88:1254–1258. doi: 10.1161/hh1201.092095. [DOI] [PubMed] [Google Scholar]
- [10].Lipsius SL, Hüser J, Blatter LA. Intracellular Ca2+ release sparks atrial pacemaker activity. News Physiol Sci. 2001;16:101–6. doi: 10.1152/physiologyonline.2001.16.3.101. [DOI] [PubMed] [Google Scholar]
- [11].Vinogradova TM, Lyashkov AE, Zhu W, Ruknudin AM, Sirenko S, Yang D, Deo S, Barlow M, Johnson S, Caffrey JL, Zhou YY, Xiao RP, Cheng H, Stern MD, Maltsev VA, Lakatta EG. High basal protein kinase A-dependent phosphorylation drives rhythmic internal Ca2+ store oscillations and spontaneous beating of cardiac pacemaker cells. Circ Res. 2006;98:505–14. doi: 10.1161/01.RES.0000204575.94040.d1. [DOI] [PubMed] [Google Scholar]
- [12].Lakatta EG, Vinogradova T, Lyashkov A, Sirenko S, Zhu W, Ruknudin A, Maltsev VA. The integration of spontaneous intracellular Ca2+ cycling and surface membrane ion channel activation entrains normal automaticity in cells of the heart’s pacemaker. Ann N Y Acad Sci. 2006;1080:178–206. doi: 10.1196/annals.1380.016. [DOI] [PubMed] [Google Scholar]
- [13].Lakatta EG, Vinogradova TM, Maltsev VA. The missing link in the mystery of normal automaticity of cardiac pacemaker cells. Ann N Y Acad Sci. 2008;1123:41–57. doi: 10.1196/annals.1420.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Maltsev VA, Lakatta EG. Normal heart rhythm is initiated and regulated by an intracellular calcium clock within pacemaker cells. Heart Lung Circ. 2007;16:335–48. doi: 10.1016/j.hlc.2007.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Tuganowski W. The influence of adenylate cyclase inhibitors on the spontaneous activity of the cardiac pacemaker. Arch Int Pharmacodyn Ther. 1977;225:275–286. [PubMed] [Google Scholar]
- [16].Tsien RW. Adrenaline-like effects of intracellular iontophoresis cyclic AMP in cardiac Pupkinje fibers. Nature (New Biol.) 1973;245:120–122. doi: 10.1038/newbio245120a0. [DOI] [PubMed] [Google Scholar]
- [17].Tuganowski W, Kopeć P. Effect of exogenous cAMP in the rabbit right auricle. Pol J Pharmacol Pharm. 1978;30:451–4. [PubMed] [Google Scholar]
- [18].Yamasaki Y, Fujiwara M, Toda N. Effects of intarcellular applied cyclic 3′5′-adenosine monophosphate on the electrical activity of sinoatrial node cells of the rabbit. J Pharmacol Exp. Ther. 1974;190:15–20. [PubMed] [Google Scholar]
- [19].Pollack GH. Cardiac pacemaking: an obligatory role of catecholamines? Science. 1977;196:731–738. doi: 10.1126/science.16342. [DOI] [PubMed] [Google Scholar]
- [20].Freeman SE, Turner RJ. The effects of l-propranolol and practolol on atrial and nodal transmembrane potentials. J Pharmacol Exp Ther. 1975;195:133–9. [PubMed] [Google Scholar]
- [21].Mattick P, Parrington J, Odia E, Simpson A, Collins T, Terrar D. Ca2+-stimulated adenylyl cyclase isoform AC1 is preferentially expressed in guinea-pig sino-atrial node cells and modulates the I(f) pacemaker current. J Physiol. 2007;582:1195–203. doi: 10.1113/jphysiol.2007.133439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Vinogradova TM, Ruknudin AM, Zhu W, Lyashkov AE, Volkova M, Boheler K, Xiao RP, Spurgeon HA, Lakatta EG. High Basal cAMP Content Markedly Elevates PKA-dependent Protein Phosphorylation and Sustains Spontaneous Beating in Rabbit Sinoatrial Nodal Pacemaker Cells (SANC) Biophysical J. 2006;155a (Abstract) [Google Scholar]
- [23].Younes A, Lyashkov AE, Graham D, Sheydina A, Volkova MV, Mitsak M, Vinogradova TM, Lukyanenko YO, Li Y, Ruknudin AM, Boheler KR, van Eyk J, Lakatta EG. Ca(2+) -stimulated basal adenylyl cyclase activity localization in membrane lipid microdomains of cardiac sinoatrial nodal pacemaker cells. J Biol Chem. 2008;283:14461–8. doi: 10.1074/jbc.M707540200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Beavo JA, Brunton LL. Cyclic nucleotide research -still expanding after half a century. Nat Rev Mol Cell Biol. 2002;3:710–718. doi: 10.1038/nrm911. [DOI] [PubMed] [Google Scholar]
- [25].Tsien RW, Giles W, Greengard P. Cyclic AMP mediates the effects of adrenaline on cardiac Pupkinje fibers. Nature (New Biol.) 1972;240:181–183. doi: 10.1038/newbio240181a0. [DOI] [PubMed] [Google Scholar]
- [26].Shahid M, Rodger IW. Chronotropic and inotropic actions of amrinone, carbazeran and isobutylmethyl xanthine: role of phosphodiesterase inhibition. Br J Pharmacol. 1989;98:291–301. doi: 10.1111/j.1476-5381.1989.tb16894.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Orito K, Takase H, Fujiki H, Mori T. Effects of toborinone (OPC-18790), a new positive inotropic agent, on action potential in guinea pig sinoatrial node: compared with milrinone and E-4031. Jpn J Pharmacol. 1996;72:79–82. doi: 10.1254/jjp.72.79. [DOI] [PubMed] [Google Scholar]
- [28].Kodama I, Kondo N, Shibata S. Effects of amrinone on the transmembrane action potential of rabbit sinus node pacemaker cells. Br.J.Pharmac. 1983;80:511–7. doi: 10.1111/j.1476-5381.1983.tb10723.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Vinogradova TM, Sirenko S, Lyashkov AE, Younes A, Li Y, Zhu W, Yang D, Ruknudin A, Spurgeon HA, Lakatta EG. Constitutive phosphodiesterase activity restricts spontaneous beating rate of cardiac pacemaker cells by suppressing local Ca2+ releases. Circ Res. 2008;102:761–769. doi: 10.1161/CIRCRESAHA.107.161679. [DOI] [PubMed] [Google Scholar]
- [30].Vinogradova TM, Lyashkov AE, Spurgeon H, Lakatta EG. Local Ca releases and spontaneous beating rate of rabbit sinoatrial node cells are controlled by interaction of basal phosphodiesterase 3 and 4 activity. Circulation Suppl. 2008;118:S343. (Abstract) [Google Scholar]
- [31].Brown H, DiFrancesco D. Voltage-clamp investigations of membrane currents underlying pace-maker activity in rabbit sino-atrial node. J Physiol. 1980;308:331–51. doi: 10.1113/jphysiol.1980.sp013474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Baruscotti M, Bucchi A, DiFrancesco D. Physiology and pharmacology of the cardiac pacemaker (“funny”) current. Pharmacol Ther. 2005;107:59–79. doi: 10.1016/j.pharmthera.2005.01.005. [DOI] [PubMed] [Google Scholar]
- [33].DiFrancesco D. Pacemaker mechanisms in cardiac tissue. Annu Rev Physiol. 1993;55:451–67. doi: 10.1146/annurev.ph.55.030193.002323. [DOI] [PubMed] [Google Scholar]
- [34].Ju YK, Saint DA, Hirst GD, Gage PW. Sodium currents in toad cardiac pacemaker cells. Journal of Membrane Biology. 1995;145:119–28. doi: 10.1007/BF00237370. [DOI] [PubMed] [Google Scholar]
- [35].Satoh H. Sino-atrial nodal cells of mammalian hearts: ionic currents and gene expression of pacemaker ionic channels. J Smooth Muscle Res. 2003;39:175–93. doi: 10.1540/jsmr.39.175. [DOI] [PubMed] [Google Scholar]
- [36].Shinagawa Y, Satoh H, Noma A. The sustained inward current and inward rectifier K+ current in pacemaker cells dissociated from rat sinoatrial node. J Physiol. 2000;523:593–605. doi: 10.1111/j.1469-7793.2000.t01-2-00593.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Noma A, Morad M, Irisawa H. Does the “Pacemaker Current” Generate the Diastolic Depolarization in the Rabbit SA Node Cells? Pfiiigers Arch. 1983;397:190–4. doi: 10.1007/BF00584356. [DOI] [PubMed] [Google Scholar]
- [38].Nikmaram MR, Boyett MR, Kodama I, Suzuki R, Honjo H. Variation in effects of Cs+, UL-FS-49, and ZD-7288 within sinoatrial node. Am J Physiol. 1997;272:H2782–92. doi: 10.1152/ajpheart.1997.272.6.H2782. [DOI] [PubMed] [Google Scholar]
- [39].Leitch SP, Sears CE, Brown HF, Paterson DJ. Effects of high potassium and the bradycardic agents ZD7288 and cesium on heart rate of rabbits and guinea pigs. J Cardiovasc Pharmacol. 1995;25:300–6. doi: 10.1097/00005344-199502000-00016. [DOI] [PubMed] [Google Scholar]
- [40].Sohn HG, Vassalle M. Cesium effects on dual pacemaker mechanisms in guinea pig sinoatrial node. J Mol Cell Cardiol. 1995;27:563–77. doi: 10.1016/s0022-2828(08)80051-x. [DOI] [PubMed] [Google Scholar]
- [41].Joung B, Tang L, Maruyama M, Han S, Chen Z, Stucky M, Jones LR, Fishbein MC, Weiss JN, Chen PS, Lin SF. Intracellular calcium dynamics and acceleration of sinus rhythm by beta-adrenergic stimulation. Circulation. 2009;119:788–96. doi: 10.1161/CIRCULATIONAHA.108.817379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Choate JK, Feldman R. Neuronal control of heart rate in isolated mouse atria. Am J Physiol Heart Circ Physiol. 2003;285:H1340–6. doi: 10.1152/ajpheart.01119.2002. [DOI] [PubMed] [Google Scholar]
- [43].Liu J, Noble PJ, Xiao G, Abdelrahman M, Dobrzynski H, Boyett MR, Lei M, Noble D. Role of pacemaking current in cardiac nodes: insights from a comparative study of sinoatrial node and atrioventricular node. Prog Biophys Mol Biol. 2008;96:294–304. doi: 10.1016/j.pbiomolbio.2007.07.009. [DOI] [PubMed] [Google Scholar]
- [44].Denyer JC, Brown HF. Pacemaking in rabbit isolated sino-atrial node cells during Cs+ block of the hyperpolarization-activated current if. J Physiol. 1990;429:401–9. doi: 10.1113/jphysiol.1990.sp018264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Vinogradova TM, Bogdanov KY, Lakatta EG. β-adrenergic stimulation modulates ryanodine receptor Ca2+ release during diastolic depolarization to accelarate pacemaker activity in rabbit sinoatrial nodal cells. Circ Res. 2002;90:73–79. doi: 10.1161/hh0102.102271. [DOI] [PubMed] [Google Scholar]
- [46].DiFrancesco D. Cardiac pacemaker I(f) current and its inhibition by heart rate-reducing agents. Curr Med Res Opin. 2005;21:1115–22. doi: 10.1185/030079905x50543. [DOI] [PubMed] [Google Scholar]
- [47].Bucchi A, Baruscotti M, Robinson RB, DiFrancesco D. Modulation of rate by autonomic agonists in SAN cells involves changes in diastolic depolarization and the pacemaker current. J Mol Cell Cardiol. 2007;43:39–48. doi: 10.1016/j.yjmcc.2007.04.017. [DOI] [PubMed] [Google Scholar]
- [48].Ono K, Shibata S, Iijima T. Pacemaker mechanism of porcine sino-atrial node cells. J Smooth Muscle Res. 2003;39:195–204. doi: 10.1540/jsmr.39.195. [DOI] [PubMed] [Google Scholar]
- [49].Verkerk AO, Wilders R, van Borren MM, Peters RJ, Broekhuis E, Lam K, Coronel R, de Bakker JM, Tan HL. Pacemaker current (I(f)) in the human sinoatrial node. Eur Heart J. 2007;28:2472–8. doi: 10.1093/eurheartj/ehm339. [DOI] [PubMed] [Google Scholar]
- [50].Sanders L, Rakovic S, Lowe M, Mattick PA, Terrar DA. Fundamental importance of Na+-Ca2+ exchange for the pacemaking mechanism in guinea-pig sino-atrial node. J Physiol. 2006;571:639–49. doi: 10.1113/jphysiol.2005.100305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Irisawa H, Brown HF, Giles W. Cardiac pacemaking in the sinoatrial node. Physiol Rev. 1993;73:197–227. doi: 10.1152/physrev.1993.73.1.197. [DOI] [PubMed] [Google Scholar]
- [52].DiFrancesco D, Tortora P. Direct activation of cardiac pacemaker channels by intracellular cyclic AMP. Nature. 1991;351:145–147. doi: 10.1038/351145a0. [DOI] [PubMed] [Google Scholar]
- [53].DiFrancesco D, Tromba C. Muscarinic control of the hyperpolarization-activated current (if) in rabbit sino-atrial node myocytes. J Physiol. 1988;405:493–510. doi: 10.1113/jphysiol.1988.sp017344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Hata T, Nishimura M, Ogino K, Uchiyama H, Watanabe Y. Electrophysiological effects of amrinone on the automaticity and membrane current system of the rabbit sinoatrial node cells. Heart Vessels. 1998;13:114–21. doi: 10.1007/BF01747828. [DOI] [PubMed] [Google Scholar]
- [55].Ishii TM, Takano M, Xie LH, et al. Molecular characterization of the hyperpolarization-activated cation channel in rabbit heart sinoatrial node. J Biol Chem. 1999;274:12,835–12,839. doi: 10.1074/jbc.274.18.12835. [DOI] [PubMed] [Google Scholar]
- [56].Herrmann S, Stieber J, Ludwig A. Pathophysiology of HCN channels. Pflugers Arch. 2007;454:517–22. doi: 10.1007/s00424-007-0224-4. [DOI] [PubMed] [Google Scholar]
- [57].Moosmang S, Stieber J, Zong X, Biel M, Hofmann F, Ludwig A. Cellular expression and functional characterization of four hyperpolarization-activated pacemaker channels in cardiac and neuronal tissues. Eur J Biochem. 2001;268:1646–1652. doi: 10.1046/j.1432-1327.2001.02036.x. [DOI] [PubMed] [Google Scholar]
- [58].Ludwig A, Budde T, Stieber J, Moosmang S, Wahl C, Holthoff K, Langebartels A, Wotjak C, Munsch T, Zong X, Feil S, Feil R, Lancel M, Chien KR, Konnerth A, Pape HC, Biel M, Hofmann F. Absence epilepsy and sinus dysrhythmia in mice lacking the pacemaker channel HCN2. EMBO J. 2003;22:216–24. doi: 10.1093/emboj/cdg032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Stieber J, Herrmann S, Feil S, Löster J, Feil R, Biel M, Hofmann F, Ludwig A. The hyperpolarization-activated channel HCN4 is required for the generation of pacemaker action potentials in the embryonic heart. Proc Natl Acad Sci. 2003;100:15235–40. doi: 10.1073/pnas.2434235100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Herrmann S, Stieber J, Stöckl G, Hofmann F, Ludwig A. HCN4 provides a ‘depolarization reserve’ and is not required for heart rate acceleration in mice. EMBO J. 2007;26:4423–32. doi: 10.1038/sj.emboj.7601868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Hoesl E, Stieber J, Herrmann S, Feil S, Tybl E, Hofmann F, Feil R, Ludwig A. Tamoxifen-inducible gene deletion in the cardiac conduction system. J Mol Cell Cardiol. 2008;45:62–9. doi: 10.1016/j.yjmcc.2008.04.008. [DOI] [PubMed] [Google Scholar]
- [62].Harzheim D, Pfeiffer KH, Fabritz L, Kremmer E, Buch T, Waisman A, Kirchhof P, Kaupp UB, Seifert R. Cardiac pacemaker function of HCN4 channels in mice is confined to embryonic development and requires cyclic AMP. EMBO J. 2008;27:692–703. doi: 10.1038/emboj.2008.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Schulze-Bahr E, Neu A, Friederich P, Kaupp UB, Breithardt G, Pongs O, Isbrandt D. Pacemaker channel dysfunction in a patient with sinus node disease. J Clin Invest. 2003;111:1537–45. doi: 10.1172/JCI16387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Milanesi R, Baruscotti M, Gnecchi-Ruscone T, DiFrancesco D. Familial sinus bradycardia associated with a mutation in the cardiac pacemaker channel. N Engl J Med. 2006;354:151–7. doi: 10.1056/NEJMoa052475. [DOI] [PubMed] [Google Scholar]
- [65].Robinson RB, Brink PR, Cohen IS, Rosen MR. I(f) and the biological pacemaker. Pharmacol Res. 2006;53:407–15. doi: 10.1016/j.phrs.2006.03.007. [DOI] [PubMed] [Google Scholar]
- [66].Rosen MR, Brink PR, Cohen IS, Robinson RB. Biological pacemakers based on I(f) Med Biol Eng Comput. 2007;45:157–66. doi: 10.1007/s11517-006-0060-2. [DOI] [PubMed] [Google Scholar]
- [67].Hagiwara N, Irisawa H, Kameyama M. Contribution of two types of calcium currents to the pacemaker potentials of rabbit sino-atrial node cells. J. Physiol. 1988;395:233–53. doi: 10.1113/jphysiol.1988.sp016916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Satoh H. Role of T-type Ca2+ channel inhibitors in the pacemaker depolarization in rabbit sino-atrial nodal cells. Gen Pharmacol. 1995;26:581–7. doi: 10.1016/0306-3623(94)00214-8. [DOI] [PubMed] [Google Scholar]
- [69].Mangoni ME, Couette B, Marger L, Bourinet E, Striessnig J, Nargeot J. Voltage-dependent calcium channels and cardiac pacemaker activity: from ionic currents to genes. Prog Biophys Mol Biol. 2006;90:38–63. doi: 10.1016/j.pbiomolbio.2005.05.003. [DOI] [PubMed] [Google Scholar]
- [70].Chen CC, Lamping KG, Nuno DW, Barres R, Prouty SJ, Lavoie JL, Cribbs LL, England SK, Sigmund CD, Weiss RM, Williamson RA, Hill JA, Campbell KP. Abnormal coronary function in mice deficient in a1H T-type Ca2+ channels. Science. 2003;302:1416–18. doi: 10.1126/science.1089268. [DOI] [PubMed] [Google Scholar]
- [71].Mangoni ME, Nargeot J. Genesis and regulation of the heart automaticity. Physiol Rev. 2008;88:919–82. doi: 10.1152/physrev.00018.2007. [DOI] [PubMed] [Google Scholar]
- [72].Mangoni ME, Traboulsie A, Leoni AL, Couette B, Marger L, Le Quang K, Kupfer E, Cohen-Solal A, Vilar J, Shin HS, Escande D, Charpentier F, Nargeot J, Lory P. Bradycardia and slowing of the atrioventricular conduction in mice lacking CaV3.1/alpha1G T-type calcium channels. Circ Res. 2006;98:1422–30. doi: 10.1161/01.RES.0000225862.14314.49. [DOI] [PubMed] [Google Scholar]
- [73].Ono K, Iijima T. Pathophysiological significance of T-type Ca2+ channels: properties and functional roles of T-type Ca2+ channels in cardiac pacemaking. J Pharmacol Sci. 2005;99:197–204. doi: 10.1254/jphs.fmj05002x2. [DOI] [PubMed] [Google Scholar]
- [74].Kobayashi T, Yamada Y, Fukao M, Tsutsuura M, Tohse N. Regulation of Cav1.2 current: interaction with intracellular molecules. J Pharmacol Sci. 2007;103:347–53. doi: 10.1254/jphs.cr0070012. [DOI] [PubMed] [Google Scholar]
- [75].Zhang Z, Xu Y, Song H, Rodriguez J, Tuteja D, Namkung Y, Shin HS, Chiamvimonvat N. Functional Roles of Ca(v)1.3 (alpha(1D)) calcium channel in sinoatrial nodes: insight gained using gene-targeted null mutant mice. Circ Res. 2002;90:981–7. doi: 10.1161/01.res.0000018003.14304.e2. [DOI] [PubMed] [Google Scholar]
- [76].Verheijck EE, van Ginneken AC, Wilders R, Bouman LN. Contribution of L-type Ca2+ current to electrical activity in sinoatrial nodal myocytes of rabbits. Am. J. Physiol. 1999;276:H1064–77. doi: 10.1152/ajpheart.1999.276.3.H1064. [DOI] [PubMed] [Google Scholar]
- [77].Tanaka H, Clark RB, Giles WR. Positive chronotropic responses of rabbit sino-atrial node cells to flash photolysis of caged isoproterenol and cyclic AMP. Proc Biol Sci. 1996;263:241–8. doi: 10.1098/rspb.1996.0038. [DOI] [PubMed] [Google Scholar]
- [78].McDonald TF, Pelzer S, Trautwein W, Pelzer DJ. Regulation and modulation of calcium channels in cardiac, skeletal, and smooth muscle cells. Physiol. Rev. 1994;74:365–507. doi: 10.1152/physrev.1994.74.2.365. [DOI] [PubMed] [Google Scholar]
- [79].Verde I, Vandecasteele G, Lezoualc’h F, Fischmeister R. Characterization of the cyclic nucleotide phosphodiesterase subtypes involved in the regulation of the L-type Ca2+ current in rat ventricular myocytes. Br J Pharmacol. 1999;127:65–74. doi: 10.1038/sj.bjp.0702506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Trautwein W, Kameyama M, Hescheler J, Hofmann F. Cardiac calcium channels and their transmitter modulation. Prog. ZooZ. 1986;33:163–82. [Google Scholar]
- [81].Fraser ID, Tavalin SJ, Lester LB, Langeberg LK, Westphal AM, Dean RA, Marrion NV, Scott JD. A novel lipid-anchored A-kinase Anchoring Protein facilitates cAMP-responsive membrane events. EMBO J. 1998;17:2261–2272. doi: 10.1093/emboj/17.8.2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Striessnig J. Pharmacology, structure and function of cardiac L-type Ca(2+) channels. Cell Physiol Biochem. 1999;9:242–69. doi: 10.1159/000016320. [DOI] [PubMed] [Google Scholar]
- [83].Hulme JT, Ahn M, Hauschka SD, Scheuer T, Catterall WA. A novel leucine zipper targets AKAP15 and cyclic AMP-dependent protein kinase to the C terminus of the skeletal muscle Ca2+ channel and modulates its function. J Biol Chem. 2002;277:4079–4087. doi: 10.1074/jbc.M109814200. [DOI] [PubMed] [Google Scholar]
- [84].Petit-Jacques J, Bois P, Bescond J, Lenfant J. Mechanism of muscarinic control of the high-threshold calcium current in rabbit sino-atrial node myocytes. Pflugers Arch. 1993;423:21–27. doi: 10.1007/BF00374956. [DOI] [PubMed] [Google Scholar]
- [85].Weidman S. Effect of current flow on the membrane potential of cardiac muscle. J.Physiol (London) 1951;115:227–236. doi: 10.1113/jphysiol.1951.sp004667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Noble D. The surprising heart: a review of recent progress in cardiac electrophysiology. J Physiol. 1984;353:1–50. doi: 10.1113/jphysiol.1984.sp015320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Sanguinetti MC, Jurkiewicz NK. Two components of cardiac delayed rectifier K+ current. Differential sensitivity to block by class III antiarrhythmic agents. J Gen Physiol. 1990;96:195–215. doi: 10.1085/jgp.96.1.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Walsh KB, Kass RS. Regulation of a heart potassium channel by protein kinase A and C. Science. 1988;242:67–9. doi: 10.1126/science.2845575. [DOI] [PubMed] [Google Scholar]
- [89].Walsh KB, Kass RS. Distinct voltage-dependent regulation of a heart-delayed IK by protein kinases A and C. Am J Physiol. 1991;261:C1081–90. doi: 10.1152/ajpcell.1991.261.6.C1081. [DOI] [PubMed] [Google Scholar]
- [90].Potet F, Scott JD, Mohammad-Panah R, Escande D, Baró I. AKAP proteins anchor cAMP-dependent protein kinase to KvLQT1/IsK channel complex. Am J Physiol Heart Circ Physiol. 2001;280:H2038–45. doi: 10.1152/ajpheart.2001.280.5.H2038. [DOI] [PubMed] [Google Scholar]
- [91].Brown HF, Kimura J, Noble D, Noble SJ, Taupignon A. The ionic currents underlying pacemaker activity in rabbit sino-atrial node: experimental results and computer simulations. Proc R Soc Lond B. 1984;222:329–347. doi: 10.1098/rspb.1984.0067. [DOI] [PubMed] [Google Scholar]
- [92].Zhou Z, Lipsius SL. Na+-Ca2+ exchange current in latent pacemaker cells isolated from cat right atrium. J. Physiol. 1993;466:263–285. [PMC free article] [PubMed] [Google Scholar]
- [93].Nicoll DA, Longoni S, Philipson KD. Molecular cloning and functional expression of the cardiac sarcolemmal Na(+)-Ca2+ exchanger. Science. 1990;250:562–5. doi: 10.1126/science.1700476. [DOI] [PubMed] [Google Scholar]
- [94].Schulze DH, Muqhal M, Lederer WJ, Ruknudin AM. Sodium/calcium exchanger (NCX1) macromolecular complex. J. Biol. Chem. 2003;278:28849–28855. doi: 10.1074/jbc.M300754200. [DOI] [PubMed] [Google Scholar]
- [95].Wei SK, Ruknudin A, Hanlon SU. Protein kinase A hyperphosphorylation increases basal current but decreases beta-adrenergic responsiveness of the sarcolemmal Na+-Ca2+ exchanger in failing pig myocytes. Circ Res. 2003;92:897–903. doi: 10.1161/01.RES.0000069701.19660.14. [DOI] [PubMed] [Google Scholar]
- [96].Ginsburg KS, Bers DM. Isoproterenol does not enhance Ca-dependent Na/Ca exchange current in intact rabbit ventricular myocytes. J. Mol. Cell Cardiol. 2005;39:972–981. doi: 10.1016/j.yjmcc.2005.09.005. [DOI] [PubMed] [Google Scholar]
- [97].Seifen E, Schaer H, Marshall JM. Effect of calcium on the membrane potentials of single pacemaker fibres in isolared rabbits atria. Nature. 1964;202:1223–4. doi: 10.1038/2021223a0. [DOI] [PubMed] [Google Scholar]
- [98].Cranefield PF. The conduction of the cardiac impulse. Futura Publ. Comp.; Mount Kisco, New York: 1975. [Google Scholar]
- [99].Hagiwara N, Irisawa H. Modulation by intracellular Ca2+ of the hyperpolarization-activated inward current in rabbit single sino-atrial node cells. J Physiol. 1989;409:121–41. doi: 10.1113/jphysiol.1989.sp017488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Satoh H. Electropharmacology of taurine on the hyperpolarization-activated inward current and the sustained inward current in spontaneously beating rat sino-atrial nodal cells. J. Pharmacol. Sci. 2003;91:229–38. doi: 10.1254/jphs.91.229. [DOI] [PubMed] [Google Scholar]
- [101].Zaza A, Maccaferri G, Mangoni M, DiFrancesco D. Intracellular calcium does not directly modulate cardiac pacemaker (if) channels. Pflugers Arch. 1991;419:662–4. doi: 10.1007/BF00370312. [DOI] [PubMed] [Google Scholar]
- [102].Rigg L, Mattick PA, Heath BM, Terrar DA. Modulation of the hyperpolarization-activated current (I(f)) by calcium and calmodulin in the guinea-pig sino-atrial node. Cardiovasc Res. 2003;57(2):497–504. doi: 10.1016/s0008-6363(02)00668-5. [DOI] [PubMed] [Google Scholar]
- [103].Kass RS, Sanguinetti MC. Inactivation of calcium channel current in the calf cardiac Purkinje fiber. Evidence for voltage- and calcium-mediated mechanisms. J. Gen. Physiol. 1984;84:705–26. doi: 10.1085/jgp.84.5.705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Isenberg G. Cardiac Purkinje fibers: the slow inward current component under the influence of modified [Ca2+]i. Pfluegers Arch. 1977;371:61–9. doi: 10.1007/BF00580773. [DOI] [PubMed] [Google Scholar]
- [105].Tseng GN, Boyden PA. Different effects of intracellular Ca and protein kinase C on cardiac T and L Ca currents. Am J Physiol. 1991;261:H364–79. doi: 10.1152/ajpheart.1991.261.2.H364. [DOI] [PubMed] [Google Scholar]
- [106].Tohse N. Calcium-sensitive delayed rectifier potassium current in guinea pig ventricular cells. Am J Physiol. 1990;258:H1200–7. doi: 10.1152/ajpheart.1990.258.4.H1200. [DOI] [PubMed] [Google Scholar]
- [107].Heath BM, Terrar DA. Protein kinase C enhances the rapidly activating delayed rectifier potassium current, IKr, through a reduction in C-type inactivation in guinea-pig ventricular myocytes. J Physiol. 2000;522:391–402. doi: 10.1111/j.1469-7793.2000.t01-2-00391.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Guo J, Ono K, Noma A. A sustained inward current activated at the diastolic potential range in rabbit sino-atrial node cells. J Physiol. 1995;483:1–13. doi: 10.1113/jphysiol.1995.sp020563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Guo J, Mitsuiye T, Noma A. The sustained inward current in sino-atrial node cells of guinea-pig heart. Pflugers Arch. 1997;433:390–6. doi: 10.1007/s004240050293. [DOI] [PubMed] [Google Scholar]
- [110].Mitsuiye T, Shinagawa Y, Noma A. Sustained inward current during pacemaker depolarization in mammalian sinoatrial node cells. Circ. Res. 2000;87:88–91. doi: 10.1161/01.res.87.2.88. [DOI] [PubMed] [Google Scholar]
- [111].Verkerk AO, Wilders R, Zegers JG, van Borren MM, Ravesloot JH, Verheijck EE. Ca(2+)-activated Cl(−) current in rabbit sinoatrial node cells. J Physiol. 2002;540:105–17. doi: 10.1113/jphysiol.2001.013184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Braun AP, Schulman H. The multifunctional calcium/calmodulin-dependent protein kinase: from form to function. Annu Rev Physiol. 1995;57:417–45. doi: 10.1146/annurev.ph.57.030195.002221. [DOI] [PubMed] [Google Scholar]
- [113].Vinogradova TM, Zhou YY, Bogdanov KY, Yang D, Kuschel M, Cheng H, Xiao RP. Sinoatrial node pacemaker activity requires Ca2+/calmodulin dependent protein kinase II activation. Circ Res. 2000;87:760–767. doi: 10.1161/01.res.87.9.760. [DOI] [PubMed] [Google Scholar]
- [114].Xiao RP, Cheng H, Lederer WJ, Suzuki T, Lakatta EG. Dual regulation of Ca2+/calmodulin-dependent kinase II activity by membrane voltage and by calcium influx. Proc Natl Acad Sci U S A. 1994;91:9659–63. doi: 10.1073/pnas.91.20.9659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Yuan W, Bers DM. Ca-dependent facilitation of cardiac Ca current is due to Ca-calmodulin-dependent protein kinase. Am J Physiol. 1994;267:H982–93. doi: 10.1152/ajpheart.1994.267.3.H982. [DOI] [PubMed] [Google Scholar]
- [116].Dzhura I, Wu Y, Colbran RJ, Balser JR, Anderson ME. Calmodulin kinase determines calcium-dependent facilitation of L-type calcium channels. Nat Cell Biol. 2000;2:173–7. doi: 10.1038/35004052. [DOI] [PubMed] [Google Scholar]
- [117].Wegener AD, Simmerman HK, Lindemann JP, Jones LR. Phospholamban phosphorylation in intact ventricles. Phosphorylation of serine 16 and threonine 17 in response to beta-adrenergic stimulation. J. Biol. Chem. 1989;264:11468–11474. [PubMed] [Google Scholar]
- [118].Xu A, Narayanan N. Ca2+/calmodulin-dependent phosphorylation of the Ca2+-ATPase, uncoupled from phospholamban, stimulates Ca2+-pumping in native cardiac sarcoplasmic reticulum. Biochem Biophys Res Commun. 1999;258:66–72. doi: 10.1006/bbrc.1999.0579. [DOI] [PubMed] [Google Scholar]
- [119].Li Y, Sirenko S, Vinogradova TM, Lyashkov AE, Zhu W, Juhaszova M, Ziman B, Wang S, Lakatta EG. High basal Ca2+/calmodulin kinase II activity modulates spontaneous sarcoplasmic reticulum Ca2+ cycling that drives normal automaticity in sinoatrial nodal cells. Circulation. 2007;116:86–87. (Abstract) [Google Scholar]
- [120].Lakatta EG. Functional implications of spontaneous sarcoplasmic reticulum Ca2+ release in the heart. Cardiovasc Res. 1992;26:193–214. doi: 10.1093/cvr/26.3.193. [DOI] [PubMed] [Google Scholar]
- [121].Musa H, Lei M, Honjo H, Jones SA, Dobrzynski H, Lancaster MK, Takagishi Y, Henderson Z, Kodama I, Boyett MR. Heterogeneous expression of Ca(2+) handling proteins in rabbit sinoatrial node. J Histochem Cytochem. 2002;50:311–24. doi: 10.1177/002215540205000303. [DOI] [PubMed] [Google Scholar]
- [122].Lyashkov AE, Juhaszova M, Dobrzynski H, Vinogradova TM, Maltsev VA, Juhasz O, Spurgeon HA, Sollott SJ, Lakatta EG. Calcium cycling protein density and functional importance to automaticity of isolated sinoatrial nodal cells are independent of cell size. Circ Res. 2007;100:1723–31. doi: 10.1161/CIRCRESAHA.107.153676. [DOI] [PubMed] [Google Scholar]
- [123].Rigg L, Heath BM, Cui Yi, Terrar DA. Localization and functional significance of ryanodine receptors during β-adrenergic stimulation in guinea-pig sino-atrial node. Cardiovasc Res. 2000;48:254–264. doi: 10.1016/s0008-6363(00)00153-x. [DOI] [PubMed] [Google Scholar]
- [124].Meissner G. Ryanodine activation and inhibition of the Ca release channel of sarcoplasmic reticulum. J Biol Chem. 1986;261:6300–6305. [PubMed] [Google Scholar]
- [125].Honjo H, Inada S, Lancaster MK, Yamamoto M, Niwa R, Jones SA, Shibata N, Mitsui K, Horiuchi T, Kamiya K, Kodama I, Boyett MR. Sarcoplasmic reticulum Ca2+ release is not a dominating factor in sinoatrial node pacemaker activity. Circ Res. 2003;92:e41–4. doi: 10.1161/01.res.0000055904.21974.be. [DOI] [PubMed] [Google Scholar]
- [126].Nett MP, Vassalle M. Obligatory role of diastolic voltage oscillations in sino-atrial node discharge. J Mol Cell Cardiol. 2003;35:1257–76. doi: 10.1016/s0022-2828(03)00236-0. [DOI] [PubMed] [Google Scholar]
- [127].Ju YK, Chu Y, Chaulet H, Lai D, Gervasio OL, Graham RM, Cannell MB, Allen DG. Store-operated Ca2+ influx and expression of TRPC genes in mouse sinoatrial node. Circ Res. 2007;100:1605–14. doi: 10.1161/CIRCRESAHA.107.152181. [DOI] [PubMed] [Google Scholar]
- [128].Masumiya H, Yamamoto H, Hemberger M, Tanaka H, Shigenobu K, Chen SR, Furukawa T. The mouse sino-atrial node expresses both the type 2 and type 3 Ca(2+) release channels/ryanodine receptors. FEBS Lett. 2003;553:141–4. doi: 10.1016/s0014-5793(03)00999-2. [DOI] [PubMed] [Google Scholar]
- [129].Nikmaram MR, Liu J, Abdelrahman M, Dobrzynski H, Boyett MR, Lei M. Characterization of the effects of ryanodine, TTX, E-4031 and 4-AP on the sinoatrial and atrioventricular nodes. Prog Biophys Mol Biol. 2008;96:452–64. doi: 10.1016/j.pbiomolbio.2007.07.003. [DOI] [PubMed] [Google Scholar]
- [130].Wu Y, Gao Z, Chen B, Koval OM, Singh MV, Guan X, Hund TJ, Kutschke W, Sarma S, Grumbach IM, Wehrens XH, Mohler PJ, Song LS, Anderson ME. Calmodulin kinase II is required for fight or flight sinoatrial node physiology. Proc Natl Acad Sci U S A. 2009;106:5972–7. doi: 10.1073/pnas.0806422106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [131].Ju YK, Allen DG. How does beta-adrenergic stimulation increase the heart rate? The role of intracellular Ca2+ release in amphibian pacemaker cells. J Physiol. 1999;516:793–804. doi: 10.1111/j.1469-7793.1999.0793u.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [132].Rose RA, Kabir MG, Backx PH. Altered Heart Rate and Sinoatrial Node Function in Mice Lacking the cAMP Regulator Phosphoinositide 3-Kinase-γ. Circ Res. 2007;101:1274–1282. doi: 10.1161/CIRCRESAHA.107.158428. [DOI] [PubMed] [Google Scholar]
- [133].Lancaster MK, Jones SA, Harrison SM, Boyett MR. Intracellular Ca2+ and pacemaking within the rabbit sinoatrial node: heterogeneity of role and control. J Physiol. 2004;556:481–94. doi: 10.1113/jphysiol.2003.057372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [134].Bucchi A, Baruscotti M, Robinson RB, DiFrancesco D. I(f)-dependent modulation of pacemaker rate mediated by cAMP in the presence of ryanodine in rabbit sino-atrial node cells. J Mol Cell Cardiol. 2003;35:905–13. doi: 10.1016/s0022-2828(03)00150-0. [DOI] [PubMed] [Google Scholar]
- [135].Tsien RW, Kass RS, Weingart R. Cellular and subcellular mechanisms of cardiac pacemaker oscillations. J Exp Biol. 1979;81:205–15. doi: 10.1242/jeb.81.1.205. [DOI] [PubMed] [Google Scholar]
- [136].Vinogradova TM, Zhou YY, Maltsev V, Lyashkov A, Stern M, Lakatta EG. Rhythmic ryanodine receptor Ca2+ releases during diastolic depolarization of sinoatrial pacemaker cells do not require membrane depolarization. Circ Res. 2004;94:802–9. doi: 10.1161/01.RES.0000122045.55331.0F. [DOI] [PubMed] [Google Scholar]
- [137].Parsons SP, Vinogradova TM, Lyashkov AE, Spurgeon HA, Stern MD, Lakatta EG, Maltsev AV. Local Ca2+ waves rather than sparks boost the subsarcolemmal Ca2+ rise in late diastole of rabbit sinoatrial node cells: characterization in two dimensions. Biophysical J. 2007;76a Abstract. [Google Scholar]
- [138].Bogdanov KY, Maltsev VA, Vinogradova TM, Lyashkov AE, Spurgeon HA, Stern MD, Lakatta EG. Membrane potential fluctuations resulting from submembrane Ca2+ releases in rabbit sinoatrial nodal cells impart an exponential phase to the late diastolic depolarization that controls their chronotropic state. Circ Res. 2006;99:979–87. doi: 10.1161/01.RES.0000247933.66532.0b. [DOI] [PubMed] [Google Scholar]
- [139].DiFrancesco D. The contribution of the ‘pacemaker’ current (if) to generation of spontaneous activity in rabbit sino-atrial node myocytes. J Physiol. 1991;434:23–40. doi: 10.1113/jphysiol.1991.sp018457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [140].Maltsev VA, Lakatta EG. Dynamic interactions of an intracellular Ca2+ clock and membrane ion channel clock underlie robust initiation and regulation of cardiac pacemaker function. Cardiovasc Res. 2008;77:274–84. doi: 10.1093/cvr/cvm058. [DOI] [PubMed] [Google Scholar]
- [141].Maltsev VA, Lakatta EG. Synergism of coupled subsarcolemmal Ca2+ clocks and sarcolemmal voltage clocks confers robust and flexible pacemaker function in a novel pacemaker cell model. Am J Physiol Heart Circ Physiol. 2009;296:H594–615. doi: 10.1152/ajpheart.01118.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [142].Bhuiyan ZA, van den Berg MP, van Tintelen JP, Bink-Boelkens MT, Wiesfeld AC, Alders M, Postma AV, van Langen I, Mannens MM, Wilde AA. Expanding spectrum of human RYR2-related disease: new electrocardiographic, structural, and genetic features. Circulation. 2007;116:1569–76. doi: 10.1161/CIRCULATIONAHA.107.711606. [DOI] [PubMed] [Google Scholar]
- [143].Le Scouarnec S, Bhasin N, Vieyres C, Hund TJ, Cunha SR, Koval O, Marionneau C, Chen B, Wu Y, Demolombe S, Song LS, Le Marec H, Probst V, Schott JJ, Anderson ME, Mohler PJ. Dysfunction in ankyrin-B-dependent ion channel and transporter targeting causes human sinus node disease. Proc Natl Acad Sci U S A. 2008;105:15617–22. doi: 10.1073/pnas.0805500105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [144].Barbuti A, DiFrancesco D. Control of cardiac rate by “funny” channels in health and disease. Ann N Y Acad Sci. 2008;1123:213–23. doi: 10.1196/annals.1420.024. [DOI] [PubMed] [Google Scholar]
- [145].Himeno Y, Sarai N, Matsuoka S, Noma A. Ionic mechanisms underlying the positive chronotropy induced by beta1-adrenergic stimulation in guinea pig sinoatrial node cells: a simulation study. J Physiol Sci. 2008;58:53–65. doi: 10.2170/physiolsci.RP015207. [DOI] [PubMed] [Google Scholar]
- [146].Hauswirth o, Noble D, Tsien RW. Adrenalin: mechanism of action on the pacemaker potential in cardiac Purkinje fibers. Science. 1968;162:916–7. doi: 10.1126/science.162.3856.916. [DOI] [PubMed] [Google Scholar]
- [147].Tsien RW. Effects of epinephrine on the pacemaker potassium current of cardiac Purkinje fibers. J. Gen Physiol. 1974;64:293–319. doi: 10.1085/jgp.64.3.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [148].Brown HF, DiFrancesco D, Noble SJ. How does adrenaline accelerate the heart? Nature. 1979;280:235–6. doi: 10.1038/280235a0. [DOI] [PubMed] [Google Scholar]
- [149].DiFrancesco D, Mangoni M. Modulation of single hyperpolarization-activated channels (if) by cAMP in the rabbit sino-atrial node. J. Physiol. 1994;474:473–82. doi: 10.1113/jphysiol.1994.sp020038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [150].Yamamoto M, Dobrzynski H, Tellez J, Niwa R, Billeter R, Honjo H, Kodama I, Boyett MR. Extended atrial conduction system characterised by the expression of the HCN4 channel and connexin45. Cardiovasc Res. 2006;72:271–81. doi: 10.1016/j.cardiores.2006.07.026. [DOI] [PubMed] [Google Scholar]
- [151].Lakatta EG, Difrancesco D. What keeps us ticking: a funny current, a calcium clock, or both? J Mol Cell Cardiol. 2009 doi: 10.1016/j.yjmcc.2009.03.022. [in press] [DOI] [PMC free article] [PubMed] [Google Scholar]
- [152].Lei M, Brown HF, Terrar DA. Modulation of delayed rectifier potassium current, iK, by isoprenaline in rabbit isolated pacemaker cells. Exp Physiol. 2000;85:27–35. [PubMed] [Google Scholar]
- [153].Freeman LC, Kass RS. Delayed rectifier potassium channels in ventricle and sinoatrial node of the guinea pig: molecular and regulatory properties. Cardiovasc Drugs Ther. 1993;(Suppl 3):627–35. doi: 10.1007/BF00877630. [DOI] [PubMed] [Google Scholar]
- [154].Ding WG, Toyoda F, Matsuura H. Blocking action of chromanol 293B on the slow component of delayed rectifier K(+) current in guinea-pig sino-atrial node cells. Br J Pharmacol. 2002;137:253–62. doi: 10.1038/sj.bjp.0704861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [155].Kurokawa J, Bankston JR, Kaihara A, Chen L, Furukawa T, Kass RS. KCNE variants reveal a critical role of the beta subunit carboxyl terminus in PKA-dependent regulation of the IKs potassium channel. Channels (Austin) 2009;3:16–24. doi: 10.4161/chan.3.1.7387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [156].Lei M, Cooper PJ, Camelliti P, Kohl P. Role of the 293b-sensitive, slowly activating delayed rectifier potassium current, IKs, in pacemaker activity of rabbit isolated sino-atrial node cells. Cardiovascular Research. 2002;53:68–79. doi: 10.1016/s0008-6363(01)00459-x. [DOI] [PubMed] [Google Scholar]
- [157].Reuter H. The dependence of slow inward current in Purkinje fibres on the extracellular calcium-concentration. J. Physiol. 1967;192:479–492. doi: 10.1113/jphysiol.1967.sp008310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [158].Reuter H, Scholz H. The regulation of the calcium conductance of cardiac muscle by adrenaline. J Physiol. 1977;264:49–62. doi: 10.1113/jphysiol.1977.sp011657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [159].Reuter H. Calcium channel modulation by neurotransmitters, enzymes and drugs. Nature. 1983;301:569–74. doi: 10.1038/301569a0. [DOI] [PubMed] [Google Scholar]
- [160].Ke Y, Lei M, Collins TP, Rakovic S, Mattick PA, Yamasaki M, Brodie MS, Terrar DA, Solaro RJ. Regulation of L-type calcium channel and delayed rectifier potassium channel activity by p21-activated kinase-1 in guinea pig sinoatrial node pacemaker cells. Circ Res. 2007;100(9):1317–27. doi: 10.1161/01.RES.0000266742.51389.a4. [DOI] [PubMed] [Google Scholar]
- [161].van der Heyden MA, Wijnhoven TJ, Opthof T. Molecular aspects of adrenergic modulation of cardiac L-type Ca2+ channels. Cardiovasc Res. 2005;65:28–39. doi: 10.1016/j.cardiores.2004.09.028. [DOI] [PubMed] [Google Scholar]
- [162].Kamp TJ, Hell JW. Regulation of cardiac L-type calcium channels by protein kinase A and protein kinase C. Circ Res. 2000;87:1095–102. doi: 10.1161/01.res.87.12.1095. [DOI] [PubMed] [Google Scholar]
- [163].Hartzell HC, Mery P-F, Fischmeister R, Szabo G. Sympathetic regulation of cardiac calcium current is due exclusively to cAMP-dependent phosphorylation. Nature Lond. 199l;351:573–6. doi: 10.1038/351573a0. [DOI] [PubMed] [Google Scholar]
- [164].Mauban JR, O’Donnell M, Warrier S, Manni S, Bond M. AKAP-scaffolding proteins and regulation of cardiac physiology. Physiology (Bethesda) 2009;24:78–87. doi: 10.1152/physiol.00041.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [165].Toyoda F, Ding WG, Matsuura H. Responses of the sustained inward current to autonomic agonists in guinea-pig sino-atrial node pacemaker cells. Br J Pharmacol. 2005;144(5):660–8. doi: 10.1038/sj.bjp.0706101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [166].Vinogradova TM, Maltsev VA, Bogdanov KY, Lyashkov AE, Lakatta EG. Rhythmic Ca2+ oscillations drive sinoatrial nodal cell pacemaker function to make the heart tick. Ann NY Acad Sci. 2005;1047:138–56. doi: 10.1196/annals.1341.013. [DOI] [PubMed] [Google Scholar]
- [167].Valdivia HH, Kaplan JH, Ellis-Davies GC, Lederer WJ. Rapid adaptation of cardiac ryanodine receptors: modulation by Mg2+ and phosphorylation. Science. 1995;267:1997–2000. doi: 10.1126/science.7701323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [168].Marx SO, Reiken S, Hisamatsu Y, Jayaraman T, Burkhoff D, Rosemblit N, Marks AR. PKA phosphorylation dissociates FKBP12.6 from the calcium release channel (ryanodine receptor): defective regulation in failing hearts. Cell. 2000;101:365–76. doi: 10.1016/s0092-8674(00)80847-8. [DOI] [PubMed] [Google Scholar]