Abstract
The immune mechanisms that provoke concomitant inflammation of synovial joints and cardiac valves in disorders such as rheumatic fever and systemic lupus erythematosus remain poorly defined. Here, we report the discovery of spontaneous endocarditis—in addition to their well-studied autoimmune arthritis—in K/BxN T cell receptor (TCR) transgenic mice. The same adaptive immune system elements were required for initiation of arthritis and endocarditis, and both diseases were dependent on autoantibodies. In contrast, the participation of key innate immune system molecules and perhaps T cells as effectors of inflammation differed between the 2 target tissues. Arthritis in K/BxN TCR transgenic mice depended primarily on complement C5 and not FcRγ-using receptors; conversely, endocarditis depended essentially on FcRγ receptors and not C5. Elucidating how a single systemic autoimmune disease engages distinct immune effector pathways to damage different target tissues is essential for optimizing the treatment of such disorders.
Keywords: autoimmunity, complement, Fc receptor, rheumatic, lupus
Many systemic autoimmune diseases affect both the synovial joints and the cardiovascular system. For example, rheumatoid arthritis and systemic lupus erythematosus (SLE), often entailing inflammatory arthritis, lead to increased risk for coronary artery disease (1, 2). Inflammation of the cardiac valves occurs in rheumatic fever following streptococcal infection (rheumatic carditis), and in SLE and the related antiphospholipid syndrome (Libman-Sacks endocarditis) (3). The immune mechanisms by which these autoantibody-associated diseases cause inflammation of synovial and endothelial tissues remain unclear.
Analyses of tissues from patients with rheumatic or autoimmune endocarditis provide descriptive rather than mechanistic insight into pathogenesis (4). Unfortunately, most existing animal models of rheumatic carditis depend on immunization with a foreign antigen such as streptococcal M protein or with its proposed mimic, cardiac myosin, and produce both endocarditis and myocarditis, but not arthritis (5). The proposed pathogenesis of endocarditis in these animal models involves 2 main steps: antibody-initiated damage and activation of the endothelium, followed by T-cell infiltration (5). A more accurate animal model comprising both endocarditis and arthritis would be very valuable for mechanistic dissections.
The K/BxN mouse model has afforded key insights into the pathogenesis of autoantibody-induced arthritis. In these mice, T lymphocytes bearing a transgene-encoded T-cell receptor (TCR) termed KRN recognize self-peptides derived from glucose-6-phosphate isomerase (GPI) and presented by the major histocompatibility complex (MHC) class II molecule Ag7 from the NOD mouse strain (6, 7). Autoreactive KRN T cells stimulate GPI-reactive B lymphocytes, leading to production of anti-GPI autoantibodies and the development of arthritis. Passive transfer of anti-GPI autoantibodies provokes arthritis in recipient mice, and depends primarily on innate immune system cells and molecules (8). The effector molecules required for K/BxN serum-transferred arthritis include the alternative pathway of complement and Fcγ receptors (9, 10), both also required for arthritis induced by injection of anticollagen antibodies (11, 12), suggesting common pathways by which antibodies provoke synovial inflammation. Here we show that the heart is a second target organ in arthritic K/BxN TCR transgenic mice and provide evidence for different immunopathogenic mechanisms in the 2 target tissues.
Results
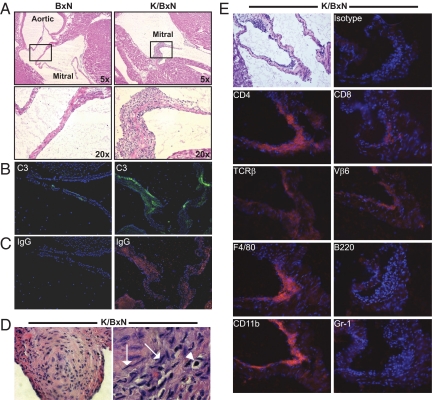
Based on the coexistence of arthritis and endocarditis in several human autoantibody-associated disorders, we asked whether K/BxN TCR transgenic mice, with high-titer autoantibodies and arthritis, might also have cardiac inflammation. Indeed, we discovered mitral valve inflammation in essentially all of them (Fig. 1A). Occasional K/BxN mice had mild aortic valve inflammation, but involvement of the tricuspid and pulmonary valves was not observed. In contrast, transgene-negative (BxN) littermates had no cardiac valve inflammation. As has been described in patients with rheumatic carditis or Libman-Sacks endocarditis (13–15), complement C3 and Ig were bound to the inflamed mitral valves of K/BxN mice (Fig. 1 B and C). In addition, the myocardium of some of them contained collections of cells resembling the Aschoff nodules and Anitschkow cells characteristic of rheumatic carditis (Fig. 1D) (16). The temporal development of endocarditis paralleled the course of arthritis in this model (6), becoming evident around 3 weeks of age and progressively worsening [supporting information (SI) Fig. S1].
Fig. 1.
Histological characterization of mitral valve inflammation in K/BxN mice. (A) Long-axis sections through the hearts of 8-week-old nonarthritic BxN mice (Left) and arthritic K/BxN mice (Right) show inflammation of the mitral valve in K/BxN mice. Hematoxylin and eosin (H&E); original magnifications are indicated. (B and C) Immunofluorescent staining of similar sections demonstrates deposition of (B) complement C3 (green) and (C) Ig IgG (red) in the inflamed mitral valve of K/BxN mice; the sections are counterstained with DAPI to detect nuclei (blue). (Objective: ×10.) (D) Sections of 2 different K/BxN hearts are shown, demonstrating characteristic nodular collections of amorphous cells similar to Aschoff bodies or nodules (Left). (Objective: ×40.) Some cells have characteristic “caterpillar”-shaped nuclei (arrows) and “owl's-eye” nuclei (arrowhead) typical of Anitschkow cells (Right). (Objective: ×100.) Though the origin of these cells is debated, the structures are well-described characteristics of rheumatic carditis in humans. (E) A mildly inflamed mitral valve from a K/BxN mouse heart is shown in the upper left (H&E). Serial sections from this same specimen were stained with biotinylated antibodies recognizing the indicated antigens (detected with red staining) and with DAPI to detect nuclei (blue). Vβ6 is the TCR β-chain encoded by the KRN transgene. The isotype control antibody displayed is rat IgG2a. (Objective: ×10.)
The inflammatory mitral valve infiltrate in K/BxN mice was composed predominantly of macrophages and T lymphocytes, including T cells bearing the KRN transgene-encoded TCR Vβ6 subunit (Fig. 1E). Although some CD8+ T cells were present, most of the infiltrating cells expressed CD4. We did not detect B cells in the inflamed valves. This predominance of T cells and macrophages is similar to the infiltrate in human rheumatic carditis (4). Notably, though synovial fluid of K/BxN mice contains primarily neutrophils (6), we did not detect this cell type in the inflamed valves.
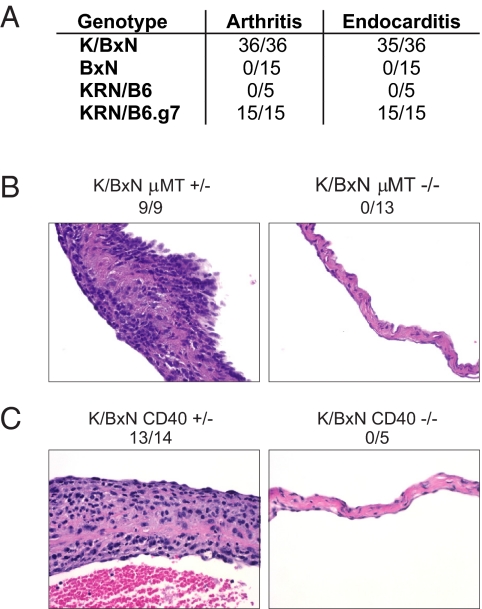
We took advantage of the genetic manipulability of the K/BxN TCR transgenic mouse system to dissect the immunologic requirements for the development of autoimmune endocarditis. As with arthritis, cardiac valve inflammation required both the KRN TCR transgenes and the NOD-derived MHC class II molecule Ag7. No other NOD-derived genes were needed, because KRN/C57BL/6 mice expressing congenic Ag7 also developed endocarditis (Fig. 2A). B cells were required for the development of cardiac valve inflammation (Fig. 2B), as they are for arthritis (6), because K/BxN mice carrying the μMT mutation got neither disease. Similarly, CD40 was needed for the development of both arthritis (8) and endocarditis (Fig. 2C). T-cell activation was unimpaired in CD40-deficient K/BxN mice (8), suggesting that the lack of endocarditis in these animals reflected impaired T/B cell interaction, compromising both antibody isotype switching and autoantibody production, as is the case with arthritis (8). Thus, several of the same elements of the adaptive immune system critical for the initiation phase of arthritis in K/BxN mice were also required for their endocarditis.
Fig. 2.
Adaptive immune requirements for endocarditis in K/BxN mice. (A) The KRN TCR transgene and the MHC molecule I-Ag7 are required for the development of arthritis and endocarditis. KRN/B6.g7 indicates KRN/B6 mice with congenically expressed Ag7. Numbers represent the incidence of arthritis and endocarditis. A valve was considered to have endocarditis if an inflammatory valve infiltrate was present. (B and C) B cells and CD40 are required for endocarditis. Mitral valves from (B) B-cell-sufficient μMT+/− and B cell-deficient μMT−/− K/BxNmice and from (C) CD40-sufficient and CD40-deficient K/BxN mice. The incidence of mitral valve inflammation in mice of each genotype is indicated. (H&E, objective: ×40.)
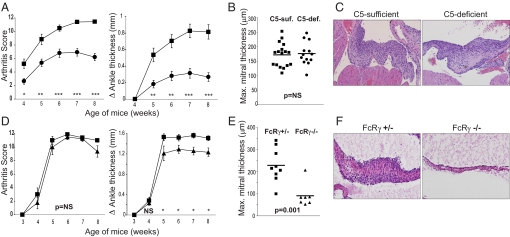
To investigate the effector pathways of endocarditis, we generated KRN TCR transgenic mice lacking either of the 2 major effector pathways by which autoantibodies mediate pathology in K/BxN serum-transferred arthritis: complement activation and Fc receptor binding (17, 18). The alternative pathway of complement activation is required for K/BxN serum-transferred arthritis, acting primarily through production of the anaphylatoxin C5a, so mice lacking C5 or the C5a receptor are resistant to serum-transferred disease (9). Here, we took advantage of a naturally occurring mutation of the C5 gene that causes C5 deficiency in NOD mice and other strains (19). B6 mice with a congenic NOD-derived interval containing the C5-deficient allele were generated and bred with KRN/B6 mice, and the resulting KRN+ C5-heterozygous progeny were mated with NOD animals to produce C5-sufficient (heterozygous for the mutation) and C5-deficient K/BxN TCR transgenic mice. The absence of C5 resulted in less severe arthritis (Fig. 3A), without affecting anti-GPI autoantibody titers (Fig. S2A). In contrast, it had no observable effect on the severity of cardiac valve inflammation in the same animals (Fig. 3 B and C). To ensure that the decrease in arthritis severity reflected an effect of C5 deficiency and not a deficit in other elements encoded in the NOD-derived congenic interval, we treated 3-week-old K/BxN mice with an anti-C5 monoclonal antibody. Arthritis severity was reduced without affecting cardiac valve inflammation (Fig. S3), recapitulating the C5-deficiency phenotype.
Fig. 3.
Differential contributions of C5 and FcRγ to arthritis and endocarditis in K/BxN mice. (A) Arthritis development is impaired in C5-deficient (circles, n = 23) relative to C5-sufficient K/BxN mice (squares, n = 26) as measured by arthritis score and change in ankle thickness as described (29). Error bars, SEM; *, P < 0.05, **, P < 0.001, ***, P < 0.0001. (B and C) Mitral valve inflammation in K/BxN mice is not affected by C5 deficiency as measured by maximum mitral thickness (B) and shown by histology (C). (D) Arthritis scores are equivalent, and only a mild decrement in ankle thickness occurs in FcRγ-deficient (triangles, n = 8) relative to FcRγ-sufficient (squares, n = 10) KRN+ Ag7+ C57BL/6 mice. Error bars, SEM; NS = not significant, *, P < 0.05. (E and F) Mitral valve inflammation is significantly reduced in FcRγ-deficient relative to FcRγ-sufficient mice as measured by maximum mitral thickness (E) and shown by histology (F). For (B) and (E), each point represents one mouse; P values were calculated using Student's t test. (H&E, objective: ×20.)
The other main pathway by which autoantibodies provoke inflammation is by binding to Fc receptors. The activating IgG Fc receptors in mice (FcγRI, FcγRIII, and FcγRIV) share the common gamma cytoplasmic signaling chain, FcRγ (Fcer1g), which is required for K/BxN serum-transferred arthritis (9, 10, 20). Intriguingly, a homozygous null mutation of FcRγ in K/BxN TCR transgenic mice had little or no effect on arthritis (Fig. 3D), whereas the development of endocarditis was substantially impaired (Fig. 3 E and F). Anti-GPI titers were equivalent in FcRγ-sufficient and FcRγ-deficient animals (Fig. S2B). Binding of complement or Ig was not observed in the noninflamed mitral valves of FcRγ-deficient mice, whereas it was unimpaired in C5-deficient mice (Fig. S4), suggesting that FcRγ-mediated binding of Ig might initiate local immune complex deposition.
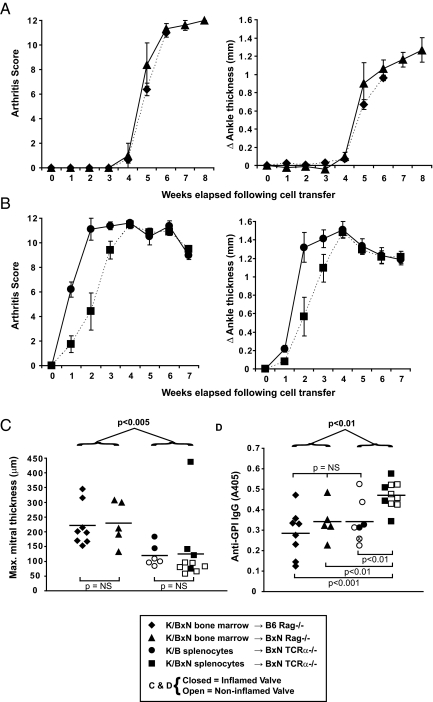
We then asked whether valve pathology could be elicited by simple transfer of K/BxN serum, which readily provokes arthritis in recipient mice (8). This proved impossible, even under exaggerated conditions in which mice were given multiple serum injections over many weeks (Fig. S5). We therefore turned to cell-transfer systems to provide mechanistic insights. Reconstitution of irradiated 7-week-old Rag-1-deficient B6 or BxN mice with bone marrow from K/BxN donors resulted in arthritis and endocarditis in all recipients (Fig. 4 A and C). The fact that the recipient mice were adults suggests that there is no unique window of susceptibility of the cardiac valves of neonatal or young mice to autoimmune attack. Also, the Ag7 MHC class II molecule that is the restriction element of the KRN TCR need not be expressed on the valve endothelium to cause carditis (or analogous joint cells to provoke arthritis), because Rag-1-deficient B6 mice reconstituted with K/BxN bone marrow express Ag7 only on bone marrow-derived cells.
Fig. 4.
Cell transfer systems to provoke arthritis and endocarditis. (A) Arthritis scores and ankle thickness measurements following transfer of bone marrow from K/BxN mice into irradiated B6 Rag-deficient (diamonds) or BxN Rag-deficient (triangles) mice (mean, SEM). For the B6-Rag recipients, the mice were killed due to wasting disease 6 weeks following transplantation. (B) Arthritis scores and ankle thickness measurements following transfer of K/B splenocytes (circles) or K/BxN splenocytes (squares) into BxN TCRα−/− mice (mean, SEM). (C) The maximal thickness of the mitral valves was determined for each mouse at the time of sacrifice. Endocarditis was defined as in Fig. 2A. (D) Anti-GPI IgG titers were determined by ELISA for each mouse at the time of sacrifice (6–8 weeks following splenocyte or bone marrow cell transfer, sera were diluted 1:1,000). For (C) and (D), closed symbols indicate mice with endocarditis, whereas open symbols indicate mice without endocarditis; for one mouse (open symbol with “x” in D), serum was available but histologic evaluation of the heart was not. Bars indicate mean values; P values for comparisons between the groups are provided (Student's t test).
We also attempted to provoke endocarditis by transferring mature splenocytes from KRN/B6 (K/B) mice or K/BxN mice into TCRα-deficient BxN recipients. Both groups of mice developed robust arthritis (Fig. 4B); however, fewer than half of the mice showed endocarditis, irrespective of the source of donor splenocytes (Fig. 4C). Endocarditis was less penetrant and less severe than in the bone marrow cell recipients, despite that the splenocyte recipients had a faster onset of arthritis, longer duration of arthritis before sacrifice (contrast Fig. 4 A and B), and higher anti-GPI IgG titers 6–8 weeks following cell transfer (Fig. 4D). These data suggest that the presence of an autoimmune response capable of producing high-titer anti-GPI autoantibodies and prolonged, severe arthritis does not necessarily result in endocarditis—some other factor(s) must be at work.
Discussion
Several human autoantibody-associated rheumatic diseases affect both the synovial joints and the cardiac valves (3). We have shown that TCR transgenic K/BxN mice develop endocarditis in addition to their well-described arthritis (6). The inflammatory heart disease in K/BxN mice shares several histopathologic similarities with endocarditis observed in human rheumatic conditions, including, predominantly, mitral valve inflammation, infiltration of lymphocytes and macrophages, and the presence of bound complement and Ig. K/BxN mice therefore offer a powerful model for dissecting how systemic autoimmunity provokes inflammation in these 2 target tissues.
Autoimmunity in K/BxN mice occurs in 2 phases: an initiation phase in which a breach of self-tolerance occurs, leading to the generation of autoantibodies, followed by an effector phase in which pathogenic autoantibodies and perhaps T cells provoke tissue inflammation (6, 8). The initiation phase depends on components of the adaptive immune system, including the KRN transgenic TCR, MHC class II molecule I-Ag7, B cells, and CD40 (6, 8). Our findings that these same cells and molecules are required for inflammation in both the joints and the cardiac valves therefore suggests that the initiation phase of autoimmunity is common to both target organs. Once initiated, however, that systemic autoimmune response engages different immune effector mechanisms to provoke pathology in the joints versus the heart.
Specifically, we have shown that complement C5 contributes to the pathogenesis of arthritis but not endocarditis in K/BxN mice. A role for C5 in arthritis in K/BxN TCR transgenic mice is not surprising, based on the known requirements for C5a and its receptor in K/BxN serum-transferred arthritis (9). C5 was not required for the development of endocarditis in K/BxN mice. One possibility is that a C5a chemotactic gradient cannot be established in the heart simply due to the high velocity of blood flow; any potential contribution of C5 to the development of endocarditis is literally washed away. This finding that C5 was dispensable for the development of endocarditis was unexpected however in view of our finding that C3, the molecule immediately upstream of C5 in the complement cascade, was deposited on the inflamed cardiac valves of K/BxN mice (and also C5-deficient K/BxN mice). Two interpretations are possible: C3 might be deposited on the valve surface but not be required for inflammation, or bound C3 might provoke inflammation via a C5-independent mechanism, perhaps by engagement of cellular receptors for C3 or its cleavage products. Differentiating these possibilities definitively will require the generation of C3-deficient K/BxN mice, a task complicated by the chromosomal proximity of the C3 gene locus to the mouse MHC (encoding the required I-Ag7 molecule). Nonetheless, it is clear that C5 is important for the development of arthritis but dispensable for the pathogenesis of endocarditis in K/BxN mice.
Conversely, the common gamma subunit of activating Fc receptors, FcRγ, is required for endocarditis but not arthritis in K/BxN TCR transgenic mice. That arthritis can develop normally in the absence of FcRγ (and to a lesser degree in the absence of C5) in K/BxN transgenic mice, whereas deficiency of either molecule completely abrogates serum-transferred arthritis, likely reflects the ≈10-fold higher anti-GPI antibody titers in the transgenic animals, and implies some redundancy in these antibody-driven inflammatory pathways (9, 10). More importantly, that the development of endocarditis depends on FcRγ provides key mechanistic insights. This finding, considered together with the fact that B cells are also required for endocarditis, suggests that the binding of autoantibodies to the valve endothelium elicits pathology via interaction with activating Fc receptors. We have provided 2 lines of evidence, however, that these bound autoantibodies alone might not be sufficient to induce endocarditis. First, passive transfer of serum from K/BxN mice reliably induced arthritis but not endocarditis. Second, adoptive transfer of splenocytes from K/B or K/BxN mice consistently induced arthritis and high-titer anti-GPI antibody titers, but only infrequently caused endocarditis. A working model in which autoantibodies “activate” the valve endothelium, and then other effectors (e.g., FcR-bearing cells and perhaps T cells) infiltrate the valve to provoke endocarditis (5) is consistent with the data we present here. This multistep model can also explain why common conditions such as viral infections that lead to the formation of circulating immune complexes do not typically provoke endocarditis—a specific cellular immune response in the valves might also be necessary.
Our finding that endocarditis is more easily induced by transfer of bone marrow cells than by transfer of splenocytes sheds light on how T cells might contribute to the development of endocarditis. One way to explain the different results with the 2 transfer systems is that the T-cell responses engendered in the 2 contexts differ in some way, impacting on the development of endocarditis. One notable difference between bone marrow cell and splenocyte transfer is that the former results in continued production of new lymphocytes of diverse repertoire, whereas the repertoire in the latter case is more restricted. It may be that a minority T-cell population, perhaps even one with a non-KRN TCR specificity, is needed for endocarditis. Differences in cytokine profiles or other phenotypic features are also possible. For example, it has recently been demonstrated that transfer of committed CD4+ T cells into lymphopenic hosts (as in our splenocyte transfer experiments) can result in reprogramming of the cytokine expression pattern of the transferred cells (21); homing properties are also known to change (22). It should also be kept in mind that other explanations, e.g., related to antibody affinity or isotype, remain possible instead, or in addition. Dissecting these possibilities is expected to provide additional insight regarding the pathogenesis of autoimmune endocarditis.
K/BxN mice represent the first genetically tractable animal model of coexisting autoantibody-induced arthritis and endocarditis, affording new insights into the mechanisms by which systemic autoimmunity can provoke endothelial inflammation. This model has revealed differential contributions of key inflammatory effector pathways in different target organs, in the context of the same systemic autoimmunity: complement C5 contributes to the development of arthritis but not of endocarditis, whereas FcRγ is critical for endocarditis but not arthritis. In a way, this finding is conceptually reminiscent of the different pathogenic pathways mediating diabetes (fas and perforin-dependent) versus neuropathy (IFN-γ dependent) in NOD and B7–2-deficient NOD mice, respectively (23), although those organ-specific diseases do not occur spontaneously in the same mouse as do arthritis and endocarditis in K/BxN mice. Thus, a single systemic autoimmune disease can damage different tissues via distinct immunopathogenic mechanisms.
Materials and Methods
Mice.
KRN TCR transgenic, C57BL/6.H2g7 (B6.g7), NOD/LtJ, CD40 (Tnfsrf5)-deficient NOD, μMT (Igh-6)-deficient, and TCR-α-deficient NOD mice were bred in our specific-pathogen-free colonies. C57BL/6.C5NOD congenic mice containing the NOD-derived interval containing 35.4 Mb (C5) to 38.4 Mb (MO2.125) of chromosome 2 were generated and backcrossed 12 generations to C57BL/6. FcRγ (Fcer1g)-deficient mice on the C57BL/6 background (24) were purchased from Taconic. C57BL/6J, CD40 (Tnfsrf5)-deficient C57BL/6J (25), μMT (Igh-6)-deficient C57BL/6J mice (26), TCR-α-deficient C57BL/6 (27), Rag-1-deficient C57BL/6J and Rag-1-deficient NOD (28) mice were purchased from the Jackson Laboratory. Mouse genotype analysis was performed by PCR. Mice were maintained under Institutional Animal Care and Use Committee-approved protocols at Harvard Medical School (protocol 3024) and at the University of Minnesota (protocol 0611A96106).
Measurement of Anti-GPI Titers and Assessment of Arthritis.
Anti-GPI titers were measured, and arthritis was assessed as described (29, 30).
Histology.
Except where indicated, 8-week-old mice were used for analysis of cardiac valve histopathology. Routine histological techniques were used for paraffin sections of formalin-fixed hearts or of hearts frozen in optimal cutting temperature (OTC) compound. Slides were stained with hematoxylin/eosin by standard protocols. The maximal thickness of mitral valves was determined by examining serial sections from each heart; for the section displaying the most inflamed mitral valve for each heart, the mitral valve was measured at its thickest point using DP-BSW software (Olympus).
Immunofluorescent Staining.
After blocking Fc receptors with 2.4G2 antibody, frozen sections were stained with biotinylated antibodies. Antibodies recognizing B220 (RA3–6B2), CD11b (M1/70), CD4 (RM4–5), CD8alpha (53–6.7), TCR beta (H57–507), Gr-1 (RB6–8C5), Vb6 (RR4–7), VCAM (429), and appropriate isotype control antibodies were all from BD Biosciences. Biotinylated anti-F4/80 (BM8) was from eBioscience. Antibodies were detected with the Vectastain ABC-AP kit with Vector Red Substrate I (Vector Labs), and slides were counterstained with DAPI to detect nuclei. Complement C3 was detected with FITC-labeled monoclonal antibody (RMC11H9; Cedarlane Labs) and bound Ig was detected with Texas Red conjugated F(ab′)2 anti-mouse IgG (Jackson ImmunoResearch). Slides were viewed on an Olympus BX51 fluorescent microscope equipped with a digital camera and DP-BSW software (Olympus).
Splenocyte and Bone Marrow Transfers.
For bone marrow transplants, Rag1-deficient 7-week-old recipient mice were irradiated with 900 Rad. Four hours after irradiation, 1 × 10e6 bone marrow cells from K/BxN donor mice were injected intravenously. Splenocyte transfers were performed as described (8). The splenocytes were injected intravenously into unirradiated TCR-α-deficient BxN F1 recipients. Hearts were harvested for histologic analysis 6–8 weeks following bone marrow or splenocyte transfer.
Anti-C5 Antibody Treatment.
K/BxN mice were given 0.6 mg of monoclonal anti-C5 antibody [derived from the BB5.1 hybridoma, gift from Dr. B. Stockinger (MRC National Institute for Medical Research, London)] (31) i.p. twice weekly starting at the age of 3 weeks. Control group K/BxN mice received nonspecific isotype control mouse IgG1 (MOPC 21; Sigma) via the same dosing regimen.
Supplementary Material
Acknowledgments.
We thank Kimie Hattori and Sindhuja Rao for assistance with mice. This work was supported by grants from the National Institutes of Health (R01 AR046580) and Young Chair funds to D. Mathis and C. Benoist, and by Joslin's National Institute of Diabetes and Digestive and Kidney Diseases-funded Diabetes and Endocrinology Research Center core facilities. Bryce Binstadt was supported by a Pfizer Postdoctoral Fellowship in Rheumatology/Immunology, University of Minnesota Department of Pediatrics funding, an Arthritis Foundation Arthritis Investigator Award, and National Institutes of Health Grant K08 AR054317.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0909132106/DCSupplemental.
References
- 1.Chung CP, et al. Increased coronary-artery atherosclerosis in rheumatoid arthritis: Relationship to disease duration and cardiovascular risk factors. Arthritis Rheum. 2005;52:3045–3053. doi: 10.1002/art.21288. [DOI] [PubMed] [Google Scholar]
- 2.Asanuma Y, et al. Premature coronary-artery atherosclerosis in systemic lupus erythematosus. N Engl J Med. 2003;349:2407–2415. doi: 10.1056/NEJMoa035611. [DOI] [PubMed] [Google Scholar]
- 3.Blank M, Aron-Maor A, Shoenfeld Y. From rheumatic fever to Libman-Sacks endocarditis: Is there any possible pathogenetic link? Lupus. 2005;14:697–701. doi: 10.1191/0961203305lu2203oa. [DOI] [PubMed] [Google Scholar]
- 4.Guilherme L, et al. Rheumatic heart disease: Proinflammatory cytokines play a role in the progression and maintenance of valvular lesions. Am J Pathol. 2004;165:1583–1591. doi: 10.1016/S0002-9440(10)63415-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Guilherme L, Kalil J, Cunningham M. Molecular mimicry in the autoimmune pathogenesis of rheumatic heart disease. Autoimmunity. 2006;39:31–39. doi: 10.1080/08916930500484674. [DOI] [PubMed] [Google Scholar]
- 6.Kouskoff V, et al. Organ-specific disease provoked by systemic autoimmunity. Cell. 1996;87:811–822. doi: 10.1016/s0092-8674(00)81989-3. [DOI] [PubMed] [Google Scholar]
- 7.Matsumoto I, Staub A, Benoist C, Mathis D. Arthritis provoked by linked T and B cell recognition of a glycolytic enzyme. Science. 1999;286:1732–1735. doi: 10.1126/science.286.5445.1732. [DOI] [PubMed] [Google Scholar]
- 8.Korganow AS, et al. From systemic T cell self-reactivity to organ-specific autoimmune disease via immunoglobulins. Immunity. 1999;10:451–461. doi: 10.1016/s1074-7613(00)80045-x. [DOI] [PubMed] [Google Scholar]
- 9.Ji H, et al. Arthritis critically dependent on innate immune system players. Immunity. 2002;16:157–168. doi: 10.1016/s1074-7613(02)00275-3. [DOI] [PubMed] [Google Scholar]
- 10.Corr M, Crain B. The role of FcgammaR signaling in the K/B x N serum transfer model of arthritis. J Immunol. 2002;169:6604–6609. doi: 10.4049/jimmunol.169.11.6604. [DOI] [PubMed] [Google Scholar]
- 11.Banda NK, et al. Alternative complement pathway activation is essential for inflammation and joint destruction in the passive transfer model of collagen-induced arthritis. J Immunol. 2006;177:1904–1912. doi: 10.4049/jimmunol.177.3.1904. [DOI] [PubMed] [Google Scholar]
- 12.Nandakumar KS, et al. Induction of arthritis by single monoclonal IgG anti-collagen type II antibodies and enhancement of arthritis in mice lacking inhibitory FcgammaRIIB. Eur J Immunol. 2003;33:2269–2277. doi: 10.1002/eji.200323810. [DOI] [PubMed] [Google Scholar]
- 13.Bidani AK, Roberts JL, Schwartz MM, Lewis EJ. Immunopathology of cardiac lesions in fatal systemic lupus erythematosus. Am J Med. 1980;69:849–858. doi: 10.1016/s0002-9343(80)80010-6. [DOI] [PubMed] [Google Scholar]
- 14.Kaplan MH, Bolande R, Rakita L, Blair J. Presence of bound immunoglobulins and complement in the myocardium in acute rheumatic fever. Association with cardiac failure. N Engl J Med. 1964;271:637–645. doi: 10.1056/NEJM196409242711301. [DOI] [PubMed] [Google Scholar]
- 15.Ziporen L, et al. Libman-Sacks endocarditis in the antiphospholipid syndrome: Immunopathologic findings in deformed heart valves. Lupus. 1996;5:196–205. doi: 10.1177/096120339600500306. [DOI] [PubMed] [Google Scholar]
- 16.Fraser WJ, Haffejee Z, Cooper K. Rheumatic Aschoff nodules revisited: An immunohistological reappraisal of the cellular component. Histopathology. 1995;27:457–461. doi: 10.1111/j.1365-2559.1995.tb00310.x. [DOI] [PubMed] [Google Scholar]
- 17.Boross P, Verbeek JS. The complex role of Fcgamma receptors in the pathology of arthritis. Springer Semin Immunopathol. 2006;28:339–350. doi: 10.1007/s00281-006-0049-9. [DOI] [PubMed] [Google Scholar]
- 18.Thurman JM, Holers VM. The central role of the alternative complement pathway in human disease. J Immunol. 2006;176:1305–1310. doi: 10.4049/jimmunol.176.3.1305. [DOI] [PubMed] [Google Scholar]
- 19.Lynch DM, Kay PH. Studies on the polymorphism of the fifth component of complement in laboratory mice. Exp Clin Immunogenet. 1995;12:253–260. [PubMed] [Google Scholar]
- 20.Nimmerjahn F, Ravetch JV. Fcgamma receptors: Old friends and new family members. Immunity. 2006;24:19–28. doi: 10.1016/j.immuni.2005.11.010. [DOI] [PubMed] [Google Scholar]
- 21.Nurieva R, Yang XO, Chung Y, Dong C. Cutting edge: In vitro generated Th17 cells maintain their cytokine expression program in normal but not lymphopenic hosts. J Immunol. 2009;182:2565–2568. doi: 10.4049/jimmunol.0803931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Min B, Yamane H, Hu-Li J, Paul WE. Spontaneous and homeostatic proliferation of CD4 T cells are regulated by different mechanisms. J Immunol. 2005;174:6039–6044. doi: 10.4049/jimmunol.174.10.6039. [DOI] [PubMed] [Google Scholar]
- 23.Bour-Jordan H, Thompson HL, Bluestone JA. Distinct effector mechanisms in the development of autoimmune neuropathy versus diabetes in nonobese diabetic mice. J Immunol. 2005;175:5649–5655. doi: 10.4049/jimmunol.175.9.5649. [DOI] [PubMed] [Google Scholar]
- 24.Takai T, et al. FcR gamma chain deletion results in pleiotrophic effector cell defects. Cell. 1994;76:519–529. doi: 10.1016/0092-8674(94)90115-5. [DOI] [PubMed] [Google Scholar]
- 25.Kawabe T, et al. The immune responses in CD40-deficient mice: Impaired immunoglobulin class switching and germinal center formation. Immunity. 1994;1:167–178. doi: 10.1016/1074-7613(94)90095-7. [DOI] [PubMed] [Google Scholar]
- 26.Kitamura D, Roes J, Kuhn R, Rajewsky K. A B cell-deficient mouse by targeted disruption of the membrane exon of the immunoglobulin mu chain gene. Nature. 1991;350:423–426. doi: 10.1038/350423a0. [DOI] [PubMed] [Google Scholar]
- 27.Lie BA, et al. Application and interpretation of transmission/disequilibrium tests: Transmission of HLA-DQ haplotypes to unaffected siblings in 526 families with type 1 diabetes. Am J Hum Genet. 2000;66:740–743. doi: 10.1086/302780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mombaerts P, et al. RAG-1-deficient mice have no mature B and T lymphocytes. Cell. 1992;68:869–877. doi: 10.1016/0092-8674(92)90030-g. [DOI] [PubMed] [Google Scholar]
- 29.Lee DM, et al. Mast cells: A cellular link between autoantibodies and inflammatory arthritis. Science. 2002;297:1689–1692. doi: 10.1126/science.1073176. [DOI] [PubMed] [Google Scholar]
- 30.Nguyen LT, Jacobs J, Mathis D, Benoist C. Where FoxP3-dependent regulatory T cells impinge on the development of inflammatory arthritis. Arthritis Rheum. 2007;56:509–520. doi: 10.1002/art.22272. [DOI] [PubMed] [Google Scholar]
- 31.Frei Y, Lambris JD, Stockinger B. Generation of a monoclonal antibody to mouse C5 application in an ELISA assay for detection of anti-C5 antibodies. Mol Cell Probes. 1987;1:141–149. doi: 10.1016/0890-8508(87)90022-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.