Abstract
Identification of drug resistance before it becomes a public health concern requires a clear distinction between what constitutes a normal and a suboptimal treatment response. A novel method of analyzing drug efficacy studies in human helminthiases is proposed and used to investigate recent claims of atypical responses to ivermectin in the treatment of River Blindness. The variability in the rate at which Onchocerca volvulus microfilariae repopulate host's skin following ivermectin treatment is quantified using an individual-based onchocerciasis mathematical model. The model estimates a single skin repopulation rate for every host sampled, allowing reports of suboptimal responses to be statistically compared with responses from populations with no prior exposure to ivermectin. Statistically faster rates of skin repopulation were observed in 3 Ghanaian villages (treated 12–17 times), despite the wide variability in repopulation rates observed in ivermectin-naïve populations. Another village previously thought to have high rates of skin repopulation was shown to be indistinguishable from the normal treatment response. The model is used to generate testable hypotheses to identify whether atypical rates of skin repopulation by microfilariae could result from low treatment coverage alone or provide evidence of decreased ivermectin efficacy. Further work linking phenotypic poor responses to treatment with parasite molecular genetics markers will be required to confirm drug resistance. Limitations of the skin-snipping method for estimating parasite load indicates that changes in the distribution of microfilarial repopulation rates, rather than their absolute values, maybe a more sensitive indicator of emerging ivermectin resistance.
Keywords: drug resistance, helminth parasites, Onchocerca volvulus, overdispersion
The recent dramatic increase in the use of mass drug administration for the control of helminth infections in humans has raised the possibility that anthelmintic drug resistance may impede the success of such control programs. Anthelmintic resistance is already a major problem in veterinary parasitic nematodes (1), and suboptimal responses to treatment have been reported in a number of human helminthiases (2–8).
Current methods of detecting such suboptimal responses to treatment are, however, relatively crude, particularly for those human infections which cannot be passaged in the laboratory or lack animal models. Adult parasites are often inaccessible within the body so drug efficacy studies rely on sampling transmission stages as a proxy for measuring parasite intensity, and as host immune responses may be involved in the drug's mode of action, in vitro assays are seldom useful (9). The fecal egg count reduction test is the standard method for measuring drug efficacy in intestinal helminths, yet it is estimated that this technique may only detect a suboptimal response when greater than 25% of the parasite population carries the resistance-conferring gene (10).
In this paper, we propose a novel technique for analyzing phenotypic studies that seek to investigate the occurrence of suboptimal anthelmintic responses. We focus on the filarial nematodes and in particular on the causal agent of River Blindness, Onchocerca volvulus, for which ivermectin is at present the only drug that can be feasibly used for mass treatment of human populations (11). The method proposed can be adapted to the study of treatment responses at an individual host level in other parasitic helminths of medical and veterinary importance.
Although at the standard dose ivermectin has a limited adulticidal efficacy against O. volvulus, it is a highly effective microfilaricide, killing the stages transmitted to the black fly vectors and temporarily preventing repopulation of the host's skin by microfilariae through an effect on female worm fertility (12). This latter effect may (13) or may not (14) be cumulative with each subsequent treatment. Recently, faster than expected skin repopulation rates have been reported in villages treated for prolonged periods, particularly in Ghana (4, 5, 8), raising controversy as to whether these observations are indicative of emerging resistance to ivermectin's effect on the release of microfilariae by the adult female worm (embryostatic effect) or due to other causes (15–17).
Typically, studies investigating drug efficacy compare percent changes in mean infection intensity at various time points after treatment in relation to the precontrol baseline in groups sampled from treatment-naïve and repeatedly treated populations (12). This approach is subject to several pitfalls that obscure interpretation, some of which have been illustrated using a simple mathematical model in supporting information (SI) Text,Expected Changes in Microfilarial Load over Multiple Rounds of Treatment. First, the slow rate of skin repopulation by microfilariae following treatment indicates that even a year after ivermectin the microfilarial population has not reached its equilibrium with the fertile adult worm population (12); therefore, comparing the percent reduction in microfilarial load after 2 or more ivermectin treatments with that in hosts receiving treatment for the first time (for whom pretreatment microfilarial loads in the denominator would be higher), may give rise to false cases of suboptimal response to treatment (type 1 error). Second, expressing changes in drug efficacy as a percent of the mean pretreatment load may produce considerable variability because at low loads small changes in absolute values may have a disproportionate effect on the percentage reduction. Third, the uncertainty surrounding the operation of a cumulative effect of ivermectin on worm fertility over multiple treatment rounds means that there may not be yearly reductions in microfilarial load unless there was a continual fall in the force of infection (Fig. S1 and SI Text). Finally, aggregate data mask individual host's variation in response to treatment, the analysis of which is crucial to distinguishing between typical and anomalous patterns.
Before a suboptimal response to treatment can be identified, it is important to quantify the range of parasitological responses observed in a wide range of populations before the widespread introduction of chemotherapy. In this paper, we analyze individual host parasitological responses to ivermectin by fitting a mathematical model to microfilarial loads assessed at various time points after treatment. The model generates a single estimate of the rate of skin repopulation for each host (whilst keeping remaining parameters constant), which allows their microfilarial load at any time point after treatment to be predicted (we have chosen one year because ivermectin treatments are usually given annually in Africa). This technique is used to quantify the individual response seen in subjects treated for the first time in clinical and early community trials (the control dataset described in SI Text, Description of the Control Dataset). We then compare statistically the estimates obtained from the control group with the microfilarial loads estimated from communities which have been repeatedly treated and suggested to have faster than expected rates of skin repopulation (the test dataset).
Results
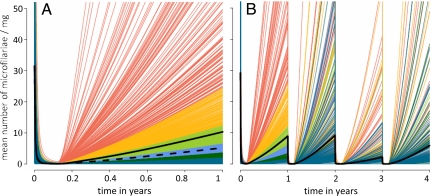
The between-host variation in posttreatment rates of skin repopulation by microfilariae is substantial even in populations previously unexposed to ivermectin. The range of responses seen in the control dataset after a single round of ivermectin is shown in Fig. 1A, with each line representing the (modeled) dynamics of skin repopulation for each individual host in the dataset. Ivermectin is a highly effective microfilaricide, reducing the number of microfilariae in the skin of all treated hosts to very low levels. From this point forward, a highly overdispersed range of responses is observed, with a small percentage of hosts exhibiting very high rates of skin repopulation, whilst the majority have relatively low microfilarial loads 12 months after treatment. By this time point, microfilarial loads in some hosts (0.4%) had reached >200 microfilariae per milligram of skin (mf mg−1), whereas 32% showed no evidence of skin repopulation. The average rate of repopulation obtained from fitting the mathematical model described in SI Text, Individual-Based Human Onchocerciasis Model, to individual data are twice as fast as that estimated by an earlier version fitted to aggregate data (12). A similarly heterogeneous response to treatment was observed in hosts for whom there were data up to 4 treatment rounds. Fig. 1B color-codes the rate of skin repopulation of individual hosts after consecutive treatments according to their response after the first one.
Fig. 1.
The variability in posttreatment microfilarial repopulation rates among hosts after (A) their first ivermectin treatment (sample size = 1369) and (B) their first 4 rounds of ivermectin treatment (sample size = 534). Individual lines represent the modeled dynamics for each host in the dataset. Lines in both panels are colored according to the cumulative distribution of responses seen at year 1: lowest 50% (dark blue), 50–60% (dark green), 60–70% (light blue), 70–80% (light green), 80–90% (yellow), and 90–100% (red). Solid thick black line shows the arithmetic mean of all of the individual responses. Dashed black line in panel (A) indicates the rate of skin repopulation estimated in [12], fitting the model to published aggregate data.
Hosts with higher microfilarial loads at baseline had faster rates of skin repopulation possibly resulting from higher adult worm burdens before chemotherapy and/or higher numbers of newly patent adult worms. On average, for every 100 mf mg−1 increase in microfilarial load at baseline, microfilarial load would be 2 mf mg−1 higher a year after treatment [relative risk = 1.019; 95% confidence interval (CI): 1.016−1.022, P < 0.001]. Although this may not appear a large increase in posttreatment microfilarial load, 2 mf mg−1 constitutes a sizable proportion of the overall number of microfilariae seen in the skin a year after treatment (on average only 10 mf mg−1, see Fig. 1A). No association was seen with host age, sex, or number of times microfilarial load was assessed after treatment, but the number of studies with information on host age and sex was relatively small. Studies investigating a population's response after more than 5 treatment rounds are unlikely to have information on microfilarial loads before the start of chemotherapy, so will have to rely on infection intensity estimates at the time of treatment. Negative binomial regression using the data of the multiply treated hosts in the control dataset showed a significant association between microfilarial loads before and a year after treatment (i.e., between microfilarial counts in Fig. 1B at rounds 1 and 2, 2 and 3, and 3 and 4. For every 100 mf mg−1 increase in microfilarial load at the time of treatment, microfilarial load would be 1.5 mf mg−1 higher a year later [the average relative risk over the 3 retreatments was 1.015 (1.004–1.026) P = 0.008]. Although significant, the effect is relatively small; Fig. 1B shows that hosts with a low rate of skin repopulation after the first round of chemotherapy may experience faster rates of skin repopulation after subsequent treatments.
In those samples obtained from community trials of repeated ivermectin treatments, host microfilarial prevalence and intensity appear to decrease over subsequent rounds (Fig. 1B, Table 1). Transmission intensity was concomitantly measured only in the Guatemalan study (18), so it is difficult to ascertain whether this is due to a reduction in the force of infection or a cumulative impact of ivermectin on female worm fertility (13, 14). No significant difference in the rate of skin repopulation was seen between rounds 1, 2, and 4 if hosts with no detectable microfilariae at the time of treatment were excluded from the analysis (Table 1). This suggests that microfilaria-positive hosts at treatment had the same rate of skin repopulation irrespective of how many treatments they had received, allowing us to directly compare the responses in the control dataset with those in the test dataset. The possible causes of the lower microfilarial load after round 3 have been discussed elsewhere (hosts contributing data from 4 consecutive rounds of chemotherapy largely come from a single study in Guatemala, see refs. 14, 18, 19).
Table 1.
Changes in skin microfilarial prevalence and load over multiple rounds of ivermectin treatment in the control dataset
| Estimated microfilarial prevalence and load 1 year post treatment x | |||||
|---|---|---|---|---|---|
| Treatment round (x) | Baseline | 1 | 2 | 3 | 4 |
| Microfilarial prevalence (%) | 100 | 63.1 (59.0, 67.0) | 56.6 (52.2, 60.7) | 34.3 (30.3, 38.4) | 47.0 (42.7, 51.3) |
| Mean microfilarial load* | 30.1 (27.9, 32.4) | 8.53 (6.99, 10.1) | 8.83 (6.60, 11.0) | 2.10 (1.59, 2.62) | 5.76 (4.15, 7.39) |
| Mean intensity in microfilaridermic hosts at time of treatment x* | — | 8.53† (6.99, 10.1) | 12.4†(9.08, 15.7) | 3.35 (2.49, 4.23) | 10.7† (7.06, 14.4) |
The individual-based onchocerciasis mathematical model is used to generate estimates (and their 95% confidence intervals) one year after each round of treatment. Microfilarial load estimates are also given that exclude hosts who had no detectable microfilariae immediately prior to chemotherapy, i.e. hosts who would normally be excluded in drug efficacy studies of populations already under mass drug administration. This component of the control dataset is restricted to hosts contributing microfilarial data prior to each of the 4 treatment rounds (sample size = 534).
*Measured as the arithmetic mean number of microfilariae per milligram of skin from two snips taken at the iliac crests (see text).
†No statistical difference between the different rounds of treatment (see Methods).
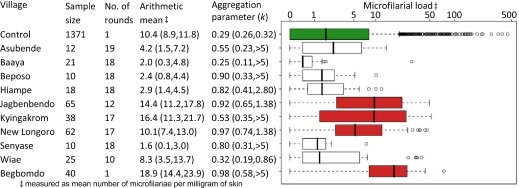
No single host from the test dataset has a particularly high rate of skin repopulation when compared to the range of responses observed in the control dataset (Fig. 2). None of the hosts from the test dataset had an estimated microfilarial load one year after treatment in the top 1% of those observed in the control group, with only 3% of hosts in the test dataset (10 individuals) having responses in the highest 5% percentile of the control dataset. However, analyzing the test data by village showed a statistically higher rate of skin repopulation in the villages of Jagbenbendo (treated 12 times), Kyingakrom (treated 17 times), and New Longoro (treated 17 times), as well as in the previously untreated village of Begbomdo (Fig. 2). One year after the last treatment, the village of New Longoro had a similar arithmetic mean to that of the control dataset but a higher median, reflecting the more evenly distributed microfilarial loads observed in New Longoro (Fig. 2) and highlighting the importance of choosing appropriate measures of central tendency.
Fig. 2.
Comparison between microfilarial load in the control and test datasets a year after ivermectin treatment. The different communities of the test dataset are grouped separately. The boxplot displays graphically the median (thick black line) and quartile range (enclosed box). Outlier results exceeding 1.5 times the interquartile range are displayed as open circles. Note the log scale on the horizontal axis. Villages in red had statistically significantly higher skin repopulation rates than the control (green) dataset (see Methods for statistical analysis). Ninety-five percent CI estimates for the arithmetic mean and aggregation parameter of the negative binomial distribution (an inverse measure of parasite overdispersion, estimated using maximum likelihood) are given in parentheses.
The high posttreatment microfilarial loads estimated for some Ghanaian villages could result from a large number of new infections due to ongoing transmission in the area. Microfilarial load varies markedly between hosts according to their age, sex, and geographical location, suggesting that differences in microfilarial load observed before the start of chemotherapy are largely caused by heterogeneity in host exposure to vectors (20). Host microfilarial load may also be influenced by human genetic factors (21) as well as immunological responses (22), whose relative contribution has not yet been quantified. The expression presented in SI Text, Estimation of the Minimum ATP Required to Explain Rates of Skin Repopulation, is used to estimate the minimum annual transmission potential (ATP, the number of infective larvae potentially received per host per year) that would be required to explain the high rates of skin repopulation estimated in the 4 villages by acquisition of new parasites alone, taking into account the different sample sizes of each village and assuming a 0 to 20% hypothetical contribution of host factors to the variability in microfilarial loads (Table 2). These values could be compared to estimates of the villages' ATPs derived from field observations. If observed ATPs were substantially lower than those in Table 2, it would provide evidence that the high rate of skin repopulation in these villages is not caused solely by acquisition of new parasites or low ivermectin coverage but due to a decreased efficacy of ivermectin in suppressing the production of live microfilariae by adult female worms.
Table 2.
Estimates for the minimum annual transmission potential (ATP) required to cause the observed rates of skin repopulation in the statistically significant villages by acquisition of new parasites (or low ivermectin coverage) alone
| Hypothetical contribution of host genetics or immunologic factors to observed microfilarial load, % | ATP required to explain microfilarial loads |
||
|---|---|---|---|
| 0% | 10% | 20% | |
| Begbomdo | 582 | 457 | 346 |
| Jagbenbendo | 611 | 484 | 371 |
| Kyingakrom | 423 | 329 | 246 |
| New Longoro | 513 | 405 | 307 |
A description of the method is given in the SI Appendix. Note that the minimum ATP estimates are for hosts who were microfilaria-positive prior to treatment, which may differ from the ATP for the whole community. ATPs represent the average number of infective larvae potentially received per host per year
The village of Begbomdo was included in the original Osei-Atweneboana et al. study (8) as an ivermectin-naïve village against which communities that had received many annual rounds of treatment could be compared. A significantly faster rate of skin repopulation was observed in hosts from Begbomdo than in the control dataset (Fig. 2). Begbomdo had a baseline microfilarial prevalence of 72%, indicating a high level of ongoing transmission. For a range of West African villages, this prevalence level would correspond to ATP values in excess of 1,000 (23). Therefore, although we do not have ATP estimates for Begbomdo, they would probably exceed the 582 infective larvae per person per year required for the observed skin repopulation rate to have occurred through high rates of reinfection alone (Table 2, making the conservative assumption that baseline differences in microfilarial loads are caused solely by heterogeneity in exposure to black fly bites). Microfilaria-positive hosts in Begbomdo also had a higher mean microfilarial load at baseline (45.1 mf mg−1) than those in the control dataset (31.5 mf mg−1), providing further evidence that this is a high transmission area. Therefore, we conclude that there is no evidence of suboptimal responses within Begbomdo [although the possibility that suboptimally responding parasites might have been imported from areas undergoing chemotherapy (such as Jagbenbendo which is only 30 km away) cannot be excluded].
Discussion
The rate at which O. volvulus microfilariae repopulate the skin following ivermectin treatment varies considerably between hosts even after a first dose. Some of this variability is likely due to the limitations of the skin snip technique in estimating the true host's microfilarial load (e.g., small area of skin sampled, low number of samples per individual). Additionally, the procedure is known to be relatively insensitive at low microfilarial loads (24) as microfilariae clump in patches within the skin (25). Considerable variation in microfilarial counts has been observed in individual hosts who were sampled multiple times on the same day (26), indicating that care should be taken when interpreting individual parasitological responses derived from a small number of snips. However, since the same procedure was used in both the control and test datasets, this uncertainty is implicitly incorporated within the analysis presented here. The population samples from the villages of Jagbenbendo, Kyingakrom, and New Longoro showed statistically significantly faster rates of skin repopulation than those in the control dataset. These 3 villages had been previously identified as being “of concern” (8) along with the village of Wiae, which this analysis did not classify as being statistically different from the control dataset. The high microfilarial loads in Begbomdo, the untreated village, are most likely due to high transmission intensity.
The sensitivity and specificity of methods for detecting decreased drug efficacy will be improved by examining individual host responses to treatment rather than the means of aggregate responses in groups of hosts. Microfilarial repopulation rates vary substantially between hosts, even those with no prior exposure to ivermectin. Detecting suboptimal responses through changes in aggregate measures of parasite intensity over time (such as the arithmetic or geometric mean) would require large numbers of microfilaria-positive hosts (27). Due to the inaccuracy of current methods for estimating infection intensity, and the overdispersed nature of helminth infections among hosts, the first signs of decreased drug efficacy may be indicated by a change in the distribution of parasite load. For example, a modest increase in parasite load across a number of hosts may be more informative than a large change in average parasite intensity, which could be heavily influenced by a single host with a high microfilarial estimate.
The impact of a decreased drug efficacy on the distribution of parasites within the treated host population will depend on the degree of parasite genetic differentiation between hosts. If all of the resistant parasites inhabited a small number of hosts, then drug resistance would initially increase parasite overdispersion in the treated host population, which may be difficult to detect as parasite populations per se are typically overdispersed. However, if resistant parasites were more evenly distributed within the host population, then drug resistance would reduce the degree of parasite overdispersion after chemotherapy, which may be easier to distinguish statistically. In human onchocerciasis, the distribution of resistant parasites will depend on the biting behavior of the black fly vectors. Parasite aggregation appears to have decreased in the villages with a high rate of skin repopulation by microfilariae (Fig. 2.), which is consistent with black flies biting members of the community at random rather than returning to the same hosts for a blood meal.
It has been proposed that high rates of skin repopulation by microfilariae may be explained by a level of reinfection with incoming parasites resulting from low ivermectin coverage in the study sites and surrounding areas (15–17). The difficulties in assessing accurately both the coverage of mass chemotherapy control programs (28) and the intensity of transmission to which individual hosts are exposed (29), may prevent the scientific community from reaching a consensus on whether a particular study constitutes evidence of suboptimal response. This work provides testable hypotheses regarding the level of reinfection that would be required for a suboptimal response to be explained by acquisition of new parasites due to low treatment coverage alone.
The reliability of the approach for detecting suboptimal responses to treatment will depend on the long-term impact of treatment on parasite and host. The method described assumes that the rate of skin repopulation is no greater after more than 10 rounds of treatment than after 1–4 rounds. This supposes that any immunological changes that may occur in the host population after a sustained reduction in the force of infection and prolonged treatment do not increase the rate of skin repopulation, parasite establishment, or survival. The opposite may in fact be the case, as ivermectin-facilitated immunity may allow hosts to fend off infection better (30), and parasite establishment may be facilitated by infection (31). The statistical power of this technique for identifying suboptimal responses to treatment will improve the more data become available for analysis within the control and test datasets.
The model assumes all parasites are identical, only differing in their rate of skin repopulation following ivermectin treatment (i.e., all parameters are kept constant with the exception of the rate of skin repopulation, which is estimated for each host separately). This assumption is based on lack of published evidence for heterogeneity in life-history parameters of O. volvulus (e.g., differences in life expectancy)—but see recent work by Bourguinat et al. (32).
Studies investigating possible suboptimal responses to treatment should select randomly among microfilaria-positive hosts (as was done in ref. 8). False suboptimal response cases can occur if only hosts with high microfilarial loads are included, as these hosts may have a faster skin repopulation rate in the first place.
One of the advantages of the technique developed in this paper is that it does not require a priori information on the treatment coverage of an area (which is difficult to estimate accurately) to identify atypical rates of skin repopulation. Also, the model is fitted to data from individuals who were all treated and followed-up longitudinally, rather than to cross-sectional data of populations subject to different coverage levels. Estimation of the host's skin repopulation rate provides a method of quantifying an individual host's response to treatment which can be correlated with the genotypic information of parasites obtained from that host (microfilariae and adult worms). A number of possible molecular markers (including beta-tubulin and P-glycoprotein among others) have been identified which could provide useful tools in identifying anthelmintic resistance before it becomes widely spread (32, 33). Linking phenotypic responses with molecular genetics markers will be required to prove that suboptimal responses are in fact due to drug-resistant parasites and not to host-related factors (3).
Even in the villages with a faster rate of skin repopulation, ivermectin is still a highly effective microfilaricidal drug, controlling morbidity and possibly reducing transmission. Ivermectin kills microfilariae equally well in all hosts of the control and test datasets. This is important as the lack of heterogeneity observed in ivermectin's microfilaricidal effect reduces the chance of this type of drug resistance developing as there is little variability within the parasite population for selection to act upon. This should, however, not create complacency and efforts should be made to contain the spread of the suboptimal responses identified in Ghana by, for instance, vector control and anti-Wolbachia therapy (34). Mathematical models can be used to investigate how changing from annual to biannual mass ivermectin distribution could compensate for faster rates of skin repopulation and help national programs achieve their objectives of morbidity control, sustained reductions in transmission, or elimination of the parasite reservoir.
The methods outlined within this paper can be extended to other human helminthiases. The models and analysis can be suitably modified to investigate treatment responses in other filariasis (through the study of microfilariae in the blood), schistosomiasis, and soil-transmitted helminthiases (by examining egg counts in feces and urine). Large datasets of responses in treatment-unexposed populations should ideally be collated for each infection and chemotherapy regimen. Mathematical models can be used to capture the range of responses to be expected in treatment-naïve populations and this range should be made publicly available (together with model assumptions) to allow possible suboptimal response cases to be rigorously investigated phenotypically and genotypically. Models can also be used to differentiate suboptimal responses to treatment from high rates of parasite reinfection (35), providing testable hypotheses as to the causes of atypical patterns and tools for early-warning identification of responses that warrant in-depth investigation. Claims of drug resistance that are not rigorously tested risk jeopardizing political and financial support to mass chemotherapy control campaigns, especially those that rely on drug donation programs. Mathematical epidemiology can provide a useful insight into the question of what constitutes evidence of drug resistance, helping move the debate from conjecture to evidence-based decision making.
Methods
Study Areas and Datasets.
A recent systematic review and metaanalysis collated all published (aggregate) data on the different effects against O. volvulus of a single dose of ivermectin in treatment-naïve population samples and represented the ensuing posttreatment dynamics by fitting a mathematical model to the means for groups of studies at each time point (12). To ascertain the variability present within each study we contacted the authors of the articles included in the review and obtained individual host microfilarial counts before and at various times after treatment (together with variables such as age and sex) for a total of 7 studies conducted in Ghana, Guatemala, Liberia, and Sierra Leone. This constitutes the control dataset comprising 1369 individuals treated for the first time, as described below and in SI Text, Description of the Control Dataset (Table S1) (19, 36–41). The test dataset consists of 240 microfilaria-positive hosts from 9 communities in Ghana treated 10–19 times, plus 40 hosts from an untreated community (see ref. 8 for a description of the villages). Rates of skin repopulation were estimated for each host by fitting the individual-based model to microfilarial load assessed at various time points after treatment (for the control dataset see Table S1, the test dataset day 90, 180, and 365). This allowed the microfilarial load one year posttreatment to be estimated for each host and to be compared statistically between the control and test datasets to determine whether the Ghanaian study (8) truly provides evidence of suboptimal responses to treatment.
Mathematical Model.
An individual-based, deterministic onchocerciasis dynamics model was fitted to microfilarial data from each host (SI Text, Individual-Based Human Onchocerciasis Model). The mathematical model comprises 3 differential equations, describing the rate of change with respect to time of the number of nonfertile and fertile female worms per host, and microfilariae per mg of skin. Parameter values applicable to a wide range of epidemiological settings, including the per capita rate of female worm fecundity were taken from (23). Fertile adult worms and microfilariae were assumed to have a constant per capita death rate, with a mean life-expectancy of 10 years and 15 months, respectively (23). Parameters regarding the effect of ivermectin on the different parasite stages were taken from ref. 12. Studies which recorded changes of microfilarial load during the first 60 days after chemotherapy indicate very little between-host variability in ivermectin's microfilaricidal efficacy, allowing the model to concentrate specifically on the variability in the rate of skin repopulation between hosts. This is captured using a single parameter which is individually estimated for each host in the population for each round of treatment. The parameter enters into the equation for microfilariae by multiplying the fecundity rate of fertile worms times the ratio of skin repopulation within that host relative to that in the mean-based model of ref. 12. Keeping the remaining 12 parameters fixed, the model estimates (using least squares) a single rate of skin repopulation for each host for each round of treatment using the 1–3 posttreatment time points available. Generating a single rate of repopulation for each host after each round of chemotherapy allows studies with samples taken at different times after treatment to be directly compared. This increases substantially the size of the control dataset for comparison with the test dataset (Table S1) and also permits investigation of any systematic changes in skin repopulation. Estimates of the microfilarial load one year after treatment were generated for each host and used within the statistical analysis (although the conclusions of the analysis would have remained the same had other time points been used, e.g., 6 months posttreatment).
Statistical Analyses.
Negative binomial regression (to account for overdispersion in microfilarial loads) was first used to test whether host characteristics (baseline microfilarial load, age, sex, number of time points at which skin snips were taken) influenced microfilarial load one year after treatment. Relative risk estimates were generated for each of the variables which were statistically significant at the 5% level.
Microfilarial estimates one year after treatment rounds 1–4 were compared for the 534 hosts who had been followed longitudinally using the nonparametric Kruskal-Wallis test. The estimated microfilarial load one year after the first treatment is used as the control dataset due to its larger sample size and to reduce type I error. If there were some form of cumulative impact of ivermectin on female worm fecundity, then the rate of skin repopulation by microfilariae would be greatest after the first round. It is thought that O. volvulus is relatively unsusceptible to ivermectin during its long prepatent period (42) which can be 6–18 months (43). Consequently, the skin repopulation rate recorded after the first treatment round could include microfilariae from newly patent parasites which were acquired before any reduction in the force of infection may have occurred. Finding statistically faster rates of microfilarial repopulation in a parasite population exposed to a lower force of infection which has received more than 10 rounds of mass treatment than in an ivermectin-naïve population with ongoing transmission should, therefore, provide robust evidence of a suboptimal response. Investigating whether this is due to heritable changes in the parasite population, the ultimate proof of drug resistance, is the essential next step.
Estimated microfilarial loads one year after treatment were compared between the control (treatment-naïve) dataset and each community of the test dataset using the non-parametric Kruskal-Wallis test. To take into consideration the small size of the samples from some of the test villages, the control dataset was repeatedly resampled 100,000 times with replacement, with a sample size equal to that of the test village in question. Rates of repopulation by microfilariae were deemed as significantly higher in a given test village if >95% of the resamples from the control dataset were below the median of the test village. The results of this analysis confirmed those of the Kruskal-Wallis test. The same conclusions were reached when the analysis was repeated using only a single posttreatment time point to estimate the rate of skin repopulation in the test dataset, indicating that the high number of time points in the test dataset was not responsible for the observed results. Ninety-five percent CI for the arithmetic mean and aggregation parameter of the negative binomial distribution (an inverse measure of parasite overdispersion) were estimated through bootstrapping (100,000 repeats).
Estimation of Rates of Acquisition of New Parasites.
By making reasonable assumptions about the relative contribution of host-specific factors (genetic or immunologic) to infection, the mathematical model can be used to estimate the minimum number of infective larvae a person should potentially receive during a year (ATP) to generate the estimated microfilarial loads. The expression describing the relationship between ATP and the baseline microfilarial load is given in SI Text, Estimation of the Minimum ATP Required to Explain Rates of Skin Repopulation. If one assumes that O. volvulus is largely unsusceptible to ivermectin during its prepatent period (43), the number of parasites reaching patency before and up to a year after chemotherapy will be the same. This enables the number of microfilariae resulting from parasite reinfection to be estimated from baseline microfilarial loads. The control dataset was sampled randomly to generate 100,000 subgroups of hosts whose ATP were estimated. The rate of skin repopulation was compared between these subgroups and the test dataset to find the minimum ATP that could have generated the data in the Ghanaian villages with statistically higher microfilarial loads one year after the last ivermectin treatment.
Supplementary Material
Acknowledgments.
We thank Beatriz Muñoz and Hugh Taylor for providing the data from Liberia and acknowledge financial support by the Medical Research Council and The Wellcome Trust, United Kingdom (to T.S.C. and M.G.B.). The Ghanaian study received support from the Ghana Government and McGill University, Montreal, Canada.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0906176106/DCSupplemental.
References
- 1.Kaplan RM. Drug resistance in nematodes of veterinary importance: A status report. Trends Parasitol. 2004;20:477–481. doi: 10.1016/j.pt.2004.08.001. [DOI] [PubMed] [Google Scholar]
- 2.Albonico M, et al. Efficacy of mebendazole and levamisole alone or in combination against intestinal nematode infections after repeated targeted mebendazole treatment in Zanzibar. Bull WHO. 2003;81:343–352. [PMC free article] [PubMed] [Google Scholar]
- 3.Ali MMM, et al. Immunocompetence may be important in the effectiveness of Mectizan (R) (ivermectin) in the treatment of human onchocerciasis. Acta Trop. 2002;84:49–53. doi: 10.1016/s0001-706x(02)00117-1. [DOI] [PubMed] [Google Scholar]
- 4.Awadzi K, et al. Thirty-month follow-up of sub-optimal responders to multiple treatments with ivermectin, in two onchocerciasis-endemic foci in Ghana. Ann Trop Med Parasitol. 2004;98:359–370. doi: 10.1179/000349804225003442. [DOI] [PubMed] [Google Scholar]
- 5.Awadzi K, et al. An investigation of persistent microfilaridermias despite multiple treatments with ivermectin, in two onchocerciasis-endemic foci in Ghana. Ann Trop Med Parasitol. 2004;98:231–249. doi: 10.1179/000349804225003253. [DOI] [PubMed] [Google Scholar]
- 6.De Clercq D, et al. Failure of mebendazole in treatment of human hookworm infections in the southern region of Mali. Am J Trop Med Hyg. 1997;57:25–30. doi: 10.4269/ajtmh.1997.57.25. [DOI] [PubMed] [Google Scholar]
- 7.Eberhard ML, Lammie PJ, Dickinson CM, Roberts JM. Evidence of nonsusceptibility to diethylcarbamazine in Wuchereria bancrofti. J Infect Dis. 1991;163:1157–1160. doi: 10.1093/infdis/163.5.1157. [DOI] [PubMed] [Google Scholar]
- 8.Osei-Atweneboana MY, Eng JK, Boakye DA, Gyapong JO, Prichard RK. Prevalence and intensity of Onchocerca volvulus infection and efficacy of ivermectin in endemic communities in Ghana: A two-phase epidemiological study. Lancet. 2007;369:2021–2029. doi: 10.1016/S0140-6736(07)60942-8. [DOI] [PubMed] [Google Scholar]
- 9.Churcher TS, Basáñez MG. Sampling strategies to detect anthelmintic resistance: The perspective of human onchocerciasis. Trends Parasitol. 2009;25:11–17. doi: 10.1016/j.pt.2008.09.011. [DOI] [PubMed] [Google Scholar]
- 10.Martin PJ, Anderson N, Jarrett RG. Detecting benzimidazole resistance with faecal egg count reduction tests and in vitro assays. Aust Vet J. 1989;66:236–240. doi: 10.1111/j.1751-0813.1989.tb13578.x. [DOI] [PubMed] [Google Scholar]
- 11.Dadzie Y, Neira M, Hopkins D. Final report of the conference on the eradicability of Onchocerciasis. Filaria J. 2003;2:2. doi: 10.1186/1475-2883-2-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Basáñez MG, et al. Effect of single-dose ivermectin on Onchocerca volvulus: A systematic review and meta-analysis. Lancet Infect Dis. 2008;8:310–322. doi: 10.1016/S1473-3099(08)70099-9. [DOI] [PubMed] [Google Scholar]
- 13.Plaisier AP, et al. Irreversible effects of ivermectin on adult parasites in onchocerciasis patients in the Onchocerciasis Control Programme in West Africa. J Infect Dis. 1995;172:204–210. doi: 10.1093/infdis/172.1.204. [DOI] [PubMed] [Google Scholar]
- 14.Bottomley C, Isham V, Collins RC, Basáñez MG. Rates of microfilarial production by Onchocerca volvulus are not cumulatively reduced by multiple ivermectin treatments. Parasitology. 2008;135:1571–1581. doi: 10.1017/S0031182008000425. [DOI] [PubMed] [Google Scholar]
- 15.Cupp E, Richards F, Lammie P, Eberhard M. Efficacy of ivermectin against Onchocerca volvulus in Ghana. Lancet. 2007;370:1123. doi: 10.1016/S0140-6736(07)61501-3. author reply 1124–1125. [DOI] [PubMed] [Google Scholar]
- 16.Mackenzie CD. Efficacy of ivermectin against Onchocerca volvulus in Ghana. Lancet. 2007;370:1123. doi: 10.1016/S0140-6736(07)61502-5. author reply 1124–1125. [DOI] [PubMed] [Google Scholar]
- 17.Remme JH, Amazigo U, Engels D, Barryson A, Yaméogo L. Efficacy of ivermectin against Onchocerca volvulus in Ghana. Lancet. 2007;370:1123–1124. doi: 10.1016/S0140-6736(07)61503-7. author reply 1124–1125. [DOI] [PubMed] [Google Scholar]
- 18.Cupp EW, et al. The effects of repetitive community-wide ivermectin treatment on transmission of Onchocerca volvulus in Guatemala. Am J Trop Med Hyg. 1992;47:170–180. doi: 10.4269/ajtmh.1992.47.170. [DOI] [PubMed] [Google Scholar]
- 19.Collins RC, et al. Ivermectin: Reduction in prevalence and infection intensity of Onchocerca volvulus following biannual treatments in five Guatemalan communities. Am J Trop Med Hyg. 1992;47:156–169. doi: 10.4269/ajtmh.1992.47.156. [DOI] [PubMed] [Google Scholar]
- 20.Filipe JAN, et al. Human infection patterns and heterogeneous exposure in river blindness. Proc Natl Acad Sci USA. 2005;102:15265–15270. doi: 10.1073/pnas.0502659102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Timmann C, et al. Human genetic resistance to Onchocerca volvulus: Evidence for linkage to chromosome 2p from an autosome-wide scan. J Infect Dis. 2008;198:427–433. doi: 10.1086/589720. [DOI] [PubMed] [Google Scholar]
- 22.Hoerauf A, Brattig N. Resistance and susceptibility in human onchocerciasis–beyond Th1 vs. Th2. Trends Parasitol. 2002;18:25–31. doi: 10.1016/s1471-4922(01)02173-0. [DOI] [PubMed] [Google Scholar]
- 23.Basáñez MG, Boussinesq M. Population biology of human onchocerciasis. Philos Trans R Soc London B. 1999;354:809–826. doi: 10.1098/rstb.1999.0433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Toé L, et al. Detection of Onchocerca volvulus infection by O-150 polymerase chain reaction analysis of skin scratches. J Infect Dis. 1998;178:282–285. doi: 10.1086/517454. [DOI] [PubMed] [Google Scholar]
- 25.Kershaw WE, Duke BOL, Budden FH. Distribution of microfilariae of Onchocerca volvulus in the skin; its relation to the skin changes and to eye lesions and blindness. Br Med J. 1954;2:724–729. doi: 10.1136/bmj.2.4890.724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Picq JJ, Jardel JP. A method of evaluating microfilaria densities of Onchocerca volvulus Leuckart, 1893, in onchoceriasis patients. Bull WHO. 1974;51:145–153. [PMC free article] [PubMed] [Google Scholar]
- 27.Fulford AJ. Dispersion and bias: Can we trust geometric means? Parasitol Today. 1994;10:446–448. doi: 10.1016/0169-4758(94)90181-3. [DOI] [PubMed] [Google Scholar]
- 28.Mathieu E, et al. Comparison of methods for estimating drug coverage for filariasis elimination, Leogane Commune, Haiti. Trans R Soc Trop Med Hyg. 2003;97:501–505. doi: 10.1016/s0035-9203(03)80006-8. [DOI] [PubMed] [Google Scholar]
- 29.Renz A, Fuglsang H, Anderson J. Studies on the dynamics of transmission of onchocerciasis in a Sudan-savanna area of North Cameroon IV. The different exposure to Simulium bites and transmission of boys and girls and men and women, and the resulting manifestations of onchocerciasis. Ann Trop Med Parasitol. 1987;81:253–262. doi: 10.1080/00034983.1987.11812118. [DOI] [PubMed] [Google Scholar]
- 30.Soboslay PT, et al. Ivermectin-facilitated immunity in onchocerciasis; activation of parasite-specific Th1-type responses with subclinical Onchocerca volvulus infection. Clin Exp Immunol. 1994;96:238–244. doi: 10.1111/j.1365-2249.1994.tb06548.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Duerr HP, Dietz K, Schulz-Key H, Büttner DW, Eichner M. Density-dependent parasite establishment suggests infection-associated immunosuppression as an important mechanism for parasite density regulation in onchocerciasis. Trans R Soc Trop Med Hyg. 2003;97:242–250. doi: 10.1016/s0035-9203(03)90132-5. [DOI] [PubMed] [Google Scholar]
- 32.Bourguinat C, et al. Genetic selection of low fertile Onchocerca volvulus by ivermectin treatment. PLoS Negl Trop Dis. 2007;1:e7. doi: 10.1371/journal.pntd.0000072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bourguinat C, et al. P-glycoprotein-like protein, a possible genetic marker for ivermectin resistance selection in Onchocerca volvulus. Mol Biochem Parasitol. 2008;158:101–111. doi: 10.1016/j.molbiopara.2007.11.017. [DOI] [PubMed] [Google Scholar]
- 34.Taylor MJ, Hoerauf A. A new approach to the treatment of filariasis. Curr Opin Infect Dis. 2001;14:727–731. doi: 10.1097/00001432-200112000-00011. [DOI] [PubMed] [Google Scholar]
- 35.Danso-Appiah A, De Vlas SJ. Interpreting low praziquantel cure rates of Schistosoma mansoni infections in Senegal. Trends Parasitol. 2002;18:125–129. doi: 10.1016/s1471-4922(01)02209-7. [DOI] [PubMed] [Google Scholar]
- 36.Awadzi K, et al. The chemotherapy of onchocerciasis. XI. A double-blind comparative study of ivermectin, diethylcarbamazine and placebo in human onchocerciasis in northern Ghana. Ann Trop Med Parasitol. 1986;80:433–442. doi: 10.1080/00034983.1986.11812044. [DOI] [PubMed] [Google Scholar]
- 37.Awadzi K, Dadzie KY, Kläger S, Gilles HM. The chemotherapy of onchocerciasis. XIII. Studies with ivermectin in onchocerciasis patients in northern Ghana, a region with long lasting vector control. Trop Med Parasitol. 1989;40:361–366. [PubMed] [Google Scholar]
- 38.Awadzi K, Opoku NO, Addy ET, Quartey BT. The chemotherapy of onchocerciasis. XIX: The clinical and laboratory tolerance of high dose ivermectin. Trop Med Parasitol. 1995;46:131–137. [PubMed] [Google Scholar]
- 39.Awadzi K, et al. The safety and efficacy of amocarzine in African onchocerciasis and the influence of ivermectin on the clinical and parasitological response to treatment. Ann Trop Med Parasitol. 1997;91:281–296. doi: 10.1080/00034989761139. [DOI] [PubMed] [Google Scholar]
- 40.Greene BM, et al. A comparison of 6-, 12-, and 24-monthly dosing with ivermectin for treatment of onchocerciasis. J Infect Dis. 1991;163:376–380. doi: 10.1093/infdis/163.2.376. [DOI] [PubMed] [Google Scholar]
- 41.Whitworth JA, Morgan D, Maude GH, Downham MD, Taylor DW. A community trial of ivermectin for onchocerciasis in Sierra Leone: Clinical and parasitological responses to the initial dose. Trans R Soc Trop Med Hyg. 1991;85:92–96. doi: 10.1016/0035-9203(91)90173-v. [DOI] [PubMed] [Google Scholar]
- 42.Boussinesq M, Chippaux JP. A controlled prospective trial of the prophylactic effect of a single dose of ivermectin against Onchocerca volvulus. Parasite. 2001;8:255–259. doi: 10.1051/parasite/2001083255. [DOI] [PubMed] [Google Scholar]
- 43.Duke BOL. The population dynamics of Onchocerca volvulus in the human host. Trop Med Parasitol. 1993;44:61–68. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.