Abstract
Type-IV P-type ATPases (P4-ATPases) are putative phospholipid translocases, or flippases, that translocate specific phospholipid substrates from the exofacial to the cytosolic leaflet of membranes to generate phospholipid asymmetry. In addition, the activity of Drs2p, a P4-ATPase from Saccharomyces cerevisiae, is required for vesicle-mediated protein transport from the Golgi and endosomes, suggesting a role for phospholipid translocation in vesicle budding. Drs2p is necessary for translocation of a fluorescent phosphatidylserine analogue across purified Golgi membranes. However, a flippase activity has not been reconstituted with purified Drs2p or any other P4-ATPase, so whether these ATPases directly pump phospholipid across the membrane bilayer is unknown. Here, we show that Drs2p can catalyze phospholipid translocation directly through purification and reconstitution of this P4-ATPase into proteoliposomes. The noncatalytic subunit, Cdc50p, also was reconstituted in the proteoliposome, although at a substoichiometric concentration relative to Drs2p. In proteoliposomes containing Drs2p, a phosphatidylserine analogue was actively flipped across the liposome bilayer to the outer leaflet in the presence of Mg2+-ATP, whereas no activity toward the phosphatidylcholine or sphingomyelin analogues was observed. This flippase activity was mediated by Drs2p, because protein-free liposomes or proteoliposomes reconstituted with a catalytically inactive form of Drs2p showed no translocation activity. These data demonstrate for the first time the reconstitution of a flippase activity with a purified P4-ATPase.
Keywords: flippase, membrane asymmetry, P4-ATPase, proteoliposome, Cdc50p
Phospholipids are asymmetrically distributed across the plasma membrane of eukaryotic cells, and the control of this membrane asymmetry is important in many cellular processes (1, 2). For example, in resting human red blood cells, phosphatidylserine (PS) and phosphatidylethanolamine (PE) are restricted primarily to the inner leaflet of the plasma membrane, whereas phosphatidylcholine (PC) and sphingomyelin (SM) are exposed on the cell surface (2, 3). Regulated exposure of PS in the outer leaflet of red blood cells and platelets provides an interface for stimulating coagulation reactions (3). Apoptotic cells also expose PS as a signal for removal by macrophages or other cells (4). In addition, phospholipid asymmetry of the bile canalicular membrane is critical to the integrity of membrane and normal bile secretion by hepatocytes (5). Loss of phospholipid asymmetry caused by mutations in the human FIC1/ATP8B1 gene may be the fundamental cause of the liver disease progressive familial intrahepatic cholestasis (6).
Membrane asymmetry is thought to be generated by proteins that transport phospholipids unidirectionally across a membrane bilayer (2, 7). ATP-dependent flippases are proposed to mediate the inward movement of phospholipid from the extracellular leaflet to the cytosolic leaflet, whereas the opposing outward transport is carried out by floppases. It has been suggested that a subset of ATP-binding cassette (ABC) transporters involved in multidrug resistance (MDR) and bile secretion, including human MDR1 P-glycoprotein/ABCB1, MDR3/ABCB4, MRP1/ABCC1, and their homologues in other organisms, catalyzes floppase activity (8, 9). Several of these ABC transporters have been purified and reconstituted into liposomes. For example, proteoliposomes formed with MDR1 P-glycoprotein purified from Chinese hamster ovary cells were shown to transport a variety of lipid analogues, including PS, PE, PC, SM, and glucosylceramide analogues bearing a fluorescent 7-nitro-2–1,3-benzoxadiazol-4-yl (NBD) group, across the liposome bilayer (10, 11). Another class of active transporters, the type-IV P-type ATPase (P4-ATPases), is proposed to catalyze the flippase activity that restricts PS and PE to the cytosolic leaflet of the plasma membrane (7–9). Slow flop of phospholipids without headgroup specificity, combined with rapid flip of PS and PE, is thought to establish the observed membrane asymmetry.
The first ATP-dependent flippase activity described was the aminophospholipid translocase in human red blood cells, which rapidly translocates spin-labeled or unmodified PS and PE in the outer leaflet of the plasma membrane to the inner leaflet (12, 13). Since the discovery of the aminophospholipid translocase, a major goal has been to define the enzyme responsible for this activity through purification and reconstitution. A flippase activity toward spin-labeled PS and PE analogues has been reconstituted with a partially purified, but unidentified, Mg2+-ATPase from human red blood cells (14). ATPase II/Atp8a1, a Mg2+-ATPase that potentially catalyzes aminophospholipid translocase activity in bovine chromaffin granules (15), has been purified to homogeneity (16, 17). Phylogenetic analysis of the ATPase II/Atp8a1 sequence indicates that it is a member of the P4-ATPase family, which includes Fic1/Atp8b1 and Drs2p from yeast (18, 19). However, a flippase activity has not been reconstituted with any purified P4-ATPase, including mammalian ATPase II/Atp8a1.
Although it is not known if a P4-ATPase in a purified form is sufficient to catalyze flippase activity, yeast P4-ATPases are necessary for flippase activities detected in the plasma membrane (20), secretory vesicles (21), and the trans-Golgi network (TGN) (22). Drs2p is localized primarily to the TGN (23) and requires a chaperone protein Cdc50p for its proper localization (24). Isolated TGN membranes containing a temperature-sensitive mutant form of Drs2p translocate NBD-PS, but not NBD-PC, to the cytosolic leaflet at permissive temperature (22). However, inactivation of this mutant Drs2p at higher temperature ablates the NBD-PS flippase activity (22). A possible interpretation of these data is that Drs2p pumps phospholipid substrates directly across membrane bilayers. Alternatively, Drs2p may pump an unidentified ion into the TGN to generate an ion gradient, which then is coupled by another transporter (an unidentified symporter) to phospholipid translocation. To gain further insight into the mechanism of phospholipid translocation, we sought to purify and reconstitute Drs2p to determine if it can pump phospholipid substrates directly across a lipid bilayer.
Toward the goal of reconstituting Drs2p, we have overexpressed Drs2p in yeast and purified it using affinity purification techniques. The ATPase activity of purified Drs2p is Mg2+-ATP dependent and sensitive to orthovanadate. After reconstitution, the Drs2p-containing proteoliposomes actively translocate NBD-PS to the outer leaflet upon addition of Mg2+-ATP, demonstrating that a purified P4-ATPase is sufficient to catalyze flippase activity.
Results
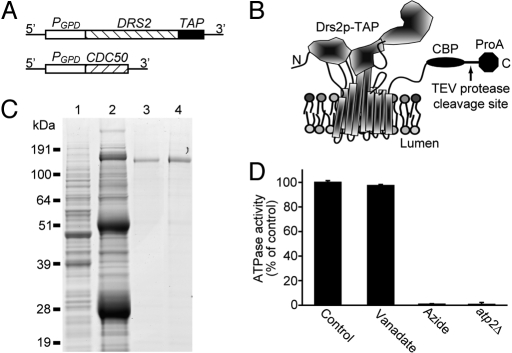
DRS2 was overexpressed in its native host Saccharomyces cerevisiae by substituting the strong glyceraldehyde-3-phosphate dehydrogenase (GPD) promoter for its endogenous promoter (25) (Fig. 1A). To facilitate purification, a tandem affinity purification (TAP) tag (26) was integrated into the 3′ end of the DRS2 gene to express C-terminally TAP-tagged Drs2p (Drs2p-TAP, Fig. 1 A and B). Drs2p-TAP was functional in vivo, because strains expressing DRS2-TAP as the sole source of Drs2p grew at 20 °C (supporting information (SI) Fig. S1). Strains carrying loss-of-function drs2 alleles cannot grow at 20 °C or below (23). Drs2p requires Cdc50p, a noncatalytic subunit and chaperone protein, for its export from the endoplasmic reticulum (ER) (24), so we also overexpressed CDC50 using the GPD promoter (Fig. 1A). These modifications led to a 10-fold increase in Drs2p expression relative to wild-type cells (Fig. S2).
Fig. 1.
Expression and purification of Drs2p-TAP. (A) Chromosomal organization of DRS2-TAP and CDC50. (B) Schematic of Drs2p-TAP modeled on the crystal structure of the sarcoplasmic reticulum Ca2+-ATPase 1 (SERCA1) in the E1 conformation (32). CBP, calmodulin-binding peptide moiety; ProA, protein A moiety. (C) Aliquots from a Drs2p-TAP purification from strain XZY10b were subject to SDS/PAGE, and the gel was stained with SimplyBlue. Lane 1, cell lysate; lane 2, proteins bound to the IgG beads; lane 3, proteins bound to the calmodulin beads; lane 4, final eluate. (D) ATPase activity of purified Drs2p-TAP preparations. The control, vanadate (100 μM), and azide (1 mM) samples were assayed using 50 ng of Drs2p-TAP purified from stain XZY10b (ATP2), and the atp2Δ sample was assayed using 100 ng of Drs2p-TAP from strain XZY38b (atp2Δ), deficient for the F1-ATPase.
For purification of Drs2p-TAP, a cell lysate prepared without detergent was centrifuged at 15,000 × g for 12 min to pellet ER membranes along with any misfolded Drs2p-TAP retained there by the quality control machinery. We used 1% polyoxyethelene 9-lauryl ether (C12E9) to solubilize Drs2p-TAP from TGN membranes that remained in the supernatant (see Materials and Methods). As seen in Fig. 1C, Drs2p-TAP was purified successfully by the TAP procedure and composed more than 90% of protein in the final eluate (lane 4). Cdc50p was not detected in this preparation by SimplyBlue staining but was identified by mass spectrometry (Table S1). Typically, 5% of Drs2p-TAP in the cell lysate was recovered in the first affinity purification step (IgG column), and 1% was recovered in the second affinity step (calmodulin column), yielding 10 μg of Drs2p/L culture (≈1011 cells).
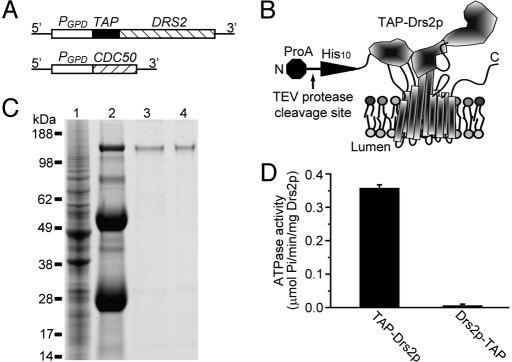
Robust ATPase activity was detected in the purified Drs2p-TAP sample. However, this ATPase activity was insensitive to orthovanadate, a commonly used P-type ATPase inhibitor (16, 17), and was completely inhibited by 1 mM azide (Fig. 1D). This result suggested that the ATPase activity was catalyzed primarily by contaminating mitochondrial F1-ATPase. Indeed, the α and β subunits of the F1-ATPase were detected as trace contaminants by mass spectrometry (Table S1), but the specific activity of F1-ATPase is very high (150 μmol ATP hydrolyzed/min/mg) (27). To test if the activity was catalyzed by the F1-ATPase, the ATP2 gene encoding the catalytic β subunit of the F1-ATPase was disrupted in the strain used for Drs2p-TAP purification. No ATPase activity could be detected in Drs2p-TAP preparations from the atp2Δ strain (Fig. 1D), even though Drs2p-TAP was recovered with a yield and purity comparable to that shown in Fig. 1C. Other ATPases also were detected at trace levels (Table S1), but their activities were below the limit of detection in these preparations. Therefore, purified Drs2p-TAP seemed to be catalytically inactive in vitro. A variety of purification and assay conditions were tested for Drs2p-TAP, including the addition of potential substrate lipids such as PS, but none of the conditions used yielded an active enzyme. However, moving the affinity tag from the C terminus to the N terminus of Drs2p (TAP-Drs2p, Fig. 2 A and B) and modifying the TAP tag to contain a decahistidine instead of the calmodulin-binding peptide as the second affinity module (Fig. 2B) allowed purification of enzymatically active Drs2p.
Fig. 2.
Expression and purification of TAP-Drs2p. (A) Chromosomal organization of TAP-DRS2 and CDC50. (B) Schematic of TAP-Drs2p. His10, decahistidine moiety; ProA, protein A moiety. (C) Aliquots from a TAP-Drs2p purification from strain XZY60m (atp2Δ) were subject to SDS/PAGE, and the gel was stained with SimplyBlue. Lane 1, cell lysate; lane 2, proteins bound to the IgG beads; lane 3, proteins bound to the Ni-NTA beads; lane 4, final eluate. (D) Comparison of activity of TAP-Drs2p and Drs2p-TAP ATPase purified from strain XZY60m (atp2Δ) and XZY38b (atp2Δ), respectively.
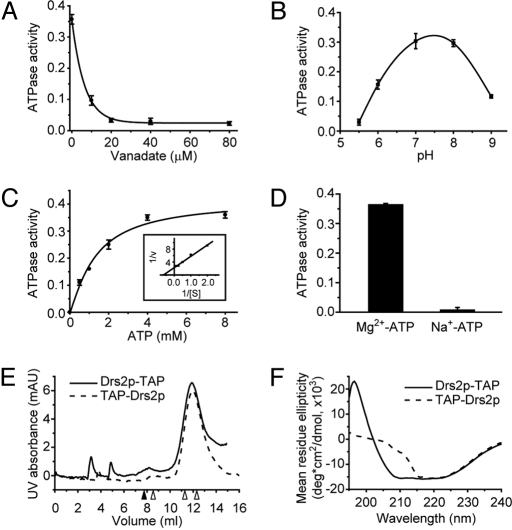
TAP-Drs2p also was purified from a strain that was deficient for the F1-ATPase, and a comparable yield and purity were obtained (compare Fig. 2C and Fig. 1C). The TAP-Drs2p preparation now displayed robust ATPase activity relative to Drs2p-TAP (Fig. 2D). The only significant difference between the 2 Drs2p preparations was the position of the tag (N-terminal versus C-terminal), indicating that the ATPase activity detected in the TAP-Drs2p sample was catalyzed by Drs2p and not by minor contaminants in the sample. Consistent with other P-type ATPases, the ATPase activity of TAP-Drs2p was sensitive to orthovanadate with an IC50 of 5 μM and was resistant to azide (Fig. 3A and Fig. S3). TAP-Drs2p showed an optimal pH of 7.5 (Fig. 3B), a Km of 1.5 ± 0.3 mM for ATP (Fig. 3C), and a Vmax of 0.45 ± 0.03 μmol Pi released/min/mg Drs2p (≈70 ± 5 ATPs hydrolyzed/min/Drs2p, Fig. 3C) under these assay conditions. The ATPase activity of TAP-Drs2p also was Mg2+-dependent (Fig. 3D), as reported for purified mammalian Atp8a1 (16, 17).
Fig. 3.
Characterization of purified Drs2p in 0.1% C12E9. (A) Sensitivity of TAP-Drs2p ATPase activity to orthovanadate (VO43−). (B) pH profile of TAP-Drs2p ATPase activity assayed as described in Materials and Methods but with varying buffers. pH 5.5 and 6, 50 mM Mes; pH 7, 8, and 9, 50 mM Tris-HCl. (C) Determination of the Km for ATP and Vmax of ATP hydrolysis for TAP-Drs2p. (Inset) Double-reciprocal plot. (D) Mg2+ dependence of TAP-Drs2p ATPase activity. y axis: units of (A) − (D) = μmol Pi released/min/mg Drs2p. (E) Size-exclusion chromatography of purified TAP-Drs2p and Drs2p-TAP using ÄKTA FPLC system and Superose 12 column from GE Healthcare. Samples were injected at 0 mL. The closed arrowhead indicates the void volume (7.77 mL), and the 3 open arrowheads (from left to right) indicate the peaks of ferritin (440 kDa), catalase (232 kDa), and aldolase (158 kDa) standards. (F) Circular dichroism spectra of purified TAP-Drs2p (40 μg/mL) and Drs2p-TAP (38 μg/mL) in 20 mM Tris-HCl (pH 7.5), 100 mM NaCl, and 0.1% C12E9.
To assess the oligomeric state of purified Drs2p, TAP-Drs2p and Drs2p-TAP were subjected to size-exclusion chromatography. The majority of both Drs2 proteins eluted as a single peak at nearly the same volume (Fig. 3E). Relative to protein standards, the mass of TAP-Drs2p was estimated to be 181 kDa and the mass of Drs2p-TAP to be 184 kDa, very close to their predicted monomer masses of 156 kDa and 160 kDa. To probe the molecular basis of Drs2p-TAP inactivity, TAP-Drs2p and Drs2p-TAP were analyzed by circular dichroism. The spectra of the 2 proteins were strikingly different, especially in the far UV region (Fig. 3F), suggesting a significant difference between their tertiary structures (Table S2). Although the molecular basis for its inactivity is not fully understood, Drs2p-TAP served as an enzymatically dead negative control in this study.
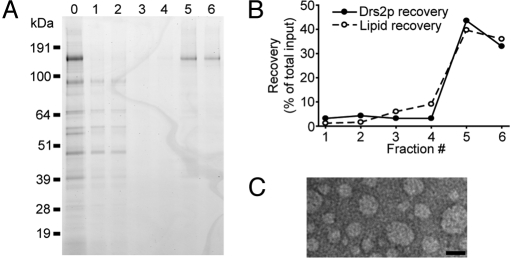
Purified TAP-Drs2p was incorporated into PC liposomes via detergent-mediated reconstitution (see Materials and Methods). To improve reconstitution efficiency by increasing the protein/lipid ratio, single-step affinity-purified TAP-Drs2p was used directly for reconstitution, because single-step affinity purification provided more Drs2p than the 2-step purification, although with lower purity (Fig. 4A, lane 0). After flotation of reconstituted samples in a glycerol step gradient, most TAP-Drs2p was found in the top fractions and co-fractionated with phospholipids (Fig. 4 A, lanes 5 and 6, and B). Importantly, most contaminating proteins from the single-step affinity purification remained at the bottom of the gradient and were well separated from the proteoliposome fractions (Fig. 4A, lanes 1 and 2). As determined by mass spectrometry, the proteoliposome fractions contained relatively pure TAP-Drs2p, comparable to that obtained from the 2-step TAP procedure (Table 1 and Table S1). Based on negative staining and visualization by electron microscopy, the size of proteoliposomes ranged from 25 to 75 nm in diameter, with a mean diameter of 40 nm (Fig. 4C). The protein/lipid ratio was 1:200 (wt/wt) in the proteoliposome samples, and we estimated that there was 1 Drs2p molecule per 2–3 liposomes. In our reconstituted samples, Drs2p was preferentially inserted into proteoliposomes with the ATPase domain facing outward, as revealed by a protease protection assay (Fig. S4). Any Drs2p reconstituted in the opposite orientation would not be stimulated by Mg2+-ATP present outside the proteoliposome.
Fig. 4.
Flotation of TAP-Drs2p proteoliposomes in a glycerol gradient. (A) Fractions collected from the glycerol gradient were subject to SDS/PAGE, and the gel was stained with SimplyBlue. Lane 0, the protein sample before reconstitution; lanes 1–6, fractions (#1–6) collected from the bottom to the top of the gradient. (B) Recovery of Drs2p and phospholipid in each fraction relative to the starting material. (C) Electron micrograph of negative-stained proteoliposome sample from fraction #5. (Scale bar, 50 nm.)
Table 1.
Proteins identified in Drs2p proteoliposomes
| Protein | Peptides recovered |
|
|---|---|---|
| N-TAP | C-TAP | |
| Drs2p | 275 | 313 |
| Cdc50p | 20 | 16 |
| Ssa1p | 11 | 8 |
| Ssa2p | 8 | 6 |
| Pma1p | 7 | 3 |
| Tdh3p | 6 | 9 |
| Psa1p | 5 | 1 |
| Hsp82p | 4 | 8 |
| Hsp26p | 3 | 2 |
| Por1p | 3 | 2 |
| Tdh2p | 3 | 5 |
| Rpl4ap | 3 | 1 |
| Rpl4bp | 3 | 0 |
| Eno1p | 3 | 0 |
| Rpl5p | 2 | 0 |
| Tub2p | 2 | 0 |
| Cdc19p | 2 | 1 |
| Rpp0p | 1 | 2 |
| Tdh1p | 1 | 1 |
| Sam1p | 1 | 3 |
| Kar2p | 1 | 1 |
| Yef3p | 1 | 2 |
| Fas1p | 1 | 2 |
Protein composition of proteoliposomes reconstituted with either TAP-Drs2p (N-TAP) or Drs2p-TAP (C-TAP) determined by MALDI-TOF mass spectrometry.
To assay for flippase activity, PS (the potential substrate), PC, or SM (control) analogues bearing a fluorescent NBD group on a short sn2 acyl chain (C6) were incorporated into PC proteoliposomes during reconstitution. The TAP-Drs2p proteoliposomes then were assayed for flippase activity using dithionite, a membrane-impermeable quenching agent, to monitor the interleaflet distribution of NBD-phospholipid (see Materials and Methods). To improve the sensitivity for detecting transbilayer flip, the NBD-phospholipid in the outer leaflet was pre-quenched with dithionite, and the proteoliposomes were re-floated in a glycerol gradient to remove the dithionite. This treatment resulted in liposomes with most fluorescent NBD-phospholipid in the inner leaflet and increased the sensitivity for detecting flip of NBD-phospholipid to the outer leaflet.
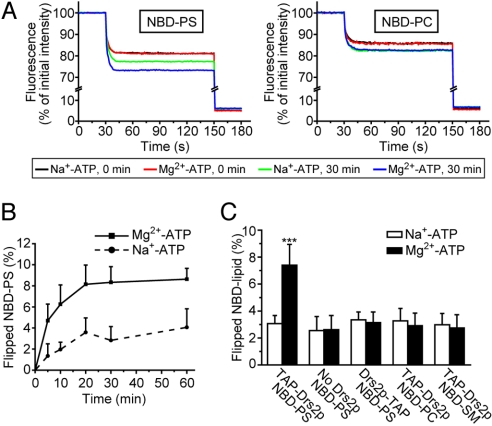
From preliminary experiments, we noticed that incubation of liposomes with ATP alone (Na+-ATP) caused a small nonspecific increase in accessibility of NBD-phospholipid to dithionite (Fig. S5). To take this effect into account, we incubated the proteoliposomes with Na+-ATP, which is not hydrolyzed by TAP-Drs2p (Fig. 3D), as a background control in the flippase assay. As seen in Fig. 5A, after a 30-min incubation, Na+-ATP caused an ≈3% net increase in NBD-PS accessibility to dithionite (green trace versus black trace). In the presence of Mg2+-ATP, significantly more NBD-PS (≈7%) was accessible to dithionite (blue trace versus red trace). The increased quenching of NBD-PS was not caused by Mg2+ alone or by contaminating Ca2+ (Fig. S5). NBD-PS was flipped across the liposome bilayer with a half-time of ≈4 min (Fig. 5B), and the initial velocity was about 0.02 μmol NBD-PS flipped/min/mg Drs2p (≈3 NBD-PS flipped/min/Drs2p). As controls, no difference between the Mg2+-ATP and Na+-ATP incubations was observed with TAP-Drs2p proteoliposomes containing NBD-PC or NBD-SM instead of NBD-PS (Fig. 5 A and C), indicating that PS is the moiety that was recognized and translocated. Moreover, if Drs2p activity simply caused increased leakiness of the proteoliposome to dithionite, we would have observed enhanced quenching of the NBD-PC or NBD-SM probes. In other control experiments, no increased quenching was observed with protein-free liposomes (No Drs2p) containing NBD-PS (Fig. 5C) or with proteoliposomes reconstituted with the enzymatically dead Drs2p-TAP and NBD-PS (Fig. 5C). These data demonstrated that the increased quenching (≈4%) of NBD-PS in TAP-Drs2p proteoliposome was mediated by a Mg2+-, ATP-, and Drs2p-dependent flippase activity specific for the PS analogue.
Fig. 5.
Reconstitution of NBD-PS flippase activity with TAP-Drs2p proteoliposomes. (A) Dithionite quenching of NBD-PS (Left) or NBD-PC (Right) fluorescence in TAP-Drs2p proteoliposomes before and after 30 min of incubation with Mg2+-ATP or Na+-ATP. Dithionite was added at 30 s to quench outer leaflet NBD-phospholipid, and Triton X-100 was added at 150 s to quench remaining inner leaflet NBD-phospholipid. (B) Time course of NBD-PS translocation. The flippase assay was conducted as described in Materials and Methods, except that the incubation time varied from 0 to 60 min as indicated. Results were averaged from 4–6 independent experiments. (C) Flippase assay with liposomes containing TAP-Drs2p, Drs2p-TAP, or no Drs2p and the indicated NBD-phospholipid was performed as described in Materials and Methods. The increase in outer leaflet NBD-phospholipid after a 30-min incubation with Mg2+-ATP or Na+-ATP was plotted. Results were averaged from 6–9 independent experiments. ***, P < 0.001.
Furthermore, mass spectrometric studies revealed that the protein composition of the TAP-Drs2p and the Drs2p-TAP proteoliposomes was nearly identical (Table 1). In both samples, Cdc50p was recovered as the second most abundant protein based on the number of peptides recovered. However, a relatively small number of Cdc50p peptides was recovered, and Cdc50p was not apparent in the gels stained by SimplyBlue. Thus, it seems that the majority of Drs2p was not complexed with Cdc50p in the reconstituted samples. Other proteins recovered in low quantities, such as heat shock proteins and proteins involved in metabolism and protein synthesis, and are common contaminants of affinity purifications, are very abundant proteins in yeast (28). Most importantly, the 2 proteoliposome samples were comparable in protein composition, with the major difference being the placement of the TAP tag on Drs2p, indicating that Drs2p activity is responsible for the difference in the flippase activity between the 2 samples.
Discussion
For more than a decade it has been proposed that Drs2p and the P4-ATPase family are phospholipid translocases or flippases, and several lines of evidence indicate that Drs2p is necessary for flipping NBD-labeled PS or PE analogues in isolated Golgi membranes and post-Golgi secretory vesicles (7–9, 18, 21, 22). However, no in vitro reconstitution of a flippase activity had been achieved with a purified P4-ATPase to test whether these pumps are sufficient to catalyze the flippase activity directly. Toward this goal, we purified Drs2p from yeast, reconstituted it into proteoliposomes, and demonstrated NBD-PS flippase activity with the purified and reconstituted enzyme.
In this study, Drs2p with affinity tags at either end (TAP-Drs2p and Drs2p-TAP) was purified. Surprisingly, although the 2 protein preparations were very comparable in yield and purity (Figs. 1C and 2C, Table 1, and Table S1), only TAP-Drs2p was catalytically active in vitro, whereas Drs2p-TAP was enzymatically dead (Figs. 2D and 5C). However, both Drs2 proteins are functional in vivo, because they support yeast growth at low temperatures (Fig. S1). By contrast, Drs2p-deficient cells exhibit a cold-sensitive growth defect (23). Drs2p-TAP seems to be folded well enough to exit the ER, because our purification method should remove most of the ER-localized Drs2p. Based on size-exclusion chromatography, both Drs2 proteins seem to be primarily monomeric in detergent solutions (Fig. 3E). Moreover, no significant difference in stability and solubility was observed between TAP-Drs2p and Drs2p-TAP (Figs. 2C and 1C). However, the tertiary structure of TAP-Drs2p was found to differ strikingly from that of Drs2p-TAP as revealed by circular dichroism (Fig. 3F). The inactive Drs2p-TAP was predicted to assume a more ordered structure than TAP-Drs2p (Table S2). P-type ATPases undergo dramatic conformational changes during the catalytic cycle, and it is possible that Drs2p-TAP is inactive because it is trapped in a single conformational state. Further work will be required to determine whether the loss of Drs2p-TAP activity in vitro resulted from the position of the C-terminal TAP tag or from the calmodulin-binding peptide versus the decahistidine module.
The ATPase activity of purified TAP-Drs2p was Mg2+-ATP-dependent and orthovanadate-sensitive (Fig. 3 D and A), consistent with the properties of purified mammalian Atp8a1 (16, 17). The specific activity of TAP-Drs2p was 0.45 ± 0.03 μmol Pi released/min/mg Drs2p (Vmax, Fig. 3C), and this activity is within the range of values reported for purified Atp8a1 (16, 17). However, we observed only a mild increase of ≈40% in the ATPase activity of purified TAP-Drs2p when incubated with PS, a potential phospholipid substrate for Drs2p, (Fig. S3). This stimulation level is marginal compared with the steep increases (greater than 10-fold) observed for purified mammalian Atp8a1 (16, 17). Activation of Atp8a1 ATPase activity by PS suggests that PS is a native substrate for Atp8a1. The modest PS stimulation of TAP-Drs2p sample may result from the co-purification of substrate phospholipid with Drs2p, so that the preparation is at nearly maximum activation. However, the specific activity of TAP-Drs2p was relatively low compared with PS-activated bovine Atp8a1 (3–9 μmol Pi released/min/mg) (16). Whether this difference reflects a species difference or whether only a portion of Drs2p molecules retained activity after purification remains to be determined.
Upon reconstitution, the TAP-Drs2p proteoliposome exhibited flippase activity that was specific for NBD-PS (Fig. 5), because no activity was observed with the PC and SM analogues (Fig. 5 A and C). TAP-Drs2p was the major but not the only protein present in the proteoliposome (Fig. 4A and Table 1). Mass spectrometry also identified several other proteins in low abundance, including Cdc50p (Table 1). Any of these proteins potentially could be responsible for the flippase activity independently or dependently of TAP-Drs2p. However, the former possibility was ruled out because a control proteoliposome sample reconstituted with the enzymatically dead Drs2p-TAP showed no NBD-PS flippase activity (Fig. 5C) and contained a protein composition almost identical to that of the TAP-Drs2p proteoliposome (Table 1). These data argue strongly that Cdc50p and minor contaminants were incapable of catalyzing flippase activity without active Drs2p.
However, our data are consistent with the possibility that Cdc50p also may be required for the flippase activity of Drs2p, because Cdc50p was present in the TAP-Drs2p proteoliposomes (Table 1). Moreover, recent work suggests that Cdc50p facilitates the catalytic cycle of Drs2p (29). In the current study, we estimated that around 40% of the liposomes would contain 1 Drs2p molecule if an equal distribution of Drs2p in liposomes is assumed. Drs2p seemed to be inserted preferentially into proteoliposomes with its ATPase domain facing outward (Fig. S4). Therefore, at most, 40% of the liposomes would contain a Drs2p molecule that has access to externally applied Mg2+-ATP, and 40% would be the upper limit of NBD-PS translocation we could achieve under these conditions. We have observed a nearly saturated Mg2+-ATP-dependent flip of 4% NBD-PS (Fig. 5), perhaps indicating that only 4% of the liposomes contain an active flippase. If the Drs2p-Cdc50p complex is essential for flippase activity, the molar concentration of Cdc50p would need to be at least 1/10 that of Drs2p to explain why we observed 1/10 the maximum expected activity. Based on mass spectrometric results, the number of Cdc50p peptides recovered in the TAP-Drs2p proteoliposome was about 1/10 that of Drs2p (Table 1). Thus, it is possible that the flippase activity reported here was catalyzed by Drs2p-Cdc50p complexes, and that Drs2p alone is insufficient to drive flippase activity. Alternatively, it is possible that Cdc50p is not required, and the suboptimal flippase activity in the proteoliposomes resulted from the physical restraint of small liposome size (40 nm in mean diameter, Fig. 4C), an unequal distribution of Drs2p in proteoliposomes, a high percentage of inactive Drs2p molecules in the TAP-Drs2p preparation, and/or the absence of other potential regulators of Drs2p activity. Further work will be required to determine the extent of the Cdc50p contribution to flippase activity. Nonetheless, the studies reported here provide a critical step forward in the biochemical characterization of a phospholipid flippase and define the minimal flippase unit as a P4-ATPase, perhaps in association with its noncatalytic subunit.
Materials and Methods
Reagents.
IgG Sepharose 6 Fast Flow, calmodulin Sepharose 4B, and ATP (> 99% purity) were from GE Healthcare. Ni-NTA agarose was from Qiagen. AcTEV Protease and SimplyBlue SafeStain (Coomassie G-250) were from Invitrogen, and Bio-Beads SM-2 was from Bio-Rad. Phospholipids and fluorescent derivatives were from Avanti Polar Lipids and were L-α-phosphatidylcholine from chicken egg (egg PC), 1-palmitoyl-2-oleoyl-sn-glycero-3-phospho-L-serine (POPS), 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine (POPC), 1-palmitoyl-2-[6-(NBD-amino)hexanoyl]-sn-glycero-3-phospho-L-serine (NBD-PS), 1-palmitoyl-2-[6-(NBD-amino)hexanoyl]-sn-glycero-3-phosphocholine (NBD-PC), and N-[6-(NBD-amino)hexanoyl]-sphingosine-1-phosphocholine (NBD-SM). Lipids were dissolved in chloroform and stored at −20 °C.
Yeast Strains and Protein Purification.
Yeast strains used for protein purification were XZY10b (MATa his3 leu2 ura3 met15 PGPD::DRS2::TAP PGPD::CDC50), XZY38b (MATa his3 leu2 ura3 met15 PGPD::DRS2::TAP PGPD::CDC50 atp2Δ::URA3), and XZY60m (MATα his3 leu2 ura3 lys2 PGPD::TAP::DRS2 PGPD::CDC50 atp2Δ::URA3), where TAP in DRS2-TAP encodes the calmodulin-binding peptide–TEV protease cleavage site–protein A tag, and TAP in TAP-DRS2 encodes the protein A–TEV protease cleavage site–decahistidine tag.
Yeast strains were grown in 1 L of 2X YPD (2% yeast extract/4% peptone/4% dextrose) medium to saturation (usually 10–15 OD600/mL) at 30 °C before harvesting by centrifugation at 5,000 × g for 5 min. All steps following harvest were performed at 4 °C unless otherwise stated. Cells were washed with 10 mM NaN3, resuspended in 20 mL of lysis buffer (40 mM Tris-HCl, pH 7.5/150 mM NaCl/10% glycerol), and lysed using an EmulsiFlex-C3 High Pressure Homogenizer (Avestin, Inc.) at 25,000 psi for 6 complete passes in the presence of protease inhibitors (500 μM benzamidine hydrochloride, 10 μM phenanthroline, 250 nM aprotinin, 3 μM pepstatin A, 4 μM leupeptin, 2 mM EDTA, 1 mM PMSF). The cell lysate was centrifuged at 15,000 × g for 12 min, and 10% C12E9 was added to the supernatant to a final concentration of 1%. Samples were mixed on an end-over-end rotator for 2 h to solubilize Drs2p. Then 400 μL of IgG Sepharose 6 Fast Flow beads (50% slurry in 50 mM potassium phosphate and 20% ethanol) prewashed with 10 mL of wash buffer (40 mM Tris-HCl, pH 7.5/150 mM NaCl/10% glycerol/0.1% C12E9) was added and rotated for 2 h, followed by a wash with 10 mL of wash buffer. The IgG beads were resuspended in 1 mL of TEV buffer (40 mM Tris-HCl, pH 7.5/150 mM NaCl/10% glycerol/0.1% C12E9/10 mM 2-mercaptoethanol/0.5 mM EDTA) with 100 units of AcTEV Protease, and Drs2p was released by rotating the mixture for 2 h at 16 °C.
In the second affinity step, Drs2p-TAP was bound to 200 μL of calmodulin Sepharose 4B beads in 5 mL of calmodulin binding buffer (40 mM Tris-HCl, pH 7.5/150 mM NaCl/10% glycerol/0.1% C12E9/1 mM imidazole/10 mM 2-mercaptoethanol/2 mM CaCl2) by rotating for 1 h, followed by a wash with 20 mL of calmodulin binding buffer, and was eluted with 1 mL of calmodulin elution buffer (40 mM Tris-HCl, pH 7.5/150 mM NaCl/10% glycerol/0.1% C12E9/6 mM EGTA). Similarly, TAP-Drs2p was incubated with 200 μL of Ni-NTA agarose beads in 5 mL of Ni-binding buffer (40 mM Tris-HCl, pH 7.5/300 mM NaC/10% glycerol/0.1% C12E9/20 mM imidazole/10 mM 2-mercaptoethanol) by rotating for 1 h, followed by a wash with 20 mL of Ni-binding buffer, and was eluted with 1 mL of Ni elution buffer (40 mM Tris-HCl, pH 7.5/150 mM NaCl/10% glycerol/0.1% C12E9/200 mM imidazole). Protein eluates were stored at −20 °C in the presence of 50% glycerol. Recovery of Drs2p was determined using Odyssey Infrared Imaging System (LI-COR, Inc.) to quantify SimplyBlue-stained bands relative to a BSA standard curve. Purified Drs2p was assayed for ATPase activity in ATPase buffer (50 mM Tris-HCl, pH 7.5/100 mM NaCl/50 mM KCl/0.1% C12E9/4 mM Na+-ATP/10 mM MgCl2) at 37 °C for 2 h, and released phosphate was measured colorimetrically using protocols previously described (17).
Proteoliposome Formation.
Proteoliposomes were formed by detergent removal using Bio-Beads SM-2 adsorption (30). All steps were performed at 4 °C unless otherwise stated. Normally, 4 mg of lipid mixture (99% egg PC and 1% NBD-phospholipid) was dried under an N2 stream and solubilized completely with 1 mL of 1% C12E9 in reconstitution buffer (40 mM Tris-HCl, pH 7.5/150 mM NaCl) at room temperature. 1 mL of TEV buffer containing ≈20 μg of Drs2p purified by single-affinity purification (IgG Sepharose 6 Fast Flow) was mixed gently with the lipid solution, and the protein-lipid-detergent solution was incubated by rotation for 1 h before SM-2 beads were added. After the addition of 100 mg of extensively washed SM-2 beads and 6 h of incubation on an end-over-end rotator, a second 200-mg portion of SM-2 beads was added and incubated for another 12 h without removing the original SM-2 beads. The supernatant containing proteoliposomes then was removed carefully and mixed with 500 mg of fresh SM-2 beads for 3 h of incubation. The resulting proteoliposome was removed and stored at 4 °C. Two hundred microliters of proteoliposomes was mixed with 200 μL of 80% glycerol and was placed at the bottom of a glycerol step gradient consisting of 40% glycerol, 300 μL 30% glycerol,400 μL 10% glycerol, and 100 μL reconstitution buffer (top layer). The samples were centrifuged in a TLS-55 rotor (Beckman Coulter, Inc.) at 50,000 rpm for 6 h, and 200-μL fractions were collected by piercing the bottom of the tube. The recovery of lipids for each fraction was calculated as (fluorescence of NBD-phospholipid in each fraction)/(initial fluorescence of total NBD-phospholipid used for reconstitution) × 100%, and Drs2p recovery was determined by (Drs2p quantified by SimplyBlue staining in each fraction)/(initial Drs2p used for reconstitution) × 100%. For flippase assays, proteoliposome fractions containing both phospholipids and Drs2p were pretreated with 10 mM dithionite for 3 min to quench most fluorescent NBD-phospholipid in the outer leaflet of proteoliposomes. Treated proteoliposomes were re-floated as described earlier in the text to separate them from dithionite.
Flippase Assay.
Flippase activity was defined by a change of interleaflet distribution of fluorescent lipid probes (NBD-phospholipids) in proteoliposomes measured using a dithionite-based quenching approach (31). For each individual measurement, 10 μL of proteoliposomes containing ≈50 ng of Drs2p and ≈10 nmol of phospholipids were incubated with either 5 mM Na+-ATP or Mg2+-ATP in 40 μL of flippase buffer (40 mM Tris-HCl, pH 7.5/200 mM NaCl) at 37 °C. At 0 and 30 min of incubation, each sample was assayed for probe distribution. Samples were diluted to 1 mL with flippase buffer in a quartz cuvette and mixed by inverting the cuvette 10 times, and the total fluorescence (FT) was recorded for 30 s in an AB2 fluorometer (SLM Instruments, Inc.) (λex = 460 nm, λem = 534 nm) to obtain a stable baseline. Then, 10 μL of 1 M dithionite dissolved in 1 M Tris (pH 10) was added to the sample and mixed to quench the fluorescent probes in the outer leaflet of liposomes, and fluorescence was recorded until a stable line was obtained (120 s, FD). The sample then was solubilized by addition of 100 μL of 10% Triton X-100, and the background fluorescence (F0) was recorded for another 30 s. The percentage of NBD-phospholipid in the outer leaflet of proteoliposomes that is accessible to dithionite quenching was calculated as (FT − FD)/(FT − F0) × 100% (defined as PO), and the percentage of NBD-phospholipid flipped to the outer leaflet over a 30-min period was calculated as PO30min − PO0min.
Supplementary Material
Acknowledgments.
We thank Amy-Joan L. Ham and W. Hayes McDonald from the Mass Spectrometry Research Center at Vanderbilt University for their assistance and Melanie D. Ohi and Melissa Chambers (Vanderbilt University) for their help with negative staining and electron microscopy. We also are grateful to Andrzej M. Krezel (Vanderbilt University) for his help with size exclusion chromatography and Paramasivam Natarajan (Vanderbilt University) for his help with circular dichroism. This project is supported by National Institutes of Health Grant R01GM062367 to T.R.G.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. A.K.M. is a guest editor invited by the Editorial Board.
This article contains supporting information online at www.pnas.org/cgi/content/full/0904293106/DCSupplemental.
References
- 1.Bretscher MS. Membrane structure: Some general principles. Science. 1973;181:622–629. doi: 10.1126/science.181.4100.622. [DOI] [PubMed] [Google Scholar]
- 2.Devaux PF. Protein involvement in transmembrane lipid asymmetry. Annu Rev Biophys Biomol Struct. 1992;21:417–439. doi: 10.1146/annurev.bb.21.060192.002221. [DOI] [PubMed] [Google Scholar]
- 3.Zwaal RF, Schroit AJ. Pathophysiologic implications of membrane phospholipid asymmetry in blood cells. Blood. 1997;89:1121–1132. [PubMed] [Google Scholar]
- 4.Williamson P, Schlegel RA. Transbilayer phospholipid movement and the clearance of apoptotic cells. Biochim Biophys Acta. 2002;1585:53–63. doi: 10.1016/s1388-1981(02)00324-4. [DOI] [PubMed] [Google Scholar]
- 5.Paulusma CC, et al. Atp8b1 deficiency in mice reduces resistance of the canalicular membrane to hydrophobic bile salts and impairs bile salt transport. Hepatology. 2006;44:195–204. doi: 10.1002/hep.21212. [DOI] [PubMed] [Google Scholar]
- 6.Folmer DE, Elferink RP, Paulusma CC. P4 ATPases—lipid flippases and their role in disease. Biochim Biophys Acta. 2009;1791:628–635. doi: 10.1016/j.bbalip.2009.02.008. [DOI] [PubMed] [Google Scholar]
- 7.Graham TR. Flippases and vesicle-mediated protein transport. Trends Cell Biol. 2004;14:670–677. doi: 10.1016/j.tcb.2004.10.008. [DOI] [PubMed] [Google Scholar]
- 8.Daleke DL. Regulation of transbilayer plasma membrane phospholipid asymmetry. J Lipid Res. 2003;44:233–242. doi: 10.1194/jlr.R200019-JLR200. [DOI] [PubMed] [Google Scholar]
- 9.Pomorski T, Holthuis JC, Herrmann A, van Meer G. Tracking down lipid flippases and their biological functions. J Cell Sci. 2004;117:805–813. doi: 10.1242/jcs.01055. [DOI] [PubMed] [Google Scholar]
- 10.Romsicki Y, Sharom FJ. Phospholipid flippase activity of the reconstituted P-glycoprotein multidrug transporter. Biochemistry. 2001;40:6937–6947. doi: 10.1021/bi0024456. [DOI] [PubMed] [Google Scholar]
- 11.Eckford PD, Sharom FJ. The reconstituted P-glycoprotein multidrug transporter is a flippase for glucosylceramide and other simple glycosphingolipids. Biochem J. 2005;389:517–526. doi: 10.1042/BJ20050047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Seigneuret M, Devaux PF. ATP-dependent asymmetric distribution of spin-labeled phospholipids in the erythrocyte membrane: Relation to shape changes. Proc Natl Acad Sci USA. 1984;81:3751–3755. doi: 10.1073/pnas.81.12.3751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Daleke DL, Huestis WH. Incorporation and translocation of aminophospholipids in human erythrocytes. Biochemistry. 1985;24:5406–5416. doi: 10.1021/bi00341a019. [DOI] [PubMed] [Google Scholar]
- 14.Auland ME, Roufogalis BD, Devaux PF, Zachowski A. Reconstitution of ATP-dependent aminophospholipid translocation in proteoliposomes. Proc Natl Acad Sci USA. 1994;91:10938–10942. doi: 10.1073/pnas.91.23.10938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zachowski A, Henry JP, Devaux PF. Control of transmembrane lipid asymmetry in chromaffin granules by an ATP-dependent protein. Nature. 1989;340:75–76. doi: 10.1038/340075a0. [DOI] [PubMed] [Google Scholar]
- 16.Ding J, et al. Identification and functional expression of four isoforms of ATPase II, the putative aminophospholipid translocase. Effect of isoform variation on the ATPase activity and phospholipid specificity. J Biol Chem. 2000;275:23378–23386. doi: 10.1074/jbc.M910319199. [DOI] [PubMed] [Google Scholar]
- 17.Paterson JK, et al. Lipid specific activation of the murine P4-ATPase Atp8a1 (ATPase II) Biochemistry. 2006;45:5367–5376. doi: 10.1021/bi052359b. [DOI] [PubMed] [Google Scholar]
- 18.Tang X, Halleck MS, Schlegel RA, Williamson P. A subfamily of P-type ATPases with aminophospholipid transporting activity. Science. 1996;272:1495–1497. doi: 10.1126/science.272.5267.1495. [DOI] [PubMed] [Google Scholar]
- 19.Axelsen KB, Palmgren MG. Evolution of substrate specificities in the P-type ATPase superfamily. J Mol Evol. 1998;46:84–101. doi: 10.1007/pl00006286. [DOI] [PubMed] [Google Scholar]
- 20.Pomorski T, et al. Drs2p-related P-type ATPases Dnf1p and Dnf2p are required for phospholipid translocation across the yeast plasma membrane and serve a role in endocytosis. Mol Biol Cell. 2003;14:1240–1254. doi: 10.1091/mbc.E02-08-0501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Alder-Baerens N, Lisman Q, Luong L, Pomorski T, Holthuis JC. Loss of P4 ATPases Drs2p and Dnf3p disrupts aminophospholipid transport and asymmetry in yeast post-Golgi secretory vesicles. Mol Biol Cell. 2006;17:1632–1642. doi: 10.1091/mbc.E05-10-0912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Natarajan P, Wang J, Hua Z, Graham TR. Drs2p-coupled aminophospholipid translocase activity in yeast Golgi membranes and relationship to in vivo function. Proc Natl Acad Sci USA. 2004;101:10614–10619. doi: 10.1073/pnas.0404146101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen CY, Ingram MF, Rosal PH, Graham TR. Role for Drs2p, a P-type ATPase and potential aminophospholipid translocase, in yeast late Golgi function. J Cell Biol. 1999;147:1223–1236. doi: 10.1083/jcb.147.6.1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Saito K, et al. Cdc50p, a protein required for polarized growth, associates with the Drs2p P-type ATPase implicated in phospholipid translocation in Saccharomyces cerevisiae. Mol Biol Cell. 2004;15:3418–3432. doi: 10.1091/mbc.E03-11-0829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Janke C, et al. A versatile toolbox for PCR-based tagging of yeast genes: New fluorescent proteins, more markers and promoter substitution cassettes. Yeast. 2004;21:947–962. doi: 10.1002/yea.1142. [DOI] [PubMed] [Google Scholar]
- 26.Rigaut G, et al. A generic protein purification method for protein complex characterization and proteome exploration. Nat Biotechnol. 1999;17:1030–1032. doi: 10.1038/13732. [DOI] [PubMed] [Google Scholar]
- 27.Gause EM, Buck MA, Douglas MG. Binding of citreoviridin to the beta subunit of the yeast F1-ATPase. J Biol Chem. 1981;256:557–559. [PubMed] [Google Scholar]
- 28.Gould KL, Ren L, Feoktistova AS, Jennings JL, Link AJ. Tandem affinity purification and identification of protein complex components. Methods. 2004;33:239–244. doi: 10.1016/j.ymeth.2003.11.019. [DOI] [PubMed] [Google Scholar]
- 29.Lenoir G, Williamson P, Puts CF, Holthuis JC. Cdc50p plays a vital role in the ATPase reaction cycle of the putative aminophospholipid transporter Drs2p. J Biol Chem. 2009;284:17956–17967. doi: 10.1074/jbc.M109.013722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gummadi SN, Hrafnsdottir S, Walent J, Watkins WE, Menon AK. Reconstitution and assay of biogenic membrane-derived phospholipid flippase activity in proteoliposomes. Methods in Molecular Biology. 2003;228:271–279. doi: 10.1385/1-59259-400-X:271. [DOI] [PubMed] [Google Scholar]
- 31.McIntyre JC, Sleight RG. Fluorescence assay for phospholipid membrane asymmetry. Biochemistry. 1991;30:11819–11827. doi: 10.1021/bi00115a012. [DOI] [PubMed] [Google Scholar]
- 32.Toyoshima C, Inesi G. Structural basis of ion pumping by Ca2+-ATPase of the sarcoplasmic reticulum. Annu Rev Biochem. 2004;73:269–292. doi: 10.1146/annurev.biochem.73.011303.073700. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.