Abstract
Sexually dimorphic brain nuclei underlie gender-specific neural functions and susceptibility to disease, but the developmental basis of dimorphisms is poorly understood. In these studies, we focused on the anteroventral periventricular nucleus (AVPV), a nucleus that is larger in females and critical for the female-typical cyclic surge pattern of luteinizing hormone (LH) release. Sex differences in the size and function of the AVPV result from apoptosis that occurs preferentially in the developing male. To identify upstream pathways responsible for sexual differentiation of the AVPV, we used targeted apoptosis microarrays and in vivo and in vitro follow-up studies. We found that the tumor necrosis factor α (TNFα)-TNF receptor 2 (TNFR2)−NFκB cell survival pathway is active in postnatal day 2 (PND2) female AVPV and repressed in male counterparts. Genes encoding key members of this pathway were expressed exclusively in GABAergic neurons. One gene in particular, TNF receptor-associated factor 2 (TRAF2)-inhibiting protein (trip), was higher in males and it inhibited both TNFα-dependent NFκB activation and bcl-2 gene expression. The male AVPV also had higher levels of bax and bad mRNA, but neither of these genes was regulated by either TNFα or TRIP. Finally, the trip gene was not expressed in the sexually dimorphic nucleus of the preoptic area (SDN-POA), a nucleus in which apoptosis is higher in females than males. These findings form the basis of a new model of sexual differentiation of the AVPV that may also apply to the development of other sexually dimorphic nuclei.
Keywords: NFkB, TNFR2, TNFα, sexual dimorphism, apoptosis
Sex differences in the morphology of brain nuclei were first observed over 30 years ago (1), but the physiological significance of these differences is known in only a few cases. The neural control of gonadotropin release in rodents is perhaps the best studied example of sex-specific physiology linked to neural dimorphisms. Females have a cyclic pattern that culminates in the preovulatory surge of luteinizing hormone (LH) release on one day of the cycle (2). This female-typical pattern of LH release is controlled by the anteroventral periventricular nucleus (AVPV) (3–6), one of the few sexually dimorphic nuclei that is larger in females. Clearly, sexual dimorphisms of the nucleus are linked to function, because developmental manipulations that alter the size of the AVPV region (7) result in inappropriate gonadotropin release patterns and infertility in adulthood (8).
Sexual differentiation of the AVPV, like that of other dimorphic nuclei, is thought to be through apoptosis (9). Consistent with this idea, cells in the developing male AVPV express higher levels of the proapoptotic gene, bax, while expression of the prosurvival gene, bcl2, is more abundant in the developing female AVPV (10). The Bcl2/Bax ratio determines whether or not apoptosis will be triggered in the AVPV, because genetic manipulations of either Bcl2 or Bax expression alter the volume of the nucleus (11, 12). Unfortunately, we still have little information on the sex-specific upstream pathway(s) that regulate either Bcl2 or Bax expression to produce sexual dimorphism of the AVPV.
One obstacle to elucidating signaling pathways responsible for apoptosis in the developing AVPV has been identifying the phenotype of steroid-sensitive neuronal targets. We recently showed that most of the AVPV neurons of the female are unique dual-phenotype GABAergic/glutamatergic (Glu) neurons that communicate estradiol (E2) signals directly to gonadotropin-releasing hormone neurons (13). These GABAergic neurons contain virtually all estrogen receptor α (ERα) and estrogen receptor β (ERβ) expression found in the AVPV region and females have twice as many of the neurons as males (13). The AVPV also has sexually dimorphic populations of neurotensin-, galanin-, substance P-, and kisspeptin-containing neurons that are necessary for maximal LH surge release and contain estrogen receptors (14, 15). Significantly, we found that expression of genes encoding these neuropeptides in the AVPV region is confined to GABAergic neurons*. Females also have more dopaminergic (DA) neurons, but these cells comprise a much smaller portion of the AVPV than GABA/Glu neurons (16) and few, if any, contain ER (17). Moreover, the number of DA neurons does not change in either Bax knockout or Bcl2 overexpressing mice, even though sex differences in the volume of the AVPV are absent in these animals (11, 12). Thus, GABA/Glu/peptidergic neurons are likely responsible for the sexual dimorphism in AVPV volume.
Taking advantage of this information, we used targeted expression microarrays to identify pathways important for sex-specific apoptosis in AVPV GABAergic neurons of developing rat brains. Genes within tumor necrosis factor α (TNFα) signaling pathways were the most highly represented in the set of sex-specific genes identified. Of these, tumor necrosis factor receptor associated factor 2-inhibiting protein (TRIP) expression showed the greatest sex differences with significantly higher expression in males. Results of in vivo and in vitro studies reported herein strongly suggest that TRIP downregulates the TNFα-TNF receptor 2 (TNFR2)–NFκB pathway, thereby decreasing bcl2 gene expression and cell survival in the male AVPV. Interestingly, males had higher levels of bad and bax gene expression that could not be explained by TNFα or TRIP signaling. These findings suggest that both TNFα-dependent and -independent pathways contribute to sexual differentiation of the AVPV.
Results
Genes Related to TNFα Signaling Differ Between Sexes in Developing AVPV.
Fourteen of 23 genes identified as sex specific on the apoptosis GEArray DNA microarrays (SABiosciences) are involved in TNFα signaling pathways or in the apoptosis pathway downstream of TNFα signaling (see SI). Expression of trip was dramatically higher in males. TRIP binds TNF receptor-associated factor 2 (TRAF2, a TNFR2 adaptor protein), thereby preventing TNFα activation of NFκB (18). Thus, TRIP and other members of the canonical NFκB pathway became the focus of our follow-up studies.
Using QPCR and Western blots, we verified that the PND2 male AVPV had significantly higher TRIP mRNA and protein levels than that of the female (Fig. 1). We also found that males had lower levels of immunoreactive NFκB p65 subunit-nuclear localization signal (Fig. 2), a finding consistent with the ability of TRIP to inhibit NFκB nuclear translocation (18). We verified that Bcl2 mRNA levels were lower in males (Fig. 3A), while levels of Bax and Bad (Fig. 3 B and C) were higher in males.
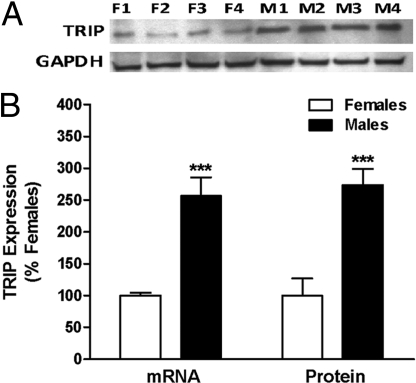
Fig. 1.
Sex differences in TRIP mRNA and protein levels in AVPV on PND2. QPCR was used to measure TRIP mRNA and Western blot analysis to measure TRIP. (A) Photomicrograph of Western blot results using 4 pools each of female (F1–F4) and male (M1–M4) AVPV with GAPDH as loading control. (B) Graphical depiction of Western and QPCR analyses of TRIP mRNA and protein. Each bar represents mean ± SEM of 4 separate samples, each of which contained AVPV tissue pooled from 4 individual animals that came from at least 3 different litters.***, P < 0.005, significantly different from female.
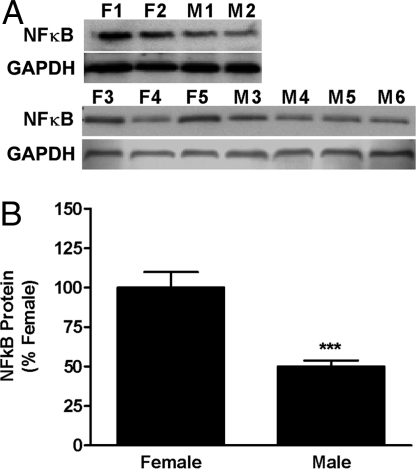
Fig. 2.
Sex differences in nuclear NFκB in AVPV on PND2. Nuclear NFκB-p65 subunit-containing heterodimers were measured in microdissections of AVPV from male and female rat pups collected on PND2. (A) Photomicrographs showing results of Westerns that were measured using densitometry. (B) Graph of results of Western blot analysis. Each bar represents mean ± SEM of 5-6 separate samples, each of which contained AVPV tissue pooled from 4 individual animals that came from at least 3 different litters. Levels were measured using Western blot analysis and GAPDH was used as an internal control. ***, P < 0.0001, significantly different from female.
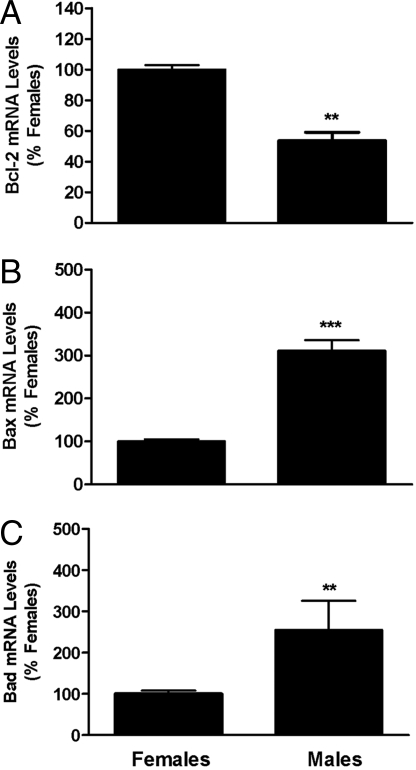
Fig. 3.
Sex differences in mRNAs encoding members of the Bcl2 family. Bcl2 (A), Bad (B), and Bax (C) mRNA levels were measured in microdissections of AVPV from male and female rat pups collected on PND2. Each bar represents mean ± SEM of 4 samples and each sample contained tissue from 4 different animals representing at least 3 different litters. Levels were measured using QPCR. GAPDH was used as an internal control. **, P < 0.01; ***, P < 0.0001, significantly different from female.
Levels of TNFα, TNFR2, and TRAF2 Gene Expression in the AVPV Did Not Differ Between Sexes.
Neither TNFα nor TNFR2 genes were identified in the microarray as sex specific, but because they are critical in the pathway upstream of TRIP, we compared expression of these genes, and the gene encoding TRAF2, in PND2 male and female AVPV. We found no sex differences in expression of any of these genes. The values were as follows (expressed as % female control values): TNFα mRNA level was 100.0 ± 21.5% in females (n = 4 pools) and 81.66 ± 13.3% in males (n = 4 pools); TNFR2 mRNA level was 100.0 ± 17.1% in females (n = 6 pools) and 114.51 ± 18.5% in males (n = 9 pools); and TRAF2 mRNA level was 100 ± 10.9% in females (n = 8 pools) and 106.9 ± 17.2% in males (n = 9 pools).
TNFR2, TRAF2, and TRIP mRNA Are Found in GABA Neurons of the AVPV.
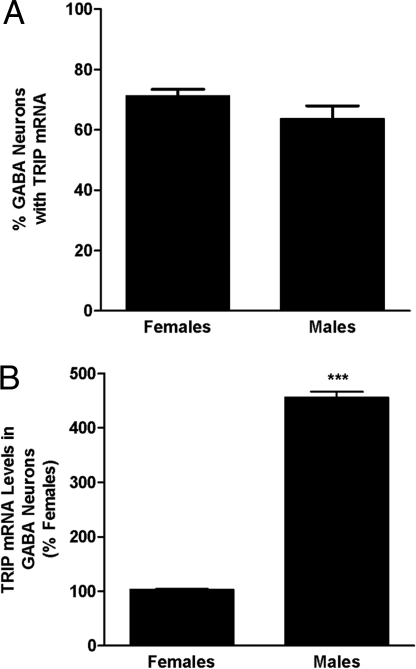
Consistent with a role of the TNFα–NFκB pathway in AVPV development, we found nearly identical distribution of mRNAs encoding TRIP, TNFR2, and TRAF2 in the PND2 preoptic area (Fig. 4A). Expression was particularly strong in the AVPV region. Results of the dual-label in situ hybridization studies showed that virtually all tnfr2, traf2, and trip gene expression in the AVPV of PND2 rats was confined to GABAergic neurons (Fig. 4B). Importantly, TRIP was absent in the SDN-POA (Fig. 4C), a GABAergic nucleus that is larger in males than in females because cell death is higher in the latter (19). Regardless of sex, ≈70% of all AVPV GABA neurons contained TRIP mRNA in PND2 animals (Fig. 5A), but the mean cellular levels of TRIP mRNA were significantly higher in GABAergic neurons of males than females (Fig. 5B).
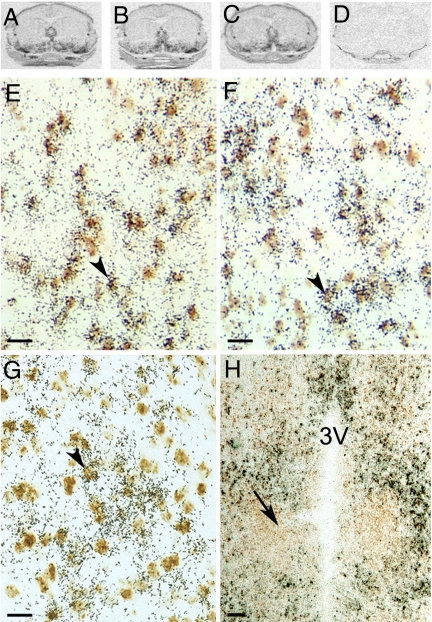
Fig. 4.
Autoradiograms of ISH for TNFR2 (A), TRAF2 (B), and TRIP (C) mRNA hybridized to 35S-labeled cRNA probes in brain POA sections that contain the AVPV of PND2 male rats. (D) Example of signal produced by sense strand control probes (TRIP). Photomicrographs showing results of dual-label ISH studies colocalizing digoxigenin-labeled cRNA probes to GAD mRNA (marker of GABA neurons; brown stain) and 35S-labeled cRNA probes to TNFR2 (E), TRAF2 (F), or TRIP (G) mRNA in POA sections of PND2 males. Arrowheads denote double-labeled cells. (Scale bar, 20 μm.) (H) Note the total absence of signal in the more caudal SDN-POA (denoted by arrow). (Scale bar, 100 μm.)
Fig. 5.
Sex differences in percentage of AVPV GABAergic neurons that contained TRIP mRNA (A) and relative levels of TRIP mRNA in AVPV GABAergic neurons (B). Each bar represents mean ± SEM of values from female (n = 4–5) and male (n = 4–5) rat pups examined on PND2. Data were obtained using computer-assisted image analysis of dual-label ISH studies. We used 35S-labeled cRNA probes for TRIP mRNA and digoxigenin-labeled cRNA probes for GAD mRNA. ***, P < 0.0001, significantly different from female.
TRIP Blocked TNFα-Induced NFκB Activation and Bcl-2 Gene Expression Without Altering Bad or Bax Expression.
Our in vivo findings suggested that TRIP interfered with a constitutively active NFκB cell survival pathway in GABAergic neurons. We tested this idea in vitro using N42 cells, a GABA/Glu hypothalamic cell line (verified by Cellutions Biosystems) that we found to express trip, traf2, and tnfr2 genes.
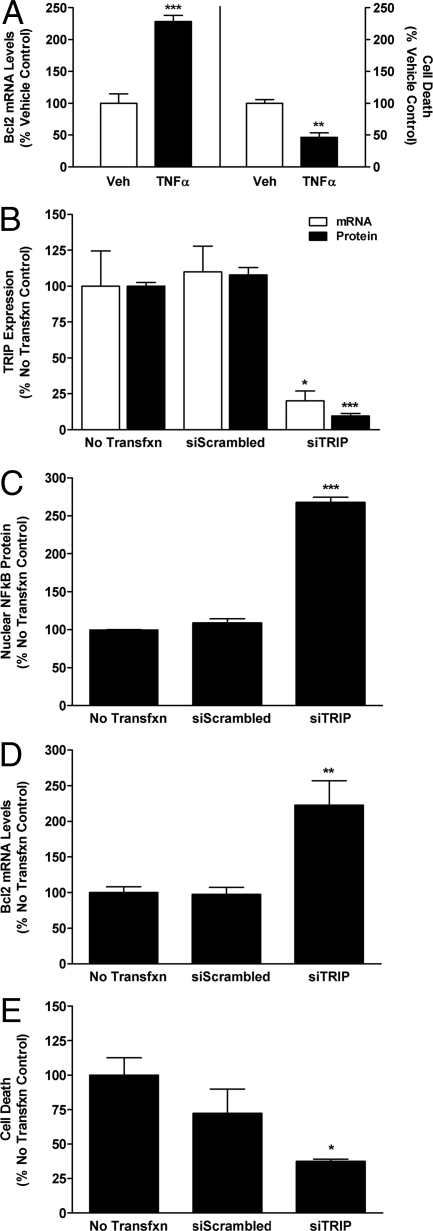
As predicted by our in vivo findings, treatment of N42 cells with TNFα significantly decreased cell death and increased Bcl-2 (Fig. 6A). However, neither Bax nor Bad mRNA levels were affected by TNFα administration (Fig. S1). Silencing of TRIP expression (Fig. 6B) increased TNFα-mediated activation of NFκB (Fig. 6C), Bcl-2 expression (Fig. 6D), and significantly decreased cell death in N42 cells (Fig. 6E). Knockdown of TRIP affected neither Bax nor Bad mRNA levels after TNFα administration (Fig. S2).
Fig. 6.
Results of in vitro studies on the effects of TNFα and TRIP in N42 hypothalamic cells. (A) Effects of TNFα or vehicle (Veh) on Bcl2 mRNA levels measured with QPCR and on cell death measured using CytoTox-Fluor cytotoxicity assay. Each bar represents mean ± SEM of 6 samples assayed in duplicate. **, P < 0.005, significantly different from Vehicle controls; ***, P < 0.001. (B) Verification of siRNA knockdown of TRIP mRNA and protein levels. Each bar represents mean ± SEM of 4 samples. (C–E) Effects of TRIP silencing on TNFα-induced NFκB-p65 subunit levels analyzed using Western blots (C), Bcl2 mRNA levels measured with QPCR (D), and cell death (E). In these studies, cells were treated with 100 U of TNFα and comparisons were made among cells without transfection (No Transfxn) or transfection for 24 h with vectors containing AllStars Negative Control siRNA (siScrambled) or vectors containing a combination of 2 siTRIP constructs, S101454649 and S101454635. (C–E) Each bar represents mean ± SEM of at least 4 samples assayed in duplicate. *, P < 0.05, significantly different from No Transfxn and siScrambled controls; **, P < 0.001; ***, P < 0.0001.
Discussion
Our findings suggest that trip, a novel gene expressed preferentially in the male AVPV, inhibits a constitutively active TNFα–TNFR2–NFκB–Bcl2 pathway thereby triggering cell death. Importantly, TNFR2, TRAF2, and TRIP were all present in GABA/Glu neurons that make up most of the sexually dimorphic AVPV. In contrast, TRIP was absent in GABAergic neurons of the SDN-POA, a very exciting finding because, unlike the AVPV, cell death in SDN-POA is higher in females (20). Interestingly, the male AVPV also had higher levels of mRNA encoding proapoptotic Bax and Bad, but upregulation of these genes appeared to be independent of TNFα and TRIP signaling. Thus, at least 2 separate pathways may play a role in sexual differentiation of the AVPV.
Our finding that most of the differentially expressed genes in the microarray studies involved TNFα signaling was somewhat surprising. TNFα is generally thought of as a proinflammatory cytokine (21, 22) and a modulator of cell survival (23–25), but it has never been linked to sex-specific apoptosis in the nervous system. The ability of TNFα to promote cell survival is predominantly through the TNFR2 receptor subtype (26). Upon ligand binding, the TRAF2 adaptor associates with the receptor and this activated complex results in degradation of IκBα enabling NFκB dimers (p50–p65) to translocate to the nucleus (27–30). TRIP binding to the TRAF2–TNFR2 complex blocks this cascade and prevents NFκB nuclear translocation (18).
We found that expression of TNFR2, TRAF2, and TRIP overlapped completely in the AVPV in a strikingly restricted pattern. Each was found predominantly in the AVPV, but also in the POA, and periventricular POA. These nuclei support the female-specific pattern of gonadotropin release and together comprise a functional unit called the rostral periventricular area of the third ventricle (RP3V) (15). Our anatomical findings reinforce the idea that TNFR2, TRAF2, and TRIP genes are important for the development of female reproductive competence.
We found no sex differences in TNFα or TNFR2 mRNA levels in the AVPV, but males had higher levels of TRIP mRNA and protein. Importantly, our in vitro work showed that TRIP inhibited NFκB activation. This is a novel finding in neural cells, but in nonneural cell lines TRIP inhibits NFκB activation by enhancing cytoplasmic sequestration of NFκB dimers (18). Support for the idea that TRIP exerts a similar effect in vivo comes from our work showing that TRIP was higher and NFκB lower in the male AVPV than in that of the female. Previous work also shows that levels of active caspase 3 (10) and apoptotic cells (31) are higher in the developing male AVPV. It is notable that we found no TRIP mRNA in the SDN-POA of males, a nucleus in which NFκB activation is higher and cell death lower in males than females (20). On the basis of this evidence, we propose that TRIP triggers male-specific cell death by inhibiting a TNFα–TNFR2–NFκB pathway.
TRIP has never before been identified in the brain, nor has it been linked to any neurodevelopmental process. In fact, this little-known protein has been studied only in NIH 3T3 fibroblast and human embryonic kidney 293 (HEK293) cells (18, 32). In line with our finding that TRIP knockdown increased survival of TNFα-treated hypothalamic N42 cells, previous work showed that TRIP overexpression decreases survival of HEK293 cells (18). In contrast, TRIP knockdown in NIH 3T3 cells enhances apoptosis (32). This discrepancy may be explained by the fact that TRIP inhibits NFκB activation in all 3 of these cell lines, but NFκB activation generally inhibits apoptosis in neural cells (25, 26, 33, 34) while inducing it in nonneural cells (35–38).
Our finding that Bcl2 mRNA levels are higher in the developing female brain than that of the male is consistent with previous work suggesting that NFκB directly regulates Bcl2 gene expression (39). It is also consistent with work of others who found higher levels of Bcl2 protein in the AVPV of developing females (10). Our in vitro data show that TNFα increases both NFκB p65 subunits and Bcl2 gene expression in N42 cells and that TRIP inhibits both. The role of Bcl-2 in sexual differentiation of the AVPV was studied previously in transgenic mice overexpressing Bcl2 (12). In these animals, the number of total neurons was increased in the male AVPV, but the sexually dimorphic population of DA neurons in this region was unaffected (12). Our data showing that all TRIP mRNA is in GABAergic neurons that make up the bulk of the AVPV may explain these findings.
In contrast to bcl2 gene expression, we found that both bax and bad expression levels were higher in PND2 males than in females. Previous work similarly demonstrated that Bax protein levels are higher in male AVPV, but that work found no differences in Bad protein levels (10). An important finding in our studies was that, unlike bcl2, neither bax nor bad gene expression was regulated by TNFα or TRIP. This suggests that masculinization of the AVPV GABA/Glu neurons involves both TNFα/TRIP-dependent downregulation of Bcl2 and TNFα/TRIP-independent upregulation of Bax and Bad. There are no studies on the role of Bad in masculinization of the AVPV and the only evidence that Bax plays a role comes from work in Bax knockout mice (11). In these animals, the number of non-DA neurons (presumably GABA/Glu neurons) increases in both males and females compared with wild-type animals. However, elimination of Bax necessarily increases the Bcl2/Bax ratio such that apoptosis is likely inhibited (40). Therefore, studies of the Bax knockout mice do not address the question of whether the TRIP-dependent and -independent pathways are cooperative. To make this determination, we need to identify the upstream signaling pathway(s) for Bax gene induction. We are currently investigating the possibility that p53 is one such signal, considering that it is a regulator of Bax gene expression (41) and found to be significantly higher in the developing male than in the female AVPV on our microarray.
In summary, our findings indicate that the TNFα–TRIP–NFκB pathway plays an important and previously unsuspected role in determining the sex-specific structure and function of the AVPV. On the basis of our findings, we propose a new model for sexual differentiation of the AVPV wherein an active cell survival pathway important for female-typical development is suppressed by increased TRIP expression in the male. The model is as follows: (i) Feminization of the AVPV depends on a constitutively active TNFα–TNFR2–NFκB–Bcl2 pathway that maintains cell survival of GABAergic neurons. (ii) Elevated TRIP expression in GABA neurons of the male AVPV inhibits NFκB activation, thereby decreasing Bcl2 gene expression. (iii) TNFα- and TRIP-independent pathways upregulate Bax and Bad to further decrease the prosurvival:proapoptosis ratio in the male AVPV. (iv) As a result, the death rate of GABA neurons is higher in the male than in the female AVPV, thus establishing sexual dimorphism and sex-specific gonadotropin release patterns. This testable model provides the opportunity to open a new frontier in the field of sexual differentiation of the brain. Studies testing this model are important because such diverse stimuli as metabolic disturbances (42), alcohol (43), hypoxia (44), pyrogenic agents (45), glucocorticoids (46), and antiinflammatory drugs (47) affect NFκB activity and may, therefore, alter sexual differentiation processes and adversely affect adult fertility.
Materials and Methods
Animals.
All studies used brains from PND2 Sprague–Dawley rat pups (Harlan) stored at −80 °C. Animal care and housing conditions were as described previously (48).
Microarray Studies.
We used rat apoptosis GEArray DNA microarrays (SABiosciences) to compare gene expression in pools of AVPV tissue microdissected from male and female pups. Microdissections from 500-μm cryosections through the extent of the AVPV were pooled from 4 individuals (from 3 different litters) for each sample and we used 4 samples of each sex. RNA from samples was isolated with the RNeasy Lipid Tissue kit (Qiagen) and then used to prepare biotinylated cRNA probes using apoptosis GEArray kit reagents. After prehybridization, we mixed 4 μg of biotinylated cRNA probe in hybridization buffer and incubated microarrays overnight at 60 °C.
After posthybridization washes, microarrays were processed for chemiluminescent detection as directed and apposed to Kodak BioMax X-ray film for 2–3 min. Resulting autoradiograms were uploaded to the SABiosciences Website for data acquisition and statistical analysis (GEArray expression analysis suite) using parameters described in SI Text. We identified as positive only genes that differed significantly (P < 0.05, unpaired t-tests) between sexes and differed in all 4 microarray sets. All genes that differed significantly showed greater than 2-fold differences between sexes.
Validation of Microarray Findings Using QPCR and Western Blots.
After reverse transcription (MMLV reverse transcriptase kit; Promega), we performed QPCR using prevalidated primer sets (SABiosciences) for mRNAs encoding rTRIP (PPR44232A), rBcl2 (PPR06577A), rBax (PPR06496B), rBad (PPR06535A), rTNFα (PPR06411E), and rTRAF2 (PPR55844A). We verified that rGAPDH (PPR06557A) did not differ between sexes and used it as an internal control for normalization. Details of QPCR are described in SI Text. The ΔΔCt method was used to analyze the data (49).
For Western blots to assess sex differences in TRIP, Bcl-2, Bax, Bad, and activated NFκB, we used AVPV tissue from 4 pools from each sex prepared as described above. Western blots (detailed in SI Text) were performed using the following antibodies: Rabbit anti-TRIP polyclonal (1:200, CeMines), rabbit anti-NFκB p65.
ISH Studies.
We obtained 12-μm cryosections through the POA of PND2 rats for single- and dual-label ISH studies. Single-label ISH was performed as described previously (50) using 35S-labeled cRNA probes for mRNAs encoding TNFR2, TRAF2, or TRIP. We generated transcriptional templates for each target using standard PCR cloning methods (13) and primers described in SI. After posthybridization washes, sections were placed against Kodak BioMax X-ray film and developed after 7 days.
For dual-label studies performed as described previously (13), we combined 35S-labeled cRNA probes to TNFR2, TRAF2, or TRIP mRNA with digoxigenin-labeled cRNA probes to GAD mRNA. To detect GABAergic cells, we used a mixture of 3 cRNA probes to GAD1 and 2 mRNAs (48). We detected digoxigenin-labeled probes with immunocytochemical methods, dipped slides in NTB emulsion (Kodak), exposed for 3 weeks, and analyzed as described previously (13). We first counted neurons positive for TRIP and GAD mRNA and then determined the number of silver grains (signal for TRIP mRNA) overlying cells with signal for GAD probes.
In Vitro Studies on the Role of TRIP in Cell Survival of GABA/Glu Neurons.
N42 cells were grown under standard conditions in Dulbecco's modified eagle medium (DMEM; Cellgro) supplemented with 10% vol/vol FBS (FBS; HyClone) and 1% vol/vol penicillin-streptomycin-glutamine.
For studies on the effects of TNFα on the nuclear translocation of NFκB, we treated N42 cells with 100 U/mL TNFα or BSA for 30 min before isolating protein for Western blots. Cells were similarly treated to determine effects of TNFα on apoptosis, but included treatment with 1 mM H2O2 as a positive control and cells incubated in growth medium with no treatment as a negative control. Cell death was assayed after 12 h using CytoTox-Fluor cytotoxicity assay kits (Promega) and a FLUOstar OPTIMA microplate reader (BMG Labtechnologies).
To silence TRIP gene expression, we used 2 siRNA constructs simultaneously (S101454649 and S101454635; Qiagen). We used a similar strategy to knockdown GAPDH (S101009379 and S101009393), our internal control. We used AllStars negative control siRNA (102728, Qiagen) and no-transfection controls. After 24 and 27 h, we isolated mRNA and protein and determined percentage knockdown by QPCR and Western blots. We used prevalidated primers from SABiosciences for mTRIP (PPM03076E) and mGAPDH (PPM02946E) for QPCR studies.
To determine the effect of TRIP knockdown on levels of NFκB, we treated transfected cells with 100 U/mL TNFα for 30 min and isolated protein after 27 h. In a separate TRIP knockdown study, we treated cells with 100 U/mL TNFα for 30 min to activate the NFκB, then added fresh medium, incubated cells for 12 h, and isolated RNA. QPCR was used to measure Bcl-2, Bax, and Bad mRNA levels (with prevalidated primers from SABiosciences; mBcl-2, PPM02918E; mBax, PPM02917E; and mBad, PPM02916E). We ran a similar study with CytoTox-Fluor cytotoxicity assay kits (Promega) to examine effects of TRIP knockdown on cell death.
Statistical Analyses.
Sex differences in levels of various mRNAs measured by QPCR, and in protein levels, numbers of GABA cells positive for TRIP mRNA and cellular levels of TRIP in GABA neurons were detected using unpaired t-tests. To probe effects of TNFα on cell death, we used 1-way ANOVA and post hoc Bonferroni's t-tests.
Supplementary Material
Acknowledgments.
This research was supported by National Institutes of Health grants HD027305 and ES013885 (to S.L.P.). K.A.I. and L.K.A. were funded by the National Science Foundation through Northeast Alliance for Graduate Education and the Professoriate fellowships.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Krishnan S, Rauhut M, Hrabovszky E, Carpenter CD, Petersen SL, GABA/glutamate neurons of the anteroventral periventricular nucleus are also peptidergic and contain opiod and noradrenergic receptors, in Thirty-Fifth Annual Meeting of the Society for Neuroscience, November 2005, Washington, DC, abstr 242.2.
This article contains supporting information online at www.pnas.org/cgi/content/full/0906293106/DCSupplemental.
References
- 1.Gorski RA, Gordon JH, Shryne JE, Southam AM. Evidence for a morphological sex difference within the medial preoptic area of the rat brain. Brain Res. 1978;148:333–346. doi: 10.1016/0006-8993(78)90723-0. [DOI] [PubMed] [Google Scholar]
- 2.Pfeiffer CA. Sexual differences of the hypophyses and their determination by the gonads. Amer J Anat. 1936;58:195–225. [Google Scholar]
- 3.Petersen SL, Barraclough CA. Suppression of spontaneous LH surges in estrogen-treated ovariectomized rats by microimplants of antiestrogens into the preoptic brain. Brain Res. 1989;484:279–289. doi: 10.1016/0006-8993(89)90371-5. [DOI] [PubMed] [Google Scholar]
- 4.Ronnekleiv OK, Kelly MJ. Luteinizing hormone-releasing hormone neuronal system during the estrous cycle of the female rat: Effects of surgically induced persistent estrus. Neuroendocrinology. 1986;43:564–576. doi: 10.1159/000124583. [DOI] [PubMed] [Google Scholar]
- 5.Wiegand SJ, Terasawa E, Bridson WE, Goy RW. Effects of discrete lesions of preoptic and suprachiasmatic structures in the female rat. Alterations in the feedback regulation of gonadotropin secretion. Neuroendocrinology. 1980;31:147–157. doi: 10.1159/000123066. [DOI] [PubMed] [Google Scholar]
- 6.Wiegand SJ, Terasawa E. Discrete lesions reveal functional heterogeneity of suprachiasmatic structures in regulation of gonadotropin secretion in the female rat. Neuroendocrinology. 1982;34:395–404. doi: 10.1159/000123335. [DOI] [PubMed] [Google Scholar]
- 7.Gorski RA. Critical role for the medial preoptic area in the sexual differentiation of the brain. Prog Brain Res. 1984;61:129–146. doi: 10.1016/S0079-6123(08)64432-5. [DOI] [PubMed] [Google Scholar]
- 8.Barraclough CA. Modifications in the CNS regulation of reproduction after exposure of prepubertal rats to steroid hormones. Recent Prog Horm Res. 1966;22:503–539. doi: 10.1016/b978-1-4831-9825-5.50016-6. [DOI] [PubMed] [Google Scholar]
- 9.Arai Y, Sekine Y, Murakami S. Estrogen and apoptosis in the developing sexually dimorphic preoptic area in female rats. Neurosci Res. 1996;25:403–407. doi: 10.1016/0168-0102(96)01070-x. [DOI] [PubMed] [Google Scholar]
- 10.Tsukahara S, Kakeyama M, Toyofuku Y. Sex differences in the level of Bcl-2 family proteins and caspase-3 activation in the sexually dimorphic nuclei of the preoptic area in postnatal rats. J Neurobiol. 2006;66:1411–1419. doi: 10.1002/neu.20276. [DOI] [PubMed] [Google Scholar]
- 11.Forger NG, et al. Deletion of Bax eliminates sex differences in the mouse forebrain. Proc Natl Acad Sci USA. 2004;101:13666–13671. doi: 10.1073/pnas.0404644101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zup SL, et al. Overexpression of bcl-2 reduces sex differences in neuron number in the brain and spinal cord. J Neurosci. 2003;23:2357–2362. doi: 10.1523/JNEUROSCI.23-06-02357.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ottem EN, Godwin JG, Krishnan S, Petersen SL. Dual-phenotype GABA/glutamate neurons in adult preoptic area: Sexual dimorphism and function. J Neurosci. 2004;24:8097–8105. doi: 10.1523/JNEUROSCI.2267-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Herbison AE. Multimodal influence of estrogen upon gonadotropin-releasing hormone neurons. Endocr Rev. 1998;19:302–330. doi: 10.1210/edrv.19.3.0332. [DOI] [PubMed] [Google Scholar]
- 15.Herbison AE. Estrogen positive feedback to gonadotropin-releasing hormone (GnRH) neurons in the rodent: The case for the rostral periventricular area of the third ventricle (RP3V) Brain Res Rev. 2008;57:277–287. doi: 10.1016/j.brainresrev.2007.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Simerly RB, Swanson LW, Handa RJ, Gorski RA. Influence of perinatal androgen on the sexually dimorphic distribution of tyrosine hydroxylase-immunoreactive cells and fibers in the anteroventral periventricular nucleus of the rat. Neuroendocrinology. 1985;40:501–510. doi: 10.1159/000124122. [DOI] [PubMed] [Google Scholar]
- 17.Herbison AE, Theodosis DT. Localization of oestrogen receptors in preoptic neurons containing neurotensin but not tyrosine hydroxylase, cholecystokinin or luteinizing hormone-releasing hormone in the male and female rat. Neuroscience. 1992;50:283–298. doi: 10.1016/0306-4522(92)90423-y. [DOI] [PubMed] [Google Scholar]
- 18.Lee SY, Lee SY, Choi Y. TRAF-interacting protein (TRIP): A novel component of the tumor necrosis factor receptor (TNFR)- and CD30-TRAF signaling complexes that inhibits TRAF2-mediated NF-kappaB activation. J Exp Med. 1997;185:1275–1285. doi: 10.1084/jem.185.7.1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tsukahara S. Sex differences and the roles of sex steroids in apoptosis of sexually dimorphic nuclei of the preoptic area in postnatal rats. J Neuroendocrinol. 2009;21:370–376. doi: 10.1111/j.1365-2826.2009.01855.x. [DOI] [PubMed] [Google Scholar]
- 20.Davis EC, Popper P, Gorski RA. The role of apoptosis in sexual differentiation of the rat sexually dimorphic nucleus of the preoptic area. Brain Res. 1996;734:10–18. [PubMed] [Google Scholar]
- 21.Fiers W. Tumor necrosis factor. Characterization at the molecular, cellular and in vivo level. FEBS Lett. 1991;285:199–212. doi: 10.1016/0014-5793(91)80803-b. [DOI] [PubMed] [Google Scholar]
- 22.Carswell EA, et al. An endotoxin-induced serum factor that causes necrosis of tumors. Proc Natl Acad Sci USA. 1975;72:3666–3670. doi: 10.1073/pnas.72.9.3666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Barger SW, et al. Tumor necrosis factors alpha and beta protect neurons against amyloid beta-peptide toxicity: evidence for involvement of a kappa B-binding factor and attenuation of peroxide and Ca2+ accumulation. Proc Natl Acad Sci USA. 1995;92:9328–9332. doi: 10.1073/pnas.92.20.9328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Houzen H, Kikuchi S, Kanno M, Shinpo K, Tashiro K. Tumor necrosis factor enhancement of transient outward potassium currents in cultured rat cortical neurons. J Neurosci Res. 1997;50:990–999. doi: 10.1002/(SICI)1097-4547(19971215)50:6<990::AID-JNR9>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 25.Mattson MP, Goodman Y, Luo H, Fu W, Furukawa K. Activation of NF-kappaB protects hippocampal neurons against oxidative stress-induced apoptosis: Evidence for induction of manganese superoxide dismutase and suppression of peroxynitrite production and protein tyrosine nitration. J Neurosci Res. 1997;49:681–697. doi: 10.1002/(SICI)1097-4547(19970915)49:6<681::AID-JNR3>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 26.Marchetti L, Klein M, Schlett K, Pfizenmaier K, Eisel UL. Tumor necrosis factor (TNF)-mediated neuroprotection against glutamate-induced excitotoxicity is enhanced by N-methyl-D-aspartate receptor activation. Essential role of a TNF receptor 2-mediated phosphatidylinositol 3-kinase-dependent NF-kappa B pathway. J Biol Chem. 2004;279:32869–32881. doi: 10.1074/jbc.M311766200. [DOI] [PubMed] [Google Scholar]
- 27.Darnay BG, Haridas V, Ni J, Moore PA, Aggarwal BB. Characterization of the intracellular domain of receptor activator of NF-kappaB (RANK). Interaction with tumor necrosis factor receptor-associated factors and activation of NF-kappab and c-Jun N-terminal kinase. J Biol Chem. 1998;273:20551–20555. doi: 10.1074/jbc.273.32.20551. [DOI] [PubMed] [Google Scholar]
- 28.Pomerantz JL, Baltimore D. NF-kappaB activation by a signaling complex containing TRAF2, TANK and TBK1, a novel IKK-related kinase. EMBO J. 1999;18:6694–6704. doi: 10.1093/emboj/18.23.6694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rothe M, et al. I-TRAF is a novel TRAF-interacting protein that regulates TRAF-mediated signal transduction. Proc Natl Acad Sci USA. 1996;93:8241–8246. doi: 10.1073/pnas.93.16.8241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yang CH, Murti A, Pfeffer LM. Interferon induces NF-kappa B-inducing kinase/tumor necrosis factor receptor-associated factor-dependent NF-kappa B activation to promote cell survival. J Biol Chem. 2005;280:31530–31536. doi: 10.1074/jbc.M503120200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sumida H, Nishizuka M, Kano Y, Arai Y. Sex differences in the anteroventral periventricular nucleus of the preoptic area and in the related effects of androgen in prenatal rats. Neurosci Lett. 1993;151:41–44. doi: 10.1016/0304-3940(93)90040-r. [DOI] [PubMed] [Google Scholar]
- 32.Park ES, et al. Early embryonic lethality caused by targeted disruption of the TRAF-interacting protein (TRIP) gene. Biochem Biophys Res Commun. 2007;363:971–977. doi: 10.1016/j.bbrc.2007.09.103. [DOI] [PubMed] [Google Scholar]
- 33.Cheng B, Christakos S, Mattson MP. Tumor necrosis factors protect neurons against metabolic-excitotoxic insults and promote maintenance of calcium homeostasis. Neuron. 1994;12:139–153. doi: 10.1016/0896-6273(94)90159-7. [DOI] [PubMed] [Google Scholar]
- 34.Tamatani M, Ogawa S, Nunez G, Tohyama M. Growth factors prevent changes in Bcl-2 and Bax expression and neuronal apoptosis induced by nitric oxide. Cell Death Differ. 1998;5:911–919. doi: 10.1038/sj.cdd.4400439. [DOI] [PubMed] [Google Scholar]
- 35.Regamey A, et al. The tumor suppressor CYLD interacts with TRIP and regulates negatively nuclear factor kappaB activation by tumor necrosis factor. J Exp Med. 2003;198:1959–1964. doi: 10.1084/jem.20031187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chen J, et al. SIRT1 protects against microglia-dependent amyloid-beta toxicity through inhibiting NF-kappaB signaling. J Biol Chem. 2005;280:40364–40374. doi: 10.1074/jbc.M509329200. [DOI] [PubMed] [Google Scholar]
- 37.John GR, Lee SC, Brosnan CF. Cytokines: Powerful regulators of glial cell activation. Neuroscientist. 2003;9:10–22. doi: 10.1177/1073858402239587. [DOI] [PubMed] [Google Scholar]
- 38.Akama KT, Albanese C, Pestell RG, Van Eldik LJ. Amyloid beta-peptide stimulates nitric oxide production in astrocytes through an NFkappaB-dependent mechanism. Proc Natl Acad Sci USA. 1998;95:5795–5800. doi: 10.1073/pnas.95.10.5795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Turco MC, et al. NF-kappaB/Rel-mediated regulation of apoptosis in hematologic malignancies and normal hematopoietic progenitors. Leukemia. 2004;18:11–17. doi: 10.1038/sj.leu.2403171. [DOI] [PubMed] [Google Scholar]
- 40.Oltvai ZN, Milliman CL, Korsmeyer SJ. Bcl-2 heterodimerizes in vivo with a conserved homolog, Bax, that accelerates programmed cell death. Cell. 1993;74:609–619. doi: 10.1016/0092-8674(93)90509-o. [DOI] [PubMed] [Google Scholar]
- 41.Chipuk JE, et al. Direct activation of Bax by p53 mediates mitochondrial membrane permeabilization and apoptosis. Science. 2004;303:1010–1014. doi: 10.1126/science.1092734. [DOI] [PubMed] [Google Scholar]
- 42.Kuhad A, Bishnoi M, Tiwari V, Chopra K. Suppression of NF-kappabeta signaling pathway by tocotrienol can prevent diabetes associated cognitive deficits. Pharmacol Biochem Behav. 2009;92:251–259. doi: 10.1016/j.pbb.2008.12.012. [DOI] [PubMed] [Google Scholar]
- 43.Lee S, Rivier C. Role played by hypothalamic nuclear factor-{kappa}B in alcohol-mediated activation of the rat hypothalamic-pituitary-adrenal axis. Endocrinology. 2005;146:2006–2014. doi: 10.1210/en.2004-1268. [DOI] [PubMed] [Google Scholar]
- 44.Royds JA, Dower SK, Qwarnstrom EE, Lewis CE. Response of tumour cells to hypoxia: Role of p53 and NFkB. Mol Pathol. 1998;51:55–61. doi: 10.1136/mp.51.2.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Deshpande R, Khalili H, Pergolizzi RG, Michael SD, Chang MD. Estradiol down-regulates LPS-induced cytokine production and NFkB activation in murine macrophages. Am J Reprod Immunol. 1997;38:46–54. doi: 10.1111/j.1600-0897.1997.tb00275.x. [DOI] [PubMed] [Google Scholar]
- 46.Evans-Storms RB, Cidlowski JA. Delineation of an antiapoptotic action of glucocorticoids in hepatoma cells: The role of nuclear factor-kappaB. Endocrinology. 2000;141:1854–1862. doi: 10.1210/endo.141.5.7466. [DOI] [PubMed] [Google Scholar]
- 47.Buckland M, Lombardi G. Aspirin and the induction of tolerance by dendritic cells. Handb Exp Pharmacol. 2009;(188):197–213. doi: 10.1007/978-3-540-71029-5_9. [DOI] [PubMed] [Google Scholar]
- 48.Hays LE, Carpenter CD, Petersen SL. Evidence that GABAergic neurons in the preoptic area of the rat brain are targets of 2,3,7,8-tetrachlorodibenzo-p-dioxin during development. Environ Health Perspect 110 Suppl. 2002;3:369–376. doi: 10.1289/ehp.02110s3369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 50.Curran-Rauhut MA, Petersen SL. Regulation of glutamic acid decarboxylase 65 and 67 gene expression by ovarian steroids: Identification of 2 functionally distinct populations of GABA neurones in the preoptic area. J Neuroendocrinol. 2002;14:310–317. doi: 10.1046/j.1365-2826.2002.00780.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.