Abstract
T cell unresponsiveness or anergy is one of the mechanisms that maintain inactivity of self-reactive lymphocytes. E3 ubiquitin ligases are important mediators of the anergic state. The RING finger E3 ligase GRAIL is thought to selectively function in anergic T cells but its mechanism of action and its role in vivo are largely unknown. We show here that genetic deletion of Grail in mice leads not only to loss of an anergic phenotype in various models but also to hyperactivation of primary CD4+ T cells. Grail−/− CD4+ T cells hyperproliferate in vitro to TCR stimulation alone or with concomitant anti-CD28 costimulation, with transient increased survival. In vitro differentiated T helper 1 cells show slight but significant hypersecretion of IFN-γ in Grail−/− mice whereas Th2 and Th17 cytokine secretions are unchanged. Consistent with defective in vitro anergy, oral tolerance is abolished in vivo in OT-II TCR transgenic Grail−/− mice fed with ovalbumin. In experimental allergic encephalitis, a model of organ-specific autoimmunity, oral tolerization with myelin basic protein was abrogated as well in Grail−/− mice. On the protein level, Grail−/− naïve T cells show no significant differences of total and phosphorylated levels of ZAP70, phospholipase Cγ1, and MAP kinases p38 and JNK but elevated baseline levels of MAP kinase ERK1/2. In summary, we define a role for GRAIL in primary T cell activation, survival, and differentiation. In addition, we formally prove an indispensable role for GRAIL in T cell anergy and oral tolerance—a promising, antigen-specific strategy to treat autoimmune diseases.
Keywords: anergy, E3 ligase, ERK, PKC theta, MAP kinase
Central (thymic) and peripheral tolerance are essential mechanisms to prevent autoimmunity (1). Because some autoreactive T cell clones escape tolerization in the thymus, peripheral mechanisms have evolved to prevent lymphocyte mediated self-destruction. T cell unresponsiveness or anergy is thought to contribute to maintenance of peripheral tolerance (2). Means of inducing T cell anergy in vitro include delivery of a calcium signal via the ionophore ionomycin. By studying this model, Macian et al. have discovered that up-regulation of anergy-associated genes is largely NFAT dependent (3). The NFAT pathway has been shown to be crucial for anergy induction. NFAT1 activation in the absence of its transcriptional partner AP-1 induces T cell anergy (3). However, the exact molecular mediators of T cell anergy induction and maintenance remain incompletely understood (2, 4).
The functional significance of anergized T cells is also not yet completely elucidated. Functional unresponsiveness of potentially autoreactive T cells seems more dangerous to the host than deleting them; this suggests that anergic T cell clones have functional (possibly tolerogenic) properties that are yet to be clearly defined. In some models, anergic T cells do not merely remain passive but acquire suppressor function (2). However, this seems not to be the default pathway after anergy induction. High dose oral tolerance favors T cell anergy and deletion in vivo whereas low dose regimens induce transferable suppression via regulatory T cell (Treg) generation (5). Anergy and active suppression have also been shown to be separate processes in a model of transplant tolerance (6). Thus, the precise role of anergic T cells that lack suppressor function needs to be studied further to fully understand its biologic relevance. To this end, we generated a genetic disruption of a putative “anergy factor” in mice.
E3 ubiquitin ligases have recently been identified as important mediators of T cell tolerance (4, 7). The transmembrane E3 ligase GRAIL (Gene related to anergy in lymphocytes) has been reported to be highly up-regulated during anergy induction compared with resting/activated T cell clones (8), and was subsequently shown to be involved in anergy induction (9). Its unique location in the recycling endosomal compartment (8) and its reportedly selective expression pattern in T cells distinguish GRAIL from other RING finger E3 ligases (Cbl-b, TRAF6, Roquin) recently implicated in immune tolerance (10–14). GRAIL is broadly expressed in various organs at high levels but tightly regulated in T cells by ubiquitin editing enzymes (8, 15). In vitro targets of GRAIL are thought to be CD40 ligand (CD40L), Rho GDP-dissociation inhibitor and most recently also tetraspanins CD151 and CD81 (16–18). The functional relevance of these findings is largely unknown, particularly, the contributions of GRAIL to general T cell biology aside from its role in anergy. We show here that genetic disruption of Grail in mice led to a variety of abnormalities in naïve, helper, and anergic T cells.
Results
Grail−/− Mice Have Normal Thymic Development and Peripheral T Cell Numbers.
We genetically targeted the highly conserved RING finger domain of GRAIL in mice to study its in vivo function (Fig. 1 A–D, and Fig. S1). Grail−/− mice were viable and born with the expected Mendelian frequency. None of the major organs showed any gross abnormalities (Fig. S2) or histologic differences. Thymic development was unaffected with equal numbers of thymocyte subsets (Fig. S3). Absolute numbers of T and B cells, dendritic cells, macrophages, neutrophils, and other differentiated cell lineages were unremarkable. Ratios of CD4+ and CD8+ T cells from spleen and lymph node (LN) were also comparable at 8 weeks of age (Fig. S4), as were naïve versus memory CD4+ or CD8+ T cell ratios (Fig. S5); there was, however, a slight increase in memory markers in aged mice.
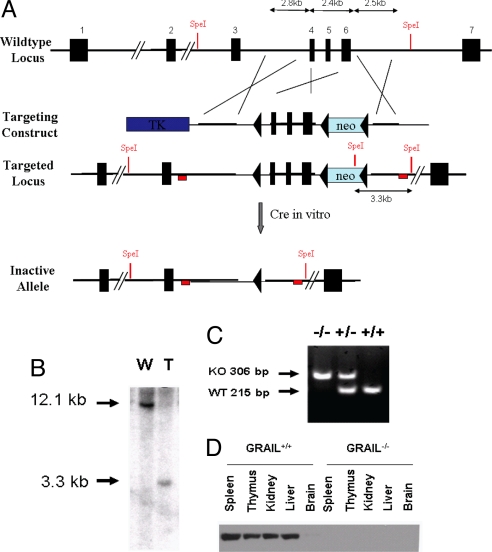
Fig. 1.
Generation of Grail-deficient mice. (A) Grail genomic locus and targeting strategy. Targeting region, 3′ and 5′ arms are depicted in Upper. Restriction enzyme sites are shown in red. The red box resembles the location of the 3′ probe used for Southern blot analysis shown in B. 5′ probe is also shown as red box next to Exon 2. The blue box indicates the HSV Thymidine Kinase (TK) negative selection marker, the green box resembles the Neomycin resistance cassette used for positive selection. (B) Southern blot analysis of ES cell clones. ES cell DNA was treated with SpeI rendering a 3.3-kb fragment at the targeted locus after detection with a specific probe designed on the 3′ end as depicted in A. W, wild-type clone; T, targeted clone. (C) Genotyping of offspring from Grail wild-type (+/+), heterozygous (+/−) and KO (+/−) mice generated after in vitro Cre treatment of ES cells containing loxP flanked Grail. (D) Western blot analysis of various tissue lysates for GRAIL expression using a C-terminal antibody for detection.
Grail−/− T Cells Are Defective in Several in Vitro Models of T Cell Anergy.
We next tested the ability of Grail−/− T cells to become anergized in response to various stimuli. This included delivery of incomplete signals either with the calcium ionophore ionomycin (Fig. 2 A–C); anergizing cytokines (TGF-β; Fig. 2D); or active suppression via regulatory T cells known to induce anergy (Fig. 2 E and F). Anergy induction was impaired in each model (Fig. 2 A–F). Both proliferation and IL-2 secretion were enhanced (Fig. 2 A–F). Interestingly, CD4+ CD62Lhigh CD25+ Treg-mediated inhibition of CD4+ CD62Lhigh CD25− responder cell proliferation was intact when tested in a standard in vitro coculture suppression assay despite high expression of Grail mRNA in this cell type (Fig. S6). However, Tregs isolated based on FOXP3 expression using a bicistronic fluorescent reporter were defective in suppressing proliferation and IL-2 production of naïve responder cells (Fig. 2 G and H) (19).
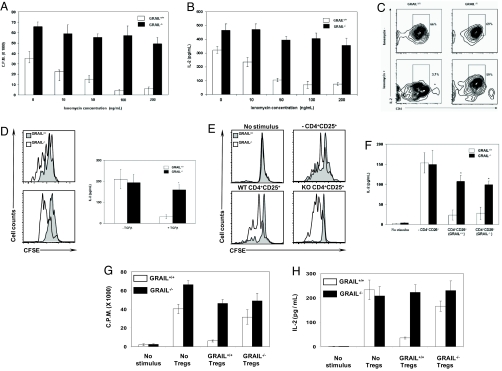
Fig. 2.
Grail-deficient T cells exhibit defective anergy induction. (A–C) Ionomycin-induced anergy. (A) Sorted splenic naïve CD4+ T cells were stimulated with anti-(α-)CD3/CD28, rested, then washed and treated with ionomycin for 12 h before restimulation. (B and C) IL-2 was measured by ELISA (B), and intracellularly by flow cytometry (C). C.P.M., counts per minute. (D) TGF-β-induced anergy. Sorted splenic naïve CD4+ T cells were stimulated with α-CD3/CD28 for 5 days either with or without TGF-β. Cells were washed, labeled with CFSE and restimulated with α-CD3/CD28. Proliferation was measured, based on CFSE dilution, after 72 h by flow cytometry (Left) and IL-2 by ELISA (Right). (E and F) Treg-mediated anergy. CFSE labeled naïve CD4+ T cells were mixed with equal ratio of CD25+ Tregs and cultured in the presence of α-CD3/CD28-coated latex beads. After 24 h, CD4+ CD25− T cells were sorted based on the CFSE staining and restimulated. Proliferation (E) and IL-2 (F) were measured as described in D. (G and H) FOXP3+ Treg suppressor cell assay. Sorted naïve CD4+ T cells were cocultured with equal ratio of CD4+ CD25+ mRFP+ Tregs obtained from Foxp3-IRES-mRFP (FIR) Grail+/+ and Grail−/− mice as described in SI Materials and Methods, and stimulated with α-CD3/CD28. Proliferation was measured by 3H-thymidine incorporation (G) and IL-2 was measured by ELISA (H).
Grail−/− Mice Cannot Be Tolerized In Vivo.
T cell anergy is an important mechanism of mucosal tolerance in vivo (3). The organism is exposed to numerous dietary and commensal antigens through the GI tract without mounting an immune response. High dose oral tolerance is known to induce T cell anergy/deletion (5). We therefore studied oral tolerance to ovalbumin using the OT-II TCR transgenic model in which Grail mRNA was highly up-regulated in CD4+ T cells from mesenteric LNs after tolerization. Remarkably, OT-II Grail−/− mice could not be tolerized in vivo despite increasing doses of antigen (Fig. 3 A–C). CD4+ T cell proliferation and IL-2 secretion was not suppressed ex vivo (Fig. 3 A–C). Oral tolerance induction was also aborted in experimental allergic encephalitis (EAE), a mouse model of the organ-specific autoimmune disease multiple sclerosis (Fig. 3D). Induction of EAE with myelin basic protein (MBP) together with an adjuvant can be ameliorated in this model by prior oral feeding of the antigen (20). Grail−/− mice, however, could not be tolerized via this route (Fig. 3D) supporting the in vivo importance of GRAIL in T cell tolerance.
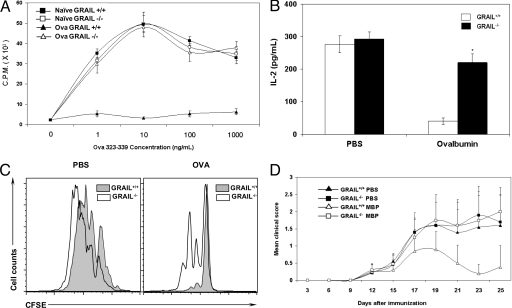
Fig. 3.
Defects in in vivo tolerance in Grail−/− mice. (A–C) OVA-induced oral tolerance. Grail+/+ and Grail−/− OT-II mice were fed with either ovalbumin protein (20 mg/mL) or PBS through drinking water for 5 days. Splenic CD4+ T cells were stimulated in vitro with OVA peptide pulsed APCs. Proliferation was measured by 3H-thymidine incorporation (A), IL-2 by ELISA (B), and CFSE dilution by flow cytometry (C). (D) Oral tolerance in EAE. Grail+/+ and Grail−/− mice (n = 10) were fed with MBP by gastric intubation. Mice were immunized s.c. with 400 μg of MBP in CFA. Bordetella pertussis toxin was injected i.p. on days 0 and 2. Disease symptoms were assessed by mean clinical score.
GRAIL Controls Activation and Death of Naïve T Cells.
We found that GRAIL mRNA is expressed in naïve, effector and memory T cell subsets (Fig. S6 A and B), suggesting a functional role beyond anergy. We therefore investigated whether GRAIL is also involved in primary T cell activation. Indeed, GRAIL regulates activation of naïve T cells at various levels (Fig. 4 A–C). Grail−/− T cells were hyperresponsive to TCR stimulation alone or with anti-CD28 (Fig. 4 A–C and Fig. S7). Interestingly, they proliferated and cycled more vigorously (Fig. 4 A–C; Fig. S8A) although there was no significant difference between amounts of IL-2 secreted at 12 h of stimulation (Fig. S8B). Direct intracellular stimulation with phorbol ester and calcium ionophore abolished the differences in proliferation (Fig. 4A). Grail−/− T cells were also hypersensitive to increasing doses of TCR stimulation (Fig. 4 B and C); however, baseline levels of intracellular calcium and TCR-induced calcium mobilization were equal in KO and wild-type T cells (Fig. 4D) suggesting that other signaling pathways downstream of TCR might be disturbed in the absence of GRAIL.
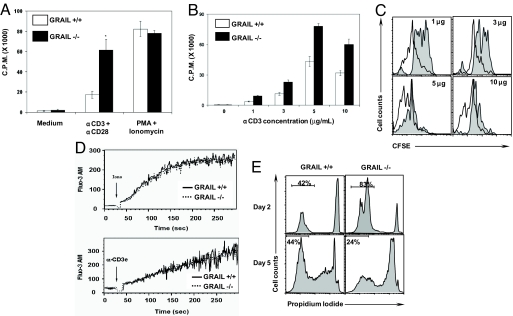
Fig. 4.
Grail−/− T cells show augmented responses to TCR stimulation. (A–C) Splenic naïve CD4+ T cells were stimulated with α-CD3 (5 μg/mL)/CD28 (A) or various concentrations (B and C), or PMA/ionomycin(A) for 48 h (A and B) or 72 h (C). Proliferation was measured by 3H-thymidine incorporation (A and B) or based on CFSE dilution by flow cytometry (C). Grail+/+, gray histograms; Grail−/−, white histograms. (D) Calcium flux at baseline and after T cell stimulation. Splenic naïve CD4+ T cells were labeled with Fluo3-AM. Cells were prewarmed and calcium release was measured either in the presence of ionomycin (Upper) or upon TCR cross linking (Lower). (E) Viability of T cells after primary stimulation. Splenic naïve CD4+ T cells were stimulated with α-CD3/CD2. At indicated time points, cell viability was measured by flow cytometry using propidium iodide exclusion.
To determine whether GRAIL is also involved in cell death in vitro, we examined viability of CD4+ T cells at various time points after activation (Fig. 4E). Although survival was enhanced after 2 days of stimulation, increased cell death was observed after 5 days (Fig. 4E), possibly due to increased consumption of growth factors although unlikely because fresh medium was added each time. Interestingly, the expression levels of Fas and Fas ligand were unchanged in Grail−/− mice.
Grail−/− Helper T Cells Overproduce IFN-γ and Are Susceptible to Cell Death.
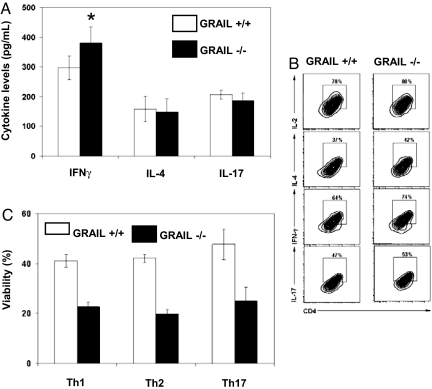
Profiling of Grail mRNA expression in T cell subsets showed not only significant levels in resting T cells but also increased levels in Th1 cells (Fig. S6 A and B). We therefore examined T helper cell differentiation and activation in vitro. We found a significant increase in the secretion of IFN-γ upon stimulation of terminally differentiated Th1 cells (Fig. 5 A and B) consistent with a functional role of increased Grail RNA expression in this T helper cell subset (Fig. S6B). Differentiation and activation of Th2 and Th17 cells were, however, not altered (Fig. 5 A and B). Interestingly, viability was reduced by ≈50% in all cultures of differentiated helper T cell subsets (Th1, Th2, Th17) from Grail−/− mice at day 5 in culture (Fig. 5C) suggesting a broader role of GRAIL in survival than mentioned above. Similar to naïve CD4+ T cells, hyperproliferation was noted during initial differentiation cultures across all helper T cell subsets. Importantly, fresh medium and cytokine cocktails were added to each differentiation culture at day 2 excluding deprivation of nutrients and growth factors to be responsible for subsequent increased cell death.
Fig. 5.
Grail−/− T cells exhibit several helper T cell abnormalities. (A and B) Helper T cell cytokine secretion. Naïve CD4+ T cells were sorted from the spleens of Grail+/+ and Grail−/− mice, and cultured for 5 days under conditions that promote Th1, Th2 and Th17 differentiation. On day 6, cells were harvested, restimulated with α-CD3 (5 μg/mL) and α-CD28 (2 μg/mL) for 24 h. Levels of respective cytokines were measured from supernatants by ELISA (A) or intracellularly by flow cytometry (B). *, P < 0.05 as described in SI Materials and Methods. (C) Viability of differentiated helper T cells. Naïve CD4+ T cells were sorted from the spleens of Grail+/+ and Grail−/− mice, and cultured for 5 days under conditions that promote Th1, Th2, and Th17 differentiation as described in SI Materials and Methods. On day 5, cell viability was measured by flow cytometry using propidium iodide exclusion. Indicated is the average viability of duplicate samples.
CD40L is an important T helper cell regulator with diverse functions including costimulation of B cells. We also measured CD40L expression on wild-type and KO T cells because ectopic expression of GRAIL in T cells down-regulates this B cell costimuatory ligand (17). However, we did not find increased levels of CD40L in Grail−/− mice either in resting or activated states (Fig. S9).
GRAIL Deficiency Increases Total and Phosphorylated Levels of ERK1/2.
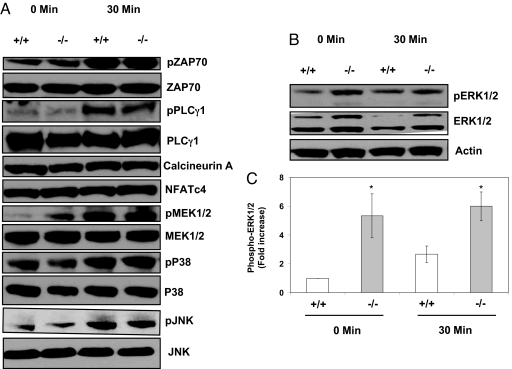
We next studied various signaling pathways involved in primary T cell activation to better understand the mechanism leading to these diverse functional abnormalities in Grail−/− mice (Fig. 6). TCR-proximal signals like ZAP-70 and PLCγ1 protein levels and phosphorylation were intact in resting and TCR-activated Grail−/− T cells (Fig. 6A). We also examined MAP kinase signaling in T cells because this pathway is crucial for T cell proliferation, differentiation and cytokine secretion (21). The MAP kinases p38 and JNK were not altered (Fig. 6A). We found, however, that ERK1/2 protein levels and phosphorylated states were elevated in Grail−/− T cells at baseline and after activation (Fig. 6 B and C). Upstream MEK1/2 levels and phosphorylation states were unchanged (Fig. 6A) suggesting that ERK protein might be the level of regulation. Recent data suggest that ERK is important for IFN-γ secretion in CD4+ T cells (22), possibly explaining the increased cytokine levels seen in stimulated Grail−/− Th1 cells (Fig. 5). We also found higher levels of protein kinase C theta (PKCθ), which is known to control T cell proliferation and survival (23–27) (Fig. S10). Immunoprecipitation experiments suggest possible interaction of GRAIL with PKCθ (Fig. S10), but these findings are often nonspecific and need further biochemical and in vivo studies for confirmation. When assessing proteins of the calcium-dependent signaling pathway, we found that calcineurin A and downstream NFAT protein levels were not different in WT and KO (Fig. 6A) consistent with normal calcium mobilization (Fig. 4D).
Fig. 6.
Grail deficiency in T cells leads to augmented levels of ERK. (A and B) Western blot analysis of signal transduction molecules. Splenic naïve CD4+ T cells were stimulated with α-CD3/CD28, harvested and lysed, and Western blot analysis was performed using antibodies against various molecules as indicated. Blots shown are representative of at least 3 experiments with identical results. (C) Densitometric analysis of ERK1/2 phosphorylation. Phospho-ERK bands as shown in Fig. 6B were quantified using densitometry. Results were normalized to control (Grail+/+ at 0 min), which was arbitrarily set to 1.0. Bars represent mean ± SD (n = 3). y axis represents fold increase in optic density to control. *, P < 0.05 (P = 0.008 for 0 min, P = 0.001 for 30 min).
Discussion
We have demonstrated that disruption of GRAIL in mice led to multiple defects in naïve, helper, and anergic T cell states, affecting survival, proliferation and cytokine secretion at various stages. Loss of T cell anergy was demonstrated in various in vitro models including ionomycin-, Treg-, and TGF-β-induced anergy. Importantly, Grail−/− mice exhibited also defective oral tolerance in vivo in two models (OT-II and EAE). On the protein level of naïve CD4+ T cells, we found that loss of GRAIL increases total levels of ERK. Of note, GRAIL localizes, at least partly, to the endosomal compartment, whereas ERK is diffusely distributed in the cytoplasm [although it is dynamically regulated and has been localized on endosomal compartments as well under certain conditions (28, 29)]. It is therefore theoretically possible that the cytoplasmic RING finger domain of GRAIL might directly regulate ERK protein levels. However, the exact molecular mechanisms how GRAIL controls naïve CD4+ T cell proliferation and anergy remain to be elucidated in further studies.
Negative regulation of PKCθ by GRAIL could potentially also explain several of the observed T cell abnormalities in Grail−/− mice, most notably the hyperproliferative phenotype of naïve T cells. It has been shown that TCR signaling required for primary T cell activation and IL-2 secretion is orchestrated by PKCθ (23, 25, 30). In addition, PKCθ is known to have dual effects on T cell apoptosis, a promoting role by inducing FasL expression and a protective role via Bcl-2/xL-dependent survival signals and/or inactivation of proapoptotic BAD (23–27, 31). Although an influence of GRAIL on PKCθ still needs to be firmly established, it is possible that the transient survival advantage of Grail−/− T cells, and 50% reduced survival of in vitro differentiated Th1, Th2, and Th17 cells could at least partially be related to this preliminary finding. The exact role of PKCθ in Th1-mediated immunity remains to be clarified (23–27). The slight, but consistent hypersecretion of IFN-γ in Grail−/− Th1 cells could possibly be due to increased ERK. Interestingly, ERK has recently been shown to play a role in IFN-γ secretion in CD4+ T cells (22). Further studies are needed to dissect which effects of GRAIL are responsible for the various abnormalities found in naïve and effector T cells from Grail−/− mice.
As discussed above, our data support a role for GRAIL in T lymphocyte activation, survival, and differentiation. In addition, we demonstrate an essential role of GRAIL in T cell anergy and in vivo tolerance using two different mouse models. Gene-targeting of the E3 ligase Cbl-b reveals also defects in T cell proliferation, anergy and in vivo tolerance (10–12). Combined deletion of GRAIL and Cbl-b is likely to create profound deficiency in anergy induction considering that each E3 ligase acts in different cellular compartments and potentially interferes with nonredundant signaling pathways. It has been shown that PKCθ protein levels are elevated in anergic T cells from Cbl-b−/− (and also Itch−/−) mice (32). It remains to be shown whether targets of E3 ligases differ in resting versus anergic T cells. ERK1/2 and PKCθ are both integrated in pathways that are altered or blocked in anergic T cells (2, 32, 33). It is therefore possible these pathways are at least indirectly controlled by GRAIL in anergic T cells.
The role of GRAIL in Tregs is less clear. It has been shown that GRAIL is sufficient for the conversion of T cells to a regulatory phenotype (34). In Grail−/− mice, primary inhibition of T cell proliferation by CD4+ CD62Lhigh CD25+ Tregs was intact whereas Treg-mediated anergy induction (measured with CFSE labeling and sorting experiments) was defective. Using a purer system with FOXP3+ CD4+ T cells sorted based on a bicistronic fluorescent reporter inserted into exon 13 of the Foxp3 gene as described in ref. 19, we could demonstrate that FOXP3+ Treg function was indeed impaired. The reason for the different outcomes in these two in vitro assays are unclear but could relate to the fact that CD25 is an imperfect marker for Tregs, and that activated Grail−/− CD4+ T cells have a heightened sensitivity to cell death. In vitro activation of previously activated CD25+ cells from Grail−/− mice could potentially lead to selective death of these cells with preferential survival of truly suppressive FOXP3+ cells in the in vitro coculture assay. This possibility has been excluded when using FOXP3 reporter mice. Further studies on the role of GRAIL in FOXP3+ Tregs will lead to a better understanding of the exact mechanisms involved. Previous studies have examined the role of PKCθ and ERK1/2 in Treg function and FOXP3 induction (35–37). It remains to be shown whether these molecules are relevant for the observed in vitro defect of FOXP3+ Tregs from Grail−/− mice, or if other proteins are involved.
CD40L is another molecule that has been shown to interact with GRAIL (17). Ectopic expression of GRAIL in naïve T cells from CD40−/− mice led to down-regulation of CD40L (17). However, we did not see increased CD40L expression in Grail−/− mice either in resting or activated states, but these differences could be due to different experimental settings and/or the fact that GRAIL was overexpressed in the prior study (17). Additional work is needed to substantiate a role for GRAIL in regulating costimulatory receptors in vitro and in vivo and its role in T cell differentiation and survival.
In summary, we established that GRAIL is an important gatekeeper of multiple T cell states including activation, survival, and differentiation. Elevated baseline levels of ERK in naïve CD4+ T cells suggest a potential role for GRAIL in setting signal thresholds. T cell homeostasis and immune tolerance are disturbed in human autoimmune diseases. Relieving these control mechanisms is known to enhance cancer immunity. It will therefore be important to investigate the role of GRAIL in other disease models and in human disease states. Further dissection of its diverse mechanisms in the activation and function of T cells will hopefully lead to a better understanding of this unique E3 ubiquitin ligase, and eventually to improvement of antigen-specific treatment strategies like oral tolerance induction.
Materials and Methods
Generation of Grail-Deficient Mice.
Using the Cre-loxP system, exons 4, 5, and 6 were targeted in ES cells (Fig. 1). Targeted ES clones were identified by Southern blot and treated with Cre in vitro, causing deletion of exons 4–6 and an out-of-frame mutation. Further details on KO generation and information on wild-type and transgenic mice are described in SI Materials and Methods .
In Vitro T Cell Studies.
For ionomycin-induced anergy, CD4+ T cells were stimulated with αCD3/CD28, rested, then cultured with or without ionomycin and restimulated. For TGF-β-induced anergy, T cells were stimulated with or without TGF-β, washed and restimulated. For Treg-induced anergy, CFSE-labeled naïve T cells were incubated with CD4+ CD25+ Tregs and αCD3/CD28, resorted and restimulated. For FOXP3+ Treg suppression, CD4+ T cells were cocultured with FOXP3+ Tregs from reporter mice described in ref. 19 and stimulated with αCD3/CD28. Further details and information on T cell proliferation and differentiation assays, calcium flux, PCR, Western blot and ELISA are described in SI Materials and Methods.
In Vivo Tolerance Models.
CD4+ T cells from ovalbumin fed OT-II transgenic mice were stimulated ex vivo with OVA peptide to assess for oral tolerance in WT and KO mice. Further details and information on oral tolerance in the EAE model are described in SI Materials and Methods.
Supplementary Material
Acknowledgments.
The authors thank K. Rajewsky (Harvard Medical School) for having provided the targeting vector pEASY-flox; L. Alexopoulou for help with the targeting construct; E. Esplugues for assistance with T cell cultures; A.-K. Robertson for help with EAE studies; L. Evangelisti, C. Hughes and J. Stein for technical assistance; C. G. Fathman (Stanford University) for sharing data; and F. Manzo for manuscript preparation. This work was supported by grants from the National Institutes of Health (to R.A.F.); a fellowship from the American Diabetes Association (to C.R.), and the Emmy Noether Program of the German Research Foundation (Deutsche Forschungsgemeinschaft) and a fellowship from the Arthritis National Research Foundation (to M.A.K.). R.A.F. is an Investigator of the Howard Hughes Medical Institute.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0908957106/DCSupplemental.
References
- 1.Walker LS, Abbas AK. The enemy within: Keeping self-reactive T cells at bay in the periphery. Nat Rev Immunol. 2002;2:11–19. doi: 10.1038/nri701. [DOI] [PubMed] [Google Scholar]
- 2.Schwartz RH. T cell anergy. Annu Rev Immunol. 2003;21:305–334. doi: 10.1146/annurev.immunol.21.120601.141110. [DOI] [PubMed] [Google Scholar]
- 3.Macian F, et al. Transcriptional mechanisms underlying lymphocyte tolerance. Cell. 2002;109:719–731. doi: 10.1016/s0092-8674(02)00767-5. [DOI] [PubMed] [Google Scholar]
- 4.Fathman CG, Lineberry NB. Molecular mechanisms of CD4+ T-cell anergy. Nat Rev Immunol. 2007;7:599–609. doi: 10.1038/nri2131. [DOI] [PubMed] [Google Scholar]
- 5.Faria AM, Weiner HL. Oral tolerance. Immunol Rev. 2005;206:232–259. doi: 10.1111/j.0105-2896.2005.00280.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kurtz J, et al. Mechanisms of early peripheral CD4 T cell tolerance induction by anti-CD154 monoclonal antibody and allogeneic bone marrow transplantation: Evidence for anergy and deletion, but not regulatory cells. Blood. 2004;103:4336–4343. doi: 10.1182/blood-2003-08-2642. [DOI] [PubMed] [Google Scholar]
- 7.Lin AE, Mak TW. The role of E3 ligases in autoimmunity and the regulation of autoreactive T cells. Curr Opin Immunol. 2007;19:665–673. doi: 10.1016/j.coi.2007.10.002. [DOI] [PubMed] [Google Scholar]
- 8.Anandasabapathy N, et al. GRAIL: An E3 ubiquitin ligase that inhibits cytokine gene transcription is expressed in anergic CD4+ T cells. Immunity. 2003;18:535–547. doi: 10.1016/s1074-7613(03)00084-0. [DOI] [PubMed] [Google Scholar]
- 9.Seroogy CM, et al. The gene related to anergy in lymphocytes, an E3 ubiquitin ligase, is necessary for anergy induction in CD4 T cells. J Immunol. 2004;173:79–85. doi: 10.4049/jimmunol.173.1.79. [DOI] [PubMed] [Google Scholar]
- 10.Bachmaier K, et al. Negative regulation of lymphocyte activation and autoimmunity by the molecular adaptor Cbl-b. Nature. 2000;403:211–216. doi: 10.1038/35003228. [DOI] [PubMed] [Google Scholar]
- 11.Chiang YJ, et al. Cbl-b regulates the CD28 dependence of T-cell activation. Nature. 2000;403:216–220. doi: 10.1038/35003235. [DOI] [PubMed] [Google Scholar]
- 12.Jeon MS, et al. Essential role of the E3 ubiquitin ligase Cbl-b in T cell anergy induction. Immunity. 2004;21:167–177. doi: 10.1016/j.immuni.2004.07.013. [DOI] [PubMed] [Google Scholar]
- 13.King CG, et al. TRAF6 is a T cell-intrinsic negative regulator required for the maintenance of immune homeostasis. Nat Med. 2006;12:1088–1092. doi: 10.1038/nm1449. [DOI] [PubMed] [Google Scholar]
- 14.Vinuesa CG, et al. A RING-type ubiquitin ligase family member required to repress follicular helper T cells and autoimmunity. Nature. 2005;435:452–458. doi: 10.1038/nature03555. [DOI] [PubMed] [Google Scholar]
- 15.Soares L, et al. Two isoforms of otubain 1 regulate T cell anergy via GRAIL. Nat Immunol. 2004;5:45–54. doi: 10.1038/ni1017. [DOI] [PubMed] [Google Scholar]
- 16.Lineberry N, Su L, Soares L, Fathman CG. The single-subunit transmembrane E3 ligase GRAIL captures and then ubiquitinates transmembrane proteins across the cell membrane. J Biol Chem. 2008;283:28497–28505. doi: 10.1074/jbc.M805092200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lineberry NB, et al. Cutting edge: The transmembrane E3 ligase GRAIL ubiquitinates the costimulatory molecule CD40 ligand during the induction of T cell anergy. J Immunol. 2008;181:1622–1626. doi: 10.4049/jimmunol.181.3.1622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Su L, Lineberry N, Huh Y, Soares L, Fathman CG. A novel E3 ubiquitin ligase substrate screen identifies Rho guanine dissociation inhibitor as a substrate of gene related to anergy in lymphocytes. J Immunol. 2006;177:7559–7566. doi: 10.4049/jimmunol.177.11.7559. [DOI] [PubMed] [Google Scholar]
- 19.Wan YY, Flavell RA. Identifying Foxp3-expressing suppressor T cells with a bicistronic reporter. Proc Natl Acad Sci USA. 2005;102:5126–5131. doi: 10.1073/pnas.0501701102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen Y, et al. Oral tolerance in myelin basic protein T-cell receptor transgenic mice: Suppression of autoimmune encephalomyelitis and dose-dependent induction of regulatory cells. Proc Natl Acad Sci USA. 1996;93:388–391. doi: 10.1073/pnas.93.1.388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dong C, Davis RJ, Flavell RA. MAP kinases in the immune response. Annu Rev Immunol. 2002;20:55–72. doi: 10.1146/annurev.immunol.20.091301.131133. [DOI] [PubMed] [Google Scholar]
- 22.Czyzyk J, Chen HC, Bottomly K, Flavell RA. p21 Ras/Impedes Mitogenic Signal Propagation Regulates Cytokine Production and Migration in CD4 T Cells. J Biol Chem. 2008;283:23004–23015. doi: 10.1074/jbc.M804084200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hayashi K, Altman A. Protein kinase C theta (PKCθ): A key player in T cell life and death. Pharmacol Res. 2007;55:537–544. doi: 10.1016/j.phrs.2007.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Isakov N, Altman A. Protein kinase C(θ) in T cell activation. Annu Rev Immunol. 2002;20:761–794. doi: 10.1146/annurev.immunol.20.100301.064807. [DOI] [PubMed] [Google Scholar]
- 25.Manicassamy S, Gupta S, Sun Z. Selective function of PKC-θ in T cells. Cell Mol Immunol. 2006;3:263–270. [PubMed] [Google Scholar]
- 26.Pfeifhofer C, et al. Protein kinase C θ affects Ca2+ mobilization and NFAT cell activation in primary mouse T cells. J Exp Med. 2003;197:1525–1535. doi: 10.1084/jem.20020234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sun Z, et al. PKC-θ is required for TCR-induced NF-κB activation in mature but not immature T lymphocytes. Nature. 2000;404:402–407. doi: 10.1038/35006090. [DOI] [PubMed] [Google Scholar]
- 28.Robertson SE, et al. Extracellular signal-regulated kinase regulates clathrin-independent endosomal trafficking. Mol Biol Cell. 2006;17:645–657. doi: 10.1091/mbc.E05-07-0662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Teis D, Wunderlich W, Huber LA. Localization of the MP1-MAPK scaffold complex to endosomes is mediated by p14 and required for signal transduction. Dev Cell. 2002;3:803–814. doi: 10.1016/s1534-5807(02)00364-7. [DOI] [PubMed] [Google Scholar]
- 30.Altman A, Villalba M. Protein kinase C-θ (PKCθ): It's all about location, location, location. Immunol Rev. 2003;192:53–63. doi: 10.1034/j.1600-065x.2003.00027.x. [DOI] [PubMed] [Google Scholar]
- 31.Manicassamy S, Sun Z. The critical role of protein kinase C-θ in Fas/Fas ligand-mediated apoptosis. J Immunol. 2007;178:312–319. doi: 10.4049/jimmunol.178.1.312. [DOI] [PubMed] [Google Scholar]
- 32.Heissmeyer V, et al. Calcineurin imposes T cell unresponsiveness through targeted proteolysis of signaling proteins. Nat Immunol. 2004;5:255–265. doi: 10.1038/ni1047. [DOI] [PubMed] [Google Scholar]
- 33.Li W, Whaley CD, Mondino A, Mueller DL. Blocked signal transduction to the ERK and JNK protein kinases in anergic CD4+ T cells. Science. 1996;271:1272–1276. doi: 10.1126/science.271.5253.1272. [DOI] [PubMed] [Google Scholar]
- 34.MacKenzie DA, et al. GRAIL is up-regulated in CD4+ CD25+ T regulatory cells and is sufficient for conversion of T cells to a regulatory phenotype. J Biol Chem. 2007;282:9696–9702. doi: 10.1074/jbc.M604192200. [DOI] [PubMed] [Google Scholar]
- 35.Gupta S, et al. Differential requirement of PKC-θ in the development and function of natural regulatory T cells. Mol Immunol. 2008;46:213–224. doi: 10.1016/j.molimm.2008.08.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hickman SP, Yang J, Thomas RM, Wells AD, Turka LA. Defective activation of protein kinase C and Ras-ERK pathways limits IL-2 production and proliferation by CD4+CD25+ regulatory T cells. J Immunol. 2006;177:2186–2194. doi: 10.4049/jimmunol.177.4.2186. [DOI] [PubMed] [Google Scholar]
- 37.Luo X, et al. Cutting edge: TGF-beta-induced expression of Foxp3 in T cells is mediated through inactivation of ERK. J Immunol. 2008;180:2757–2761. doi: 10.4049/jimmunol.180.5.2757. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.