Abstract
Several different families of DNA viruses encode proteins that inactivate the cellular retinoblastoma tumor suppressor protein (pRb), which normally functions to bind E2F transcription factors and restrict expression of genes necessary for cellular processes including DNA replication. Human cytomegalovirus (HCMV) UL97, a protein kinase functionally orthologous to cellular cyclin-dependent kinases, phosphorylates pRb on inactivating residues during HCMV infection. To assess if such phosphorylation is biologically relevant, we tested whether the human papillomavirus type 16 E7 protein, which inactivates pRb family proteins by direct binding and destabilization, could substitute for UL97 during HCMV infection. In the absence of UL97, expression of wild-type E7 protein, but not a mutant E7 unable to bind pRb family proteins, restored E2F-responsive cellular gene expression, late viral gene expression, and viral DNA synthesis to levels normally observed during wild-type virus infection of quiescent cells. UL97-null mutants exhibited more pronounced defects in virus production and DNA synthesis in quiescent cells as compared to serum-fed, cycling cells. E7 expression substantially enhanced infectious virus production in quiescent cells, but did not complement the defects observed during UL97-null virus infection of cycling cells. Thus, a primary role of UL97 is to inactivate pRb family proteins during infection of quiescent cells, and this inactivation likely abets virus replication by induction of cellular E2F-responsive genes. Our findings have implications for human cytomegalovirus disease and for drugs that target UL97.
Keywords: E2F, cyclin-dependent kinase, cell cycle, antiviral drugs, retinoblastoma protein
The pathway controlled by the retinoblastoma (pRb) family of tumor suppressors is dysregulated in most if not all human cancers [reviewed in (1)]. pRb proteins inhibit the ability of the E2F transcription factors to activate the expression of genes required for many important cellular processes, including DNA replication. During normal cell cycle progression, phosphorylation of pRb proteins by cyclin dependent kinases (Cdks) leads to the disruption of pRb-E2F complexes and the entry of cells into the S phase, where DNA is synthesized. Certain viruses with DNA genomes specifically target pRb proteins for inactivation, at least in part to facilitate the high level genome replication required for efficient virus production (2, 3). One such virus is human papillomavirus (HPV), of which certain subtypes cause cervical cancer (2). The HPV16 E7 protein binds and destabilizes pRb family proteins, liberating E2F to activate the expression of genes required for DNA synthesis (4–6).
Recently, we discovered a different way by which viruses inactivate pRb: direct phosphorylation by a virally encoded kinase (7). The human cytomegalovirus (HCMV) protein kinase encoded by the UL97 gene is necessary and sufficient for pRb phosphorylation on inactivating residues in vitro (7) and in cells (7, 8). HCMV is a ubiquitous human pathogen that establishes a lifelong infection, which is typically asymptomatic in healthy individuals [reviewed in (9)]. Nonetheless, HCMV is a leading viral cause of birth defects and life threatening disease in immunocompromised patients, and UL97 is an important target for drugs to ameliorate HCMV disease (10–14).
HCMV mutants lacking UL97 produce approximately 10 to 1,000 fold fewer infectious particles than wild-type virus during replication in cultured cells (7, 15–18). Specific defects of these mutants during viral replication include reduced viral DNA synthesis, impaired exit of capsids from the nucleus (nuclear egress), and altered intracellular localization of virion components; pharmacological inhibition of UL97 results in similar phenotypes (8, 10, 15, 17, 19, 20). Our discovery that UL97 directly phosphorylates pRb prompted us to examine the roles that both UL97 and pRb play during HCMV infection. To determine whether UL97-mediated inactivation of pRb was important during HCMV infection, we asked if the pRb-inactivating protein from HPV16 could complement HCMV replication defects observed in the absence of UL97.
Results
The Human Papillomavirus 16 E7 Protein Expressed from a Recombinant Human Cytomegalovirus Inactivates and Degrades pRb.
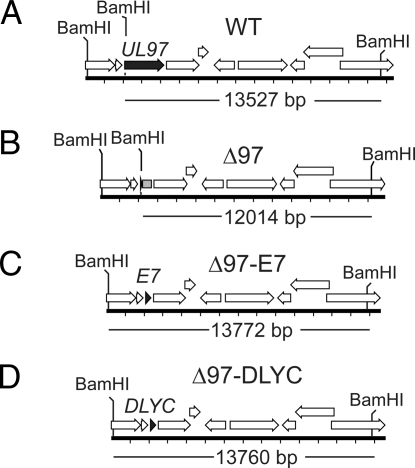
UL97-null HCMV fails to induce pRb phosphorylation and shows defects in the production of infectious virus progeny (7, 8, 14–17, 20). We wanted to determine whether UL97 functionally inactivates pRb and if heterologous pRb inactivation in cells infected with UL97-null virus would rescue deficiencies of UL97-null HCMV. To this end, we used E. coli ‘recombineering’ techniques on an infectious bacterial artificial chromosome (BAC) clone of HCMV strain AD169 (21) to engineer viruses in which UL97 protein-coding sequences were replaced by either a wild-type allele of the HPV16 E7 oncoprotein, or a ΔDLYC mutant allele encoding an HPV16 E7 that lacks an LxCxE motif essential for inactivation of pRb (22, 23). These manipulations resulted in two UL97-null viruses: Δ97-E7 and Δ97-DLYC (Fig. 1). Restriction digests (Fig. S1) and sequencing of resulting BAC clones confirmed the genomic integrity of these recombinant viruses. Electroporation of these BAC clones into permissive human fibroblast cells allowed for the recovery of infectious virus.
Fig. 1.
UL97-null HCMVs that express wild-type or mutant forms of the E7 oncoprotein of HPV16. Schematic of the coding content of the UL95–UL105 region of the viruses used in this study. (A) Wild-type HCMV strain AD169 (WT); BamHI sites are labeled and an arrow representing the UL97 ORF is shown in black (top). (B) The same region of Δ97, a UL97 deletion virus is shown. A remnant 37-amino acid ORF, comprised of the first 23 amino acids of UL97 and an additional 14 amino acids attributed to a frameshift after the deletion, is shown in black; a gray box represents a remnant UL97 coding region not predicted to be translated due to a naturally occurring stop codon. (C) Δ97-E7, a UL97-null HCMV that encodes the E7 protein of HPV16 (E7, black arrow) in place of UL97. (D) Δ97-DLYC, a UL97-null HCMV that encodes a ΔDLYC mutant form of HPV16 E7 (DLYC, black arrow) in place of UL97.
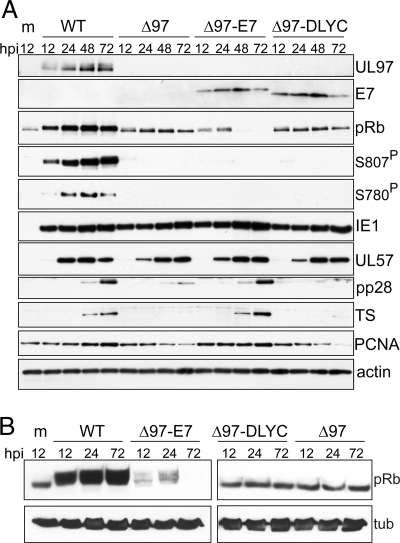
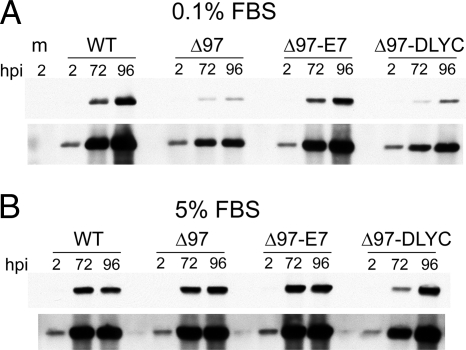
Upon infection of subconfluent, serum-starved (quiescent) human fibroblasts, the appropriate expression patterns of UL97 and E7 proteins were observed by Western blot (Fig. 2A). Furthermore, in mock-infected cells, or in cells infected with a previously characterized UL97-null virus, Δ97 (18), pRb remained in a hypophosphorylated state, while infection with wild-type virus (WT) resulted in pRb hyperphosphorylation (Fig. 2 A and B), as previously reported (7, 8, 24). In cells infected with Δ97-E7, pRb levels were substantially decreased, and there was some conversion of hypophosphorylated pRb into the hyperphosphorylated form at early time points (Fig. 2 A and B). These changes were not observed in cells infected with Δ97-DLYC, where pRb remained stable and hypophosphorylated throughout infection.
Fig. 2.
Analysis of viral and cellular protein expression in quiescent cells. Western blot analyses of cellular and viral protein expression in cells infected with the viruses used in this study. (A) Serum-deprived human fibroblasts were infected at an MOI of 1 PFU/cell with WT HCMV (WT), UL97-null HCMV (Δ97), or UL97-null HCMV expressing either wild-type (Δ97-E7) or ΔDLYC mutant (Δ97-DLYC) forms of HPV16 E7. Lysates prepared at the indicated time points (hpi) were compared for the expression of the following viral gene products: the immediate early protein IE1–72 (IE1); UL57, an early protein; and pp28, a late protein. Expression levels of TS and PCNA were measured as examples of E2F-responsive cellular genes. Expression of UL97, E7, pRb were also monitored as controls, as was beta-actin, to indicate protein loading. Phosphospecific antibodies were used to monitor for the presence of pRb phosphorylated at Ser-807/811 (S807P) and at Ser-780 (S780P). (B) Serum-starved cells maintained in 0.1% FBS were either mock-infected (m) or infected with the same viruses and lysed at the indicated time points (hpi) following infection and analyzed for pRb expression and mobility by Western blot. Beta tubulin (tub) reactivity was determined as a loading control.
Induction of E2F-Responsive Cellular Genes and Viral Genes.
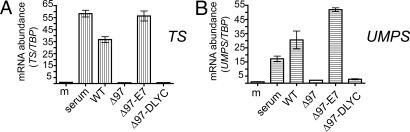
Hypophosphorylated (active) pRb prevents the expression of E2F-responsive genes (1). Previous work has shown that HCMV infection induces E2F-responsive gene expression (25–27), but the viral protein(s) responsible have not been conclusively identified. In subconfluent, serum-starved cells, we found that the re-addition of serum or WT infection caused an increase in the steady state levels of transcripts encoding thymidylate synthase (TS) and uracil monophosphate synthase (UMPS) (Fig. 3). The TS promoter is known be highly E2F-responsive (28) and the UMPS promoter has been shown to bind E2F-1 and E2F-4 (29). Δ97 and Δ97-DLYC, which were unable to induce pRb phosphorylation (Fig. 2), failed to induce the expression of these two transcripts (Fig. 3). However, infection with Δ97-E7 caused accumulation of TS and UMPS transcripts to levels similar to those during WT infection or after re-addition of serum (Fig. 3).
Fig. 3.
Comparison of mRNA levels for TS and UMPS. Analysis of mRNA levels for two genes involved in cellular DNA synthesis whose promoters bind E2F transcription factors. (A) RT-qPCR was used to compare mRNA levels at 48 hpi for TS and TATA-binding protein (TBP) in serum-deprived cells which were either mock-infected or infected with the indicated viruses. RNA from a set of cells that were serum stimulated in parallel and harvested at 24 h post-stimulation was used as a positive control for TS induction. TS mRNA levels are displayed as normalized values after correcting for differences in TBP expression. (B) mRNA levels at 48 hpi for UMPS were compared, as above. For both panels, the data represent the average measurement from three replicates per condition, with error bars representing standard deviations.
Like all herpesviruses, productive infection with HCMV initiates a temporal cascade of gene expression where immediate early genes are first expressed, followed next by early genes, and finally by late genes. We found that the expression of proteins encoded by immediate early gene UL123 (IE1) and the early gene UL57 was similar after infection of quiescent cells with WT, Δ97, Δ97-E7, and Δ97-DLYC viruses (Fig. 2A). However, expression of the pp28 protein encoded by the late gene UL99 was markedly reduced under conditions where both UL97 and wild-type E7 were absent, and where pRb remained present in its hypophosphorylated (active) form (Fig. 2A). The defect in late gene expression correlated with a decrease in protein expression for two E2F-responsive cellular DNA synthesis genes, TS and proliferating cell nuclear antigen (PCNA) (Fig. 2A). A similar defect in pp28 expression that correlated with decreased pRb phosphorylation and TS expression was observed with an HCMV point mutant expressing a catalytically deficient UL97 (K355Q), but no such defect was seen with a rescued virus derived from this mutant (Q335K) (Fig. S2A).
HPV16 E7 Expression increases Infectious Virion Production and Viral DNA Synthesis in Quiescent Cells Infected with UL97-Null HCMV.
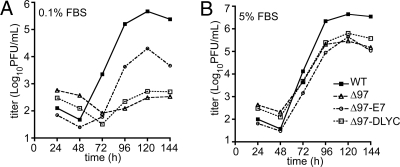
Because HPV16 E7 complemented defects in E2F-responsive gene expression and viral late gene expression observed in the absence of UL97, we asked if this papillomavirus protein could increase the yield of infectious UL97-null viruses. We found that Δ97 exhibited a replication defect of as much as 1,000-fold in quiescent cells (Fig. 4A), and no less than ≈100-fold in repeated experiments. Moreover, the UL97 point mutant virus (K355Q) replicated indistinguishably from Δ97 under these conditions (Fig. S3A). Expression of wild-type E7, but not the ΔDLYC mutant, was able to rescue infectious virus production substantially (50-fold in Fig. 4A). Thus, E7 can partially, but not fully, substitute for the loss of UL97 during HCMV infection of quiescent cells.
Fig. 4.
Complementation of a severe replication defect in quiescent cells of UL97-null HCMV by wild-type HPV E7. Replication kinetics experiments comparing viral replication efficiency in quiescent and cycling cells. (A) Serum-deprived cells, maintained in medium containing 0.1% FBS were infected with WT HCMV (WT), UL97-null HCMV (Δ97), or UL97-null HCMV expressing either wild-type (Δ97-E7) or ΔDLYC mutant (Δ97-DLYC) forms of HPV16 E7 at an MOI of 1 PFU/cell and compared for yield of infectious virus. (B) Asynchronously dividing cells, maintained in the presence of 5% FBS, were infected as above and compared for yield of infectious virus. For both panels, because counts from duplicate titrations did not vary by more than 2-fold for any data points, error bars are not shown.
As has been previously reported by others as data not shown (20), UL97-null viruses produced higher titers in serum-fed cycling cells than in quiescent cells, consistently showing only a ≈10-fold replication defect relative to WT in cycling cells (Fig. 4B). Interestingly, E7 expression was unable to complement this smaller defect of Δ97 in cycling cells (Fig. 4B).
To control for the possibility that HPV16 E7 expression nonspecifically enhances replication of wild-type HCMV, we prepared two viruses, WT-E7 and WT-DLYC, which in addition to expressing UL97 from its native locus, express either the wild-type or the ΔDLYC mutant form of HPV16 E7, respectively, under the control of a duplicated UL97 promoter in the unique short (US) region of the HCMV genome (Fig. S4) from an intergenic locus between US9 and US10, a site that has been used by others to express heterologous genes (30).
We confirmed that these viruses express E7 (Fig. S2B). To examine the biological activity of the E7 expressed by these viruses, we used the UL97 inhibitor maribavir (10). In quiescent cells, treatment with 1 μM maribavir reduced the yield of parental WT and WT-DLYC by approximately 100-fold, but reduced the yield of WT-E7 by less than 10-fold (Fig. S3B). Maribavir treatment inhibited pRb phosphorylation in WT- and WT-DLYC-infected cells; however, in WT-E7-infected cells, as expected, pRb levels were drastically reduced, which correlated with increased expression of TS and pp28 (Fig. S2A), akin to what was seen in Δ97-E7-infected cells (Fig. 2A). Furthermore, under serum-deprivation conditions, WT-E7 replicated to yield three-fold lower levels of infectious virus than its parental WT (Fig. S3B) and similar results were seen during replication in 5% FBS (Fig. S3C). Thus, the complementation of the Δ97 defect by HPV16 E7 is not due to nonspecific enhancement of HCMV replication.
UL97 has been previously reported to be important for viral DNA synthesis (10, 17). As noted above, in the absence of UL97 activity or E7, we detected reduced levels of pp28, a viral protein whose expression requires viral DNA synthesis (31) (Fig. 2A and Fig. S2A). While it is possible that UL97 may have a direct effect on HCMV late gene expression, we thought it unlikely that a HPV protein would, and therefore sought an alternative explanation for this observation. Since many E2F regulated cellular genes have clear roles in cellular DNA synthesis (1), we decided to investigate whether differences in viral DNA synthesis might correlate with the complementation of the UL97-null phenotype by E7 that we observed in quiescent cells.
Southern blotting experiments revealed that in quiescent cells, viral DNA synthesis during lytic infection with either Δ97 or Δ97-DLYC was substantially reduced compared to that seen with WT or Δ97-E7 (Fig. 5A). Thus, the ΔDLYC mutant form of E7 was apparently unable to complement the viral DNA synthesis defect observed in the absence of UL97, while wild-type E7 was able to restore viral DNA synthesis to wild-type levels (Fig. 5A). In contrast, the DNA synthesis defect of UL97-null viruses was not as dramatic in cycling cells, and expression of wild-type E7 did not appear to provide any notable advantage in viral DNA synthesis under these conditions (Fig. 5B).
Fig. 5.
Comparison of viral DNA synthesis in dividing versus non-dividing cells by Southern blot. Southern blot analysis of viral DNA synthesis in non-dividing, serum-deprived cells and asynchronously dividing cells maintained in 5% FBS. (A) Equal quantities of total DNA, prepared at the indicated time points from quiescent cells maintained in 0.1% FBS which were infected with WT HCMV (WT), UL97-null HCMV (Δ97), or UL97-null HCMV expressing either wild-type (Δ97-E7) or ΔDLYC mutant (Δ97-DLYC) forms of HPV16 E7, were analyzed by Southern blot using a probe against a viral gene (UL83). (B) Samples from a parallel experiment in asynchronously dividing cells maintained in 5% FBS were subjected to Southern blot analysis, as above. In both panels, a longer exposure is shown immediately beneath the normal exposure so that levels of DNA from input virus can be observed.
We thus conclude that the HPV16 E7 protein, through its ability to inactivate pRb family members, can replace some, but not all of the functions of the HCMV UL97 protein. Thus, pRb family protein inactivation by UL97 protein kinase is required for efficient HCMV replication in quiescent cells. Furthermore, our results suggest that a function of pRb-inactivation by UL97 is to facilitate viral DNA synthesis, most likely by activating the expression of E2F-responsive cellular genes.
Discussion
Papillomaviruses and cytomegaloviruses each face the challenge of amplifying their genomes in non-dividing cells that are incompetent for rapid and efficient DNA synthesis. These two viruses have evolved independent mechanisms to inactivate the pRb tumor suppressor protein and activate E2F-mediated gene expression. HPV16 E7 binds and destabilizes pRb, and HCMV UL97 phosphorylates pRb. In quiescent cells, expression of wild-type HPV16 E7 by UL97-null HCMV restored viral DNA synthesis and gene expression to wild-type or near wild-type levels, and enhanced production of infectious virus substantially.
However, HPV16 E7 failed to fully complement UL97-null HCMV in production of infectious particles, and HPV16 E7 did not appear to enhance replication of UL97-null virus in cycling cells, which may underscore the relevance of functions of UL97 other than inactivation of pRb family members. In particular, UL97-mediated phosphorylation of lamin A/C has been posited to play a role in altering the nuclear lamina to promote nuclear egress (15, 32–34). A defect in nuclear egress largely explains the replication defect of UL97-null mutants in cycling cells (15), which would in turn explain why HPV16 E7 expression does not complement the loss of UL97 in these cells. It is also possible that toxicity from HPV16 E7 expression [reviewed in (35)] may account for some portion of the replication defect observed for Δ97-E7 in quiescent cells. Further studies will be needed to demonstrate whether the failure to phosphorylate lamin A/C accounts for the UL97-null replication defect in quiescent cells that cannot be complemented by HPV16 E7 expression.
There is considerable variability reported in the literature regarding the degree and nature of replication defects caused by genetic ablation or pharmacological inhibition of UL97, and some of this variability appears to depend on cell type and culture conditions (10, 14–18, 36). Nonetheless, we are unaware of any previously presented data directly comparing the replication of viruses lacking UL97 activity in dividing versus resting cells. Our results suggest that at least some of the variability in the literature may be explained by the relative proportion, at the time of infection, of resting cells, which contain hypophosphorylated, active pRb, and cycling cells, which contain inactivated pRb. Indeed, we have observed variability in yield of UL97-null viruses that correlated with cultivation of cells in different lots of serum. Other researchers have used serum-fed confluent cells in their assays (10, 36, 37). Since confluent cells can express increased levels of Cdk inhibitor proteins that prevent cellular kinases from efficiently inactivating pRb (38, 39), such conditions might also be expected to exacerbate pRb-dependent replication defects of UL97-null mutants.
While this manuscript was being completed, Gill et al. reported that HCMV mutants bearing mutations in UL97 that alter putative pRb binding motifs replicated well in human fibroblasts (40). However, the viruses were assayed in serum-fed cells and these mutations were not shown to abolish pRb inactivation.
As recently reviewed (3), HCMV encodes at least three other genes that have been reported to antagonize the function of pRb family proteins or otherwise up-regulate E2F-responsive cellular genes; these include IE1, IE2, and pp71. Since the viruses used in this study were wild-type with regard to IE1, IE2, and pp71, our results suggest that pRb family member inactivation by these proteins is functionally or temporally distinct from that mediated by UL97, or by HPV16 E7, when expressed in place of UL97. Moreover, our results show that UL97 is responsible for activating at least a subset of E2F-responsive genes when HCMV infects quiescent cells. Thus, it seems likely that UL97-null viruses grow better in serum-fed cycling cells than in quiescent cells because serum induces E2F-responsive gene expression.
Unlike cells infected with Δ97 or Δ97-DLYC, where pRb remains hypophosphorylated, there was evidence of pRb phosphorylation in cells infected with Δ97-E7 (Fig. 2 A and B). Because HCMV encodes no other conventional protein kinases, and because E7 is not known to have kinase activity, such phosphorylation might be attributed to increased activity of Cdk2-cyclin E complexes following pRb inactivation by E7, as cyclin E expression is up-regulated following pRb inactivation (41–43) and Cdk2-cyclin E activity has been shown to be induced by HCMV infection (24, 44). Moreover, HPV16 E7 preferentially associates with and destabilizes hypophosphorylated pRb (45). Nonetheless, precisely why Cdks might phosphorylate pRb in cells infected with Δ97-E7 but fail to do so in Δ97 and Δ97-DLYC infected cells remains unclear.
To successfully infect and spread within its natural host, HCMV is likely to depend on its ability to replicate in non-dividing cells. Our results argue that a major role of UL97 is to inactivate pRb family proteins in non-dividing cells, where these proteins are not readily inactivated by host cell kinases. Since the vast preponderance of cells in an adult vertebrate are quiescent and contain unduplicated DNA (46), the role of UL97 as an inactivator of pRb family proteins is likely to be of great relevance for HCMV disease.
UL97 is an important drug target in therapeutic strategies against HCMV infection (10–13). Maribavir, a specific inhibitor of UL97 kinase activity, is currently being evaluated for approval as an antiviral agent against HCMV disease (10, 11, 47, 48). Thus, the results presented here help establish a likely mechanism by which inhibition of UL97 may provide its therapeutic efficacy in patients (11, 48). Furthermore, our data suggest that UL97 inhibition may be more efficacious at treating HCMV disease caused by viral infection of resting cells, and less efficacious when disease results from infection of dividing cells. It is also of interest that investigators have reported an association between HCMV infection and certain cancers (49), particularly glioblastoma (50–52), a brain tumor. While a role for HCMV in the etiology of any human malignancy remains to be demonstrated, the ability of UL97 to inactivate an important tumor suppressor provides an impetus to consider that UL97 may potentially act as an oncogene, and that UL97 inhibitors such as maribavir might conceivably inhibit tumor progression in certain cancers.
Materials and Methods
Plasmids, Cells, and Viruses.
Sequences encoding wild-type and ΔDLYC mutant E7 were each PCR amplified from pOZ-C E7 and pOZ E7 DLYC (53) and adapted for “en passant” mutagenesis, as previously described (18, 54). Details are provided in SI Materials and Methods. Δ97, a recombinant HCMV bearing a large deletion in UL97, has been previously described (18). All HCMV infection experiments were performed in human foreskin fibroblasts (ATCC) at an MOI of 1 PFU/cell, as previously described (18), with the exception that all virus preparations used in this study were concentrated by ultracentrifugation and resuspended in Dulbecco's modified Eagle's Medium (DMEM) containing 0.1% FBS before use. Ultracentrifugation of virus was performed as described previously (55), except 25 mM Na-HEPES pH 7.4 was used instead of Tris-HCl to buffer the 20% D-(+) sorbitol cushion. In experiments where serum-deprivation was used, cells were maintained in DMEM containing 0.1% FBS and 20 μg/mL gentamicin from 48 h before infection until the end of the experiment.
Construction of Recombinant Viruses.
Recombinant viruses were constructed using two-step Red, “en passant” recombination (54), as described previously (18). Δ97-E7 and Δ97-DLYC were constructed from AD169rv (21), a BAC clone of HCMV strain AD169. WT-E7 and WT-DLYC were derived from pBAC/AD169, a close predecessor of pAD/Cre (56). See Table S1 for a list of primers used, and SI Materials and Methods for further details.
Western Blotting.
Western blotting was carried out as described previously (7, 18). To detect E7, monoclonal antibodies ED19 (57) or ED17 (Santa Cruz Biotechnology), in combination with 8C9 (Invitrogen), were used. pRb, tubulin, UL97, IE1, UL44, and actin were detected as previously described (7, 18, 33). UL57 and pp28 were detected using mouse monoclonal antibodies (Virusys Inc). TS and PCNA were detected using monoclonal antibodies TS 106 (Abcam Inc.), and PC10 (Santa Cruz Biotechnology), respectively.
RNA Quantification.
To quantify mRNA levels of TS, UMPS, and TBP, reverse-transcriptase (RT) qPCR was performed on DNase treated total RNA isolated from infected cells using TaqMan Gene Expression Assay (Applied Biosystems) primer and probe sets for TS, UMPS, and TBP, as per the manufacturer's recommendations. Additional details are provided in SI Materials and Methods.
Viral Replication Assays.
Replication kinetics assays were performed as described previously (18), except supernatants of infected cells were collected without first scraping cells. Where indicated, maribavir was used at 1 μM.
Southern Blotting.
Southern blots were performed with chemiluminescent detection, as described previously (58). A probe that encompassed the entire UL83 ORF was used to detect viral DNA. Additional details are provided in SI Materials and Methods.
Supplementary Material
Acknowledgments.
We thank Blair L. Strang (Harvard Medical School), Courtney G. Havens (Harvard Medical School), and Timothy F. Kowalik (University of Massachussets Medical School, Worcester, MA) for helpful discussions; Ulrich Koszinowski (Max von Pettenkofer Institute, Munich, Germany) for AD169rv; Greg A. Smith (Northwestern University) for the generous gift of E. coli strain GS1783; Dong Yu and Thomas Shenk (Princeton University, Princeton, NJ) for pBAC/AD169; Gloria Komazin-Meredith (Harvard Medical School) for pp28-LUC; and Sandrine Boissel (Harvard Medical School) and Miranda Grace (Harvard Medical School) for expert technical assistance. R.F.K. is a Burroughs Wellcome Fund Investigator in the Pathogenesis of Infectious Disease. This work was supported by National Institutes of Health Grants RO1-AI26077 (to D.M.C.), R56-AI64703 (to R.F.K.), R01-CA066980 (to K.M.); National Institutes of Health Training Grants T32-CA009135–31 (to A.J.H.), T32-GM007215 (to A.J.H.), and T32-AI07245 (to J.P.K.); and National Institutes of Health Ruth L. Kirschstein National Research Service Award Postdoctoral Fellowship F32-AI075766 (to J.P.K.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0901521106/DCSupplemental.
References
- 1.Nevins JR. The Rb/E2F pathway and cancer. Hum Mol Genet. 2001;10:699–703. doi: 10.1093/hmg/10.7.699. [DOI] [PubMed] [Google Scholar]
- 2.Helt AM, Galloway DA. Mechanisms by which DNA tumor virus oncoproteins target the Rb family of pocket proteins. Carcinogenesis. 2003;24:159–169. doi: 10.1093/carcin/24.2.159. [DOI] [PubMed] [Google Scholar]
- 3.Hume AJ, Kalejta RF. Regulation of the retinoblastoma proteins by the human herpesviruses. Cell Div. 2009;4:1. doi: 10.1186/1747-1028-4-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boyer SN, Wazer DE, Band V. E7 protein of human papilloma virus-16 induces degradation of retinoblastoma protein through the ubiquitin-proteasome pathway. Cancer Res. 1996;56:4620–4624. [PubMed] [Google Scholar]
- 5.Dyson N, Howley PM, Munger K, Harlow E. The human papilloma virus-16 E7 oncoprotein is able to bind to the retinoblastoma gene product. Science. 1989;243:934–937. doi: 10.1126/science.2537532. [DOI] [PubMed] [Google Scholar]
- 6.Jones DL, Munger K. Analysis of the p53-mediated G1 growth arrest pathway in cells expressing the human papillomavirus type 16 E7 oncoprotein. J Virol. 1997;71:2905–2912. doi: 10.1128/jvi.71.4.2905-2912.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hume AJ, et al. Phosphorylation of retinoblastoma protein by viral protein with cyclin-dependent kinase function. Science. 2008;320:797–799. doi: 10.1126/science.1152095. [DOI] [PubMed] [Google Scholar]
- 8.Prichard MN, et al. Human cytomegalovirus UL97 kinase activity is required for the hyperphosphorylation of retinoblastoma protein and inhibits the formation of nuclear aggresomes. J Virol. 2008;82:5054–5067. doi: 10.1128/JVI.02174-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mocarski ES, Shenk T, Pass RF. Cytomegaloviruses. In: Knipe DM, Howley PM, editors. Fields' Virology. 5th Ed. Philadelphia: Wolters Kluwer Health/Lippincott Williams & Wilkins; 2007. pp. 2701–2772. [Google Scholar]
- 10.Biron KK, et al. Potent and selective inhibition of human cytomegalovirus replication by 1263W94, a benzimidazole L-riboside with a unique mode of action. Antimicrob Agents Chemother. 2002;46:2365–2372. doi: 10.1128/AAC.46.8.2365-2372.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lalezari JP, et al. Phase I dose escalation trial evaluating the pharmacokinetics, anti-human cytomegalovirus (HCMV) activity, and safety of 1263W94 in human immunodeficiency virus-infected men with asymptomatic HCMV shedding. Antimicrob Agents Chemother. 2002;46:2969–2976. doi: 10.1128/AAC.46.9.2969-2976.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Littler E, Stuart AD, Chee MS. Human cytomegalovirus UL97 open reading frame encodes a protein that phosphorylates the antiviral nucleoside analogue ganciclovir. Nature. 1992;358:160–162. doi: 10.1038/358160a0. [DOI] [PubMed] [Google Scholar]
- 13.Sullivan V, et al. A protein kinase homologue controls phosphorylation of ganciclovir in human cytomegalovirus-infected cells. Nature. 1992;358:162–164. doi: 10.1038/358162a0. [DOI] [PubMed] [Google Scholar]
- 14.Dunn W, et al. Functional profiling of a human cytomegalovirus genome. Proc Natl Acad Sci USA. 2003;100:14223–14228. doi: 10.1073/pnas.2334032100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Krosky PM, Baek MC, Coen DM. The human cytomegalovirus UL97 protein kinase, an antiviral drug target, is required at the stage of nuclear egress. J Virol. 2003;77:905–914. doi: 10.1128/JVI.77.2.905-914.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Prichard MN, et al. A recombinant human cytomegalovirus with a large deletion in UL97 has a severe replication deficiency. J Virol. 1999;73:5663–5670. doi: 10.1128/jvi.73.7.5663-5670.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wolf DG, Courcelle CT, Prichard MN, Mocarski ES. Distinct and separate roles for herpesvirus-conserved UL97 kinase in cytomegalovirus DNA synthesis and encapsidation. Proc Natl Acad Sci USA. 2001;98:1895–1900. doi: 10.1073/pnas.98.4.1895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kamil JP, Coen DM. Human cytomegalovirus protein kinase UL97 forms a complex with the tegument phosphoprotein pp65. J Virol. 2007;81:10659–10668. doi: 10.1128/JVI.00497-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Azzeh M, Honigman A, Taraboulos A, Rouvinski A, Wolf DG. Structural changes in human cytomegalovirus cytoplasmic assembly sites in the absence of UL97 kinase activity. Virology. 2006;354:69–79. doi: 10.1016/j.virol.2006.05.037. [DOI] [PubMed] [Google Scholar]
- 20.Prichard MN, Britt WJ, Daily SL, Hartline CB, Kern ER. Human cytomegalovirus UL97 kinase is required for the normal intranuclear distribution of pp65 and virion morphogenesis. J Virol. 2005;79:15494–15502. doi: 10.1128/JVI.79.24.15494-15502.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hobom U, Brune W, Messerle M, Hahn G, Koszinowski UH. Fast screening procedures for random transposon libraries of cloned herpesvirus genomes: Mutational analysis of human cytomegalovirus envelope glycoprotein genes. J Virol. 2000;74:7720–7729. doi: 10.1128/jvi.74.17.7720-7729.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jones DL, Thompson DA, Munger K. Destabilization of the RB tumor suppressor protein and stabilization of p53 contribute to HPV type 16 E7-induced apoptosis. Virology. 1997;239:97–107. doi: 10.1006/viro.1997.8851. [DOI] [PubMed] [Google Scholar]
- 23.Phelps WC, Munger K, Yee CL, Barnes JA, Howley PM. Structure-function analysis of the human papillomavirus type 16 E7 oncoprotein. J Virol. 1992;66:2418–2427. doi: 10.1128/jvi.66.4.2418-2427.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jault FM, et al. Cytomegalovirus infection induces high levels of cyclins, phosphorylated Rb, and p53, leading to cell cycle arrest. J Virol. 1995;69:6697–6704. doi: 10.1128/jvi.69.11.6697-6704.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wade M, Kowalik TF, Mudryj M, Huang ES, Azizkhan JC. E2F mediates dihydrofolate reductase promoter activation and multiprotein complex formation in human cytomegalovirus infection. Mol Cell Biol. 1992;12:4364–4374. doi: 10.1128/mcb.12.10.4364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Browne EP, Wing B, Coleman D, Shenk T. Altered cellular mRNA levels in human cytomegalovirus-infected fibroblasts: Viral block to the accumulation of antiviral mRNAs. J Virol. 2001;75:12319–12330. doi: 10.1128/JVI.75.24.12319-12330.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Song YJ, Stinski MF. Effect of the human cytomegalovirus IE86 protein on expression of E2F-responsive genes: A DNA microarray analysis. Proc Natl Acad Sci USA. 2002;99:2836–2841. doi: 10.1073/pnas.052010099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.DeGregori J, Kowalik T, Nevins JR. Cellular targets for activation by the E2F1 transcription factor include DNA synthesis- and G1/S-regulatory genes. Mol Cell Biol. 1995;15:4215–4224. doi: 10.1128/mcb.15.8.4215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ren B, et al. E2F integrates cell cycle progression with DNA repair, replication, and G(2)/M checkpoints. Genes Dev. 2002;16:245–256. doi: 10.1101/gad.949802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Marschall M, Freitag M, Weiler S, Sorg G, Stamminger T. Recombinant green fluorescent protein-expressing human cytomegalovirus as a tool for screening antiviral agents. Antimicrob Agents Chemother. 2000;44:1588–1597. doi: 10.1128/aac.44.6.1588-1597.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Depto AS, Stenberg RM. Functional analysis of the true late human cytomegalovirus pp28 upstream promoter: Cis-acting elements and viral trans-acting proteins necessary for promoter activation. J Virol. 1992;66:3241–3246. doi: 10.1128/jvi.66.5.3241-3246.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Marschall M, et al. Cellular p32 recruits cytomegalovirus kinase pUL97 to redistribute the nuclear lamina. J Biol Chem. 2005;280:33357–33367. doi: 10.1074/jbc.M502672200. [DOI] [PubMed] [Google Scholar]
- 33.Hamirally S, et al. Viral mimicry of Cdc2/cyclin-dependent kinase 1 mediates disruption of nuclear lamina during human cytomegalovirus nuclear egress. PLoS Pathog. 2009;5:e1000275. doi: 10.1371/journal.ppat.1000275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lee CP, et al. Epstein-Barr virus BGLF4 kinase induces disassembly of the nuclear lamina to facilitate virion production. J Virol. 2008;82:11913–11926. doi: 10.1128/JVI.01100-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.O'Brien V. Viruses and apoptosis. J Gen Virol. 1998;79:1833–1845. doi: 10.1099/0022-1317-79-8-1833. [DOI] [PubMed] [Google Scholar]
- 36.Chou S, Van Wechel LC, Marousek GI. Effect of cell culture conditions on the anticytomegalovirus activity of maribavir. Antimicrob Agents Chemother. 2006;50:2557–2559. doi: 10.1128/AAC.00207-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hertel L, Chou S, Mocarski ES. Viral and cell cycle-regulated kinases in cytomegalovirus-induced pseudomitosis and replication. PLoS pathogens. 2007;3:e6. doi: 10.1371/journal.ppat.0030006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Polyak K, et al. p27Kip1, a cyclin-Cdk inhibitor, links transforming growth factor-beta and contact inhibition to cell cycle arrest. Genes Dev. 1994;8:9–22. doi: 10.1101/gad.8.1.9. [DOI] [PubMed] [Google Scholar]
- 39.Reynisdottir I, Polyak K, Iavarone A, Massague J. Kip/Cip and Ink4 Cdk inhibitors cooperate to induce cell cycle arrest in response to TGF-beta. Genes Dev. 1995;9:1831–1845. doi: 10.1101/gad.9.15.1831. [DOI] [PubMed] [Google Scholar]
- 40.Gill RB, Frederick SL, Hartline CB, Chou S, Prichard MN. Conserved retinoblastoma protein-binding motif in human cytomegalovirus UL97 kinase minimally impacts viral replication but affects susceptibility to maribavir. Virology J. 2009;6:9. doi: 10.1186/1743-422X-6-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Botz J, et al. Cell cycle regulation of the murine cyclin E gene depends on an E2F binding site in the promoter. Mol Cell Biol. 1996;16:3401–3409. doi: 10.1128/mcb.16.7.3401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Geng Y, et al. Regulation of cyclin E transcription by E2Fs and retinoblastoma protein. Oncogene. 1996;12:1173–1180. [PubMed] [Google Scholar]
- 43.Ohtani K, DeGregori J, Nevins JR. Regulation of the cyclin E gene by transcription factor E2F1. Proc Natl Acad Sci USA. 1995;92:12146–12150. doi: 10.1073/pnas.92.26.12146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bresnahan WA, Boldogh I, Thompson EA, Albrecht T. Human cytomegalovirus inhibits cellular DNA synthesis and arrests productively infected cells in late G1. Virology. 1996;224:150–160. doi: 10.1006/viro.1996.0516. [DOI] [PubMed] [Google Scholar]
- 45.Dyson N, Guida P, Munger K, Harlow E. Homologous sequences in adenovirus E1A and human papillomavirus E7 proteins mediate interaction with the same set of cellular proteins. J Virol. 1992;66:6893–6902. doi: 10.1128/jvi.66.12.6893-6902.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pardee AB. G1 events and regulation of cell proliferation. Science. 1989;246:603–608. doi: 10.1126/science.2683075. [DOI] [PubMed] [Google Scholar]
- 47.Wang LH, et al. Phase I safety and pharmacokinetic trials of 1263W94, a novel oral anti-human cytomegalovirus agent, in healthy and human immunodeficiency virus-infected subjects. Antimicrob Agents Chemother. 2003;47:1334–1342. doi: 10.1128/AAC.47.4.1334-1342.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Trofe J, Pote L, Wade E, Blumberg E, Bloom RD. Maribavir: A novel antiviral agent with activity against cytomegalovirus. Ann Pharmacother. 2008;42:1447–1457. doi: 10.1345/aph.1L065. [DOI] [PubMed] [Google Scholar]
- 49.Michaelis M, Doerr HW, Cinatl J. The story of human cytomegalovirus and cancer: Increasing evidence and open questions. Neoplasia. 2009;11:1–9. doi: 10.1593/neo.81178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Prins RM, Cloughesy TF, Liau LM. Cytomegalovirus immunity after vaccination with autologous glioblastoma lysate. N Engl J Med. 2008;359:539–541. doi: 10.1056/NEJMc0804818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Scheurer ME, Bondy ML, Aldape KD, Albrecht T, El-Zein R. Detection of human cytomegalovirus in different histological types of gliomas. Acta Neuropathol. 2008;116:79–86. doi: 10.1007/s00401-008-0359-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cobbs CS, et al. Human cytomegalovirus infection and expression in human malignant glioma. Cancer Res. 2002;62:3347–3350. [PubMed] [Google Scholar]
- 53.Huh KW, et al. Association of the human papillomavirus type 16 E7 oncoprotein with the 600-kDa retinoblastoma protein-associated factor, p600. Proc Natl Acad Sci USA. 2005;102:11492–11497. doi: 10.1073/pnas.0505337102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tischer BK, von Einem J, Kaufer B, Osterrieder N. Two-step red-mediated recombination for versatile high-efficiency markerless DNA manipulation in Escherichia coli. Biotechniques. 2006;40:191–197. doi: 10.2144/000112096. [DOI] [PubMed] [Google Scholar]
- 55.Blankenship CA, Shenk T. Mutant human cytomegalovirus lacking the immediate-early TRS1 coding region exhibits a late defect. J Virol. 2002;76:12290–12299. doi: 10.1128/JVI.76.23.12290-12299.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yu D, Smith GA, Enquist LW, Shenk T. Construction of a self-excisable bacterial artificial chromosome containing the human cytomegalovirus genome and mutagenesis of the diploid TRL/IRL13 gene. J Virol. 2002;76:2316–2328. doi: 10.1128/jvi.76.5.2316-2328.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Nguyen CL, Eichwald C, Nibert ML, Munger K. Human papillomavirus type 16 E7 oncoprotein associates with the centrosomal component gamma-tubulin. J Virol. 2007;81:13533–13543. doi: 10.1128/JVI.01669-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Jurak I, Brune W. Induction of apoptosis limits cytomegalovirus cross-species infection. EMBO J. 2006;25:2634–2642. doi: 10.1038/sj.emboj.7601133. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.