Abstract
High consumption of cruciferous vegetables is associated with a reduced risk of prostate cancer in epidemiological studies. There is preliminary evidence that sulforaphane, derived from glucoraphanin found in a number of crucifers, may prevent and induce regression of prostate cancer and other malignancies in preclinical models, but the mechanisms that may explain these effects are not fully defined. Recent reports show that sulforaphane may impair prostate cancer growth through inhibition of histone deacetylases, which are up-regulated in cancer. Indeed, one of these enzymes, histone deacetylase 6 (HDAC6), influences the acetylation state of a key androgen receptor (AR) chaperone, HSP90. AR is the central signaling pathway in prostate cancer, and its inhibition is used for both prevention and treatment of this disease. However, it is not known whether the effects of sulforaphane involve suppression of AR. We hypothesized that sulforaphane treatment would lead to hyperacetylation of HSP90 and that this would destabilize AR and attenuate AR signaling. We confirmed this by demonstrating that sulforaphane enhances HSP90 acetylation, thereby inhibiting its association with AR. Moreover, AR is subsequently degraded in the proteasome, which leads to reduced AR target gene expression and reduced AR occupancy at its target genes. Finally, sulforaphane inhibits HDAC6 deacetylase activity, and the effects of sulforaphane on AR protein are abrogated by overexpression of HDAC6 and mimicked by HDAC6 siRNA. The inactivation by sulforaphane of HDAC6-mediated HSP90 deacetylation and consequent attenuation of AR signaling represents a newly defined mechanism that may help explain this agent's effects in prostate cancer.
Keywords: HSP90, acetylation, ERG
High consumption of cruciferous vegetables is associated with a lower risk of prostate cancer in epidemiological studies, although the precise constituents that may mediate this observation are unknown (1–4). Sulforaphane, a derivative of glucoraphanin found in crucifers, has heterogeneous biological activities including Phase 2 enzyme induction, cell cycle arrest, and apoptosis, but data on pathways that mediate effects on cell growth, survival, and differentiation are incomplete (5). Of note, several reports show that sulforaphane treatment of prostate cancer cells in vitro leads to reduced prostate cancer cell survival, and treatment of xenograft implants or transgenic animal models of prostate cancer with sulforaphane inhibits tumor formation and metastases (6–8).
Recently, sulforaphane was shown to inhibit histone deacetylase (HDAC) proteins, which are up-regulated in cancer (6, 9–11). Despite their name, some HDAC proteins remove acetyl groups from histone proteins whereas others act on non-histone proteins. Histone deacetylation reduces gene expression whereas non-histone protein deacetylation changes protein function (11). Histone deacetylase 6 (HDAC6) is a cytoplasmic non-histone protein deacetylase whose substrates include alpha-tubulin and the HSP90 chaperone protein (12–14). When HDAC6 deacetylates HSP90, this leads to activation of HSP90, enhanced binding of HSP90 to client proteins including the androgen receptor (AR) protein, and consequently attenuated degradation of client proteins including AR (13). Conversely, HSP90 hyperacetylation after treatment with certain HDAC inhibitors results in dissociation of HSP90 from client proteins such as AR and AR degradation (12, 13, 15, 16).
Indeed, AR, which is activated by androgens, is the most therapeutically relevant target in malignant prostate epithelial cells (17). Both preclinical studies and clinical trials have demonstrated the usefulness and effectiveness of targeting AR with hormonal agents, which act by reducing androgen production or binding to AR for prostate cancer prevention and treatment (17–19). Hormonal agents targeting AR in recurrent prostate cancer are commonly used but are not curative, and resistance mechanisms including AR gene amplification, AR mutations, and up-regulation of pathways that activate AR independent of androgens eventually develop in patients (20). In addition, recent work also demonstrates that androgen levels sufficient for AR activation persist in prostate cancer cells in man despite the use of hormonal agents, and that AR transcript variants, which encode for AR proteins, which are active even in the absence of androgens, exist (21, 22).
Thus, the destabilization and degradation of AR protein represents a rational strategy to interfere with AR signaling and to overcome the aforementioned resistance mechanisms. The present study was designed to test the hypothesis that sulforaphane would inhibit the function of HDAC6, and that this would suppress the stability or function of AR. Sulforaphane's ability to suppress HDAC6 was unknown, and its effects on AR signaling remained poorly characterized. We describe herein experimental results that confirm that sulforaphane destabilizes AR protein and disrupts AR signaling by inactivating HDAC6.
Results
Sulforaphane Treatment Increases HSP90 Acetylation and Leads to Dissociation of AR from HSP90.
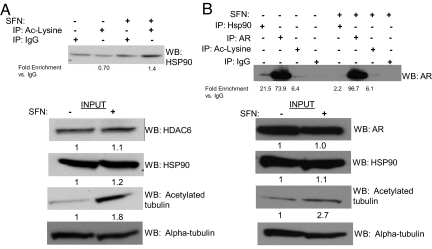
We treated LNCaP prostate cancer cells with sulforaphane and showed increased enrichment of acetylated lysines on HSP90 compared with vehicle 4 h after treatment (Fig. 1A). In the input samples, HSP90, HDAC6, and alpha-tubulin levels were similar between vehicle and sulforaphane-treated cells; levels of acetylated alpha-tubulin, a HDAC6 target, increased in sulforaphane-treated samples, indicating inhibition of protein deacetylation (Fig. 1A). Similar results were seen in VCaP prostate cancer cells (Fig. S1). Sulforaphane treatment also disrupted the interaction between HSP90 and AR (Fig. 1B). The AR immunoprecipitation followed by Western blotting for AR demonstrates similar AR protein levels between vehicle (lane 2) and sulforaphane-treated (lane 6) cells in the setting of decreased HSP90–AR interaction with sulforaphane treatment (lanes 1 and 5). Levels of acetylation of the androgen receptor were similar in both conditions (lanes 3 and 7). In the input samples, levels of HSP90, AR, and alpha-tubulin were similar between vehicle and sulforaphane-treated cells whereas sulforaphane increased levels of acetylated alpha-tubulin (Fig. 1B).
Fig. 1.
Sulforaphane treatment of prostate cancer cells increases HSP90 acetyation and dissociates it from AR. (A and B) Immunoprecipitations were carried out after treatment with sulforaphane (SFN) 20 μM or vehicle for 4 h followed by a Western blot for (A) HSP90 and (B) AR. Enrichment was quantified for each immunoprecipitation. Inputs were probed with the indicated antibodies by Western blot and were quantified.
Sulforaphane Treatment Lowers AR Protein Levels.
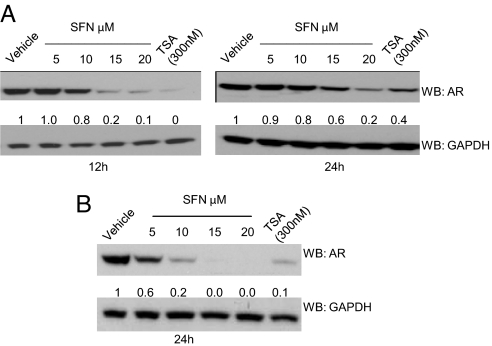
Using later time points, we treated LNCaP cells with sulforaphane and found that AR protein levels decreased at 12 h for the highest doses of sulforaphane (10–20 μM) (Fig. 2A). In VCaP cells, at 24 h, AR protein levels declined for all sulforaphane dose levels (Fig. 2B). Similar results were seen for both cell lines with the HDAC inhibitor trichostatin A (TSA) and the triterpenoid CDDO-Imidazole that, like sulforaphane, acts via the Nrf2-Keap1 pathway (Fig. S2). AR transcripts were not consistently suppressed by sulforaphane treatment, emphasizing that posttranscriptional mechanisms are involved (Fig. S3). Thus, across both AR-expressing cell lines, sulforaphane treatment reduced AR proteins levels.
Fig. 2.
Sulforaphane treatment of prostate cancer cells lowers AR protein levels. (A) Western blot of protein lysates from LNCaP cells treated with vehicle, increasing doses of sulforaphane, or TSA (trichostatin A) at the indicated time points. (B) Western blot of protein lysates from VCaP cells at 24 h. AR and GAPDH levels by Western blot were quantified.
Sulforaphane Treatment Lowers AR Target Gene Expression.
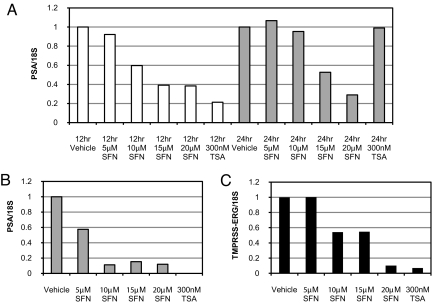
We next determined the functional consequences of AR protein depletion by performing real-time PCR for several AR target genes, PSA and the TMPRSS2-ERG gene fusion, which is present in VCaP cells (Fig. 3 A–C). There was a dose-dependent reduction in gene expression in both cell lines. Similar results were seen with TSA. Thus, there is a high concordance between reduced AR protein levels and reduced AR target gene expression in prostate cancer cells.
Fig. 3.
Sulforaphane treatment of prostate cancer cells reduces AR target gene expression. (A) Real-time PCR of PSA expression from LNCaP cells treated with vehicle, increasing doses of sulforaphane, or TSA at the indicated time points. (B and C) Real-time PCR of PSA (B) and TMPRSS2-ERG (C) gene expression from VCaP cells at 24 h. The vehicle-treated sample was set to 1. 18S was used as an endogenous control in all assays.
Sulforaphane Treatment Reduces AR Occupancy at Its Androgen Response Elements (AREs) and Lowers ERG Protein Levels.
To determine whether AR protein depletion from its target genes' AREs was responsible for reduced target gene expression, we performed chromatin immunoprecipitation (ChIP). Sulforaphane treatment reduced enrichment of AR at the PSA ARE in both cell lines (Fig. S4 A and B). Similarly, AR enrichment was decreased with sulforaphane treatment at the TMPRSS2 ARE (Fig. S4C). A Western blot with VCaP protein lysates at this same time point confirms near-absent levels of AR and ERG proteins after sulforaphane treatment (Fig. S4D).
Proteasome Inhibitor Treatment Rescues AR Protein from Sulforaphane-Induced Degradation.
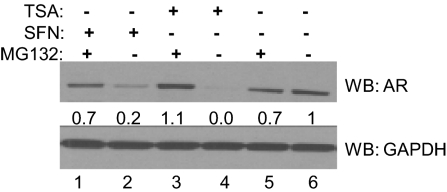
Because it is known that hyperacetylation of HSP90 inhibits its function and targets its client proteins to the proteasome for posttranslational degradation, we assessed the effects of treatment with sulforaphane and TSA with the proteasome inhibitor MG132 (Fig. 4). Treatment with sulforaphane (lane 2) or TSA (lane 4) in the absence of MG132 reduced AR protein levels versus the vehicle-treated cells (lane 6). However, simultaneous treatment with MG132 and either sulforaphane (lane 1) or TSA (lane 3) restored the AR steady-state level close to that seen in the vehicle-treated control (lane 5).
Fig. 4.
Proteasome inhibitor treatment of prostate cancer cells rescues AR protein from sulforaphane treatment. LNCaP cancer cells were treated for 24 h with 20 μM sulforaphane with or without 10 μM MG132, 300 nM TSA with or without 10 μM MG132, 10 μM MG132, or vehicle. AR levels and GAPDH levels by Western blot were quantified.
Sulforaphane Inhibits HDAC6 Enzymatic Function.
We determined recombinant HDAC6 enzymatic activity on its tubulin substrate in a cell-free tubulin deacetylase assay (Fig. 5). Incubation of vehicle-treated recombinant HDAC6 with tubulin dimers (lane 2) reduced levels of acetylated tubulin versus the vehicle-treated tubulin dimers incubated without HDAC6 (lane 1), which indicated that the HDAC6 enzyme was functional. However, incubation of HDAC6 with sulforaphane (lanes 3 and 4) or TSA (lane 5) led to decreased HDAC6 deacetylase activity as evidenced by increased levels of alpha-tubulin compared with the vehicle-treated HDAC6 control (lane 2).
Fig. 5.
Sulforaphane treatment inhibits HDAC6. Tubulin dimers were either incubated without recombinant HDAC6 or with recombinant HDAC6 in the presence of vehicle, sulforaphane, or TSA. Levels of HDAC6, acetylated tubulin, and tubulin by Western blot were quantified.
HDAC6 Overexpression Rescues AR and HDAC6 Proteins from Sulforaphane Treatment.
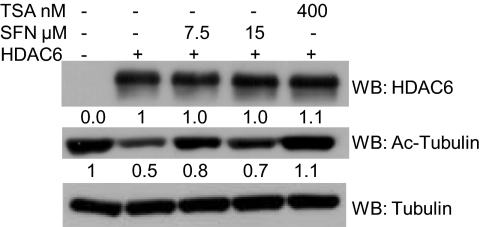
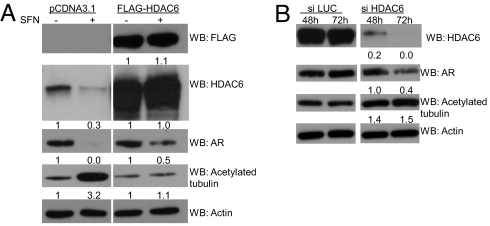
We overexpressed empty vector or FLAG-HDAC6 in LNCaP cells followed by treatment with sulforaphane or vehicle (Fig. 6A). Overexpression of HDAC6 led to a detectable anti-FLAG signal and higher levels of HDAC6 versus empty-vector-transfected cells, whereas levels of acetylated alpha-tubulin declined, indicating the presence of functional HDAC6 protein. Surprisingly, in empty-vector-transfected cells, HDAC6 protein levels were reduced with sulforaphane treatment; although sulforaphane does reduce HDAC6 transcript levels, this does not fully account for the observed HDAC6 protein depletion (Fig. S5A). Concomitant proteasome inhibitor and sulforaphane treatment (lane 4) restored HDAC6 protein levels versus sulforaphane-treated cells (lane 3) whereas TSA had minimal effects on HDAC6 protein levels (lanes 5 and 6) (Fig. S6). Additionally, in empty-vector-transfected cells, sulforaphane treatment lowered AR protein and increased acetylation of alpha-tubulin. However, in HDAC6-overexpressing, sulforaphane-treated cells, HDAC6 protein expression was restored, AR protein depletion was attenuated (without changing AR transcript levels), and alpha-tubulin acetylation was blunted versus the effects seen in empty-vector, sulforaphane-treated cells (Fig. 6A, Fig. S5B).
Fig. 6.
Ectopic overexpression of HDAC6 attenuates sulforaphane-mediated AR and HDAC6 protein depletion, and HDAC6 siRNA recapitulates the findings seen with sulforaphane. (A) LNCaP cells were transfected with pCDNA3.1 or FLAG-HDAC6. Cells were then treated with either vehicle or 15 μM sulforaphane. Intensity values for bands by Western blot in the respective vehicle controls were set to 1. (B) LNCaP cells were transfected with siRNA to either the luciferase gene (si LUC) or HDAC6 gene (si HDAC6). Levels of protein expression by Western blot were quantified, and the bands from the HDAC6 siRNA samples were compared to the luciferase control samples from the same time point.
HDAC6 siRNA Recapitulates Sulforaphane's Effect on AR Protein Levels.
We transiently transfected LNCaP cells with either a control or HDAC6 siRNA. HDAC6 siRNA depleted cells of HDAC6, which was most pronounced at 72 h (Fig. 6B). At this later time point, depletion of AR protein and acetylation of alpha-tubulin in the HDAC6 knockdown cells were most pronounced versus the control siRNA cells (Fig. 6B).
Discussion
High consumption of cruciferous vegetables is associated with a lower risk of prostate cancer development, but the precise constituents that may mediate this association are unknown. One possible candidate is sulforaphane, derived from glucoraphanin found in crucifers, which has been shown to have effects as a chemopreventive and anticancer agent in multiple preclinical systems through Phase 2 enzyme induction, cell cycle arrest, and apoptosis (1–4, 8, 23–33). However, the molecular mechanisms that underlie the previously observed antitumor effects of sulforaphane in prostate cancer models have not been fully clarified.
The AR protein is the central, therapeutically relevant pathway in prostate cancer, and hormonal deprivation strategies are commonly used in the treatment of all phases of this disease and work by interfering with androgen production or binding to AR, which is an activating event (17). Thus, interference with the state of AR activation or AR protein levels represents a rational strategy to ablate AR signaling for the prevention and treatment of this disease (17–19).
We show here that sulforaphane treatment increases acetylation of two HDAC6 target proteins: HSP90 and alpha-tubulin. At early time points, in the setting of unchanged HDAC6 protein levels, HSP90 becomes hyperacetylated, and the AR protein dissociates from HSP90, which matches an earlier report with the HDAC inhibitor LAQ824 (Fig. 1, Fig S1) (15). At later time points, after treatment with sulforaphane or the HDAC inhibitor TSA, AR protein levels decline, and this decline is attenuated by inhibition of proteasomal degradation, which suggests that sulforaphane treatment targets AR for proteolytic degradation (Figs. 2 and 4). This same effect has been demonstrated for other HSP90 client proteins including ErbB2, bcr-abl and c-kit after interference with HSP90 function with pharmacological HDAC inhibitors (12, 15, 34).
Although AR protein levels declined without reductions in AR transcript levels for most doses of sulforaphane, for the highest doses of sulforaphane (20 μM in LNCaP cells and 15–20 μM in VCaP cells) AR transcript levels also declined. We found that the HDAC inhibitor TSA also suppressed AR transcripts, a response that has been reported by others and with other HDAC inhibitors (Fig. S3) (35, 36). Consequently, the overall effect of sulforaphane and other agents with HDAC inhibitory properties on AR levels may not be solely posttranscriptional. It is clear, however, that a posttranslational effect is a critical control point in light of our observations that AR protein levels are rescued by simultaneous sulforaphane and proteasome inhibitor treatment or overexpression of HDAC6, and that most sulforaphane doses do not lower AR transcript levels (Figs. 4 and 6 and Figs. S3 and S5).
We confirmed that the disappearance of AR corresponded to reduced AR binding to and expression of its target genes (Fig. 3, Fig. S4). The reduced enrichment of AR at its target genes by ChIP demonstrates that AR depletion is mediating the reduced AR target gene expression. Notably, we found that levels of TMPRSS2-ERG, a fusion of the AR-regulated TMPRSS2 gene and the ERG transcription factor, which is commonly overexpressed in human prostate cancer, are reduced (37). ERG overexpression in normal prostate cells leads to enhanced invasiveness and growth, and ERG knockdown in VCaP prostate cancer cells by siRNA leads to decreased invasiveness (38). ERG overexpression under an AR-regulated promoter in a transgenic murine model was recently shown by two independent groups to transform prostate cells and to induce formation of prostate cancer precursor lesions highlighting ERG's importance in early stages of prostate tumorigenesis (38, 39). Thus, therapies such as sulforaphane, which deplete cells of AR and ERG protein, may hold promise for the prevention and treatment of prostate cancer.
We focused on HDAC6 as a key candidate for a sulforaphane target because others have shown that HDAC6, specifically, interacts with HSP90 and alpha-tubulin and deacetylates these proteins and that hyperacetylation of HSP90 cripples its chaperone function and leads to client protein dissociation and degradation (12–16). We demonstrated, by using a cell-free system, that incubation of recombinant HDAC6 with sulforaphane inhibits HDAC6 deacetylase activity. In cells, we showed that sulforaphane treatment inhibits HDAC6 function without changing its levels at early time points (4 h) (Figs. 1 and 5). However, at later time points (16 h), we have shown that sulforaphane leads to reduced HDAC6 protein levels (Fig. 6A, Fig. S6). The related compound CDDO-Imidazole also depletes HDAC6 in a dose-dependent manner, which parallels reduced AR protein levels and increased tubulin acetylation (Fig. S2). CDDO-Imidazole was previously shown to deplete Her2Neu protein levels in breast cancer cells through proteasomal degradation, although the effect of this agent on HDAC6 was not explored in that report (40). Given that CDDO-Imidazole, like sulforaphane, acts via the Nrf2 pathway, it is possible that the effect of these agents on HDAC6 protein levels is mediated by activation of gene targets of the Nrf2–Keap1 pathway.
There are several possible mechanisms for the reduced HDAC6 protein levels (beyond reduced HDAC6 transcript levels) (Fig. 6A, Fig. S5A); however, our data with proteasome inhibitor rescue suggests that proteasomal degradation is principal (Fig. S6). The importance of reduced HDAC6 levels with sulforaphane treatment is highlighted by the fact that overexpression of HDAC6 protein, in the presence of sulforaphane, reverses sulforaphane's effect on depleting HDAC6 and AR proteins and increasing acetylation of alpha-tubulin compared with empty-vector-transfected, sulforaphane-treated cells (Fig. 6A).
Finally, that HDAC6 depletion by siRNA recapitulates the sulforaphane treatment effects and leads to reduced AR protein further corroborates that the effects we have seen with sulforaphane are mediated by inactivation of HDAC6 (Fig. 6B). While it is possible that sulforaphane-mediated HSP90 hyperacetylation and consequent AR degradation occur via inhibition of HSP90 deacetylases besides HDAC6, although none are known, we can state that the influence of this agent on HDAC6 function and protein expression accounts, at least in part, for the influence of sulforaphane on the expression of the AR protein (Figs. 5 and 6).
Prostate cancer remains a common and sometimes lethal cancer, and it is clear that the AR protein is an important target in all phases of this disease (17, 20). Although the preclinical data and limited human data for sulforaphane is promising, including reports of inhibition of histone deacetylase activity and histone hyperacetylation and gene reactivation in xenograft tumors and intact tissues in animal models and peripheral blood mononuclear cells in man, our work elucidates unique mechanisms of action of sulforaphane through non-histone protein deacetylase inhibition-increased protein acetylation of HDAC6 targets such as alpha-tubulin and HSP90, inhibition or reduced levels of HDAC6 protein, and consequently reduced levels of AR protein and target genes including PSA and TMPRSS2-ERG (Fig. 7) (7, 9, 10). Further human testing will be necessary before suggesting whether sulforaphane may have a role in human prostate cancer prevention or therapy, but the studies we describe herein provide a strong rationale and, by clarifying the AR/HSP90/HDAC6 complex as a target of this agent, provide opportunities to conduct target validation studies.
Fig. 7.
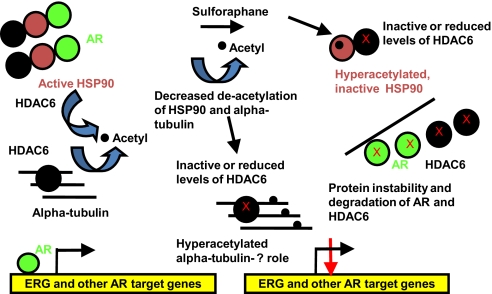
Model of sulforaphane attenuation of AR signaling via HDAC6 inactivation. Normally, HSP90 is deacetylated by HDAC6, which enables it to chaperone client proteins such as AR. HDAC6 also deacetylates alpha-tubulin. With sulforaphane treatment, HDAC6 is inhibited or targeted for protein degradation. This leads to hyperacetylated alpha-tubulin; the functional sigificance of this remains unclear. The HDAC6 inactivation with sulforaphane also leads to hyperacetylated, inactive HSP90 protein, which dissociates from AR, and AR is then targeted for protein degradation. Consequently, AR binding to its target gene androgen response elements (ARE), including TMPRSS2-ERG, is diminished, which reduces their expression.
Methods
Cell Culture and Drug Treatment.
LNCaP and VCaP cells were grown according to American Type Culture Collection instructions. Sulforaphane (SFN, #S4441 Sigma), MG-132 (#PI-102–0005, Biomol), and CDDO-Imidazole (National Cancer Institute/Developmental Therapeutics Program Open Chemical Repository) were resuspended in DMSO. Trichostatin A (TSA, #T8552 Sigma) was resuspended in 100% ethanol. Vehicle-treated cells received the respective vehicle controls.
Immunoprecipitation and Immunoblot Analysis.
Immunoprecipitation and immunoblotting experiments were performed as described (41). X-ray exposures were scanned as uncompressed images and bands were quantified by densitometry with Quantity One software (Bio-Rad). Intensity values were normalized to those of the endogenous controls. The vehicle sample was set to 1. Enrichment was calculated as: (intensity IP/intensity input)/ (intensity IgG/intensity input) or intensity IP/input (Fig. S1). See SI Methods for the antibodies used.
Real-time PCR (RNA extraction, cDNA, Real-time PCR).
Cells were lysed in TRIzol (Invitrogen) and then purified with the RNAEasy Kit (Qiagen). One microgram of RNA was reverse-transcribed using the Omniscript RT kit (Qiagen). See SI Methods for primer and PCR information. Standard curves were generated by measuring expression of target genes and 18S rRNA in serial dilutions of a mock-treated control. All samples were run in triplicate. The vehicle-treated sample was set to 1.
Chromatin Immunoprecipitation.
Cross-linking and sonication.
Cells were cross-linked with formaldehyde and reactions were stopped with glycine. Crosslinked cells were resuspended in IP buffer with SigmaFast protease inhibitor tablets (Sigma) and sonicated on ice by using a Branson Digital Sonifer model 450.
Matrix ChIP.
Sonicated cellular lysates were used for Matrix ChIP (42). Immunoprecipitations were performed without adding an antibody or with 500 ng of an anti-AR antibody.
PCR.
PCRs were amplified using a thermocycler (see SI Methods), and products were run on 2% NaBO3 agarose gels containing GelStar dye (Cambrex) for visualization (43, 44). Bands were imaged for densitometry by using a Gel Doc XR UV transilluminator and Quantity One software (Bio-Rad).
HDAC6 Tubulin Deacetylase (TDAC) Assay.
Two micrograms of recombinant HDAC6 (Biomol) were used in a cell-free tubulin deacetylase assay with 25 μg of MAP-rich polymerized tubules (Cytoskeleton), similar to Hubbert et al. (14).
HDAC6 Overexpression.
LNCaP cells were transiently transfected with pCDNA3.1 empty vector (Invitrogen) or FLAG-HDAC6 (Addgene) by using Lipofectamine LTX and Plus reagents (Invitrogen). Forty-eight hours later, media was replaced for a 16-h treatment with 15 μM sulforaphane or vehicle.
HDAC6 Knockdown.
LNCaP cells were transfected with siRNA by using DharmaFECT 3 transfection reagent for a final concentration of 100 nM (Dharmacon) (14). Cells were harvested at indicated time points posttransfection. Sequences are listed in SI Methods.
Supplementary Material
Acknowledgments.
We thank Grover Bagby, MD, Julien Licchesi, PhD, David Wang, MD, PhD, and Tomasz Beer, MD for constructive comments on this manuscript. We also thank Susanne McGlothlin for her help in preparing and submitting this manuscript. This work was funded by the Flight Attendant Medical Research Institute and National Institutes of Health award number 1KL2 RR024141 01 through the Oregon Clinical and Translational Research Institute, Grant UL1 RR024140 from the National Center for Research Resources, a component of the National Institutes of Health (NIH), and NIH Roadmap for Medical Research.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0908908106/DCSupplemental.
References
- 1.Cohen JH, Kristal AR, Stanford JL. Fruit and vegetable intakes and prostate cancer risk. J Natl Cancer Inst. 2000;92:61–68. doi: 10.1093/jnci/92.1.61. [DOI] [PubMed] [Google Scholar]
- 2.Kolonel LN, et al. Vegetables, fruits, legumes and prostate cancer: A multiethnic case-control study. Cancer Epidemiol Biomarkers Prev. 2000;9:795–804. [PubMed] [Google Scholar]
- 3.Kristal AR, Lampe JW. Brassica vegetables and prostate cancer risk: A review of the epidemiological evidence. Nutr Cancer. 2002;42:1–9. doi: 10.1207/S15327914NC421_1. [DOI] [PubMed] [Google Scholar]
- 4.Giovannucci E, Rimm EB, Liu Y, Stampfer MJ, Willett WC. A prospective study of cruciferous vegetables and prostate cancer. Cancer Epidemiol Biomarkers Prev. 2003;12:1403–1409. [PubMed] [Google Scholar]
- 5.Zhang Y. Cancer-preventive isothiocyanates: Measurement of human exposure and mechanism of action. Mutat Res. 2004;555:173–190. doi: 10.1016/j.mrfmmm.2004.04.017. [DOI] [PubMed] [Google Scholar]
- 6.Myzak MC, Hardin K, Wang R, Dashwood RH, Ho E. Sulforaphane inhibits histone deacetylase activity in BPH-1, LnCaP and PC-3 prostate epithelial cells. Carcinogenesis. 2006;27:811–819. doi: 10.1093/carcin/bgi265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Myzak MC, Tong P, Dashwood WM, Dashwood RH, Ho E. Sulforaphane retards the growth of human PC-3 xenografts and inhibits HDAC activity in human subjects. Exp Biol Med (Maywood) 2007;232:227–234. [PMC free article] [PubMed] [Google Scholar]
- 8.Singh SV, et al. Sulforaphane inhibits prostate carcinogenesis and pulmonary metastasis in TRAMP mice in association with increased cytotoxicity of natural killer cells. Cancer Res. 2009;69:2117–2125. doi: 10.1158/0008-5472.CAN-08-3502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Myzak MC, Dashwood WM, Orner GA, Ho E, Dashwood RH. Sulforaphane inhibits histone deacetylase in vivo and suppresses tumorigenesis in Apc-minus mice. FASEB J. 2006;20:506–508. doi: 10.1096/fj.05-4785fje. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Myzak MC, Karplus PA, Chung FL, Dashwood RH. A novel mechanism of chemoprotection by sulforaphane: Inhibition of histone deacetylase. Cancer Res. 2004;64:5767–5774. doi: 10.1158/0008-5472.CAN-04-1326. [DOI] [PubMed] [Google Scholar]
- 11.Marks P, et al. Histone deacetylases and cancer: Causes and therapies. Nat Rev Cancer. 2001;1:194–202. doi: 10.1038/35106079. [DOI] [PubMed] [Google Scholar]
- 12.Bali P, et al. Inhibition of histone deacetylase 6 acetylates and disrupts the chaperone function of heat shock protein 90: A novel basis for antileukemia activity of histone deacetylase inhibitors. J Biol Chem. 2005;280:26729–26734. doi: 10.1074/jbc.C500186200. [DOI] [PubMed] [Google Scholar]
- 13.Kovacs JJ, et al. HDAC6 regulates Hsp90 acetylation and chaperone-dependent activation of glucocorticoid receptor. Mol Cell. 2005;18:601–607. doi: 10.1016/j.molcel.2005.04.021. [DOI] [PubMed] [Google Scholar]
- 14.Hubbert C, et al. HDAC6 is a microtubule-associated deacetylase. Nature. 2002;417:455–458. doi: 10.1038/417455a. [DOI] [PubMed] [Google Scholar]
- 15.Chen L, et al. Chemical ablation of androgen receptor in prostate cancer cells by the histone deacetylase inhibitor LAQ824. Mol Cancer Ther. 2005;4:1311–1319. doi: 10.1158/1535-7163.MCT-04-0287. [DOI] [PubMed] [Google Scholar]
- 16.Scroggins BT, et al. An acetylation site in the middle domain of Hsp90 regulates chaperone function. Mol Cell. 2007;25:151–159. doi: 10.1016/j.molcel.2006.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Taplin ME. Drug insight: Role of the androgen receptor in the development and progression of prostate cancer. Nat Clin Pract Oncol. 2007;4:236–244. doi: 10.1038/ncponc0765. [DOI] [PubMed] [Google Scholar]
- 18.Thompson IM, et al. The influence of finasteride on the development of prostate cancer. N Engl J Med. 2003;349:215–224. doi: 10.1056/NEJMoa030660. [DOI] [PubMed] [Google Scholar]
- 19.Nair B, Wilt T, MacDonald R, Rutks I. Early versus deferred androgen suppression in the treatment of advanced prostatic cancer. Cochrane Database Syst Rev. 2002:CD003506. doi: 10.1002/14651858.CD003506. [DOI] [PubMed] [Google Scholar]
- 20.Scher HI, Sawyers CL. Biology of progressive, castration-resistant prostate cancer: Directed therapies targeting the androgen-receptor signaling axis. J Clin Oncol. 2005;23:8253–8261. doi: 10.1200/JCO.2005.03.4777. [DOI] [PubMed] [Google Scholar]
- 21.Mostaghel EA, et al. Intraprostatic androgens and androgen-regulated gene expression persist after testosterone suppression: Therapeutic implications for castration-resistant prostate cancer. Cancer Res. 2007;67:5033–5041. doi: 10.1158/0008-5472.CAN-06-3332. [DOI] [PubMed] [Google Scholar]
- 22.Hu R, et al. Ligand-independent androgen receptor variants derived from splicing of cryptic exons signify hormone-refractory prostate cancer. Cancer Res. 2009;69:16–22. doi: 10.1158/0008-5472.CAN-08-2764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chung FL, Conaway CC, Rao CV, Reddy BS. Chemoprevention of colonic aberrant crypt foci in Fischer rats by sulforaphane and phenethyl isothiocyanate. Carcinogenesis. 2000;21:2287–2291. doi: 10.1093/carcin/21.12.2287. [DOI] [PubMed] [Google Scholar]
- 24.Fahey JW, Zhang Y, Talalay P. Broccoli sprouts: An exceptionally rich source of inducers of enzymes that protect against chemical carcinogens. Proc Natl Acad Sci USA. 1997;94:10367–10372. doi: 10.1073/pnas.94.19.10367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang Y, Kensler TW, Cho CG, Posner GH, Talalay P. Anticarcinogenic activities of sulforaphane and structurally related synthetic norbornyl isothiocyanates. Proc Natl Acad Sci USA. 1994;91:3147–3150. doi: 10.1073/pnas.91.8.3147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bonnesen C, Eggleston IM, Hayes JD. Dietary indoles and isothiocyanates that are generated from cruciferous vegetables can both stimulate apoptosis and confer protection against DNA damage in human colon cell lines. Cancer Res. 2001;61:6120–6130. [PubMed] [Google Scholar]
- 27.Fimognari C, et al. Cyclin D3 and p53 mediate sulforaphane-induced cell cycle delay and apoptosis in non-transformed human T lymphocytes. Cell Mol Life Sci. 2002;59:2004–2012. doi: 10.1007/PL00012523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fimognari C, et al. Growth inhibition, cell-cycle arrest and apoptosis in human T-cell leukemia by the isothiocyanate sulforaphane. Carcinogenesis. 2002;23:581–586. doi: 10.1093/carcin/23.4.581. [DOI] [PubMed] [Google Scholar]
- 29.Gamet-Payrastre L, et al. Sulforaphane, a naturally occurring isothiocyanate, induces cell cycle arrest and apoptosis in HT29 human colon cancer cells. Cancer Res. 2000;60:1426–1433. [PubMed] [Google Scholar]
- 30.Gamet-Payrastre L, et al. Selective cytostatic and cytotoxic effects of glucosinolates hydrolysis products on human colon cancer cells in vitro. Anticancer Drugs. 1998;9:141–148. doi: 10.1097/00001813-199802000-00005. [DOI] [PubMed] [Google Scholar]
- 31.Gingras D, et al. Induction of medulloblastoma cell apoptosis by sulforaphane, a dietary anticarcinogen from Brassica vegetables. Cancer Lett. 2004;203:35–43. doi: 10.1016/j.canlet.2003.08.025. [DOI] [PubMed] [Google Scholar]
- 32.Singh AV, Xiao D, Lew KL, Dhir R, Singh SV. Sulforaphane induces caspase-mediated apoptosis in cultured PC-3 human prostate cancer cells and retards growth of PC-3 xenografts in vivo. Carcinogenesis. 2004;25:83–90. doi: 10.1093/carcin/bgg178. [DOI] [PubMed] [Google Scholar]
- 33.Wang L, et al. Targeting cell cycle machinery as a molecular mechanism of sulforaphane in prostate cancer prevention. Int J Oncol. 2004;24:187–192. [PubMed] [Google Scholar]
- 34.Bauer S, Yu LK, Demetri GD, Fletcher JA. Heat shock protein 90 inhibition in imatinib-resistant gastrointestinal stromal tumor. Cancer Res. 2006;66:9153–9161. doi: 10.1158/0008-5472.CAN-06-0165. [DOI] [PubMed] [Google Scholar]
- 35.Marrocco DL, et al. Suberoylanilide hydroxamic acid (vorinostat) represses androgen receptor expression and acts synergistically with an androgen receptor antagonist to inhibit prostate cancer cell proliferation. Mol Cancer Ther. 2007;6:51–60. doi: 10.1158/1535-7163.MCT-06-0144. [DOI] [PubMed] [Google Scholar]
- 36.Rokhlin OW, et al. Mechanisms of cell death induced by histone deacetylase inhibitors in androgen receptor-positive prostate cancer cells. Mol Cancer Res. 2006;4:113–123. doi: 10.1158/1541-7786.MCR-05-0085. [DOI] [PubMed] [Google Scholar]
- 37.Tomlins SA, et al. Recurrent fusion of TMPRSS2 and ETS transcription factor genes in prostate cancer. Science. 2005;310:644–648. doi: 10.1126/science.1117679. [DOI] [PubMed] [Google Scholar]
- 38.Tomlins SA, et al. Role of the TMPRSS2-ERG gene fusion in prostate cancer. Neoplasia. 2008;10:177–188. doi: 10.1593/neo.07822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Klezovitch O, et al. A causal role for ERG in neoplastic transformation of prostate epithelium. Proc Natl Acad Sci USA. 2008;105:2105–2110. doi: 10.1073/pnas.0711711105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Konopleva M, et al. Synthetic triterpenoid 2-cyano-3,12-dioxooleana-1,9-dien-28-oic acid induces growth arrest in HER2-overexpressing breast cancer cells. Mol Cancer Ther. 2006;5:317–328. doi: 10.1158/1535-7163.MCT-05-0350. [DOI] [PubMed] [Google Scholar]
- 41.Qian DZ, et al. Targeting tumor angiogenesis with histone deacetylase inhibitors: The hydroxamic acid derivative LBH589. Clin Cancer Res. 2006;12:634–642. doi: 10.1158/1078-0432.CCR-05-1132. [DOI] [PubMed] [Google Scholar]
- 42.Flanagin S, Nelson JD, Castner DG, Denisenko O, Bomsztyk K. Microplate-based chromatin immunoprecipitation method, Matrix ChIP: A platform to study signaling of complex genomic events. Nucleic Acids Res. 2008;36:e17. doi: 10.1093/nar/gkn001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang Q, et al. A hierarchical network of transcription factors governs androgen receptor-dependent prostate cancer growth. Mol Cell. 2007;27:380–392. doi: 10.1016/j.molcel.2007.05.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yoon HG, Wong J. The corepressors silencing mediator of retinoid and thyroid hormone receptor and nuclear receptor corepressor are involved in agonist- and antagonist-regulated transcription by androgen receptor. Mol Endocrinol. 2006;20:1048–1060. doi: 10.1210/me.2005-0324. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.