Abstract
Mucosal dendritic cells have been implicated in the capture, storage, and transmission of HIV to CD4+ T cells as well as in the promotion of HIV replication in activated CD4+ T cells during the cognate T-cell and DC interaction. We report that HIV induces human genital mucosal epithelial cells to produce thymic stromal lymphopoietin (TSLP) via activation of the NFκB signaling pathway. The TSLP secreted by HIV exposed epithelial cells activated DC, which promoted proliferation and HIV-1 replication of co-cultured autologous CD4+ T cells. In rhesus macaques, we observed dramatic increases in TSLP expression concurrent with an increase in viral replication in the vaginal tissues within the first 2 weeks after vaginal SIV exposure. These data suggest that HIV-mediated TSLP production by mucosal epithelial cells is a critical trigger for DC-mediated amplification of HIV-infection in activated CD4+ T cells. The cross talk between mucosal epithelial cells and DC, mediated by HIV-induced TSLP, may be an important mechanism for the high rate of HIV infection in women through the vaginal mucosa.
Keywords: Dendritic cells, HIV
More than half of all new HIV type 1 (HIV-1) infections are acquired by women through intravaginal exposure (1–5). Even though genital mucosal epithelial cells express low to negligible levels of the receptors for HIV and the mucosal microenvironment is laden with soluble antiviral factors, productive infection of T cells and macrophages does occur at these sites and effectively spreads to distant lymphoid tissues, most rapidly to the gut (6–10). Dendritic cells (DCs) in the genital mucosal tissues can capture HIV and migrate to the draining lymph nodes where the virus is transmitted to CD4+ T cells (11, 12). However, the first host cell type in the mucosal surface to sense HIV infection, trigger immune activation, and promote HIV amplification and spread remains unknown. We and others have previously shown that thymic stromal lymphopoietin (TSLP), an epithelial cell-derived interleukin 7 (IL-7)-like cytokine, is expressed in response to microbial infection or allergen exposure (13, 14). TSLP potently activates human myeloid DCs (mDCs), which display a remarkable ability to induce homeostatic expansion of naïve CD4+ T cells (13, 14). These studies suggest that TSLP links the communication between epithelial cells and the DCs of the immune system at a molecular level. We therefore hypothesized that HIV entry at the mucosal tissues stimulates epithelial cells to produce TSLP, which may activate DCs, leading to recruitment and expansion of CD4+ T-cell targets for HIV infection. We obtained evidence for HIV induced human epithelial cells to produce TSLP via the NFκB signaling pathway in vitro. In rhesus macaques inoculated with simian immunodeficiency virus (SIV) by the vaginal route, we observed dramatic increases in TSLP expression and SIV replication in the vaginal tissues within the first 2 weeks after virus exposure.
Results
HIV Induces Production of TSLP in Epithelial Cells.
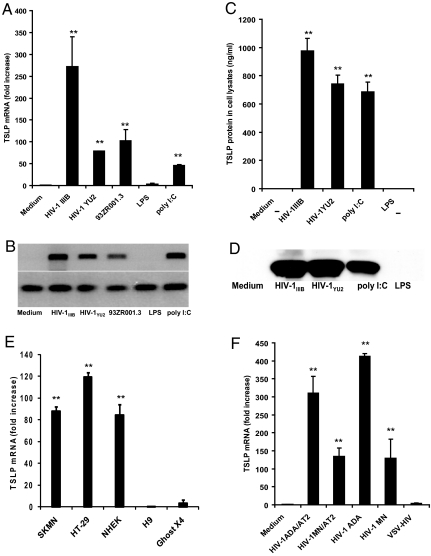
To investigate whether HIV induces TSLP production by epithelial cells, we cultured human cervical epithelial cells (C33A) with HIV for 12–18 h at 37 °C and analyzed the cell extracts as well as the culture supernatants for TSLP. Compared with the medium control, the T-cell tropic virus HIV-1IIIB (X4 strain), macrophage tropic HIV-1YU2 (R5 strain), and a primary clade D HIV-1 isolate 93ZR001.3 were all able to induce C33A cells to produce high levels of TSLP mRNA as measured by quantitative PCR (Fig. 1A) and by RT-PCR (Fig. 1B). The TSLP expression was also confirmed at the protein level by ELISA (Fig. 1C) and Western blot analyses (Fig. 1D). As described in the literature, TSLP expression at the RNA and protein levels was also observed with poly I:C but not lipopolysaccharide (LPS) (15). The HIV-induced increase in TSLP expression was observed in several different human epithelial cell lines and primary human keratinocytes, but not in other cell types such as the fibroblasts and T cells (Fig. 1E). As shown in Fig. 1F, significantly high levels of TSLP expression were observed in the C33A cells cultured with both X4 and R5 strains of HIV-1 (HIV-1MN and HIV-1ADA, respectively), that are infectious as well as inactivated by treatment with aldrithiol-2 (AT-2), a reagent shown to covalently modify the essential zinc fingers in the nucleocapsid (NC) protein of HIV-1 thereby arresting the viral life cycle before initiation of reverse transcription (16, 17). On the other hand, no TSLP expression was observed in cells cultured with HIV-1 that is pseudotyped with Vesicular Stomatitis Virus (VSV) envelope (VSV-HIV). These results demonstrate that exposure to diverse HIV-1 strains, both infectious and noninfectious, can result in efficient induction of TSLP expression in a variety of epithelial cells, including primary human keratinocytes.
Fig. 1.
HIV induces TSLP expression in cervical epithelial cells. Increased levels of TSLP mRNA were detected in cervical epithelial cells (C33A) exposed to HIV-1IIIB, HIV-1YU2, the primary clade D HIV isolate 93ZR001.3 (equivalent to 60–70 × 103 cpm of RT activity) or poly IC (5 μg/mL) when compared to medium alone or in cells cultured with LPS (5 μg/mL), as assessed by quantitative real time PCR (A) and RT PCR (B). **, P < 0.01 comparing TSLP mRNA levels in HIV-treated cells to LPS-treated cells. Increased levels of TSLP protein were detected in the lysates of the C33A cells exposed to HIV-1IIIB, HIV-1YU2, or poly I:C by ELISA (C) and also by Western blot analysis (D). **, P < 0.01 comparing TSLP mRNA levels in HIV-treated cells to LPS-treated cells. Increased levels of TSLP mRNA were detected in neuronal and intestinal epithelial cells (SKMN and HT-29, respectively) as well as in primary normal human epithelial keratinocytes (NHEK), but not in non-epithelial cells such as human T lymphoblastoid cells (H9) and fibroblast-like human osteosarcoma tumor cells (GHOST X4) (E). **, P < 0.01 comparing TSLP mRNA levels in epithelial cells to GHOST X4 cells. Increased levels of TSLP mRNA were detected in cervical epithelial cells (C33A) cultured with infectious R5 and X4 viruses, (HIV-1ADA and HIV-1MN, respectively) as well as non-infectious AT-2 treated viruses (HIV-1ADA and HIV-1MN, respectively), when compared to medium alone or cells cultured with VSV-pseudotyped HIV (VSV-HIV) (F). **, P < 0.01 comparing TSLP mRNA levels in HIV-treated cells to VSV-HIV-treated cells. All of the viruses were used at concentrations equivalent to 60–70 × 103 cpm of RT activity, and data presented are average values of three independent experiments.
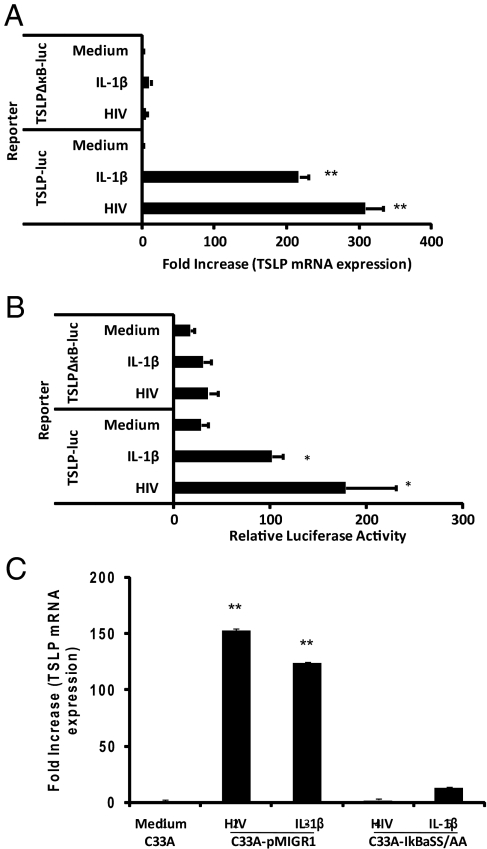
Transcriptional Activation of the Human TSLP Promoter by HIV Includes NFκB Activation.
It has recently been shown that TSLP expression can be induced in airway epithelial cells by pro-inflammatory mediators (IL-1β, TNF-α) and agonists for toll-like receptors 2, 8, and 9 (TLR2, TLR8, and TLR9, respectively), via the induction of NFκB, and an NFκB-binding site was also identified within the human TSLP (hTSLP) promoter (18). We investigated whether the mechanism by which HIV induces TSLP expression in epithelial cells involves NFκB signaling. Cervical epithelial cells (C33A) were transfected with a luciferase reporter plasmid containing either the full-length hTSLP promoter which contains an NFκB-binding site 3.7 kb upstream of the start of transcription (TSLP-luc), or a mutated NFκB-binding motif (TSLPΔκB-luc) constructed by site-directed mutagenesis (18). The transfected cells were then incubated with HIV or IL-1β, as the positive control reagent, and TSLP mRNA expression and luciferase activity were determined. Compared with the medium control, HIV was able to induce an increase in TSLP mRNA expression (309-fold) and the hTSLP promoter activity (178-fold) to levels similar to those observed after stimulation with IL-1β (Fig. 2 A and B). On the other hand, no significant TSLP mRNA expression or luciferase activity were observed in C33A cells transfected with the construct containing the mutated NFκB-binding site and exposed to HIV or IL-1β suggesting that HIV-induced TSLP expression, similar to that of IL-1β, involves the NFκB signaling (Fig. 2 A and B). Additionally we constructed retroviral vectors expressing the NF-κB superrepressor, IκBαSS/AA, or a control sequence pMIGR1 and transfected C33A cells to derive C33A-IκBαSS/AA and C33A-pMIGR1 cell lines, respectively. Expression of IκBαSS/AA, but not pMIGR1, resulted in the abrogation of TSLP expression in response to HIV as well as IL-1β in these cells (Fig. 2C). Together, these results further support the involvement of the NFκB signaling pathway in the transcriptional activation of HIV induced TSLP expression.
Fig. 2.
Transcriptional activation of the human TSLP promoter by HIV includes NFκB activation. Increased levels of TSLP mRNA were detected in cervical epithelial cells (C33A) transfected with wild-type human TSLP promoter plasmid exposed to HIV-1IIIB and IL-1β when compared to medium alone or in cells transfected with a mutated NFκB-binding site as assessed by quantitative real-time PCR (A). **, P < 0.01 comparing TSLP mRNA levels in epithelial cells (C33A) transfected with wild-type human TSLP promoter plasmid to cells transfected with a mutated NFκB-binding site. Increased levels of luciferase activity were detected in cervical epithelial cells (C33A) transfected with wild-type human TSLP promoter-driven luciferase reporter plasmid (hTSLP-luc) exposed to HIV-1IIIB and IL-1β when compared to medium alone or in cells transfected with hTSLP-luc plasmid containing a mutated NFκB-binding site (TSLPΔκB-luc) (B). *, P < 0.05, statistically significant difference compared with cells transfected with hTSLP-luc plasmid containing a mutated NFκB-binding site. TSLP expression, as determined by real-time PCR, in response to HIV-1IIIB or IL-1β was abrogated in C33A cells transfected with a retroviral vector expressing an NFκB superrepressor, IκBαSS/AA, (C33A-IκBαSS/AA), but not in those transfected with a control vector, pMIGR1, (C33A-pMIGR1) (C). **, P < 0.01 comparing TSLP mRNA levels in epithelial cells (C33A) transfected with a control vector to cells transfected with a retroviral vector expressing an NFκB superrepressor. The data presented are average values of three independent experiments.
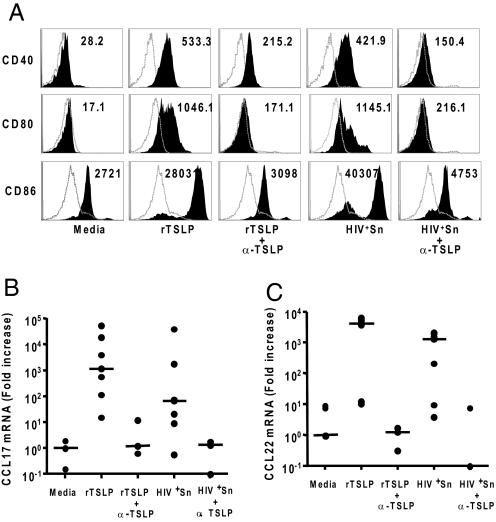
TSLP from HIV-Exposed Epithelial Cells Activates Human CD11c+ Myeloid Dendritic Cells.
TSLP has two key functional effects on human myeloid DCs (mDCs) that include upregulation of costimulatory molecules CD80 and CD86, and induction of thymus and activation regulated chemokine (TARC or CCL17) and macrophage-derived chemokine (MDC or CCL22) secretion (14, 19). To determine the biological activity of TSLP in the supernatants of HIV exposed epithelial cells, we cultured CD11c+ mDCs isolated from human peripheral blood mononuclear cells (PBMCs) with supernatants of epithelial cells incubated with HIV (HIV+Sn), recombinant TSLP (rTSLP) at 100 ng/mL, or culture medium (Fig. 3). We observed significant upregulation of CD80, CD86, and CD40 expression on mDCs after 24 h of culture with HIV+Sn, similar to that with rTSLP (Fig. 3A). The ability of HIV+Sn and rTSLP to upregulate activation marker expression could be blocked by neutralizing TSLP antibodies (Fig. 3A). In addition, rTSLP and HIV+Sn stimulated mDC to produce high levels of chemokines CCL17 and CCL22 at both the mRNA and protein levels (Fig. 3 B and C and Fig. S1 A and B). This biological activity of TSLP within the HIV+Sn, along with that of rTSLP could also be blocked by neutralizing TSLP antibodies (Fig. 3 B and C and Fig. S1 A and B).
Fig. 3.
TSLP within the supernatant from epithelial cells cultured with HIV potently activates human CD11c+ myeloid dendritic cells (mDCs) and induces production of CCL17 and CCL22. Recombinant TSLP (rTSLP) and supernatant from C33A cells cultured with HIV-1IIIB (HIV+Sn) potently upregulated surface expression of CD40, CD80, and CD86 on CD11c+ mDCs, relative to that from cells cultured with tissue culture medium only (A). The open histograms represent the isotype control antibody treatment and the filled histograms represent staining for antibodies to the specific DC activation markers. Numbers indicate the mean fluorescence intensity (MFI). Treatment of mDC isolated by fluorescence sorting from the PBMC samples of multiple donors with rTSLP or HIV+Sn resulted in the expression of high amounts of the chemokines CCL17 and CCL22 as determined by quantitative real time PCR analyses of the mRNA (B and C). The mDCs isolated from multiple donors were pretreated with TSLP-specific antibody (α-TSLP) or an isotype control antibody before stimulation with culture medium, rTSLP or supernatants collected from C33A cells cultured with HIV-1IIIB (HIV+Sn), and the expression levels of the activation markers CD40, CD80, and CD86 were determined by flow cytometry (A) and that of chemokines CCL17 and CCL22 by quantitative RT-PCR (B and C).
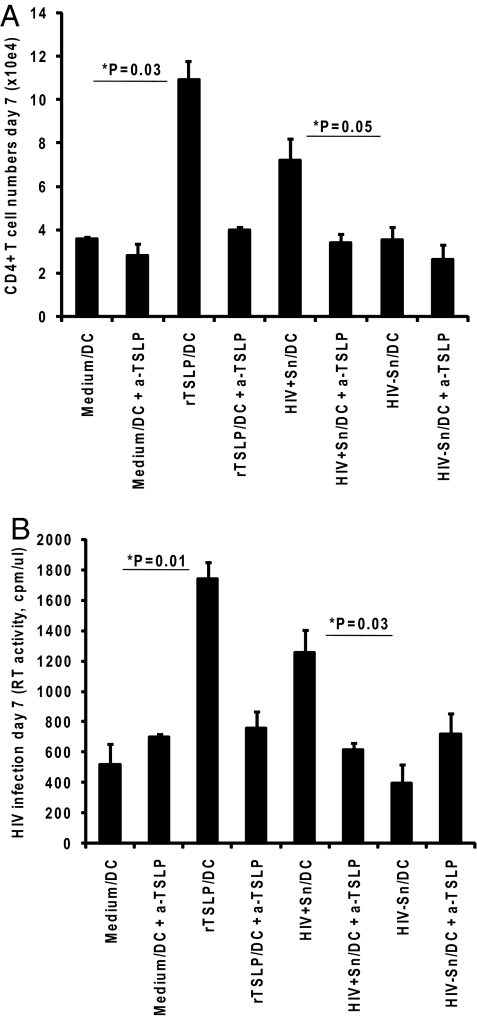
Myeloid DCs Activated by TSLP from HIV-Exposed Epithelial Cells Induce Naïve CD4+ T-Cell Expansion and HIV Infection.
To further delineate the biological activity of the HIV-induced TSLP, mDCs activated by the culture supernatants of HIV-exposed epithelial cells (HIV+Sn/DCs) were co-cultured at a 1:1 ratio for 7 days with autologous, naïve CD4+ T cells. We found that, relative to that of DCs treated either with medium (medium/DCs) or HIV−Sn (HIV−Sn/DCs), both rTSLP-treated DCs (rTSLP/DCs) and HIV+Sn/DCs induced a significant expansion of CD4+ T cells by day 7 (Fig. 4A). Increases in the numbers of CD4+ T cells on day 7, as determined by enumerating the total viable cells using the Trypan-blue dye exclusion method, were 1.8-fold for co-cultures with HIV+Sn/DCs and 3.04-fold for those with rTSLP/DCs. Additionally, significant levels of CD4+ T-cell proliferation were observed in co-cultures with rTSLP/DCs and HIV+Sn/DCs, compared to that with medium/DCs and HIV−Sn/DCs, respectively, as determined by the [3H]thymidine incorporation and CFSE staining methodologies (Fig. S2 A and B). A key feature of TSLP-DC is their ability to trigger the homeostatic proliferation of inflammatory Th2 CD4+ T cells that produce the Th2 cytokines IL-4, IL-5, and IL-13 along with the traditional Th1 cytokine TNF-α (19). We observed high level expression of each of these cytokines, but not IL-10 or IFN-γ, in the CD4+ T cells co-cultured with HIV+Sn/DCs, similar to that seen with rTSLP/DCs (Fig. S3). Addition of HIV to the co-cultures resulted in significant increases in infection, as determined by the analysis of the reverse transcriptase (RT) activity, with HIV+Sn/DCs and rTSLP/DCs when compared to respective control co-cultures of HIV−Sn/DCs and medium/DCs (Fig. 4C). These changes amounted to 3.2- and 3.4-fold increases in the levels of infection by day 7 in the co-cultures of T cells with HIV+Sn/DCs and rTSLP/DCs, respectively, compared to that with HIV−Sn/DCs or medium/DCs. The increases in the numbers as well as HIV infection of naïve autologous CD4+ T cells in co-cultures with DCs activated by rTSLP or the HIV+Sn could be blocked by neutralizing TSLP antibodies (Fig. 4 A and B). Similar results showing increased infection of co-cultured T cells were observed when HIV-1YU2, an R5 strain of HIV-1 was used (Fig. S4).
Fig. 4.
mDC activated by HIV-induced TSLP from epithelial cells promote naïve autologous CD4+ T-cell proliferation and increased HIV infection. Naïve CD4+ T cells were co-cultured with mDCs that were activated by preincubating with the culture medium, rTSLP, or supernatants collected from C33A cells cultured with or without HIV-1IIIB (HIV+Sn and HIV−Sn, respectively), and increased numbers of viable cells on day 7 were determined by Trypan blue dye-exclusion (A). A separate set of co-cultures were infected with HIV-1IIIB for seven days and the amount of virus produced in the supernatants was measured by estimating the RT activity on day 7 (B). Error bars represent standard deviation values for triplicate cultures, and data shown are average values of three independent experiments.
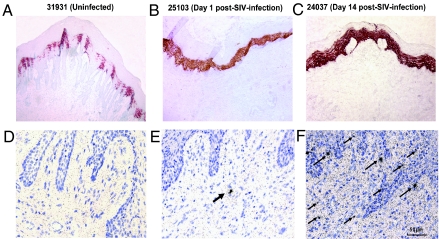
TSLP Is Expressed in the Vaginal Tissues of Rhesus Macaques Infected with Simian Immunodeficiency Virus, SIVmac251.
We investigated whether the HIV-induced TSLP expression observed in the in vitro studies with human epithelial cells also occurs after in vivo infection within the mucosal sites of viral entry in the nonhuman primate model by studying vaginal tissues from rhesus macaques intravaginally inoculated with the simian immunodeficiency virus, SIVmac251. For this, we took advantage of rhesus monkey vaginal tissue samples available from an earlier study where the animals were infected by the vaginal route with SIVmac251 and vaginal samples were obtained after necropsy of animals at various time points post-challenge (20). Immunohistochemistry analyses demonstrated high levels of TSLP in the vaginal mucosa starting as early as day 1 post-inoculation (post-SIV), relative to that seen before infection (Fig. 5 A and B and Fig. S5 A–F). The TSLP expression levels remained high at day 14 as assessed in one animal (Fig. 5C). The levels of TSLP expression correlated with a 41- and 99-fold increase in TSLP mRNA expression at days 1 and 14, respectively, compared to that in animals before infection as determined by quantitative real time PCR analyses of the mRNA isolated from the tissue samples (Fig. S6). This increase in TSLP expression coincided with a large increase in viral RNA positive cells by day 14 post-SIV, relative to day 1 and 0 (uninfected) in these tissues as determined by in situ hybridization (ISH) analyses (Fig. 5 D–F). Viral RNA copy analyses reported previously for these monkeys was below the detectable level in uninfected animals while post-SIV infection the levels ranged between 4 × 102 and 3.6 × 104 for day 1 and between 5.0 × 105 and 6.0 × 105 for days 9 through 14 (20). Thus, there was a rapid and sustained increase in TSLP expression coinciding with SIV infection in the vaginal mucosa of rhesus macaques after SIV inoculation by the vaginal route. While expression of a number of inflammatory mediators, cytokines and antiviral effector molecules within the vaginal tissues has been reported after vaginal SIV inoculation (21), our in vitro studies showed HIV-induced TSLP induces DC activation, because neutralization of TSLP within the HIV-exposed epithelial cell culture supernatants abrogated DC activation as well as homeostatic expansion and HIV replication of CD4+ T cells in co-cultures. Together, our in vitro data from HIV-exposed human epithelial cells and the results from vaginal tissues in the non-human primates exposed to SIV by the vaginal route suggest that HIV/SIV can effectively manipulate the mucosal microenviroment to ensure local infection by inducing epithelial cells to produce TSLP.
Fig. 5.
Increased expression of TSLP in vaginal mucosal tissues from rhesus macaques after vaginal SIV infection. Samples collected from the vaginal tissues of monkeys before infection (A, uninfected) and days 1 (B) and 14 (C) post-infection with SIVmac251 were used for immunohistochemical staining for TSLP showed low levels of TSLP in the normal vaginal tissues, but higher levels after 1 and 14 days post-SIV exposure. The sequential samples were also subjected to in situ hybridization (ISH) analyses using radiolabeled riboprobes, and low numbers of SIV RNA+ cells were observed at day 1 and much increased numbers at day 14 postinfection (shown with black arrows in E and F), relative to tissue from an uninfected monkey (D).
Discussion
In this study, we demonstrate that diverse HIV-1 strains, either live or inactivated, induce TSLP production by human epithelial cells, including primary human keratinocytes, in culture. TSLP released by epithelial cells in response to HIV strongly activates human myeloid DC, which potently induced a robust homeostatic proliferation of CD4+ T cells and promoted HIV replication in these activated T cells. These data suggest that epithelial cells represent the first target for HIV to trigger DC-mediated immune activation that facilitates viral replication in CD4+ T cells during early vaginal HIV infection. This hypothesis is further supported by data from the in vivo experiments showing that inoculation of SIV into the vaginal tract of rhesus monkeys induced rapid upregulation of TSLP in epithelial cells that coincided with high levels of infected cells in situ.
Literature reports suggest that genital epithelial cells cannot be infected by HIV and may only permit transcytosis through epithelial cell surface proteins enabling infection of the nearby DCs and CD4+ T cells (22–26). In our studies we observed induction of TSLP expression by both live and inactivated HIV-1 strains. It has been reported that certain microbial products that mimic double-stranded RNA, such as poly I:C, induce TSLP production by interacting with toll-like receptors (TLR), specifically TLR3, on the epithelial cells (15). In fact, in our studies we did observe TSLP production by the different epithelial cells used in response to stimulation with poly I:C, however, we believe the capacity of HIV to induce TSLP as demonstrated in our studies is independent of TLR3 interaction because the infection process of HIV does not involve a double-stranded RNA step and AT2-treated HIV-1 strains incapable of viral replication were effective in inducing TSLP production in the epithelial cells. It is possible that HIV may induce TSLP expression through TLR7 or TLR8 interactions since both receptors are known to recognize single-stranded RNA within the cellular compartment and both upregulate the NFκB signaling pathway which we have shown is involved in HIV induced TSLP expression, similar to that reported after stimulation with certain inflammatory cytokines and TLR ligands (18).
Studies of SIV infection in the vaginal tissues of rhesus macaques showed only a few SIV-infected cells between days 1–4 post-infection, but substantial increase beyond day 4 with a concurrent increase at the distant lymphoid tissues (20). Thus, the outcome of HIV exposure at the vaginal mucosa, which exhibits low pH and contains several antiviral soluble factors, is dependent on the extent to which the virus can manipulate the mucosal tissue microenvironment within the first few days to ensure virus dissemination locally. Results from the present investigation suggest that induction of TSLP production by the mucosal epithelial cells may be a strategy adopted by HIV to successfully maneuver the hostile vaginal mucosal microenvironment through DC-mediated recruitment of CD4+ T-cell targets.
It has been shown that antiviral CD8 T-cell responses were generated within the vaginal mucosal tissues of rhesus macaques infected by the vaginal route, but at a slower rate and to a much lower level compared to the peak viral loads and this trend was even more pronounced in the gut (27). Based on these observations, it has been suggested that the antiviral immunity at the vaginal mucosa, as measured by analyzing for CD8 T-cell response is “too little and too late” to provide protection in terms of clearance of infection and prevention of CD4+ T-cell loss. However, it has not been clear what changes within the mucosal microenvironment subsequent to viral exposure facilitate kinetics of virus infection over that of antiviral host immune responses. In this study we observed that virus-induced TSLP expression paralleled SIV infection within the vaginal tissues over the first 2 weeks post-infection. These data, together with the results from in vitro studies support virus-induced TSLP production by the mucosal epithelial cells as a critical event for DC-mediated expansion of HIV infected CD4+ T cells during vaginal HIV transmission.
Historically, the role of TSLP has been documented in the study of allergic diseases, but here we show that TSLP may be an important player in the acute phase of HIV-1 infection by creating an environment that is conducive for sustaining the small dose of the initial virus inoculum that crosses the mucosal barrier. This raises the possibility of targeting TSLP as a strategy against mucosal HIV-1 transmission and/or TSLP as a potential modulator of antigen-specific immune responses induced by candidate vaccines delivered at these mucosal viral entry sites.
Materials and Methods
Cell Culture and Production of Virus Stock.
Epithelial cell lines representing cervical (C33A), neuronal (SKMN), and intestinal (HT29) origin were obtained from ATCC and maintained in DMEM supplemented with 10% FBS (FBS) and the appropriate antibiotics. Adult normal human epidermal keratinocytes (NHEK) were obtained from Lonza Biosciences and maintained in KGM-2 media. The Ghost X4 cells were obtained through the AIDS Research and Reference Reagent Program, Division of AIDS, National Institute of Allergy and Infectious Diseases, National Institutes of Health and maintained in DMEM supplemented with 10% FBS (FBS) and the appropriate antibiotics. Chronically HIV-infected H9 cells (H9/HIV-1IIIB) were maintained in RPMI-1640 medium supplemented with 10% FBS. Primary human peripheral blood mononuclear cells (PBMC) were isolated by the standard Ficoll-Hypaque density gradient separation method from blood samples purchased from Gulf Coast Blood Center (Houston, TX). A plasmid encoding the HIV-1 proviral DNA with a deletion in the envelope region, pMenv(−), and another plasmid encoding HIV-1 env sequence representing clade D virus 93ZR001.3 and the R5 virus YU2, were obtained through the AIDS Research and Reference Reagent Program, Division of AIDS, NIAID, NIH. The plasmid MD.6 encoding the VSV envelope glycoprotein was obtained from Dr. Inder Verma, Salk Institute, San Diego, CA. The AT-2 treated and untreated HIV-1 strains ADA and MN were obtained from Dr. Jeff Lifson, Laboratory of Retroviral Pathogenesis, AIDS Vaccine Program, NCI-Frederick Cancer Research and Development Center, Frederick, MD.
DC Activation and Viability.
The CD11c+ myeloid DCs cultured with medium, rTSLP, HIV−Sn, or HIV+Sn for 24 h were collected and resuspended in EDTA-containing medium. Viability of the DCs was determined by the standard Trypan blue dye-exclusion method. To determine the activation status, the DCs were stained with PE-conjugated mouse anti-human mAbs to CD40, CD80 and CD86 and an IgG1 isotype control (all from BD Biosciences) and analyzed on an LSR II flow cytometer (BD Biosciences) using flowjo software (version 8.3.4, Tree Star). The dead cells were excluded from the analyses by using the violet Live/Dead stain kit (Invitrogen). The DC culture supernatants were collected at 24 h, frozen at −80 °C, and analyzed within 3 months with protein ELISA kits for TARC and MDC (R&D Systems). In some experiments, RNA samples prepared from the cultured mDCs were used for gene expression analyses.
mDC-CD4+ T-Cell Co-Cultures.
Activated mDC were co-cultured with 2.5 to 5.0 × 104 purified autologous naïve CD4+ T cells (DC:T cell ratio, 1:1; SI Materials and Methods) in a round bottomed 96-well culture plates for 7 days. In some experiments, HIV-1IIIB was added to the co-cultures overnight, and the cells were washed three times to remove any free viral particles. The co-cultures were resuspended in RPMI medium containing 10% FBS. The mixture was then incubated for 5 min in 37 °C in 10% FBS-RPMI 1640 and the cells were pelleted by centrifugation. The cells were subsequently washed three times with 10% FBS-RPMI 1640. On days 4 and 7, the viable cell counts were determined by the standard Trypan blue dye-exclusion method. In the co-cultures where HIV is added, the relative levels of infection were determined by assaying for reverse transcriptase (RT) activity in the culture supernatants.
In Situ Hybridization for Detection of SIV RNA-Positive Cells.
The in situ hybridization analysis (ISH) was performed on the vaginal tissue sections using 35S-labeled SIV riboprobes as described earlier (29), with modifications. Radioactive probes had a specific activity of >3.5 × 108 cpm/μg as determined by the in vitro transcription labeling of the SIV gag and env genes. The hybridization solution contained radiolabeled SIV probes at a total concentration of 1.5 × 106 cpm/100 μL. The riboprobe mixture in the hybridization buffer was layered over each tissue section. The slides were coated with LM-1 autoradiographic emulsion (Amersham) and allowed to develop at 4 °C for 14 days. Controls for ISH included: (i) matched tissues from SIV-uninfected rhesus monkeys, (ii) tissues from SIV-infected rhesus monkeys with high virus loads (positive control), (iii) serial tissue sections hybridized with SIV sense riboprobes, and (iv) omission of the probe in the hybridization mixture.
SIV RNA Measurement.
Tissue RNA samples were analyzed for viral RNA (vRNA) by a quantitative branched DNA (bDNA) assay (30) and reported as viral RNA copy numbers per microgram total tissue RNA. The detection limit of this assay is 125 copies of vRNA. To evaluate the specificity of the tissue assay, samples were collected from three animals that had not been exposed to SIV (21). Excluding one spurious result, average values for the bDNA assay were 113 copies/μg tissues RNA in the uninfected animals. Thus, we set the cut-off for the assay at 200 copies/μg tissue RNA, the average + 2 standard deviation (SD) values. Tissue sample with less than 200 copies of vRNA/μg total tissue RNA were reported as negative.
Immunohistochemistry.
The vaginal tissue sections were incubated with either rat anti-human TSLP (mAb 12F3, DNAX), mouse anti-human CD4 (mAb M-T477, BD PharMingen) or mouse anti-human CD11c (mAb AHS1153, Biosource) at room temperature for 1 h in PBS. The slides were washed with PBS twice and incubated with biotinylated secondary antibody for 30 min (PK-4004, Vector Laboratories) before washing and treatment with avidin-peroxidase complex reagents for 30 min (PK-4004, Vector Laboratories). Subsequently, the slides were washed and incubated with the substrate SK-4200, which stained red, or SK-4100, which stained brown (Vector Laboratories).
Real-Time Quantitative RT-PCR.
Epithelial cells exposed to different HIV-1 strains or mDC subjected various treatments were lysed and mRNA was extracted with an RNeasy kit (Qiagen). Reverse transcription was done with SuperScript II (Invitrogen), and the cDNA samples were analyzed by real-time quantitative PCR assay with an ABI Prism 7500 Sequence Detection system (Applied Biosystems). Reactions were incubated for 2 min at 50 °C, denatured for 10 min at 95 °C, and subjected to 40 two-step amplification cycles with annealing-extension for 60 °C for 1 min followed by denaturation at 95 °C for 15 s. For the analysis of human TSLP and human GAPDH real time PCR probes were purchased directly from the manufacturer (SuperArray). For the analysis of human TARC and MDC, the primer sequences were as follows: TARC: 5′-CATGGCCCCACTGAAGATG-3′ and 5′-CCTGGAGCAGTCCTCAGATGTC-3′ and MDC: 5′-GCATGGCTCGCCTACAGACT-3′ and 5′-CAGACGGTAACGGACGTAATCA-3′.
ELISA.
Concentrations of the TSLP protein in cell-free supernatants and cell lysates were measured using a specific ELISA kit (R&D Systems). The minimal detection limit for this kit is set to 31.25 pg/mL.
Western Blot Analysis.
Aliquots of 2 × 107 epithelial cells exposed to HIV were subjected to lysis in 2× Laemmli buffer. The lysates were then mixed with 4× loading buffer and resolved on a 17.5% SDS polyacrylamide gel followed by Western blot analyses using rat anti-TSLP (mAb 12F3, DNAX) at a dilution of 1:1,000 as the primary antibody and horseradish peroxidase labeled donkey anti-rat IgG H&L secondary antibody (diluted 1:10,000) and the femto fluorometric system (Pierce Biotechnology).
Transfection and Luciferase Assay.
Cervical epithelial cells (3 × 105) were transfected, using the calcium phosphate method, with 1 μg luciferase reporter plasmid driven by wild-type or mutated TSLP promoter and 15 ng control renilla luciferase reporter driven by a constitutive thymidine kinase promoter (pRL-tk-luc) (Promega). The TSLP promoter plasmids were obtained from Steven F. Ziegler at the University Of Washington School Of Medicine. The cells were cultured for 48 h and then exposed to IL-1β and HIV overnight. Cells were harvested and lysed in 100 μL lysis buffer. Luciferase activity was measured using a Lumat LB9507 luminometer to determine whether HIV exposure of epithelial cells resulted in an induction of luciferase expression as robust as that previously seen with IL-1β. Relative luciferase activity was calculated as a ratio of relative light units to relative renilla luciferase units. In each experiment, samples were analyzed in triplicate and each experiment was repeated at least three times.
Generation of C33A Cells Expressing an NF-κB Superrepressor.
An IkBa mutant harboring mutations in its phosphorylation sites, serines 32 and 36 (named IkBaSS/AA), was cloned into the retroviral vector pMIGR1 (provided by Warren Pear, Abramson Family Cancer Research Institute). Since the IkBaSS/AA is resistant to inducible degradation (32), it functions as a superrepressor of NF-κB. To produce recombinant retroviruses, pMIGR1-IkBaSS/AA or pMIGR1 vector control was transiently transfected into 293 cells along with the packaging plasmid pCL-Ampho and the VSV-G plasmid, as previously described (33). The recombinant viruses were used to infect C33A cells, which were used as the bulk of cells in the experiments.
Statistical Analyses.
Using the Student t test the P values were calculated to determine the significance of fold increase in CD4+T cells and HIV infection (RT activity).
Supplementary Material
Acknowledgments.
We thank Margaret L Kripke from UT MDACC for advice and assistance with manuscript preparation and Dr. Warren Pear from the University of Pennsylvania for providing the pMIGR1 vector. This work was funded by National Institute of Allergy and Infectious Diseases Grants AI 42694 and 46969 (to K.J.S.) R01 AI061645–01 and U19 AI071130–01 (to Y.-J.L.). All of the cell culture media were produced by the Central Media laboratory, which is funded by National Institutes of Health Grant CA 16672.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0907347106/DCSupplemental.
References
- 1.World Health Organization. HIV AIDS Epidemic Update. Geneva, Switzerland: WHO; 2007. Available at http://www.who.int/ [Google Scholar]
- 2.Galvin SR, Cohen MS. The role of sexually transmitted diseases in HIV transmission. Nat Rev Microbiol. 2004;2:33–42. doi: 10.1038/nrmicro794. [DOI] [PubMed] [Google Scholar]
- 3.Pope M, Haase AT. Transmission, acute HIV-1 infection and the quest for strategies to prevent infection. Nat Med. 2003;9:847–852. doi: 10.1038/nm0703-847. [DOI] [PubMed] [Google Scholar]
- 4.Quinn TC, et al. Viral load and heterosexual transmission of human immunodeficiency virus type 1. N Engl J Med. 2000;342:921–929. doi: 10.1056/NEJM200003303421303. [DOI] [PubMed] [Google Scholar]
- 5.Røttingen JA, et al. A systematic review of the epidemiologic interactions between classic sexually transmitted diseases and HIV: How much really is known? Sex Transm Dis. 2001;28:579–597. doi: 10.1097/00007435-200110000-00005. [DOI] [PubMed] [Google Scholar]
- 6.Li Q, et al. Peak SIV replication in resting memory CD4+ T cells depletes gut lamina propria CD4+ T cells. Nature. 2005;435:1148–1152. doi: 10.1038/nature03513. [DOI] [PubMed] [Google Scholar]
- 7.Brenchley JM, et al. HIV disease: Fallout from a mucosal catastrophe? Nat Immunol. 2006;7:235–239. doi: 10.1038/ni1316. [DOI] [PubMed] [Google Scholar]
- 8.Guadalupe M, et al. Severe CD4+ T-cell depletion in gut lymphoid tissue during primary human immunodeficiency virus type 1 infection and substantial delay in restoration following highly active antiretroviral therapy. J Virol. 2003;2007:11708–11717. doi: 10.1128/JVI.77.21.11708-11717.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sankaran S, et al. Rapid onset of intestinal epithelial barrier dysfunction in primary human immunodeficiency virus infection is driven by an imbalance between immune response and mucosal repair and regeneration. J Virol. 2008;82:538–545. doi: 10.1128/JVI.01449-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Veazy R, Lackner AA. HIV swiftly guts the immune system. Nat Med. 2005;11:469–470. doi: 10.1038/nm0505-469. [DOI] [PubMed] [Google Scholar]
- 11.Lore K, Larsson M. The role of dendritic cells in the pathogenesis of HIV-1 infection. APMIS. 2003;111:776–788. doi: 10.1034/j.1600-0463.2003.11107809.x. [DOI] [PubMed] [Google Scholar]
- 12.Wu L, Vineet NK. Dendritic-cell interactions with HIV: Infection and viral dissemination. Nat Rev Immunol. 2006;6:859–898. doi: 10.1038/nri1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu YJ. Thymic stomal lymphopoietin: Master switch for allergic inflammation. J Exp Med. 2006;203:269–273. doi: 10.1084/jem.20051745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ziegler SF, Liu YJ. Thymic stromal lymphopoietin in normal and pathogenic T cell development and function. Nat Immunol. 2006;7:709–714. doi: 10.1038/ni1360. [DOI] [PubMed] [Google Scholar]
- 15.Kato A, et al. TLR3- and Th2 cytokine-dependent production of thymic stromal lymphopoietin in human airway epithelial cells. J Immunol. 2007;179:1080–1087. doi: 10.4049/jimmunol.179.2.1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Frank I, et al. Infectious and whole inactivated simian immunodeficiency viurses interact similarly with primate dendritic cells (DCs): Differential intracellular fate of virions in mature and immature DCs. J Virol. 2002;76:2936–2951. doi: 10.1128/JVI.76.6.2936-2951.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rossio JL, et al. Inactivation of human immunodeficiency virus type 1 infectivity with preservation of confirmational and functional integrity of the virion surface proteins. J Virol. 1998;72:7992–8001. doi: 10.1128/jvi.72.10.7992-8001.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee HC, Ziegler SF. Inducible expression of the proallergic cytokine thymic stromal lymphopoietin in airway epithelial cells is controlled by NFkB. Proc Natl Acad Sci USA. 2007;104:914–919. doi: 10.1073/pnas.0607305104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Watanabe N, et al. Human thymic stromal lymphopoietin promotes dendritic cell- mediated CD4+ T cell homeostatic expansion. Nat Immunol. 2004;5:426–434. doi: 10.1038/ni1048. [DOI] [PubMed] [Google Scholar]
- 20.Miller CJ, et al. Propagation and dissemination of infection after vaginal transmission of simian immunodeficiency virus. J Virol. 2005;79:9217–9227. doi: 10.1128/JVI.79.14.9217-9227.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Abel K, et al. Temporal and anatomic relationship between virus replication and cytokine gene expression after vaginal simian immunodeficiency virus infection. J Virol. 2005;79:12164–12172. doi: 10.1128/JVI.79.19.12164-12172.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Argyris EG, et al. Human immunodeficiency virus type 1 enters primary human brain microvascular endothelial cells by a mechanism involving cell surface proteoglycans independent of lipid rafts. J Virol. 2003;77:12140–12151. doi: 10.1128/JVI.77.22.12140-12151.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rimoldi M, et al. Intestinal immune homeostasis is regulated by the crosstalk between epithelial cells and dendritic cells. Nat Immunol. 2005;6:507–514. doi: 10.1038/ni1192. [DOI] [PubMed] [Google Scholar]
- 24.Stoddard E, et al. gp340 expressed on human genital epithelia binds HIV-1 envelope protein and facilitates viral transmission. J Immunol. 2007;179:3126–3132. doi: 10.4049/jimmunol.179.5.3126. [DOI] [PubMed] [Google Scholar]
- 25.Bobardt M, et al. Syndecan captures, protects, and transmits HIV to T lymphocytes. Immunity. 2003;18:27–39. doi: 10.1016/s1074-7613(02)00504-6. [DOI] [PubMed] [Google Scholar]
- 26.Mondor I, et al. Human immunodeficiency virus type 1 attachment to HeLa CD4 cells is CD4 independent and gp120 dependent and requires cell surface heparans. J Virol. 1998;72:3623–3634. doi: 10.1128/jvi.72.5.3623-3634.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Reynolds M, et al. CD8+ T-lymphocyte response to major immunodominant epitopes after vaginal exposure to simian immunodeficiency virus: Too late and too little. J Virol. 2005;79:9228–9235. doi: 10.1128/JVI.79.14.9228-9235.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Soumelis V, et al. Human epithelial cells trigger dendirtic cell-mediated allergic inflammation by producing TSLP. Nat Immunol. 2002;3:673–680. doi: 10.1038/ni805. [DOI] [PubMed] [Google Scholar]
- 29.Haase AT, et al. Detection of viral nucleic acids by in situ hybridization. In: Maramorosch K, Koprowski H, editors. Methods in Virology. New York: Academic; 1984. pp. 189–226. [Google Scholar]
- 30.Dailey PJ, et al. Quantitation of simian immunodeficiency virus (SIV) RNA in plasma of acute and chronically infected macaques using a branched DNA (bDNA) signal amplification assay. 13th Annu. Symp. Nonhum. Primate Models of AIDS; Monterey, CA. 1995. [Google Scholar]
- 31.Nehete P, et al. A post-CD4-binding step involving interaction of the V3 region of viral gp120 with host cell surface glycosphingolipids is common to entry and infection by diverse HIV-1 strains. Antiviral Res. 2002;56:233–251. doi: 10.1016/s0166-3542(02)00130-4. [DOI] [PubMed] [Google Scholar]
- 32.Good L, et al. Multiple structural domains within IκBα are required for its inducible degradation by both cytokines and phosphatase inhibitors. Biochem Biophys Res Commun. 1996;223:123–128. doi: 10.1006/bbrc.1996.0856. [DOI] [PubMed] [Google Scholar]
- 33.Cvijic ME, et al. Study of T-cell signaling by somatic cell mutagenesis and complementation cloning. J Immunol Methods. 2003;278:293–304. doi: 10.1016/s0022-1759(03)00191-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.