Vision begins when light enters the eye and falls onto the neural retina, a thin tissue in the back of the eye. In the neural retina, photoreceptor cells (cones for high visual acuity; rods for night vision) capture and transform the light into electrophysiological signals, which are relayed by retinal ganglion cells to the brain. Lining the neural retina is a single-layered, often darkly pigmented, transporting epithelium—the retinal pigment epithelium (RPE)—that forms the outer blood–retinal barrier and regulates retinal physiology. The RPE interacts intimately with cones and rods, and together they constitute the outer retina. Atrophy of the RPE or photoreceptors leads to vision loss, as in age-related macular degeneration, a leading cause of blindness in people age 65 and older in developed countries (1). How the humanretina forms is both scientifically fascinating and clinically important because such knowledge may lead to better diagnosis, prevention, and treatment of visual impairment. However, direct examinations of the molecular events unfolding as a cell begins its journey to becoming a retinal cell in our own species have been limited; current knowledge is mostly extrapolated from studies of other mammals, mice in particular, as well as a range of nonmammalian vertebrates. This landscape is changing, as a study by Meyer et al. (2) in this issue of PNAS offers a way to look into early events in human retinogenesis.
Meyer et al. (2) report that a targeted and stepwise differentiation process channels human embryonic stem (ES) cells and induced pluripotent stem (iPS) cells through a sequence of stages that mimic retinogenesis during vertebrate embryonic development to eventually differentiating into RPE cells and cones, end products similar to those reported by others (3–5). During this stepwise differentiation process, the pluripotent stem cells progressively narrow their potential fates to the eye field, then to the optic vesicle, the optic cup, retinal progenitors, and finally to differentiated cells of the retina (Fig. 1). As the cells journey through these stages, they express regulatory genes that define the corresponding stage. For instance, cells at the eye field-equivalent stage express a number of eye-specifying genes (6), including Pax6, Rx, and Six3. This is followed by the expression of Mitf, a gene widely expressed in the optic vesicle and shown to be important for promoting and maintaining RPE properties (7–9). As the process continues, the population of Mitf+ cells decreases, while the population of cells expressing Chx10 increases, reminiscent of the “optic vesicle → optic cup” transformation, during which the identities of RPE vs. neural retina are established by key regulators including Mitf (pro-RPE) and Chx10 (pro-neural retina) (10, 11). Further, experimental attenuation of fibroblast growth factor (FGF) signaling leads to expansion of Mitf+ (RPE) cell population at the expense of the Chx10+ (retina) cells, an outcome consistent with the classic phenomenon of FGF-stimulated “RPE → neural retina” transdifferentiation in early chick and rodent embryos (12). By confirming landmark events in early retinogenesis in vertebrates, the results show that the time has come to learn some of the molecular underpinning as pluripotent stem cells of our own species forfeit other potential pathways to become specialized into cells of the retina.
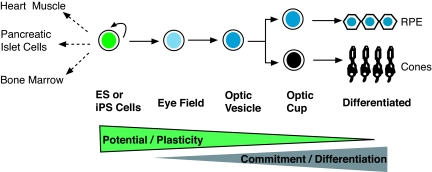
Fig. 1.
Pluripotent stem cells on the path to differentiating into retinal cells. Human ES and iPS cells have the potential to differentiate into any specialized cells, such as those in the heart, pancreas, or bone marrow. When subjected to the targeted and stepwise differentiation process described by Meyer et al. (2), these pluripotent stem cells embark on a special journey to becoming RPE cells and cones of the retina. This process is sequential, going through stages of eye field, optic vesicle, optic cup, and final cell differentiation. As cells travel along the path, their potential/plasticity decreases, whereas their commitment to differentiating into specific cell types increases. This stepwise process resembles the process in mammalian embryogenesis.
Meyer et al. (2) observed the genesis of cones in their experiments. During vertebrate development, cone genesis follows that of retinal ganglion cells, the first-born. The cone genesis from ES/iPS cells could reflect the cone pathway as a default for the stem cell-derived retinal progenitors, or the culture conditions favoring cone genesis. After all, embryonic retinogenesis takes place in a three-dimensional environment, where various cellular, molecular, and electrophysiological cues are spatially and temporally coordinated. More than likely, many of these cues are missing from the in vitro system. Converting the current two-dimensional culture model to three dimensions, along with supplementing culture medium with factors, may increase the utility of the in vitro system in dissecting the detailed events in human retinogenesis. In addition, it may facilitate the identification of intrinsic and extrinsic factors that steer progenitor cells to differentiate into specific types of retinal cells for future cell replacement.
Besides opening a way to examine the molecular detail of human retinogenesis, the in vitro modeling system also offers an opportunity to scrutinize molecular differences between human and model organisms. Highlighting one such difference is the lack of macula in the mouse retina. Importantly, not only is the macula responsible for high visual acuity, its degeneration is a serious and common cause of blindness. Identification of those factors that contribute to making the human retina unique will facilitate effective translation of findings from animal studies into clinical practice.
Notably, iPS cells can undergo the same stepwise fate restriction or commitment to give rise to RPE cells and cones. This paves the road for elucidating the molecular mechanism underlying RPE or cone degenerations that result from genetic changes and for developing patient-specific therapeutic strategies.
The generation of specific types of retinal cells from ES cells and especially from iPS cells is of high potential impact on the development of cell replacement therapies. Indeed, human pluripotent stem cells can give rise to, besides RPE cells and cones (2–5), inner retinal neurons with functional glutamate receptors (3) and photoreceptors that restore some visual function in genetically modified mice after cell transplantation (4). Together, these studies support the prospect of a scheme of “iPS → eye field → retina” for autologous cell transplantation (Fig. 2).
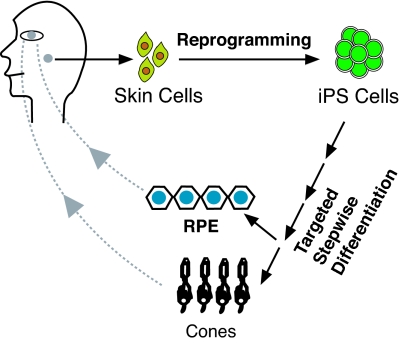
Fig. 2.
A scheme of “iPS → eye field → retina” for autologous cell transplantation. Skin keratinocytes are reprogrammed to give rise to iPS cells, which are subjected to the targeted stepwise differentiation process (2) to produce RPE cells or cones. The autologous RPE or cones can then be transplanted into the patient with atrophy of RPE or cones because of nongenetic causes. For genetic causes, gene therapy (15, 16) needs to be incorporated.
Acknowledgments.
The research of S.-Z.W. is supported by National Institutes of Health/National Eye Institute Grant EY011640.
Footnotes
The author declares no conflict of interest.
See companion article on page 16698.
References
- 1.Curcio CA, Medeiros NE, Millican CL. Photoreceptor loss in age-related macular degeneration. Invest Ophthalmol Vis Sci. 1996;37:1236–1249. [PubMed] [Google Scholar]
- 2.Meyer JS, et al. Modeling early retinal development with human embryonic and induced pluripotent stem cells. Proc Natl Acad Sci USA. 2009;106:16698–16703. doi: 10.1073/pnas.0905245106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Osakada F, et al. Toward the generation of rod and cone photoreceptors from mouse, monkey and human embryonic stem cells. Nat Biotechnol. 2008;26:215–224. doi: 10.1038/nbt1384. [DOI] [PubMed] [Google Scholar]
- 4.Osakada F, Ikeda H, Sasai Y, Takahashi M. Stepwise differentiation of pluripotent stem cells into retinal cells. Nat Protoc. 2009;4:811–824. doi: 10.1038/nprot.2009.51. [DOI] [PubMed] [Google Scholar]
- 5.Hirami Y, et al. Generation of retinal cells from mouse and human induced pluripotent stem cells. Neurosci Lett. 2009;458:126–131. doi: 10.1016/j.neulet.2009.04.035. [DOI] [PubMed] [Google Scholar]
- 6.Zaghloul NA, Yan B, Moody SA. Step-wise specification of retinal stem cells during normal embryogenesis. Biol Cell. 2005;97:321–337. doi: 10.1042/BC20040521. [DOI] [PubMed] [Google Scholar]
- 7.Mochii M, et al. Role of mitf in differentiation and transdifferentiation of chicken pigmented epithelial cell. Dev Biol. 1998;193:47–62. doi: 10.1006/dbio.1997.8800. [DOI] [PubMed] [Google Scholar]
- 8.Bumsted KM, Barnstable CJ. Dorsal retinal pigment epithelium differentiates as neural retina in the microphthalmia (mi/mi) mouse. Invest Ophthalmol Vis Sci. 2000;41:903–908. [PubMed] [Google Scholar]
- 9.Nguyen M, Arnheiter H. Signaling and transcriptional regulation in early mammalian eye development: a link between FGF and MITF. Development. 2000;127:3581–3591. doi: 10.1242/dev.127.16.3581. [DOI] [PubMed] [Google Scholar]
- 10.Rowan S, Chen CM, Young TL, Fisher DE, Cepko CL. Transdifferentiation of the retina into pigmented cells in ocular retardation mice defines a new function of the homeodomain gene Chx10. Development. 2004;131:5139–5152. doi: 10.1242/dev.01300. [DOI] [PubMed] [Google Scholar]
- 11.Horsford DJ, et al. Chx10 repression of Mitf is required for the maintenance of mammalian neuroretinal identity. Development. 2005;132:177–187. doi: 10.1242/dev.01571. [DOI] [PubMed] [Google Scholar]
- 12.Reh TA, Levine EM. Multipotential stem cells and progenitors in the vertebrate retina. J Neurobiol. 1998;36:206–220. [PubMed] [Google Scholar]
- 13.Lamba DA, Karl MO, Ware CB, Reh TA. Efficient generation of retinal progenitor cells from human embryonic stem cells. Proc Natl Acad Sci USA. 2006;103:12769–12774. doi: 10.1073/pnas.0601990103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lamba DA, Gust J, Reh TA. Transplantation of human embryonic stem cell-derived photoreceptors restores some visual function in Crx-deficient mice. Cell Stem Cell. 2009;9:73–79. doi: 10.1016/j.stem.2008.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Maguire AM, et al. Safety and efficacy of gene transfer for Leber's congenital amaurosis. N Engl J Med. 2008;358:2240–2248. doi: 10.1056/NEJMoa0802315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cideciyan AV, et al. Human RPE65 gene therapy for leber congenital amaurosis: Persistence of early visual improvements and safety at 1 year. Hum Gene Ther. 2009 Jul 7; doi: 10.1098/hum.2009.086. [DOI] [PMC free article] [PubMed] [Google Scholar]