Abstract
Mediator is a large, multisubunit complex that is essential for transcription of mRNA by RNA Pol II in eukaryotes and is believed to bridge transcriptional activators and the general transcription machinery. However, several recent studies suggest that the requirement for Mediator during transcriptional activation is not universal, but rather activator dependent, and may be indirect for some genes. Here we have investigated Mediator association with several constitutively transcribed genes in yeast by comparing a yeast strain that harbors a temperature-sensitive mutation in an essential Mediator subunit, Srb4, with its wild-type (WT) counterpart. We find modest association of Mediator with constitutively active genes and show that this association is strongly decreased in srb4 ts yeast, whereas association with a nontranscribed region or repressed gene promoters is lower and unaffected in the mutant yeast. The tail module of Mediator remains associated with ribosomal protein (RP) gene promoters in srb4 ts yeast, while subunits from the head and middle modules are lost. Tail module association at Rap1-dependent gene promoters is lost in rap1 ts yeast, indicating that Rap1 is required for Mediator recruitment at these gene promoters and that its recruitment occurs via the tail module. Pol II association is also rapidly and severely affected in srb4 ts yeast, indicating that Mediator is directly required for pol II association at constitutively transcribed genes. Our results are consistent with Mediator functioning as a general transcription factor in yeast.
Keywords: Rap1, transcription
Transcription of mRNA coding genes in eukaryotes involves the action of coregulator complexes, in addition to activators and the general transcription machinery. Activators, binding to sites upstream of the core promoter (up to a few hundred bp distant in yeast, and considerably farther in metazoans), interact with the general transcription machinery with the help of coactivator complexes. Two types of coactivator complexes have been identified that can act as a bridge between activators and the general transcription machinery. One type comprises the TAF (TBP-associated factor)-containing complexes, which include the TFIID and SAGA complexes, and the other is the Mediator complex (1, 2).
Mediator was discovered in the yeast Saccharomyces cerevisiae through the observation that purified activators and the general transcription machinery were not sufficient for activated transcription in vitro (3). Biochemical purification identified Mediator as a large complex that allowed activator-dependent transcription in vitro, while results from genetic experiments also suggested a role for Mediator as coactivator. Subsequent investigations supported this view, and the importance of Mediator in mRNA transcription has been demonstrated both for individual genes and on a genomewide basis (1, 4–6). Mediator is structurally and functionally conserved across eukaryotes, comprising 25–30 subunits (7, 8). Structural and biochemical studies have revealed that Mediator exists in an extended conformation, with 3 distinct modules termed the head, middle, and tail; subunits from the individual modules often share genetic properties (9, 10). In vivo, Mediator appears to be a direct target of activators and is recruited to gene promoters at the preinitiation stage of transcription (11–16). Mediator in turn facilitates recruitment of the preinitiation complex (PIC) to promoters (17, 18). Recruitment of pol II is probably achieved in part by direct contacts between the carboxyl-terminal domain (CTD) of the Rpb1 subunit and the head and middle modules of Mediator (19, 20). In addition, there is evidence that Mediator participates in “basal,” or nonactivated transcription (6, 21, 22).
A large body of work has led to the general view that Mediator acts as a global regulator of transcription by communicating regulatory information between gene-specific activators and the general transcription machinery (2). One early and key piece of evidence for this view was the finding that in a med17/srb4 ts mutant yeast strain, the vast majority (over 90%) of pol II transcribed genes show decreased transcription at the restrictive temperature (4, 6). However, the role of Mediator as a general transcription factor has been questioned in some recent reports. In a study in which chromatin immunoprecipitation (ChIP) was used to examine association of Mediator with gene promoters in yeast, it was reported that although high levels of Mediator subunits were observed associated with genes activated by heat shock or growth in galactose medium, association with constitutively transcribed genes, including the highly expressed RP genes, was not significantly above background (23). Another recent study in mammals reported that activation of the SNAT2 gene by its activator ATF4 does not lead to enhanced recruitment of Mediator to its promoter (24). Similarly, Deato and colleagues reported that during skeletal muscle differentiation, several Mediator subunits are severely reduced or absent in myotubes, and correspondingly, the myogenin promoter requires a special cofactor TAF3/TRF3 for transcriptional activation in vitro but does not require Mediator (25). In contrast to these reports, another study found Mediator to be associated with promoters of many inactive and active genes in S. cerevisiae (26). Clearly, a consensus has not yet been reached on the general role of Mediator in transcription in vivo.
To address the general requirement for Mediator in transcriptional activation of pol II transcribed genes, we have investigated Mediator association with constitutively transcribed genes in yeast, using a more sensitive approach than was used previously. Specifically, we have performed ChIP to examine Mediator association with promoters of constitutively transcribed genes in wild-type (WT) yeast and in a med17/srb4 ts mutant yeast strain. By comparing association at the restrictive temperature, which causes dissociation of the head module of Mediator (27, 28), we show that Mediator is associated with constitutively transcribed genes and is required for pol II recruitment to these genes. Our results are consistent with the view of Mediator as a component of the core transcription machinery that is nearly universally required for transcription of mRNA genes.
Results
Mediator Associates Specifically with Several Constitutively Active Genes.
To analyze Mediator association with constitutively active genes, we have used a med17/srb4 ts mutant yeast strain. Med17/Srb4 is a subunit of the head module of Mediator and is encoded by an essential gene in yeast. In yeast harboring the temperature-sensitive mutation srb4–138, the head module of Mediator is disrupted at 37 °C, and mRNA transcription is decreased to less than 20% of WT levels within 30 min of temperature shift and to less than 10% of WT levels within 4 h (4, 6, 27). We reasoned that by comparing Mediator association at promoter regions in WT and srb4 ts yeast, relative to association with a control nontranscribed region, even low levels of functionally significant association could be detected. To test this hypothesis, we generated isogenic WT and srb4 ts strains from yeast expressing Myc13-tagged Med18/Srb5 or Med15/Gal11 or TAP-tagged Med14/Rgr1 (29). The WT and mutant strains were grown in complete synthetic medium (CSM) at 25 °C, rapidly shifted to 37 °C, and incubated at 37 °C for 45 min to inactivate the Srb4 subunit in the srb4 ts mutant before carrying out ChIP. Serine was added to the medium to induce the CHA1 gene to provide a positive control for Mediator recruitment (29). Association of Mediator subunits with both core promoter (−100 bp to +100 bp) and upstream activator sequence (UAS) regions (spanning Rap1 binding sites from 300 to 650 bp upstream of the starting ATG for the RP genes and CDC19, and between 150 bp and 250 bp upstream of the starting ATG for CHA1) was quantified by real time PCR and was normalized to input DNA and to a nontranscribed region of ChrV.
Results of ChIP assays showed only modestly enriched association of Srb5 and Rgr1 with the constitutively transcribed genes examined here (RPS11B, RPL12A, RPS21B, and CDC19) relative to the ChrV control, in general agreement with previous findings (23) (Fig. 1) (note that a log2 scale is used in the figures). Importantly, the association of Mediator with constitutively transcribed gene promoters, and with the induced CHA1 promoter, was substantially reduced in the srb4 ts mutant (Fig. 1 A and C). Association of Srb5 was also strongly decreased at the UAS region in the srb4 ts mutant (Fig. 1B). This decrease was not caused by decreased Srb5 levels in the cell, as Western blotting showed equivalent levels in wild-type and srb4 ts yeast (supporting information (SI) Fig. S1). Surprisingly, although Rgr1 dissociation was seen from the core promoter regions of all transcribed genes examined in srb4 ts yeast (Fig. 1C), this effect was markedly reduced or absent at the UAS region (Fig. 1D). This difference in dissociation of Rgr1 was observed at CHA1 as well. Nonetheless, the lower association seen in srb4 ts yeast at core promoters for both Srb5 and Rgr1, and at the UAS region for Srb5, indicates that association of Srb5 and Rgr1 with these genes depends on Mediator integrity more than their association with the ChrV control does, suggesting the latter represents nonspecific association or background immunoprecipitation.
Fig. 1.
Mediator associates specifically with constitutively transcribed genes. (A and B) Association of Srb5 from the head module of Mediator with the core promoter and UAS of the indicated genes was analyzed by ChIP. WT and srb4 ts mutant strains expressing Myc13-tagged Srb5 were grown at 25 °C and shifted to 37 °C for 45 min before carrying out ChIP. ChIPed DNA was analyzed by real time PCR. The bars represent log2 of the IP/input ratios for the indicated genes, normalized to log2 of IP/input for ChrV, as described in Materials and Methods. (C and D) Association of Rgr1 from the middle module of Mediator in WT and srb4 ts yeast strains expressing TAP-tagged Rgr1 was analyzed as described for Srb5. Error bars represent standard deviation. Each experiment was performed 3–4 times.
Previous studies on Mediator association with constitutively active genes, performed using yeast grown in YPD medium (23) or CSM (26), came to different conclusions. We therefore also analyzed Srb5 association by carrying out ChIP on WT and srb4 ts yeast grown in YPD medium and compared the results with those obtained from yeast grown in CSM. We observed results similar to those shown in Fig. 1 for Srb5 association using yeast grown in YPD medium (Fig. S2), suggesting that these growth conditions have little effect on Mediator association with the genes assayed here.
These ChIP results indicate that the head and middle modules of Mediator are associated specifically with constitutively transcribed gene promoters, consistent with a widespread involvement of Mediator in transcription.
In addition to its association with active genes, Mediator has been reported to be present at some inactive gene promoters (26) and at nontranscribed regions of the yeast genome (30). To analyze Mediator presence on inactive genes, we used ChIP to monitor Srb5 and Rgr1 association with the SPO13 and PLM2 genes, neither of which is expressed under the experimental conditions used in this study (31). No significant enrichment was observed by ChIP either for Srb5 or Rgr1 at the SPO13 or PLM2 core promoter regions in wild-type yeast (Fig. 1 A and C). Furthermore, no decrease in association was observed in the srb4 ts mutant. We conclude that Mediator does not associate with the inactive promoters of SPO13 and PLM2, thus further confirming that the low level of Mediator enrichment observed on constitutively transcribed genes shown in Fig. 1 is functionally significant.
Mediator Recruitment at RP Gene Promoters Is Rap1 Dependent.
Previous studies in yeast, Drosophila, and human cells have indicated direct interaction of Mediator subunits with enhancer-bound activators, pointing to a general role of Mediator as a coactivator (32, 33). If Mediator plays a functional role at RP genes and other constitutively transcribed genes in yeast, we would therefore expect its presence to depend on the activators for those genes. To test this idea, we asked whether Mediator association is activator dependent. All 3 RP gene promoters and CDC19 have Rap1 binding sites in their upstream regions (34, 35). At RP gene promoters, Rap1 is required for association of Fhl1 and Ifh1 and concomitant activation (36). To test whether Mediator association with these genes depends on Rap1, we constructed a rap1 ts mutant yeast strain expressing Myc13-tagged Srb5 and then analyzed association of Srb5 by ChIP after a temperature shift to 37 °C for 1 h. The results show that Srb5 association with RPS11B, RPL12A, and RPS21B is greatly reduced in rap1 ts yeast compared to WT, both at core promoter (Fig. 2A) and UAS regions (Fig. 2B). No loss was observed at CHA1 in the rap1 ts mutant, verifying selective dependence on Rap1. Although the CDC19 promoter has a Rap1 binding site, little if any dependence on Rap1 was seen for Srb5 recruitment at this promoter, indicating that Mediator recruitment is independent of Rap1 at CDC19. Consistent with this result, expression of CDC19 was not altered at nonpermissive temperature in rap1 ts mutant yeast (37).
Fig. 2.
Srb5 association is selectively affected at RP genes in rap1 ts yeast. Association of Srb5 from the head module of Mediator was analyzed in rap1 ts and WT yeast at the core promoter (A) and UAS regions (B) of the indicated genes. WT and rap1 ts mutant yeast strains expressing Myc13-tagged Srb5 were grown at 25 °C and shifted to 37 °C for 1 h before carrying out ChIP. ChIPed DNA was analyzed by real time PCR. The bars represent log2 of IP/input ratios for the indicated genes, normalized to log2 of IP/input ratios for ChrV. Error bars represent standard deviation. Each experiment was performed 3–4 times.
These results demonstrate that Rap1 is required to recruit Mediator to Rap1-dependent RP genes.
Independent Recruitment of the Tail Module of Mediator.
Previously we showed that in srb4 ts mutant yeast at nonpermissive temperature, recruitment of Srb5 and Rgr1 from the head and middle modules of Mediator to the induced CHA1 promoter was reduced, while recruitment of Gal11 from the tail module was unimpaired (29). To examine Gal11 association with constitutively transcribed gene promoters, we again used WT and srb4 ts yeast strains expressing a Myc13-tagged Gal11 subunit and performed ChIP from yeast after a 45-min shift to 37 °C. We found low levels of Gal11 at all constitutively transcribed genes examined here (about 2-fold higher than seen at the nontranscribed ChrV region), with no reduction in srb4 ts mutant yeast compared to WT (Fig. 3 A and B). Similar association was observed at core promoter and UAS regions.
Fig. 3.
The association of Gal11 remains unaffected in srb4 ts mutant strain at nonpermissive temperature but is selectively affected at RP gene promoters in rap1 ts mutant yeast. (A–D) Association of Gal11 from the tail module of Mediator with the core promoter and UAS of the indicated genes was analyzed by ChIP. WT, srb4 ts, and rap1 ts mutant strains expressing Myc13-tagged Gal11 were grown at 25 °C and shifted to 37 °C for 45 min (srb4 ts and WT) or 1 h (rap1 ts and WT) before carrying out ChIP. ChIPed DNA was analyzed by real time PCR. The bars represent log2 of IP/input ratios normalized to log2 of IP/input ratios for ChrV. Error bars indicate standard deviation. Each experiment was performed 3–4 times.
These data could reflect recruitment of the tail module independently of the head and middle modules of Mediator, or they could indicate that the tail module does not associate with the genes that we have examined. To distinguish between these alternatives, we analyzed Gal11 association with ribosomal and glycolytic gene promoters in rap1 ts mutant yeast by introducing the Myc13-tagged Gal11 subunit into this yeast strain. Several previous studies suggest that different activators can interact with the tail module of Mediator (36, 41, 42). If Rap1-dependent recruitment of Mediator at RP gene promoters similarly depends on the tail module, we should see reduction in the association of tail module in a rap1 ts strain in contrast to the srb4 ts mutant strain, where we observed no change.
ChIP performed at 37 °C showed severe reduction in the association of Gal11 at all 3 RP gene promoters in rap1 ts compared to WT yeast strains (Fig. 3 C and D). No change in Gal11 association was observed at the CHA1 promoter between WT and rap1 ts yeast, indicating that the effect of the rap1 ts mutant is specific to the RP gene promoters. Another subunit from the tail module of Mediator, Med2, also showed reduced association in rap1 ts yeast compared to WT, confirming Rap1-dependent recruitment of the tail module to RP genes (Fig. S3 A and B).
Taken together, these results demonstrate that Rap1 recruits Mediator to RP gene promoters by interacting, directly or indirectly, with its tail module. Our findings also confirm the continued presence of the tail module at these genes at 37 °C in srb4 ts mutant yeast, despite the loss of association of middle and head module subunits.
Pol II Recruitment Is Rapidly and Severely Affected at Constitutively Transcribed Gene Promoters in srb4 ts Mutant Yeast.
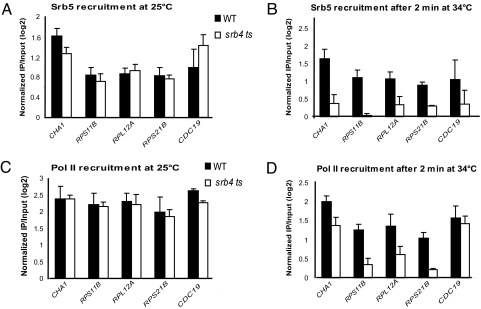
Although the large majority of genes transcribed by pol II show decreased transcription in srb4 ts yeast at 37 °C (6), it is possible that some genes are affected indirectly (23). For such genes, we would expect to see a delayed effect on pol II association. To examine this possibility, we monitored pol II association with gene promoters at short intervals after temperature shift. Shifting temperature to 37 °C led to rapidly reduced association of Rpb3 for the 3 RP gene promoters examined even in WT yeast, consistent with earlier work demonstrating effects of mild heat shock on both transcription and stability of mRNA encoding ribosomal proteins (Fig. S4) (38). To reduce effects of stress response, we therefore shifted cells to 34 °C instead of 37 °C (39). At 25 °C, Srb5 association with core promoter regions was indistinguishable for wild-type and srb4 ts yeast for all 5 genes examined and was substantially reduced in srb4 ts yeast within 2 min after shifting to 34 °C (Fig. 4 A and B). As expected, association remained low in srb4 ts relative to wild-type yeast at later time points (10 min and 30 min after temperature shift) (Fig. S5 A and B), and was not as greatly affected as at 37 °C (compare Fig. 1A to Fig. S5B). Similarly, pol II association was the same in wild type and srb4 ts yeast at 25 °C, was substantially reduced in srb4 ts yeast after 2 min shift to 34 °C (Fig. 4 C and D), and remained at lower levels than in wild type after 10 min and 30 min at 37 °C (Fig. S5 C and D). These results strongly suggest that Mediator has a direct role in recruitment of pol II at these and likely the majority of constitutively transcribed genes in yeast.
Fig. 4.
Rapid loss of Mediator and pol II association at constitutively transcribed genes in srb4 ts mutant yeast at nonpermissive temperature. Association of Srb5 from the head module of Mediator (A and B) and of the Rpb3 subunit of pol II (C and D) with the core promoters of the indicated genes was determined by ChIP in WT and srb4 ts yeast at 25 °C or after 2 min at 34 °C, as indicated. ChIPed DNA was analyzed by real time PCR. The bars represent log2 of IP/input ratios for the indicated genes normalized to log2 of IP/input ratios for the tRNA gene promoter, tQ(UUG)C. Error bars indicate standard deviation. Each experiment was performed 3–4 times.
Discussion
The yeast Mediator complex was discovered as an activity that could facilitate activator-dependent transcription by RNA pol II in vitro, and individual components of the complex were shown to be the products of genes that had been identified in genetic screens as contributing to transcriptional activation in vivo. These findings strongly suggested that Mediator is a bona fide coactivator in vivo. Subsequent work showed that loss of Mediator function in the srb4 ts mutant led to loss of the preponderance of pol II transcription in yeast, leading to a widely accepted view that Mediator, like general transcription factors such as TFIID, participates in most pol II transcription. This notion was further supported by recent findings that Mediator can also stimulate basal transcription in vitro (21).
Consistent with these findings, Mediator is recruited to the promoters of many inducible genes in both yeast and higher eukaryotes (18, 29, 40, 41). However, it remained possible that Mediator does not function directly at all genes, and that transcriptional defects caused by loss of Mediator could be the result of indirect effects at many genes. In support of the latter view, Mediator association was found not to be significantly different from the background level at a large proportion of yeast promoters in cells grown in YPD (23, 42). In contrast, another study found Mediator to be associated with upstream regions of a large number (≈1,200) of genes, and with coding sequences of many genes, in yeast grown in CSM (26). Similarly, Mediator association with a large number of both intergenic regions and coding sequences was reported in fission yeast (Schizosaccharomyces pombe) (43). More recently, comparison of Mediator association at promoters in yeast grown in YPD and in CSM suggested that although differences in growth conditions could account for a small proportion of the discrepant results published previously, the greater portion of the discrepancy could not be explained in this way (42).
Here, we have examined Mediator association with several constitutively active genes—3 RP genes and CDC19, also known as PYK1—by comparing ChIP of Mediator subunits in WT and srb4 ts yeast at the restrictive temperature. Normalization to a nontranscribed region of chromosome V controls for nonspecific effects, for example nontargeted association of Mediator to open regions of chromatin. Nontargeted effects were indeed seen in these ChIP experiments, as they have been in others; stronger ChIP signals are typically observed at control regions in the presence of antibody than in its absence (data not shown). However, if the measured association of Mediator subunits to promoter regions occurred through such nonspecific effects, we would expect to see proportional losses at all such regions. In contrast, we observed increased loss of Mediator head and middle subunits (Srb5 and Rgr1) in srb4 ts compared to WT yeast at the gene promoters examined, relative to ChrV (and also relative to a tRNA gene; data not shown). These findings strongly suggest that Mediator is stabilized at these promoters by additional protein–protein interactions whose loss in the mutant yeast results in a greater decrease in association than is seen at control regions. This finding therefore indicates specific recruitment, and therefore a likely functional role, of Mediator at constitutively active genes in yeast. Consistent with this interpretation, we also observe reduced association of Mediator in rap1 ts yeast specifically at genes under control of Rap1. In further support of a direct, functional role of Mediator at constitutively active genes, we observed decreased pol II association within 10 min of inactivation of Mediator, and in some cases within 2 min, strongly arguing for direct effects.
Mediator has in some cases been found more closely associated with UAS regions than proximal promoter regions, consistent with activator-dependent recruitment (15, 44). We found association of Srb5 with both UAS and core promoter regions, even when these regions were separated by over 500 bp (Fig. 1 A and B). A recent genomewide localization study also reported a relatively broad distribution of Mediator binding, encompassing about 200 bp upstream of the transcription start site (45). This breadth could reflect a range of preferred binding sites at different promoters or, alternatively, could indicate that Mediator is distinctly present at both UAS and proximal promoter regions (perhaps at different times) of active genes. There is precedence for this idea in studies of the HO promoter in yeast and heat shock promoters in Drosophila (44, 46). Interestingly, Rgr1 association with the core promoter regions of the genes examined here was substantially decreased in srb4 ts yeast, but association of Rgr1 with the UAS region showed a less marked decrease (Fig. 1 C and D). Rgr1 bridges the middle and tail modules of Mediator (10). One possible explanation for our observations is that Rgr1 interactions with other tail module subunits are not sufficient to retain Rgr1 at core promoter regions, while the combination of interactions with other tail subunits and interactions with the activator allow it to be retained at the UAS region in srb4 ts yeast. Alternatively, a change in conformation of the Mediator complex could occur as it moves (if this is the case) from the UAS to the core promoter region, thereby altering Rgr1 interactions. Additional investigations will be needed to address this issue.
Mediator complex has also been reported to be associated with the promoters of inactive genes in yeast, suggesting that it may prime genes for activation (26). We did not observe any detectable Mediator association with the inactive genes that we examined (Fig. 1). At least 1 of the genes (ZRT2) denoted as inactive in the study of Andrau et al., and found to be associated with Mediator, is active in yeast growing in CSM according to microarray data (31). Another gene, ROX1, was indicated as being inactive under the anaerobic growth conditions used in the study, but the experimental conditions reported did not indicate that anaerobic growth conditions were actually used (26). Lack of transcriptional activity was, however, supported by very low pol II association in ChIP measurements with these genes. Further investigation will be needed to understand whether and to what extent Mediator associates with the promoters of inactive genes.
Previously we reported recruitment of the tail module of Mediator to the CHA1 promoter, even in the absence of head and middle module recruitment, in srb4 ts yeast (29). Here we find similar independent recruitment of the tail module to 3 RP genes and to CDC19 in srb4 ts yeast, despite loss of head and middle module recruitment. Similarly, Hinnebusch and coworkers observed continued Gcn4-mediated recruitment of the tail module in sin4Δ yeast, while the head and middle modules were no longer recruited to the genes monitored (47). We observed loss of tail module association at RP genes in rap1 ts yeast, consistent with previous studies suggesting that the tail module is the principal target of activators (17, 32). However, subunits from all 3 modules of Mediator were found to show decreased association with genes activated by Gal4 in srb4 ts yeast (27, 28). The extent to which recruitment of Mediator depends primarily on the tail module on a genomewide basis and whether the tail module exerts additional functions that are independent of the middle and head modules remain to be seen.
In summary, we have shown here that Mediator associates with several constitutively expressed genes in yeast and is likely to contribute directly to recruitment of pol II. Mediator is evidently targeted to RP genes by a Rap1-dependent mechanism via the tail module, and the tail module can stably associate with gene promoters independently of the middle and head modules. Our results support the notion of Mediator as a general, although possibly not universal, transcription factor (1, 21).
Materials and Methods
Yeast Strains and Growth.
Yeast cultures were grown on complete synthetic medium lacking appropriate amino acids (6.7 g/L YNB w/o AA, 2% glucose, and dropout mix) or YPD (20 g/L bactopeptone, 10 g/L yeast extract, 2% glucose, 0.15 g/L L-tryptophan). Rapid temperature shifts were achieved by addition of prewarmed media to cultures grown at 25 °C. Transformation of yeast was performed using a standard lithium acetate method (48).
Strains used in this study are listed in Table S1. Strain RMY419 was derived from CBY10037 (a generous gift of Charlie Boone, University of Toronto) by replacing the chromosomal SRB5 gene with SRB5–13MYC::HIS3, using standard methods (49). Strain RMY420 was derived from CBY10037 by replacing the chromosomal MED2 gene with MED2-TAP::HIS3. Strain RMY421 was derived from CBY10037 by replacing the chromosomal GAL11 gene with GAL11–13MYC::HIS3MX.
Chromatin Immunoprecipitation.
ChIP was performed essentially as described (29) except that incubation at 37 °C following temperature shift was done for 45 min before crosslinking unless otherwise noted. The CHA1 gene was induced at 25 °C 30 min before the temperature shift. For ChIP of Myc13-tagged proteins, whole cell extract was incubated with ≈2 μg of anti-Myc antibody (Roche Applied Science). For ChIP of TAP-tagged proteins, antibody to protein A was used (Sigma).
Quantitations were done by real time PCR; log2 ratios of IP/input are depicted in the figures after subtracting log ratios obtained for a nontranscribed region of ChrV or tRNA, as indicated in the figure legends. Primer sequences are listed in Table S2.
Supplementary Material
Acknowledgments.
We thank Alan Hinnebusch and Charlie Boone for providing yeast strains and Joe Wade for critically reading the manuscript and for helpful discussions. We also acknowledge the Wadsworth Center Molecular Genetics Core. This work was supported by National Science Foundation grants MCB0517825 and MCB0641776 (to R.H.M.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0905103106/DCSupplemental.
References
- 1.Kornberg RD. Mediator and the mechanism of transcriptional activation. Trends Biochem Sci. 2005;30:235–239. doi: 10.1016/j.tibs.2005.03.011. [DOI] [PubMed] [Google Scholar]
- 2.Taatjes DJ, Marr MT, Tjian R. Regulatory diversity among metazoan co-activator complexes. Nat Rev Mol Cell Biol. 2004;5:403–410. doi: 10.1038/nrm1369. [DOI] [PubMed] [Google Scholar]
- 3.Flanagan PM, Kelleher Rd, Sayre MH, Tschochner H, Kornberg RD. A mediator required for activation of RNA polymerase II transcription in vitro. Nature. 1991;350:436–438. doi: 10.1038/350436a0. [DOI] [PubMed] [Google Scholar]
- 4.Holstege FC, et al. Dissecting the regulatory circuitry of a eukaryotic genome. Cell. 1998;95:717–728. doi: 10.1016/s0092-8674(00)81641-4. [DOI] [PubMed] [Google Scholar]
- 5.Myers LC, Gustafsson CM, Hayashibara KC, Brown PO, Kornberg RD. Mediator protein mutations that selectively abolish activated transcription [see comments] Proc Natl Acad Sci USA. 1999;96:67–72. doi: 10.1073/pnas.96.1.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thompson CM, Young RA. General requirement for RNA polymerase II holoenzymes in vivo. Proc Natl Acad Sci USA. 1995;92:4587–4590. doi: 10.1073/pnas.92.10.4587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boube M, Joulia L, Cribbs DL, Bourbon HM. Evidence for a mediator of RNA polymerase II transcriptional regulation conserved from yeast to man. Cell. 2002;110:143–151. doi: 10.1016/s0092-8674(02)00830-9. [DOI] [PubMed] [Google Scholar]
- 8.Sato S, et al. A set of consensus mammalian mediator subunits identified by multidimensional protein identification technology. Mol Cell. 2004;14:685–691. doi: 10.1016/j.molcel.2004.05.006. [DOI] [PubMed] [Google Scholar]
- 9.Dotson MR, et al. Structural organization of yeast and mammalian mediator complexes. Proc Natl Acad Sci USA. 2000;97:14307–14310. doi: 10.1073/pnas.260489497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guglielmi B, et al. A high resolution protein interaction map of the yeast Mediator complex. Nucleic Acids Res. 2004;32:5379–5391. doi: 10.1093/nar/gkh878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bhaumik SR, Raha T, Aiello DP, Green MR. In vivo target of a transcriptional activator revealed by fluorescence resonance energy transfer. Genes Dev. 2004;18:333–343. doi: 10.1101/gad.1148404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bryant GO, Ptashne M. Independent recruitment in vivo by gal4 of two complexes required for transcription. Mol Cell. 2003;11:1301–1309. doi: 10.1016/s1097-2765(03)00144-8. [DOI] [PubMed] [Google Scholar]
- 13.Govind CK, Yoon S, Qiu H, Govind S, Hinnebusch AG. Simultaneous recruitment of coactivators by Gcn4p stimulates multiple steps of transcription in vivo. Mol Cell Biol. 2005;25:5626–5638. doi: 10.1128/MCB.25.13.5626-5638.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Koh SS, Ansari AZ, Ptashne M, Young RA. An activator target in the RNA polymerase II holoenzyme. Mol Cell. 1998;1:895–904. doi: 10.1016/s1097-2765(00)80088-x. [DOI] [PubMed] [Google Scholar]
- 15.Kuras L, Borggrefe T, Kornberg RD. Association of the Mediator complex with enhancers of active genes. Proc Natl Acad Sci USA. 2003;100:13887–13891. doi: 10.1073/pnas.2036346100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Natarajan K, Jackson BM, Zhou H, Winston F, Hinnebusch AG. Transcriptional activation by Gcn4p involves independent interactions with the SWI/SNF complex and the SRB/mediator. Mol Cell. 1999;4:657–664. doi: 10.1016/s1097-2765(00)80217-8. [DOI] [PubMed] [Google Scholar]
- 17.Han SJ, et al. Activator-specific requirement of yeast mediator proteins for RNA polymerase II transcriptional activation. Mol Cell Biol. 1999;19:979–988. doi: 10.1128/mcb.19.2.979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Qiu H, et al. An array of coactivators is required for optimal recruitment of TATA binding protein and RNA polymerase II by promoter-bound Gcn4p. Mol Cell Biol. 2004;24:4104–4117. doi: 10.1128/MCB.24.10.4104-4117.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Asturias FJ, Jiang YW, Myers LC, Gustafsson CM, Kornberg RD. Conserved structures of mediator and RNA polymerase II holoenzyme. Science. 1999;283:985–987. doi: 10.1126/science.283.5404.985. [DOI] [PubMed] [Google Scholar]
- 20.Davis JA, Takagi Y, Kornberg RD, Asturias FA. Structure of the yeast RNA polymerase II holoenzyme: Mediator conformation and polymerase interaction. Mol Cell. 2002;10:409–415. doi: 10.1016/s1097-2765(02)00598-1. [DOI] [PubMed] [Google Scholar]
- 21.Takagi Y, Kornberg RD. Mediator as a general transcription factor. J Biol Chem. 2006;281:80–89. doi: 10.1074/jbc.M508253200. [DOI] [PubMed] [Google Scholar]
- 22.Kim YJ, Bjorklund S, Li Y, Sayre MH, Kornberg RD. A multiprotein mediator of transcriptional activation and its interaction with the C-terminal repeat domain of RNA polymerase II. Cell. 1994;77:599–608. doi: 10.1016/0092-8674(94)90221-6. [DOI] [PubMed] [Google Scholar]
- 23.Fan X, Chou DM, Struhl K. Activator-specific recruitment of Mediator in vivo. Nat Struct Mol Biol. 2006;13:117–120. doi: 10.1038/nsmb1049. [DOI] [PubMed] [Google Scholar]
- 24.Thiaville MM, et al. Activated transcription via mammalian amino acid response elements does not require enhanced recruitment of the Mediator complex. Nucleic Acids Res. 2008;36:5571–5580. doi: 10.1093/nar/gkn538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Deato MD, et al. MyoD targets TAF3/TRF3 to activate myogenin transcription. Mol Cell. 2008;32:96–105. doi: 10.1016/j.molcel.2008.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Andrau JC, et al. Genome-wide location of the coactivator mediator: Binding without activation and transient Cdk8 interaction on DNA. Mol Cell. 2006;22:179–192. doi: 10.1016/j.molcel.2006.03.023. [DOI] [PubMed] [Google Scholar]
- 27.Linder T, Zhu X, Baraznenok V, Gustafsson CM. The classical srb4–138 mutant allele causes dissociation of yeast Mediator. Biochem Biophys Res Commun. 2006;349:948–953. doi: 10.1016/j.bbrc.2006.08.099. [DOI] [PubMed] [Google Scholar]
- 28.Takagi Y, et al. Head module control of mediator interactions. Mol Cell. 2006;23:355–364. doi: 10.1016/j.molcel.2006.06.007. [DOI] [PubMed] [Google Scholar]
- 29.He Q, Battistella L, Morse RH. Mediator requirement downstream of chromatin remodeling during transcriptional activation of CHA1 in yeast. J Biol Chem. 2008;283:5276–5286. doi: 10.1074/jbc.M708266200. [DOI] [PubMed] [Google Scholar]
- 30.Esnault C, et al. Mediator-dependent recruitment of TFIIH modules in preinitiation complex. Mol Cell. 2008;31:337–346. doi: 10.1016/j.molcel.2008.06.021. [DOI] [PubMed] [Google Scholar]
- 31.Yu C, Palumbo MJ, Lawrence CE, Morse RH. Contribution of the histone H3 and H4 amino termini to Gcn4p- and Gcn5p-mediated transcription in yeast. J Biol Chem. 2006;281:9755–9764. doi: 10.1074/jbc.M513178200. [DOI] [PubMed] [Google Scholar]
- 32.Kim TW, et al. MED16 and MED23 of Mediator are coactivators of lipopolysaccharide- and heat-shock-induced transcriptional activators. Proc Natl Acad Sci USA. 2004;101:12153–12158. doi: 10.1073/pnas.0401985101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kim YJ, Lis JT. Interactions between subunits of Drosophila Mediator and activator proteins. Trends Biochem Sci. 2005;30:245–249. doi: 10.1016/j.tibs.2005.03.010. [DOI] [PubMed] [Google Scholar]
- 34.Lascaris RF, Mager WH, Planta RJ. DNA-binding requirements of the yeast protein Rap1p as selected in silico from ribosomal protein gene promoter sequences. Bioinformatics. 1999;15:267–277. doi: 10.1093/bioinformatics/15.4.267. [DOI] [PubMed] [Google Scholar]
- 35.McNeil JB, Dykshoorn P, Huy JN, Small S. The DNA-binding protein RAP1 is required for efficient transcriptional activation of the yeast PYK glycolytic gene. Curr Genet. 1990;18:405–412. doi: 10.1007/BF00309909. [DOI] [PubMed] [Google Scholar]
- 36.Wade JT, Hall DB, Struhl K. The transcription factor Ifh1 is a key regulator of yeast ribosomal protein genes. Nature. 2004;432:1054–1058. doi: 10.1038/nature03175. [DOI] [PubMed] [Google Scholar]
- 37.Yarragudi A, Parfrey LW, Morse RH. Genome-wide analysis of transcriptional dependence and probable target sites for Abf1 and Rap1 in Saccharomyces cerevisiae. Nucleic Acids Res. 2007;35:193–202. doi: 10.1093/nar/gkl1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Grigull J, Mnaimneh S, Pootoolal J, Robinson MD, Hughes TR. Genome-wide analysis of mRNA stability using transcription inhibitors and microarrays reveals posttranscriptional control of ribosome biogenesis factors. Mol Cell Biol. 2004;24:5534–5547. doi: 10.1128/MCB.24.12.5534-5547.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gasch AP, et al. Genomic expression programs in the response of yeast cells to environmental changes. Mol Biol Cell. 2000;11:4241–4257. doi: 10.1091/mbc.11.12.4241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Malik S, Roeder RG. Dynamic regulation of pol II transcription by the mammalian Mediator complex. Trends Biochem Sci. 2005;30:256–263. doi: 10.1016/j.tibs.2005.03.009. [DOI] [PubMed] [Google Scholar]
- 41.Leroy C, Cormier L, Kuras L. Independent recruitment of mediator and SAGA by the activator Met4. Mol Cell Biol. 2006;26:3149–3163. doi: 10.1128/MCB.26.8.3149-3163.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fan X, Struhl K. Where does mediator bind in vivo? PLoS ONE. 2009;4:e5029. doi: 10.1371/journal.pone.0005029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhu X, et al. Genome-wide occupancy profile of mediator and the Srb8–11 module reveals interactions with coding regions. Mol Cell. 2006;22:169–178. doi: 10.1016/j.molcel.2006.03.032. [DOI] [PubMed] [Google Scholar]
- 44.Park JM, Werner J, Kim JM, Lis JT, Kim YJ. Mediator, not holoenzyme, is directly recruited to the heat shock promoter by HSF upon heat shock. Mol Cell. 2001;8:9–19. doi: 10.1016/s1097-2765(01)00296-9. [DOI] [PubMed] [Google Scholar]
- 45.Venters BJ, Pugh BF. A canonical promoter organization of the transcription machinery and its regulators in the Saccharomyces genome. Genome Res. 2009;19:360–371. doi: 10.1101/gr.084970.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bhoite LT, Yu Y, Stillman DJ. The Swi5 activator recruits the Mediator complex to the HO promoter without RNA polymerase II. Genes Dev. 2001;15:2457–2469. doi: 10.1101/gad.921601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhang F, Sumibcay L, Hinnebusch AG, Swanson MJ. A triad of subunits from the Gal11/tail domain of Srb mediator is an in vivo target of transcriptional activator Gcn4p. Mol Cell Biol. 2004;24:6871–6886. doi: 10.1128/MCB.24.15.6871-6886.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hill J, Donald KA, Griffiths DE. DMSO-enhanced whole cell yeast transformation. Nucleic Acids Res. 1991;19:5791. doi: 10.1093/nar/19.20.5791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Longtine MS, et al. Additional modules for versatile and economical PCR-based gene deletion and modification in Saccharomyces cerevisiae. Yeast. 1998;14:953–961. doi: 10.1002/(SICI)1097-0061(199807)14:10<953::AID-YEA293>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.