Abstract
If the amygdala is involved in shaping perceptual experience when affectively significant visual items are encountered, responses in this structure should be correlated with both visual cortex responses and behavioral reports. Here, we investigated how affective significance shapes visual perception during an attentional blink paradigm combined with aversive conditioning. Behaviorally, following aversive learning, affectively significant scenes (CS+) were better detected than neutral (CS−) ones. In terms of mean brain responses, both amygdala and visual cortical responses were stronger during CS+ relative to CS− trials. Increased brain responses in these regions were associated with improved behavioral performance across participants and followed a mediation-like pattern. Importantly, the mediation pattern was observed in a trial-by-trial analysis, revealing that the specific pattern of trial-by-trial variability in brain responses was closely related to single-trial behavioral performance. Furthermore, the influence of the amygdala on visual cortical responses was consistent with a mediation, although partial, via frontal brain regions. Our results thus suggest that affective significance potentially determines the fate of a visual item during competitive interactions by enhancing sensory processing through both direct and indirect paths. In so doing, the amygdala helps separate the significant from the mundane.
Keywords: attentional blink, aversive conditioning, emotion, fMRI
A critical function of the amygdala appears to be to segregate the neural representations of the significant from the mundane (1, 2). One manner by which the amygdala carries out this critical function is by modulating mnemonic processes (3). A second manner by which the amygdala accomplishes this is by shaping perceptual experience directly. For example, classical conditioning can enhance perceptual sensitivity and stimulus salience by retuning sensory cortex in accordance with a stimulus's behavioral importance. These plastic changes depend on modulatory signals that originate in the amygdala (4). Converging evidence has been reported in human conditioning studies, which indicate that aversive learning modulates the sensory representation of affectively significant stimuli in a manner that is paralleled by behavioral benefits in detection and discrimination performance (5–7).
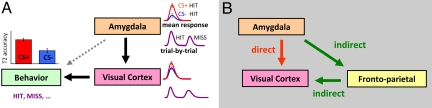
Although the amygdala is believed to shape perceptual experience, the mechanisms by which this is accomplished remain poorly understood. For instance, although lesion data support a causal link between the amygdala and enhanced perception (2) and between the amygdala and increased sensory responses (8), the precise link between amygdala responses, increased sensory responses, and enhanced perception is unclear. Here, we reasoned that if the amygdala is involved in shaping perceptual experience when affectively significant visual items are encountered, responses in this structure should be correlated with both visual cortex responses and behavioral reports. Furthermore, we hypothesized that, during a challenging visual task, the relationship between amygdala responses and behavior would be mediated by the visual cortex—given that the latter is directly involved in visual perception per se. In other words, whereas individual differences in visual performance would be expected to be predicted by amygdala responses, this relationship would be strongly dependent on visual cortical responses (see Fig. 1A).
Fig. 1.
Network interactions. (A) The effect of the amygdala on behavioral performance was hypothesized to be mediated via visual cortex. The mediation was anticipated both in the case that mean evoked responses were linked to mean accuracy and, importantly, when trial-by-trial fluctuations in brain responses and behavior were considered. (B) The contribution of amygdala responses to visual cortical responses was hypothesized to take place via both direct and indirect pathways.
A growing body of work suggests that the impact of the amygdala on behavior depends on the availability of processing resources (9–11). We therefore further hypothesized that the relationship between evoked amygdala and visual cortical responses would depend, at least in part, on frontoparietal regions involved in attentional processing. To test this prediction, a second mediation model was evaluated (Fig. 1B) in which both a direct path between the amygdala and visual cortex and an indirect pathway involving frontoparietal areas were included (alternative models are, of course, possible and are discussed in the SI Text).
For correlational (i.e., noncausal) methods such as functional MRI (fMRI) and single-unit recordings, the strongest and most direct link between brain activity and behavior involves the trial-by-trial relationship between neural signals and behavioral choice. Accordingly, we reasoned that if the amygdala is critical in shaping perceptual experience when affectively significant visual items are encountered, moment-to-moment fluctuations in evoked responses in this structure should be correlated to fluctuations in both visual cortex responses and behavioral reports. Importantly, the mediation-like interactions between the amygdala, visual cortex, and behavior were anticipated to occur on a trial-by-trial manner, consistent with the notion that the amygdala directly contributes to shaping visual responses and perception (see Fig. 1A).
Results
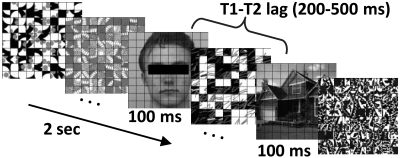
To test the above model, subjects performed an attentional blink (AB) task during fMRI scanning and were asked to detect two target objects presented among distracters in a rapid serial visual presentation stream (RSVP). Typically, a subject's detection of the second target (T2) is significantly impaired when it closely follows the first target (T1) (12), an effect that is decreased when T2 is an affectively significant item (13, 14). To investigate trial-by-trial responses, a slow event-related design (14–18-s trials) was used. As others before (15), we capitalized on the existence of category-related responses in ventral visual cortex to faces (fusiform gyrus; FG) and scenes (parahippocampal gyrus; PHG) to help separate responses to T1 (faces) and T2 (scenes) (Fig. 2). In particular, the PHG responds strongly to houses/buildings and quite weakly to faces (16), allowing for the separation of T2-evoked responses. The experimental session begun with an initial learning phase during which houses (CS+) or buildings (CS−; counterbalanced) were paired with shock (50% contingency). Subsequently, participants completed additional AB runs and a “localizer run” involving a 1-back memory task with faces and scenes. To focus our hypotheses and the tests performed, we analyzed our data in terms of regions of interest (ROIs), which focused on the amygdala, PHG in visual cortex, and frontoparietal regions.
Fig. 2.
Experimental design. Subjects performed the attentional blink task, which involved reporting two target stimuli (T1, face; T2, scene) among a stream containing 18 distractors. Note that the eyes were not obscured during the actual experiment.
Mean Responses: Performance and Behavior.
We first describe our findings in terms of mean responses and then describe those based on trial-by-trial analyses.
Learning Phase.
Confirming the effectiveness of fear conditioning, during the learning phase, stronger skin conductance responses (SCRs) were evoked by CS+ relative to CS− scenes [Fig. S1A; t (29) = 4.91, P < 0.001]. Similarly, stronger fMRI responses to CS+ relative to CS− scenes were observed in the right (R) amygdala and R PHG ROIs [Fig. S1 B and C; t (29) = 2.06, P < 0.05; t (29) = 2.50, P < 0.05, respectively].
Behavioral Performance During the AB Phase.
Before scanning, during a behavioral session not involving conditioning, the T1-T2 lag was calibrated for each individual so as to yield 60–65% detection accuracy for T2 performance. During fMRI scanning, T2 performance during CS− trials was 61.9% (SE = 1.9) and 71.9% (SE = 1.4) during CS+ trials [t (29) = 5.83, P < 0.001]. Mean accuracy for T2 trials that did not contain a scene was 97.4% (SE = 0.8). See SI Text and Fig. S2 for additional details of behavioral performance.
T2-Related Responses in Visual Cortex.
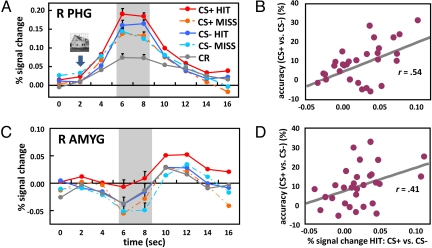
Visual responses in the PHG ROI were analyzed according to a two conditioning (CS+, CS−) by two perceptual decision (hit, miss) repeated-measures ANOVA (Fig. 3A). For the R PHG, the results revealed significant main effects of conditioning [F (1, 29) = 4.26, P < 0.05] and perceptual decision [F (1, 29) = 15.18, P < 0.005] and, importantly, a significant statistical interaction [F (1, 29) = 8.83, P < 0.01], which reflected greater differential responses during the CS+ relative to the CS− condition. Subsequent tests revealed that evoked responses during hit trials were stronger than during miss trials for both CS+ and CS− conditions [CS+: t (29) = 4.49, P < 0.001; CS−: t (29) = 2.68, P < 0.05, respectively]. In addition, simple-effect analyses revealed that responses evoked during hit trials were stronger for CS+ vs. CS− [t (29) = 3.93, P < 0.001], whereas no significant difference was observed during miss trials [t (29) = 0.48, n.s.]. The latter result is important, because it reveals that affective modulation of evoked responses was tied to visual perception, i.e., occurred only when subjects correctly detected T2 scenes. For the L PHG, only a significant main effect of perceptual decision [F (1, 16) = 6.21, P < 0.05] was detected (Fig. S3A). As the R PHG was more robustly engaged in our task, subsequent analyses focused on the right hemisphere (see SI Text for control analyses involving the FG).
Fig. 3.
PHG and amygdala (AMYG) responses. (A) Average time-courses of evoked responses in the right PHG ROI as a function of experimental condition. Evoked responses of individual trials were based on the average of time points at 6 and 8 s post-trial onset (see shaded area), i.e., 4–6 following T2 presentation. (B) Scatterplot illustrating the correlation between evoked responses in the right PHG ROI and behavioral performance across participants. (C) Average time-courses of evoked responses in the right amygdala ROI as a function of experimental condition. (D) Scatterplot illustrating the correlation between evoked responses in the right amygdala and behavioral performance across participants. Error bars in panels A and C denote the standard within-subject error term (30). R, right.
To initially explore the relationship across individuals between responses evoked in visual cortex and T2 behavioral performance, we conducted a correlation analysis involving the R PHG. Differential responses to hits (CS+ vs. CS−) were significantly correlated with improvements in T2 performance (CS+ vs. CS−) [Fig. 3B; r (30) = 0.54, P < 0.01]. This result is consistent with the notion that visual responses in the R PHG are closely related to the behavioral enhancement in AB performance that was observed as a function of affective significance.
T2-Related Responses in the Amygdala.
Only the right amygdala was robustly activated during our task (see SI Text), so analyses were performed on the right ROI only. As in visual cortex, responses were analyzed according to a two conditioning (CS+, CS−) by two perceptual decision (hit, miss) repeated-measures ANOVA (Fig. 3C). Note that during the shaded response window of Fig. 3C, responses were negative going, consistent with findings that amygdala responses decrease relative to low-level baselines during effortful mental operation, like those involving attention (17) (see SI Text for additional discussion). Nevertheless, differential responses in the amygdala followed a similar pattern as in visual cortex. The results revealed a significant main effect of perceptual decision [F (1, 29) = 11.13, P < 0.005] with the trend of a main effect of conditioning [F (1, 29) = 3.07, P = 0.09], although a significant statistical interaction was not detected [F (1, 29) = 0.97, n.s.]. However, because the trial-by-trial analysis revealed an interaction-like pattern (see below), follow up t-tests were performed, which indicated that the contrast of hit vs. miss trials was significant for CS+ trials [t (29) = 3.98, P < 0.001] but not for CS− trials [t (29) = 1.41, n.s.]. Finally, the contrast between CS+ vs. CS− hits revealed a significant difference [t (29) = 2.65, P < 0.05]. No significant differences were observed between miss trials [t (29) = 0.49, n.s.], indicating that differential responses were not produced unless a T2 scene was correctly reported.
As in the case of visual cortex, hit-related responses evoked in the amygdala were correlated with T2 behavioral performance across participants, namely, increased responses in the amygdala (CS+ vs. CS−) were significantly correlated with improved behavioral performance (CS+ vs. CS−) [r (30) = 0.41, P < 0.05] (Fig. 3D). In addition, responses in the amygdala during hit trials (CS+ vs. CS−) were positively correlated with responses in the R PHG during these trials (CS+ vs. CS−) [r (30) = 0.60, P < 0.001].
ROI Analysis of Frontoparietal Regions.
Because of the sluggish nature of fMRI responses, T1-related processes cannot be clearly dissociated from T2-related responses in regions of the frontoparietal cortex that are sensitive to fluctuations in attentional demands. Accordingly, because our central goal was to probe how affective learning influences the AB, our analysis of frontoparietal ROIs, in addition to other sites that were robustly engaged by our task, focused on two specific contrasts: CS+ vs. CS− hits and CS+ vs. CS− misses (Figs. S3 and S4; see Table S1 for a complete list). When hit trials were contrasted across conditions (CS+ vs. CS−), significant differences were observed in the R inferior parietal lobule (IPL) [t (29) = 2.99, P < 0.01], L/R middle frontal gyrus (MFG) [t (29) = 3.32, P < 0.01; t (29) = 3.21, P < 0.01, respectively], R superior frontal gyrus (SFG) [t (29) = 2.38, P < 0.05], and L anterior insula [t (29) = 3.65, P < 0.01]. No region showed significant differences when miss trials were contrasted (Ps > 0.2), again indicating that differential responses were not produced unless a T2 scene was correctly reported.
Network Interactions.
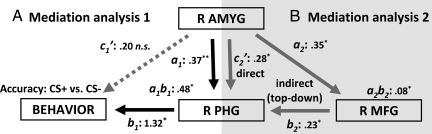
In the correlation analysis above, we observed that, across participants, responses in the amygdala were significantly correlated with behavioral performance (Fig. 3D). Using statistical mediation anaysis (Fig. S5) (31), we then tested the hypothesis that the effect of the amygdala on behavior was mediated via visual cortex (Fig. 1A). Evidence for this relationship would be provided if the strength of the path between the amygdala and behavior were significantly reduced once PHG responses were taken into account. To formally test this hypothesis, we performed a mediation analysis by using responses from the R amygdala (predictor) and the PHG (mediator), in addition to behavioral performance (outcome). Because we wanted to test if these interactions subserved the improvement in T2 performance with affectively significant stimuli, the variables entered into the analysis considered CS+ and CS− differences during hit trials. As shown in Fig. 4A, the path between the amygdala and PHG (path a1; a1 = 0.37, P < 0.01) and the path between the PHG and behavior (path b1; b1 = 1.32, P < 0.05), after controlling for amygdala responses, were statistically significant. Critically, the mediation effect also was significant (a1b1 = 0.48, P < 0.05). However, the direct effect from the amygdala to behavior was not significant after controlling for the effect of the R PHG (c′ = 0.20, n.s.), indicating that the PHG mediated (i.e., statistically nonsignificant c′) the relationship between the amygdala and the behavioral benefits of affective significance on scene detection.
Fig. 4.
Mediation analysis at the level of mean responses. (A) Path analysis was used to test the hypothesis that the effect of the amygdala on behavior was mediated by the PHG. For all variables, differences between CS+ and CS− hits were used. A significant mediation was detected (i.e., significant a1b1), such that the c′ path (effect from the predictor to the outcome after controlling for a mediator effect) was not statistically significant. The statistical significance of path coefficients was determined via bootstrapping (see SI Text). The dotted line indicates a path that was not statistically significant. (B) Path analysis was used to test the hypothesis that the effect of the amygdala on PHG responses was mediated via the MFG. Other conventions as in panel A. Both direct and indirect paths were statistically significant. *P < 0.05, **P < 0.01, two-tailed. R, right; AMYG, amygdala.
Frontoparietal regions have been proposed to constitute the neural locus of capacity-limited processes thought to underlie the AB. Accordingly, we hypothesized that the impact of the amygdala on visual cortex was potentially mediated via frontoparietal regions. Another possibility is that the contributions of the amygdala are better understood as involving both direct (amygdala → PHG) and indirect (amygdala → frontoparietal → PHG) effects (Fig. 1B). We explored two frontoparietal regions, the R MFG and R IPL, because they (i) were modulated by affective significance (CS+ vs. CS− hit) and (ii) exhibited significant correlations with the R amygdala (Ps < .05); both of these regions have also been implicated in the AB (18). Both the direct path (c2′ = 0.28, P < 0.05) and the indirect paths were statistically significant (a2 = 0.35, P < 0.05; b2 = 0.23, P < 0.05; a2b2 = 0.08, P < 0.05), indicating that both a direct effect of the amygdala on the PHG and an indirect effect of the amygdala on the PHG that involved the MFG were identified (Fig. 4B). A structural equation model in which the paths from Fig. 4 A and B were estimated simultaneously revealed similar findings (Fig. S6). When the R IPL was considered in a similar mediation analysis, a mediation effect was not observed (ab = 0.01, n.s.).
Moment-to-Moment Fluctuations in Behavior and Brain Responses.
We now turn to the goal of predicting moment-to-moment T2 decisions from single-trial fMRI responses.
Visual Cortex.
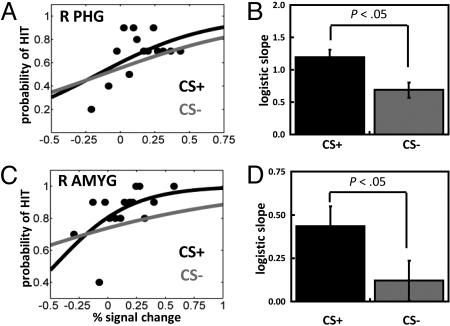
The previous analyses investigated relationships between brain and behavior by considering mean responses for each trial type. We reasoned, however, that if fluctuations in visual cortex responses determine T2 detection performance, trial-by-trial response amplitude of PHG responses should predict behavioral reports. In addition, because performance was better during the CS+ relative to the CS− condition, this relationship should be stronger for the former. To evaluate these predictions, we performed logistic regression analysis and modeled the probability of a hit trial as a function of single-trial amplitude. The mean logistic regression slopes, which represent the strength of the predictive effect, were significantly greater than zero for both CS+ [mean slope: 1.19, SE = 0.29; t (29) = 4.10, P < 0.001] and CS− trials [mean slope: 0.69, SE = 0.21; t (29) = 3.26, P < 0.01], indicating that trial-by-trial fluctuations in fMRI signals reliably predicted perceptual T2 decisions (Fig. 5 A and B). Importantly, a direct comparison of the CS+ and CS− condition revealed that the predictive power of the logistic regression fit was stronger during CS+ relative to CS− scenes [t (29) = 2.22, P < 0.05].
Fig. 5.
Trial-by-trial analysis between hit vs. miss trials. (A) Logistic regression analysis of evoked responses in the right PHG ROI as a function of affective significance (CS+ and CS−) for a representative individual. The slope of the logistic fit indicates the strength of the predictive effect. For clarity, only binned data for the CS+ condition are shown (black dots). (B) Mean logistic slopes across individuals for the PHG. (C) Same analysis as in (A) but for the right amygdala (AMYG) ROI. (D) Mean logistic slopes across individuals for the amygdala. Error bars in panels B and D denote the standard within-subject error term (30).
Amygdala.
An analogous trial-by-trial analysis was performed for the amygdala. The mean logistic regression slope was significantly greater than zero for CS+ trials [mean slope: 0.49; t (29) = 3.82, P < 0.001], but not for CS− trials [mean slope: 0.15; t (29) = 1.55, n.s.], indicating that trial-by-trial fluctuations in fMRI signals in the amygdala contributed to perceptual T2 decisions more robustly when T2 stimuli were affectively significant [paired t-test: t (29) = 2.23, P < 0.05; Fig. 5 C and D]. Note that because logistic regression slopes evaluate the relationship between hits and misses, this result indicates that an interaction-type pattern was present in our data (see SI Text for further discussion).
Network Interactions.
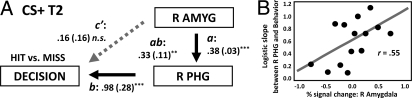
The mediation analyses presented above focused on explaining the relationship between mean differential responses in the amygdala and visual cortex and mean behavioral accuracy across individuals. If the above network interactions subserve behavioral performance as hypothesized, they should be observed in a moment-to-moment basis. Importantly, the mediation would be expected to be predictive of the behavioral outcome on individual trials, namely, whether or not a subject correctly detected a target scene (hit vs. miss). Accordingly, for every participant, behavioral reports were explained via simple and multiple logistic regression analyses (see SI Text). The path coefficients thus obtained were then tested at the group level. Trial-by-trial responses in the amygdala reliably predicted behavioral decisions during the CS+ condition [c = 0.49, SE = 0.13; t (29) = 3.82, P < 0.001]. As obtained in the previous mean-response mediation analysis (Fig. 4A), the effect of the amygdala on behavioral decisions was mediated by the R PHG [ab = 0.33, SE = 0.11; t (29) = 3.13, P < 0.01] (Fig. 6A). In other words, the direct effect of the amygdala was not significant after controlling for the effect of the PHG (c′ = 0.16, SE = 0.16, n.s.). A similar analysis was not carried out for the CS− condition because the amygdala did not significantly predict behavioral decisions in this case (c = 0.15, SE = 0.10, n.s.).
Fig. 6.
Trial-by-trial mediation analysis. (A) Mediation analysis involving the amygdala, PHG, and behavior was performed based on individual trials. The results indicated that the trial-by-trial link between amygdala responses and behavior (hit vs. miss) was mediated via the PHG. Conventions and abbreviations as in Fig. 4. Both path strength and standard errors (in parentheses) are provided (statistical significance was evaluated via one-sample t tests across participants). (B) The strength of the visual cortex to behavior relationship (as indexed via the slope of the logistic fit) was correlated with the magnitude of evoked responses in the amygdala: The stronger the response in the amygdala, the tighter the relationship between visual responses and behavior. **P < 0.01, ***P < 0.005, two-tailed.
Amygdala Responses and the Slope of the Visual Cortex to Behavior Relationship.
We further reasoned that if the amygdala shapes perception, the strength of the predictive effect between visual cortex and behavior should depend on the strength of amygdala signals. To test this hypothesis, trials across participants were pooled together and binned according to response magnitude (see SI Text and Fig. S7). The same trials were then used, and trial-by-trial logistic regressions involving the PHG and behavior were determined for every bin. Fig. 6B displays the results, which revealed that as response strength in the amygdala increased, the slope of the logistic regression was steeper [r (14) = .55, P < 0.05], i.e., the link between brain responses in visual cortex and behavior was tighter.
Discussion
In this study, we investigated how affective significance shapes visual perception during the AB paradigm. Our first goal was to characterize how mean signal changes in amygdala responses were related to changes in visual cortical responses and behavioral performance. Our second goal was to characterize these relationships on a moment-by-moment basis so that fluctuations in fMRI responses could be linked to variability in behavioral performance. Our main findings can be summarized as follows. Behaviorally, affectively significant scenes (CS+) were better detected than neutral (CS−) ones. In terms of mean brain responses, both amygdala and visual cortical responses were stronger during CS+ relative to CS− trials. Increased brain responses in these regions were associated with improved behavioral performance across participants and followed a mediation-like pattern. Importantly, the mediation pattern was observed in a trial-by-trial analysis, revealing that the specific pattern of trial-by-trial variability in brain responses was closely related to single-trial behavioral performance.
AB with Affective Stimuli.
Following affective learning, participants showed better visual detection of affectively significant stimuli (CS+ scenes) relative to neutral stimuli (CS− scenes), even though processing resources were taxed due to the detection of the initial target stimulus. Thus, although T1-related attentional demands were equivalent during both trial types, affectively significant stimuli were less susceptible to the AB than neutral ones, consistent with a growing body of studies that have showed that both emotional words and faces more effectively vie for limited resources during the AB paradigm (2, 13, 14). Because we used physically identical stimuli (across participants), the observed behavioral effect can be attributed to the different learning histories and not simply to differences in, say, visual features between emotional and neutral stimuli (see also reference 19).
Perception and the Strength of Amygdala and Sensory Representations.
Consistent with a large body of data (20), responses in the amygdala were stronger during CS+ relative to CS− trials. Interestingly, differential amygdala responses between hit and miss trials were reliably detected during CS+ trials but not for CS− trials. For both CS+ and CS− conditions, stronger evoked responses were observed in visual cortex during correct (hit) than incorrect (miss) trials, results that support the notion that the strength of the sensory representation plays a critical role in determining the fate of stimuli in the AB (15, 21). It is noteworthy that the comparison between CS+ and CS− miss trials did not reveal significant differential responses in the amygdala or PHG (or elsewhere in the brain). It thus appears that the affective nature of a stimulus itself does not guarantee differential responses, contrary to suggestions of stronger automaticity of emotion-laden stimuli (22). More generally, this finding suggests that affective perception is indeed under the control of attentional mechanisms during temporal “bottleneck” conditions, in addition to during spatial competition conditions (10, 23).
The analyses in terms of mean signal changes were complemented by trial-by-trial analyses that revealed that trial-by-trial fMRI signal amplitude in both the amygdala and sensory cortex (PHG) reliably predicted perceptual decisions (hit vs. miss). Combined, these results indicate that affective learning strengthened an item's representation of CS+ scenes, such that they reached awareness more reliably. In particular, the trial-by-trial link between visual responses and behavior demonstrates the importance of the strength of sensory representations in determining the fate of T2 stimuli in the AB (15).
How Is Visual Perception Shaped by Affective Significance?
The goal of the present investigation was to probe not only how evoked responses in individual regions were linked to behavioral performance during the AB, but, importantly, to advance our understanding of how network interactions impact visual perception. On the one hand, we were interested in probing how interactions between the amygdala and visual cortex were related to behavioral performance. On the other hand, we were also interested in understanding how these regions interacted with frontoparietal sites. At the level of mean responses, the results of our path analyses were consistent with the overall proposal of Fig. 1. Specifically, the impact of the amygdala on behavior was consistent with a mediation via visual cortex, and the influence of the amygdala on visual cortical responses was consistent with a mediation, although partial, via frontoparietal regions.
In the first case, we considered the amygdala to be a “source” region (note the arrow direction in Fig. 1A). This was done because, in our study, affective significance was obtained via aversive conditioning, which is known to be dependent on the amygdala in both nonhuman species and humans (24, 25). In our study, during an initial, separate experimental phase, participants were conditioned to a specific category of scenes (houses or buildings) and during the subsequent AB task phase, the two stimulus categories were used as CS+ and CS− stimuli. Accordingly, we hypothesized that the prior effect of learning history would lead to increased processing in visual cortex. Importantly, the hypothesized direction in Fig. 1A was informed by lesion data that directly supports the notion that increased responses in visual cortex depend on the integrity of the amygdala (8) and is also consistent with anatomical data that amygdala efferents project to anterior portions of the visual cortex (26).
Similar considerations also apply to the interactions suggested in Fig. 1B. In this case, the direct connection between the amygdala and visual cortex was supplemented by an additional “indirect” link via frontoparietal regions. It has been suggested that frontoparietal regions are involved in the control of visual competition, including competitive interactions during the AB, and that they may be critical “bottleneck” sites (27). Note that the “indirect” pathway in Fig. 1B is also indirect in the sense that the amygdala does not appear to strongly connect to these regions monosynaptically (28). Taken together, our results are consistent with the mediation-like interactions outlined in Fig. 1. Naturally, our analyses do not allow us to infer causal relationships, and other interaction schemes are possible (see additional analysis in SI Text and Fig. S8). For instance, the role of frontoparietal regions may be better viewed as one of “gating” the link between the amygdala and visual cortex, namely, a more moderation-like interaction pattern.
Evidence that the interactions between the amygdala and visual cortex subserve behavior during the AB was further supported by path analysis at the trial-by-trial level. Finally, our analyses revealed that the variability of responses in the amygdala was directly related to the strength of the visual cortex-to-behavior relationship. In summary, our results suggest that affective significance determines the fate of a visual item during competitive interactions by enhancing sensory processing through both direct and indirect paths. In so doing, the amygdala helps separate the significant from the mundane and shapes our visual world.
Methods
For additional information, please see SI Text.
Subjects.
Thirty right-handed subjects participated in the experiment and provided informed consent, as approved by the Institutional Review Board of Indiana University, Bloomington, IN.
Behavioral Experiment.
The behavioral AB experiment, which was administered prior for scanning, consisted of both single- and dual-task conditions and was used to calibrate task difficulty for the subsequent fMRI sessions. During the dual task, participants were asked to search for two targets presented among 18 distractor items in an RSVP (Fig. 2). The first target (T1) was a face image, and the second target (T2) was a scene image. Each RSVP item was displayed for 100 ms. The T1 task involved identifying one of three potential target faces (“Andy,” “Bill,” and “Chad”). The T2 task involved a categorization of scene stimuli (house, building, or no-scene). Three main trial types were investigated: Hit, correct trial containing a house/building stimulus; miss, no-scene response for trials containing a house/building stimulus; correct reject (cr), correct trial that did not contain a house/building (i.e., containing a distractor at the T2 position). During the single-task condition, the same trial structure was used, except that the T1 image was replaced with a distractor stimulus and the final T1-related decision display was removed.
fMRI Experiment.
After the behavioral session, participants finished two to three fMRI sessions. Each session involved behavioral training (which occurred during the anatomical scan), affective learning (one run), dual tasks (12 runs), and a functional localizer task (one run). During conditioning, either houses or buildings were paired with mild electrical stimulation, thereby generating CS+ and CS− stimuli (counterbalanced across subjects). After fear conditioning, participants performed dual-task trials (i.e., the AB paradigm), which were identical to those during the behavioral session, except that a slow event-related design was used with trials occurring every 14, 16, or 18 s. For each participant, the initial temporal lag between the first and second targets was individually set to the lag that yielded 60–65% accuracy during the previous behavioral session. Performance during the fMRI session was monitored on a per-run basis, and task difficulty was further calibrated (via changes in T1-T2 lag) to maintain T2 detection accuracy around 60–65% for the CS− condition. At the end of the first fMRI session, participants performed one additional functional localizer with novel faces and scenes to help determine ROIs in the PHG and FG.
MRI Data Acquisition and Analysis.
MRI data were acquired using a 3T TRIO scanner (Siemens Medical Systems). Data analysis used the AFNI package (29) and other tools. Statistical tests for our experimental hypotheses were conducted within a set of target ROIs that included the amygdala, PHG, FG, and additional frontoparietal regions.
Supplementary Material
Acknowledgments.
We thank Andrew Bauer for assistance with figures. Support for this work was provided in part by the National Institute of Mental Health (award R01 MH071589 to L.P.) and the Indiana METACyt Initiative of Indiana University, funded in part through a major grant from the Lilly Endowment, Inc.
Footnotes
The authors declare no conflicts of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0904551106/DCSupplemental.
References
- 1.Adolphs R, Tranel D. Emotion recognition and the human amygdala. In: Aggleton JP, editor. The Amygdala: A Functional Analysis. New York, NY: Oxford University Press; 2000. pp. 587–630. [Google Scholar]
- 2.Anderson AK, Phelps EA. Lesions of the human amygdala impair enhanced perception of emotionally salient events. Nature. 2001;411:305–309. doi: 10.1038/35077083. [DOI] [PubMed] [Google Scholar]
- 3.Cahill L, McGaugh JL. Modulation of memory storage. Curr Opin Neurobiol. 1996;6:237–242. doi: 10.1016/s0959-4388(96)80078-x. [DOI] [PubMed] [Google Scholar]
- 4.Weinberger NM. Specific long-term memory traces in primary auditory cortex. Nat Rev Neurosci. 2004;5:279–290. doi: 10.1038/nrn1366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Morris JS, Friston KJ, Dolan RJ. Experience-dependent modulation of tonotopic neural responses in human auditory cortex. Proc Biol Sci. 1998;265:649–657. doi: 10.1098/rspb.1998.0343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li W, Howard JD, Parrish TB, Gottfried JA. Aversive learning enhances perceptual and cortical discrimination of indiscriminable odor cues. Science. 2008;319:1842–1845. doi: 10.1126/science.1152837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Padmala S, Pessoa L. Affective learning enhances visual detection and responses in primary visual cortex. J Neurosci. 2008;28:6202–6210. doi: 10.1523/JNEUROSCI.1233-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vuilleumier P, Richardson MP, Armony JL, Driver J, Dolan RJ. Distant influences of amygdala lesion on visual cortical activation during emotional face processing. Nat Neurosci. 2004;7:1271–1278. doi: 10.1038/nn1341. [DOI] [PubMed] [Google Scholar]
- 9.Pessoa L, McKenna M, Gutierrez E, Ungerleider LG. Neural processing of emotional faces requires attention. Proc Natl Acad Sci USA. 2002;99:11458–11463. doi: 10.1073/pnas.172403899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lim SL, Padmala S, Pessoa L. Affective learning modulates spatial competition during low-load attentional conditions. Neuropsychologia. 2008;46:1267–1278. doi: 10.1016/j.neuropsychologia.2007.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Holmes A, Vuilleumier P, Eimer M. The processing of emotional facial expression is gated by spatial attention: evidence from event-related brain potentials. Cogn Brain Res. 2003;16:174–184. doi: 10.1016/s0926-6410(02)00268-9. [DOI] [PubMed] [Google Scholar]
- 12.Raymond JE, Shapiro KL, Arnell KM. Temporary suppression of visual processing in an RSVP task: An attentional blink? J Exp Psychol Hum Percept Perform. 1992;18:849–860. doi: 10.1037//0096-1523.18.3.849. [DOI] [PubMed] [Google Scholar]
- 13.Anderson AK. Affective influences on the attentional dynamics supporting awareness. J Exp Psychol Gen. 2005;134:258–281. doi: 10.1037/0096-3445.134.2.258. [DOI] [PubMed] [Google Scholar]
- 14.De Martino B, Kalisch R, Rees G, Dolan RJ. Enhanced processing of threat stimuli under limited attentional resources. Cereb Cortex. 2009;19:127–133. doi: 10.1093/cercor/bhn062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Marois R, Yi D-J, Chun MM. The neural fate of consciously perceived and missed events in the attentional blink. Neuron. 2004;41:465–472. doi: 10.1016/s0896-6273(04)00012-1. [DOI] [PubMed] [Google Scholar]
- 16.Epstein R, Harris A, Stanley D, Kanwisher N. The parahippocampal place area: Recognition, navigation, or encoding? Neuron. 1999;23:115–125. doi: 10.1016/s0896-6273(00)80758-8. [DOI] [PubMed] [Google Scholar]
- 17.Drevets WC, Raichle ME. Reciprocal suppression of regional cerebral blood flow during emotional versus higher cognitive processes: Implications for interactions between emotion and cognition. Cogn Emot. 1998;12:353–385. [Google Scholar]
- 18.Marois R, Chun MM, Gore JC. Neural correlates of the attentional blink. Neuron. 2000;28:299–308. doi: 10.1016/s0896-6273(00)00104-5. [DOI] [PubMed] [Google Scholar]
- 19.Milders M, Sahraie A, Logan S, Donnellon N. Awareness of faces is modulated by their emotional meaning. Emotion. 2006;6:10–17. doi: 10.1037/1528-3542.6.1.10. [DOI] [PubMed] [Google Scholar]
- 20.Buchel C, Morris J, Dolan RJ, Friston KJ. Brain systems mediating aversive conditioning: An event-related fMRI study. Neuron. 1998;20:947–957. doi: 10.1016/s0896-6273(00)80476-6. [DOI] [PubMed] [Google Scholar]
- 21.Williams MA, Visser TA, Cunnington R, Mattingley JB. Attenuation of neural responses in primary visual cortex during the attentional blink. J Neurosci. 2008;28:9890–9894. doi: 10.1523/JNEUROSCI.3057-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Öhman A, Wiens S. On the automaticity of autonomic responses in emotion: An evolutionary perspective. In: Davidson RJ, Scherer KR, Goldsmith HH, editors. Handbook of Affective Sciences. New York, NY: Oxford University Press; 2003. pp. 256–275. [Google Scholar]
- 23.Silvert L, et al. Influence of attentional demands on the processing of emotional facial expressions in the amygdala. Neuroimage. 2007;38:357–366. doi: 10.1016/j.neuroimage.2007.07.023. [DOI] [PubMed] [Google Scholar]
- 24.LeDoux JE, Iwata J, Cicchetti P, Reis DJ. Different projections of the central amygdaloid nucleus mediate autonomic and behavioral correlates of conditioned fear. J Neurosci. 1988;8:2517–2529. doi: 10.1523/JNEUROSCI.08-07-02517.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.LaBar KS, LeDoux JE, Spencer DD, Phelps EA. Impaired fear conditioning following unilateral temporal lobectomy in humans. J Neurosci. 1995;15:6846–6855. doi: 10.1523/JNEUROSCI.15-10-06846.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Amaral DG, Behniea H, Kelly JL. Topographic organization of projections from the amygdala to the visual cortex in the macaque monkey. Neuroscience. 2003;118:1099–1120. doi: 10.1016/s0306-4522(02)01001-1. [DOI] [PubMed] [Google Scholar]
- 27.Marois R, Ivanoff J. Capacity limits of information processing in the brain. Trends Cogn Sci. 2005;9:296–305. doi: 10.1016/j.tics.2005.04.010. [DOI] [PubMed] [Google Scholar]
- 28.Ghashghaei HT, Hilgetag CC, Barbas H. Sequence of information processing for emotions based on the anatomic dialogue between prefrontal cortex and amygdala. Neuroimage. 2007;34:905–923. doi: 10.1016/j.neuroimage.2006.09.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cox RW. AFNI: Software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res. 1996;29:162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- 30.Loftus GR, Masson ME. Using confidence intervals in within-subject designs. Psychon Bull Rev. 1994;1:476–490. doi: 10.3758/BF03210951. [DOI] [PubMed] [Google Scholar]
- 31.Wager TD, Davidson ML, Hughes BL, Lindquist MA, Ochsner KN. Prefrontal-subcortical pathways mediating successful emotion regulation. Neuron. 2008;59:1037–1050. doi: 10.1016/j.neuron.2008.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.