Abstract
Helminths are multicellular eukaryotic parasites that infect over one quarter of the world’s population. Through coevolution with the human immune system, these organisms have learned to exploit immunoregulatory pathways, resulting in asymptomatic tolerance of infections in many individuals. When infections and the resulting immune responses become dysregulated, however, acute and chronic pathologies often develop. A recent international meeting focused on how these parasites modulate host immunity and how control of parasitic and immunopathological disease might be achieved.
The intestinal helminth H. polygyrus.
IMAGE COURTESY OF CONSTANCE FINNEY.
It is widely accepted that helminths and their antigens inherently induce T helper (Th) 2 responses, and it is likely that Th2 immunity evolved in response to infections with these parasites. In February 2009, scientists from 32 countries assembled in Tahoe City, CA for the Keystone symposium on Pathogenesis and Immune Regulation in Helminth Infections (February 1–5, 2009), organized by Rick Maizels, Maria Yazdanbakhsh, and Thomas Wynn, to discuss how immune responses develop in response to helminth infections and how these infections are (or are not) controlled. The discussions provided new insights into the cellular players, cytokines, and effector molecules that help initiate, and subsequently limit, immune responses during helminth infection.
How the human immune system fights off—or learns to live with—worm infections has been extensively studied, but many questions remain unanswered. On the host side, the coordinate functions of multiple cell types, including phagocytes, granulocytes, B cells, Th2 cells, and regulatory T (T reg) cells, dictate the outcome of infection.
Getting things started: Th2 cells and beyond
IL-4–producing Th2 cells are perhaps the most extensively studied cells in the context of worm infections, and generating an effective Th2 response has long been thought to require the early production of interleukin (IL)-4. Contrary to this idea, however, Graham Le Gros (Wellington, New Zealand) showed that neither IL-4 nor signal transducer and activator of transcription (STAT)-6, a key component of the IL-4R signaling pathway, was essential for the development of a Th2 response in vivo. Activation of an IL-4–linked GFP reporter construct was intact in STAT-6–deficient mice infected with Nippostrongylus brasiliensis (King et al., 2008; van Panhuys et al., 2008), suggesting that other factors, whether host- or parasite-derived, are critical for Th2 polarization in vivo.
Another Th2-inducing cytokine, thymic stromal lymphopoietin (TSLP), may also be dispensable for generating Th2 responses against certain helminth infections. In noninfectious settings, such as allergy, Th2 responses are largely TSLP dependent. But in the absence of a functional TSLP receptor, mice infected with Heligmosomoides polygyrus, N. brasiliensis, or Schistosoma mansoni (Fig. 1). still generated strong Th2 responses (Nicola Harris, Lausanne, Switzerland; Massacand et al., 2009; Ramalingam et al., 2009). This rule does not hold true for all worm infections, however, as TSLP is essential for the development of Th2 responses against Trichuris muris (Taylor et al., 2009a). A key difference between these parasites appears to be the ability of H. polygyrus and N. brasiliensis, but not T. muris, to inhibit the production of inflammatory cytokines, such as IL-6, IL-12, and TNF, from dendritic cells (DCs) in response to Toll-like receptor (TLR) ligation. T. muris may thus require TSLP to help restrain IL-12 production before a Th2 response can develop, whereas other parasites inhibit cytokine production directly, thereby circumventing this pathway.
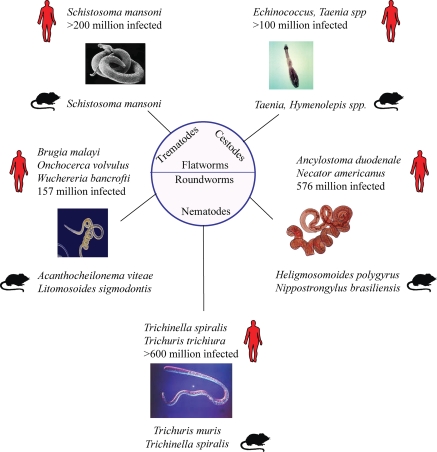
Figure 1.
Helminth infections. A summary of the major helminth infections of humans and their mouse model counterparts. Estimates of human infection numbers are taken from Hotez et al. (2008). Note that the schematic does not include highly prevalent helminth infections such as Ascaris lumbricoides (807 million estimated infections), other important human parasites, or the prominent veterinary helminth organisms.
Where and how Th2 cells are generated has been the subject of intense scrutiny. Using IL-4-GFP (“4get”) reporter mice to track cells that produce IL-4 in response to infection, multiple groups have found that follicular T helper (Tfh) cells are the predominant IL-4–producing cells in responding lymph nodes (Richard Locksley, San Francisco, CA and Edward Pearce, Saranac Lake, NY; Reinhardt et al., 2009; Zaretsky et al., 2009). Similar findings have also been reported by Markus Mohrs (King and Mohrs, 2009). This may not seem surprising, considering the well-established role of IL-4 in B cell activation and Ig class switching, but it nevertheless raised questions about the precise definition of a Th2 cell. Locksley argued that conventional Th2 cells may function primarily within tissues to mediate classical allergic-like inflammation, such as eosinophil recruitment, whereas Tfh cells function in the follicles to deliver help to B cells. Pearce offered further perspective on the Th2 population in Schistosoma infection by showing that Th2 cells lose responsiveness as chronic disease develops, a process mediated by increased expression of GRAIL, an E3 ubiquitin ligase implicated in the development of T cell anergy (Taylor et al., 2009b).
These and other studies have taught us a great deal about the host requirements for generating Th2 responses against helminths. But far less is known about what components of the parasites are required to initiate the host response. New data from Markus Mohrs (Saranac Lake, NY) revealed that the Th2-inducing potential of schistosome eggs is due in part to the action of omega-1, a glycoprotein with ribonuclease activity that is released from eggs under physiological conditions (Everts et al., 2009). Purified omega-1 induced strong Th2 responses in mice, even in those lacking the IL-4 receptor. Omega-1 also inhibited DC activation in response to lipopolysaccharide, suggesting that the egg protein acts at the initial stages of response (Steinfelder et al., 2009). Production of omega-1 likely helps sustain infection, as the subsequent Th2 response stimulates the formation of protective granulomas around the eggs. The hunt is now on to identify the cellular receptor for omega-1.
Like most helminth infections, schistosomiasis elicits a predominant CD4+ Th2 cell response, and mice depend on this response to survive infection. In the absence of IL-4, mice develop hepatotoxicity, endotoxemia, and severe cachexia, which together contribute to the death of the animal. However, some strains of mice, such as CBA, are more prone to developing Th1 and Th17 responses, and thus develop severe pathology in response to S. mansoni infection (Miguel Stadecker and Mara Shainheit, Boston, MA). DCs from CBA mice produced more IL-12p40 and IL-6 in response to live schistosome eggs than did DCs from C57BL/6 mice. CBA DCs also stimulated stronger IL-17 production from transgenic T cells specific for the egg antigen Sm-p40. In vitro experiments suggested that the increased Th17 response requires both IL-1 and IL-23 (Rutitzky et al., 2008) and that the Th17 response is responsible for the severe egg-induced inflammatory response seen in infected CBA mice (Shainheit et al., 2008). Co-infection with the intestinal nematode H. polygyrus decreased IL-17 and interferon (IFN)-γ production in CBA mice, perhaps helping to explain the low incidence of autoimmune diseases in regions where helminth infections are endemic (Bazzone et al., 2008).
Keeping potentially dangerous Th1 responses at bay in T. muris infection depends on activation of the NF-κB signaling pathway in intestinal epithelial cells (IECs), according to David Artis (Philadelphia, PA). In mice with an IEC-specific ablation of the classical NF-κB pathway, DCs produced excess inflammatory cytokines leading to the development of a nonprotective Th1 response (Zaph et al., 2007). Normally, the DC-triggered induction of a Th2 response depends on the NF-κB–dependent production of TSLP and IL-25 from IECs (Zaph et al., 2008; Taylor et al., 2009a).
Applying the brakes: T reg cells
Generating a Th2 response to parasite infections is essential, but controlling that response is equally imperative. Some of this control is provided by T reg cells, which were shown to dampen Th2 responses during H. polygyrus infection (Rick Maizels, Edinburgh, Scotland, UK; Wilson et al., 2005). In this model, the parasite itself drives the differentiation of T reg cells from naive CD4+ T cells by secreting a product that binds to host TGF-β receptors. Inhibiting this interaction in vivo decreased the intensity of infection, presumably as a result of more effective Th2 responses, illustrating the evolutionary advantage of this pathway for the parasite.
Induction of T reg cells is also needed for Litomosoides sigmodontis to establish long-lasting infections. When T reg cells were depleted in susceptible mouse strains, infection was cleared (Nienke van der Werf and Matthew Taylor, Edinburgh, Scotland, UK; Taylor et al., 2009). In chronic infection, subsequent reactivation of hyporesponsive Th2 cells, which express high levels of GITR, CTLA-4, and PD-1, required CTLA-4 blockade or co-stimulation of the cells through GITR. Indeed, GITR ligation was essential for the development of Th2 responses during this infection. Recent data indicates that GITR also promotes the generation of Th2 responses during the initial immune-priming stage of infection and that blocking the PD-1 pathway enhances parasite killing, illustrating the opposing roles of these pathways in anti-helminth immunity.
T reg cells also suppress host immune responses in humans, presumably allowing parasites to establish chronic infections (Maizels and Yazdanbakhsh, 2003). Indeed, schistosome-infected individuals in Gabon had increased numbers of circulating T reg cells compared with their uninfected neighbors (Maria Yazdanbakhsh, Leiden, Netherlands and Ayola Akim Adegnika, Lambaréné, Gabon). And helminth-induced T reg cells may have more potent suppressive activity than those from healthy individuals. T reg cells from geohelminth-infected individuals in Indonesia, for example, were more effective at suppressing proliferation and IFN-γ production by effector T cells in response to malaria antigens and BCG than T reg cells from healthy individuals (Yazdanbakhsh and Taniawati Supali, Jakarta, Indonesia). Tom Nutman (Bethesda, MD) also reported diminished production of cytokines in response to malaria antigens in peripheral blood cells from helminth/malaria coinfected individuals. This inhibition was driven primarily by the suppressive cytokine IL-10 (Metenou et al., 2009)
Maintaining balance: alternatively activated macrophages
Robust Th2 responses are required to clear helminth infections, but Th2 responses that overshoot can be dangerous. Although Th1 and Th17 responses develop in response to acute S. mansoni infections in most mouse strains, they quickly wane and are replaced by Th2 responses. Although this transition is initially beneficial to the host, persistent Th2 responses can contribute to liver pathology and hepatosplenic disease (Wynn, 2007).
Prior studies had suggested that alternatively activated macrophages (AAMs), which are triggered by Th2 responses, promote pathology during chronic infection (Wynn, 2008). But more recent data show that AAMs actually help to dampen immune responses in schistosomiasis and to inhibit the development of severe hepatosplenic disease (Tom Wynn, Bethesda, MD). The protective action of AAMs depended on expression of arginase-1 (Arg-1), as mice whose macrophages lack Arg-1 failed to suppress Th2 responses and egg-induced inflammation. As a result, the mice developed fibrosis (Pesce et al., 2009a). The protective effect of AAMs is likely to involve the arginase-dependent depletion of l-arginine, which is required to maintain T cell proliferation and Th2 responses (Pesce et al., 2009a). Consistently, macrophages from mice lacking the cationic amino acid transporter 2 (Cat2), which regulates l-arginine transport into cells, also had increased Arg1 activity and reduced Th2 activity. Surprisingly, however, Cat2-deficient mice developed severe pathology despite their reduced Th2 responses, perhaps owing to enhanced Arg1 activity in fibroblasts (Thompson et al., 2008).
Another product of AAMs, resistin-like molecule-α (RELMα/Fizz1/Retnla), helps to control inflammation in response to S. mansoni. RELMα can also be produced by other cell types, as macrophages, eosinophils, and epithelial cells produced the molecule after exposure to S. mansoni eggs in the lung (Fig. 2; Meera Nair, Philadelphia, PA). Mice lacking RELMα developed severe egg-induced inflammation in the lung and liver that was associated with an enhanced Th2 response and could be reversed by treating the mice with recombinant RELMα (Nair et al., 2009; Pesce et al., 2009b).
Figure 2.
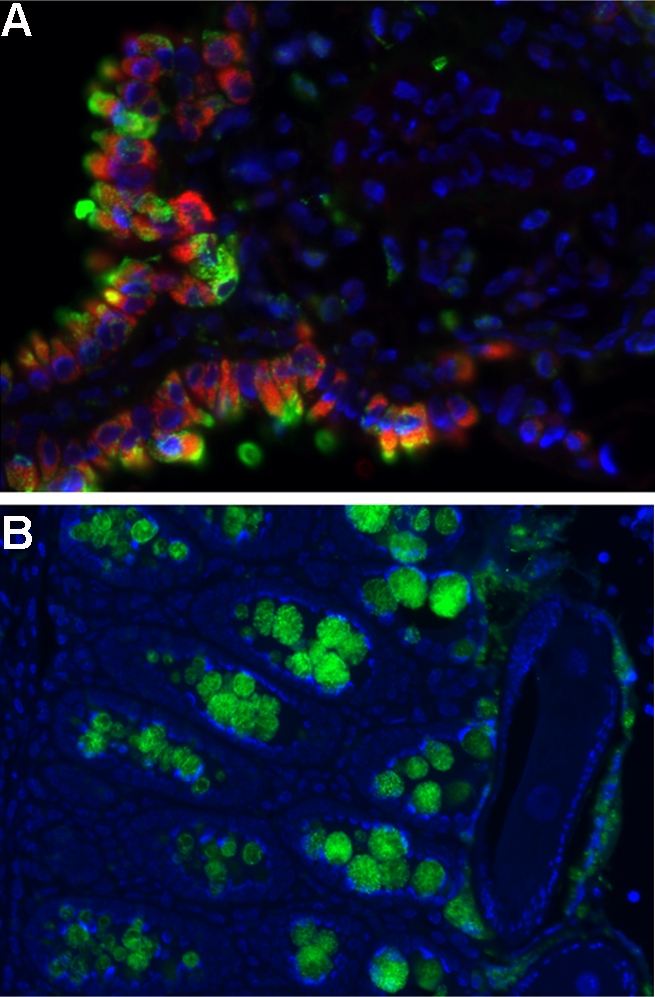
Expression of RELM proteins after exposure to helminth parasites. RELMα (green) is expressed by mannose receptor–positive cells (red) in the lungs of C57BL/6 mice challenged i.v. with S. mansoni eggs (A), and RELMβ is expressed by goblet cells of the gastrointestinal tract in C57BL/6 mice infected orally with T. muris eggs (B). Image courtesy of Meera Nair and David Artis, University of Pennsylvania.
The dominant cellular sources of RELMα may depend on the stimulus and route of exposure. For example, after S. mansoni infection, or during primary pulmonary granuloma formation, RELMα was predominantly produced by eosinophils rather than macrophages (Tom Wynn, Bethesda, MD; Pesce et al., 2009b). The role of AAM-derived RELMα should thus be clarified through studies with cell-specific deletions of Relmα. A related molecule, RELMβ, was shown to be required for expulsion of N. brasiliensis and H. polygyrus, which both reside in the intestinal lumen, but was not required for expulsion of T. spiralis, which lives within epithelial cells (De’Broski Herbert, Cincinnati, OH). RELMβ appears to inhibit the ability of worms to feed on host tissues resulting in reduced ATP content and worm fecundity.
AAMs may also have a hand in the direct killing of parasites, as clodronate liposome-mediated depletion of AAMs dramatically inhibited killing of Brugia malayi in mice (Judith Allen, Edinburgh, Scotland, UK). However, parasite killing was also impaired in mice receiving PBS-loaded liposomes or latex beads, which compromise macrophage function without reducing cell numbers or the expression of AAM-associated genes (Arg1, RELMα, and Ym1). This suggests that macrophages are critical for parasite killing, but that alternative activation may not be required. Macrophage activation by Th2 cytokines might instead function primarily to regulate immune responses and to facilitate tissue repair rather than to promote parasite killing. Given that helminths cause considerable damage while migrating through tissues, particularly the gut and lung, Allen and colleagues proposed a model in which the immune system repairs helminth-induced damage more rapidly after secondary worm exposure in an IL-4/IL-13–dependent manner, accelerating the return of homeostasis to critical tissues (Loke et al., 2007; Mylonas et al., 2009). Whether helminth-elicited AAMs play a role in the immune-regulation in models of chronic inflammation awaits further study.
New cells on the scene: basophils
Basophils are newly recognized players in the immune response to helminth infections. These cells, along with eosinophils, are known to produce IL-4 during allergic responses and certain helminth infections (Fig. 3), but whether these cells were essential for clearing helminths was not clear. The critical importance of these cells was demonstrated in part by Locksley, who showed that allergic responses and worm expulsion (N. brasiliensis) did not rely on IL-4 production by T cells, as these functions were intact in mice whose T cells lacked CD4+ IL-4 and IL-13. Parasite expulsion was also intact in mice lacking mast cells (KitW-sh) or eosinophils (Gata-1 mutant). But basophil depletion (using anti-MAR-1 antibodies) hindered worm clearance. The ability of basophils to produce IL-4 is likely to be critical during both primary and secondary responses. Indeed, although schistosome-infected mice lacking IL-4 and IL-13 succumbed to infection, provision of IL-4 by non-T cells was sufficient to rescue them. And depleting basophils from immunodeficient mice that had been injected with IL-4/13-deficient lymphocytes and then infected with N. brasiliensis attenuated eosinophil recruitment and worm expulsion (David Voehringer, Munich, Germany; Ohnmacht and Voehringer, 2009).
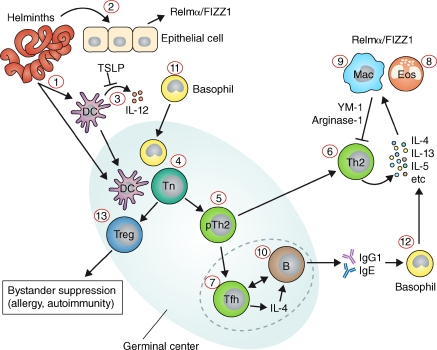
Figure 3.
Cellular interactions in the immune response to helminths. Helminths are complex eukaryotic pathogens with multiple life stages that can affect different tissues within the parasitized host. Helminth-derived antigens can drain directly to secondary lymphoid organs or interact with cells at the site of infection, including DCs (1) or epithelial cells (2). Many helminth antigens inhibit the production of proinflammatory cytokines by DCs (3), and trigger the production of cytokines such as TSLP from epithelial cells, which inhibits IL-12 production by DCs. Antigen-carrying DCs activate naive Th cells (4), which proliferate and differentiate into precursor Th2 cells (5). pTh2 cells can then become Th2 cells (6) or Tfh cells (7). Th2 cells release various cytokines, including IL-5, which drives eosinophilia (8), and IL-4/-13, which stimulate AAMs (9). AAMs in turn produce molecules such as arginase-1 and YM-1, which dampen Th2 responses. Tfh produce IL-4 and provide help to B cells for IgG1 and IgE production (10). During the early response to helminth infections, MHC class II–expressing basophils (11) enter the reactive secondary lymphoid organs and may help to polarize the Th2 response. IgE can then activate basophils (12), which in turn produce cytokines that activate alternative macrophages. Helminth infections can also promote the development of T reg cell responses (13).
Basophils were also found in the draining lymph nodes of mice infected with T. muris. The Artis group set out to ask which antigen-presenting cells were critical in this model using mice in which MHC class II expression was restricted to DCs. These mice could not generate protective Th2 responses to T. muris, indicating the need for at least one other antigen-presenting cell type. Using IL-4-GFP (“4get”) reporter mice, they spotted IL-4–producing, MHC class II+ basophils in the draining lymph nodes. When these cells were depleted, Th2 responses were impaired. Basophil responses were boosted by the cytokine TSLP, which was produced primarily by intestinal epithelial cells, and both cell types were required for an optimal Th2 response (Perrigoue et al., 2009). The effects of basophils on Th2 induction appear to be age-dependent, according to Padraic Fallon (Dublin, Ireland), who showed that impaired basophil function in juvenile mice (<21 d of age) resulted in insufficient IL-4 production, poor Th2 generation, and an inability to expel N. brasiliensis.
B cells, antibodies, and symbionts
B cells and antibodies are essential in combatting some, but not all, helminth infections. Bill Gause (Newark, NJ) demonstrated that both macrophages and B cells were required to prevent H. polygyrus from invading gut tissues within the first several days of a memory response. But B cells were not required for effective expulsion of N. brasiliensis, suggesting that the importance of B cells depends on the infecting parasite.
High levels of parasite-specific IgE correlate with resistance to disease induced by S. mansoni infection (Butterworth et al., 1992), although what triggers IgE production and how these antibodies protect the host remain elusive. The protective role of IgE was studied in ongoing field studies, which showed that repeated treatment with the drug praziquantel over 3–6 yr accelerated the development of resistance to reinfection. Resistance correlated with increased levels of antigen-specific IgE and increased expression of CD23, the low-affinity IgE receptor, on B cells (Dan Colley, Athens, GA; Mwinzi et al., 2009).
Some helminths, such as the filarial parasites, are infected with symbiotic Wolbachia bacteria, which has led to therapeutic use of doxycycline antibiotics to eliminate both endosymbionts and the worms they depend on. Achim Hoerauf (Bonn, Germany) showed that TLR-dependent neutrophilic responses in filarial infections are stimulated by Wolbachia rather than the worm itself. Growth of tissue-dwelling filarial parasites also appears to be enhanced by angiogenic factors such as CCL17 (TARC) and VEGF-A, and the latter was promoted by the symbiotic bacteria.
Worm influences on allergy, autoimmunity, and vaccines
Infection with helminths can be beneficial to the host, as the Th2 and T reg cell responses that develop in response to helminths can suppress allergic and autoimmune responses (Wilson and Maizels, 2004). For example, helminth infections may contribute to a decreased incidence of type I diabetes. Illustrating this, Marc Hubner and Edward Mitre (Bethesda, MD) reported that infection with L. sigmodontis or injection of L. sigmodontis antigens prevented diabetes in NOD mice (Hübner et al., 2009). Protection was associated with increased Th2 responses and T reg cell numbers, rather than decreased pathogenic Th17 responses. Somewhat surprisingly, protection did not require the signature Th2 cytokine IL-4. Infection with S. mansoni also protected against diabetes in NOD mice, as discussed by Anne Cooke (Cambridge, England, UK). CD25+ T reg cells played a crucial role in this model, as transfer of CD25+ cells from infected mice protected against disease. And in vitro studies demonstrated that schistosome egg antigens triggered the TGF-β–dependent expression of FoxP3 in naive NOD T cells (Zaccone et al., 2009), suggesting that inducible T reg cells may help prevent disease in this system.
Allergic responses are also muted by helminth infections (Maizels, 2005). Exposure to the intestinal worm N. brasiliensis or worm extracts decreased allergen-induced airway inflammation in mice sensitized to ovalbumin (OVA), including decreased eosinophilia, airway hyperreactivity, and OVA-specific IgG1 and IgE production (Klaus Erb, Germany). The reduction in airway inflammation was attributed to the induction of T reg cells and depended on IL-10 production. Although somewhat counterintuitive, the protection against Th2-type allergic responses went hand-in-hand with increased worm-specific Th2 responses, which might have been predicted to worsen the allergic response. How N. brasiliensis infection inhibits allergic responses without compromising helminth-specific Th2 immunity is not yet known.
Echoing the critical role of IL-10 in suppressing inflammation, H. polygyrus infection of IL-10–deficient mice relieved the spontaneous colonic inflammation that normally develops in these mice, according to Joel Weinstock (Boston, MA). Turning to a mouse model of OVA-induced colitis, Weinstock showed that H. polygyrus infection reduced colitis with fewer OVA-specific Th1 and Th17 cells accumulating in the lamina propria (Elliott et al., 2008). In this model, TGF-β–producing cells expressing TLR4 also suppressed inflammation in response to lipopolysaccharide.
Whether the protective effect of helminth infections in mice can be extended to humans remains to be demonstrated on a broad scale. However, an impressive example of the power of these infections to suppress autoimmunity was provided by P’ng Loke (San Francisco, CA), who described a patient suffering from ulcerative colitis who relieved his intestinal inflammation by infecting himself with the intestinal worm Trichuris trichiura. The cytokine IL-22, which was expressed in a distinct population of CD4+ T cells in the lamina propria of this patient, may have helped control inflammation. Similarly, CD4+ T cell–derived IL-22 protects against inflammatory bowel disease in mice (Zenewicz et al., 2008).
A study of hookworm-infected individuals in Papua New Guinea showed that infected individuals tended toward higher IgE production although they did not develop overt allergies (Daniel Blount, Nottingham, England, UK). The practical potential of helminth infections in alleviating allergies and autoimmunity is not yet clear. The Nottingham group has completed phase I studies showing that low-dose hookworm infections are safe in humans and do not exacerbate symptoms in rhinitis patients during hay fever season (Blount et al., 2009; Feary et al., 2009). Trials are now under way to assess the potential benefits of this treatment in patients with Crohn’s disease and multiple sclerosis. In an independent study, infection with the hookworm Necator americanus was shown to reduce the symptoms of Crohn’s disease (Danielle Smyth, Brisbane, Australia; Croese et al., 2006). This group also found that secretory antigens from the dog hookworm Ancylostoma caninum alleviated dextran sulfate sodium–induced colitis in mice with reduced production of Th1 and Th17 cytokines, suggesting that a beneficial effect might be achieved without active infection.
An excellent example of a helminth-derived therapeutic was presented by Billy Harnett (Glasgow, Scotland, UK) on the filarial nematode protein ES-62. This secreted protease bears crucial phosphorylcholine sidechains that interact with B cells, macrophages, mast cells, and DCs, and modulate TLR-linked signaling pathways (Harnett and Harnett, 2008). ES-62 can suppress immune responsiveness in vivo, in both collagen-induced arthritis and OVA-specific allergic airway inflammation.
These studies highlight the potentially beneficial side effects of helminth infection, but there is also a downside. By the same means that helminth infections benefit the host—i.e., by dampening potentially damaging inflammation—they also blunt responses to vaccines. Children infected with helminths, for example, generated relatively poor multifunctional T cell responses (coproduction of IL-2, TNF, and IFN-γ) when vaccinated against influenza (Yazdanbakhsh, Leiden, Netherlands and Adegnika, Lambaréné, Gabon). Similarly, responses to vaccines against Salmonella were suppressed by preexisting intestinal helminth infection (Cathryn Nagler, Chicago, IL). Suboptimal responses in orally vaccinated mice were not caused by impaired antigen recognition, as activated, antigen-specific CD4+ T cells accumulated normally in the draining lymph nodes, but rather by increased antigen-specific, IL-10–producing CD4+ T cells—most likely T reg cells. As one of the main goals of global health organizations is to broaden vaccine coverage with a focus on geographical regions where gastrointestinal helminth infections are endemic, efforts to eliminate or broadly control helminth infections may be required to maximize vaccine efficacy. And large-scale studies are needed to assess the full impact of helminth infections on the clinical course of coendemic infections, as well as inflammatory conditions such as allergy and autoimmunity.
Finally, there is an urgent agenda to develop protective anti-helminth vaccines, which are conspicuously absent from the world’s public health armamentarium. Peter Hotez (Washington, DC) outlined progress made using the most advanced vaccines for human hookworm, including Na-ASP-2, Na-GST-1, and Na-APR-1 (Loukas et al., 2006), with the latest updates on trials in endemic areas. The immunological responses in vaccinated subjects will also be invaluable in identifying beneficial and protective responses and distinguishing them from the regulatory responses induced by helminth parasites.
Concluding remarks
These studies have revealed groundbreaking new findings about the biology of helminth infections and how mammalian hosts respond to them, providing a much clearer conceptual grasp of the balance between the immune reactivity and regulation that controls the outcome of helminth infections. Throughout the meeting, it was clear that laboratory models and field studies shared common themes, and it was gratifying to see laboratory findings being translated into human therapies. Perhaps the realization that the same regulatory pathways that are induced by helminth infections can also limit vaccine efficacy and interfere with immunity to heterologous infections will inject new energy into the effort to use antihelmintic treatments to maximize protection against the major childhood microbial diseases.
Acknowledgments
The authors are grateful for the generous support provided by the Keystone Symposia Global Health Series, funded by the Bill and Melinda Gates Foundation. We are also grateful to the Burroughs Wellcome Fund, the United European Gastroenterology Federation, Cell Press, SCYNEXIS, and the National Institute of Allergy and Infectious Diseases for additional financial support for this meeting.
References
- Bazzone L.E., Smith P.M., Rutitzky L.I., Shainheit M.G., Urban J.F., Setiawan T., Blum A.M., Weinstock J.V., Stadecker M.J. 2008. Coinfection with the intestinal nematode Heligmosomoides polygyrus markedly reduces hepatic egg-induced immunopathology and proinflammatory cytokines in mouse models of severe schistosomiasis.Infect. Immun. 76:5164–5172 doi:10.1128/IAI.00673-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blount D., Hooi D., Feary J., Venn A., Telford G., Brown A., Britton J., Pritchard D. 2009. Immunological profiles of persons recruited for a randomized, placebo-controlled clinical trial of hookworm infection.Am. J. Trop. Med. Hyg. In press [DOI] [PubMed] [Google Scholar]
- Butterworth A.E., Dunne D.W., Fulford A.J., Thorne K.J., Gachuhi K., Ouma J.H., Sturrock R.F. 1992. Human immunity to Schistosoma mansoni: observations on mechanisms, and implications for control.Immunol. Invest. 21:391–407 doi:10.3109/08820139209069381 [DOI] [PubMed] [Google Scholar]
- Croese J., O’neil J., Masson J., Cooke S., Melrose W., Pritchard D., Speare R. 2006. A proof of concept study establishing Necator americanus in Crohn’s patients and reservoir donors.Gut. 55:136–137 doi:10.1136/gut.2005.079129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott D.E., Metwali A., Leung J., Setiawan T., Blum A.M., Ince M.N., Bazzone L.E., Stadecker M.J., Urban J.F., Jr., Weinstock J.V. 2008. Colonization with Heligmosomoides polygyrus suppresses mucosal IL-17 production.J. Immunol. 181:2414–2419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everts B., Perona-Wright G., Smits H.H., Hokke C.H., van der Ham A.J., Fitzsimmons C.M., Doenhoff M.J., van der Bosch J., Mohrs K., Haas H., et al. 2009. Omega-1, a glycoprotein secreted by Schistosoma mansoni eggs, drives Th2 responses.J. Exp. Med. 206:1673–1680 doi:10.1084/jem.20082460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feary J., Venn A., Brown A., Hooi D., Falcone F.H., Mortimer K., Pritchard D.I., Britton J. 2009. Safety of hookworm infection in individuals with measurable airway responsiveness: a randomized placebo-controlled feasibility study.Clin. Exp. Allergy. 39:1060–1068 doi:10.1111/j.1365-2222.2009.03187.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harnett W., Harnett M.M. 2008. Therapeutic immunomodulators from nematode parasites.Expert Rev. Mol. Med. 10:e18 doi:10.1017/S1462399408000720 [DOI] [PubMed] [Google Scholar]
- Hotez P.J., Brindley P.J., Bethony J.M., King C.H., Pearce E.J., Jacobson J. 2008. Helminth infections: the great neglected tropical diseases.J. Clin. Invest. 118:1311–1321 doi:10.1172/JCI34261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hübner M.P., Stocker J.T., Mitre E. 2009. Inhibition of type 1 diabetes in filaria-infected non-obese diabetic mice is associated with a T helper type 2 shift and induction of FoxP3+ regulatory T cells.Immunology. 127:512–522 doi:10.1111/j.1365-2567.2008.02958.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- King I.L., Mohrs M. 2009. IL-4–producing CD4+ T cells in reactive lymph nodes during helminth infection are T follicular helper cells.J. Exp. Med. 206:1001–1007 doi:10.1084/jem.20090313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- King S.B., Knorn A.M., Ohnmacht C., Voehringer D. 2008. Accumulation of effector CD4 T cells during type 2 immune responses is negatively regulated by Stat6.J. Immunol. 180:754–763 [DOI] [PubMed] [Google Scholar]
- Loke P., Gallagher I., Nair M.G., Zang X., Brombacher F., Mohrs M., Allison J.P., Allen J.E. 2007. Alternative activation is an innate response to injury that requires CD4+ T cells to be sustained during chronic infection.J. Immunol. 179:3926–3936 [DOI] [PubMed] [Google Scholar]
- Loukas A., Bethony J., Brooker S., Hotez P. 2006. Hookworm vaccines: past, present, and future.Lancet Infect. Dis. 6:733–741 doi:10.1016/S1473-3099(06)70630-2 [DOI] [PubMed] [Google Scholar]
- Maizels R.M. 2005. Infections and allergy - helminths, hygiene and host immune regulation.Curr. Opin. Immunol. 17:656–661 doi:10.1016/j.coi.2005.09.001 [DOI] [PubMed] [Google Scholar]
- Maizels R.M., Yazdanbakhsh M. 2003. Immune regulation by helminth parasites: cellular and molecular mechanisms.Nat. Rev. Immunol. 3:733–743 doi:10.1038/nri1183 [DOI] [PubMed] [Google Scholar]
- Massacand J.C., Stettler R.C., Meier R., Humphreys N.E., Grencis R.K., Marsland B.J., Harris N.L. 2009. Helminth products bypass the need for TSLP in Th2 immune responses by directly modulating dendritic cell function.Proc. Natl. Acad. Sci. USA. 106:13968–13973 doi:10.1073/pnas.0906367106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metenou S., Dembélé B., Konate S., Dolo H., Coulibaly S.Y., Coulibaly Y.I., Diallo A.A., Soumaoro L., Coulibaly M.E., Sanogo D., et al. 2009. Patent filarial infection modulates malaria-specific type 1 cytokine responses in an IL-10-dependent manner in a filaria/malaria-coinfected population.J. Immunol. 183:916–924 doi:10.4049/jimmunol.0900257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mwinzi P.N., Ganley-Leal L., Black C.L., Secor W.E., Karanja D.M., Colley D.G. 2009. Circulating CD23+ B cell subset correlates with the development of resistance to Schistosoma mansoni reinfection in occupationally exposed adults who have undergone multiple treatments.J. Infect. Dis. 199:272–279 doi:10.1086/595792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mylonas K.J., Nair M.G., Prieto-Lafuente L., Paape D., Allen J.E. 2009. Alternatively activated macrophages elicited by helminth infection can be reprogrammed to enable microbial killing.J. Immunol. 182:3084–3094 doi:10.4049/jimmunol.0803463 [DOI] [PubMed] [Google Scholar]
- Nair M.G., Du Y., Perrigoue J.G., Zaph C., Taylor J.J., Goldschmidt M., Swain G.P., Yancopoulos G.D., Valenzuela D.M., Murphy A., et al. 2009. Alternatively activated macrophage-derived RELM-alpha is a negative regulator of type 2 inflammation in the lung.J. Exp. Med. 206:937–952 doi:10.1084/jem.20082048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohnmacht C., Voehringer D. 2009. Basophil effector function and homeostasis during helminth infection.Blood. 113:2816–2825 doi:10.1182/blood-2008-05-154773 [DOI] [PubMed] [Google Scholar]
- Perrigoue J.G., Saenz S.A., Siracusa M.C., Allenspach E.J., Taylor B.C., Giacomin P.R., Nair M.G., Du Y., Zaph C., van Rooijen N., et al. 2009. MHC class II-dependent basophil-CD4+ T cell interactions promote T(H)2 cytokine-dependent immunity.Nat. Immunol. 10:697–705 doi:10.1038/ni.1740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pesce J.T., Ramalingam T.R., Mentink-Kane M.M., Wilson M.S., El Kasmi K.C., Smith A.M., Thompson R.W., Cheever A.W., Murray P.J., Wynn T.A. 2009a. Arginase-1-expressing macrophages suppress Th2 cytokine-driven inflammation and fibrosis.PLoS Pathog. 5:e1000371 doi:10.1371/journal.ppat.1000371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pesce J.T., Ramalingam T.R., Wilson M.S., Mentink-Kane M.M., Thompson R.W., Cheever A.W., Urban J.F., Jr., Wynn T.A. 2009b. Retnla (relmalpha/fizz1) suppresses helminth-induced Th2-type immunity.PLoS Pathog. 5:e1000393 doi:10.1371/journal.ppat.1000393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramalingam T.R., Pesce J.T., Mentink-Kane M.M., Madala S., Cheever A.W., Comeau M.R., Ziegler S.F., Wynn T.A. 2009. Regulation of helminth-induced Th2 responses by thymic stromal lymphopoietin.J. Immunol. 182:6452–6459 doi:10.4049/jimmunol.0900181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinhardt R.L., Liang H.E., Locksley R.M. 2009. Cytokine-secreting follicular T cells shape the antibody repertoire.Nat. Immunol. 10:385–393 doi:10.1038/ni.1715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutitzky L.I., Bazzone L., Shainheit M.G., Joyce-Shaikh B., Cua D.J., Stadecker M.J. 2008. IL-23 is required for the development of severe egg-induced immunopathology in schistosomiasis and for lesional expression of IL-17.J. Immunol. 180:2486–2495 [DOI] [PubMed] [Google Scholar]
- Shainheit M.G., Smith P.M., Bazzone L.E., Wang A.C., Rutitzky L.I., Stadecker M.J. 2008. Dendritic cell IL-23 and IL-1 production in response to schistosome eggs induces Th17 cells in a mouse strain prone to severe immunopathology.J. Immunol. 181:8559–8567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinfelder S., Andersen J.F., Cannons J.L., Feng C.G., Joshi M., Dwyer D., Caspar P., Schwartzberg P.L., Sher A., Jankovic D. 2009. The major component in schistosome eggs responsible for conditioning dendritic cells for Th2 polarization is a T2 ribonuclease (omega-1).J. Exp. Med. 206:1681–1690 doi:10.1084/jem.20082462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor M.D., van der Werf N., Harris A., Graham A.L., Bain O., Allen J.E., Maizels R.M. 2009. Early recruitment of natural CD4+ Foxp3+ Treg cells by infective larvae determines the outcome of filarial infection.Eur. J. Immunol. 39:192–206 doi:10.1002/eji.200838727 [DOI] [PubMed] [Google Scholar]
- Taylor B.C., Zaph C., Troy A.E., Du Y., Guild K.J., Comeau M.R., Artis D. 2009a. TSLP regulates intestinal immunity and inflammation in mouse models of helminth infection and colitis.J. Exp. Med. 206:655–667 doi:10.1084/jem.20081499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor J.J., Krawczyk C.M., Mohrs M., Pearce E.J. 2009b. Th2 cell hyporesponsiveness during chronic murine schistosomiasis is cell intrinsic and linked to GRAIL expression.J. Clin. Invest. 119:1019–1028 doi:10.1172/JCI36534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson R.W., Pesce J.T., Ramalingam T., Wilson M.S., White S., Cheever A.W., Ricklefs S.M., Porcella S.F., Li L., Ellies L.G., Wynn T.A. 2008. Cationic amino acid transporter-2 regulates immunity by modulating arginase activity.PLoS Pathog. 4:e1000023 doi:10.1371/journal.ppat.1000023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Panhuys N., Tang S.C., Prout M., Camberis M., Scarlett D., Roberts J., Hu-Li J., Paul W.E., Le Gros G. 2008. In vivo studies fail to reveal a role for IL-4 or STAT6 signaling in Th2 lymphocyte differentiation.Proc. Natl. Acad. Sci. USA. 105:12423–12428 doi:10.1073/pnas.0806372105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson M.S., Maizels R.M. 2004. Regulation of allergy and autoimmunity in helminth infection.Clin. Rev. Allergy Immunol. 26:35–50 doi:10.1385/CRIAI:26:1:35 [DOI] [PubMed] [Google Scholar]
- Wilson M.S., Taylor M.D., Balic A., Finney C.A.M., Lamb J.R., Maizels R.M. 2005. Suppression of allergic airway inflammation by helminth-induced regulatory T cells.J. Exp. Med. 202:1199–1212 doi:10.1084/jem.20042572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wynn T.A. 2007. Common and unique mechanisms regulate fibrosis in various fibroproliferative diseases.J. Clin. Invest. 117:524–529 doi:10.1172/JCI31487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wynn T.A. 2008. Cellular and molecular mechanisms of fibrosis.J. Pathol. 214:199–210 doi:10.1002/path.2277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaccone P., Burton O., Miller N., Jones F.M., Dunne D.W., Cooke A. 2009. Schistosoma mansoni egg antigens induce Treg that participate in diabetes prevention in NOD mice.Eur. J. Immunol. 39:1098–1107 doi:10.1002/eji.200838871 [DOI] [PubMed] [Google Scholar]
- Zaph C., Troy A.E., Taylor B.C., Berman-Booty L.D., Guild K.J., Du Y., Yost E.A., Gruber A.D., May M.J., Greten F.R., et al. 2007. Epithelial-cell-intrinsic IKK-beta expression regulates intestinal immune homeostasis.Nature. 446:552–556 doi:10.1038/nature05590 [DOI] [PubMed] [Google Scholar]
- Zaph C., Du Y., Saenz S.A., Nair M.G., Perrigoue J.G., Taylor B.C., Troy A.E., Kobuley D.E., Kastelein R.A., Cua D.J., et al. 2008. Commensal-dependent expression of IL-25 regulates the IL-23-IL-17 axis in the intestine.J. Exp. Med. 205:2191–2198 doi:10.1084/jem.20080720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaretsky A.G., Taylor J.J., King I.L., Marshall F.A., Mohrs M., Pearce E.J. 2009. T follicular helper cells differentiate from Th2 cells in response to helminth antigens.J. Exp. Med. 206:991–999 doi:10.1084/jem.20090303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zenewicz L.A., Yancopoulos G.D., Valenzuela D.M., Murphy A.J., Stevens S., Flavell R.A. 2008. Innate and adaptive interleukin-22 protects mice from inflammatory bowel disease.Immunity. 29:947–957 doi:10.1016/j.immuni.2008.11.003 [DOI] [PMC free article] [PubMed] [Google Scholar]