Abstract
Atherosclerosis is an inflammatory vascular disease responsible for the first cause of mortality worldwide. Recent studies have clearly highlighted the critical role of the immunoinflammatory balance in the modulation of disease development and progression. However, the immunoregulatory pathways that control atherosclerosis remain largely unknown. We show that loss of suppressor of cytokine signaling (SOCS) 3 in T cells increases both interleukin (IL)-17 and IL-10 production, induces an antiinflammatory macrophage phenotype, and leads to unexpected IL-17–dependent reduction in lesion development and vascular inflammation. In vivo administration of IL-17 reduces endothelial vascular cell adhesion molecule–1 expression and vascular T cell infiltration, and significantly limits atherosclerotic lesion development. In contrast, overexpression of SOCS3 in T cells reduces IL-17 and accelerates atherosclerosis. We also show that in human lesions, increased levels of signal transducer and activator of transcription (STAT) 3 phosphorylation and IL-17 are associated with a stable plaque phenotype. These results identify novel SOCS3-controlled IL-17 regulatory pathways in atherosclerosis and may have important implications for the understanding of the increased susceptibility to vascular inflammation in patients with dominant-negative STAT3 mutations and defective Th17 cell differentiation.
The immunoinflammatory response plays a prominent role in driving atherosclerotic lesion development, progression, and complications (Binder et al., 2002; Hansson and Libby, 2006; Tedgui and Mallat, 2006; Weber et al., 2008). Defining the direct roles of specific immune regulatory pathways in the modulation of atherosclerosis is certainly of considerable interest (Tedgui and Mallat, 2006).
Suppressor of cytokine signaling (SOCS) proteins are key physiological regulators of both innate and adaptive immunity, and control the development of various immunoinflammatory diseases (Yoshimura et al., 2007). SOCS3 is expressed in atherosclerotic lesions, and the current paradigm suggests an atheroprotective role through inhibition of STAT3 signaling and the suppression of proinflammatory responses (Tang et al., 2005; Gharavi et al., 2007; Ortiz-Muñoz et al., 2009). However, its direct role in the control of the immune response of atherosclerosis is still largely unknown.
Recent studies have addressed the role of T cell–specific SOCS3 expression on T cell differentiation and cytokine production. Intriguingly, one study reported preferential Th3- and/or Tr1-like differentiation and reduced Th1 polarization in mice lacking SOCS3 expression in T cells (Kinjyo et al., 2006). However, others have reported a preferential promotion of Th17 in the absence of T cell–specific SOCS3 expression (Chen et al., 2006), consistent with the critical role of STAT3 activation in Th17 development (for review see Dong, 2008). Tr1-related responses have been associated with the reduction of atherosclerosis (Maron et al., 2002; Mallat et al., 2003), whereas recent studies have indirectly associated IL-17 production with potentially proatherogenic responses (Eid et al., 2009). Still, the direct roles of SOCS3 and IL-17 production in the modulation of vascular inflammation and atherosclerotic lesion development remain unknown. The involvement of SOCS3- and IL-17–related signaling pathways in various inflammatory diseases (Bettelli et al., 2007; Yoshimura et al., 2007) will certainly promote the development of therapeutic strategies aiming at the modulation of these pathways to limit disease severity and progression. Whether modulation of SOCS3 and IL-17 production would similarly alter the inflammatory process related to atherosclerosis remains unknown. We have therefore designed a series of experiments to directly assess the roles of T cell–specific SOCS3 and (SOCS3-controlled) IL-17 in the modulation of vascular inflammation and atherosclerotic lesion development.
RESULTS AND DISCUSSION
SOCS3 expression in T cells significantly affects atherosclerotic lesion development
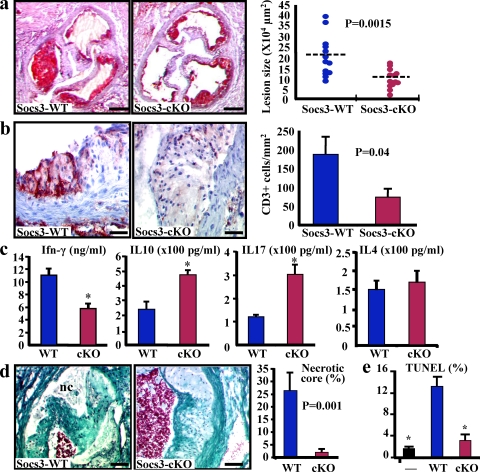
We first examined the effect of SOCS3 deletion in T cells on the development of atherosclerosis. We reconstituted low-density lipoprotein receptor–deficient (Ldlr−/−) mice with either a WT bone marrow from SOCS3flox/flox mice (the reconstituted mice are designated SOCS3-WT) or a bone marrow from mice with T cell–specific deletion in SOCS3 (SOCS3flox/flox:Lck-Cre) generated by a conditional gene targeting approach using a Cre-loxP system (designated SOCS3-cKO; Yasukawa et al., 2003; Kinjyo et al., 2006). Phosphorylated (P)-STAT3 was readily detectable in atherosclerotic lesions, and we found a marked increase in P-STAT3 in spleen-derived T cells from SOCS3-cKO mice compared with SOCS-WT mice (Fig. S1), indicating an efficient and functional deletion of SOCS3 in T cells. We therefore quantified lesion size after 6 wk on a high fat diet. We found an unexpected 50% reduction of aortic sinus lesion size in SOCS3-cKO mice compared with controls (Fig. 1 a), despite similar plasma cholesterol levels (18.4 ± 0.7 vs. 17.6 ± 0.5 g/liter; P = 0.32). We observed a similar protection against atherosclerosis at the levels of the aortic sinus and the descending thoracic aorta in a separate set of old Ldlr−/− mice reconstituted with SOCS3-cKO bone marrow (Fig. S1 d).
Figure 1.
SOCS3 deletion in T cells promotes IL-17 and IL-10 production, inhibits macrophage apoptosis, and limits atherosclerotic lesion development. (a) Atherosclerotic lesion size in the aortic root of chimeric Ldlr−/− SOCS3-WT or SOCS-cKO mice. Data are representative of four independent experiments throughout this work. Dashed lines indicate mean values. (b) Lesion T cell infiltration (red staining; mean values ± SEM). Data are representative of three experiments. (c) Reduced IFN-γ but increased IL-10 and IL-17 production in supernatants of CD3-stimulated CD4+ T cells (means ± SEM of five mice per group and two experiments; *, P < 0.05). (d) Necrotic core (nc; trichrome staining) size (means ± SEM) in the aortic root. Representative of two experiments and 13 mice per group. (e) Macrophage apoptosis (TUNEL staining) after coincubation with CD4+ cells from SOCS3-WT or SOCS-cKO mice (means ± SEM). Representative of three experiments (*, P < 0.05). Bars: (a) 250 µm; (b) 30 µm; (d) 60 µm.
We then tested the effect of SOCS3 overexpression in T cells on the development of atherosclerosis (Fig. S1 e). We reconstituted Apoe−/−/Rag2−/− mice with purified CD4+ cells recovered from either WT or SOCS3-transgenic (Tg) mice (Seki et al., 2003). As expected, we found reduced P-STAT3 in SOCS3-Tg T cells (unpublished data). After 6 wk of a high fat diet, spleen-derived CD4+ cells of mice transferred with CD4+ SOCS3-Tg cells showed reduced production of IL-17 and IL-10 but enhanced production of IL-4 (Fig. S1 e). This is consistent with previous studies that showed reduced Th17 and preferential Th2 cell differentiation of T cells isolated from SOCS3-Tg mice (Seki et al., 2003; Tanaka et al., 2008). Interestingly, we found a fourfold increase of lesion size in Apoe−/−/Rag2−/− mice transferred with CD4+ SOCS3-Tg cells compared with controls (Fig. S1 e), despite similar plasma cholesterol levels (12.1 ± 1.6 vs. 14.1 ± 0.78 g/liter; P = 0.25). Thus, SOCS3 expression in T cells significantly affects atherosclerotic lesion development.
SOCS3 deletion in T cells enhances IL-10 and IL-17 production
The reduction of atherosclerosis in SOCS3-cKO mice was associated with a significant decrease of T cell infiltration within the lesions (Fig. 1 b), suggesting a modulation of the T cell phenotype. We found no difference in the number and suppressive function of natural CD4+CD25+Foxp3+ regulatory T cells between the two groups of mice (Fig. S2). Thus, we analyzed cytokine production by purified spleen-derived CD4+ cells. We observed a significant reduction of IFN-γ production and an increase of IL-10 by cells recovered from SOCS3-cKO mice (Fig. 1 c), which was consistent with previous results showing preferential Th3- and/or Tr1-like differentiation and reduced Th1 polarization in mice lacking SOCS3 expression in T cells (Kinjyo et al., 2006). However, we also detected a threefold increase in IL-17 production by the purified SOCS3-deficient CD4+ cells compared with controls, consistent with the critical role of STAT3 activation in Th17 development (Fig. 1 c; Chen et al. 2006; Dong, 2008). Flow cytometry analysis on freshly isolated cells showed no cells expressing both IL-17 and IL-10, suggesting that in vivo, IL-10 and IL-17 are produced by distinct T cells, which is in agreement with a recent study showing that RORγt+ T cells producing both IL-10 and IL-17 could not be observed in vivo (Lochner et al., 2008). Consistent with enhanced IL-17 production, we found increased IL-6 but reduced IL-27 levels in the circulating blood of SOCS3-cKO mice (Fig. S2 c). We also found increased production of IL-6 by cultured SOCS3-cKO splenocytes. IL-6 neutralization did not alter IL-17 production, indicating that the Th17 profile of SOCS3-cKO cells was independent of IL-6 (Fig. S2 d). Thus, our results indicate that in the absence of SOCS3 expression in T cells, there is a preferential switch toward increased production of both IL-10 and IL-17, which helps explain seemingly divergent previous results on Th3, Tr1, and Th17 cells.
SOCS3 deletion in T cells induces an antiinflammatory phenotype in macrophages
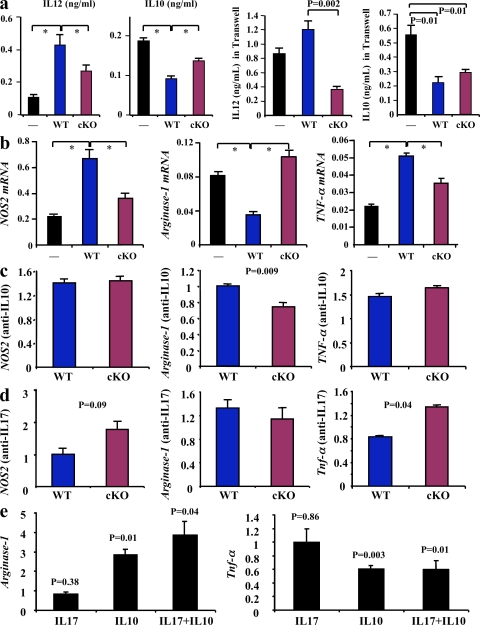
It was remarkable that the loss of SOCS3 in one cell type has produced significant effects on lesion development, where many other cell types are involved. Thus, we hypothesized that the T cell cytokine profile induced by SOCS3 deletion may modulate the inflammatory response of other cell types. In this regard, the innate immune system plays a critical role in atherogenesis (Hansson and Libby, 2006). Therefore, we examined the impact of SOCS3 deletion in T cells on macrophage infiltration and activation. The extent of lesion macrophage accumulation was not altered in SOCS3-cKO compared with SOCS3-WT mice (35,022 ± 6,308 vs. 36,635 ± 5,223 µm2; P = 0.84). We hypothesized that this could be related to a differential modulation of macrophage survival within the lesions. Interestingly, we found a marked reduction in the size of the necrotic core in lesions of SOCS3-cKO mice (Fig. 1 d). This was confirmed by the reduction of lesion Tdt-mediated dUTP-biotin nick-end labeling (TUNEL) staining (unpublished data) and a protection against macrophage apoptosis when bone marrow–derived macrophages were incubated in the presence of SOCS3-cKO CD4+ cells compared with SOCS3-WT CD4+ cells (Fig. 1 e). Macrophage death within the lesions is highly promoted by proinflammatory signals. We found a significant reduction of IL-12 and an increase in IL-10 production by macrophages after coincubation with SOCS3-cKO CD4+ cells compared with coincubation with SOCS3-WT CD4+ cells (Fig. 2 a). In addition, we found a significant reduction in the expression of NOS2 and TNF, and an increase of arginase-1 after coincubation of macrophages with SOCS3-cKO T cells compared with SOCS3-WT cells, consistent with a limitation of M1 phenotype and the preservation of an antiinflammatory potential (Fig. 2 a). These effects persisted when T cells and macrophages were physically separated using Transwells, suggesting the involvement of soluble factors (Fig. 2 a). Neutralization of IL-10 production and, to a lesser extent IL-17, prevented some of the antiinflammatory effects (Fig. 2, c and d), whereas incubation with IL-10 was associated with an antiinflammatory phenotype that persisted after supplementation with IL-17 (Fig. 2 e). Our results indicate that SOCS3 signaling in T cells not only affects the T cell phenotype but also regulates the production of pro- and antiinflammatory/atherogenic mediators by macrophages. This is consistent with a study showing that STAT3 activity in tumor cells mediates immune evasion through blockade of inflammatory signal production by the innate and adaptive immune system (Wang et al., 2004). Further studies will be necessary to identify the soluble factors responsible for the full change in macrophage phenotype.
Figure 2.
SOCS3 deletion in T cells promotes an antiinflammatory macrophage phenotype. (a) IL-12 and IL-10 production by macrophages after coincubation with CD4+ cells from SOCS3-WT or SOCS-cKO mice in the presence or absence of Transwells. Mean values ± SEM of three to four mice per group are shown (*, P < 0.05). (b) TNF-α, NOS2, and arginase-1 mRNA expression (arbitrary units relative to GAPDH mRNA expression) by macrophages after coincubation with CD4+ cells. Mean values ± SEM of three to four mice per group and three independent experiments are shown (*, P < 0.05). (c and d) Quantitative analysis (relative to incubation without neutralizing antibodies) of NOS2, arginase-1, and TNF-α mRNA expression in macrophages after coincubation with CD4+ cells in the presence of either anti–IL-10 (c) or anti–IL-17 (d) neutralizing antibodies. (e) Quantitative analysis (relative to control without incubation with recombinant cytokines) of arginase-1 and TNF-α mRNA expression in macrophages after incubation with IL-10, IL-17 or IL-10, and IL-17. Values in c–e represent mean values ± SEM of three mice per group and three different experiments.
Neutralization of IL-17 abrogates atheroprotection in mice with T cell–specific SOCS3 deletion and enhances vascular inflammation
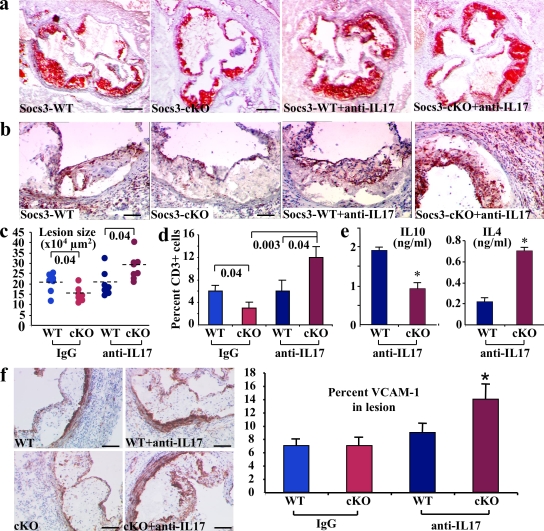
The reduction of atherosclerosis in SOCS3-cKO mice was associated with a reduction in IFN-γ and an increase in both IL-10 and IL-17. The pro- and antiatherogenic roles of IFN-γ and IL-10, respectively, have been extensively addressed in previous studies (Tedgui and Mallat, 2006). However, the role of IL-17 in atherosclerosis is still unexplored. Thus, we examined the direct role of IL-17 production in the atheroprotective effect of T cell–specific SOCS3 deletion. We generated additional series of chimeric Ldlr−/− mice that were put on a high fat diet and were treated with either a mouse monoclonal anti–IL-17A neutralizing antibody (Uyttenhove and Van Snick, 2006; Uyttenhove et al., 2007) or an isotype-matched control for 6 wk (see Materials and methods). Interestingly, neutralization of IL-17 did not alter lesion size in SOCS3-WT mice, which are highly Th1 biased and produce low levels of IL-17, but totally abrogated the atheroprotective effect of T cell–specific SOCS3 deletion and led to a marked increase of lesion formation (Fig. 3, a and c). Acceleration of lesion development was associated with a marked increase in vascular inflammation, as revealed by increased vascular cell adhesion molecule (VCAM)–1 expression (Fig. 3 f), marked T cell infiltration within the lesions and the adventitia of anti–IL-17–treated SOCS3-cKO mice (Fig. 3, b and d), and a switch toward reduced IL-10 and increased IL-4 production by spleen-derived CD4+ cells (Fig. 3 e) but no change in IFN-γ (15.4 ± 0.5 vs. 12.7 ± 1.1 ng/ml in SOCS3-WT and SOCS3-cKO, respectively). Regulatory T cell function was not altered by anti–IL-17 treatment (not depicted), and the proatherogenic effect of IL-17 neutralization in SOCS3-cKO mice was not prevented by IL-10 supplementation (Fig. S3). These results identify an unprecedented role for SOCS3-controlled IL-17 in the control of vascular inflammation and T cell accumulation within atherosclerotic lesions, which profoundly affects lesion development. Our results are in agreement with a study showing that IL-17 neutralization was associated with enhanced T cell infiltration in a model of allergic asthma (Schnyder-Candrian et al., 2006).
Figure 3.
SOCS3-controlled IL-17 production protects against vascular inflammation and atherosclerotic lesion development. Representative photomicrographs (a and b) and quantitative analysis (c and d) of atherosclerotic lesion size (a and c) and lesion T cell infiltration (b and d) in the aortic root of chimeric Ldlr−/− SOCS3-WT or SOCS-cKO mice treated either with a neutralizing anti–IL-17A antibody (anti–IL-17) or an isotype-matched control (IgG; n = 7–8 mice per group). The anti–IL-17 in vivo experiment was repeated twice. p-values are shown in c and d. Dashed lines indicate mean values. (e) Increased IL-4 but reduced IL-10 production in supernatants of CD3-stimulated CD4+ T cells recovered at the time of sacrifice from chimeric Ldlr−/− mice treated with a neutralizing anti–IL-17A antibody. Means ± SEM of five mice per group performed in triplicates are shown (*, P < 0.05). (f) VCAM-1 expression in atherosclerotic lesions (n = 6–8 mice per group and two separate experiments; *, P < 0.05). Bars: (a) 250 µm; (b) 100 µm; (f) 130 µm.
In vivo administration of IL-17 to Ldlr−/− mice reduces endothelial VCAM-1 expression, vascular T cell infiltration, and atherosclerotic lesion development
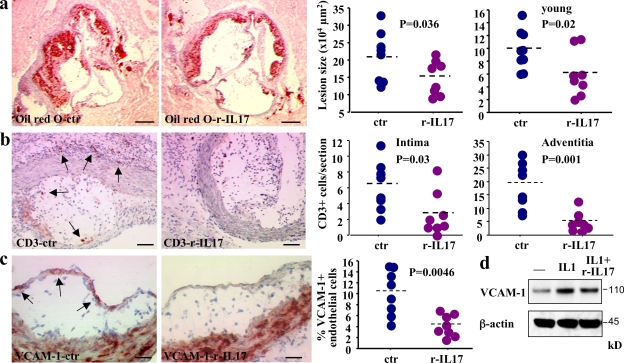
Neutralization of IL-17 accelerated atherosclerosis in SOCS3-cKO mice but had no effect on lesion development in SOCS3-WT mice (Fig. 3). We reasoned that this apparent discrepancy might be caused by the low level of IL-17 production by SOCS3-WT T cells (highly Th1 biased on a C57BL/6 background) and that supplementation of SOCS3-WT mice with IL-17 might inhibit lesion development. Thus, we fed 17-wk-old female Ldlr−/− mice a high fat diet and treated them with either rIL-17 or control mouse serum albumin. Administration of rIL-17 led to a significant elevation of circulating IL-17 levels (Fig. S3 c) and to a moderate but significant elevation of IL-6 production (27.5 ± 18.7 vs. 1 ± 0.6 pg/ml; P = 0.008) comparable to levels detected in SOCS3-cKO mice, suggesting biological effects. We found no change in T cell cytokine profile (Fig. S3 d) or splenocyte inflammatory response (unpublished data) in mice treated with rIL-17, indicating that at this dosage, IL-17 did not promote inflammatory cell activation. Interestingly, we found a significant reduction of atherosclerotic lesion development in mice supplemented with rIL-17 (Fig. 4 a). We reproduced the results in a different set of younger 12-wk-old female Ldlr−/− mice (Fig. 4 a). Of note, atheroprotection was associated with a marked inhibition of vascular inflammation as revealed by a significant inhibition of vascular T cell infiltration (Fig. 4 b) and a 60% reduction of endothelial VCAM-1 expression (Fig. 4 c). rIL-17 also reduced IL-1–induced endothelial VCAM-1 expression in vitro (Fig. 4 d). These results indicate that IL-17 inhibits high fat–induced vascular inflammation and atherosclerotic lesion development.
Figure 4.
Supplementation with IL-17 reduces vascular inflammation and limits atherosclerotic lesion development. (a) Atherosclerotic lesion size in the aortic root of 17-wk-old female chimeric Ldlr −/− SOCS3-WT mice fed a high fat diet and treated with rIL-17 or control serum albumin for 5 wk. Two different experiments are depicted with eight to nine animals in each group. Young animals were 12 wk old. (b) Lesion and adventitial T cell infiltration in 17-wk-old animals. Similar results were obtained in young animals (not depicted). (c) Endothelial VCAM-1 expression in IL-17–treated 17-wk-old mice. Results represent two separate experiments. (d) One representative example out of three different experiments of a Western blot showing reduction of IL-1–induced VCAM-1 expression after mouse endothelial cells are incubated with IL-17. Dashed lines indicate mean values. Bars: (a) 250 µm; (b) 50 µm; (c) 25 µm.
Vascular expression of IL-17 and P-Stat3 is associated with plaque stability
We then examined IL-17 expression in mouse atherosclerotic arteries (Ldlr−/− mice reconstituted with SOCS3-WT or SOCS3-cKO) using immunohistochemistry. As expected, we were unable to detect IL-17 expression in T cells of SOCS3-WT mice (not depicted), and only occasional IL-17+ inflammatory cells were detected in lesions of SOCS3-cKO mice (Fig. 5, left, a and b), which may be explained, at least in part, by the low number of activated SOCS3-deficient T cells that infiltrated the lesions. Unexpectedly, we found a marked staining of IL-17 in medial smooth muscle cells (SMCs) of plaque-free areas (which was confirmed in normal arteries; Fig. 5, left, e), and the staining appeared to be rapidly lost in medial SMCs underlying early lipid lesions (Fig. 5, left, c and d). We confirmed the detection of IL-17 in normal mouse aorta by Western blotting using a different antibody (unpublished data). These results show that C57BL/6 Ldlr−/− mice, which are Th1 biased, produce low levels of IL-17 in T cells and that vascular wall production of IL-17 decreases during the development of atherosclerosis, suggesting a relationship between SMC differentiation and IL-17 expression.
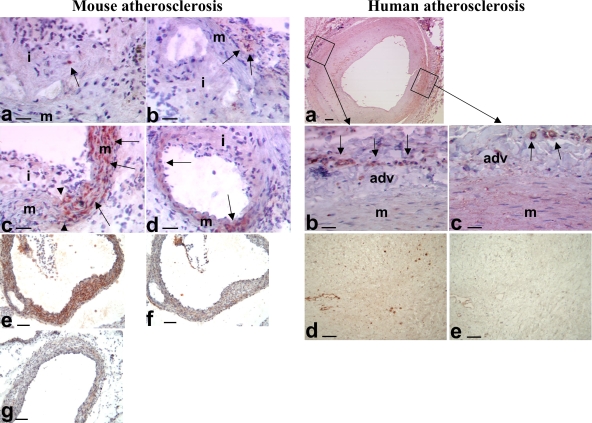
Figure 5.
Expression of IL-17 in atherosclerotic vessels. (left) Mouse atherosclerosis. (a and b) Occasional staining for IL-17 (red, arrows) in inflammatory cells within the intima (i) and adventitia (arrows in b) of SOCS3-cKO mice. (c and d) Representative examples of staining for IL-17 (red, arrows) in the media (m) of the aortic sinus (c) and the coronary arteries (d) of SOCS3-cKO mice. Note that IL-17 staining in media was detected in plaque-free areas (right of the arrowheads) and was lost in areas where plaques have developed (left of the arrowheads). (e–g) Staining for IL-17 (red/brown) in the media of the aortic sinus of WT (e and f) or IL-17A–deficient (g) mice. Note that IL-17 staining almost disappeared after adsorption with the IL-17–specific peptide (f) or in vessels of IL-17A–deficient mice (g). Data are representative of at least 10 different mouse arteries. Bars: (a–d) 45 µm; (e–g) 90 µm. (right) Human atherosclerosis. (a) IL-17 staining in the media (m) and adventitia (adv) of carotid plaques. (b) The image is from an area underlying an advanced plaque. It shows IL-17 staining in adventitial vessels (arrows), but the media has lost its IL-17 staining. (c) The image is from an area underlying an early intimal thickening. It shows staining for IL-17 in the adventitial vessels (arrows) and still diffuse moderate IL-17 staining within the media. (d) Another example of IL-17 staining (brown) in an advanced carotid plaque. (e) Disappearance of IL-17 staining after adsorption with the IL-17–specific peptide. Data are representative of at least 10 different human arteries. Bars: (a) 250 µm; (b and c) 30 µm; (d and e) 45 µm.
Finally, we studied human carotid atherosclerotic arteries retrieved from patients undergoing carotid endarterectomy. Patients characteristics are given in Table S1. P-Stat3 and IL-17 expression were readily detectable in CD3+ cells of human plaques (Fig. 6), although not all CD3+ cells expressed IL-17 or P-Stat3 (Fig. 6). We could also detect P-Stat3 in other cell types, as expected (Fig. 6, and not depicted). Interestingly, we also detected IL-17 expression in vascular SMCs, and consistent with our data in mice, we found a clear decrease in its expression by medial SMCs underlying advanced lesions (Fig. 5, right). Interestingly, semiquantitative assessment of P-Stat3 and IL-17 expression showed increased levels of these two markers in plaques with fibrous (stable) compared with atheromatous (unstable) phenotype (Fig. 6). Consistent with this finding, increased levels of P-Stat3 were significantly associated with a low macrophage content, and increased levels of IL-17 were associated with a lower macrophage infiltration and a higher SMC content (Fig. 6). No relation was found between Stat3 or P-Stat1 levels and plaque phenotype (unpublished data).
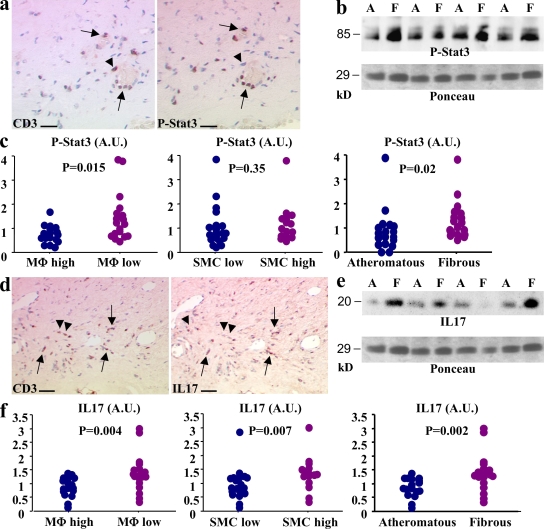
Figure 6.
Stat3 phosphorylation and IL-17 expression in human carotid atherosclerotic plaques are associated with markers of plaque stability. (a) Stat3 phosphorylation (P-Stat3) in CD3+ cells of human carotid atherosclerotic plaques (arrows). Arrowheads show a CD3+ cell with no P-Stat3. (b) Detection of P-Stat3 in protein extracts from atheromatous (A) or fibrous (F) carotid plaques using Western blotting. Ponceau red is shown for estimation of sample loading. (c) Semiquantitative analysis of P-Stat3 content of carotid plaques and relation to plaque macrophage (MΦ) content, plaque SMC content, and plaque phenotype (atheromatous or fibrous). (d) IL-17 expression in CD3+ cells of human carotid atherosclerotic plaques (arrows). Arrowheads show two CD3+ cells with no IL-17 expression. (e) Detection of IL-17 in protein extracts from atheromatous (A) or fibrous (F) carotid plaques using Western blotting (the same blot shown for P-Stat3 is shown for IL-17). (e) Semiquantitative analysis of the IL-17 content of carotid plaques and the relation to plaque macrophage content, plaque SMC content, and plaque phenotype (atheromatous or fibrous). Overall, 40 different atherosclerotic plaques from 5 separate experiments were studied. Bars: (a) 30 µm; (c) 60 µm. A.U., arbitrary units.
In conclusion, we show that endogenous expression of SOCS3 in T cells interrupts a major regulatory pathway in atherosclerosis through inhibition of IL-17 production. Interestingly, patients have been identified with defective STAT3 signaling (Minegishi et al., 2007) and IL-17 production (Ma et al., 2008; Milner et al., 2008), and recent observational studies have reported the abnormal occurrence of vascular inflammation (van der Meer et al., 2006; Ling et al., 2007) in this setting despite the absence of classical cardiovascular risk factors. Although the reported vascular abnormalities consisted mainly of inflammatory vascular aneurysms, some cases of coronary atherosclerosis have been reported in these young individuals (Freeman et al., 2007). Thus, our results may have important implications for the understanding of the pathophysiological mechanisms of vascular inflammation in humans and identify novel targets for disease modulation.
MATERIALS AND METHODS
Animals.
We subjected 12- or 24-wk-old C57BL/6 Ldlr−/− female mice to medullar aplasia by 9.5 Gy of lethal total body irradiation. We repopulated the mice with an intravenous injection of bone marrow cells isolated from femurs and tibias of female C57BL/6 SOCS3-WT mice (SOCS3flox/flox) or littermate mice with a T cell–specific deletion in SOCS3 (SOCS3flox/flox:Lck-Cre) generated by a conditional gene targeting approach using a Cre-loxP system (Yasukawa et al., 2003). The SOCS3fl/fl-Lck-Cre mice were 87.5% C57BL/6 and 12.5% 129 and were intercrossed for >10 generations, indicating that they were on a homogeneous background. After 4 wk of recovery, mice were fed a high fat diet containing 15% fat, 1.25% cholesterol, and 0% cholate for 6 wk. In some experiments, the reconstituted mice received an i.p. injection of either purified neutralizing anti–IL-17A–specific antibody (200 µg/mouse, twice per week; Fig. S4; Uyttenhove and Van Snick, 2006; Uyttenhove et al., 2007) or IgG1 control for 6 wk. In others experiments using anti–IL-17A antibody or IgG control, we injected i.p. the reconstituted SOCS3flox/flox:Lck-Cre mice with recombinant mouse IL-10 (eBioscience) diluted in PBS 0.05% mouse albumin (2 µg/mouse, twice per week) or PBS 0.05% albumin for 6 wk. In another set of experiments, we treated i.p. 12- or 17-wk-old C57BL/6 Ldlr−/− female mice with recombinant mouse IL-17A (eBioscience) diluted in PBS 0.05% mouse albumin (2 µg/mouse, twice per week) or PBS 0.05% albumin during 5 wk of a high fat diet. Finally, 6-wk-old male C57BL/6 Apoe−/−/Rag2−/− mice (provided by P. Gourdy, Institut National de la Santé et de la Recherche Médicale, Toulouse, France) were transferred intravenously with 3 × 106 CD4+ cells recovered from either C57BL/6 SOCS3-WT or C57BL/6 SOCS3-overexpressing Tg (SOCS3-Tg; Seki et al., 2003), and were put on high fat diet for 6 wk. Experiments were conducted according to the French veterinary guidelines and those formulated by the European Community for experimental animal use (L358-86/609EEC), and were approved by the Institut National de la Santé et de la Recherche Médicale.
Extent and composition of atherosclerotic lesions.
Quantification of lesion size and composition was performed as previously described (Taleb et al., 2007). TUNEL staining was performed on fixed cryostat sections as previously described (Ait-Oufella et al., 2007). Anti–mouse VCAM-1 antibody (clone 429) was obtained from BD. Tyrosine 705 phosphorylation of Stat3 was detected in the plaque using rabbit anti–P-Stat3 antibody (Cell Signaling Technology). IL-17 staining in the plaque was performed using a validated anti–human/mouse IL-17 antibody from Santa Cruz Biotechnology, Inc. (Takahashi et al., 2008). The IL-17A–deficient mice were provided by Y. Iwakura (University of Tokyo, Tokyo, Japan).
Cell recovery and purification, culture, proliferation, and cytokine assays.
CD11c+, CD4+CD25+, and CD4+CD25− cells were purified and processed for cell proliferation assays and cytokine production as previously described in detail (Taleb et al., 2007). In some experiments (Fig. S3), 2 × 105 CD4+ T cells per well were stimulated in anti-CD3–coated (2 µg/ml) and anti-CD28–coated (2 µg/ml) microplates for 48 h in the absence of CD11c+ cells. IL-4, IL-10, IL-17, and IFN-γ productions in the supernatants were measured using specific ELISAs (BD and R&D Systems).
Flow cytometry.
Splenocytes were labeled with FITC-conjugated anti-CD4 (clone GK1.5; Miltenyi Biotec), and intracellular Foxp3 staining was performed using PE-conjugated anti–mouse/rat Foxp3 (PFJK-16s; eBioscience) according to the manufacturer's instructions (eBioscience). Anti–P-Stat3 staining was performed on CD4+ T cells stimulated with 100 ng/ml IL-6 for 15 min according to the manufacturer's protocol (Cell Signaling Technology). Labeled cells were then analyzed by flow cytometry on a flow cytometer (Epics XL; Beckman Coulter). IL-17 and IL-10 staining was performed using cytokine secretion assay kits (Miltenyi Biotec).
Western blot analysis.
CD4+ cells were stimulated with coated anti-CD3 and anti-CD28 antibodies (2 µg/ml each) for 60 min. Cells were then processed for Western blot analyses. Membranes were probed with antibodies directed against SOCS3 (clone 1B2; MBL International), P-Stat3 and Stat3 protein (Cell Signaling Technology). On saphenous vein endothelial cell extracts, we quantified VCAM-1 expression by using a specific antibody (R&D Systems). On human plaque extracts, P-Stat3, Stat3, and P-Stat1 staining was performed using specific antibodies purchased from Cell Signaling Technology. Human IL-17 was detected using a monoclonal antibody (clone 41802; R&D Systems).
T cell–macrophage co-cultures.
Macrophages were prepared from mouse bone marrow. Differentiated macrophages were then cultured in 6-well plates either without T cells or with WT or SOCS3-cKO T cells (106 cells per well) for 24 h in the presence of 1 µg/ml of soluble anti-CD3 antibody, after which the T cells were removed and the different cultures were washed twice with PBS and the macrophages were stimulated for 24 h with 1 µg/ml LPS and 100 U/ml IFN-γ. In some experiments, SOCS3-WT or SOCS3-cKO T cells were incubated in the presence of the neutralizing antibodies anti–IL-10 (2 µg/ml; R&D Systems) or anti-IL-17 (10 µg/ml; Uyttenhove and Van Snick, 2006), or their corresponding isotype-matched controls. In other experiments, 10 ng/ml of the recombinant proteins IL-10 and/or IL-17 (eBioscience) were added on macrophages for 24 h. Quantitative real-time PCR was performed on an ABI PRISM 7700 (Applied Biosystems) in triplicates. Cycle threshold for GAPDH (primers: GAPDH-R, 5′-CGTCCCGTAGACAAAATGGTGAA-3′; GAPDH-L, 5′-GCCGTGAGTGGAGTCATACTGGAACA-3′) was used to normalize gene expression. Primer sequences of M1 macrophage markers (NOS2 and TNF-α) and M2 marker (arginase-1) are as follows: NOS2-R, 5′-CCAAGCCCTCACCTACTTCC-3′; NOS2-L, 5′-CTCTGAGGGCTGACACAAGG-3′; TNF-α–R, 5′-GTAGCCCACGTCGTAGCAAAC-3′; TNF-α–L, 5′-CTGGCACCACTAGTTGGTTGTC-3′; Arg-1–R, 5′-CTCCAAGCCAAAGTCCTTAGAG-3′; Arg-1–L, 5′-AGGAGCTGTCATTAGGGACATC-3′). PCR conditions were 10 min at 95°C; 35 cycles of 95°C for 15 s, 60°C for 20 s, and 72°C for 20 s; and a final extension at 72°C for 20 s. On supernatants, ELISAs were performed to measure IL-10 and IL-12 (BD) of the different conditions. TUNEL staining was performed on macrophages to analyze apoptosis.
Human carotid plaques.
The processing and examination of the dissected atherosclerotic plaques has been described previously (Ho et al., 1995; Verhoeven et al., 2004). All stainings were examined microscopically and plaque characteristics were scored semiquantitatively, as described previously (Verhoeven et al., 2004). In brief, no or minor (categorized as “low”) macrophage and SMC infiltration represents absent or minimal staining with few clustered cells, whereas moderate and heavy represent moderate or heavy staining with larger areas of clustered cells (categorized as “high”). The size of the lipid core was estimated as a percentage of total plaque area with a division in three categories: <10% (fibrous plaques), 10–40% (fibroatheromatous plaques), and >40% (atheromatous plaques). Recently, we have demonstrated that our semiquantitative analysis of atherosclerotic plaque histology is well reproducible, both intra- and interobserver (Hellings et al., 2007). 40 carotid atherosclerotic plaques (Table S1) were used for determination of P-Stat3, Stat3, P-Stat1, Stat1, and IL-17 expression. Some of these were also used for immunohistochemical staining. The plaques were from the Athero-Express study, a longitudinal vascular biobank study in which participants provided written informed consent, and the study was approved by the Medical Ethics Committee of the University Medical Center Utrecht. Other plaques used for immunohistochemical staining were previously described (Boddaert et al., 2005).
Statistical analysis.
Values are expressed as means ± SEM. Differences between values were examined using nonparametric Mann-Whitney or Kruskal-Wallis tests and were considered significant at P < 0.05.
Online supplemental material.
Fig. S1 shows the efficiency of SOCS3 deletion and the effects of SOCS3 deletion or overexpression in T cells on atherosclerosis. Fig. S2 shows regulatory T cell number and function, and cytokine levels in chimeric SOCS-WT and SOCS3-cKO mice. Fig. S3 shows complementary IL-10 and IL-17 supplementation experiments. Fig. S4 shows characterization of the MM17F3 anti–IL-17 antibody. Table S1 shows patient characteristics. Online supplemental material is available at http://www.jem.org/cgi/content/full/jem.20090545/DC1.
Acknowledgments
The IL-17A–deficient mice were provided by Y. Iwakura (University of Tokyo, Tokyo, Japan). We are indebted to P. Gourdy for providing the Apoe−/−/Rag2−/− mice, to R. Merval for help in ELISA measurements, to J. Vilard for expert technical assistance in quantitative PCR experiments, and to D. Macé for his valuable technical support. We are also indebted to M. Pla and M. Chopin for animal care.
This work was supported by the Institut National de la Santé et de la Recherche Médicale; by the transatlantic Leducq Immunoregulatory Network; the European Union Seventh Framework program TOLERAGE; the European Vascular Genomics Network; the Fund of Scientific Medical Research; the Belgian Federal Service for Scientific Technical and Cultural Affairs (C. Uyttenhove and J. Van Snick); grants-in-aid from the Ministry of Education, Culture, Sports, Science, and Technology of Japan; and the Program for Promotion of Fundamental Studies in Health Sciences of the National Institute of Biomedical Innovation (A. Yoshimura). Z. Mallat is a recipient of a Contrat d'Interface from Assistance Publique–Hôpitaux de Paris.
The authors have no conflicting financial interests.
Footnotes
Abbreviations used:
- P
- phosphorylated
- SMC
- smooth muscle cell
- SOCS
- suppressor of cytokine signaling
- Tg
- transgenic
- TUNEL
- Tdt-mediated dUTP-biotin nick-end labeling
- VCAM
- vascular cell adhesion molecule
References
- Ait-Oufella H., Kinugawa K., Zoll J., Simon T., Boddaert J., Heeneman S., Blanc-Brude O., Barateau V., Potteaux S., Merval R., et al. 2007. Lactadherin defciency leads to apoptotic cell accumulation and accelerated atherosclerosis in mice.Circulation. 115:2168–2177 doi:10.1161/CIRCULATIONAHA.106.662080 [DOI] [PubMed] [Google Scholar]
- Bettelli E., Oukka M., Kuchroo V.K. 2007. T(H)-17 cells in the circle of immunity and autoimmunity.Nat. Immunol. 8:345–350 doi:10.1038/ni0407-345 [DOI] [PubMed] [Google Scholar]
- Binder C.J., Chang M.K., Shaw P.X., Miller Y.I., Hartvigsen K., Dewan A., Witztum J.L. 2002. Innate and acquired immunity in atherogenesis.Nat. Med. 8:1218–1226 doi:10.1038/nm1102-1218 [DOI] [PubMed] [Google Scholar]
- Boddaert J., Mallat Z., Fornes P., Esposito B., Lecomte D., Verny M., Tedgui A., Belmin J. 2005. Age and gender effects on apoptosis in the human coronary arterial wall.Mech. Ageing Dev. 126:678–684 doi:10.1016/j.mad.2005.01.001 [DOI] [PubMed] [Google Scholar]
- Chen Z., Laurence A., Kanno Y., Pacher-Zavisin M., Zhu B.M., Tato C., Yoshimura A., Hennighausen L., O'Shea J.J. 2006. Selective regulatory function of Socs3 in the formation of IL-17-secreting T cells.Proc. Natl. Acad. Sci. USA. 103:8137–8142 doi:10.1073/pnas.0600666103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong C. 2008. TH17 cells in development: an updated view of their molecular identity and genetic programming.Nat. Rev. Immunol. 8:337–348 doi:10.1038/nri2295 [DOI] [PubMed] [Google Scholar]
- Eid R.E., Rao D.A., Zhou J., Lo S.F., Ranjbaran H., Gallo A., Sokol S.I., Pfau S., Pober J.S., Tellides G. 2009. Interleukin-17 and interferon-gamma are produced concomitantly by human coronary artery-infiltrating T cells and act synergistically on vascular smooth muscle cells.Circulation. 119:1424–1432 doi:10.1161/CIRCULATIONAHA.108.827618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman A.F., Kleiner D.E., Nadiminti H., Davis J., Quezado M., Anderson V., Puck J.M., Holland S.M. 2007. Causes of death in hyper-IgE syndrome.J. Allergy Clin. Immunol. 119:1234–1240 doi:10.1016/j.jaci.2006.12.666 [DOI] [PubMed] [Google Scholar]
- Gharavi N.M., Alva J.A., Mouillesseaux K.P., Lai C., Yeh M., Yeung W., Johnson J., Szeto W.L., Hong L., Fishbein M., et al. 2007. Role of the Jak/STAT pathway in the regulation of interleukin-8 transcription by oxidized phospholipids in vitro and in atherosclerosis in vivo.J. Biol. Chem. 282:31460–31468 doi:10.1074/jbc.M704267200 [DOI] [PubMed] [Google Scholar]
- Hansson G.K., Libby P. 2006. The immune response in atherosclerosis: a double-edged sword.Nat. Rev. Immunol. 6:508–519 doi:10.1038/nri1882 [DOI] [PubMed] [Google Scholar]
- Hellings W.E., Pasterkamp G., Vollebregt A., Seldenrijk C.A., De Vries J.P., Velema E., De Kleijn D.P., Moll F.L. 2007. Intraobserver and interobserver variability and spatial differences in histologic examination of carotid endarterectomy specimens.J. Vasc. Surg. 46:1147–1154 doi:10.1016/j.jvs.2007.08.018 [DOI] [PubMed] [Google Scholar]
- Ho G.H., Moll F.L., Joosten P.P., van de Pavoordt E.D., Overtoom T.T. 1995. The Mollring Cutter remote endarterectomy: preliminary experience with a new endovascular technique for treatment of occlusive superficial femoral artery disease.J. Endovasc. Surg. 2:278–287 doi:10.1583/1074-6218(1995)002<0278:TMCTRE>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Kinjyo I., Inoue H., Hamano S., Fukuyama S., Yoshimura T., Koga K., Takaki H., Himeno K., Takaesu G., Kobayashi T., Yoshimura A. 2006. Loss of SOCS3 in T helper cells resulted in reduced immune responses and hyperproduction of interleukin 10 and transforming growth factor–β1.J. Exp. Med. 203:1021–1031 doi:10.1084/jem.20052333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling J.C., Freeman A.F., Gharib A.M., Arai A.E., Lederman R.J., Rosing D.R., Holland S.M. 2007. Coronary artery aneurysms in patients with hyper IgE recurrent infection syndrome.Clin. Immunol. 122:255–258 doi:10.1016/j.clim.2006.10.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lochner M., Peduto L., Cherrier M., Sawa S., Langa F., Varona R., Riethmacher D., Si-Tahar M., Di Santo J.P., Eberl G. 2008. In vivo equilibrium of proinflammatory IL-17+ and regulatory IL-10+ Foxp3+ RORγt+ T cells.J. Exp. Med. 205:1381–1393 doi:10.1084/jem.20080034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma C.S., Chew G.Y., Simpson N., Priyadarshi A., Wong M., Grimbacher B., Fulcher D.A., Tangye S.G., Cook M.C. 2008. Deficiency of Th17 cells in hyper IgE syndrome due to mutations in STAT3.J. Exp. Med. 205:1551–1557 doi:10.1084/jem.20080218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallat Z., Gojova A., Brun V., Esposito B., Fournier N., Cottrez F., Tedgui A., Groux H. 2003. Induction of a regulatory T cell type 1 response reduces the development of atherosclerosis in apolipoprotein E-knockout mice.Circulation. 108:1232–1237 doi:10.1161/01.CIR.0000089083.61317.A1 [DOI] [PubMed] [Google Scholar]
- Maron R., Sukhova G., Faria A.M., Hoffmann E., Mach F., Libby P., Weiner H.L. 2002. Mucosal administration of heat shock protein-65 decreases atherosclerosis and inflammation in aortic arch of low-density lipoprotein receptor-deficient mice.Circulation. 106:1708–1715 doi:10.1161/01.CIR.0000029750.99462.30 [DOI] [PubMed] [Google Scholar]
- Milner J.D., Brenchley J.M., Laurence A., Freeman A.F., Hill B.J., Elias K.M., Kanno Y., Spalding C., Elloumi H.Z., Paulson M.L., et al. 2008. Impaired T(H)17 cell differentiation in subjects with autosomal dominant hyper-IgE syndrome.Nature. 452:773–776 doi:10.1038/nature06764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minegishi Y., Saito M., Tsuchiya S., Tsuge I., Takada H., Hara T., Kawamura N., Ariga T., Pasic S., Stojkovic O., et al. 2007. Dominant-negative mutations in the DNA-binding domain of STAT3 cause hyper-IgE syndrome.Nature. 448:1058–1062 doi:10.1038/nature06096 [DOI] [PubMed] [Google Scholar]
- Ortiz-Muñoz G., Martin-Ventura J.L., Hernandez-Vargas P., Mallavia B., Lopez-Parra V., Lopez-Franco O., Muñoz-Garcia B., Fernandez-Vizarra P., Ortega L., Egido J., Gomez-Guerrero C. 2009. Suppressors of cytokine signaling modulate JAK/STAT-mediated cell responses during atherosclerosis.Arterioscler. Thromb. Vasc. Biol. 29:525–531 doi:10.1161/ATVBAHA.108.173781 [DOI] [PubMed] [Google Scholar]
- Schnyder-Candrian S., Togbe D., Couillin I., Mercier I., Brombacher F., Quesniaux V., Fossiez F., Ryffel B., Schnyder B. 2006. Interleukin-17 is a negative regulator of established allergic asthma.J. Exp. Med. 203:2715–2725 doi:10.1084/jem.20061401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seki Y., Inoue H., Nagata N., Hayashi K., Fukuyama S., Matsumoto K., Komine O., Hamano S., Himeno K., Inagaki-Ohara K., et al. 2003. SOCS-3 regulates onset and maintenance of T(H)2-mediated allergic responses.Nat. Med. 9:1047–1054 doi:10.1038/nm896 [DOI] [PubMed] [Google Scholar]
- Takahashi N., Vanlaere I., de Rycke R., Cauwels A., Joosten L.A., Lubberts E., van den Berg W.B., Libert C. 2008. IL-17 produced by Paneth cells drives TNF-induced shock.J. Exp. Med. 205:1755–1761 doi:10.1084/jem.20080588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taleb S., Herbin O., Ait-Oufella H., Verreth W., Gourdy P., Barateau V., Merval R., Esposito B., Clément K., Holvoet P., et al. 2007. Defective leptin/leptin receptor signaling improves regulatory T cell immune response and protects mice from atherosclerosis.Arterioscler. Thromb. Vasc. Biol. 27:2691–2698 doi:10.1161/ATVBAHA.107.149567 [DOI] [PubMed] [Google Scholar]
- Tanaka K., Ichiyama K., Hashimoto M., Yoshida H., Takimoto T., Takaesu G., Torisu T., Hanada T., Yasukawa H., Fukuyama S., et al. 2008. Loss of suppressor of cytokine signaling 1 in helper T cells leads to defective Th17 differentiation by enhancing antagonistic effects of IFN-gamma on STAT3 and Smads.J. Immunol. 180:3746–3756 [DOI] [PubMed] [Google Scholar]
- Tang J., Kozaki K., Farr A.G., Martin P.J., Lindahl P., Betsholtz C., Raines E.W. 2005. The absence of platelet-derived growth factor-B in circulating cells promotes immune and inflammatory responses in atherosclerosis-prone ApoE−/− mice.Am. J. Pathol. 167:901–912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tedgui A., Mallat Z. 2006. Cytokines in atherosclerosis: pathogenic and regulatory pathways.Physiol. Rev. 86:515–581 doi:10.1152/physrev.00024.2005 [DOI] [PubMed] [Google Scholar]
- Uyttenhove C., Van Snick J. 2006. Development of an anti-IL-17A auto-vaccine that prevents experimental auto-immune encephalomyelitis.Eur. J. Immunol. 36:2868–2874 doi:10.1002/eji.200636662 [DOI] [PubMed] [Google Scholar]
- Uyttenhove C., Sommereyns C., Théate I., Michiels T., Van Snick J. 2007. Anti-IL-17A autovaccination prevents clinical and histological manifestations of experimental autoimmune encephalomyelitis.Ann. NY Acad. Sci. 1110:330–336 doi:10.1196/annals.1423.035 [DOI] [PubMed] [Google Scholar]
- van der Meer J.W., Weemaes C.M., van Krieken J.H., Blomjous C.E., van Die C.E., Netea M.G., Bredie S.J. 2006. Critical aneurysmal dilatation of the thoracic aorta in young adolescents with variant hyperimmunoglobulin E syndrome.J. Intern. Med. 259:615–618 [DOI] [PubMed] [Google Scholar]
- Verhoeven B.A., Velema E., Schoneveld A.H., de Vries J.P., de Bruin P., Seldenrijk C.A., de Kleijn D.P., Busser E., van der Graaf Y., Moll F., Pasterkamp G. 2004. Athero-express: differential atherosclerotic plaque expression of mRNA and protein in relation to cardiovascular events and patient characteristics. Rationale and design.Eur. J. Epidemiol. 19:1127–1133 doi:10.1007/s10564-004-2304-6 [DOI] [PubMed] [Google Scholar]
- Wang T., Niu G., Kortylewski M., Burdelya L., Shain K., Zhang S., Bhattacharya R., Gabrilovich D., Heller R., Coppola D., et al. 2004. Regulation of the innate and adaptive immune responses by Stat-3 signaling in tumor cells.Nat. Med. 10:48–54 doi:10.1038/nm976 [DOI] [PubMed] [Google Scholar]
- Weber C., Zernecke A., Libby P. 2008. The multifaceted contributions of leukocyte subsets to atherosclerosis: lessons from mouse models.Nat. Rev. Immunol. 8:802–815 doi:10.1038/nri2415 [DOI] [PubMed] [Google Scholar]
- Yasukawa H., Ohishi M., Mori H., Murakami M., Chinen T., Aki D., Hanada T., Takeda K., Akira S., Hoshijima M., et al. 2003. IL-6 induces an anti-inflammatory response in the absence of SOCS3 in macrophages.Nat. Immunol. 4:551–556 doi:10.1038/ni938 [DOI] [PubMed] [Google Scholar]
- Yoshimura A., Naka T., Kubo M. 2007. SOCS proteins, cytokine signalling and immune regulation.Nat. Rev. Immunol. 7:454–465 doi:10.1038/nri2093 [DOI] [PubMed] [Google Scholar]