Abstract
T helper cells secreting interleukin (IL)-17 (Th17 cells) play a crucial role in autoimmune diseases like multiple sclerosis (MS). Th17 differentiation, which is induced by a combination of transforming growth factor (TGF)-β/IL-6 or IL-21, requires expression of the transcription factor retinoic acid receptor–related orphan receptor γt (RORγt). We identify the nuclear receptor peroxisome proliferator–activated receptor γ (PPARγ) as a key negative regulator of human and mouse Th17 differentiation. PPARγ activation in CD4+ T cells selectively suppressed Th17 differentiation, but not differentiation into Th1, Th2, or regulatory T cells. Control of Th17 differentiation by PPARγ involved inhibition of TGF-β/IL-6–induced expression of RORγt in T cells. Pharmacologic activation of PPARγ prevented removal of the silencing mediator for retinoid and thyroid hormone receptors corepressor from the RORγt promoter in T cells, thus interfering with RORγt transcription. Both T cell–specific PPARγ knockout and endogenous ligand activation revealed the physiological role of PPARγ for continuous T cell–intrinsic control of Th17 differentiation and development of autoimmunity. Importantly, human CD4+ T cells from healthy controls and MS patients were strongly susceptible to PPARγ-mediated suppression of Th17 differentiation. In summary, we report a PPARγ-mediated T cell–intrinsic molecular mechanism that selectively controls Th17 differentiation in mice and in humans and that is amenable to pharmacologic modulation. We therefore propose that PPARγ represents a promising molecular target for specific immunointervention in Th17-mediated autoimmune diseases such as MS.
CD4+ T helper (Th) cells differentiate into discrete subsets, which can be discriminated on the basis of their cytokine expression profiles. Besides the “classical” CD4+ T cell subsets (i.e., Th1, Th2, and regulatory T cells), a new subset characterized by secretion of IL-17 was identified (Harrington et al., 2005; Park et al., 2005). Th17 cells provide protection in certain infections, but more importantly, have been linked to development of autoimmunity, a function previously assigned to Th1 cells (Bettelli et al., 2007). Th17 cells mediate pathology in several mouse models of autoimmunity, such as experimental autoimmune encephalomyelitis (EAE), inflammatory bowel disease, and collagen-induced arthritis (Cua et al., 2003; Murphy et al., 2003; Yen et al., 2006). Recent studies have addressed the role of Th17 cells in human autoimmunity (Lock et al., 2002; Tzartos et al., 2008). Th17 differentiation critically depends on TGF-β, together with proinflammatory cytokines such as IL-6 or IL-21 (Ivanov et al., 2006; Yang et al., 2008). The key transcription factor for Th17 differentiation is retinoic acid (RA) receptor–related orphan receptor γt (RORγt; Ivanov et al., 2006; Manel et al., 2008). However, little information exists on the T cell–intrinsic molecular mechanisms controlling RORγt activity, thus contributing to control of Th17-mediated autoimmunity.
We and others have previously shown that the nuclear receptor peroxisome proliferator–activated receptor γ (PPARγ) is a negative regulator of dendritic cell maturation and function, thereby contributing to CD4+ T cell anergy in vivo (Klotz et al., 2007; Szatmari et al., 2007). PPARγ has also been reported to influence the function of Th cell clones (Clark et al., 2000); however, the influence of PPARγ on Th differentiation has not yet been addressed. Upon ligand binding, PPARγ heterodimerizes with the retinoid X receptor and binds to the PPAR response elements (PPRE) located in the promotor region of target genes (Pascual et al., 2005; Glass and Ogawa, 2006). Additionally, the antiinflammatory effects of PPARγ are mediated by negative interference with proinflammatory cell signaling, e.g., stabilization of corepressor complexes, such as nuclear corepressor (NCoR) and silencing mediator for retinoid and thyroid hormone receptors (SMRT; Pascual et al., 2005; Straus and Glass, 2007). PPARγ agonists include endogenous ligands such as the linoleic acid derivative 13s-hydroxyoctadecadienoic acid (HODE) produced by 12/15-lipoxygenase, as well as several synthetic agonistic ligands such as the antidiabetic thiazolidinediones, e.g., pioglitazone (PIO; Huang et al., 1999; Straus and Glass, 2007). Previous studies demonstrated a beneficial role of PPARγ in EAE (Niino et al., 2001; Diab et al., 2002; Feinstein et al., 2002). These findings prompted us to address the question of whether PPARγ is involved in the T cell–intrinsic control of Th17 responses.
RESULTS AND DISCUSSION
Control of Th17 differentiation by PPARγ
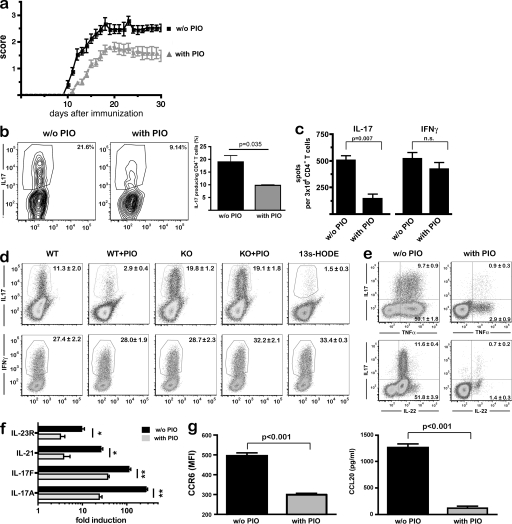
We first investigated the influence of PPARγ on the Th17 responses during MOG-induced EAE. Pharmacological activation of PPARγ with PIO in vivo ameliorated the disease course over the entire observation period (Fig. 1 a), as previously reported (Niino et al., 2001; Diab et al., 2002; Feinstein et al., 2002). Importantly, CD4+ T cells isolated from the central nervous system (CNS) of PIO-treated EAE mice at day 17 after disease induction produced significantly less IL-17A after PMA/ionomycin restimulation (Fig. 1 b). This prompted us to investigate in more detail the influence of PPARγ on the function of autoreactive MOG-specific T cells. We therefore determined the frequency of antigen-specific Th17 cells in the CNS by ELISpot. After MOG35-55-peptide specific stimulation of equal numbers of CNS-derived T cells, we observed a strong reduction in antigen-specific IL-17 producing, but interestingly not IFN-γ producing, CD4+ T cells (Fig. 1). In light of these results, we next investigated the influence of PPARγ on CD4+ Th differentiation. To focus exclusively on the effect of PPARγ in T cells, we used stimulation with αCD3/αCD28 in the absence of antigen-presenting cells. Interestingly, PPARγ activation by PIO selectively inhibited Th17 differentiation induced by TGF-β and IL-6, whereas IL-12–induced Th1 differentiation was completely unaffected (Fig. 1 d; Fig. S1). To obtain unequivocal evidence for the role of PPARγ for Th17 differentiation, we generated T cell–specific PPARγ knockout mice by crossing CD4-Cre mice with mice carrying loxP sites within the PPARγ gene (CD4-PPARγKO; Fig. S2). In the absence of PPARγ, Th17 differentiation was strongly increased when compared with wild-type CD4+ T cells (Fig. 1 d), indicating that PPARγ serves as a T cell–intrinsic brake of Th17 differentiation under physiological conditions. Accordingly, Th1 differentiation was not altered in CD4-PPARγKO T cells (Fig. 1 d). Interestingly, the endogenous PPARγ agonist 13s-HODE, a linoleic acid derivative (Huang et al., 1999), equally suppressed Th17, but not Th1, differentiation (Fig. 1 d), further indicating that PPARγ activity limits Th17 differentiation under physiological conditions. Given the fact that 12/15-lipoxygenase is expressed in T cells (Vanderhoek, 1988), it is reasonable to assume that endogenous ligands produced by T cells themselves serve as a brake for Th17 differentiation in an autocrine fashion. Also, PPARγ ligand production by antigen-presenting cells may contribute to local control of Th17 differentiation (Huang et al., 1999).
Figure 1.
Control of Th17 differentiation by PPARγ. (a) MOG-EAE was induced in PIO or vehicle-treated wild-type mice (n = 6 per group, 3 experiments), and the clinical disease score was assessed daily. (b) In a separate experiment, mice were sacrificed at the peak of disease (day 18), CD4+ T cells were isolated from the CNS, restimulated with PMA/ionomycin, and analyzed by flow cytometry gated on CD4+ T cells; representative dot plots and mean results ± SEM from four animals per group are shown; data are from two experiments. (c) CD4+ T cells from the CNS were restimulated with MOG35-55-loaded DCs, and numbers of IL-17 and of IFN-γ–producing cells per 3 × 104 CD4+ T cells were determined by ELISpot analysis. Graphs denote mean ± SEM of all animals (n = 6 per group, 2 experiments). (d) Purified CD4+ T cells from CD4-PPARγKO mice or WT littermates were treated with PIO or the endogenous PPARγ agonist 13s-HODE, and Th17 differentiation was induced by stimulation for 72 h (top row). Alternatively, Th1 differentiation was induced for 72 h (bottom row). Cytokine-producing cells were determined by flow cytometry after PMA/ionomycin restimulation. Only living cells were analyzed by using LIVE/DEAD stain and exclusion of autofluorescence (x axis). Numbers denote mean percentage ± SEM. (e) Th17 differentiation was induced as above and TNF, IL-17A, and IL-22 expression were determined by flow cytometry. Numbers denote mean percentage ± SEM. (f) Th17 differentiation was induced as above; after 72 h expression of IL-17A, IL-17F, IL-21, and IL-23R were measured by quantitative real-time RT-PCR normalized to β-actin levels. (g) Additionally, CCR6-expression was determined by flow cytometry; CCL20-release was assessed by ELISA. (d–g) One out of at least three independent experiments is shown.
We further substantiated the inhibitory effect of PPARγ on Th17 differentiation by investigating other classical markers of Th17 cells. In addition to IL-17A, we found that PPARγ activation by PIO suppressed expression of TNF and IL-22 (Fig. 1 e), as well as IL-17F, IL-21, and IL-23R, in T cells (Fig. 1 f). Likewise, expression of the chemokine receptor CCR6 and its ligand CCL20 were also strongly controlled by PPARγ activation (Fig. 1 g). This demonstrates that PPARγ, indeed, influenced differentiation of Th17 cells rather than merely suppressing IL-17A production.
Selectivity of PPARγ for Th17 differentiation
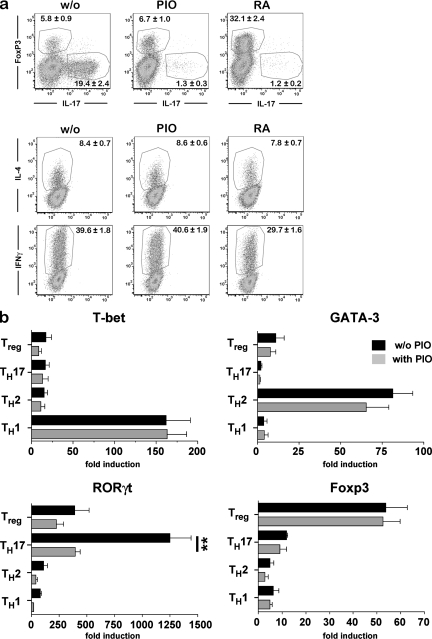
To further characterize the specificity of PPARγ on the differentiation of Th17 cells, we evaluated the effect of PIO on cytokine-induced CD4+ T cell differentiation into Th1, Th2, or regulatory T cells. Importantly, PIO did not modulate TGF-β–mediated induction of Foxp3+ regulatory T cells, IL-4–mediated induction of Th2 cells, or IL-12–mediated induction of Th1 cells (Fig. 2 a). This is in contrast to the effect of RA, which is a natural ligand of the nuclear RA receptor (Chambon, 1994) that has been shown to reciprocally regulate Th17 and regulatory T cell differentiation (Mucida et al., 2007). In direct comparison, RA and PIO both efficiently suppressed Th17 differentiation, whereas RA but not PIO induced TGF-β–mediated expression of Foxp3 (Fig. 2 a). Accordingly, CD4-PPARγKO T cells did not show altered TGF-β–mediated Foxp3-induction (unpublished data). A further distinction between RA and PIO was observed on Th1 differentiation, as RA slightly but significantly impeded IL-12–mediated induction of IFN-γ expression in T cells (Fig. 2 a), as has been previously reported (Iwata et al., 2003). Collectively, these data indicate that distinct molecular mechanisms were involved in PPARγ-mediated, as compared with RA-mediated, control of T cell differentiation.
Figure 2.
Selectivity of PPARγ for Th17 differentiation. (a) CD4+ T cells were subjected to Th1, Th2, Th17, and regulatory T cell differentiation protocols, as described in the Materials and methods section, and the influence of RA and PIO on the induction of lineage markers was determined by flow cytometry and analyzed as described in Materials and methods. (b) CD4+ T cell differentiation was induced as described in Materials and methods, and the influence of PIO on expression of the lineage-determining transcription factors T-bet, GATA-3, RORγt, and Foxp3 was determined by quantitative real-time PCR and normalized to β-actin levels after 48 h. Data in a and b are representative of at least three independent experiments.
We next investigated whether PPARγ also affected expression of the key transcription factors determining CD4+ T cell differentiation. PPARγ-activation selectively suppressed TGF-β/IL-6–mediated expression of RORγt, the transcription factor required for Th17 induction, whereas the expression of the transcription factors determining Th1, Th2, and regulatory T cell differentiation, i.e., T-bet, GATA-3, and FoxP3, was not influenced by PIO (Fig. 2 b), again confirming that PPARγ acted specifically on the differentiation of Th17 cells. Other transcriptional regulators have been reported to influence Th17 differentiation. Foxp3 has been shown to directly antagonize RORγt activity, and thus prevent Th17 differentiation (Zhou et al., 2008). Furthermore, several groups have reported that the aryl hydrocarbon receptor elicits either regulatory T cell or Th17 responses when activated by distinct ligands; however, the underlying mechanisms do not seem to involve RORγt regulation (Quintana et al., 2008; Veldhoen et al., 2008). Additionally, the nuclear orphan receptor NR2F6 seems to regulate Th17-dependent autoimmunity, but with no apparent involvement of RORγt (Hermann-Kleiter et al., 2008). It can therefore be concluded that several receptors are involved in the T cell–intrinsic control of Th17-responses, but that the molecular pathways involved in these processes are distinct. Even among the family of PPARs, the regulatory effect on Th17 differentiation is not a general feature, as lack of PPARα in T cells did not result in altered IL-17 expression levels (Dunn et al., 2007).
PPARγ inhibits Th17 differentiation by controlling RORγt induction
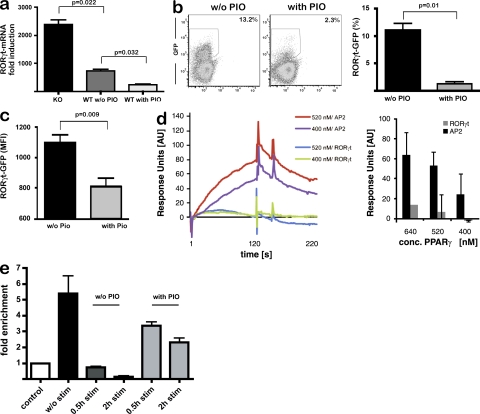
We next evaluated whether PPARγ influenced RORγt expression in T cells. In PPARγKO T cells, we observed enhanced cytokine-induced RORγt induction compared with PPARγWT T cells (Fig. 3 a). The suppressive effect of PPARγ activation by PIO on the one hand and the increased expression of RORγt in PPARγKO T cells on the other hand illustrate the dynamic range of PPARγ-mediated control of Th17 differentiation. We substantiated the influence of PPARγ activation on RORγt expression using reporter mice, which express GFP under control of the RORc(γt) promoter (Lochner et al., 2008). In such T cells, we observed that PIO strongly reduced TGF-β/IL-6–mediated GFP-expression (Fig. 3 b). Importantly, both the frequency of GFPpos T cells and the mean fluorescence intensity of GFP-expressing T cells were reduced by PIO (Fig. 3, b and c). These results indicated that most CD4+ T cells failed to express RORγt under the influence of PPARγ activation, thus giving rise to less Th17 cells. Furthermore, the decreased mean fluorescence intensity of GFP in PIO-treated RORc(γt) reporter T cells (Fig. 3 c) revealed that upon PPARγ activation there was less GFP, i.e., RORγt, on a per cell basis, suggesting that PPARγ reduced RORγt transcription on a single-cell level.
Figure 3.
PPARγ inhibits Th17 differentiation by controlling RORγt induction. (a) Th17 differentiation from PPARγKO and wild-type T cells was induced as described in Materials and methods; RORγt expression was determined by quantitative real-time PCR and normalized to β-actin levels. (b and c) CD4+ T cells from Rorc(γt)-GFPTG reporter mice were treated with PIO, and Th17 differentiation was induced. After 14 h, GFP− expression was assessed by flow cytometry and analyzed for frequency of GFPpos cells (b) and for MFI of GFP-expressing cells (c). One out of three independent experiments is shown. (d) PPARγ was recombinantly expressed (Fig. S3 a), and interaction of recombinant PPARγ with the murine RORγt promoter was determined by surface plasmon resonance analysis. Sensograms show the binding of indicated concentrations of PPARγ at either the RORγt promoter or the murine AP2 promoter containing a bona fide PPRE site as positive control; shown are a representative sensogram (left) and a quantitative analysis (right). The bar graph shows mean ± SEM from three independent experiments. (e) Signal-dependent clearance of SMRT from the RORγt promoter is prevented by PIO. ChIP experiments were performed for SMRT in mock-treated CD4+ T cells and in CD4+ T cells stimulated with TGF-β/IL6 in the presence or absence of PIO. ChIP assay was performed with αSMRT or IgG for control of specificity. Immunoprecipitated DNA was analyzed by quantitative PCR using primers specific for the RORγt promoter; as control, binding of SMRT to a nonrelated DNA control (exon 1 of the RORγ gene) was investigated and set as 1. Two independent experiments were performed, and mean results ± SEM are shown.
The control of PPARγ over RORγt transcription led us to examine whether the RORγt promoter contained a bona fide PPARγ-binding site (PPRE), which might permit direct interaction of PPARγ with the RORγt promoter. Bioinformatic analysis did not reveal any known PPRE sequence within the mouse RORγt promoter (unpublished data). In addition, we excluded direct interaction of PPARγ with the RORγt promoter by examining the binding of recombinant PPARγ to the full-length RORγt promoter using surface plasmon resonance analysis. In contrast to strong and specific binding of PPARγ to the AP2 promoter, which contains a PPRE site (Frohnert et al., 1999), we did not observe significant binding to the RORγt promoter (Fig. 3 d).
The lack of a high-affinity PPARγ binding site in the RORγt promoter raised the possibility that PPARγ might negatively regulate RORγt transcription through a transrepression mechanism that does not require direct DNA binding. One such mechanism involves the ability of ligand-activated PPARγ to inhibit signal-dependent clearance of NCoR or SMRT corepressor complexes from promoters of regulated genes (Pascual et al., 2005; Ghisletti et al., 2009). To investigate this possibility, we used chromatin immunoprecipitation (ChIP) to screen of genomic sequences surrounding the RORγt promoter (unpublished data) for corepressor binding. These studies revealed the binding of the corepressor SMRT, but not NCoR, at the RORγt promoter in unstimulated mouse CD4+ T cells (Fig. 3 e and not depicted). Importantly, stimulation of CD4+ T cells with TGF-β and IL-6 resulted in rapid and nearly complete loss of SMRT from the RORγt promoter (Fig. 3 e), indicating that SMRT clearance precedes RORγt activation. Interestingly, this cytokine-induced clearance of SMRT from the RORγt promoter was prevented by the PPARγ agonist PIO (Fig. 3 e). These data suggest that the retention of SMRT results in persistent repression of RORγt in the presence of activating cytokines, and are consistent with prior studies demonstrating that PPARγ suppresses activation of inflammatory response genes in macrophages by preventing NCoR/SMRT turnover (Ghisletti et al., 2009). Interference of SMRT clearance from the RORγt promoter thus provides a previously unrecognized mechanism by which ligand-activated PPARγ may control Th17 differentiation in T cells. However, these findings do not exclude other mechanisms, such as modulation of STAT3 or IRF4 signaling (Nurieva et al., 2007; Huber et al., 2008).
PPARγ in T cells controls CNS autoimmunity and restricts Th17 differentiation in vivo
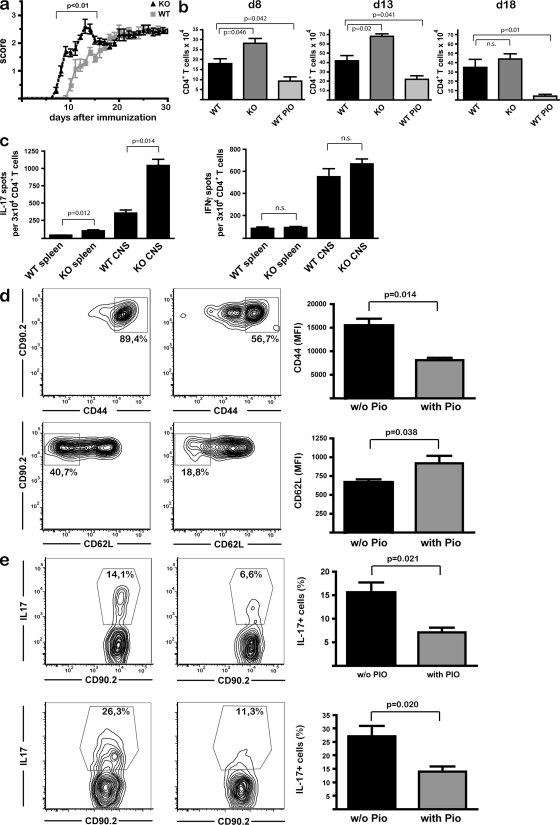
To analyze whether PPARγ is involved in T cell–intrinsic control of CNS autoimmunity, we induced EAE in CD4-PPARγKO mice and wild-type littermates. CD4-PPARγKO mice showed a significantly earlier onset and aggravated disease course during the initial T cell–dependent phase of disease until d15 (Fig. 4 a). However, this difference was not observed in the effector phase, when disease activity is mainly determined by a local inflammatory response within the CNS governed by microglial cells (Heppner et al., 2005). Disease activity in CD4-PPARγKO mice directly correlated with the total numbers of infiltrating CD4+ T cells in the CNS (Fig. 4 b). Both at the beginning of clinical disease activity (day 8), and at the peak of disease in CD4-PPARγKO mice (day 13), we found significantly increased total CD4+ T cell numbers in the CNS. Later (day 18) disease score and T cell influx were not different from wild-type littermates. As expected, PIO-treated wild-type mice exhibited decreased T cell numbers within the CNS at all time points investigated (Fig. 4 b). Importantly, at the peak of disease in CD4-PPARγKO mice, the frequency of MOG35-55 peptide-specific, IL-17–producing CD4+ T cells in the CNS was increased by threefold, which, together with the increase in T cell influx, enhanced the numbers of IL-17–producing autoreactive T cells within the target organ by nearly fivefold (Fig. 4, b and c). In contrast, there was no alteration in antigen-specific IFN-γ–producing CD4+ T cells in these mice (Fig. 4 c).
Figure 4.
PPARγ in T cells controls CNS autoimmunity and restricts Th17 differentiation in vivo. (a) MOG-EAE was induced in CD4-PPARγKO mice and CD4-PPARγWT littermates (n = 8 per group, 3 experiments), and the clinical disease score was assessed daily. (b) In a separate experiment, mice were sacrificed at indicated time points and mononuclear cells derived from the CNS of KO mice and WT littermates (± PIO) were analyzed by flow cytometry. Mean results from n = 4 animals per group and time point ± SEM are shown; data are from 2 experiments. (c) CD4+ T cells from spleens and CNS of each animal were restimulated with MOG35-55-loaded DCs, and numbers of IL-17 and of IFN-γ–producing cells per 3 × 104 CD4+ T cells were determined by ELISpot analysis. Graphs denote mean ± SEM of all animals (8 per group; 3 experiments). (d and e) 106 CD90.2+ OT-II cells were adoptively transferred into congenic mice treated with PIO or vehicle alone (w/o Pio), followed by s.c. immunization with OVA/CFA (100 µg OVA/mouse). CD44 and CD62L expression levels, as well as IL-17A production upon restimulation with PMA/ionomycin, were assessed by flow cytometry at day 4. Six animals per group; shown are representative plots and mean results ± SEM, from two experiments.
The clinical symptoms and antigen-specific Th17 responses in CD4-PPARγKO mice both revealed that the kinetics of CNS autoimmunity in vivo were modulated by PPARγ in a T cell–intrinsic fashion. There was pronounced accumulation of IL-17–producing T cells in the CNS compared with the spleen in CD4-PPARγKO mice (Fig. 4 c), which may be caused by guided entry of Th17 cells into the CNS. A recent study demonstrated that CCR6-expressing Th17 cells function as “pioneer” cells, enabling immune cell entry into the CNS at the beginning of CNS autoimmunity (Reboldi et al., 2009). In this regard, the control of expression of both CCR6 and its ligand CCL20 by PPARγ activation (Fig. 1 g) may explain the decreased influx of T cells and the reduced disease activity in the CNS of PIO-treated wild-type mice. The protective effect of PIO on disease activity was greatly diminished in CD4-PPARγKO mice (Fig. S4 b), thus excluding off-target effects that had been reported previously (Chawla et al., 2001) and further demonstrating that PPARγ expression in T cells was required for full protective effect of PIO on CNS autoimmunity. The observation that PPARγ activation in vivo did not entirely protect from EAE development, despite its profound effect on Th17 differentiation, lends support for a key but not exclusive role of Th17 cells in CNS inflammation, as previously reported (Yang et al., 2009). The persistent Th1 responses, which were not altered by PPARγ activation, may explain persistent disease activity, despite diminished Th17 responses.
As we also observed a significant increase in antigen-specific Th17 cell numbers in the spleen in CD4-PPARγKO mice at the peak of disease (Fig. 4 c), we next asked whether PPARγ influenced Th17 differentiation in vivo at early time points. To this end, we adoptively transferred CD90.2+ CD4+ T cells from OT-II mice, followed by immunization with OVA in CFA. Importantly, PIO treatment of these mice strongly interfered with the expression of activation markers (Fig. 4 d) and IL-17 production (Fig. 4 e) by the adoptively transferred T cells 4 d after immunization; this persisted for longer than 4 d (day 7; not depicted), demonstrating that PPARγ controls antigen-specific Th17 differentiation in vivo.
Collectively, the entire range of PPARγ-sensitive control of Th17 differentiation in vivo and CNS autoimmunity is reflected by the combination of pharmacological PPARγ activation on the one hand and by the absence of PPARγ-activity in CD4-PPARγKO mice on the other hand.
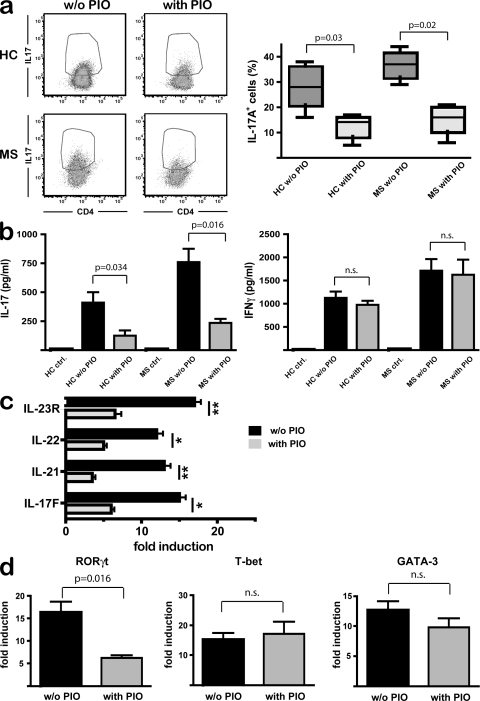
PPARγ selectively controls Th17 differentiation in T cells from healthy controls (HCs) and MS patients
The protective effects of PPARγ on both clinical manifestation and Th17-responses during EAE prompted us to investigate whether T cells from HCs and MS patients were susceptible to treatment with PPARγ agonists. Again, we focused on the effect of PPARγ activation on T cells by using direct stimulation with TGF-β/IL-21 in the absence of antigen-presenting cells. Pharmacologic PPARγ activation reduced the frequency of IL-17A–producing CD45RA+ CD4+ T cells both in HC and MS patients (Fig. 5 a). Although in our experiments there was no apparent difference in Th17 differentiation between HC and MS patients in vitro, it is important to note that PIO-treatment was equally effective in potent suppression of IL-17A release from T cells (Fig. 5 b). Moreover, no influence of PIO was observed during IFN-γ production (Fig. 5 b). Pharmacologic PPARγ activation prevented Th17 differentiation, as demonstrated by diminished expression of the Th17 markers IL-17F, IL-21, IL-22, and IL-23R upon PIO treatment (Fig. 5 c). Importantly, the specific effect of PPARγ activation on Th17 induction in human CD4+ T cells was further illustrated by selective regulation of RORγt expression, whereas T-bet and GATA-3 expression were not altered by PIO (Fig. 5 d).
Figure 5.
PPARγ selectively controls Th17 differentiation in T cells from HCs and MS patients. CD45RA+ CD4+ T cells from HC (n = 5) and from relapsing-remitting MS patient (n = 7) were treated with PIO and stimulated as described in Materials and methods. (a) IL-17A+ cells from HC and MS-patients after restimulation with PMA/ionomycin were assessed by flow cytometry. (b) IL-17A and IFN-γ secretion were determined by ELISA. Graphs show mean percentages ± SEM from the separate experiments (n = 7). (c) CD4+ T cells were activated as in a, and expression of IL-17F, IL-21, IL-22, and IL-23R was measured by real-time RT-PCR normalized to β-actin levels. (d) CD4+ T cells were stimulated as described, and expression of RORγt, T-bet, and GATA-3 after 72 h was measured by real-time PCR and normalized to β-actin levels. (c and d) One representative dataset out of three is shown.
In summary, we identify PPARγ as a defined molecular target to selectively modulate Th17 differentiation in a T cell–intrinsic fashion, which opens up new possibilities for specific immunointervention in Th17-mediated autoimmune diseases such as MS.
MATERIALS AND METHODS
Mice.
CD4-specific PPARγ knockout mice with the genotype PPARγfl/fl CD4-Cre+/− (i.e., CD4-PPARγKO mice) were generated by crossing PPARγfl/fl mice (He et al., 2003) with CD4-Cre+/− transgenic mice expressing Cre recombinase under control of the CD4 enhancer/promoter/silencer (Lee et al., 2001). Expression of Cre recombinase in CD4-expressing T cells leads to recombination at two loxP sites flanking exons two and three of the PPARγ gene, thus resulting in a T cell–specific PPARγ knockout (Fig. S1). We did not observe any alteration in immune cell frequencies in these mice (Fig. S1). CD90.2+ CD4-TCR transgenic OT II mice specific for the peptide ova323-339, BAC-transgenic Rorc(γt)-GFPTG mice, and C57BL/6 mice (Charles River Laboratories) were maintained under specific pathogen–free conditions. All animal experiments were performed according to the guidelines of the animal ethics committee and were approved by the government authorities of Nordrhein-Westfalen, Germany.
Cell culture and adoptive cell transfer.
PBMCs were obtained from the peripheral blood of healthy volunteers or from patients with clinically definite relapsing-remitting MS according to the McDonald criteria, approved by the local Ethics Committee. CD4+CD45RA+CD45RO−CD25− T cells were isolated by immunomagnetic cell separation using an AutoMACS (Miltenyi Biotec) and stimulated with plate-bound 1.5 µg/ml αCD3 antibody (OKT3), 1 µg/ml αCD28 antibody (28.2), 2.5 ng/ml TGF-β (R&D Systems) and 12.5 ng/ml IL-21 (Cell Systems) for 7 d in serum-free X-VIVO 15 medium (Biowhittaker; Yang et al., 2008). 10 µM PIO (Enzo Biochem, Inc.) was added when indicated. Mouse splenic CD4+ T cells were isolated by immunomagnetic separation using CD4-MACS beads (Miltenyi Biotec) and stimulated with plate-bound 4 µg/ml αCD3 antibody (145-2C11) and 4 µg/ml αCD28 antibody (3751) together with 5 ng/ml TGF-β and 20 ng/ml IL-6 (PeproTech) for Th17 differentiation; with IL-12 (10 ng/ml) for Th1 differentiation; with IL-4 (10 ng/ml) for Th2 differentiation or with TGF-β alone (5 ng/ml) for regulatory T cell differentiation. In one experiment, MACS-isolated splenic DCs from B6 mice were cocultured with T cells in the presence of antigen (10 µg/ml ova323-339). The endogenous PPARγ agonist 13s-HODE (Cayman Chemicals) was used at a concentration of 10 µM. All-trans RA (Sigma-Aldrich) was used at a 1 µM concentration. TCR transgenic CD4+ T cells from OTII mice bearing the congenic marker CD90.1+ were isolated and 106 cells were adoptively transferred by bolus i.v. injection in 200 µl PBS into wild-type CD90.2+ congenic mice.
EAE.
EAE was induced by s.c. injecting 50 µg MOG35-55 peptide (BIOTREND) emulsified in CFA (Difco) with 8 mg/ml heat-inactivated Mycobacterium tuberculosis and two i.p. injections of 200ng Bordetella pertussis toxin (List Biologicals) on days 0 and 2. Clinical assessment of EAE was performed daily using a scale ranging from 0 to 6: 0, clinically normal; 1, reduced tone of tail; 2, ataxia and/or slight hind-limb paresis; 3, severe hind-limb paresis; 4, hind limb plegia; 5, tetraparesis; 6, moribund/dead animals. Cell analysis from spleens and CNS was performed as indicated.
Real-time RT-PCR.
Cells were washed with ice-cold PBS, and RNA extraction was performed using the RNeasy mini kit (QIAGEN) according to the manufacturer’s protocol. Reverse transcription of RNA was performed with SuperScript III (Invitrogen). cDNA was analyzed using FAM-labeled TaqMan probes obtained from Applied Biosystems and used according to the manufacturer’s recommendations. mRNA expression levels of RORγt, T-bet, GATA-3, and Foxp3, as well as the Th17 markers IL-17A, IL17F, IL-21, IL-22, and IL-23R, were assessed using gene-specific primers. Gene expression was assessed in triplicates and normalized to β-actin. Amplification of cDNA was performed on an AbiPrism 7900 HT cycler (Applied Biosystems).
Cytokine detection.
Mouse IL-17A and Foxp3 protein expression were examined by intracellular staining according to the manufacturer’s protocol. MOG-specific IL-17 and IFN-γ production was analyzed by specific ELISpot assays according to the manufacturer’s procedures (R&D Systems), and spot numbers were counted with an automated ELISpot reader (BIOREADER-2000). Human IL-17A and IFN-γ protein levels from cell culture supernatants were determined by ELISA (R&D Systems).
ChIP experiments.
ChIP assays were performed as previously described (Pascual et al., 2005). Th17 differentiation was induced for the indicated time points before cross-linking for 10 min with 1% formaldehyde. Anti-SMRT (ABR) or control rabbit IgG (Santa Cruz Biotechnology, Inc.) were used for immunoprecipitation. A 150-bp region of the RORγt promoter was amplified spanning the most proximal transcription start site. Quantitative PCR was performed with SYBR-GreenER (Invitrogen) and analyzed on a 7200 real time PCR system (ABI).
Surface plasmon resonance analysis.
6xHIS-PPARγ was recombinantly expressed in the bacterial strain Escherichia coli BL21, eluted, and desalted using a PD-10 column and 10% glycerol in PBS. The promoter sequences of mouse RORγt and mouse AP2 were amplified by PCR using the oligonucleotides (5′-GCTTCCCAATGGACACTTGCAAG-3′ and 5′-AGGACAGCACACAGCTGGCAGTGG-3′ for RORγt; and 5′-TCTAGAAGGAAGAACCAGGG-3′ and 5′-AGGCAGAAATGCACATTTCACC-3′ for AP2). For each reaction, one primer was biotinylated at the 5′ end. As negative control, a 2-kb fragment of the mouse mannose receptor was amplified. For SPR analysis of promotor binding, a Biacore 3000 (GE Healthcare) was used. In brief, 700–1,000 RU of 5′-biotinylated variants of the respective dsDNAs were immobilized on the surface of a SA-sensorchip (GE Healthcare) according to the manufacturer’s instructions. As negative control, 2 kb of the mouse mannose receptor was used. PBS (pH 7.3) was used as running buffer, and the regeneration of the surface was accomplished by injecting 1 M Urea for 30 s. All measurements were done at a flow rate of 30 µl/min, and protein injections at indicated concentrations were conducted for 2 min using the “inject” mode. Measurements were done in the presence of 0.001 mg/ml heparin to reduce unspecific binding.
Online supplemental material.
Fig. S1 shows the Th17 differentation of highly purified naive CD4+ T cells. Fig. S2 shows the generation of T cell–specific PPARγ knockout mice and phenotypic characterization of immune cells. Fig. S3 shows the purification of recombinantly expressed full-length murine PPARγ; surface plasmon resonance analysis of PPARγ-binding to PPRE-oligonucleotides; and effect of PPARγ on SMRT-binding to the RORγt promoter in the absence of Th17 inducing conditions. Fig. S4 shows the effect of the CD4-Cre transgene on EAE disease course and the absence of a protective PIO effect in CD4-PPARγKO mice. Online supplemental material is available at http://www.jem.org/cgi/content/full/jem.20082771/DC1.
Acknowledgments
We would like to acknowledge the excellent technical assistance of N. Kuhn, C. Mandel, J. Collier, and J. Birke. Moreover, we acknowledge the assistance of the Flow Cytometry Core Facility at the Institute of Molecular Medicine and Experimental Immunology, University of Bonn, supported in part by SFB 704. We also acknowledge F. Frommer in helping to establish EAE-models.
This work was funded by SFB 704, BONFOR, KFO 177.
The authors have no conflicting financial interest.
Footnotes
Abbreviations used:
- ChIP
- chromatin immunoprecipitation
- CNS
- central nervous system
- EAE
- experimental autoimmune encephalomyelitis
- HC
- healthy control
- HODE
- hydroxyoctadecadienoic acid
- MS
- multiple sclerosis
- NCoR
- nuclear corepressor
- PIO
- pioglitazone
- PPAR γ
- peroxisome proliferator–activated receptor γ
- PPRE
- PPAR response element
- RA
- retinoic acid
- RORγt
- RA receptor–related orphan receptor γt
- SMRT
- silencing mediator for retinoid and thyroid hormone receptors
References
- Bettelli E., Korn T., Kuchroo V.K. 2007. Th17: the third member of the effector T cell trilogy.Curr. Opin. Immunol. 19:652–657 doi:10.1016/j.coi.2007.07.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambon P. 1994. The retinoid signaling pathway: molecular and genetic analyses.Semin. Cell Biol. 5:115–125 doi:10.1006/scel.1994.1015 [DOI] [PubMed] [Google Scholar]
- Chawla A., Barak Y., Nagy L., Liao D., Tontonoz P., Evans R.M. 2001. PPAR-gamma dependent and independent effects on macrophage-gene expression in lipid metabolism and inflammation.Nat. Med. 7:48–52 doi:10.1038/83336 [DOI] [PubMed] [Google Scholar]
- Clark R.B., Bishop-Bailey D., Estrada-Hernandez T., Hla T., Puddington L., Padula S.J. 2000. The nuclear receptor PPAR gamma and immunoregulation: PPAR gamma mediates inhibition of helper T cell responses.J. Immunol. 164:1364–1371 [DOI] [PubMed] [Google Scholar]
- Cua D.J., Sherlock J., Chen Y., Murphy C.A., Joyce B., Seymour B., Lucian L., To W., Kwan S., Churakova T., et al. 2003. Interleukin-23 rather than interleukin-12 is the critical cytokine for autoimmune inflammation of the brain.Nature. 421:744–748 doi:10.1038/nature01355 [DOI] [PubMed] [Google Scholar]
- Diab A., Deng C., Smith J.D., Hussain R.Z., Phanavanh B., Lovett-Racke A.E., Drew P.D., Racke M.K. 2002. Peroxisome proliferator-activated receptor-gamma agonist 15-deoxy-Delta(12,14)-prostaglandin J(2) ameliorates experimental autoimmune encephalomyelitis.J. Immunol. 168:2508–2515 [DOI] [PubMed] [Google Scholar]
- Dunn S.E., Ousman S.S., Sobel R.A., Zuniga L., Baranzini S.E., Youssef S., Crowell A., Loh J., Oksenberg J., Steinman L. 2007. Peroxisome proliferator-activated receptor (PPAR)alpha expression in T cells mediates gender differences in development of T cell-mediated autoimmunity.J. Exp. Med. 204:321–330 doi:10.1084/jem.20061839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinstein D.L., Galea E., Gavrilyuk V., Brosnan C.F., Whitacre C.C., Dumitrescu-Ozimek L., Landreth G.E., Pershadsingh H.A., Weinberg G., Heneka M.T. 2002. Peroxisome proliferator-activated receptor-gamma agonists prevent experimental autoimmune encephalomyelitis.Ann. Neurol. 51:694–702 doi:10.1002/ana.10206 [DOI] [PubMed] [Google Scholar]
- Frohnert B.I., Hui T.Y., Bernlohr D.A. 1999. Identification of a functional peroxisome proliferator-responsive element in the murine fatty acid transport protein gene.J. Biol. Chem. 274:3970–3977 doi:10.1074/jbc.274.7.3970 [DOI] [PubMed] [Google Scholar]
- Ghisletti S., Huang W., Jepsen K., Benner C., Hardiman G., Rosenfeld M.G., Glass C.K. 2009. Cooperative NCoR/SMRT interactions establish a corepressor-based strategy for integration of inflammatory and anti-inflammatory signaling pathways.Genes Dev. 23:681–693 doi:10.1101/gad.1773109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glass C.K., Ogawa S. 2006. Combinatorial roles of nuclear receptors in inflammation and immunity.Natl. Rev. Immunol. 6:44–55 [DOI] [PubMed] [Google Scholar]
- Harrington L.E., Hatton R.D., Mangan P.R., Turner H., Murphy T.L., Murphy K.M., Weaver C.T. 2005. Interleukin 17-producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages.Nat. Immunol. 6:1123–1132 doi:10.1038/ni1254 [DOI] [PubMed] [Google Scholar]
- He W., Barak Y., Hevener A., Olson P., Liao D., Le J., Nelson M., Ong E., Olefsky J.M., Evans R.M. 2003. Adipose-specific peroxisome proliferator-activated receptor gamma knockout causes insulin resistance in fat and liver but not in muscle.Proc. Natl. Acad. Sci. USA. 100:15712–15717 doi:10.1073/pnas.2536828100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heppner F.L., Greter M., Marino D., Falsig J., Raivich G., Hövelmeyer N., Waisman A., Rülicke T., Prinz M., Priller J., et al. 2005. Experimental autoimmune encephalomyelitis repressed by microglial paralysis.Nat. Med. 11:146–152 doi:10.1038/nm1177 [DOI] [PubMed] [Google Scholar]
- Hermann-Kleiter N., Gruber T., Lutz-Nicoladoni C., Thuille N., Fresser F., Labi V., Schiefermeier N., Warnecke M., Huber L., Villunger A., et al. 2008. The nuclear orphan receptor NR2F6 suppresses lymphocyte activation and T helper 17-dependent autoimmunity.Immunity. 29:205–216 doi:10.1016/j.immuni.2008.06.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang J.T., Welch J.S., Ricote M., Binder C.J., Willson T.M., Kelly C., Witztum J.L., Funk C.D., Conrad D., Glass C.K. 1999. Interleukin-4-dependent production of PPAR-gamma ligands in macrophages by 12/15-lipoxygenase.Nature. 400:378–382 doi:10.1038/22572 [DOI] [PubMed] [Google Scholar]
- Huber M., Brüstle A., Reinhard K., Guralnik A., Walter G., Mahiny A., von Löw E., Lohoff M. 2008. IRF4 is essential for IL-21-mediated induction, amplification, and stabilization of the Th17 phenotype.Proc. Natl. Acad. Sci. USA. 105:20846–20851 doi:10.1073/pnas.0809077106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanov I.I., McKenzie B.S., Zhou L., Tadokoro C.E., Lepelley A., Lafaille J.J., Cua D.J., Littman D.R. 2006. The orphan nuclear receptor RORgammat directs the differentiation program of proinflammatory IL-17+ T helper cells.Cell. 126:1121–1133 doi:10.1016/j.cell.2006.07.035 [DOI] [PubMed] [Google Scholar]
- Iwata M., Eshima Y., Kagechika H. 2003. Retinoic acids exert direct effects on T cells to suppress Th1 development and enhance Th2 development via retinoic acid receptors.Int. Immunol. 15:1017–1025 doi:10.1093/intimm/dxg101 [DOI] [PubMed] [Google Scholar]
- Klotz L., Dani I., Edenhofer F., Nolden L., Evert B., Paul B., Kolanus W., Klockgether T., Knolle P., Diehl L. 2007. Peroxisome proliferator-activated receptor gamma control of dendritic cell function contributes to development of CD4+ T cell anergy.J. Immunol. 178:2122–2131 [DOI] [PubMed] [Google Scholar]
- Lee P.P., Fitzpatrick D.R., Beard C., Jessup H.K., Lehar S., Makar K.W., Pérez-Melgosa M., Sweetser M.T., Schlissel M.S., Nguyen S., et al. 2001. A critical role for Dnmt1 and DNA methylation in T cell development, function, and survival.Immunity. 15:763–774 doi:10.1016/S1074-7613(01)00227-8 [DOI] [PubMed] [Google Scholar]
- Lochner M., Peduto L., Cherrier M., Sawa S., Langa F., Varona R., Riethmacher D., Si-Tahar M., Di Santo J.P., Eberl G. 2008. In vivo equilibrium of proinflammatory IL-17+ and regulatory IL-10+ Foxp3+ RORγt+ T cells.J. Exp. Med. 205:1381–1393 doi:10.1084/jem.20080034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lock C., Hermans G., Pedotti R., Brendolan A., Schadt E., Garren H., Langer-Gould A., Strober S., Cannella B., Allard J., et al. 2002. Gene-microarray analysis of multiple sclerosis lesions yields new targets validated in autoimmune encephalomyelitis.Nat. Med. 8:500–508 doi:10.1038/nm0502-500 [DOI] [PubMed] [Google Scholar]
- Manel N., Unutmaz D., Littman D.R. 2008. The differentiation of human T(H)-17 cells requires transforming growth factor-beta and induction of the nuclear receptor RORgammat.Nat. Immunol. 9:641–649 doi:10.1038/ni.1610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mucida D., Park Y., Kim G., Turovskaya O., Scott I., Kronenberg M., Cheroutre H. 2007. Reciprocal TH17 and regulatory T cell differentiation mediated by retinoic acid.Science. 317:256–260 doi:10.1126/science.1145697 [DOI] [PubMed] [Google Scholar]
- Murphy C.A., Langrish C.L., Chen Y., Blumenschein W., McClanahan T., Kastelein R.A., Sedgwick J.D., Cua D.J. 2003. Divergent pro- and antiinflammatory roles for IL-23 and IL-12 in joint autoimmune inflammation.J. Exp. Med. 198:1951–1957 doi:10.1084/jem.20030896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niino M., Iwabuchi K., Kikuchi S., Ato M., Morohashi T., Ogata A., Tashiro K., Onoé K. 2001. Amelioration of experimental autoimmune encephalomyelitis in C57BL/6 mice by an agonist of peroxisome proliferator-activated receptor-gamma.J. Neuroimmunol. 116:40–48 doi:10.1016/S0165-5728(01)00285-5 [DOI] [PubMed] [Google Scholar]
- Nurieva R., Yang X.O., Martinez G., Zhang Y., Panopoulos A.D., Ma L., Schluns K., Tian Q., Watowich S.S., Jetten A.M., Dong C. 2007. Essential autocrine regulation by IL-21 in the generation of inflammatory T cells.Nature. 448:480–483 doi:10.1038/nature05969 [DOI] [PubMed] [Google Scholar]
- Park H., Li Z., Yang X.O., Chang S.H., Nurieva R., Wang Y.H., Wang Y., Hood L., Zhu Z., Tian Q., Dong C. 2005. A distinct lineage of CD4 T cells regulates tissue inflammation by producing interleukin 17.Nat. Immunol. 6:1133–1141 doi:10.1038/ni1261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascual G., Fong A.L., Ogawa S., Gamliel A., Li A.C., Perissi V., Rose D.W., Willson T.M., Rosenfeld M.G., Glass C.K. 2005. A SUMOylation-dependent pathway mediates transrepression of inflammatory response genes by PPAR-gamma.Nature. 437:759–763 doi:10.1038/nature03988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quintana F.J., Basso A.S., Iglesias A.H., Korn T., Farez M.F., Bettelli E., Caccamo M., Oukka M., Weiner H.L. 2008. Control of T(reg) and T(H)17 cell differentiation by the aryl hydrocarbon receptor.Nature. 453:65–71 doi:10.1038/nature06880 [DOI] [PubMed] [Google Scholar]
- Reboldi A., Coisne C., Baumjohann D., Benvenuto F., Bottinelli D., Lira S., Uccelli A., Lanzavecchia A., Engelhardt B., Sallusto F. 2009. C-C chemokine receptor 6-regulated entry of TH-17 cells into the CNS through the choroid plexus is required for the initiation of EAE.Nat. Immunol. 10:514–523 doi:10.1038/ni.1716 [DOI] [PubMed] [Google Scholar]
- Straus D.S., Glass C.K. 2007. Anti-inflammatory actions of PPAR ligands: new insights on cellular and molecular mechanisms.Trends Immunol. 28:551–558 doi:10.1016/j.it.2007.09.003 [DOI] [PubMed] [Google Scholar]
- Szatmari I., Töröcsik D., Agostini M., Nagy T., Gurnell M., Barta E., Chatterjee K., Nagy L. 2007. PPARgamma regulates the function of human dendritic cells primarily by altering lipid metabolism.Blood. 110:3271–3280 doi:10.1182/blood-2007-06-096222 [DOI] [PubMed] [Google Scholar]
- Tzartos J.S., Friese M.A., Craner M.J., Palace J., Newcombe J., Esiri M.M., Fugger L. 2008. Interleukin-17 production in central nervous system-infiltrating T cells and glial cells is associated with active disease in multiple sclerosis.Am. J. Pathol. 172:146–155 doi:10.2353/ajpath.2008.070690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanderhoek J.Y. 1988. Role of the 15-lipoxygenase in the immune system.Ann. N. Y. Acad. Sci. 524:240–251 doi:10.1111/j.1749-6632.1988.tb38547.x [DOI] [PubMed] [Google Scholar]
- Veldhoen M., Hirota K., Westendorf A.M., Buer J., Dumoutier L., Renauld J.C., Stockinger B. 2008. The aryl hydrocarbon receptor links TH17-cell-mediated autoimmunity to environmental toxins.Nature. 453:106–109 doi:10.1038/nature06881 [DOI] [PubMed] [Google Scholar]
- Yang L., Anderson D.E., Baecher-Allan C., Hastings W.D., Bettelli E., Oukka M., Kuchroo V.K., Hafler D.A. 2008. IL-21 and TGF-beta are required for differentiation of human T(H)17 cells.Nature. 454:350–352 doi:10.1038/nature07021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y., Weiner J., Liu Y., Smith A.J., Huss D.J., Winger R., Peng H., Cravens P.D., Racke M.K., Lovett-Racke A.E. 2009. T-bet is essential for encephalitogenicity of both Th1 and Th17 cells.J. Exp. Med. 206:1549–1564 doi:10.1084/jem.20082584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yen D., Cheung J., Scheerens H., Poulet F., McClanahan T., McKenzie B., Kleinschek M.A., Owyang A., Mattson J., Blumenschein W., et al. 2006. IL-23 is essential for T cell-mediated colitis and promotes inflammation via IL-17 and IL-6.J. Clin. Invest. 116:1310–1316 doi:10.1172/JCI21404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou L., Lopes J.E., Chong M.M., Ivanov I.I., Min R., Victora G.D., Shen Y., Du J., Rubtsov Y.P., Rudensky A.Y., et al. 2008. TGF-beta-induced Foxp3 inhibits T(H)17 cell differentiation by antagonizing RORgammat function.Nature. 453:236–240 doi:10.1038/nature06878 [DOI] [PMC free article] [PubMed] [Google Scholar]