Abstract
Whether thymic stromal lymphopoietin (TSLP) directly induces potent human CD4+ T cell proliferation and Th2 differentiation is unknown. We report that resting and activated CD4+ T cells expressed high levels of IL-7 receptor a chain but very low levels of TSLP receptor (TSLPR) when compared with levels expressed in myeloid dendritic cells (mDCs). This was confirmed by immunohistology and flow cytometry analyses showing that only a subset of mDCs, with more activated phenotypes, expressed TSLPR in human tonsils in vivo. IL-7 induced strong STAT1, -3, and -5 activation and promoted the proliferation of naive CD4+ T cells in the presence of anti-CD3 and anti-CD28 monoclonal antibodies, whereas TSLP induced weak STAT5 activation, associated with marginally improved cell survival and proliferation, but failed to induce cell expansion and Th2 differentiation. The effect of TSLP on enhancing strong human T cell proliferation was observed only when sorted naive CD4+ T cells were cultured with mDCs at levels as low as 0.5%. TSLP could only induce naive CD4+ T cells to differentiate into Th2 cells in the presence of allogeneic mDCs. These results demonstrate that IL-7 and TSLP use different mechanisms to regulate human CD4+ T cell homeostasis.
In peripheral lymphoid tissues, the number and repertoire diversity of T cells are maintained throughout life by a process called T cell homeostasis (Stockinger et al., 2006; Surh et al., 2006; Boyman et al., 2007). In mice, the homeostatic maintenance of naive and memory CD4+ T cells and of naive CD8+ T cells has been shown to require both TCR signaling by the self-peptide–MHC and cytokines such as IL-7 (Goldrath and Bevan, 1999; Murali-Krishna et al., 1999; Tan et al., 2001; Ge et al., 2002; Surh and Sprent, 2002). The homeostasis of CD8+ memory T cells can be maintained by cytokines such as IL-7, IL-15, and thymic stromal lymphopoietin (TSLP; Goldrath and Bevan, 1999; Schluns et al., 2000; Tan et al., 2001; Ge et al., 2002; Surh and Sprent, 2002; Akamatsu et al., 2008). Previous studies have demonstrated that IL-7 can directly promote the survival and proliferation of activated human CD4+ T cells (Geginat et al., 2001; Rochman et al., 2007). Recently, a clinical study demonstrated that IL-7 can induce the expansion of human CD4+ T cells in vivo (Sportès et al., 2008).
Although it is well established that IL-7 directly acts on CD4+ T cells, the relative importance of TSLP versus IL-7 in direct regulation of human CD4+ T cell activation and Th2 differentiation is unclear. We have previously shown that TSLP can induce Th2 differentiation of naive CD4+ T cells (Soumelis et al., 2002) or robust homeostatic expansion of naive and Th2 memory CD4+ T cells indirectly via activating mDCs in humans (Watanabe et al., 2004; Ito et al., 2005; Wang et al., 2006). A recent study showed that human CD4+ T cells preactivated through TCR responded potently to TSLP and that this response could be further enhanced by IL-2 (Rochman et al., 2007). Studies using TSLP receptor (TSLPR)–deficient mice have clearly demonstrated that TSLPR expression by CD4+ T cells is essential for TSLP-mediated CD4+ T cell expansion and Th2 differentiation in vivo (Al-Shami et al., 2004; Zhou et al., 2005; He et al., 2008). The ability of mouse TSLP to directly act on CD4+ T cells was also demonstrated in vitro (Omori and Ziegler, 2007).
To determine whether the target cells of TSLP differ between humans and mice and whether TSLP and IL-7 have an equal potency in inducing human CD4+ T cell proliferation, expansion, and differentiation, we undertook four experimental approaches. First, we performed a comprehensive analysis of the expression profile of TSLPR, IL-7Rα, and IL-2R common γ (γc) chain transcripts in a variety of human immune cell types. Second, TSLPR chain protein expression was analyzed by flow cytometry using a newly generated monoclonal antibody. Third, we measured proliferation of highly purified human naive CD4+ T cells when cultured with TSLP in the presence of anti-CD3 and anti-CD28 mAbs, with or without the addition of myeloid DCs (mDCs) at 1:200 or 1:400 mDC/T cell ratios. And fourth, we analyzed the in vivo expression of TSLPR in human tonsils by immunohistology and flow cytometry. We report in this paper that resting and activated CD4+ T cells expressed high levels of IL-7Rα but very low levels of TSLPR when compared with levels expressed in mDCs, as was shown by Rochman et al. (2007). TSLPR expression on activated mDCs is at least 10× higher than that expressed by activated CD4+ T cells. In human tonsils, only a subset of mDCs was found to express TSLPR in vivo. We show that human IL-7 induces much stronger T cell activation, survival, and proliferation than TSLP. TSLP induces strong T cell proliferation only in the presence of mDCs. These data suggest that IL-7 and TSLP use different mechanisms to regulate CD4+ T cell homeostasis, which underscores differences in TSLP biology between mice and humans.
RESULTS AND DISCUSSION
Human mDCs and CD4+ T cells preferentially express TSLPR and IL-7R complexes, respectively
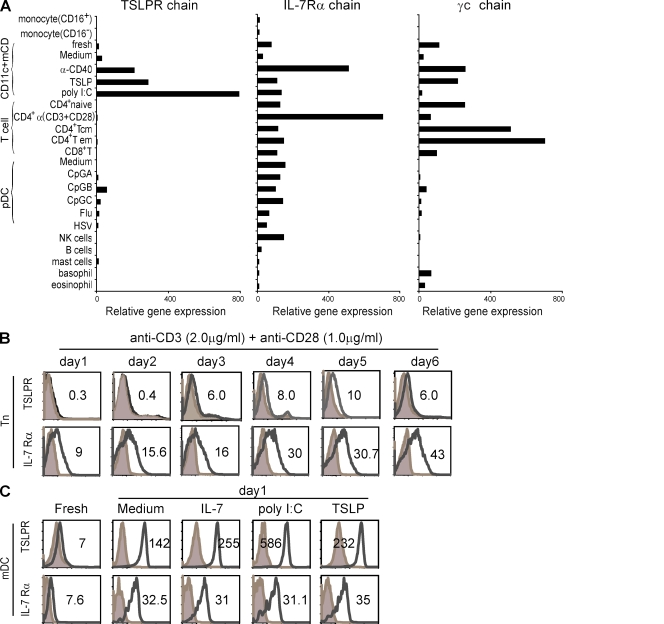
The TSLPR complex consists of the IL-7Rα chain and the TSLPR chain, which is a homologue of γc chain (Pandey et al., 2000; Park et al., 2000; Reche et al., 2001). We performed a detailed microarray analysis of messenger RNA (mRNA) expression for the TSLPR, IL-7Rα, and γc chains in a variety of human immune cell types to assess their potential to respond to TSLP or IL-7 (Fig. 1 A). The quality of this microarray data has been validated at the protein and functional levels by previous studies (Ito et al., 2005; Wang et al., 2006). We found that TSLPR chain mRNA was highly expressed by mDCs, especially after activation by anti-CD40 mAb, TSLP, or poly I:C. Activated plasmacytoid DCs (pDCs) and CD34+ human hematopoietic progenitor-derived mast cells were found to express low levels of TSLPR chain mRNA. TSLPR chain expression was undetectable in peripheral CD4+ naive, memory, and effector T cells, total CD8+ T cells, NK cells, and B cells. Interestingly, human naive CD4+ T cells expressed very low levels of TSLPR chain mRNA after anti-CD3 and anti-CD28 mAb activation, confirming a recent study by Rochman et al. (2007). The majority of human immune cell types expressed the IL-7Rα chain (Fig. 1 A). Unlike human T cells, which expressed high levels of the IL-7Rα chain, human B cells expressed undetectable levels of IL-7Rα chain mRNA. This is consistent with previous studies showing that IL-7 is dispensable for human B cell development and B cell–mediated immune responses (Noguchi et al., 1993; Russell et al., 1995; Puel et al., 1998). Our current results confirm those of previous studies showing that mDCs and mast cells are the major cell types that express the TSLPR complex and respond to TSLP (Reche et al., 2001; Allakhverdi et al., 2007). In addition, pDCs can express the TSLPR complex upon CpG-A, -B, -C, Flu, or HSV activation (Fig. 1 A).
Figure 1.
TSLPR complex expression on human immune cells. (A) Microarray profile of TSLPR and IL-7R complex mRNA expression in human immune cells is shown. Purification of cells and microarray analysis was conducted as previously described (Ito et al., 2005). Cells include the following: CD16+/− monocytes, CD4+CD11c+ mDCs, naive CD4+ T cells, central memory CD4+ T cells, effector memory CD4+ T cells, CD8+ T cells, CD123+BDCA2+pDCs, CD56+ NK cells, CD19+ B cells, CD34+ mast cells, basophils, and eosinophils. Nonstimulated and stimulated CD4+CD11c+ mDCs, pDCs, and naive CD4+ T cells were analyzed as well. The final expression output was normalized with the numerical value of one representing the estimated threshold of basal expression. Bars show the relative mRNA expression of the TSLPR, IL-7Rα, and IL-2Rγ chains in each cell subtype. (B) FACS analysis of TSLPR complex expression in naive CD4+ T cells after indicated days of anti-CD3 and anti-CD28 mAb stimulation is shown. (C) FACS analysis of TSLPR complex expression in freshly isolated, unstimulated, or stimulated CD4+CD11c+ mDCs is shown. In B and C, numbers state the mean fluorescence intensity (MFI) of receptor minus isotype control. Filled histograms represent isotype control, and open histograms represent the staining of the receptor indicated at the left of each histogram. Stimulating conditions are shown at the top of each histogram. Data represents one of five experiments.
To further compare TSLPR expression in CD4+ T cells and mDCs at the protein level, we generated a panel of mAbs against the human TSLPR chain for use in flow cytometry. We found that resting naive CD4+ T cells expressed undetectable levels of the TSLPR chain, whereas activated CD4+ T cells expressed very low levels of the TSLPR chain at days 3, 4, 5, and 6 after anti-CD3 and anti-CD28 mAb activation, as shown by Rochman et al. (2007; Fig. 1 B). In contrast, naive CD4+ T cells expressed high levels of the IL-7Rα chain, which was further upregulated upon activation (Fig. 1 B). Moreover, freshly isolated mDCs expressed detectable levels of the TSLPR chain and IL-7Rα chain, and the expression levels of both were dramatically increased upon culture in medium alone or with IL-7, poly I:C, or TSLP (Fig. 1 C).
IL-7, but not TSLP, directly promotes strong naive CD4+ T cell proliferation
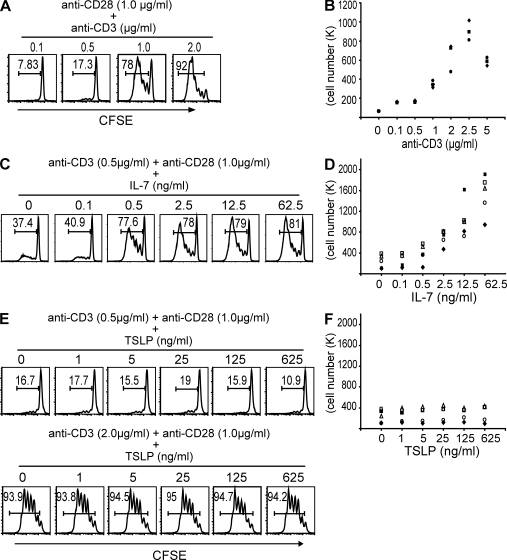
To determine whether TSLP can directly promote the proliferation of naive CD4+ T cells activated by anti-CD3 and anti-CD28 mAbs, we first carefully titrated the concentration of anti-CD3 mAb to determine suboptimal and optimal doses that could be used to measure the effect of IL-7 or TSLP. Naive CD4+ T cells were purified by a cell sorter to a purity >99%, labeled with CFSE, and then cultured with immobilized anti-CD3 and soluble anti-CD28 mAbs for 7 d. Cell proliferation was measured by FACS and counting the total number of cells. As shown in Fig. 2 (A and B), anti-CD3 mAb at 0.1 or 0.5 µg/ml was unable to induce T cell proliferation in the presence of 1 µg/ml of anti-CD28 mAb. However, 1 µg/ml of anti-CD3 mAb could induce significant proliferation of naive CD4+ T cells, and 2 µg/ml of anti-CD3 mAb could induce the maximum level of proliferation. We then choose the 0.5-µg/ml suboptimal dose of anti-CD3 mAb to test the effect of IL-7 and TSLP on promoting naive CD4+ T cell proliferation. We found that IL-7 at 0.5–62.5 ng/ml could strongly promote the proliferation of naive CD4+ T cells activated with 0.5 µg/ml of anti-CD3 and 1 µg/ml of anti-CD28 mAbs in a dose-dependent fashion, based on CFSE-labeling experiments (Fig. 2 C) and total viable cell counts (Fig. 2 D). However, TSLP at 1–625 ng/ml failed to promote the proliferation of naive CD4+ T cells under the same culture condition or when cultured with the 2-µg/ml optimal dose of anti-CD3 mAb (Fig. 2, E and F). We occasionally observed that TSLP weakly promotes CD4+ T cell proliferation (Fig. 3 A), which was indicated by a small increase in the percentage of CFSE-labeled cells from 13.9 to 20.7%. In contrast, IL-7 increases the percentage of CFSE-labeled cells from 13.9 to 86.4% in the same experiment.
Figure 2.
IL-7, and not TSLP, has an additive effect on naive CD4+ T cell proliferation triggered by anti-CD3 and anti-CD28 mAbs. CFSE-labeled purified naive CD4+ T cells were cultured for 7 d in the presence of anti-CD28 and anti-CD3 mAbs alone or with either IL-7 or TSLP. The doses of anti-CD28, anti-CD3, IL-7, and TSLP are indicated. T cell proliferation was measured by FACS analysis on day 4 and by cell count on day 7 of culture. For the FACS results, numbers in each square state the percentage of proliferated T cells. For the cell count, data points represent the total number of expanded T cells of a donor, each represented by a different symbol. (A and B) The effect of increasing doses of anti-CD3 mAb on naive CD4+ T cell proliferation is shown. Data represent one of three independent experiments (A) and three donors (B). (C and D) The effect of increasing doses of IL-7 on naive CD4+ T cell proliferation in the presence of anti-CD28 and the suboptimal dose of anti-CD3 mAbs is shown. Data represent one of five independent experiments (C) and five donors (D). (E and F) The effect of increasing doses of TSLP on naive CD4+ T cell proliferation in the presence of anti-CD28 mAb and either the suboptimal or optimal dose of anti-CD3 mAb is shown. Data represent one of five independent experiments (E) and five donors (F).
Figure 3.
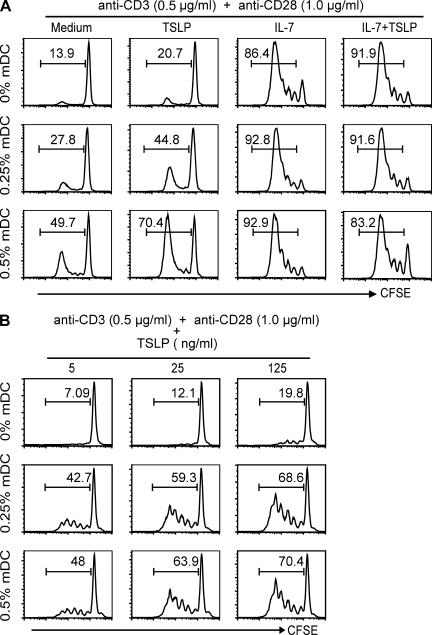
TSLP stimulates naive CD4+ T cell proliferation through mDCs. CFSE-labeled purified naive CD4+ T cells with or without autologous mDCs were cultured for 7 d with anti-CD3 and anti-CD28 mAbs alone or in the presence of TSLP, IL-7, or TSLP plus IL-7. The levels of mDCs and the doses of anti-CD28, anti-CD3, IL-7, and TSLP are indicated. Proliferation of T cells was detected by FACS on day 4 of culture; the percentage of proliferated T cells is indicated in each square. (A) The effect of IL-7 or TSLP on naive CD4+ T cell proliferation with or without autologous mDCs is shown. Data represent one of five independent experiments. (B) The effect of increasing doses of TSLP on naive CD4+ T cell proliferation with or without autologous mDCs is shown. Data represent one of five independent experiments.
TSLP mediates T cell homeostasis indirectly through mDCs
Our data demonstrate that human CD4+ T cells express very low levels of TSLPR upon activation with anti-CD3 and anti-CD28 mAbs, confirming a recent study (Rochman et al., 2007). However, we failed to observe that activated CD4+ T cells respond potently to TSLP (Rochman et al., 2007), which is in contrast to their robust response to IL-7 (Fig. 2 E). Because our previous studies have shown that mDCs activated by TSLP potently induce the proliferation of CD4+ T cells at a DC/T cell ratio as low as 1:150 (Soumelis et al., 2002; Watanabe et al., 2004), we next investigated whether addition of a few mDCs could allow TSLP to promote T cell proliferation in the presence of anti-CD3 and anti-CD28 mAbs. We found that the presence of 0.25 and 0.5% of mDCs allowed TSLP to significantly enhance the proliferation of naive CD4+ T cells cultured with anti-CD3 and anti-CD28 mAbs (Fig. 3 A). We also found that TSLPs enhanced naive CD4+ T cell proliferation in a dose-dependent fashion when they were cultured with 0.25 or 0.5% mDCs in the presence of anti-CD3 and anti-CD28 mAbs (Fig. 3 B).
TSLP does not directly induce human naive CD4+ T cells to differentiate into Th2 cells
A recent study in mice suggested that TSLP has the ability to induce naive CD4+ T cells to differentiate into Th2 cells (Omori and Ziegler, 2007). To determine whether TSLP can directly induce human naive CD4+ T cells to differentiate into Th2 cells, purified naive CD4+ T cells were mixed with either fresh autologous mDCs at a 1:200 mDC/T cell ratio or with no mDCs and then cultured with anti-CD3 and anti-CD28 mAbs only or in the presence of TSLP, IL-7, anti–IFN-γ mAb and IL-4, or IFN-γ and anti–IL-4 mAb for 7 d (Fig. S1, A and B). Meanwhile, purified naive CD4+ T cells were cocultured for 7 d with either pretriggered autologous mDCs at a 1:2 mDC/T cell ratio or pretriggered allogenic mDCs at a 1:5 mDC/T cell ratio. Both autologous and allogenic mDCs had been triggered with TSLP or medium for 24 h before coculturing with the T cells (Fig. S1, C and D). After culture, T cells were reactivated with anti-CD3 and anti-CD28 mAbs for 24 h, and the culture medium was collected to measure levels of secreted cytokines by ELISA. We found that TSLP was unable to prime naive CD4+ T cells or T cells mixed with autologous mDCs at a 1:200 mDC/T cell ratio to secrete Th2 cytokines in the presence of anti-CD3 and anti-CD28 mAbs (Fig. S1, A and B). In addition, TSLP could not induce naive CD4+ T cells to differentiate into Th2 cells when they were cocultured with pretriggered autologous mDCs at a 1:2 mDC/T cell ratio (Fig. S1 C). This is consistent with the results of our previous study in which TSLP-activated autologous mDCs induced only homeostatic proliferation of naive CD4+ T cells and not Th2 differentiation (Watanabe et al., 2004; Wang et al., 2006). TSLP only induced strong Th2 differentiation of the cultured naive CD4+ T cells in the presence of pretriggered allogeneic mDCs at a 1:5 mDC/T cell ratio (Fig. S1 D), confirming the results of our previous studies (Watanabe et al., 2004; Wang et al., 2006).
TSLP induced weak STAT1 and STAT5 activation and improved cell survival of naive CD4+ T cells
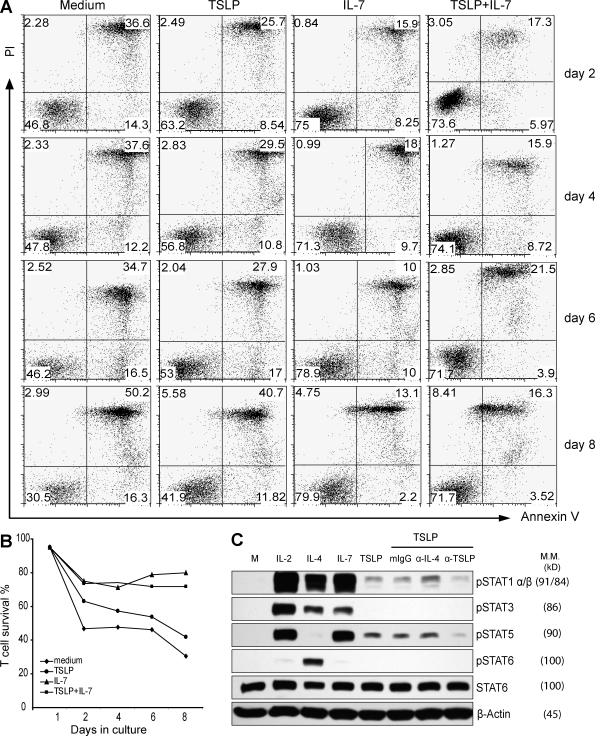
Because anti-CD3 and anti-CD28 mAbs can induce low levels of TSLPR expression in naive CD4+ T cells, we further examined whether TSLP could induce the signal transduced by the TSLPR complex. Naive CD4+ T cells were activated by anti-CD3 and anti-CD28 mAbs for 4 d and then cultured with medium alone, with IL-2, IL-4, IL-7, TSLP alone, or in the presence of an isotype control or neutralizing mAbs against IL-4 or TSLP. Activation of STAT family molecules in the T cells after 20 min of culture was examined by Western blot analysis. We found that all of the cytokines investigated, IL-2, IL-4, and IL-7, induced strong phosphorylation of multiple STAT proteins (Fig. 4 C). In contrast, TSLP only induced a very weak phosphorylation of STAT1 and STAT5, which was blocked by the TSLP-neutralizing mAb but not by the IL-4–neutralizing mAb or the isotype control, which confirms a recent study (Rochman et al., 2007).
Figure 4.
TSLP activates weak STAT1 and STAT5 phosphorylation and maintains activated CD4+ T cell viability. (A) Naive CD4+ T cells, preactivated with anti-CD3 and anti-CD28 mAbs, were restimulated with medium alone or with IL-7, TSLP, or TSLP plus IL-7 for 8 d. Every 2 d during culture, cells were stained with FITC–annexin V and PI and then analyzed by FACS. Numbers in the bottom right and left quarters state the percentage of apoptotic and viable cells, respectively. Numbers in the top right quarters state the percentage of dead cells. Data represent one of five independent experiments. (B) Survival curve based on the percentage of viable cells from experiments described in A. Data represents one of five independent experiments. (C) Naive CD4+ T cells, preactivated with anti-CD3 and anti-CD28 mAbs, were restimulated with medium alone (M) or with IL-2, IL-4, IL-7, TSLP, or TSLP with an isotype control mAb or neutralizing mAbs against IL-4 or TSLP. Activated STAT family molecules were detected by Western blotting with β-actin serving as an internal control. The molecular mass (M.M.) of each protein is shown parenthetically. Data represent one of four experiments.
Because STAT5 activation in T cells has been linked with improved cell survival that is uncoupled with cell proliferation (Isaksen et al., 2002), we further examined whether TSLP could improve the survival of naive CD4+ T cells activated by anti-CD3 and anti-CD28 mAbs. Naive CD4+ T cells were cultured with anti-CD3 and anti-CD28 mAbs for 4 d. Cells were washed and then cultured for 8 d with medium alone or with IL-7, TSLP, or TSLP and IL-7 (Fig. 4, A and B). Viable and apoptotic cells were analyzed by propidium iodide (PI) and annexin V staining at different time points of culture (Fig. 4 A). We found that IL-7 could greatly maintain the survival of activated CD4+ T cells for up to 8 d, whereas TSLP could only marginally improve the survival of activated CD4+ T cells (Fig. 4, A and B).
A subset of mDC with a more activated phenotype expresses TSLPR in human tonsils in vivo
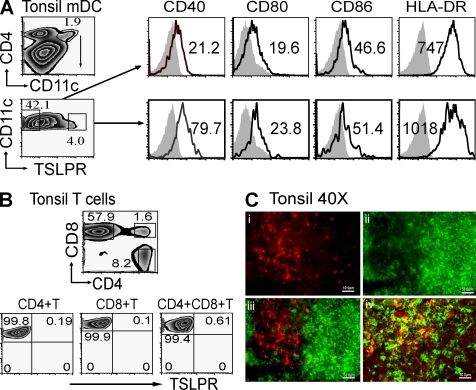
To determine TSLPR-expressing cell types in vivo, we performed flow cytometry and immunohistology analyses of cells in human tonsils. We found that a subset of CD4+CD11c+lineage− (CD3, CD14, CD16, CD19, and CD56) mDCs in human tonsils (3.96%) expressed TSLPR (Fig. 5 A). The TSLPR-positive mDCs expressed higher levels of CD40, CD80, CD86, and HLA-DR than the TSLPR-negative mDCs (Fig. 5 A). All the T cell subsets tested, including CD4+, CD8+, and CD4+CD8+ T cells in human tonsils, did not express detectable levels of TSLPR (Fig. 5 B). By immunohistology, TSLPR-expressing cells were found in the T cell areas or interfollicular areas of human tonsils, which represent a subset of CD11c+ DCs (Fig. 5 C).
Figure 5.
TSLPR expression in human tonsils. (A) FACS analysis of TSLPR, CD40, CD80, CD86, and HLA-DR expression in freshly isolated CD4+CD11c+ mDCs in tonsils is shown. (B) FACS analysis of TSLPR expression in freshly isolated CD4+, CD8+, or CD4+CD8+ T cells in tonsils is shown. (C) Identification of TSLPR-positive cell population in human tonsil tissue by immunohistology is shown. In A, numbers in each histogram state the MFI of receptor minus isotype control. Filled histograms represent isotype control, and open histograms represent the staining of the molecules indicated at the top of each histogram. In B, numbers in each square represents the percentage of cell subsets expressing indicated surface markers. Data represent one of five experiments. In C (i), red cells represent TSLPR-positive population in tonsils. In C (ii), green cells represent tonsil CD20+ B cells; in C (iii), TSLPR-positive cells (red) localize at paracortical zone between the lymphoid follicles (green); in C (iv) among CD11c+ mDCs (green) and TSLPR-positive cells (red), there is a certain population expressing both CD11c and TSLPR (yellow); (i,ii,iii, and iv) magnification, X400. Bars, 50.0 µm.
In this study, we demonstrate that, unlike IL-7, TSLP does not potently promote proliferation of human naive CD4+ T cells activated by anti-CD3 and anti-CD28 mAbs. TSLPR signaling in activated human naive CD4+ T cells led to only very weak activation of STAT1 and STAT5, as was recently demonstrated by Rochman et al. (2007), and marginally improved cell survival. In contrast, signaling from the IL-7R complex in activated naive CD4+ T cells led to robust activation of STAT1, STAT3, and STAT5, strong T cell proliferation, and prolonged T cell survival.
Both in vitro and in vivo data of our present study shows that human mDCs express very high levels of the TSLPR complex. TSLPR expression levels correlate with the function of TSLP. We have previously demonstrated that TSLP-primed DCs can induce the robust expansion of naive CD4+ T cells in both allogenic and autologous systems. In the present study, we found that even at the very low mDC/T cell ratio of 1:200 TSLP could strongly promote the proliferation of naive CD4+ T cells in culture with anti-CD3 and anti-CD28 mAbs. These results indicate that human IL-7 and TSLP use two different but complementary mechanisms to regulate peripheral T cell homeostasis. IL-7 has a potent and direct effect on T cell activation and displays a limited effect on mDCs, and TSLP predominantly acts on mDCs and has a moderate effect directly on T cells.
MATERIALS AND METHODS
Microarray analysis.
Microarray analysis was performed as described previously (Ito et al., 2005).
mDC isolation and culture.
The institutional review board for human research at The University of Texas M.D. Anderson Cancer Center (Houston, TX) approved this study. A FACSAria (BD) was used to purify (>99%) CD4+CD11c+lineage− mDCs from buffy coats (Gulf Coast Regional Blood Center, Houston, TX) or human tonsils (Texas Children’s Hospital, Houston, TX). The mDCs were cultured in RPMI medium (Invitrogen) containing 2% human AB serum, alone or with 15 ng/ml TSLP (Ito et al., 2005), 10 µg/ml poly I:C (InvivoGen), or 10 ng/ml IL-7 (R&D Systems) for 24 h.
Naive T cell isolation, culture, and proliferation assays.
CD4+CD45RA+CD45RO− naive T cells were isolated as described previously (Wang et al., 2006). In some assays, the purified naive CD4+ T cells were cultured in RPMI medium containing 4% human AB serum for 7 d in the presence of 2.0 µg/ml of immobilized anti-CD3 and 1.0 µg/ml of soluble anti-CD28 mAbs (BD) unless stated otherwise. CFSE-labeled naive CD4+ T cells were cultured with anti-CD28 and 0.1–5.0 µg/ml of anti-CD3 mAbs alone or with either 0.1–62.5 ng/ml IL-7 or 1–625 ng/ml TSLP. CFSE-labeled naive CD4+ T cells were mixed with autologous CD4+CD11c+ mDCs at either a 1:200 or 1:400 mDC/T cell ratio or mixed without mDCs, and then cultured with anti-CD3 and anti-CD28 mAbs alone or with 0.5 ng/ml IL-7, 5–125 ng/ml TSLP, or 0.5 ng/ml IL-7 and 5–125 ng/ml TSLP. T cell proliferation was measured using a FACSCalibur (BD) and by cell count.
TSLPR complex expression in mDCs and T cells.
Freshly isolated (Fig. 1) or stimulated naive CD4+ T cells and untreated or stimulated mDCs were stained with biotin-labeled anti-TSLPR antibody (2D10; Rochman et al., 2007) or PE–anti–IL-7Rα antibody (Beckman Coulter). Cells were washed, stained with APC-streptavidin, and then analyzed with a FACSCalibur.
Th2 cytokine production analyses.
Purified naive CD4+ T cells or T cells mixed with autologous mDCs at a 1:200 mDC/T cell ratio were cultured with anti-CD3 and anti-CD28 mAbs alone or with TSLP, IL-7, TSLP plus IL-7, anti-IL-4 plus IFN-γ, and anti–IFN-γ plus IL-4 conditions (Fig. S1). Meanwhile, T cells were cocultured with either autologous or allogenic mDCs, both pretriggered with either TSLP or medium for 7 d (Watanabe et al., 2004). After culturing, the T cells were restimulated with anti-CD3 and anti-CD28 mAbs for 24 h. Medium was collected and used to measure IL-4, IL-5, IL-13, and IFN-γ levels by ELISA (all kits from R&D Systems).
Annexin V analyses.
Naive CD4+ T cells were TCR triggered for 4 d. Cells were recultured in medium alone or with TSLP, IL-7, or TSLP and IL-7 for 8 d. Every 2 d, the preactivated T cells were stained with FITC–annexin V and PI (BD) and then analyzed with a FACSCalibur.
Western blot analysis.
Naive CD4+ T cells were cultured with anti-CD3 and anti-CD28 mAbs for 4 d and then stimulated with medium alone or with IL-2, IL-4, IL-7, TSLP alone, or in the presence of isotype control or neutralizing mAb against IL-4 or TSLP for 20 min. The activation of STAT1, STAT3, STAT5, and STAT6 was detected by Western blotting using antibodies against the tyrosine-phosphorylated forms of STAT1, STAT3, STAT5, and STAT6 (Cell Signaling Technology). Antibodies against STAT6 (Santa Cruz Biotechnology, Inc.) and β-actin (Sigma-Aldrich) were used in the analysis as well.
Immunohistology.
6-µm frozen sections of human tonsil were fixed with cold acetone for 10 min and then incubated with mouse anti–human TSLPR mAb (3G11; our laboratory) at room temperature for 1 h in PBS. The slides were washed with PBS three times and incubated with red fluorescent-labeled secondary antibody (Invitrogen) for 30 min. This was followed by anti-CD20 or anti-CD11c mAbs (BD) staining, and green fluorescent-labeled secondary antibody (Invitrogen) was applied.
Online supplemental material.
Fig. S1 demonstrates that TSLP cannot directly stimulate naive CD4+ T cells to produce Th2 cytokines. Online supplemental material is available at http://www.jem.org/cgi/content/full/jem.20090153/DC1.
Acknowledgments
We thank the flow cytometry facility (supported by National Cancer Institute grant P30CA16672) for cell sorting. We thank Dr. Li Zhang for the suggestion of microarray data analysis. We thank Melissa Wentz and Virginia Mohlere for critical reading of the manuscript, and Dr. Warren Leonard for helpful discussion and critical reading of the manuscript.
This work was supported by the National Institutes of Health grant R01 AI062888-01 awarded to Y.-J. Liu.
The authors have no conflicting financial interests.
Footnotes
Abbreviations used:
- γc
- common γ
- mDC
- myeloid DC
- MFI
- mean fluorescence intensity
- mRNA
- messenger RNA
- pDC
- plasmacytoid DC
- PI
- propidium iodide
- TSLP
- thymic stromal lymphopoietin
References
- Akamatsu T., Watanabe N., Kido M., Saga K., Tanaka J., Kuzushima K., Nishio A., Chiba T. 2008. Human TSLP directly enhances expansion of CD8+ T cells.Clin. Exp. Immunol. 154:98–106 doi:10.1111/j.1365-2249.2008.03731.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Shami A., Spolski R., Kelly J., Fry T., Schwartzberg P.L., Pandey A., Mackall C.L., Leonard W.J. 2004. A role for thymic stromal lymphopoietin in CD4(+) T cell development.J. Exp. Med. 200:159–168 doi:10.1084/jem.20031975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allakhverdi Z., Comeau M.R., Jessup H.K., Yoon B.R., Brewer A., Chartier S., Paquette N., Ziegler S.F., Sarfati M., Delespesse G. 2007. Thymic stromal lymphopoietin is released by human epithelial cells in response to microbes, trauma, or inflammation and potently activates mast cells.J. Exp. Med. 204:253–258 doi:10.1084/jem.20062211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyman O., Purton J.F., Surh C.D., Sprent J. 2007. Cytokines and T-cell homeostasis.Curr. Opin. Immunol. 19:320–326 doi:10.1016/j.coi.2007.04.015 [DOI] [PubMed] [Google Scholar]
- Ge Q., Palliser D., Eisen H.N., Chen J. 2002. Homeostatic T cell proliferation in a T cell-dendritic cell coculture system.Proc. Natl. Acad. Sci. USA. 99:2983–2988 doi:10.1073/pnas.052714199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geginat J., Sallusto F., Lanzavecchia A. 2001. Cytokine-driven proliferation and differentiation of human naive, central memory, and effector memory CD4(+) T cells.J. Exp. Med. 194:1711–1719 doi:10.1084/jem.194.12.1711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldrath A.W., Bevan M.J. 1999. Low-affinity ligands for the TCR drive proliferation of mature CD8+ T cells in lymphopenic hosts.Immunity. 11:183–190 doi:10.1016/S1074-7613(00)80093-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- He R., Oyoshi M.K., Garibyan L., Kumar L., Ziegler S.F., Geha R.S. 2008. TSLP acts on infiltrating effector T cells to drive allergic skin inflammation.Proc. Natl. Acad. Sci. USA. 105:11875–11880 doi:10.1073/pnas.0801532105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isaksen D.E., Baumann H., Zhou B., Nivollet S., Farr A.G., Levin S.D., Ziegler S.F. 2002. Uncoupling of proliferation and Stat5 activation in thymic stromal lymphopoietin-mediated signal transduction.J. Immunol. 168:3288–3294 [DOI] [PubMed] [Google Scholar]
- Ito T., Wang Y.H., Duramad O., Hori T., Delespesse G.J., Watanabe N., Qin F.X., Yao Z., Cao W., Liu Y.J. 2005. TSLP-activated dendritic cells induce an inflammatory T helper type 2 cell response through OX40 ligand.J. Exp. Med. 202:1213–1223 doi:10.1084/jem.20051135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murali-Krishna K., Lau L.L., Sambhara S., Lemonnier F., Altman J., Ahmed R. 1999. Persistence of memory CD8 T cells in MHC class I-deficient mice.Science. 286:1377–1381 doi:10.1126/science.286.5443.1377 [DOI] [PubMed] [Google Scholar]
- Noguchi M., Nakamura Y., Russell S.M., Ziegler S.F., Tsang M., Cao X., Leonard W.J. 1993. Interleukin-2 receptor gamma chain: a functional component of the interleukin-7 receptor.Science. 262:1877–1880 doi:10.1126/science.8266077 [DOI] [PubMed] [Google Scholar]
- Omori M., Ziegler S. 2007. Induction of IL-4 expression in CD4(+) T cells by thymic stromal lymphopoietin.J. Immunol. 178:1396–1404 [DOI] [PubMed] [Google Scholar]
- Pandey A., Ozaki K., Baumann H., Levin S.D., Puel A., Farr A.G., Ziegler S.F., Leonard W.J., Lodish H.F. 2000. Cloning of a receptor subunit required for signaling by thymic stromal lymphopoietin.Nat. Immunol. 1:59–64 doi:10.1038/76923 [DOI] [PubMed] [Google Scholar]
- Park L.S., Martin U., Garka K., Gliniak B., Di Santo J.P., Muller W., Largaespada D.A., Copeland N.G., Jenkins N.A., Farr A.G., et al. 2000. Cloning of the murine thymic stromal lymphopoietin (TSLP) receptor: Formation of a functional heteromeric complex requires interleukin 7 receptor.J. Exp. Med. 192:659–670 doi:10.1084/jem.192.5.659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puel A., Ziegler S.F., Buckley R.H., Leonard W.J. 1998. Defective IL7R expression in T(-)B(+)NK(+) severe combined immunodeficiency.Nat. Genet. 20:394–397 doi:10.1038/3877 [DOI] [PubMed] [Google Scholar]
- Reche P.A., Soumelis V., Gorman D.M., Clifford T., Liu Mr M., Travis M., Zurawski S.M., Johnston J., Liu Y.J., Spits H., et al. 2001. Human thymic stromal lymphopoietin preferentially stimulates myeloid cells.J. Immunol. 167:336–343 [DOI] [PubMed] [Google Scholar]
- Rochman I., Watanabe N., Arima K., Liu Y.J., Leonard W.J. 2007. Cutting edge: direct action of thymic stromal lymphopoietin on activated human CD4+ T cells.J. Immunol. 178:6720–6724 [DOI] [PubMed] [Google Scholar]
- Russell S.M., Tayebi N., Nakajima H., Riedy M.C., Roberts J.L., Aman M.J., Migone T.S., Noguchi M., Markert M.L., Buckley R.H., et al. 1995. Mutation of Jak3 in a patient with SCID: essential role of Jak3 in lymphoid development.Science. 270:797–800 doi:10.1126/science.270.5237.797 [DOI] [PubMed] [Google Scholar]
- Schluns K.S., Kieper W.C., Jameson S.C., Lefrançois L. 2000. Interleukin-7 mediates the homeostasis of naïve and memory CD8 T cells in vivo.Nat. Immunol. 1:426–432 doi:10.1038/80868 [DOI] [PubMed] [Google Scholar]
- Soumelis V., Reche P.A., Kanzler H., Yuan W., Edward G., Homey B., Gilliet M., Ho S., Antonenko S., Lauerma A., et al. 2002. Human epithelial cells trigger dendritic cell mediated allergic inflammation by producing TSLP.Nat. Immunol. 3:673–680 [DOI] [PubMed] [Google Scholar]
- Sportès C., Hakim F.T., Memon S.A., Zhang H., Chua K.S., Brown M.R., Fleisher T.A., Krumlauf M.C., Babb R.R., Chow C.K., et al. 2008. Administration of rhIL-7 in humans increases in vivo TCR repertoire diversity by preferential expansion of naive T cell subsets.J. Exp. Med. 205:1701–1714 doi:10.1084/jem.20071681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stockinger B., Bourgeois C., Kassiotis G. 2006. CD4+ memory T cells: functional differentiation and homeostasis.Immunol. Rev. 211:39–48 doi:10.1111/j.0105-2896.2006.00381.x [DOI] [PubMed] [Google Scholar]
- Surh C.D., Sprent J. 2002. Regulation of naïve and memory T-cell homeostasis.Microbes Infect. 4:51–56 doi:10.1016/S1286-4579(01)01509-X [DOI] [PubMed] [Google Scholar]
- Surh C.D., Boyman O., Purton J.F., Sprent J. 2006. Homeostasis of memory T cells.Immunol. Rev. 211:154–163 doi:10.1111/j.0105-2896.2006.00401.x [DOI] [PubMed] [Google Scholar]
- Tan J.T., Dudl E., LeRoy E., Murray R., Sprent J., Weinberg K.I., Surh C.D. 2001. IL-7 is critical for homeostatic proliferation and survival of naive T cells.Proc. Natl. Acad. Sci. USA. 98:8732–8737 doi:10.1073/pnas.161126098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y.H., Ito T., Wang Y.H., Homey B., Watanabe N., Martin R., Barnes C.J., McIntyre B.W., Gilliet M., Kumar R., et al. 2006. Maintenance and polarization of human TH2 central memory T cells by thymic stromal lymphopoietin-activated dendritic cells.Immunity. 24:827–838 doi:10.1016/j.immuni.2006.03.019 [DOI] [PubMed] [Google Scholar]
- Watanabe N., Hanabuchi S., Soumelis V., Yuan W., Ho S., de Waal Malefyt R., Liu Y.J. 2004. Human thymic stromal lymphopoietin promotes dendritic cell-mediated CD4+ T cell homeostatic expansion.Nat. Immunol. 5:426–434 doi:10.1038/ni1048 [DOI] [PubMed] [Google Scholar]
- Zhou B., Comeau M.R., De Smedt T., Liggitt H.D., Dahl M.E., Lewis D.B., Gyarmati D., Aye T., Campbell D.J., Ziegler S.F. 2005. Thymic stromal lymphopoietin as a key initiator of allergic airway inflammation in mice.Nat. Immunol. 6:1047–1053 doi:10.1038/ni1247 [DOI] [PubMed] [Google Scholar]