Abstract
Epstein-Barr virus–encoded small RNA (EBER) is nonpolyadenylated, noncoding RNA that forms stem-loop structure by intermolecular base-pairing, giving rise to double-stranded RNA (dsRNA)–like molecules, and exists abundantly in EBV-infected cells. Here, we report that EBER induces signaling from the Toll-like receptor 3 (TLR3), which is a sensor of viral double-stranded RNA (dsRNA) and induces type I IFN and proinflammatory cytokines. A substantial amount of EBER, which was sufficient to induce signaling from TLR3, was released from EBV-infected cells, and the majority of the released EBER existed as a complex with a cellular EBER-binding protein La, suggesting that EBER was released from the cells by active secretion of La. Sera from patients with infectious mononucleosis (IM), chronic active EBV infection (CAEBV), and EBV-associated hemophagocytic lymphohistiocytosis (EBV-HLH), whose general symptoms are caused by proinflammatory cytokines contained EBER, and addition of RNA purified from the sera into culture medium induced signaling from TLR3 in EBV-transformed lymphocytes and peripheral mononuclear cells. Furthermore, DCs treated with EBER showed mature phenotype and antigen presentation capacity. These findings suggest that EBER, which is released from EBV-infected cells, is responsible for immune activation by EBV, inducing type I IFN and proinflammatory cytokines. EBER-induced activation of innate immunity would account for immunopathologic diseases caused by active EBV infection.
Epstein-Barr virus (EBV) is a ubiquitous human herpesvirus that infects >90% of the population. Primary EBV infection is generally asymptomatic; however, when the infection is delayed until adolescence or later, ∼50% of cases manifest as infectious mononucleosis (IM). IM is characterized by the expansion of reactive T cells and is most likely to be an immunopathologic disease whose general symptoms are caused by proinflammatory cytokines, such as IL-1, IFN-γ, and TNF (Rickinson and Kieff, 2001). Chronic active EBV infection (CAEBV) and EBV-associated hemophagocytic lymphohistiocytosis (EBV-HLH) are also active EBV infections with persistent or recurrent IM-like symptoms. EBV-HLH is characterized by an EBV infection in T cells and the systemic release of proinflammatory cytokines, which subsequently causes hemophagocytosis of blood cells through the activation of macrophages (Kikuta et al., 1993; Rickinson and Kieff, 2001).
The EBV noncoding RNAs, EBV-encoded RNA 1 (EBER1) and EBER2, are 167 and 172 nt long, respectively, and are expected to form dsRNA-like structures (Rosa et al., 1981). EBER is the most abundant viral transcript in latently EBV-infected cells (Rymo, 1979), and binds to several cellular proteins including RNA-activated protein kinase (PKR; Clarke et al., 1991), ribosomal protein 22 (L22; Toczyski et al., 1994), lupus erythematosis–associated antigen (La; Lerner et al., 1981), and retinoic acid–inducible gene I (RIG-I; Samanta et al., 2006).
Here, we report that EBER exists in the sera of patients with active EBV infections and induces type I IFN and inflammatory cytokines through TLR3-mediated signaling. This may account for the pathogenesis of active EBV infections that are characterized by cytokinemia.
RESULTS AND DISCUSSION
EBER is present in the culture supernatants of EBV-infected cells
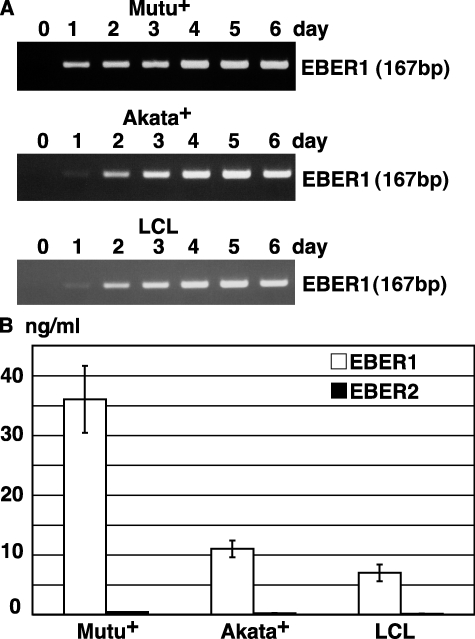
RT-PCR assays have revealed that EBER is present in the culture supernatants of the Burkitt's lymphoma–derived EBV-positive cell lines Mutu+ (Gregory et al., 1990) and Akata+ (Takada, 1984), and the EBV-transformed lymphoblastoid cell lines (LCLs). EBER was detected on day 1 of the culture, and its expression peaked on day 4 (Fig. 1 A). In a real-time PCR assay, 15–35 ng/ml EBER1 was released into the culture supernatants, whereas EBER2 was only faintly detected (Fig. 1 B).
Figure 1.
EBER1 is released into the culture supernatants of EBV-infected Mutu+, Akata+ and LCL. (A) RT-PCR of EBER1. Cells (2 × 105 cells/ml) were cultured for the designated number of days. Total RNA was extracted from 1 ml culture supernatant and subjected to 25 cycles of RT-PCR to detect EBER1. Three or more independent experiments were performed. (B) Quantitative RT-PCR of EBER1 and EBER2. Cells (2 × 105) were cultured in 1 ml medium for 4 d, and total RNA was extracted from the culture supernatant and subjected to RT-RCR for the detection of EBER1 and EBER2. Error bars indicate the SD of duplicate wells. The data presented are representative of three independent experiments.
EBER1 induces signaling from TLR3
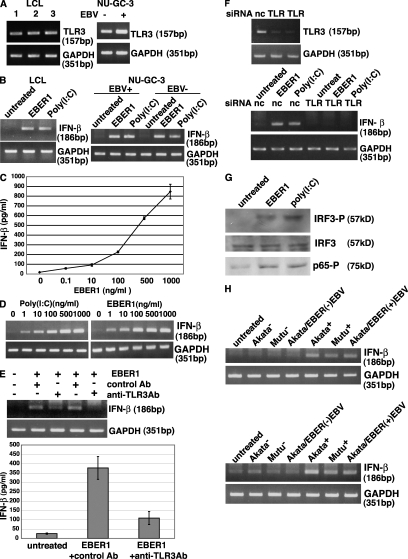
To investigate the role of the EBER1 that was released from EBV-infected cells, we first examined whether in vitro–synthesized EBER1 could induce signaling from TLR3. RT-PCR assays indicated that LCLs and gastric carcinoma–derived NU-GC-3 cells (Akiyama et al., 1988) expressed TLR3 (Fig. 2 A).The addition of in vitro–synthesized EBER1 into culture medium induced IFN-β in LCLs and EBV-positive and -negative NU-GC-3 cells (Fig. 2 B; Imai et al., 1998). A similar result was also obtained by the TLR3 agonist poly(I:C). An ELISA indicated that IFN-β production was dependent on the amount of EBER1 that was added to the culture supernatants; we found that 0.1 ng/ml EBER1 was sufficient to induce the release of IFN-β (Fig. 2 C). Next, we evaluated the efficiency of EBER1 to induce IFN-β expression in LCLs comparing with poly(I:C). No difference in efficiency was observed between EBER1 and poly(I:C) by RT-PCR (Fig. 2 D).
Figure 2.
EBER1 activates signaling through TLR3. (A) Detection of TLR3 in three LCL clones and EBV-infected and uninfected NU-GC-3 cells. Total RNA (0.1 µg) was subjected to 30 cycles of RT-PCR to detect TLR3. RT-PCR for GAPDH was used as an internal control. Three or more independent experiments were performed for each assay. (B) Effect of in vitro–synthesized EBER1 on the expression of IFN-β. LCLs and EBV-positive and –negative NU-GC-3 cells were treated with 0.5 µg/ml in vitro–synthesized EBER1 or poly(I:C) and were cultured for 14 h. Total RNA (0.1 µg) was subjected to 30 cycles of RT-PCR to detect IFN-β. RT-PCR for GAPDH was used as an internal control. Three or more independent experiments were performed. (C) Dose response of the effect of in vitro–synthesized EBER1 on the expression of IFN-β. LCLs (4 × 105 cells/ml) were treated with 0.1–1,000 ng/ml EBER1 and cultured for 14 h. IFN-β in culture supernatants was quantified by ELISA. Error bars indicate the S.D. of duplicate wells. The data presented are representative of three independent experiments. (D) Efficiency of EBER1 and poly(I:C) to induce IFN-β expression in LCLs. LCLs (4 × 105 cells/ml) were treated with 1–1,000 ng/ml EBER1 and cultured for 14 h. IFN-β induction was analyzed by RT-PCR. Three or more independent experiments were performed for each assay. (E) Effect of an anti-TLR3 antibody on EBER1-induced IFN-β production. LCLs were preincubated with the anti-TLR3 antibody for 30 min at 37°C, before being treated with 0.5 µg/ml of EBER1 and incubated for 14 h. The culture was analyzed for IFN-β induction by RT-PCR (upper panel) and ELISA (lower panel). Error bars indicate the SD of duplicate wells. The data presented are representative of three independent experiments. (F) Effect of TLR3 knockdown on EBER1-induced IFN-β production. Negative control siRNA (nc) or TLR3-siRNA (TLR) were transfected into EBER-knockout EBV-infected AGS cells. After 48 h, cells were treated with EBER1 or poly(I:C) and IFN-β induction was analyzed by RT-PCR (bottom). Efficiency of TLR3 silencing was analyzed by RT-PCR (top). Three or more independent experiments were performed for each assay. (E) Effect of EBER1 on the downstream signals of TLR3, IRF3, and NF-κB. LCLs were treated with 2.5 µg/ml in vitro–synthesized EBER1 or poly(I:C) and cultured for 3 h before the phosphorylation of IRF3, and NF-κB was examined by immunoblotting using antibodies against phosphorylated IRF3, total IRF3, and phosphorylated p65. The data presented are representative of three independent experiments. (F) Effect of culture supernatants from EBER-positive cells on the expression of IFN-β. The study includes EBV-positive and –negative Mutu cells, EBV-positive and –negative Akata cells, and EBV-negative Akata cells that were stably infected with EBER-positive EBV or EBER-knockout EBV. The cells (2 × 105 cells/ml) were cultured for 4 d and then the culture supernatants were harvested. LCLs (4 × 105) were treated with 1 ml culture supernatants (top) or RNA extracted from 1 ml culture supernatants in 1 ml culture medium (bottom) for 14 h. RNA (0.1 µg) was subjected to 30 cycles of RT-PCR to detect IFN-β. Three or more independent experiments were performed for each assay.
When LCLs were pretreated with an anti-TLR3 antibody (Matsumoto et al., 2002), and then treated with EBER1, the effect of EBER1 on the induction of IFN-β was markedly reduced (Fig. 2 E). In addition, TLR3 knockdown by siRNA resulted in reduced induction of IFN (Fig. 2 F), indicating that EBER1 induces IFN through TLR3. IFN-regulatory factor 3 (IRF3) and NF-κB function downstream of the TLR3 signaling pathway (Akira and Takeda, 2004). As shown in Fig. 2 G, both IRF3 and NF-κB were phosphorylated upon treatment of the cells with EBER1 or poly(I:C).
We next examined whether the EBER1 that was released into culture supernatants could induce the expression of IFN. Cell culture supernatant was harvested on day 4 and added to the culture medium of LCLs. After 14 h of cultivation, the induction of IFN-β in LCLs was determined by RT-PCR. The results indicated that IFN was induced in EBV-positive cells, but not in EBV-negative cells or EBER-knockout EBV-infected cells (Fig. 2 H).
EBER1 is detected as a complex with La in the culture supernatants, and the complex can induce TLR3 signaling
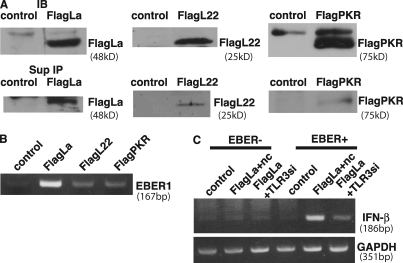
The stable presence of EBER1 in culture supernatants suggested that EBER1 was bound by some proteins, thus being protected from degradation by nucleases. We then examined whether the EBER1 that was present in culture supernatants existed as a complex with EBER-binding cellular proteins. Flag-tagged L22, La, and PKR were transfected into Mutu+ cells, and their interaction with EBER1 in culture supernatants was examined by coimmunoprecipitation assays. Although flag-tagged L22, La, and PKR were expressed equally in transfected cells, only La could be strongly immunoprecipitated from the culture supernatant (Fig. 3 A). RT-PCR indicated that EBER1 preferentially coimmunoprecipitated with La (Fig. 3 B). The presence of La in culture supernatants suggested that La was actively secreted from living cells rather than passively released from dead cells. These results indicate that EBER1 was released from EBV-infected cells as a complex with La. To further assess whether EBER can activate TLR3 in complexes with La, we used immunoprecipitates from culture supernatants of EBV-positive or EBER-knockout EBV-infected cells for stimulation. EBV-positive and EBER-knockout EBV-infected AGS cells were transfected with Flag-La, and immunoprecipitates from culture supernatants were added into a culture medium of EBER-knockout EBV-infected AGS cells. As shown in Fig. 3 C, IFN-β was clearly induced by treatment with immunoprecipitates from culture supernatants of EBV-positive cells, whereas the IFN induction was reduced by TLR3 knockdown. In contrast, no IFN induction was observed in the cells treated with immunoprecipitates from culture supernatants of EBER-knockout EBV-infected cells. These results suggest that EBER1 can induce TLR3 signaling in complexes with La.
Figure 3.
EBER is detected as a complex with La in the culture supernatants and the complex is stimulatory for TLR3. (A) Detection of La, L22, and PKR in culture supernatants. Mutu+ cells (1 × 106) were transfected with Flag-tagged La, L22, or PKR plasmid and cultured for 48 h. Flag-tagged La, L22, and PKR were detected by immunoblotting of the cell lysates (top) and immunoprecipitation of culture supernatants (bottom) using an anti-Flag antibody. Three or more independent experiments were performed for each assay. (B) Detection of EBER1 in the immunoprecipitates. RNA was extracted from the immunoprecipitates and subjected to 30 cycles of RT-PCR to detect EBER1. Three independent experiments were performed. (C) Effect of immunoprecipitates from culture supernatants on IFN-β induction. EBER-positive (EBER+) or EBER-knockout (EBER−) EBV-infected AGS cells were transfected with control (control) or Flag-tagged La plasmid (Flag La) and cultured for 48 h, followed by immunoprecipitation of culture supernatants using anti-Flag antibody. Both EBER− and EBER+ immunoprecipitates were added into the media of EBER-knockout EBV-infected AGS cells transfected with negative control siRNA (Flag La+nc) or TLR3 siRNA (Flag La+TLR3si), and IFN-β induction was analyzed by RT-PCR. The data presented are representative of three independent experiments.
EBER1 exists in sera from patients with active EBV infections and induces the production of type I IFN and inflammatory cytokines
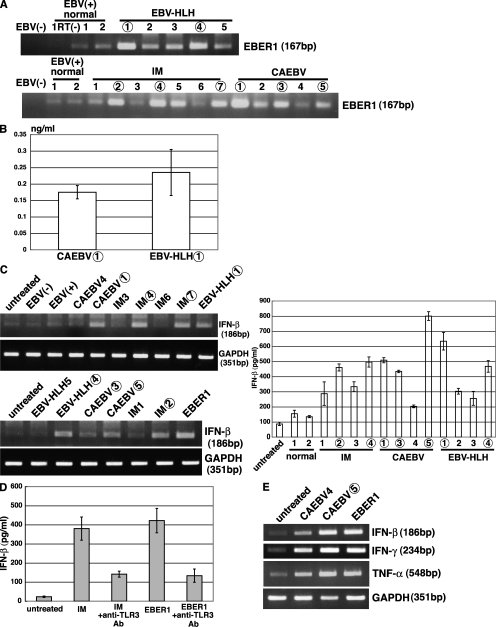
Subsequently, we examined the presence of EBER1 in the sera of patients with acute EBV infections, such as IM, CAEBV, and EBV-HLH. We also investigated the role of EBER on the activation of TLR3. Results from RT-PCR assays revealed that EBER1 was detected in patient sera and also in the sera from healthy individuals (Fig. 4 A); however, the level of EBER1 was much higher in patient sera than that obtained from healthy individuals. Our real-time PCR assays indicated that the sera contained 0.17 to 0.24 ng/ml of EBER1 (Fig. 4 B), which was sufficient to induce signaling from TLR3 (Fig. 2 C). Because we had demonstrated that sera nonspecifically induced IFN and cytokines in LCLs, we stimulated LCLs with RNA that had been purified from that sera. As shown in Fig. 4 C, RNAs from the patient sera, which contained a higher amount of EBER1, induced the release of IFN-β when added to the culture medium of LCLs. The quantification of IFN-β by ELISA indicated that all of the patient sera that we examined induced more IFN-β (200–800 pg/ml) than that induced by the sera from healthy individuals. To confirm that IFN-β induction by patient sera was mediated thorough TLR3, LCLs were pretreated with an anti-TLR3 antibody and treated with RNA from a patient serum with IM. The results demonstrate that there was a marked reduction in the RNA-induced release of IFN-β (Fig. 4 D), indicating that the RNA from serum induced the expression of IFN through TLR3. We also examined whether RNAs from the patient sera could induce type I IFN and proinflammatory cytokines such as IFN-γ and TNF in PBMCs. As shown in Fig. 4 E, RNAs purified from CAEBV, which contained a high level of EBER1, induced the expression of IFN-β, IFN-γ, and TNF.
Figure 4.
EBER1 exists in sera from patients with active EBV infections and induces the production of type I IFN and inflammatory cytokines. (A) Detection of EBER1 by RT-PCR in patient sera. RNA was extracted from 100 µl sera or plasma from patients with IM, CAEBV, and EBV-HLH, and from EBV-positive and –negative healthy donors. EBER1 was detected by 35 cycles of RT-PCR. Sample numbers in A–C correspond to each other; samples that are followed by a number in an open circle contain higher amounts of EBER1 than those with a number alone. Three or more independent experiments were performed. (B) Quantification of EBER1 in patient sera. RNA was extracted from the sera and subjected to real-time RT-PCR to detect EBER1. Error bars indicate the SD of duplicate wells. The data presented are representative of three independent experiments. (C) Induction of IFN-β production by RNA extracted from patient sera. LCLs (4 × 105) were treated with RNA that had been extracted from 100 µl sera in 1 ml culture medium, incubated for 14 h, and subjected to 30 cycles of RT-PCR to detect IFN-β (left) or ELISA of the culture supernatants for detection of IFN-β (right). The data of ELISA are shown as the means ± SD of duplicate determination and representative results of three independent experiments are shown. (D) Effect of an anti-TLR3 antibody on serum-induced IFN-β production. LCLs (4 × 105) were preincubated with the anti-TLR3 antibody for 30 min at 37°C, before being treated with RNA extracted from 100 µl of serum from a patient with IM or 1.0 µg in vitro–synthesized EBER1 as a positive control in 1 ml culture medium, and cultured for 14 h. Production of IFN-β was determined by ELISA of the culture supernatants. Error bars indicate the S.D. of duplicate wells. The data presented are representative of three independent experiments. (E) Effect of the RNA from patients sera on the induction of IFN-β and proinflammatory cytokines. Human PBMCs (1 × 106) were treated with RNA extracted from 100 µl patients sera or 0.5 µg in vitro–synthesized EBER1 in 1 ml culture medium and cultured for 14 h. The induction of IFN-β and proinflammatory cytokines was determined by 30 cycles of RT-PCR. Three independent experiments were performed.
EBER1 induces mature surface phenotype and antigen-presenting capacity of DCs
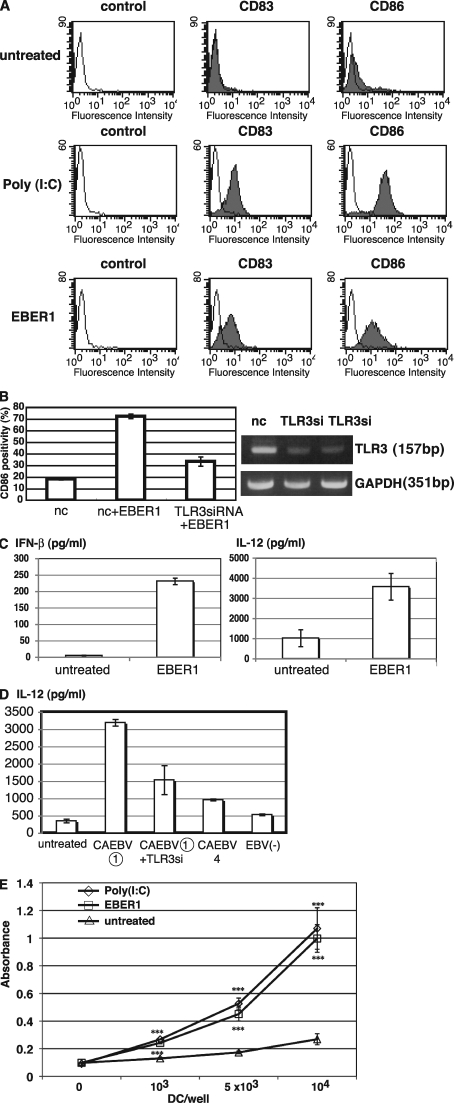
Finally, we investigated the effect of EBER1 on DC function to clarify whether EBER1-mediated signaling is sufficient for induction of immune responses. We treated immature DCs with EBER1 or poly(I:C) for 24 h and analyzed the surface markers of matured DC by flow cytometry. Treatment with EBER1, as well as poly(I:C), resulted in an increase of CD83 and CD86 levels, indicating that EBER1 induces maturation of DCs (Fig. 5 A). Because CD86 up-regulation by EBER1 was clearly reduced by TLR3 siRNA, EBER1-mediated DC maturation is dependent on TLR3 (Fig. 5 B). We next investigated cytokine production by DCs in response to EBER1. As shown in Fig. 5 C, IFN-β and IL-12 production by DCs was induced by EBER1, indicating EBER1-mediated activation of DCs. Furthermore, sera from patients containing a high level of EBER1 induced IL-12 production by DCs in a TLR3-dependent manner, whereas sera containing a low level of EBER1 or EBV-negative sera could not, suggesting that EBER1 in sera is stimulatory for DCs through TLR3 signaling (Fig. 5 D). To assess whether DCs with maturation induced via EBER1 have the capacity of antigen presentation, DCs treated with EBER1 or poly(I:C) were used for allo MLR assay. The stimulatory properties of EBER1- or poly(I:C)-treated DCs were compared with those of untreated immature DCs. As shown in Fig. 5 E, EBER1- or poly(I:C)-treated DCs induced comparable allo mixed lymphocyte reactions that were most markedly seen with 10,000 DCs/wells, in a 1:10 stimulator/responder ratios. Therefore, EBER1-treated DCs were potent inducers of primary allo T cell responses.
Figure 5.
EBER1 induces maturation of DC and subsequent antigen presentation. (A) Effect of EBER1 on phenotype of DCs. DCs were prepared from PBMCs and untreated or treated with either poly(I:C) (10 µg/ml) or EBER1 (10 µg/ml). Surface markers of matured DCs, CD83, and CD86 were measured by flow cytometry. As negative control, cells were stained with mouse IgG. The data are representative of three independent experiments. (B) Effect of TLR3 knockdown on EBER1-mediated DC maturation. DCs were transfected with TLR3 siRNA or control siRNA (nc) and were stimulated with EBER1. CD86 positivity (%) was analyzed by flow cytometry (left). Efficiency of TLR3 silencing was analyzed by RT-PCR (right). Data are shown as the means ± SD of duplicate determination and representative results of three independent experiments are shown. (C) EBER1-induced cytokine production by DCs. DCs were treated with EBER1, and IFN-β or IL-12p40 production were measured by ELISA. Error bars indicate the SD of duplicate wells, and the data presented are representative of three independent experiments. (D) Effect of sera from patients on TLR3-mediated IL-12 production by DCs. DCs were transfected with negative control siRNA or TLR3 siRNA (TLR3si) for 48 h, and then stimulated with sera from patients with CAEBV containing high amounts of EBER1 (CAEBV (1)) and IL-12 production was measured by ELISA. Sera form patients with CAEBV containing low amounts of EBER1 (CAEBV4) or EBV-negative (EBV(−)) healthy donor were also used for stimulation. Data are shown as the means ± SD of duplicate determination and representative results of three independent experiments are shown. (E) Allogenic MLR. DCs treated with either poly(I:C) or EBER1 were used as stimulator cells. Untreated immature DC were also used as stimulator cells. Allogenic PBMCs (1 × 105) were used as responder cells in triplicate cultures. Proliferation of alloreactive T cells was determined by cell proliferation assay. Data are shown as the means ± SD of triplicate determination and representative results of three independent experiments are shown. Statistical significance differences between groups were evaluated by Student's t test. ***, P < 0.001.
In this study, we have demonstrated that EBER1 is released from EBV-infected cells and activates signaling from the TLR3. We have also demonstrated that sera from patients with active EBV infections such as IM, CAEBV, and EBV-HLH contained a large amount of EBER1, which was sufficient to activate TLR3 signaling, subsequently resulting in the induction of type I IFN and proinflammatory cytokines. Furthermore, EBER1-treated DCs could induce primary immune response, suggesting that during active infection, EBER1-mediated TLR3 stimulation is responsible for immune activation by EBV. TLR3 is predominantly expressed on DCs (Doyle et al., 2003; Akira and Takeda, 2004); circulating EBER1 could induce the activation of DCs and subsequent T cell activation, leading to the systemic production of proinflammatory cytokines. EBER1-mediated immune response would be needed for host defense, therefore IM, which is characterized as a CTL response against polyclonal proliferation of EBV-infected B cells usually follows a self-limited course. In EBV-HLH, EBV-infected cells are mainly CD8+ T cells, whereas CD4+ T or NK cells are infected in CAEBV (Kasahara et al., 2001). Given that CD8+ T cells and NK cells express TLR3 and are activated by TLR3 signals (Schmidt et al., 2004; Tabiasco et al., 2006), TLR3-expressing T cells and NK cells could be activated by EBER1 through TLR3 and produce proinflammatory cytokines. Our findings suggest that immunopathologic diseases that are caused by active EBV infections could be attributed to TLR3-mediated cytokinemia by EBER1, and that circulating EBER1 could be a potential target for therapeutic agents. Because it has been reported that plasmacytoid DCs are involved in anti-EBV immunity by the secretion of IFN and T cell activation through TLR9 pathways (Lim et al., 2007), TLR3 would collaborate with TLR9 during primary EBV infection.
Recent studies have demonstrated that activation of TLR3 by viral dsRNA contributes to viral pathogenesis. For example, TLR3-mediated inflammatory responses by viral dsRNA contribute to the development of lethal encephalitis by facilitating virus entry into the brain during West Nile virus infection (Wang et al., 2004). Rotavirus genomic dsRNA induces severe injury in the small intestine of mice in a TLR3-dependent manner (Zhou et al., 2007). These findings, along with ours, suggest that activation of TLR3 induces not only protective effects against viral infection but also effects that contribute to viral pathogenesis.
It was reported that EBER1 mostly binds to L22 (Toczyski and Steitz, 1993). Therefore, if EBER1 is released because of cell death, it would be released with Flag-L22 rather than other Flag-tagged proteins from the cell, in which those proteins are equally expressed. However, the majority of the EBER1 that was released from the cells existed in a complex with Flag-La, whereas other EBER-binding proteins, such as PKR and L22 were faintly detected. This led us to hypothesize that EBER1 is released from the cells by active secretion of La. Because La has been reported to be secreted as an exosome (Kapsogeorgou et al., 2005), EBER1 might also be secreted in this manner by binding to La. Alternatively, if La is more stable than L22 in extracellular state, EBER1 released by cell death could be strongly detected with La. Interestingly, we also demonstrated that EBER1 stimulates TLR3 in complexes with La protein. Further study is needed to clarify how the EBER1–protein complex is recognized by TLR3 and the mechanism of EBER1 release.
In contrast to EBER1, EBER2 was faintly detected in the culture supernatants of EBV-infected cells or in sera from patients with active EBV infections. EBER2 has a shorter half-life than EBER1, which may account for the preferential detection of EBER1 in our experiments (Clarke et al., 1992).
MATERIALS AND METHODS
Cell culture and reagents.
LCLs, the EBV-positive Burkitt's lymphoma cell lines Akata+ (Takada, 1984) and Mutu+ (Gregory et al., 1990), and the EBV-negative cell lines Akata− (Shimizu et al., 1994) and Mutu− (Nanbo et al., 2002) were cultured in RPMI 1640 media (Sigma-Aldrich) supplemented with 10% FBS (Invitrogen) and antibiotics. Akata− cells that had been reinfected with recombinant EBER-positive EBV (Akata/EBER+EBV; Shimizu et al., 1996) or EBER-knockout EBV (Akata/EBER−EBV; Yajima et al., 2005) were cultured in RPMI 1640 media containing 700 µg/ml G418 (Sigma-Aldrich). EBV-infected (EBV+) and neomycin-resistant gene transfected (EBV−) NU-GC-3 cells and EBER-positive EBV or EBER-knockout EBV-infected AGS cells were cultured in Dulbecco's modified Eagle's medium (Sigma-Aldrich) supplemented with 10% FBS, antibiotics, and 500 µg/ml G418. The anti-Flag antibody and mouse IgG1 were purchased from Sigma-Aldrich. The anti-TLR3 antibody was obtained from eBioscience, and the anti-phospho IRF3 antibody, anti-IRF3 antibody, and anti-phospho p65 antibody were obtained from Cell Signaling Technology. poly(I:C) and polymyxin B were purchased from EMD. Anti-CD83 antibody and anti-CD86 antibody were obtained from Beckman Coulter and Ancell Co., respectively. Human recombinant GM-CSF and IL-4 were purchased from PeproTech.
RNA extraction and RT-PCR.
Total RNA was extracted using Trizol reagent (Invitrogen) and was treated with DNaseI (Invitrogen). To analyze the release of EBER, RNA was extracted from 1 ml culture media or 100 µl serum and plasma. Reverse transcription was performed using SuperScript II reverse transcription (Invitrogen) and oligo-dT primer (Promega) or sequence-specific primers for EBER1 and EBER2. The sequence of the primers used for PCR was as follows. EBER1 (5′-AGGACCTACGCTGCCCTAGA-3′, 5′-AAAACATGCGGACCAGC-3′), EBER2 (5′-AGGGACAGCCGTTGCCCTAGTGGTTTCGGA-3′, 5′-AAAACAGCGGACAAGCCGAATACC-3′), TLR3 (5′-TCACTTGCTCATTCTCCCTT-3′, 5′-GACCTCTCCATTCCTGGC-3′), IFN−β (5′-GATTCATCGAGCACTGGCTGG-3′, 5′-CTTCAGGTAATGCAGAATCC-3′), IFN-γ (5′-CAGGTCATTCAGATGTAGCG-3′, 5′-GCTTTTCGAAGTCATCTCG-3′), TNF (5′-CTTCTGCCTGCTGCACTTTGGA-3′, 5′-TCCCAAAGTAGACCTGCCCAGA-3′), and GAPDH (5′-GCCTCCTGCACCACCAACTG-3′, 5′-CGACGCCTGCTTCACCACCTTCT-3′).
Quantification of EBER1.
EBER1 was prepared by in vitro transcription as previously described (Samanta et al., 2006). Serial dilutions of EBER1 (0.1 ng/ml to 1.0 µg/ml) were made in culture media and total RNA was extracted and used for real-time PCR using the LightCycler system (Roche). These results were used as a standard for measuring the amount of EBER1 in culture supernatant.
Analysis of TLR3 activation.
In vitro–synthesized EBER1 or poly(I:C) and RNAs that had been prepared from culture supernatants or human sera were mixed with Lipofectamine 2000 (Invitrogen) for 15 min in Opti-MEM (Invitrogen) before being used to stimulate the cells. The reagents were incubated in the tissue culture medium for 1 h before being removed by washing. To neutralize TLR3, cells were pretreated with an anti-TLR3 antibody (40 µg/ml) for 30 min at 37°C. The antibody was then removed before the TLR3 stimulation.
Measurement of cytokine production.
Cell culture supernatants were collected and analyzed for IFN-β and IL-12 p40 production using a human IFN-β ELISA kit (PBL Biomedical Laboratories) or OptEIA human IL-12 (p40) ELISA kit (BD) according to the manufacturer's protocol.
Immunoblotting.
Cells were lysed with 1% NP-40 lysis buffer and the cell lysates were subjected to SDS-PAGE and subsequent electrotransfer onto nitrocellulose membranes. The membranes were blocked with 5% bovine serum albumin (Sigma-Aldrich) or nonfat milk in TBS containing 0.05% Tween 20, and were subsequently treated with the primary antibodies for phospho-IRF3, IRF-3, phospho-p65, and the Flag tag.
Analysis of EBER-protein interactions by coimmunoprecipitation.
Mutu cells (2 × 106) were transfected with Flag-La, Flag-L22, and Flag-PKR expression plasmids by electroporation. After 48 h, 1 ml culture supernatant was harvested and incubated with 3.5 µg anti-Flag mouse monoclonal antibody for 14 h at 4°C. After the addition of 50 µl of protein G–Sepharose (GE Healthcare), mixtures were incubated at 4°C for 3 h. Sepharose beads were pelleted and washed twice with lysis buffer (50 mM Tris-HCl, pH 7.4, 150 mM NaCl, 1% Nonidet P-40, 0.5% deoxycholate, and 20% protease/inhibitor) and three times with wash buffer (50 mM Tris-HCl, pH 7.4, 200 mM NaCl, 0.1% Nonidet P-40, 0.05% deoxycholate, 10% protease/inhibitor mix, 100 units/ml RNasin, 0.4% vanadyl ribonucleoside complex, and 1 mM dithiothreitol) by end-over-end rotation for 10 min. Pellets were dissolved in SDS loading buffer and subjected to immunoblotting. For RNA extraction, pellets were dissolved in 100 µl of lysis buffer and digested with 30 µg of proteinase K for 30 min at 50°C with the addition of 0.1% SDS, before being dissolved in Trizol reagent. Extracted RNA was analyzed by RT-PCR for the expression of EBER1.
Flow cytometry.
Cells were washed with FACS buffer (PBS containing 0.1% BSA, 0.1% NaN3) and stained with anti-CD83 antibody (1 µg), anti-CD86 antibody (0.5 µg), or mouse IgG1 (1 µg) together with human IgG (10 µg) for 30 min at 4°C. After washing twice with FACS buffer, cells were incubated with FITC-labeled secondary antibody (American Qualex) for 30 min at 4°C, and then analyzed on a FACSCalibur (BD).
Preparation and stimulation of DCs.
The institutional committee at Hokkaido University approved the use of human blood samples for this study. CD14+ monocytes were isolated from human PBMC using a MACS system (Miltenyi Biotec). Immature DCs were generated from monocytes by culture for 6 d in RPMI 1640 medium supplemented with 10% heat-inactivated FCS (SAFC Biosciences) in the presence of 500 IU/ml recombinant human GM-CSF and 100 IU/ml recombinant human IL-4. Immature DCs (106 cells/ml) were treated with poly(I:C) (10 µg/ ml) or synthesized EBER1 (10 µg/ml) for 24 h. Both reagents were treated with polymyxin B (5 µg/ml) at 37°C for 1 h before stimulation.
TLR3 knockdown experiment.
TLR3 stealth RNAi (Invitrogen) or stealth RNAi negative control (Invitrogen) was transfected using Lipofectamine RNAiMAX (Invitrogen) according to the manufacturer's protocol. Successful transfection was confirmed using the BLOCK-iT Alexa Fluor Red Fluorescent Oligo (Invitrogen). Monocytes were cultured for 4 d before being transfected (106 cells/well, 24 well plate) on day 4 and again on day 5 with 70 nM siRNA. On day 6 (48 h after the first transfection), cells were used for stimulation. AGS cells (105 cells/well, 24-well plate) were transfected with 10 nM siRNAs and used for stimulation after 48 h.
Mixed lymphocyte reaction (MLR) assay.
After 24 h stimulation with poly(I:C) or EBER1, DCs were treated with 50 µg/ml mitomycin C (MMC). Allo-PBMC were isolated by Histopaque density gradient separation of blood collected from healthy donors. Serial dilutions (104 to 103 cells/well) of MMC-treated DCs were cultured in triplicate with 105 PBMCs in 96-well round-bottom plates for 3 d, and PBMC proliferation was measured using CellTiter 96 nonradioactive cell proliferation assay kit (Promega).
Acknowledgments
We thank Y. Ando for technical assistance.
This work was supported by grants-in-aid from the Ministry of Education, Science, Sports, Culture and Technology, Japan (D. Iwakiri and K. Takada) and by the Takeda Science Foundation and the Akiyama Foundation (D. Iwakiri).
The authors have no conflicting financial interests.
Footnotes
Abbreviations used:
- CAEBV
- chronic active EBV infection
- dsRNA
- double-stranded RNA
- EBER
- EBV-encoded small RNA
- EBV-HLH
- EBV-associated hemophagocytic lymphohistiocytosis
- IM
- infectious mononucleosis
- IRF
- IFN-regulatory factor
- La
- lupus erythematosis-associated antigen
- LCL
- lymphoblastoid cell line
- PKR
- RNA-activated protein kinase
- RIG-I
- retinoic acid–inducible gene-I
- TLR
- Toll-like receptor
References
- Akira S., Takeda K. 2004. Toll-like receptor signalling.Nat. Rev. Immunol. 4:499–511 doi:10.1038/nri1391 [DOI] [PubMed] [Google Scholar]
- Akiyama S., Amo H., Watanabe T., Matsuyama M., Sakamoto J., Imaizumi M., Ichihashi H., Kondo T., Takagi H. 1988. Characteristics of three human gastric cancer cell lines, NU-GC-2, NU-GC-3 and NU-GC-4.Jpn. J. Surg. 18:438–446 doi:10.1007/BF02471470 [DOI] [PubMed] [Google Scholar]
- Clarke P.A., Schwemmle M., Schickinger J., Hilse K., Clemens M.J. 1991. Binding of Epstein-Barr virus small RNA EBER-1 to the double-stranded RNA-activated protein kinase DAI.Nucleic Acids Res. 19:243–248 doi:10.1093/nar/19.2.243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke P.A., Sharp N.A., Clemens M.J. 1992. Expression of genes for the Epstein-Barr virus small RNAs EBER-1 and EBER-2 in Daudi Burkitt's lymphoma cells: effects of interferon treatment.J. Gen. Virol. 73:3169–3175 doi:10.1099/0022-1317-73-12-3169 [DOI] [PubMed] [Google Scholar]
- Doyle S.E., O'Connell R., Vaidya S.A., Chow E.K., Yee K., Cheng G. 2003. Toll-like receptor 3 mediates a more potent antiviral response than Toll-like receptor 4.J. Immunol. 170:3565–3571 [DOI] [PubMed] [Google Scholar]
- Gregory C.D., Rowe M., Rickinson A.B. 1990. Different Epstein-Barr virus-B cell interactions in phenotypically distinct clones of a Burkitt's lymphoma cell line.J. Gen. Virol. 71:1481–1495 doi:10.1099/0022-1317-71-7-1481 [DOI] [PubMed] [Google Scholar]
- Imai S., Nishikawa J., Takada K. 1998. Cell-to-cell contact as an efficient mode of Epstein-Barr virus infection of diverse human epithelial cells.J. Virol. 72:4371–4378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapsogeorgou E.K., Abu-Helu R.F., Moutsopoulos H.M., Manoussakis M.N. 2005. Salivary gland epithelial cell exosomes: a source of autoantigenic ribonucleoproteins.Arthritis Rheum. 52:1517–1521 doi:10.1002/art.21005 [DOI] [PubMed] [Google Scholar]
- Kasahara Y., Yachie A., Takei K., Kanegane C., Okada K., Ohta K., Seki H., Igarashi N., Maruhashi K., Katayama K., et al. 2001. Differential cellular targets of Epstein-Barr virus (EBV) infection between acute EBV-associated hemophagocytic lymphohistiocytosis and chronic active EBV infection.Blood. 98:1882–1888 doi:10.1182/blood.V98.6.1882 [DOI] [PubMed] [Google Scholar]
- Kikuta H., Sakiyama Y., Matsumoto S., Oh-Ishi T., Nakano T., Nagashima T., Oka T., Hironaka T., Hirai K. 1993. Fatal Epstein-Barr virus-associated hemophagocytic syndrome.Blood. 82:3259–3264 [PubMed] [Google Scholar]
- Lerner M.R., Andrews N.C., Miller G., Steitz J.A. 1981. Two small RNAs encoded by Epstein-Barr virus and complexed with protein are precipitated by antibodies from patients with systemic lupus erythematosus.Proc. Natl. Acad. Sci. USA. 78:805–809 doi:10.1073/pnas.78.2.805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim W.H., Kireta S., Russ G.R., Coates P.T. 2007. Human plasmacytoid dendritic cells regulate immune responses to Epstein-Barr virus (EBV) infection and delay EBV-related mortality in humanized NOD-SCID mice.Blood. 109:1043–1050 doi:10.1182/blood-2005-12-024802 [DOI] [PubMed] [Google Scholar]
- Matsumoto M., Kikkawa S., Kohase M., Miyake K., Seya T. 2002. Establishment of a monoclonal antibody against human Toll-like receptor 3 that blocks double-stranded RNA-mediated signaling.Biochem. Biophys. Res. Commun. 293:1364–1369 doi:10.1016/S0006-291X(02)00380-7 [DOI] [PubMed] [Google Scholar]
- Nanbo A., Inoue K., Adachi-Takasawa K., Takada K. 2002. Epstein-Barr virus RNA confers resistance to interferon-α-induced apoptosis in Burkitt's lymphoma.EMBO J. 21:954–965 doi:10.1093/emboj/21.5.954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rickinson A.B., Kieff E. 2001. Epstein-Barr virus. Fields Virology. Fourth edition Fields B.N., Knipe D.M., Howley P.M., Lippincott Williams & Wilkins, Philadelphia: 2575–2627 [Google Scholar]
- Rosa M.D., Gottlieb E., Lerner M.R., Steitz J.A. 1981. Striking similarities are exhibited by two small Epstein-Barr virus-encoded ribonucleic acids and the adenovirus-associated ribonucleic acids VAI and VAII.Mol. Cell. Biol. 1:785–796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rymo L. 1979. Identification of transcribed regions of Epstein-Barr virus DNA in Burkitt lymphoma-derived cells.J. Virol. 32:8–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samanta M., Iwakiri D., Kanda T., Imaizumi T., Takada K. 2006. EB virus-encoded RNAs are recognized by RIG-I and activate signaling to induce type I IFN.EMBO J. 25:4207–4214 doi:10.1038/sj.emboj.7601314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt K.N., Leung B., Kwong M., Zarember K.A., Satyal S., Navas T.A., Wang F., Godowski P.J. 2004. APC-independent activation of NK cells by the Toll-like receptor 3 agonist double-stranded RNA.J. Immunol. 172:138–143 [DOI] [PubMed] [Google Scholar]
- Shimizu N., Tanabe-Tochikura A., Kuroiwa Y., Takada K. 1994. Isolation of Epstein-Barr virus (EBV)-negative cell clones from the EBV-positive Burkitt's lymphoma (BL) line Akata: malignant phenotypes of BL cells are dependent on EBV.J. Virol. 68:6069–6073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu N., Yoshiyama H., Takada K. 1996. Clonal propagation of Epstein-Barr virus (EBV) recombinants in EBV-negative Akata cells.J. Virol. 70:7260–7263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabiasco J., Devêvre E., Rufer N., Salaun B., Cerottini J.C., Speiser D., Romero P. 2006. Human effector CD8+ T lymphocytes express TLR3 as a functional coreceptor.J. Immunol. 177:8708–8713 [DOI] [PubMed] [Google Scholar]
- Takada K. 1984. Cross-linking of cell surface immunoglobulins induces Epstein-Barr virus in Burkitt lymphoma lines.Int. J. Cancer. 33:27–32 doi:10.1002/ijc.2910330106 [DOI] [PubMed] [Google Scholar]
- Toczyski D.P., Steitz J.A. 1993. The cellular RNA-binding protein EAP recognizes a conserved stem-loop in the Epstein-Barr virus small RNA EBER 1.Mol. Cell. Biol. 13:703–710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toczyski D.P., Matera A.G., Ward D.C., Steitz J.A. 1994. The Epstein-Barr virus (EBV) small RNA EBER1 binds and relocalizes ribosomal protein L22 in EBV-infected human B lymphocytes.Proc. Natl. Acad. Sci. USA. 91:3463–3467 doi:10.1073/pnas.91.8.3463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang T., Town T., Alexopoulou L., Anderson J.F., Fikrig E., Flavell R.A. 2004. Toll-like receptor 3 mediates West Nile virus entry into the brain causing lethal encephalitis.Nat. Med. 10:1366–1373 doi:10.1038/nm1140 [DOI] [PubMed] [Google Scholar]
- Yajima M., Kanda T., Takada K. 2005. Critical role of Epstein-Barr Virus (EBV)-encoded RNA in efficient EBV-induced B-lymphocyte growth transformation.J. Virol. 79:4298–4307 doi:10.1128/JVI.79.7.4298-4307.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou R., Wei H., Sun R., Tian Z. 2007. Recognition of double-stranded RNA by TLR3 induces severe small intestinal injury in mice.J. Immunol. 178:4548–4556 [DOI] [PubMed] [Google Scholar]