Abstract
Charcot-Marie-Tooth type 1A (CMT1A) neuropathies linked to the misexpression of peripheral myelin protein 22 (PMP22) are progressive demyelinating disorders of the peripheral nervous system. In this study we asked whether dietary restriction by intermittent fasting (IF) could alleviate the neuropathic phenotype in the Trembler J (TrJ) mouse model of CMT1A. Our results show that neuropathic mice kept on a five month long IF regimen had improved locomotor performance compared to ad libitum (AL) fed littermates. The functional benefits of this dietary intervention are associated with an increased expression of myelin proteins combined with a thicker myelin sheath, less redundant basal lamina, and a reduction in aberrant Schwann cell proliferation. These morphological improvements are accompanied by a decrease in PMP22 protein aggregates, and enhanced expression of cytosolic chaperones and constituents of the autophagy-lysosomal pathway. These results indicate that dietary restriction is beneficial for peripheral nerve function in TrJ neuropathic mice, as it promotes the maintenance of locomotor performance.
Keywords: neuropathy, myelin, dietary regimen, autophagy, chaperones, Schwann cells
Introduction
Alterations in peripheral myelin protein 22 (PMP22) gene expression are linked to heritable demyelinating peripheral neuropathies, a heterogeneous group of diseases that lead to progressive myelin instability and slowed nerve conduction velocity (Suter and Snipes, 1995). Affected individuals present with sensory and motor disturbances, muscle weakness and atrophy. In humans, the autosomal dominantly inherited Charcot-Marie-Tooth (CMT) disease is most frequently associated with duplication of a 1.5 Mb region on chromosome 17, including the intact PMP22 gene (CMT1A). In a smaller group of CMT1A, and in the severe Dejerine-Sottas Syndrome (DSS) patients, single amino acid substitutions in PMP22 are present (Sanders et al., 2001). The spontaneous mutant Trembler J (TrJ) mouse carries the identical L16P amino acid substitution in the peripheral myelin protein 22 gene as has been identified in patients with CMT1A (Suter et al., 1992; Valentijn et al., 1992) and models the behavioral and histopathological phenotypes of the disease (Notterpek and Tolwani, 1999).
The pathological findings in neuropathic mouse nerves include degenerating axonal profiles, incomplete myelination, excessive proliferation of Schwann cells and redundant basal lamina (Henry et al., 1983). How the abnormal expression of PMP22 leads to such a complex phenotype remains unclear, but altered processing and turnover rate of the mutated protein within Schwann cells are likely to play roles. Studies in Schwann cells indicate that ~80% of the newly-synthesized wild type (Wt) protein is rapidly degraded by the proteasome, presumably due to misfolding (Pareek et al., 1997; Notterpek et al., 1999). In the disease states, when one copy of PMP22 is mutated, the fraction destined for proteasomal degradation is increased, which overwhelms the proteasome and leads to protein aggregate formation (Fortun et al., 2003). Data from multiple laboratories support the involvement of protein mistrafficking and aggregation in PMP22 neuropathies (Isaacs et al., 2002; Ryan et al., 2002; Tobler et al., 2002; Fortun et al., 2003; Fortun et al., 2006), a finding that is supported by studies of nerve biopsies from patients with PMP22 gene duplication, and point mutations (Nishimura et al., 1996; Hanemann et al., 2000). Cytosolic mislocalization of PMP22 alters protein homeostasis within Schwann cells and leads to the accumulation of ubiquitin, myelin proteins, chaperones and components of the proteasome near and within the aggregates (Ryan et al., 2002; Fortun et al., 2003; Fortun et al., 2006). Therefore, approaches to prevent the accumulation of misfolded PMP22 and/or facilitate its removal could benefit Schwann cell biology and alleviate the neuropathic phenotype.
Dietary restrictions, including intermittent fasting (IF), provide a non-pharmacological approach to improve the ability of cells to enhance endogenous protective mechanisms in order to resist neurodegenerative disease and aging-associated alterations (Martin et al., 2006; Sharma and Kaur, 2007). Chaperone production and protein degradation through the autophagy-lysosomal system are enhanced in a variety of organisms and tissues by dietary restriction (Mizushima et al., 2004; Wohlgemuth et al., 2007; Steinkraus et al., 2008), however it is unknown if the peripheral nervous system responds to this intervention. Here we show that a five month long IF regimen enhanced these two protein homeostatic mechanisms within peripheral nerves of TrJ mice and alleviated neuropathic behavioral, morphological phenotypes.
Materials and methods
Mouse colonies and experimental design
Wild-type (Wt) and heterozygous Trembler J (TrJ) mice on the C57Bl/6J background (The Jackson laboratory, Bar Harbor, ME) used in the experiments were the offspring from our breeding colony maintained in the McKnight Brain Institute Animal Facility under specific pathogen-free (SPF) conditions, on a 7:00 AM-7:00 PM light cycle. The University of Florida Institutional Animal Care and Use Committee has approved the use of laboratory animals for these studies. For genotyping of the mice, DNA was isolated from tail biopsies of less than ten day old pups and used for PCR (Notterpek et al., 1997). Age-matched male TrJ and Wt mice were used for all experiments. Until nine weeks of age, the standard rodent Teklad LM-485 mouse/rat chow (Harlan laboratory, Indianapolis, IN) was provided ad libitum (AL). After 9 weeks, the animals were assigned to four different groups: Wt-AL, TrJ-AL, Wt-IF, or TrJ-IF. The procedures we used for the feeding regimen follows the protocol of Anson and colleagues that also utilized male C57BL/6 mice (Anson et al., 2003). Mice on an IF regimen had access to food every other day. Food was provided or removed between 3-4 PM every day. Water was available at all times. We measured body weight every two weeks, coinciding with the fed state of the IF group. Over a two year period, using the same experimental design we conducted three independent trials with four to ten mice in each group. At the end of each trial, we collected the sciatic nerves from each mouse for biochemical and morphological studies, as described below.
Rotarod test
All mice were tested monthly on the rotarod (Ugo Basile, Camerio VA, Italy) according to established procedures (Crawley, 1999). Mice were trained for two subsequent days with three trials per day of 60 sec at a fixed speed of 5 rpm, with 30 min rest between each trial. On the third testing day, the animals performed 3 trials on the accelerating rotarod after having been fed the night before. The rotational velocity of the rod was linearly increased from 4-36 rpm, over 300 seconds. There was a 1 hr rest period between each trial. The time each mouse spent on the rotarod before falling was recorded and analyzed.
Grip strength test
Forelimb grip strength was measured as tension force (g) on the Chatillon DFE series Digital Force Gauge apparatus (Chatillon Systems, AMETEK Inc., Florida, USA) attached to a stainless steel triangular ring. Only TrJ animals were tested. Each mouse was placed with its forelimbs on the middle of the ring and was gently pulled horizontally backward by the base of its tail until the grip was released. Forelimb grip strength was calculated from the average of 5 trials for each mouse.
Morphological studies
After five months on each diet regimen, the animals were sacrificed and a ~5 mm piece of the proximal end of the left sciatic nerve from each mouse was processed for morphological analyses (Fortun et al., 2003). Samples were immersion fixed in 2% paraformaldehyde and 1% glutaraldehyde in Tyrode’s buffer (pH 7.4), treated with 2% OsO4 in 0.1 M sodium cacodylate (pH 7.5), dehydrated and embedded in Spurr’s medium. Thick sections were stained with toluidine blue and surveyed by light microscopy using a SPOT camera (Diagnostic Instruments, Sterling Heights, MI) attached to a Nikon Eclipse E800 microscope (Melville, NY). For the morphometric studies, four mice per condition were evaluated for distribution of fiber diameter (axon with myelin, 1000 fibers/animal), myelin sheath thickness (150 fibers/animal) and g ratio (150 fibers/ animal) on light level images (Passage et al., 2004; Jeronimo et al., 2005). We analyzed the data using the public domain NIH Image J program. Total area occupied by nerve fibers was determined as a percentage of nerve cross-sectional area. The percentage of myelinated fibers was calculated by counting myelinated fibers as compared to total number of fibers in three fixed areas of view (0.04 mm2 per field) per animal (Jeronimo et al., 2005).
Primary antibodies
Unless otherwise indicated, the same antibodies were used to detect the studied proteins in the nerve sections and the protein lysates. Rabbit anti-Atg7, LC3 (Cell Signaling technology, Inc., Danvers, MA) and p62 (Biomol, Plymouth Meeting, PA) antibodies were utilized as markers for autophagy. Protein chaperone antibodies included rabbit anti-αB-crystallin, heat shock protein 40 (HSP40), HSP70, and rat anti-HSC70 (Fortun et al., 2003). Antibodies against the mouse lysosomal associated membrane protein 1 (LAMP1) (clone 1D4B, Developmental Studies Hybridoma Bank, Iowa City, IA) and cathepsin D (Cortex Biochem, Inc., San Leandro, CA) were used as lysosomal pathway markers (Notterpek et al., 1997). Antibodies against myelin proteins included rat (for Western blot), or rabbit (for immunohistochemistry) anti-myelin basic protein (MBP) (both from Chemicon, Temencula, CA, USA) and mouse anti-protein zero (P0) (Archelos et al., 1993). To detect PMP22, we used a rabbit polyclonal antibody developed against a peptide corresponding to the second extracellular loop of the rat protein (Pareek et al., 1997; Fortun et al., 2006). Mouse anti-glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (protein loading control; clone 1D4, EnCor Biotechnology Inc., Alachua, FL), rabbit anti-ubiquitin (Dako, Carpinteria, CA), rat anti-CD11b (AbD Serotec, Raleigh, NC) and mouse phospho-histone H3 (pHH3) (Chemicon) were obtained from the indicated commercial suppliers.
Biochemical analyses
Frozen sciatic nerves (at least from three animals per condition) were crushed in liquid nitrogen and solubilized in lysis buffer (62.5 mM Tris, pH 6.8, 10.0% glycerol, 3.0% SDS) supplemented with a complete, EDTA-free protease inhibitor cocktail (Roche Diagnostics, Indianapolis, IN). The protein content of the lysates was determined using the BCA kit (Pierce, Rockford, IL). Samples (20 μg, or 5 μg for myelin proteins) were fractionated by electrophoresis using polyacrylamide gels then transferred to nitrocellulose (0.45 μm pore size; Bio-Rad, Hercules, CA) or PVDF (Biorad, for LC3 and ubiquitin) membranes. After blocking in 5% nonfat dry milk in TBST, membranes were incubated with the indicated primary antibodies overnight at 4°C. Bound primary antibodies were detected by incubation with anti-rabbit, anti-mouse (Cell Signaling), anti-rat or anti-goat (Sigma-Aldrich) horseradish peroxidase (HRP) conjugated secondary antibodies. Membranes then were reacted with an enhanced chemiluminescent substrate (Perkin Elmer, Boston, MA). The reproducibility of the findings were verified on three to five independent Western blots for each tested antigen, using nerve samples from different mice. We used a GS-800 densitometer (Bio-Rad) to digitally image the films.
Immunolabeling of nerve sections
A portion of each freshly-removed left sciatic nerve was frozen by immersion in liquid nitrogen-cooled N-methyl butane. Frozen sections (5 μm thickness) were dried for one hr on Superfrost/Plus microslides (Fisher Scientific, Pittsburg, PA), followed by fixation with 1% paraformaldehyde and 90% ethanol for 2 minutes (for PMP22) or 4% paraformaldehye and phosphate buffered saline (PBS) for 10 minutes followed by permeabilization with 0.2% TritonX-100 in PBS for 15 min (for MBP and HSP70) (Notterpek et al., 1997), all at 25°C. After blocking in PBS containing 20% normal goat serum, nerve sections were incubated with primary antibodies, including rabbit anti-PMP22, rabbit anti-myelin basic protein (MBP) (Chemicon) and rat anti-HSC70 (Stressgen), overnight at 4°C. Appropriate secondary antibodies, including Alexa Fluor 594 goat anti-rabbit and anti-mouse IgG and Alexa Fluor 488 goat anti-rat IgG (all from Molecular Probes, Eugene, OR) were then added for 1-2 hr. Hoechst dye (Molecular Probes) was included in the secondary antibody solution at 10 μg/ml to visualize nuclei. Coverslips were mounted by using the ProLong Antifade kit (Molecular Probes). Samples were imaged with a SPOT camera attached to a Nikon Eclipse E800 microscope and were formatted for printing by using Adobe Photoshop 5.5.
Statistical analysis
ANOVA analyses to establish main effects and interactions of the behavioral studies were performed using StatView (SAS Institute, Cary, NC). Follow-up ANOVAs and/or Scheffe post hoc comparisons were employed to determine specific differences. We used Microsoft Excel to perform a Student’s t-test. A p value less than 0.05 was considered statistically significant.
Results
Intermittent fasting improves the locomotor performance and nerve morphology of neuropathic mice
To determine if a five month long IF regimen could slow demyelination in neuropathic animals, at nine weeks of age we assigned a cohort of Wt and heterozygous TrJ male mice to either an AL or every other day feeding schedule, on a standard rodent chow. As indicated by the body weight measurements (Fig. 1A), both groups of mice gained weight at a similar rate with a tendency for a treatment effect (p=0.7) in the absence of genotypic differences (see Supplemental Table 1A). This result is consistent with a previous report (Anson et al., 2003) and suggests that the animals consume nearly the same amount of total calories on the IF regimen as AL fed mice. To monitor the locomotor capacities of the mice at the beginning and during the trial we used the rotarod (McIlwain et al., 2001). Previous studies indicate that by 8-weeks of age transgenic rodent models of CMT1A have severe impairment performing the complex motor and balance tasks of the rotarod (Sereda et al., 1996; Passage et al., 2004). At the beginning of the trial, Wt mice remain on the rotarod for an average of 250 seconds, while TrJ mice stay on for only about 100 seconds (Fig. 1B). After one month on the regimen, the performance of Wt mice transiently decreases, but then recovers by two months, with no residual impairment over the remainder of the study. This temporary lag of performance of Wt mice may be due to a period of adjustment to the feeding schedule. After three months on the intervention, the neuropathic animals begin to show an improvement in locomotor performance by remaining on the rotarod for longer periods, a trend that is significant at the four and five month time points. A repeated measures ANOVA across the five months of testing indicates a significant genotype difference (p<0.0001) and interaction of treatment with time of testing (p<0.0001). Examination within each genotype reveals the interaction is mainly due to increased performance over time for TrJ mice (p<0.0001) (see Supplementary Tables 1B, C, D). The improvement in the motor behavior of diet restricted neuropathic mice is supported by an approximately 25% increase in forelimb grip strength (Fig. 1C) which is detected as tension force.
Figure 1. A reduction in meal frequency improves the locomotor performance of neuropathic mice.

A, Male wild-type (Wt) and heterozygous TrJ mice were used for these studies. Body weight measured every other week during the study indicates a significant increase for all four groups (p<0.0001 ANOVA, n=9 mice per condition; error bars represent SEM). B, Performance of the mice on the rotarod shows a main effect of genotype (p<0.001 ANOVA; error bars represent SEM) and a difference across trials (p<0.05 ANOVA). The performance of TrJ mice after four months on the regimen significantly improved (Post hoc ANOVA, p<0.001). Statistical analysis is presented in Supplementary Table 2. C, After five months on the regimen, the IF animals demonstrate improvement in forelimb strength (***p<0.001 ANOVA, n=6). Results are presented using Tukey box-and-whisker plots, where the box represents the inter-quartile range; the horizontal line indicates median value and error bars represent maximum and minimum values.
Histopathological changes of peripheral nerves in TrJ mice include atrophy of nerve fibers, thinned myelin, Schwann cell over-proliferation and redundant, loose basal lamina (Henry et al., 1983; Atanasoski et al., 2002; Misko et al., 2002). On cross-sectional views of sciatic nerves from seven month old mice these abnormalities are pronounced when comparing samples from AL fed TrJ with Wt (Fig. 2, panels on left). In comparison, myelinated fibers are evident in neuropathic nerves from the IF group suggesting beneficial influence of the intervention on nerve histology. Since the Schwann cell basal lamina plays a crucial role in the myelination of peripheral nerves (Court et al., 2006), we examined axon-Schwann cell units on thin sections (Fig. 2, insets). Onion-bulb formations, representing parallel basement membranes that surround Schwann cells are common in nerves of TrJ mice (Fig. 2, arrows in inset, bottom left) (Henry et al., 1983; Notterpek and Tolwani, 1999). However, in samples from animals on the intervention, axonal myelin appears thicker and the basal lamina layers of the Schwann cells are less redundant (Fig. 2, arrowheads in inset in TrJ-IF). Thus, a five month long IF regimen notably attenuates a number of detrimental histopathological changes in peripheral nerves of neuropathic mice, including axonal degeneration, demyelination and basal lamina abnormalities.
Figure 2. Myelinated axons are maintained in peripheral nerves of diet restricted neuropathic mice.
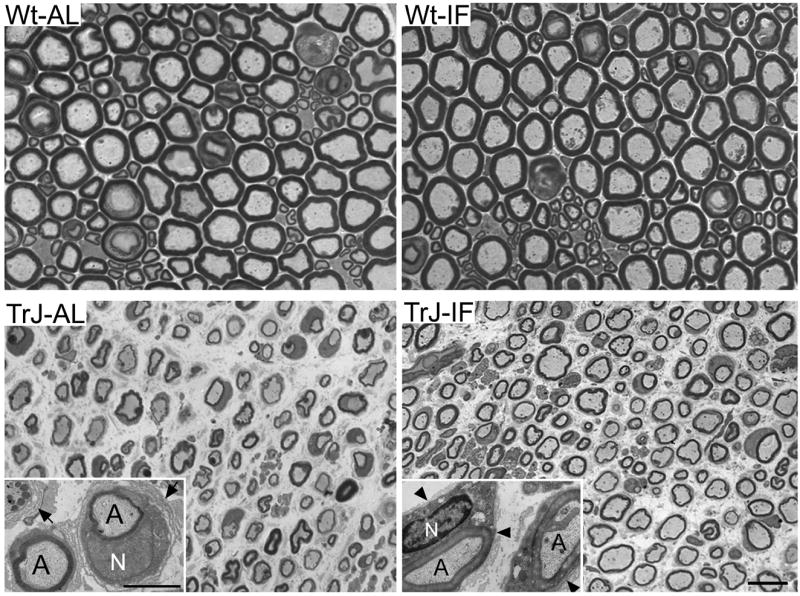
Cross-sectional views of proximal sciatic nerves from AL and intermittently fed Wt and TrJ mice are shown. On thin sections from AL fed neuropathic animals (left, inset), poorly myelinated axons (A) and multiple layers of loose basal lamina (arrows) are visible. In mice that had undergone an IF regimen (right), axonal myelination is thicker and the multiple basal lamina layers of the Schwann cells is replaced by a single tight basal lamina (arrowheads). N:nucleus. Micron bar: 10 μm and 2 μm (insets).
To substantiate the visibly improved nerve morphology in diet restricted neuropathic mice, we performed a number of morphometrical quantifications on randomly selected cross-sectional micrographs (Fig. 3) (Jeronimo et al., 2005). While the IF regimen has no striking influence on nerve tissue morphology of Wt mice, it markedly improves the cross-sectional area occupied by nerve fibers (*p<0.05) (Fig. 3A) in samples from affected mice. Similar to findings in a previous therapeutic study of neuropathic mice (Passage et al., 2004), the percentage of myelinated fibers also significantly improved (**p<0.01) (Fig. 3B), indicating attenuation of the ongoing demyelination (Notterpek et al., 1997). Moreover, in nerves of intermittently fed TrJ rodents the mean diameter of nerve fibers is increased (Fig. 3C), as also evident on the cross-sections in Fig. 2. Morphometric analysis of four thousand fibers from four mice per condition reveal that the increase in mean fiber diameter is statistically significant in both Wt and neuropathic mice (***p<0.001) on the dietary intervention (Supplemental Table 2A). We also measured myelin thickness within these samples and found a thicker myelin in TrJ nerves upon intermittent feeding (Fig. 3D). Similar to the increase in axonal diameters, the effect on myelin thickness is significant in both genotypes (***p<0.001; Supplemental Table 2B). In agreement, the ratios of the axon to fiber (axon and myelin together) diameters (referred to as g ratios) show significant decrease in both genotypes with intermittent feeding (***p<0.001; Fig. 2F, Supplemental Table 2C). These results indicate that intermittent feeding ameliorates behavioral and morphological deficits in TrJ neuropathic mice.
Figure 3. Morphometric analyses of sciatic nerves from AL and diet restricted mice.

A, In samples from neuropathic mice, the cross-sectional area occupied by nerve fibers is improved in response to the regimen (*p<0.05). B, The percentage of myelinated vs. total fibers within nerves of neuropathic mice on the IF regimen is higher, as compared to AL fed (**p<0.01). A, B: n=4 mice; error bars represent SEM. C, The distribution of fiber diameters in micrometer is shown (horizontal lines indicate mean values). The IF regimen increases the mean fiber diameter in nerves from both Wt and TrJ mice as compared to AL fed (***p<0.001, n=4 mice). D, The thickness of myelin sheath is increased in nerves from mice on the regimen as compared to AL fed (***p<0.001). E, Quantification of the median g-ratios (axon diameter/fiber diameter) of individual nerve fibers (n=150) indicates a significantly improved myelination in nerves from mice on the IF regimen, as compared to AL fed (***p<0.001). D and E, Results are presented using Tukey box-and-whisker plots, where the box represents the inter-quartile range; the horizontal line indicates median value and error bars represent maximum and minimum values. Statistical analysis is presented in Supplementary Table 2.
Myelin protein expression is enhanced by intermittent dietary restriction
The marked demyelination by six months of age in TrJ mice is accompanied by a severely reduced steady-state expression of myelin proteins (Notterpek et al., 1997). To determine whether the IF regimen had an influence on the expression of these proteins, the left sciatic nerve from each mouse was processed for biochemical analyses. In agreement with the improved axonal myelination (Figs. 2, 3), the levels of the myelin proteins; P0, PMP22 and MBP, are enhanced in nerves of Wt and TrJ mice upon IF intervention (Fig. 4A). These changes, however, are more pronounced in samples from neuropathic mice fed intermittently.
Figure 4. Steady-state expression of myelin proteins and cytosolic chaperones is enhanced by the dietary regimen.

A-B, Whole sciatic nerve lysates (20 μg/lane) from Wt and TrJ mice were probed with the indicated anti-myelin protein (P0, PMP22 and MBP), or anti-heat shock protein (Hsp) antibodies. GAPDH is shown as a protein loading control. Molecular mass in kDa, on left. C, On longitudinal sections of sciatic nerves from intermittently fed neuropathic animals, MBP-positive myelin internodes are prominent in IF mice (arrows). Co-staining the sections with anti-HSC70 antibody reveals increased expression of this cytosolic chaperone within Schwann cells (arrows). Nuclei are stained with Hoechst dye (blue). Samples from Wt mice are shown in the insets for comparison. Micron bar, 20 μm.
Heat shock proteins (HSPs) appear to play key roles in the folding and processing of myelin proteins (Homma et al., 2007; Rangaraju et al., 2008), and are known to respond to dietary modulation within the central nervous system (CNS) (Martin et al., 2006; Sharma and Kaur, 2007). Therefore, we examined the levels of heat shock protein 70 (HSP70) and 40 (HSP40), and the small chaperone αB-crystallin (Fig. 4B). In samples from Wt mice, the expression of protein chaperones is enhanced in response to IF, indicating that peripheral nerves respond to dietary modulation in a similar fashion as non-neural tissues and the CNS (Colotti et al., 2005; Martin et al., 2006). As we previously reported, the steady-state levels of cytosolic chaperones are elevated in nerves of neuropathic TrJ mice, likely due to their recruitment to protein aggregates (Fortun et al., 2003). In response to IF intervention, their levels are slightly further increased, particularly that of HSP70. To confirm the changes in myelin protein and chaperone expression, we double immunostained longitudinal sciatic nerve sections with rabbit anti-MBP and rat anti-HSC70 antibodies (Fig. 4C). In agreement with our previous report (Fortun et al., 2003) and the biochemical data (Fig. 4B), HSC70-like reactivity is low in nerve tissue from Wt animals, with a slight increase in response to the diet restriction (Fig. 4C, insets). In nerves from AL-fed TrJ mice, MBP-positive myelin tracks are sparse and the HSC70-like reactivity is slightly elevated compared to Wt (insets) (Fortun et al., 2003). In response to the dietary modulation, myelinated segments become prominent and HSC70-like immunoreactivity is noticeably enhanced within Schwann cells (arrows). We confirmed the increase in Hsp70-like reactivity by staining adjacent section with the same rabbit anti-Hsp70 antibody that was used for the Western blot (Fig. 4B).
While myelinated Schwann cells are postmitotic, they re-enter the cell cycle and proliferate due to ongoing demyelination and remyelination in nerves of CMT1A patients and rodent models (Notterpek and Tolwani, 1999; Atanasoski et al., 2002). In agreement with myelin defects in TrJ animals (Martini and Schachner, 1997; Amici et al., 2006), the neurotrophin receptor p75 and the mitotic marker phospho-histone H3 (pHH3) are strongly expressed (Fig. 5A). Strikingly, both of these indicators of Schwann cell dedifferentiation and demyelination are attenuated by the IF regimen (Fig. 5A). We also counted the number of Hoechst-positive nuclei per fixed nerve tissue area, and found a highly significant reduction in overall cell number in response to diet restriction (***p<0.001; Fig. 5B). Therefore, a five month IF regimen attenuates aberrant Schwann cell proliferation in nerves of neuropathic mice and promotes the maintenance of the differentiated, myelinated phenotype.
Figure 5. The IF regimen support the maintenance of the differentiated Schwann cell phenotype.
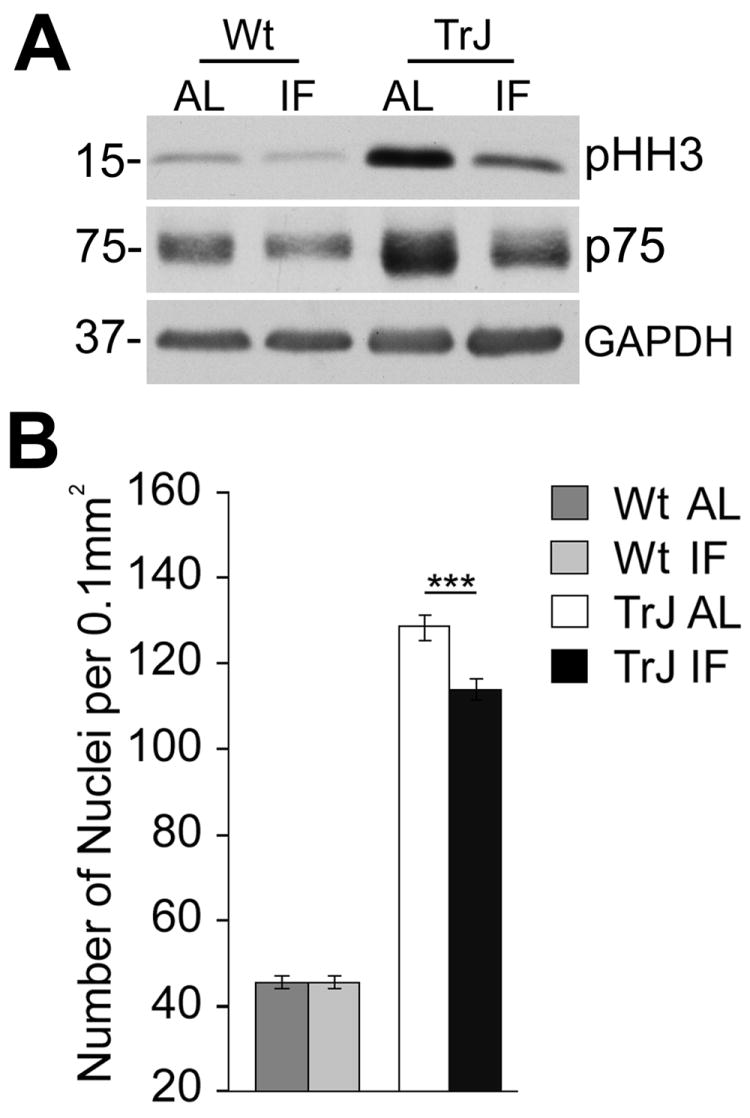
A, Whole sciatic nerve lysates (20 μg/lane) from Wt and TrJ mice were probed with anti-p75 and pHH3 antibodies. GAPDH is shown as a protein loading control. Molecular mass in kDa, on left. B, In neuropathic nerves on the IF regimen, the number of Schwann cell nuclei per fixed area of tissue is decreased significantly (***p<0.001 ANOVA, n=3 mice).
Protein degradation mechanisms are influenced by intermittent feeding
Given that disease-linked forms of PMP22 are retained within the Schwann cell cytosol and accumulate in ubiquitin-reactive protein aggregates (Nishimura et al., 1996; Hanemann et al., 2000; Sanders et al., 2001; Fortun et al., 2003), we asked whether this abnormality was affected by the dietary intervention. In peripheral nerves of Wt mice, PMP22-like immunoreactivity is detected along internodal myelin segments, and is consistent among samples from the AL and IF groups (Fig. 6, upper panels). As we have shown before (Fortun et al., 2003), in neuropathic nerves PMP22 is retained near the nuclei of Schwann cells, an abnormal location for this tetraspan myelin protein. In comparison, PMP22-positive internodal myelin segments and fewer Schwann cells with protein aggregates are detected in IF-fed TrJ mice (Fig. 6A, bottom panels). This localization of PMP22 is in agreement with the improved myelination observed in IF-fed neuropathic mice (Fig. 2-4). We next quantified PMP22-reactive protein aggregates per fixed area of nerve tissue and found that the dietary intervention reduces the frequency of protein aggregates by almost fifty percent (Fig. 6B). In agreement, the levels of poly-ubiquitinated proteins within nerve lysates from neuropathic mice are also reduced (Fig. 6C).
Figure 6. Fewer PMP22 aggregates within nerves of neuropathic mice on the IF regimen.

A, On longitudinal nerve sections from Wt mice fed AL or intermittently, PMP22 is detected along internodal segments of myelin (arrowheads). PMP22-containing protein aggregates are visible within neuropathic samples (arrows). Myelin-like PMP22 immunoreactivity is visible in nerves from neuropathic mice on the regimen (TrJ-IF) (arrowheads). Nuclei are stained with Hoechst dye (blue). Micron bar, 20 μm. B, The frequency of PMP22 protein aggregates is significantly reduced in the intermittently fed TrJ mice as compared to AL fed (***p<0.001 ANOVA, n=4; error bars represent SEM). C, Western blot analyses on total nerve lysates (20 μg/lane) reveal that the dietary regimen dramatically attenuates the accumulation of poly-ubiquitinated (pUbi) proteins in neuropathic samples, while mono-ubiquitinated (mUbi) substrate levels are maintained across genotype and diet. D, The levels of the autophagy pathway markers, Atg7, LC3 and p62 are shown. The lipidation of LC3, as visualized by the presence of LC3 II, is evident in samples from TrJ mice on the regimen. E, The expression of LAMP1 and cathepsin D is influenced by the genotype and stimulated by IF regimen. C-E, GAPDH is shown as a protein loading control. Molecular mass in kDa, on left.
A mechanism that responds to the accumulation of protein aggregates and can take part in their removal is the autophagy-lysosomal pathway (Fortun et al., 2003; Fortun et al., 2007; Sarkar et al., 2008). The expression of autophagy-associated proteins, Atg7 and LC3, are enhanced by the dietary intervention, while the steady state levels of p62, a protein that serves as a link between LC3 and ubiquitinated substrates (Klionsky et al., 2008), is reduced (Fig. 6D). The observed changes in the expression of these three molecules indicate that autophagy is enhanced within the nerves of IF neuropathic mice. The prominent autophagic response of neuropathic mice to the regimen, as compared to Wt, may reflect the already activated state of this pathway (Fortun et al., 2003). The degradation of autophagic cargo, such as protein aggregates, occurs within the lysosomes (Klionsky et al., 2008). Therefore, we examined the levels of LAMP1 and cathepsin D (CatD) within our samples (Fig. 6E). While there is a genotypic effect between Wt and TrJ mice (Notterpek et al., 1997), the dietary modulation further enhances the expression of these proteins, indicating elevated lysosomal activity. Together, these findings suggest that an induced autophagy-lysosomal pathway may contribute to the improved phenotype of TrJ neuropathic mice, likely by removing the misfolded, aggregation-prone mutated PMP22 (Tobler et al., 2002) and other poly-ubiquitinated proteins.
Attenuation of inflammatory aspects of the neuropathy by diet
Hereditary demyelinating neuropathies show overlapping pathology with inflammatory neuropathies, whereby affected nerves contain a higher number of macrophages (Misko et al., 2002; Wang Ip et al., 2006). To examine if the IF regimen impacts this aspect of the disease, we examined the presence of CD11b+ macrophages within the sciatic nerve (Fig. 7A). In agreement with a previous report (Misko et al., 2002), nerves of AL-fed TrJ animals contain numerous macrophages, however, their abundance decreases in intermittently fed mice. Quantification of three independent experiments reveals an over fifty percent reduction in CD11b+ cells in samples from TrJ mice on the dietary intervention, as compared to AL fed (***p<0.001 ANOVA; Fig. 6B). Furthermore, the levels of endogenous immunoglobulins are decreased (Fig. 6C), demonstrating that the IF regimen reduces the immunologic involvement in the neuropathy.
Figure 7. Immunologic markers in nerve samples from normal and neuropathic mice.

A, Longitudinal nerve sections from TrJ mice on the AL and IF regimen were immunostained with an anti-CD11b antibody. Nuclei are labeled with Hoechst dye (blue). Micron bar, 20 μm. B, Quantification of the number of CD11b positive cells in a fixed area of nerve tissue (***p<0.001 ANOVA, n=3). C, Western blot analysis of whole nerve lysates (20 μg/lane) with an anti-mouse IgG antibody is shown. The heavy (HC) and light chains (LC) of immunoglobulin G are shown. GAPDH serves as a protein loading control. Molecular mass in kDa, on left.
Discussion
The findings presented here reveal that enhancing endogenous protein homeostatic mechanisms within nerve tissue with a five month IF regimen significantly attenuates neuropathic behavioral and morphological phenotypes in a spontaneous mouse model of CMT1A. This dietary modulation is well-tolerated by TrJ mice and is associated with improved locomotor performance on the rotarod and increased grip strength. The benefits to the behavioral phenotype are paralleled by pronounced positive morphological changes within peripheral nerves, including increases in nerve fiber density, myelin thickness and axonal diameter. The dietary regimen partially alleviated the demyelinating phenotype of the neuropathy and supported the expression of myelin proteins. We observed a decrease in aberrant Schwann cell proliferation and in macrophage infiltration, both of which are characteristic features of CMT neuropathies (Misko et al., 2002; Wang Ip et al., 2006). Our studies indicate that the IF regimen enhanced multiple endogenous protective mechanisms within peripheral nerves, including the chaperone and the autophagy-lysosomal pathways, and prevented the inflammatory aspects and degenerative changes associated with the neuropathy.
While there have been major advances in understanding the genetics of hereditary neuropathies, treatment options for affected individuals are limited. The available transgenic and spontaneous animal models therefore serve as invaluable tools for studies aimed at understanding the cellular pathogenesis of the disease and to identify potential therapeutic targets. In a previous study, therapeutic administration of a progesterone antagonist between ages P5 and P49 in male PMP22 overexpressor transgenic rats reduced steady-state levels of PMP22 mRNA by 15%, which was sufficient to improve the disease phenotype (Sereda et al., 2003). In PMP22 overexpressor C22 mice (Huxley et al., 1996), ascorbic acid treatment between 2-5 months of age partially corrected the locomotor pathology by promoting the remyelination of axon fibers (Passage et al., 2004). The precise molecular mechanism by which this anti-oxidant improved the neuropathic phenotype is unknown but likely involved decreasing the expression level of PMP22 (Kaya et al., 2007). Ascorbic acid is now in clinical trials for CMT neuropathies (Pareyson et al., 2006). Finally, a ninety day treatment with curcumin improved the motor performance and the histopathology of TrJ mice, when initiated in newborns (Khajavi et al., 2007). Similar to our findings, these reports show that peripheral nerves of young mice respond to dietary supplements and modulations, although it is yet to be determined if these results will be reproducible in an older cohort of rodents. While the described regimen alleviates the neuropathy in affected mice, further studies will be required to determine how the human nervous system, particularly in patients with neurological diseases, may respond to long-term intermittent fasting.
We chose dietary restriction to alleviate the neuropathic phenotype of TrJ mice based on the ability of this approach to induce both chaperones and autophagy (Mizushima et al., 2004; Steinkraus et al., 2008), and knowledge concerning the subcellular trafficking of PMP22. The Wt PMP22, a substrate for proteasomal degradation, is an aggregation-prone hydrophobic protein with a low folding efficiency (Pareek et al., 1997; Notterpek et al., 1999; Sanders et al., 2001). When one copy of the gene is mutated, the pool of PMP22 destined for the proteasome is increased, which leads to protein aggregate formation (Fortun et al., 2003; Fortun et al., 2005; Fortun et al., 2006). The accumulation of misfolded PMP22 within Schwann cells interferes with the activity of the ubiquitin-proteasome system (Bence et al., 2001; Fortun et al., 2005), and serves as a nucleation site for the aggregation of protein chaperones, such as HSP70 and αB-crystallin (Ryan et al., 2002; Fortun et al., 2005; Fortun et al., 2007). The entrapment of chaperones and proteasomal constituents within protein aggregates alters protein metabolic networks that function to ensure proper folding of newly-synthesized proteins, as well as the rapid degradation of misfolded proteins and short-lived regulatory molecules. Studies from various organisms indicate that such gradual decrease in chaperones by their recruitment to protein aggregates does not induce an increase in chaperone synthesis (Soti and Csermely, 2003). Since myelinating Schwann cells are highly metabolically active, they are likely sensitive to such alterations which will impact their ability to maintain myelin homeostasis. Therefore, exogenous stimulation of chaperones to promote the folding and trafficking of PMP22 to the plasma membrane, and/or its redirection for proteasomal degradation may be a promising therapeutic avenue. Global induction of molecular chaperones can be achieved by brief exposure to stressful conditions, such as caloric restriction, or modulation of heat shock factor 1 (Morimoto et al., 1997). We recently reported that pharmacologic induction of the heat shock response improves myelin synthesis and the processing of PMP22 in organotypic explant cultures from neuropathic mice (Rangaraju et al., 2008). Similarly, here we show that increasing the endogenous levels of HSP70 within peripheral nerves is beneficial for myelination.
Autophagic protein degradation is an additional pathway that impacts the processing of PMP22 (Fortun et al., 2003; Fortun et al., 2007) and responds to dietary restriction (Mizushima et al., 2004). Our results show that the IF regimen is effective in stimulating the autophagy-lysosomal pathway in peripheral nerves, which is reflected by reduction in poly-ubiquitinated substrates and induction autophagic markers (Fig. 5). Compounds that stimulate autophagic protein degradation are under investigation and may provide benefits in a variety of protein misfolding disorders (Rubinsztein, 2006; Klionsky et al., 2008). Rapamycin, a specific inhibitor of mTOR kinase, has been shown to regulate autophagy in cells from yeast to human (Inoki et al., 2005) and can reduce the accumulation of poly-Q aggregates by routing the aggregates for lysosomal degradation via autophagy (Sarkar et al., 2008). Nonetheless, as combined stimulation of HSPs and autophagy appears to be more beneficial for the processing of PMP22 than chaperone or autophagic stimulation alone (Fortun et al., 2007), we chose the dietary approach which has the potential to activate both pathways. As our data reveals, this regimen has a pronounced influence on peripheral nerves and supported the maintenance of the differentiated Schwann cell phenotype (Fig. 3). We propose, that this benefit was a consequence of improved myelin stability and better axo-glial contacts, which is observed both biochemically and morphologically in TrJ mice on the regimen. Using the same study protocol, we detected similar morphological improvements in peripheral nerves of PMP22 overexpressor C22 mice (Huxley et al., 1996) on the IF regimen, however the behavioral assessments are less consistent (data not shown). Because of the greater variability in the behavioral phenotypes in C22 mice, future studies will require a larger cohort of mice and/or more sensitive measures of motor performance.
Dietary restrictions are effective in increasing longevity and preventing degenerative changes associated with disease (Colotti et al., 2005; Martin et al., 2006; Sharma and Kaur, 2007). For example, caloric restriction attenuated Aβ-deposition and decreased astrocytic activation in a transgenic rodent model of Alzheimer’s disease (Patel et al., 2005). The mechanism underlying these beneficial effects are unclear, but likely involved multiple molecular pathways of neuroprotection, including the stress response, increased production of neurotrophic factors, ketone bodies and changes in glucose/insulin signaling pathways (Martin et al., 2006). In comparison to the CNS, the influence of this intervention, particularly of the IF regimen, on peripheral nerve health and function has not been studied in detail. In a functional study, increased physical performance of mice on restricted diet was noted and attributed to behavior to avert risks to life (Ishihara et al., 2005). As in our Wt mice, the regimen did not induce significant changes in motor behavior (Fig. 1), the improvement of neuropathic mice are rather due to benefits to neural function. Since early transplantation studies with tissue from TrJ mice unequivocally established that the neuropathy is due to a primary disorder of Schwann cells (Aguayo et al., 1977; Perkins et al., 1981), we focused our study on peripheral nerve proteins. Besides the myelin, stress and immunologic mechanisms, we investigated possible changes in the expression of sirtuins and neurotrophins within the nerve tissue, both of which have been linked to caloric restriction (Cohen et al., 2004; Martin et al., 2006). Additional pathways that could have been influenced by the IF regimen include ER-associated protein quality control mechanisms, changes in protein translation and proteasomal protein degradation (CITE??). With the available samples however our results are inconclusive so far. Finally, besides the direct influence of IF on the nerves, skeletal muscle-derived factors, such as neurotrophins may also have contributed to the observed improvement. In future studies, we plan on exploring this possibility.
Our results clearly show biochemical, morphological and behavioral improvements in TrJ neuropathic mice in response to a five month long dietary restriction. The benefits of this regimen are mediated in part by increased expression heat shock and autophagy-lysosomal proteins within peripheral nerves. These results support further work in developing and identifying compounds that stimulate protein homeostatic mechanisms, particularly the chaperone and autophagy-lysosomal pathways for treatment of CMT1A and other protein aggregate-associated diseases.
Supplementary Material
Acknowledgments
These studies were supported in part by the National Muscular Dystrophy Association and US National Institutes of Health grants NS041012 to LN, CA095552 to WAD, and AG14979 and the Evelyn F. McKnight Brain Research Foundation to TCF. The authors wish to thank Debbie Akin for technical assistance with the morphological studies. We also thank Dr. Natalia Felitsyn for critical reading of the manuscript, and members of the Notterpek lab for helpful discussions.
Abbreviations used
- PMP22
peripheral myelin protein 22
- SCs
Schwann cells
- PNS
peripheral nervous system
- CMT
Charcot-Marie-Tooth disease
- Wt
wild-type
- TrJ
Trembler J
- SDS
sodium dodecyl sulfate
- FITC
fluoro-isothyoceine
- HSPs
heat shock proteins
- HSP70
heat shock protein 70
- LAMP1
lysosomal associated membrane protein 1
- MBP
myelin basic protein
- P0
protein zero
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aguayo AJ, Kasarjian J, Skamene E, Kongshavn P, Bray GM. Myelination of mouse axons by Schwann cells transplanted from normal and abnormal human nerves. Nature. 1977;268:753–755. doi: 10.1038/268753a0. [DOI] [PubMed] [Google Scholar]
- Amici SA, Dunn WA, Jr, Murphy AJ, Adams NC, Gale NW, Valenzuela DM, Yancopoulos GD, Notterpek L. Peripheral myelin protein 22 is in complex with alpha6beta4 integrin, and its absence alters the Schwann cell basal lamina. J Neurosci. 2006;26:1179–1189. doi: 10.1523/JNEUROSCI.2618-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anson RM, Guo Z, de Cabo R, Iyun T, Rios M, Hagepanos A, Ingram DK, Lane MA, Mattson MP. Intermittent fasting dissociates beneficial effects of dietary restriction on glucose metabolism and neuronal resistance to injury from calorie intake. Proc Natl Acad Sci U S A. 2003;100:6216–6220. doi: 10.1073/pnas.1035720100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Archelos JJ, Roggenbuck K, Schneider-Schaulies J, Toyka KV, Hartung HP. Detection and quantification of antibodies to the extracellular domain of P0 during experimental allergic neuritis. J Neurol Sci. 1993;117:197–205. doi: 10.1016/0022-510x(93)90174-w. [DOI] [PubMed] [Google Scholar]
- Atanasoski S, Scherer SS, Nave KA, Suter U. Proliferation of Schwann cells and regulation of cyclin D1 expression in an animal model of Charcot-Marie-Tooth disease type 1A. J Neurosci Res. 2002;67:443–449. doi: 10.1002/jnr.10133. [DOI] [PubMed] [Google Scholar]
- Bence NF, Sampat RM, Kopito RR. Impairment of the ubiquitin-proteasome system by protein aggregation. Science. 2001;292:1552–1555. doi: 10.1126/science.292.5521.1552. [DOI] [PubMed] [Google Scholar]
- Cohen HY, Miller C, Bitterman KJ, Wall NR, Hekking B, Kessler B, Howitz KT, Gorospe M, de Cabo R, Sinclair DA. Calorie restriction promotes mammalian cell survival by inducing the SIRT1 deacetylase. Science. 2004;305:390–392. doi: 10.1126/science.1099196. [DOI] [PubMed] [Google Scholar]
- Colotti C, Cavallini G, Vitale RL, Donati A, Maltinti M, Del Ry S, Bergamini E, Giannessi D. Effects of aging and anti-aging caloric restrictions on carbonyl and heat shock protein levels and expression. Biogerontology. 2005;6:397–406. doi: 10.1007/s10522-005-4906-z. [DOI] [PubMed] [Google Scholar]
- Court FA, Wrabetz L, Feltri ML. Basal lamina: Schwann cells wrap to the rhythm of space-time. Curr Opin Neurobiol. 2006;16:501–507. doi: 10.1016/j.conb.2006.08.005. [DOI] [PubMed] [Google Scholar]
- Crawley JN. Behavioral phenotyping of transgenic and knockout mice: experimental design and evaluation of general health, sensory functions, motor abilities, and specific behavioral tests. Brain Res. 1999;835:18–26. doi: 10.1016/s0006-8993(98)01258-x. [DOI] [PubMed] [Google Scholar]
- Fortun J, Dunn WA, Jr, Joy S, Li J, Notterpek L. Emerging role for autophagy in the removal of aggresomes in Schwann cells. J Neurosci. 2003;23:10672–10680. doi: 10.1523/JNEUROSCI.23-33-10672.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fortun J, Li J, Go J, Fenstermaker A, Fletcher BS, Notterpek L. Impaired proteasome activity and accumulation of ubiquitinated substrates in a hereditary neuropathy model. J Neurochem. 2005;92:1531–1541. doi: 10.1111/j.1471-4159.2004.02987.x. [DOI] [PubMed] [Google Scholar]
- Fortun J, Go JC, Li J, Amici SA, Dunn WA, Jr, Notterpek L. Alterations in degradative pathways and protein aggregation in a neuropathy model based on PMP22 overexpression. Neurobiol Dis. 2006;22:153–164. doi: 10.1016/j.nbd.2005.10.010. [DOI] [PubMed] [Google Scholar]
- Fortun J, Verrier JD, Go JC, Madorsky I, Dunn WA, Notterpek L. The formation of peripheral myelin protein 22 aggregates is hindered by the enhancement of autophagy and expression of cytoplasmic chaperones. Neurobiol Dis. 2007;25:252–265. doi: 10.1016/j.nbd.2006.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanemann CO, D’Urso D, Gabreels-Festen AA, Muller HW. Mutation-dependent alteration in cellular distribution of peripheral myelin protein 22 in nerve biopsies from Charcot-Marie-Tooth type 1A. Brain. 2000;123(Pt 5):1001–1006. doi: 10.1093/brain/123.5.1001. [DOI] [PubMed] [Google Scholar]
- Henry EW, Cowen JS, Sidman RL. Comparison of Trembler and Trembler-J mouse phenotypes: varying severity of peripheral hypomyelination. J Neuropathol Exp Neurol. 1983;42:688–706. doi: 10.1097/00005072-198311000-00008. [DOI] [PubMed] [Google Scholar]
- Homma S, Jin X, Wang G, Tu N, Min J, Yanasak N, Mivechi NF. Demyelination, astrogliosis, and accumulation of ubiquitinated proteins, hallmarks of CNS disease in hsf1-deficient mice. J Neurosci. 2007;27:7974–7986. doi: 10.1523/JNEUROSCI.0006-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huxley C, Passage E, Manson A, Putzu G, Figarella-Branger D, Pellissier JF, Fontes M. Construction of a mouse model of Charcot-Marie-Tooth disease type 1A by pronuclear injection of human YAC DNA. Hum Mol Genet. 1996;5:563–569. doi: 10.1093/hmg/5.5.563. [DOI] [PubMed] [Google Scholar]
- Inoki K, Ouyang H, Li Y, Guan KL. Signaling by target of rapamycin proteins in cell growth control. Microbiol Mol Biol Rev. 2005;69:79–100. doi: 10.1128/MMBR.69.1.79-100.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isaacs AM, Jeans A, Oliver PL, Vizor L, Brown SD, Hunter AJ, Davies KE. Identification of a new Pmp22 mouse mutant and trafficking analysis of a Pmp22 allelic series suggesting that protein aggregates may be protective in Pmp22-associated peripheral neuropathy. Mol Cell Neurosci. 2002;21:114–125. doi: 10.1006/mcne.2002.1158. [DOI] [PubMed] [Google Scholar]
- Ishihara H, Wenying F, Kouda K, Nakamura H, Kohno H, Nishio N, Sonoda Y. Effects of dietary restriction on physical performance in mice. J Physiol Anthropol Appl Human Sci. 2005;24:209–213. doi: 10.2114/jpa.24.209. [DOI] [PubMed] [Google Scholar]
- Jeronimo A, Jeronimo CA, Rodrigues Filho OA, Sanada LS, Fazan VP. Microscopic anatomy of the sural nerve in the postnatal developing rat: a longitudinal and lateral symmetry study. J Anat. 2005;206:93–99. doi: 10.1111/j.0021-8782.2005.00368.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaya F, Belin S, Bourgeois P, Micaleff J, Blin O, Fontes M. Ascorbic acid inhibits PMP22 expression by reducing cAMP levels. Neuromuscul Disord. 2007;17:248–253. doi: 10.1016/j.nmd.2006.12.008. [DOI] [PubMed] [Google Scholar]
- Khajavi M, Shiga K, Wiszniewski W, He F, Shaw CA, Yan J, Wensel TG, Snipes GJ, Lupski JR. Oral curcumin mitigates the clinical and neuropathologic phenotype of the Trembler-J mouse: a potential therapy for inherited neuropathy. Am J Hum Genet. 2007;81:438–453. doi: 10.1086/519926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klionsky DJ, Abeliovich H, Agostinis P, Agrawal DK, Aliev G, Askew DS, Baba M, Baehrecke EH, Bahr BA, Ballabio A, Bamber BA, Bassham DC, Bergamini E, Bi X, Biard-Piechaczyk M, Blum JS, Bredesen DE, Brodsky JL, Brumell JH, Brunk UT, Bursch W, Camougrand N, Cebollero E, Cecconi F, Chen Y, Chin LS, Choi A, Chu CT, Chung J, Clarke PG, Clark RS, Clarke SG, Clave C, Cleveland JL, Codogno P, Colombo MI, Coto-Montes A, Cregg JM, Cuervo AM, Debnath J, Demarchi F, Dennis PB, Dennis PA, Deretic V, Devenish RJ, Di Sano F, Dice JF, Difiglia M, Dinesh-Kumar S, Distelhorst CW, Djavaheri-Mergny M, Dorsey FC, Droge W, Dron M, Dunn WA, Jr, Duszenko M, Eissa NT, Elazar Z, Esclatine A, Eskelinen EL, Fesus L, Finley KD, Fuentes JM, Fueyo J, Fujisaki K, Galliot B, Gao FB, Gewirtz DA, Gibson SB, Gohla A, Goldberg AL, Gonzalez R, Gonzalez-Estevez C, Gorski S, Gottlieb RA, Haussinger D, He YW, Heidenreich K, Hill JA, Hoyer-Hansen M, Hu X, Huang WP, Iwasaki A, Jaattela M, Jackson WT, Jiang X, Jin S, Johansen T, Jung JU, Kadowaki M, Kang C, Kelekar A, Kessel DH, Kiel JA, Kim HP, Kimchi A, Kinsella TJ, Kiselyov K, Kitamoto K, Knecht E, et al. Guidelines for the use and interpretation of assays for monitoring autophagy in higher eukaryotes. Autophagy. 2008;4:151–175. doi: 10.4161/auto.5338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin B, Mattson MP, Maudsley S. Caloric restriction and intermittent fasting: two potential diets for successful brain aging. Ageing Res Rev. 2006;5:332–353. doi: 10.1016/j.arr.2006.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martini R, Schachner M. Molecular bases of myelin formation as revealed by investigations on mice deficient in glial cell surface molecules. Glia. 1997;19:298–310. [PubMed] [Google Scholar]
- McIlwain KL, Merriweather MY, Yuva-Paylor LA, Paylor R. The use of behavioral test batteries: effects of training history. Physiol Behav. 2001;73:705–717. doi: 10.1016/s0031-9384(01)00528-5. [DOI] [PubMed] [Google Scholar]
- Misko A, Ferguson T, Notterpek L. Matrix metalloproteinase mediated degradation of basement membrane proteins in Trembler J neuropathy nerves. J Neurochem. 2002;83:885–894. doi: 10.1046/j.1471-4159.2002.01200.x. [DOI] [PubMed] [Google Scholar]
- Mizushima N, Yamamoto A, Matsui M, Yoshimori T, Ohsumi Y. In vivo analysis of autophagy in response to nutrient starvation using transgenic mice expressing a fluorescent autophagosome marker. Mol Biol Cell. 2004;15:1101–1111. doi: 10.1091/mbc.E03-09-0704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morimoto RI, Kline MP, Bimston DN, Cotto JJ. The heat-shock response: regulation and function of heat-shock proteins and molecular chaperones. Essays Biochem. 1997;32:17–29. [PubMed] [Google Scholar]
- Nishimura T, Yoshikawa H, Fujimura H, Sakoda S, Yanagihara T. Accumulation of peripheral myelin protein 22 in onion bulbs and Schwann cells of biopsied nerves from patients with Charcot-Marie-Tooth disease type 1A. Acta Neuropathol. 1996;92:454–460. doi: 10.1007/s004010050546. [DOI] [PubMed] [Google Scholar]
- Notterpek L, Tolwani RJ. Experimental models of peripheral neuropathies. Lab Anim Sci. 1999;49:588–599. [PubMed] [Google Scholar]
- Notterpek L, Shooter EM, Snipes GJ. Upregulation of the endosomal-lysosomal pathway in the trembler-J neuropathy. J Neurosci. 1997;17:4190–4200. doi: 10.1523/JNEUROSCI.17-11-04190.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Notterpek L, Ryan MC, Tobler AR, Shooter EM. PMP22 accumulation in aggresomes: implications for CMT1A pathology. Neurobiol Dis. 1999;6:450–460. doi: 10.1006/nbdi.1999.0274. [DOI] [PubMed] [Google Scholar]
- Pareek S, Notterpek L, Snipes GJ, Naef R, Sossin W, Laliberte J, Iacampo S, Suter U, Shooter EM, Murphy RA. Neurons promote the translocation of peripheral myelin protein 22 into myelin. J Neurosci. 1997;17:7754–7762. doi: 10.1523/JNEUROSCI.17-20-07754.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pareyson D, Schenone A, Fabrizi GM, Santoro L, Padua L, Quattrone A, Vita G, Gemignani F, Visioli F, Solari A. A multicenter, randomized, double-blind, placebo-controlled trial of long-term ascorbic acid treatment in Charcot-Marie-Tooth disease type 1A (CMT-TRIAAL): the study protocol [EudraCT no.: 2006-000032-27] Pharmacol Res. 2006;54:436–441. doi: 10.1016/j.phrs.2006.09.001. [DOI] [PubMed] [Google Scholar]
- Passage E, Norreel JC, Noack-Fraissignes P, Sanguedolce V, Pizant J, Thirion X, Robaglia-Schlupp A, Pellissier JF, Fontes M. Ascorbic acid treatment corrects the phenotype of a mouse model of Charcot-Marie-Tooth disease. Nat Med. 2004;10:396–401. doi: 10.1038/nm1023. [DOI] [PubMed] [Google Scholar]
- Patel NV, Gordon MN, Connor KE, Good RA, Engelman RW, Mason J, Morgan DG, Morgan TE, Finch CE. Caloric restriction attenuates Abeta-deposition in Alzheimer transgenic models. Neurobiol Aging. 2005;26:995–1000. doi: 10.1016/j.neurobiolaging.2004.09.014. [DOI] [PubMed] [Google Scholar]
- Perkins CS, Aguayo AJ, Bray GM. Behavior of schwann cells from trembler mouse unmyelinated fibers transplanted into myelinated nerves. Exp Neurol. 1981;71:515–526. doi: 10.1016/0014-4886(81)90029-7. [DOI] [PubMed] [Google Scholar]
- Rangaraju S, Madorsky I, Pileggi JG, Kamal A, Notterpek L. Pharmacological induction of the heat shock response improves myelination in a neuropathic model. Neurobiol Dis. 2008 doi: 10.1016/j.nbd.2008.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubinsztein DC. The roles of intracellular protein-degradation pathways in neurodegeneration. Nature. 2006;443:780–786. doi: 10.1038/nature05291. [DOI] [PubMed] [Google Scholar]
- Ryan MC, Shooter EM, Notterpek L. Aggresome formation in neuropathy models based on peripheral myelin protein 22 mutations. Neurobiol Dis. 2002;10:109–118. doi: 10.1006/nbdi.2002.0500. [DOI] [PubMed] [Google Scholar]
- Sanders CR, Ismail-Beigi F, McEnery MW. Mutations of peripheral myelin protein 22 result in defective trafficking through mechanisms which may be common to diseases involving tetraspan membrane proteins. Biochemistry. 2001;40:9453–9459. doi: 10.1021/bi010894f. [DOI] [PubMed] [Google Scholar]
- Sarkar S, Ravikumar B, Floto RA, Rubinsztein DC. Rapamycin and mTOR-independent autophagy inducers ameliorate toxicity of polyglutamine-expanded huntingtin and related proteinopathies. Cell Death Differ. 2008 doi: 10.1038/cdd.2008.110. [DOI] [PubMed] [Google Scholar]
- Sereda M, Griffiths I, Puhlhofer A, Stewart H, Rossner MJ, Zimmerman F, Magyar JP, Schneider A, Hund E, Meinck HM, Suter U, Nave KA. A transgenic rat model of Charcot-Marie-Tooth disease. Neuron. 1996;16:1049–1060. doi: 10.1016/s0896-6273(00)80128-2. [DOI] [PubMed] [Google Scholar]
- Sereda MW, Meyer zu Horste G, Suter U, Uzma N, Nave KA. Therapeutic administration of progesterone antagonist in a model of Charcot-Marie-Tooth disease (CMT-1A) Nat Med. 2003;9:1533–1537. doi: 10.1038/nm957. [DOI] [PubMed] [Google Scholar]
- Sharma S, Kaur G. Intermittent dietary restriction as a practical intervention in aging. Ann N Y Acad Sci. 2007;1114:419–427. doi: 10.1196/annals.1396.031. [DOI] [PubMed] [Google Scholar]
- Soti C, Csermely P. Aging and molecular chaperones. Exp Gerontol. 2003;38:1037–1040. doi: 10.1016/s0531-5565(03)00185-2. [DOI] [PubMed] [Google Scholar]
- Steinkraus KA, Smith ED, Davis C, Carr D, Pendergrass WR, Sutphin GL, Kennedy BK, Kaeberlein M. Dietary restriction suppresses proteotoxicity and enhances longevity by an hsf-1-dependent mechanism in Caenorhabditis elegans. Aging Cell. 2008;7:394–404. doi: 10.1111/j.1474-9726.2008.00385.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suter U, Snipes GJ. Biology and genetics of hereditary motor and sensory neuropathies. Annu Rev Neurosci. 1995;18:45–75. doi: 10.1146/annurev.ne.18.030195.000401. [DOI] [PubMed] [Google Scholar]
- Suter U, Moskow JJ, Welcher AA, Snipes GJ, Kosaras B, Sidman RL, Buchberg AM, Shooter EM. A leucine-to-proline mutation in the putative first transmembrane domain of the 22-kDa peripheral myelin protein in the trembler-J mouse. Proc Natl Acad Sci U S A. 1992;89:4382–4386. doi: 10.1073/pnas.89.10.4382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobler AR, Liu N, Mueller L, Shooter EM. Differential aggregation of the Trembler and Trembler J mutants of peripheral myelin protein 22. Proc Natl Acad Sci U S A. 2002;99:483–488. doi: 10.1073/pnas.012593399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valentijn LJ, Baas F, Wolterman RA, Hoogendijk JE, van den Bosch NH, Zorn I, Gabreels-Festen AW, de Visser M, Bolhuis PA. Identical point mutations of PMP-22 in Trembler-J mouse and Charcot-Marie-Tooth disease type 1A. Nat Genet. 1992;2:288–291. doi: 10.1038/ng1292-288. [DOI] [PubMed] [Google Scholar]
- Wang Ip C, Kroner A, Fischer S, Berghoff M, Kobsar I, Maurer M, Martini R. Role of immune cells in animal models for inherited peripheral neuropathies. Neuromolecular Med. 2006;8:175–190. doi: 10.1385/nmm:8:1-2:175. [DOI] [PubMed] [Google Scholar]
- Wohlgemuth SE, Julian D, Akin DE, Fried J, Toscano K, Leeuwenburgh C, Dunn WA., Jr Autophagy in the heart and liver during normal aging and calorie restriction. Rejuvenation Res. 2007;10:281–292. doi: 10.1089/rej.2006.0535. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


