Abstract
The systemic rotenone model of Parkinson's disease (PD) accurately replicates many aspects of the pathology of human PD and has provided insights into the pathogenesis of PD. The major limitation of the rotenone model has been its variability, both in terms of the percentage of animals that develop a clear-cut nigrostriatal lesion and the extent of that lesion. The goal here was to develop an improved and highly reproducible rotenone model of PD. In these studies, male Lewis rats in three age groups (3, 7 or 12-14 months) were administered rotenone (2.75 or 3.0 mg/kg/day) in a specialized vehicle by daily intraperitoneal injection. All rotenone-treated animals developed bradykinesia, postural instability, and/or rigidity, which were reversed by apomorphine, consistent with a lesion of the nigrostriatal dopamine system. Animals were sacrificed when the PD phenotype became debilitating. Rotenone treatment caused a 45% loss of tyrosine hydroxylase-positive substantia nigra neurons and a commensurate loss of striatal dopamine. Additionally, in rotenone-treated animals, α-synuclein and poly-ubiquitin positive aggregates were observed in dopamine neurons of the substantia nigra. In summary, this version of the rotenone model is highly reproducible and may provide an excellent tool to test new neuroprotective strategies.
Keywords: Parkinson's disease, rotenone, neurodegeneration, dopamine, postural instability, behavior
Introduction
Neurodegenerative diseases are difficult to model in vitro and in vivo. While many models successfully reproduce certain features of the disease, their predictive value for neuroprotective interventions is uncertain, and the number of relevant outcome variables that can be assessed is limited. Therefore, there is a pressing need for improvement of current models and development of new models.
Parkinson's disease (PD) is the most common neurodegenerative movement disorder. It is characterized clinically by bradykinesia, resting tremor, rigidity and postural instability. Pathological features of PD include loss of dopamine neurons in the substantia nigra and the presence of Lewy bodies in surviving dopamine neurons (Forno, 1996). Familial PD accounts for about 10% of cases, while ‘sporadic’ or ‘idiopathic’ PD accounts for the rest. While the causes of sporadic PD remain unknown, epidemiological studies suggest that exposures to pesticides, metals and solvents are contributing risk factors (Di Monte, 2001; Fall et al., 1999; Gash et al., 2008; Gorell et al., 1997; Gorell et al., 1998; Hageman et al., 1999; Kuhn et al., 1998; Liou et al., 1997; Pezzoli et al., 1996; Semchuk et al., 1993). In general, most sporadic cases are believed to stem from a combination of environmental exposures and individual genetic susceptibility.
Several useful animal models of PD exist, each with its own advantages and disadvantages. The ideal model, one that recapitulates all of the relevant clinical and pathological features of the disease and is predictive of successful neuroprotective regimens in humans does not yet exist. Nevertheless, the rotenone model of PD provides certain advantages in modeling the pathogenesis of PD (Betarbet et al., 2000). In this model, systemic inhibition of mitochondrial complex I produces selective degeneration of the nigrostriatal dopamine system and reproduces key pathological features of clinical PD. Indeed, rotenone administration affects many of the pathogenic pathways including: oxidative stress, alpha-synuclein phosphorylation and aggregation and Lewy pathology, DJ-1 acidification and translocation, proteasomal dysfunction and nigral iron accumulation (Betarbet et al., 2006). While now several years old, the model is still relatively new compared to the rodent 6-hydroxydopamine (6-OHDA) and 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP models), both of which are still undergoing refinement in an attempt to more closely model the clinical disease (Fleming et al., 2005; Ohashi et al., 2006; Rojo et al., 2006; Tadaiesky et al., 2008). The main limitations of the rotenone model have been variability (i) in the percentage of animals that develop a nigrostriatal dopaminergic lesion, (ii) lesion magnitude, and (iii) lesion location and distribution within the striatum. Most commonly, rotenone has been delivered intravenously or subcutaneously, using osmotic pumps. However, chronic daily intraperitoneal injection of rotenone in a natural oil has been reported to elicit L-DOPA-responsive locomotor deficits and neurochemical abnormalities characteristic of PD (Alam and Schmidt, 2002; Alam and Schmidt, 2004). In this regimen, mortality was high (Biehlmaier et al., 2007)) and its histological features have not been described.
The goal of this study was to develop and characterize a reproducible, consistent rotenone model of PD. Here we present the experimental details of the model and characterization of its histological, neurochemical, and behavioral features.
Methods
Animals and supplies
Male Lewis rats were used for all experiments (Hilltop Lab Animals, Inc., Scottdale, PA, USA). Animals were utilized at the following age groups: ‘young adult’ (∼3 months), ‘adult’ (∼7 months) and ‘middle-aged’ (12-14 months). All chemicals were obtained from Sigma-Aldrich (St. Louis, MO, USA) unless otherwise noted. All experiments utilizing animals were approved by the Institutional Animal Care and Use Committee of the University of Pittsburgh.
Dose and frequency determination
Pilot studies were conducted with lower doses (2.0-2.5 mg/kg/day; dissolved in 100% Miglyol 812) to determine the optimal rotenone dose. To determine the optimal rotenone dosing frequency, systemic complex I inhibition was calculated. Male Lewis rats (n = 3 per time-point) were injected with rotenone (2.0 mg/kg, i.p.) and sacrificed at 0, 4, 8, 16, or 32 h after injection. Liver mitochondria were then isolated from rats using differential centrifugation described in the manufacturers protocol (MitoProfile® Benchtop Mitochondria Isolation Kit for Tissue, MitoSciences). Complex I activity was measured in rat liver mitochondria using a protocol adapted from (Janssen et al., 2007). Briefly, liver mitochondrial protein (15μg) was incubated in assay buffer (25 mM potassium phosphate buffer pH 7.8, 3.5 g/L BSA, 70μM DCIP, 6 μM decylubiquinone, 1μM Antimycin A) at 37°C. The reaction was started by adding NADH (200μM). Electron transfer from oxidation of NADH to decylubiquinone to the final electron acceptor 2,6-dichloroindophenol (DCIP) is followed by measuring DCIP reduction spectrophotometrically at 600nm). DCIP reduction was recorded for 20 min. Rotenone (1 μM) sensitive complex I activity was normalized to background DCIP reduction and expressed as μg of DCIP reduced/min/μg of protein.
Rotenone treatment
The rotenone solution was first prepared as a 50× stock in 100% dimethylsulfoxide (DMSO) and diluted in medium-chain triglyceride, Miglyol 812 N (Sasol North America, Inc., Houston, TX, USA; distributed by Warner Graham, Baltimore MD, USA) to obtain a final concentration of 2.75 or 3.0 mg/mL rotenone in 98% Miglyol 812 N, 2% DMSO. Vortexing the solution creates a stable emulsion of the DMSO containing rotenone and Miglyol 812N. The solution was made fresh 2-3 times/week and stored in an amber septa vial protected from light and inverted several times before each injection to eliminate the possibility of settling. The solution was administered at 1 mL/kg and control animals received the vehicle only. Experimental groups were comprised of at least 5-7 animals, with additional animals in the young-adult group for behavioral testing. Rotenone treatment of the three different age groups occurred at different times.
Experimental endpoints
Permanent bilateral denervation of the nigrostriatal dopamine system reproduces the essential motor features of the clinical PD. However, deficits associated with severe dopamine depletion can become debilitating (Cenci et al., 2002; Ungerstedt, 1971). Therefore, animals were monitored twice daily for the appearance of parkinsonian features, including bradykinesia, postural instability/gait disturbances, and rigidity. When the behavioral phenotype became debilitating-limiting mobility, feeding or grooming - animals were euthanized by CO2 exposure. Brains were rapidly removed and bisected mid-sagittally. One hemisphere was post-fixed in 4% paraformaldehyde for 7 days and then placed in 30% sucrose for at least 3 days, until infiltration was complete. The striatum from the other half of the brain was dissected on ice, flash frozen and then stored at -80°C until processed for neurochemical analysis. Alternatively, for brains wholly dedicated to histological analysis (adult animals), some animals were perfusion-fixed, first with 50 mL ice-cold 0.1 M PBS, and then with 250-350 mL 4% paraformaldehyde. These brains were then post-fixed overnight and transferred to 30% sucrose, where they were stored until fully infiltrated and further processed.
Neurobehavioral analysis
During daily handling, animals were assessed for the emergence of a PD phenotype, which included bradykinesia, postural instability/unsteady gait and rigidity. For all quantified behavioral tests, the experimenter was blinded until the analysis was complete.
Rearing behavior
When placed in a clear cylinder, rats will engage in exploratory behavior, including rearing (Fleming et al., 2004; Schallert and Tillerson, 2000). During rearing, behavior, the forelimbs will contact the wall of the cylinder. For this test, the rat was placed in a clear plexiglass cylinder (height = 30 cm, diameter = 20 cm) for 5 minutes. The test was conducted under low red-light conditions (10 lux), video-recorded, and during video playback, the number of rears was quantified. To be classified as a rear, the animal had to raise forelimbs above shoulder level and make contact with the cylinder wall with either one or both forelimbs. Removal of both forelimbs from the cylinder wall and contact with the table surface was required before another rear was scored. This test has been successfully used previously to assess behavioral deficits in the rats receiving subcutaneous or intravenous rotenone (Fleming et al., 2004).
Postural instability test
Postural instability is a hallmark feature of PD. Recently a test to quantify postural instability was developed in a unilateral 6-OHDA rat PD model (Woodlee et al., 2008). In the current study, the test was conducted as previously reported, except that both forelimbs were averaged together because rotenone produces bilateral symptoms. Briefly, the animal was held vertically, while one forelimb was allowed to contact the table surface, which was lined with medium-grit sand paper. The animal's center of gravity was then advanced until the animal initiated a “catch-up” step. The displacement distance required for the animal to regain the center of gravity was recorded. At each time-point 3 trials for each forelimb were recorded and the average reported.
Apomorphine challenge
To determine if observed behavioral deficits were dopamine-dependent, the dopamine agonist apomorphine was administered (1 mg/kg, sc) and behavioral tests were repeated. Apomorphine has previously been found to ameliorate hypokinesia, rigidity and tremor in a bilateral 6-OHDA (a specific dopaminergic neurotoxin) model (Jolicoeur et al., 1991). The rearing trial was conducted 5 minutes after apomorphine administration and the postural instability test was conducted after 10 minutes.
Immunohistochemistry
Brains were cut coronally on a frozen sliding microtome at 35 μm and stored in cryoprotectant at -20°C until use. Sections used for single-chromagen development were removed from cryoprotectant, washed in PBS 6× for 5 min each then treated with 3% hydrogen peroxide for 10 minutes followed by 3 PBS washes and blocked for 1 hour in 10% normal donkey serum, containing 0.3% triton X100. For stereological assessment of dopaminergic neurons, the sections were incubated in a primary antibody for tyrosine hydroxylase (1:3000, mouse anti-tyrosine hydroxylase; Millipore #MAB318; Bilerica, MA, USA) for 72 hours at 4°C plus 2 hours at room temperature to obtain optimal antibody penetration. The sections were then washed in PBS 3× 5 min and incubated at room temperature for 1 hour in biotinylated donkey anti-mouse secondary antibody (1:200, Jackson Immuno Research; West Grove, PA, USA). The sections were then washed 3× in PBS and incubated in Advidin-Biotin Complex solution (Vectastain, Vector Labs; Burligame, CA, USA). After washing in PBS, the sections were developed using DAB (Vector Labs). Sections used for stereological analysis, were then washed 3 times in PBS and mounted directly onto slides using Aquamount (Lerner Labs, Pittsburgh, PA, USA). For sections used for general histology, the tissue was mounted onto slides after staining, air dried, dehydrated using graded alcohols and Histoclear (National Diagnostics, Atlanta, GA, USA), followed by mounting with Histomount (National Diagnostics). For immunofluorescence, the staining was done as above except using primary antibodies for α-synuclein (AB5334P, Millipore, Billerica, MA, USA, 1:3000), poly-ubiquitin (Biomol, Plymouth Meeting, PA, USA, PW8805, 1:500), tyrosine hydroxylase, dopamine and cAMP-regulated phosphoprotein (DARPP-32; AB1656, Millipore, 1:500)or pSer19-tyrosine hydroxylase (AB5425, Millipore, 1:3000) and one of the following fluorescent secondary antibodies was used: Cy3-conjugated anti-mouse (Jackson Immuno), Cy5-conjugated anti-rabbit (Jackson Immuno), Alexa 488-conjugated anti-sheep (Molecular Probes) (1:500). These sections were then washed and mounted using gelvatol mounting media. To remove unaggregated protein, some sections were pretreated with proteinase K (2.5 μg/mL, 10 minutes) or formic acid (100%, 10 minutes) before immunohistochemistry.
Unbiased stereology
Cell counting was performed by an experimenter blinded to the treatment group using Stereo Investigator (MBF Bioscience, Williston, VT, USA). One in six sections was used to estimate the number of TH neurons in the entire substantia nigra (including the reticulata portion) of one hemisphere (middle-aged animals). Cells that were clearly stained for TH with a visible nucleus were counted. The counting frame was 45 × 45 × 13 μm (height × width × dissector height) and the sampling grid was 125 × 125 μm. The average final section thickness was 16.1 um, making the average guard zone 1.5 μm for the top and bottom of the section (the dissector was placed in the middle of the section). Stereological parameters were optimized based on a pilot study in both control and treated animals to produce a rigorous estimate of nigral dopamine neurons. The CE Gunderson (m = 1) values were < 0.1 for all animals.
Neurochemistry
Striatal dopamine and metabolites were assessed for young-adult and middle-aged animals. Adult animals were perfused-fixed for optimal histology and neurochemistry for this group was not assessed. Striatal tissue samples were sonicated on-ice in 0.1 M perchloric acid and centrifuged for 30 min at 16,100 × g. The supernatant was then collected and filtered in Costar Spin-X 0.22 μM nylon membrane polypropylene centrifuge tubes at 1,000 × g and then stored at -80°C. The supernatant was then injected into a Waters 2695 HPLC separation module (Waters Corp., Milford, MA, USA). The samples were injected using an autosampler within the module and the sample temperature was 4 °C. The HPLC mobile phase consisted of 0.06 M sodium phosphate monobasic, 0.03 M citric acid, 8% methanol, 1.075 mM 1-octanesulfonic acid, 0.1 mM ethylenediaminetetraacetic acid, 2 mM sodium chloride, pH 3.5. The flow rate was 1.0 mL/minute. Neurotransmitters were separated on a Waters XBridge C18 4.6 × 150 mm column with a 3.5 μm particle size and detected on a Waters 2465 electrochemical detector with a glassy carbon electrode set at 750 mV referenced to an ISAAC electrode. The separation and detection occurred at 28 °C. Neurotransmitters were quantified using a standard curve generated from injection of standards of the highest available purity.
Statistical analyses
For comparison of survival curves, the log-rank test was used. Two groups of data where analyzed by the Student's t-test. Behavioral comparisons of pre- and post-apomorphine data were analyzed by a paired Student's t-test. Three groups of data were analyzed by ANOVA with a Tukey post hoc test, when the ANOVA reached significance. Three groups of data for preplanned comparisons were analyzed by student's t-test with Bonferroni correction. For all tests, p<0.05 was deemed significant.
Results
Development of parkinsonian symptoms
In a pilot study lower doses (2.0-2.5 mg/kg/day) for up to 60 days produced subtle behavioral features, without progression to a severe debilitating phenotype. Data from animals treated at 2.75 or 3.0 mg/kg.day are reported here. A systemic complex I inhibition assay, conducted at 2.0 mg/kg confirmed that rotenone dosing once per 24 hours was appropriate (maximum inhibition at 8 hours and recovery by 32 hours; Supplementary Figure 1). Rotenone treatment caused moderate weight loss which was similar in magnitude across age groups (Figure 1A-C). All chronically treated animals (2.75 and 3.0 mg/kg/day) experienced parkinsonian symptoms (excluding those with injection-related mortality). Additionally, all animals eventually exhibited a severe and debilitating PD-phenotype. None of the vehicle-treated controls exhibited parkinsonian symptoms. Animals were euthanized when bradykinesia, postural instability/gait disturbances and rigidity became debilitating. The time-course of the behavioral phenotype was influenced by both age and rotenone dose, and the dose effect was significant in adult rats [Figure 1D-F; median survival time: 6 vs. 10 days after rotenone treatment; 2.75 (n = 6) vs. 3.0 (n = 6) mg/kg/day, p<0.05 log rank test]. Young-adult animals exhibited the most variability in time from beginning rotenone treatment to onset of severe parkinsonian symptoms. In general, older (“middle-aged”) animals appeared to be more sensitive to rotenone than younger animals as evidenced by more rapid development of the lesion (Figure 1D-F).
Figure 1.
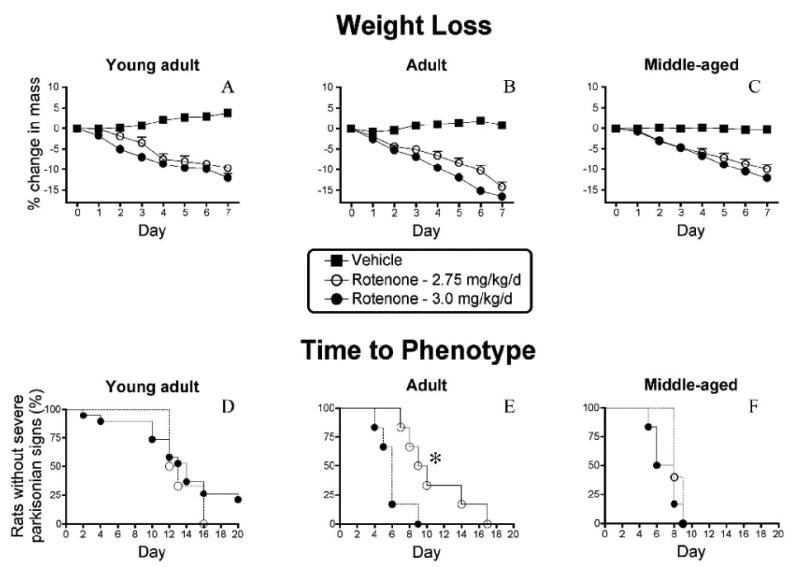
Daily intraperitoneal rotenone elicits moderate weight loss and a parkinsonian phenotype. A-C.,Percent change in mass from day 0 in (A) young-adult rats (∼ 3 months old; control, n = 13; 2.75 mg/kg/day, n = 7; 3.0, n = 19), (B) adult rats (∼7 months; control, n = 5; 2.75 mg/kg/day, n = 7; 3.0, n = 6) and (C) middle-aged rats (12-14 months, control, n = 5; 2.75 mg/kg/day, n = 5; 3.0, n = 6) treated with rotenone at 2.75 mg/kg/day (empty circles), 3.0 mg/kg/day (filled circles) and control rats (filled squares). D-F, Survival curves (development of debilitating PD phenotype characterized by severe bradykinesia, rigidity, and postural instability) of young-adult (D), adult (E) and middle-aged (F) rats treated with rotenone (2.75 or 3.0 mg/kg/day) and control rats. Animals were euthanized when severe parkinsonian symptoms developed. Mortality occurring immediately after the injection (in the absence of parkinsonian symptoms and typically <10%) was excluded from analysis. Note that the percent mass change is relatively similar for all age groups at specific time-points and dosages. Young-adult animals exhibit the most variability in temporal development of parkinsonian phenotype. Adult animals exhibit a dose-dependent response to rotenone (* = p<0.05, Log rank test for trend). Middle-aged animals also appear to be the most sensitive to rotenone.
Mortality
Mortality occurring within minutes of an injection likely resulted from needle misplacement or settling of rotenone (i.e., accidental overdose). Careful needle placement and vortexing between each injection significantly reduced such mortality. Animals that died within minutes of an injection and that did not experience parkinsonian symptoms were excluded from analysis. Overall mortality related to the injection was low (∼ 10% for all treatment groups), was not dose dependent, and decreased with each cohort, as technique improved (vortexing of solution between each injection and the use of 2% DMSO to ensure solubility). Unanticipated mortality from parkinsonian symptoms was eliminated by carefully monitoring animals twice daily; rats were euthanized when the phenotype became debilitating. Severe dopamine depletion (>95%) in the unilateral 6-hydroxydopamine model produces spontaneous behavioral deficits that are apparent with careful examination (Cannon et al., 2005; Cannon et al., 2006). However, bilateral dopamine depletion at even a more modest magnitude (>80%) significantly decreases spontaneous activity and severe (>95%) bilateral depletion can be debilitating causing akinesia, adipsia, and aphagia (Cenci et al., 2002; Deumens et al., 2002; Sakai and Gash, 1994; Schallert et al., 1978; Ungerstedt, 1971). Indeed, once the parkinsonian phenotype presented, it was progressive in nature and eventually became debilitating in all animals.
Behavioral deficits
Daily intraperitoneal rotenone produced progressive behavioral deficits (Figure 2). Rotenone-treated animals exhibited postural instability (Figure 2A), which was significant when assessed after 6 days of treatment (p<0.001, student's t-test; rotenone: n = 13, control: n=7) and after 9 days (p<0.001). These motor deficits were responsive to the dopamine receptor agonist apomorphine (Figure 2B). Postural instability decreased with apomorphine after 6 days of rotenone treatment (rotenone-treated vs. rotenone-treated + apomorphine; p<0.001, paired student's t-test) and after 9 days (p<0.01).
Figure 2.
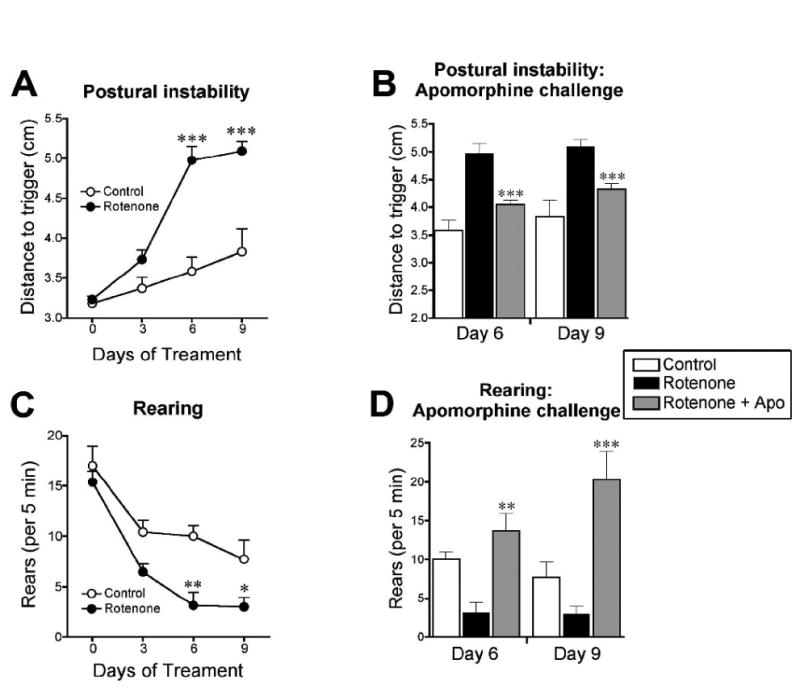
Neurobehavioral assessment of rotenone-treated rats. Daily intraperitoneal rotenone (3.0 mg/kg/day) in young-adult rats elicits progressive motor deficits that are responsive to a dopamine agonist. A, Postural instability test at pretest, and at 3, 6, and 9 days of rotenone treatment (empty circles represent control, n = 7; filled circles represent rotenone-treated, n = 13). ***p<0.001, Student's t-test. B, Postural instability test before and after apomorphine (1 mg/kg) challenge. ***p<0.001, paired Student's t-test (pre-apomorphine versus post-apomorphine). C, Rearing behavior (# of rears per 5 min). *p<0.05, **p<0.01, Student's t-test. D, Rearing before and after apomorphine challenge. **p<0.01, ***p<0.001, paired Student's t-test (pre-apomorphine versus post-apomorphine).
Rotenone also decreased rearing behavior (# of rears per 5 min), reaching significance after 6 days of rotenone treatment [3 ± 1 (n = 13) vs. 10 ± 1 (n = 7); **p<0.01, rotenone vs. control; rears ± S.E.M] and after 9 days (3 ± 1 vs. 8 ± 2; *p<0.05). These deficits were also reversed by apomorphine after 6 days of rotenone treatment [3 ± 1 (n = 13) vs. 14 ± 2 (n = 13); rotenone-treated vs. rotenone-treated + apomorphine; **p<0.01, paired student's t-test] and after 9 days (3 ± 1 vs. 20 ± 4; ***p<0.001).
Loss of dopamine terminals
Daily intraperitoneal rotenone administration (3.0 mg/kg/day) elicits a lesion of the dorsolateral striatal dopamine terminals (Figure 3). The lesions were strikingly similar in size and distribution across animals. High power magnification revealed the presence of hypertrophied (presumably dysfunctional) dopaminergic terminals (Figure 4), both near the lesion edge and also in the dorsolateral striatum of animals that received a lower dose of rotenone (2.75 mg/kg/day) and that had no overt lesion. The lesion was highly selective for dopamine terminals as there was no damage to striatal cell bodies in regions where there was loss of tyrosine hydroxylase positive terminals (Figure 5A-F; cresyl violet staining). Striatal DARPP-32 staining also indicates that there is no overt loss of striatal dopamine-receptive neurons or morphological changes in this cell population (Figure 6). Indeed, high magnification images indicate that in rotenone treated animals (Fig 5Fi), there is no evidence of morphological alterations of striatal neurons or infiltration of inflammatory cells compared to controls (Fig 5Ci).
Figure 3.
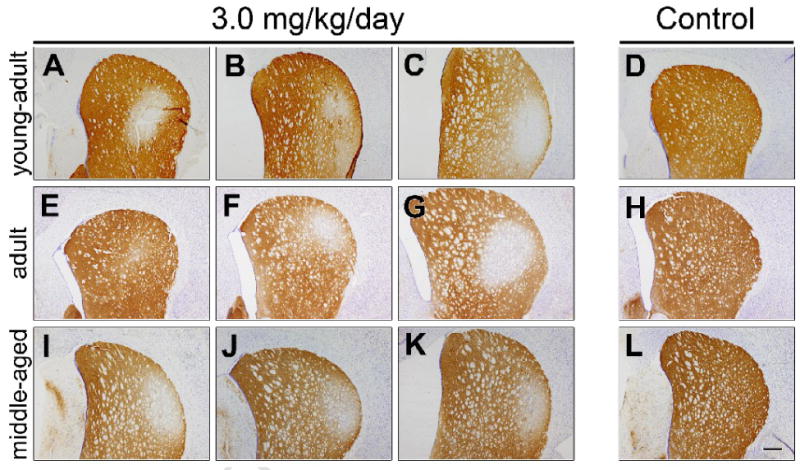
Intraperitoneal rotenone elicits dopamine terminal loss in the dorsolateral striatum. Representative images (from 3 treated animals in each group) of striatal tyrosine hydroxylase immunohistochemistry in young-adult (A-D), adult (E-H), and middle-aged (I-L) rats after daily intraperitoneal rotenone (3.0 mg/kg/day, A-C, E-G, I-K) or vehicle (D,H,L). Note that the lesions are strikingly similar in magnitude and localization across animals, particularly in the middle-aged animals. Bar = 500 μm.
Figure 4.
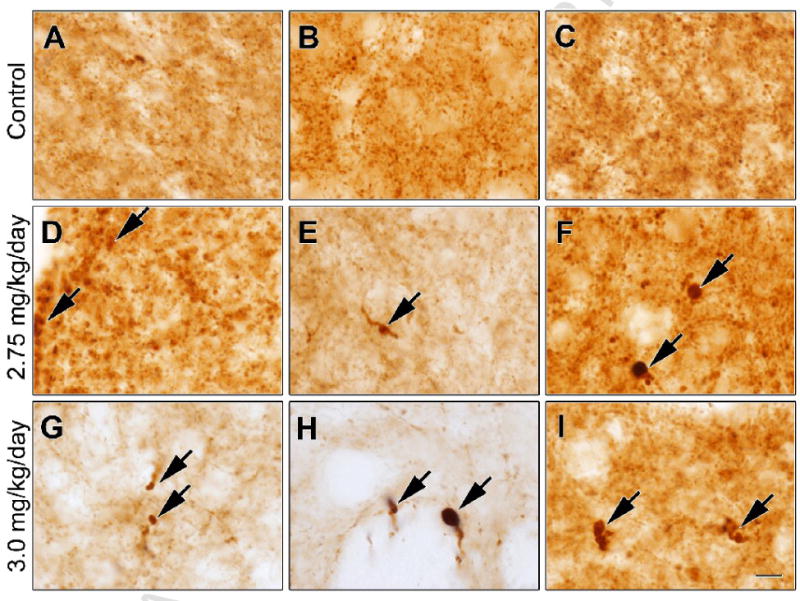
Rotenone elicits striatal dopamine terminal hypertrophy. Representative high magnification images of dorsolateral striatal tyrosine hydroxylase immunohistochemistry in vehicle (A-C) or rotenone [2.75 mg/kg/day (D-F), 3.0 mg/kg/day (G-I)] treated middle-aged animals. Arrows indicate hypertrophied and likely dysfunctional dopaminergic terminals. Hypertrophied terminals were present in all treated animals, both near the edge of the lesion in animals treated at 3.0 mg/kg and also in animals treated at 2.75 mg/kg rotenone, without an overt nigrostriatal lesion. Bar = 10 μm.
Figure 5.
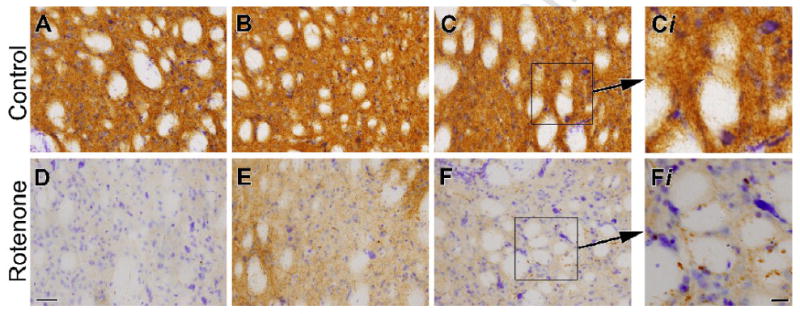
Sparing of striatal neurons after intraperitoneal rotenone. Images show Nissl staining and tyrosine immunohistochemistry in the dorsolateral striatum of control (A-C) and rotenone-treated middle-aged rats (3.0 mg/kg/day; D-F). Rotenone-treated animals exhibit a striking loss of striatal tyrosine hydroxylase immunoreactivity, while striatal cell bodies are spared, as evidenced by persistence of Nissl stained striatal neurons. Bar = 50 μm. High magnification inserts (Ci and Fi) show no alterations in striatal cell morphology or evidence of inflammatory cell infiltration in rotenone treated (Fi) compared to control (Ci). Bar = 25 μm.
Figure 6.
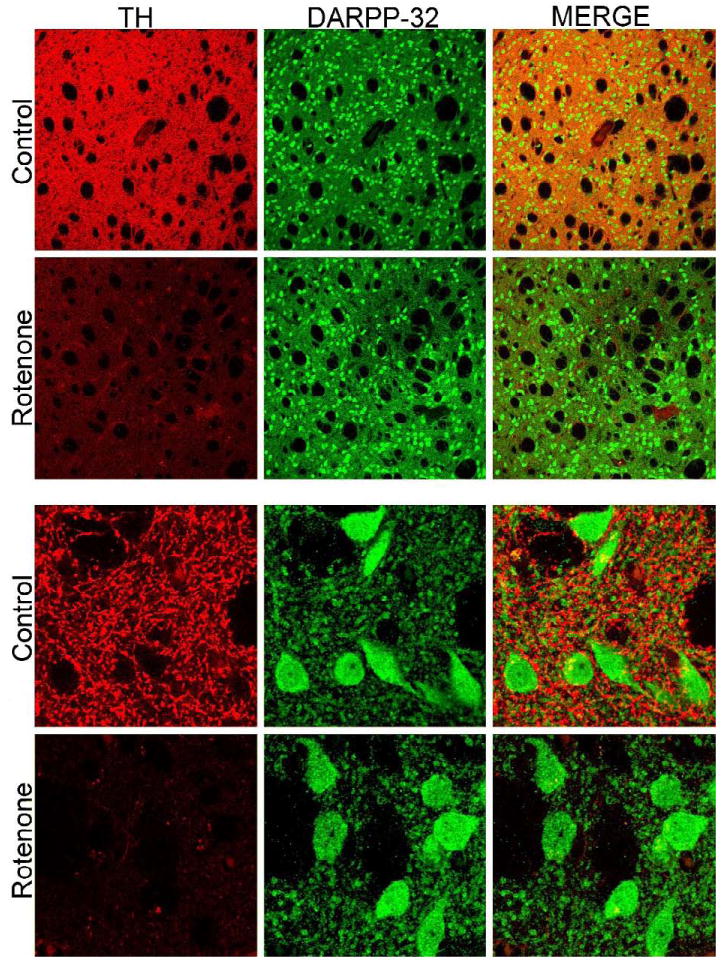
Striatal DARPP-32 staining. Striatal staining for tyrosine hydroxylase (TH) and DARPP-32. Low magnification images show that there is loss of TH+ terminals in rotenone treated animals with no overt loss of DARPP-32+ striatal cell bodies. High magnification images show that DARPP-32+ neurons do not exhibit morphological alterations in rotenone treated animals.
Dopamine depletion
Loss of dopamine occurred in both young-adult [43.1 ± 9.0 vs. 74.6 ± 6.5 ng/mg protein; rotenone (3.0 mg/kg/day; n = 7) vs. control (n = 6); p<0.05, student's t-test] and middle-aged animals [49.0 ± 12.3 (n = 5) vs. 91.6 ± 7.0 (n = 5); p<0.05] after daily intraperitoneal rotenone administration (Figure 7). Dopamine metabolite turnover [(DOPAC + HVA)/DA) was also increased in young-adult (0.77 ± 0.11 vs. 0.25 vs. 0.01; p<0.001) and middle-aged (0.77 ± 0.09 vs. 0.24 ± 0.02; p<0.001) rotenone-treated animals.
Figure 7.
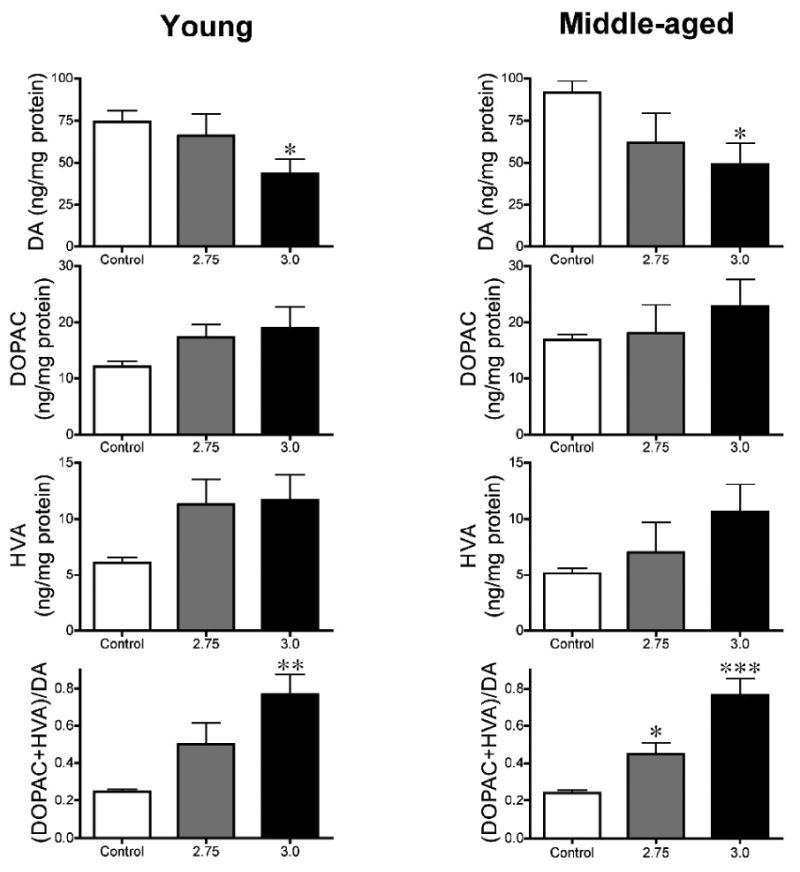
Intraperitoneal rotenone causes striatal dopamine depletion in young and middle-aged rats. Dopamine (DA), 3,4-dihydroxyphenylacetic acid (DOPAC), homovanillic acid (HVA) (ng/mg protein), and dopamine turnover [(DOPAC+HVA)/DA] in rotenone- (2.75, 3.0 mg/kg/day) and vehicle-treated rats. n = 5-7 per group. Note that alterations in catecholamine levels are similar in both age groups. * p<0.025, ** p<0.01, *** p<0.001, compared to control. Student's t-test with Bonferroni correction for multiple comparisons.
Loss of neurons in the substantia nigra
Daily intraperitoneal rotenone resulted in a loss of tyrosine hydroxylase positive neurons of the substantia nigra at both 2.75 and 3.0 mg/kg day in middle-aged rats (Figure 8; p<0.01 at each rotenone dose, Tukey's post hoc test). Because there was no difference in the magnitude of dopamine cell loss between rotenone treatment groups, these groups were merged for further statistical analysis. Overall, unbiased stereology indicated a substantial and significant loss of tyrosine hydroxylase-positive neurons in rotenone-treated rats (9,921 ± 720 vs. 18,010 ± 1,593; # of estimated tyrosine hydroxylase neurons/hemisphere ± S.E.M; p<0.001, student's t-test).
Figure 8.
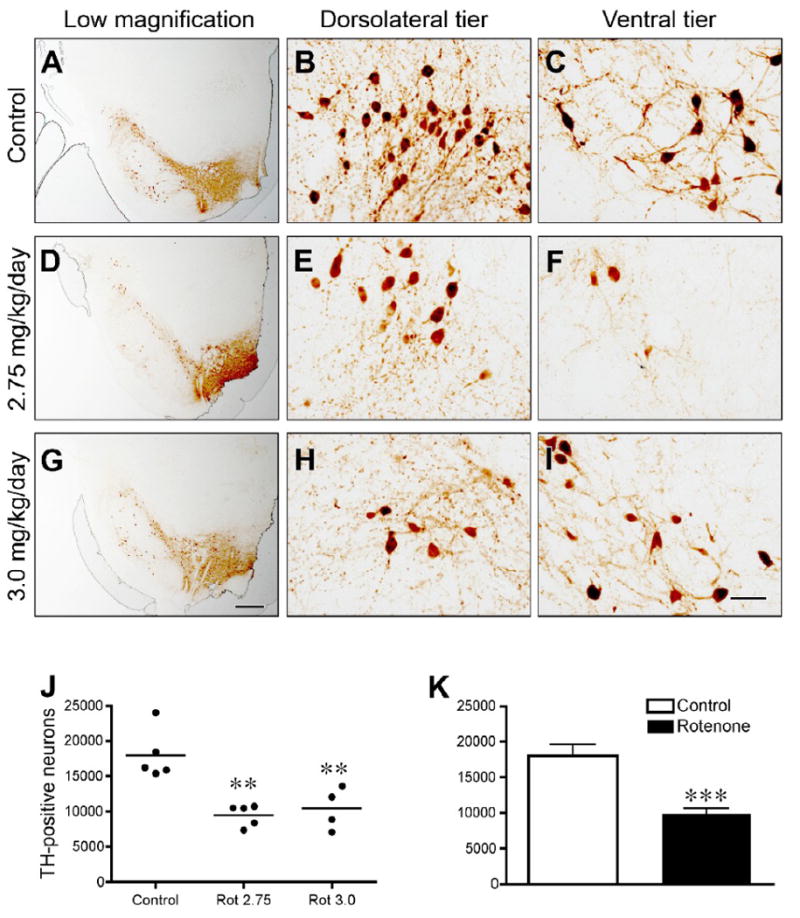
Intraperitoneal rotenone results in the loss of nigral tyrosine hydroxylase- (TH) positive neurons in middle-aged rats. Tyrosine hydroxylase immunohistochemistry (A-I) in control (A-C), and rotenone-treated rats [2.75 mg/kg/day (D-F) or 3.0 mg/kg/day (G-I)]. Low magnification images show overall cell loss and pruning of processes (A,D,G) (bar = 500 μm), medium magnification images in dorsolateral and ventral regions of nigra show cell loss, morphological changes, and loss of processes in these particularly vulnerable areas (bar = 50 μm). Unbiased stereological cell counts (J,K) show quantitative measurement of cell loss. ANOVA with Tukey's post hoc test indicated that both rotenone doses caused significant loss of dopamine neurons. Because no differences were found between animals treated at 2.75 or 3.0 mg/kg/day (J), these groups were merged and subjected to a student's t-test (K). **p<0.01; ***p<0.001 compared to control.
α-Synuclein aggregation
Intracellular aggregates of α-synuclein in dopamine neurons, a pathological feature of human PD, were replicated in dopamine neurons in the rotenone model. Increases in proteinase K-resistant (Figure 9 A, B) and formic acid-resistant (Figure 9 C, D) α-synuclein immunoreactivity were observed in the substantia nigra of rotenone-treated rats compared to controls. Confocal microscopy revealed the presence of intracellular α-synuclein and poly-ubiquitin-positive inclusions in rotenone-treated rats (Figure 8 E-L).
Figure 9.
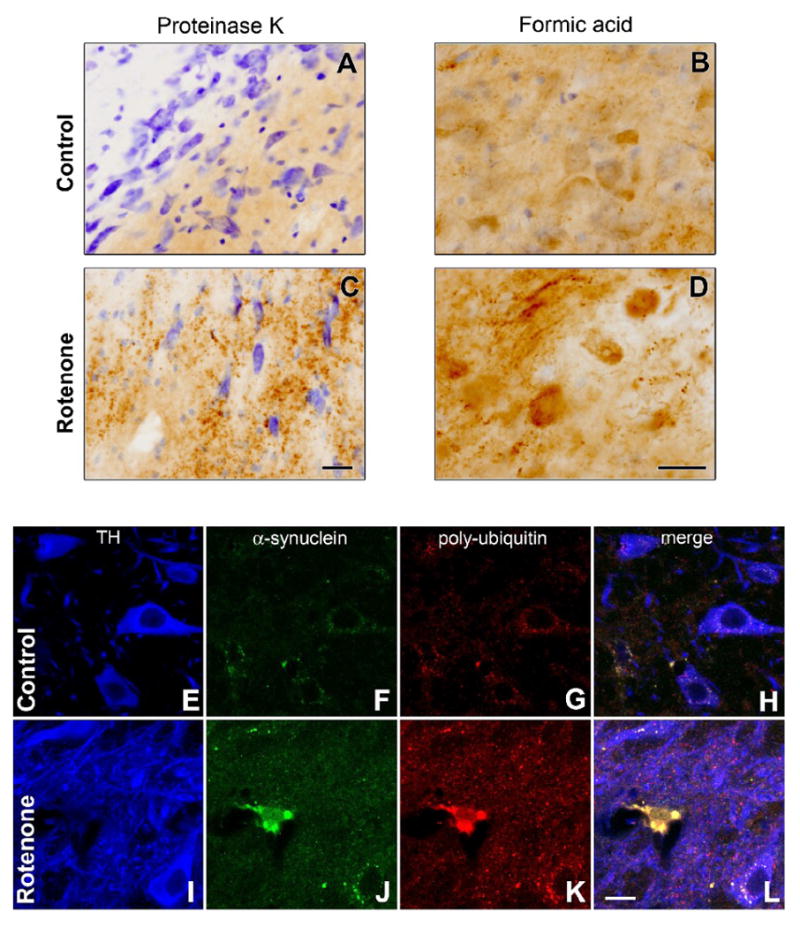
Intraperitoneal rotenone-induces α-synuclein and poly-ubiquitin accumulation and aggregation. Nigral α-synuclein immunohistochemistry in proteinase K pretreated (A,C) and formic acid pretreated tissue sections (B,D) from control (A,B) or rotenone-treated (3.0 mg/kg/day, C,D) animals (bars = 25 μm). E-L, Confocal microscopy of triple-labeled immunofluorescence for α-synuclein (α-syn; E,I), poly-ubiquitin (poly-ubiq; F,J), and pSer19-tyrosine hydroxylase (TH; G,K) and merged image (H,L) in a control (E-H) and rotenone-treated rat (I-L). E-L (bar = 10 μm). In the rotenone-treated rat, a dopamine neuron can be seen to contain multiple, large, round inclusions that stain for both α-synuclein and poly-ubiquitin.
Discussion
In developing neuroprotective or ‘disease-modifying’ treatments for PD, it has proven extremely difficult to translate positive results in animal studies to success in human clinical trials (Lang, 2006). In this regard, there is a pressing need for new and improved animal models of the disease. The rotenone model of PD reproduces many features of PD, including systemic mitochondrial impairment, oxidative damage, microglial activation, selective nigrostriatal dopaminergic degeneration, L-DOPA-responsive motor deficits, α-synuclein accumulation and aggregation with formation of Lewy body-like inclusions, impairment of ubiquitin-proteasome function, acidification and mitochondrial translocation of DJ-1, iron accumulation in substantia nigra and gastrointestinal dysfunction associated with α-synuclein accumulation and aggregation (Alam and Schmidt, 2004; Betarbet et al., 2006; Betarbet et al., 2000; Sakai and Gash, 1994). Thus, aside from preservation of dopaminergic neurons and related motor function – which are the most commonly employed endpoints in neuroprotection studies – there are numerous other relevant endpoints (e.g., α-synuclein accumulation and aggregation, ubiquitin-proteasome function or gastrointestinal dysfunction) that can be assessed in the rotenone model. Unfortunately, the use of this model for neuroprotective studies has been severely hampered by its variability. Here we report that daily intraperitoneal rotenone administration produces a highly reproducible lesion of the nigrostriatal dopamine system associated with α-synuclein pathology – a model that should be well-suited for the assessment of pathogenic pathways and experimental therapeutic interventions.
Previous studies using intraperitoneal injection of rotenone reported dopamine depletion and L-DOPA-responsive locomotor abnormalities (Alam and Schmidt, 2002; Alam and Schmidt, 2004); however, in those studies mortality was high (Biehlmaier et al., 2007; Werner J. Schmidt, personal communication) and the pathological features of the model were not characterized. We now provide further evidence of progressive locomotor deficits after rotenone treatment. These behavioral deficits were responsive to the dopamine agonist, apomorphine, indicating that loss of dopamine contributes to the deficits. While rearing deficits have been reported previously in the rotenone model (Fleming et al., 2004), variability was high, likely due to the low percentage of animals that exhibited histological evidence of a lesion to the nigrostriatal dopamine system. In our regimen, however, rearing proved to be a useful behavioral endpoint. Additionally, the postural instability test, which was recently developed in the unilateral 6-OHDA rat model of PD (Woodlee et al., 2008), was also useful in the characterization of a bilateral PD model. Interestingly, rotenone animals exhibited significant improvement after apomorphine, which was not the case in 6-OHDA treated animals. In that report, dopamine depletion was likely high (∼95%), given that 10 μg of 6-OHDA was administered into the medial forebrain bundle (Cannon et al., 2005). Therefore, it may be that apomorphine was unable to produce improvement with a lesion of such magnitude. The use of this test to quantify postural instability is of particular interest, because postural instability is one of the cardinal manifestations of PD and unsteady gait is one of the first noticeable behavioral phenotypes in this model.
Intravenous or subcutaneous rotenone administration typically produces nigrostriatal dopamine system lesions in one-third of animals (Betarbet et al., 2000). In contrast, here we report lesions in all animals treated with rotenone. Despite the fact that the current rotenone protocol is highly reproducible in terms of the ultimate behavioral phenotype and pathological features, there is still variability in the time from beginning rotenone treatment to the onset of a severe parkinsonian phenotype. This temporal variability in phenotype development was most apparent in the youngest animals. Indeed, older animals appeared to develop the phenotype more rapidly than younger animals. Rarely, animals exhibited behavioral resistance, or apparent decline followed by recovery, even while receiving daily rotenone injections (data not shown). A solution to this resistance was to increase the dose of rotenone by 10% per week after 30 days at 3.0 mg/kg/day. Under this regimen, the behavioral phenotype was observed in all animals. Alternatively, the use of older animals produces the phenotype with much less temporal variability. Interestingly, the dose-response curve for rotenone appears to be steep, as lower doses (2.0-2.5 mg/kg/day) for up to 60 days produce subtle behavioral features, without progression to a severe debilitating phenotype. A rotenone administration frequency of 24 hours was validated by a systemic complex I activity assay assessing rotenone–induced inhibition.
The debilitating phenotype observed includes bradykinesia, rigidity and postural instability. Postural instability in the form of an unsteady gait is one of the first signs of an ensuing severe parkinsonian phenotype. In this model, it should be noted that a trained observer should monitor the animals at least twice daily. While specialized behavioral tests are required to quantify behavioral deficits in unilaterally 6-OHDA lesioned animals (>95% dopamine depletion), even modest bilateral depletions can significantly impair motor function (Sakai and Gash, 1994). It should also be noted that we have achieved a severe PD phenotype with <50% striatal dopamine depletion. This is a much smaller magnitude of depletion than required to achieve such a phenotype through 6-OHDA-elicted bilateral dopamine depletion (Deumens et al., 2002; Sakai and Gash, 1994). This observation may, in part, be explained by the focal nature of the striatal dopamine terminal loss, which was localized to the dorsolateral striatum. In this region, dopamine terminals appeared to be completely destroyed, and undoubtedly, the magnitude of dopamine loss was much greater here. However, neurochemical analysis was performed on a homogenate of the entire striatum, so the focal loss of dopamine was underestimated.
Loss of striatal dopamine terminals was readily apparent in animals treated at 3.0 mg/kg/day. However, animals treated at lower dose rotenone (2.75 mg/kg/day) achieved a debilitating PD phenotype, without an overt striatal lesion in all animals. Interestingly, there was little difference in the magnitude of nigral dopamine cell loss between the dosage groups. It may have been that in animals treated at lower doses, terminal sprouting occurred from surviving dopamine neurons as a response to rotenone.
Daily intraperitoneal rotenone caused striatal dopamine depletion, degeneration of nigrostriatal dopamine terminals, and loss of tyrosine hydroxylase-positive neurons of the substantia nigra. Remarkably, the magnitude and distribution of the lesion was very similar across animals. In contrast, rotenone administration via osmotic pumps was previously found to produce lesions with a wide range of magnitude, and the striatal distribution ranged from a diffuse, widespread decrease in tyrosine hydroxylase immunoreactivity to a focal loss of staining; the precise location of the terminal lesion within the striatum also varied (Betarbet et al., 2000). With intraperitoneal administration, the loss of terminals is typically localized to the dorsolateral striatum and relatively focal in nature. Unbiased stereology showed that nigral dopamine cell loss was substantial, and it was particularly apparent in the lateral and ventral portions, the same regions that appear to be most vulnerable in human PD (Fearnley and Lees, 1991; Gibb and Lees, 1991).
As described with intravenous and subcutaneous administration of rotenone by osmotic pump, we have found that intraperitoneal rotenone also causes α-synuclein accumulation and aggregation in substantia nigra (Betarbet et al., 2006; Betarbet et al., 2000; Sherer et al., 2003). Furthermore, in the regimen described here, we found intraneuronal, cytoplasmic inclusions containing α-synuclein and poly-ubiquitin which have a morphology very similar to human Lewy bodies. As noted above, this feature of the model, together with other relevant endpoints, such as inflammation, oxidative damage, ubiquitin-proteasome function and iron accumulation, make the rotenone model particularly attractive for studying disease-modifying therapies.
In summary we have presented a behavioral, neurochemical, and histological characterization of the neurological effects of chronic intraperitoneal rotenone. The ability of this environmental neurotoxicant to produce a consistent model of PD that recapitulates many key features of pathology and pathogenesis should be of great use in testing experimental therapeutics and examining gene-environment interactions.
Supplementary Material
Acknowledgments
We dedicate this paper to Professor Werner J. Schmidt (deceased), who generously provided us with unpublished technical details of his studies. This work was funded by a grant from the Picower Foundation (J.T.G.), a postdoctoral fellowship (J.R.C.) and an Advanced Research Center grant (J.T.G.) from the American Parkinson Disease Association, and an NIH training grant (J.R.C.; T32 MH18273, PI: Michael J. Zigmond). We thank the rodent behavioral activity core at the University of Pittsburgh for aid in conducting the behavioral studies.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alam M, Schmidt WJ. Rotenone destroys dopaminergic neurons and induces parkinsonian symptoms in rats. Behav Brain Res. 2002;136:317–24. doi: 10.1016/s0166-4328(02)00180-8. [DOI] [PubMed] [Google Scholar]
- Alam M, Schmidt WJ. L-DOPA reverses the hypokinetic behaviour and rigidity in rotenone-treated rats. Behav Brain Res. 2004;153:439–46. doi: 10.1016/j.bbr.2003.12.021. [DOI] [PubMed] [Google Scholar]
- Betarbet R, Canet-Aviles RM, Sherer TB, Mastroberardino PG, McLendon C, Kim JH, Lund S, Na HM, Taylor G, Bence NF, Kopito R, Seo BB, Yagi T, Yagi A, Klinefelter G, Cookson MR, Greenamyre JT. Intersecting pathways to neurodegeneration in Parkinson's disease: effects of the pesticide rotenone on DJ-1, alpha-synuclein, and the ubiquitin-proteasome system. Neurobiol Dis. 2006;22:404–20. doi: 10.1016/j.nbd.2005.12.003. [DOI] [PubMed] [Google Scholar]
- Betarbet R, Sherer TB, MacKenzie G, Garcia-Osuna M, Panov AV, Greenamyre JT. Chronic systemic pesticide exposure reproduces features of Parkinson's disease. Nat Neurosci. 2000;3:1301–6. doi: 10.1038/81834. [DOI] [PubMed] [Google Scholar]
- Biehlmaier O, Alam M, Schmidt WJ. A rat model of Parkinsonism shows depletion of dopamine in the retina. Neurochem Int. 2007;50:189–95. doi: 10.1016/j.neuint.2006.08.001. [DOI] [PubMed] [Google Scholar]
- Cannon JR, Keep RF, Hua Y, Richardson RJ, Schallert T, Xi G. Thrombin preconditioning provides protection in a 6-hydroxydopamine Parkinson's disease model. Neurosci Lett. 2005;373:189–94. doi: 10.1016/j.neulet.2004.10.089. [DOI] [PubMed] [Google Scholar]
- Cannon JR, Keep RF, Schallert T, Hua Y, Richardson RJ, Xi G. Protease-activated receptor-1 mediates protection elicited by thrombin preconditioning in a rat 6-hydroxydopamine model of Parkinson's disease. Brain Res. 2006;1116:177–86. doi: 10.1016/j.brainres.2006.07.094. [DOI] [PubMed] [Google Scholar]
- Cenci MA, Whishaw IQ, Schallert T. Animal models of neurological deficits: how relevant is the rat? Nat Rev Neurosci. 2002;3:574–9. doi: 10.1038/nrn877. [DOI] [PubMed] [Google Scholar]
- Deumens R, Blokland A, Prickaerts J. Modeling Parkinson's disease in rats: an evaluation of 6-OHDA lesions of the nigrostriatal pathway. Exp Neurol. 2002;175:303–17. doi: 10.1006/exnr.2002.7891. [DOI] [PubMed] [Google Scholar]
- Di Monte DA. The role of environmental agents in Parkinson's disease. clinical neuroscience research. 2001;1:419–426. [Google Scholar]
- Fall PA, Fredrikson M, Axelson O, Granerus AK. Nutritional and occupational factors influencing the risk of Parkinson's disease: a case-control study in southeastern. Sweden Mov Disord. 1999;14:28–37. doi: 10.1002/1531-8257(199901)14:1<28::aid-mds1007>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- Fearnley JM, Lees AJ. Ageing and Parkinson's disease: substantia nigra regional selectivity. Brain. 1991;114(Pt 5):2283–301. doi: 10.1093/brain/114.5.2283. [DOI] [PubMed] [Google Scholar]
- Fleming SM, Delville Y, Schallert T. An intermittent, controlled-rate, slow progressive degeneration model of Parkinson's disease: antiparkinson effects of Sinemet and protective effects of methylphenidate. Behav Brain Res. 2005;156:201–13. doi: 10.1016/j.bbr.2004.05.024. [DOI] [PubMed] [Google Scholar]
- Fleming SM, Zhu C, Fernagut PO, Mehta A, DiCarlo CD, Seaman RL, Chesselet MF. Behavioral and immunohistochemical effects of chronic intravenous and subcutaneous infusions of varying doses of rotenone. Exp Neurol. 2004;187:418–29. doi: 10.1016/j.expneurol.2004.01.023. [DOI] [PubMed] [Google Scholar]
- Forno LS. Neuropathology of Parkinson's disease. J Neuropathol Exp Neurol. 1996;55:259–72. doi: 10.1097/00005072-199603000-00001. [DOI] [PubMed] [Google Scholar]
- Gash DM, Rutland K, Hudson NL, Sullivan PG, Bing G, Cass WA, Pandya JD, Liu M, Choi DY, Hunter RL, Gerhardt GA, Smith CD, Slevin JT, Prince TS. Trichloroethylene: Parkinsonism and complex 1 mitochondrial neurotoxicity. Ann Neurol. 2008;63:184–92. doi: 10.1002/ana.21288. [DOI] [PubMed] [Google Scholar]
- Gibb WR, Lees AJ. Anatomy, pigmentation, ventral and dorsal subpopulations of the substantia nigra, and differential cell death in Parkinson's disease. J Neurol Neurosurg Psychiatry. 1991;54:388–96. doi: 10.1136/jnnp.54.5.388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorell JM, Johnson CC, Rybicki BA, Peterson EL, Kortsha GX, Brown GG, Richardson RJ. Occupational exposures to metals as risk factors for Parkinson's disease. Neurology. 1997;48:650–8. doi: 10.1212/wnl.48.3.650. [DOI] [PubMed] [Google Scholar]
- Gorell JM, Johnson CC, Rybicki BA, Peterson EL, Richardson RJ. The risk of Parkinson's disease with exposure to pesticides, farming, well water, and rural living. Neurology. 1998;50:1346–50. doi: 10.1212/wnl.50.5.1346. [DOI] [PubMed] [Google Scholar]
- Hageman G, van der Hoek J, van Hout M, van der Laan G, Steur EJ, de Bruin W, Herholz K. Parkinsonism, pyramidal signs, polyneuropathy, and cognitive decline after long-term occupational solvent exposure. J Neurol. 1999;246:198–206. doi: 10.1007/s004150050334. [DOI] [PubMed] [Google Scholar]
- Janssen AJ, Trijbels FJ, Sengers RC, Smeitink JA, van den Heuvel LP, Wintjes LT, Stoltenborg-Hogenkamp BJ, Rodenburg RJ. Spectrophotometric assay for complex I of the respiratory chain in tissue samples and cultured fibroblasts. Clin Chem. 2007;53:729–34. doi: 10.1373/clinchem.2006.078873. [DOI] [PubMed] [Google Scholar]
- Jolicoeur FB, Rivest R, Drumheller A. Hypokinesia, rigidity, and tremor induced by hypothalamic 6-OHDA lesions in the rat. Brain Res Bull. 1991;26:317–20. doi: 10.1016/0361-9230(91)90245-f. [DOI] [PubMed] [Google Scholar]
- Kuhn W, Winkel R, Woitalla D, Meves S, Przuntek H, Muller T. High prevalence of parkinsonism after occupational exposure to lead-sulfate batteries. Neurology. 1998;50:1885–6. doi: 10.1212/wnl.50.6.1885. [DOI] [PubMed] [Google Scholar]
- Lang AE. Neuroprotection in Parkinson's disease: and now for something completely different? Lancet Neurol. 2006;5:990–1. doi: 10.1016/S1474-4422(06)70605-6. [DOI] [PubMed] [Google Scholar]
- Liou HH, Tsai MC, Chen CJ, Jeng JS, Chang YC, Chen SY, Chen RC. Environmental risk factors and Parkinson's disease: a case-control study in Taiwan. Neurology. 1997;48:1583–8. doi: 10.1212/wnl.48.6.1583. [DOI] [PubMed] [Google Scholar]
- Ohashi S, Mori A, Kurihara N, Mitsumoto Y, Nakai M. Age-related severity of dopaminergic neurodegeneration to MPTP neurotoxicity causes motor dysfunction in C57BL/6 mice. Neurosci Lett. 2006;401:183–7. doi: 10.1016/j.neulet.2006.03.017. [DOI] [PubMed] [Google Scholar]
- Pezzoli G, Strada O, Silani V, Zecchinelli A, Perbellini L, Javoy-Agid F, Ghidoni P, Motti ED, Masini T, Scarlato G, Agid Y, Hirsch EC. Clinical and pathological features in hydrocarbon-induced parkinsonism. Ann Neurol. 1996;40:922–5. doi: 10.1002/ana.410400616. [DOI] [PubMed] [Google Scholar]
- Rojo AI, Montero C, Salazar M, Close RM, Fernandez-Ruiz J, Sanchez-Gonzalez MA, de Sagarra MR, Jackson-Lewis V, Cavada C, Cuadrado A. Persistent penetration of MPTP through the nasal route induces Parkinson's disease in mice. Eur J Neurosci. 2006;24:1874–84. doi: 10.1111/j.1460-9568.2006.05060.x. [DOI] [PubMed] [Google Scholar]
- Sakai K, Gash DM. Effect of bilateral 6-OHDA lesions of the substantia nigra on locomotor activity in the rat. Brain Res. 1994;633:144–50. doi: 10.1016/0006-8993(94)91533-4. [DOI] [PubMed] [Google Scholar]
- Schallert T, Tillerson JL. Intervention strategies for degeneration of dopamine neurons in parkinsonism: Optimizing behavioral assessment of outcome. In: Emerich DF, et al., editors. Central Nervous System Diseases: Innovative animal models from lab to clinic. Humana Press; Totowa: 2000. pp. 131–151. [Google Scholar]
- Schallert T, Whishaw IQ, Ramirez VD, Teitelbaum P. Compulsive, abnormal walking caused by anticholinergics in akinetic, 6-hydroxydopamine-treated rats. Science. 1978;199:1461–3. doi: 10.1126/science.564552. [DOI] [PubMed] [Google Scholar]
- Semchuk KM, Love EJ, Lee RG. Parkinson's disease: a test of the multifactorial etiologic hypothesis. Neurology. 1993;43:1173–80. doi: 10.1212/wnl.43.6.1173. [DOI] [PubMed] [Google Scholar]
- Sherer TB, Kim JH, Betarbet R, Greenamyre JT. Subcutaneous rotenone exposure causes highly selective dopaminergic degeneration and alpha-synuclein aggregation. Exp Neurol. 2003;179:9–16. doi: 10.1006/exnr.2002.8072. [DOI] [PubMed] [Google Scholar]
- Tadaiesky MT, Dombrowski PA, Figueiredo CP, Cargnin-Ferreira E, Da Cunha C, Takahashi RN. Emotional, cognitive and neurochemical alterations in a premotor stage model of Parkinson's disease. Neuroscience. 2008;156:830–40. doi: 10.1016/j.neuroscience.2008.08.035. [DOI] [PubMed] [Google Scholar]
- Ungerstedt U. Adipsia and aphagia after 6-hydroxydopamine induced degeneration of the nigro-striatal dopamine system. Acta Physiol Scand Suppl. 1971;367:95–122. doi: 10.1111/j.1365-201x.1971.tb11001.x. [DOI] [PubMed] [Google Scholar]
- Woodlee MT, Kane JR, Chang J, Cormack LK, Schallert T. Enhanced function in the good forelimb of hemi-parkinson rats: compensatory adaptation for contralateral postural instability? Exp Neurol. 2008;211:511–7. doi: 10.1016/j.expneurol.2008.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


