Abstract
Background and Purpose
TIA patients are at increased risk for stroke, cardiovascular events, and death, yet little is known about whether these risks differ for men and women. We determined whether there are sex-based differences in these outcomes 30-days and one year following TIA using a national sample of elderly patients.
Methods
Rates of 30-day and one year hospitalization for TIA (ICD-9 435), stroke (ICD-9 433, 434, 436), coronary artery disease (CAD; ICD-9 410–414), all-cause readmission, and mortality were determined for fee-for-service Medicare patients 65 years of age or older discharged with a TIA in 2002. Cox proportional hazards models and random effects logistic models compared outcomes with risk-adjustment for demographics, medical history, comorbidities, and prior hospitalizations.
Results
The study included 122,063 TIA hospitalizations (mean age 79.0±7.6 years; 62% women; 86% White). Men were younger, but had higher rates of cardiac comorbidities than women. Women had lower unadjusted rates of stroke, CAD, and mortality at 30-days and one year following TIA admission. These relationships persisted in risk-adjusted analyses at 30-days for stroke (HR=0.70, 95% CI 0.64–0.77), CAD (0.86, 0.74–1.00), and mortality (OR=0.74, 0.68–0.82), as well as at one year for stroke (HR=0.85, 0.81–0.89), CAD (0.81, 0.77–0.86), and mortality (OR=0.78, 0.75–0.81).
Conclusion
These data suggest that women have a better prognosis than men within the first year following hospital discharge for a TIA. Additional research is needed to identify factors that may explain these sex-related differences in outcomes.
Keywords: transient ischemic attack (TIA), outcomes, sex
Introduction
Stroke is the third leading cause of death in the U.S. and a leading cause of disability. Because of their longer lifespan, women have a higher stroke burden than men, accounting for 61% of all stroke deaths in 2004.1 Transient ischemic attack (TIA) precedes approximately 15–23% of strokes,2, 3 and carries a 90-day stroke risk of 9% to 17%.2 In addition to stroke, TIA patients are also at increased risk for recurrent TIA, coronary artery disease, and death.3–7 With an estimated 240,000 TIAs diagnosed annually in the United States,8 TIA represents an important target for secondary stroke prevention, and is an indicator of a patient’s risk for myocardial infarction and vascular death.9–13 Rapid evaluation and treatment of TIA patients can reduce subsequent morbidity.5, 14
Relatively little is known about whether elderly women and men have comparable risks for recurrent events and mortality within the first year following TIA. Findings from studies that examined potential sex-related differences in outcomes following TIA are inconsistent.4, 6–8, 15–21 Some report a lower unadjusted 7-day stroke risk for women as well as reduced, risk-adjusted long-term rates of stroke, death, and the composite outcome of stroke, MI, or death.7, 15, 20 Others report no sex-related differences in unadjusted4, 17–19, 21 or risk-adjusted8, 16 analyses of stroke and cardiac risk. Many of these studies combined TIA and stroke in the index cohort and in the assessment of outcomes, 7, 15, 16 were conducted in European populations,7, 15, 17, 19–21 used clinical trial cohorts,7, 15 or were restricted to specific U.S. geographic areas.4, 8, 16, 18 Thus, only limited information is available reflecting both early and long-term prognosis following TIA in a U.S. national cohort of elderly patients. In the present study, we compared 30-day and one-year outcomes for elderly women and men following hospital discharge for TIA in a U.S. national cohort. Outcomes included hospitalization for recurrent TIA, stroke, cardiovascular events, all-cause rehospitalization, and mortality.
Methods
Study Population
The study population included all fee-for-service (FFS) Medicare beneficiaries 65 years or older hospitalized with a primary discharge diagnosis of TIA from January 1, 2002, through December 31, 2002 who were identified based on the International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM 435.x). Data were obtained from the Medicare Provider Analysis and Review (MEDPAR) files that included demographic information, primary and secondary discharge diagnosis codes, and procedure codes for each hospitalization. We excluded patients who were under 65 years of age, discharged from non-acute care facilities, or transferred from another facility. We further limited the cohort to patients with at least 12 months of continuous fee-for-service status prior to the index TIA hospitalization to allow assessment of comorbid conditions.
Outcomes
Rehospitalization rates at 30-days and one year following the index TIA hospital discharge were determined for recurrent TIA (ICD-9 435), ischemic stroke (ICD-9 433, 434, 436), coronary artery disease (CAD; ICD-9 410–414), and all-cause readmission. Mortality at 30-days and one year were obtained from the Medicare Enrollment Database. The accuracy of ascertainment of vital status using these data resources is high for this age group.22 Length of stay was determined for each index hospitalization and discharge disposition was categorized as home, skilled nursing facility, or other location.
Covariates
Comorbidities were identified from MEDPAR files in the year prior to the index hospitalization to avoid misclassifying pre-existing conditions as complications. Demographic characteristics included age, sex, and race. Medical history included previous diagnosis of cancer, dementia, chronic obstructive pulmonary disease (COPD), ischemic stroke, diabetes, smoking status, hypertension, acute myocardial infarction (AMI), congestive heart failure (CHF), atrial fibrillation (AF), prior receipt of coronary artery bypass graft surgery (CABG) or percutaneous transluminal coronary angioplasty (PTCA), Deyo score for comorbidities (≥3),23 and number of hospitalizations in the year prior to the index TIA (dichotomized as <2 or ≥2).
Statistical Analysis
Bivariate analyses were conducted to compare patient characteristics by sex using t-tests for continuous variables and chi-squared statistics for categorical variables. Readmission rates were compared using Cox proportional hazards models with censoring for deaths. Random effects logistic models were used to compare 30-day and one-year mortality from admission by sex taking into account the correlation among patients treated at the same hospital. Models were adjusted for demographics, medical history, comorbidities, and prior hospital events. Risk-adjusted survival curves were used to illustrate the timing of follow-up events by sex. All analyses were conducted using SAS version 9.1.3 (SAS Institute Inc., Cary, North Carolina).
Results
The final study cohort included 122,063 patients (mean age 79.0±7.6 years; 86% White) with a discharge code of TIA in 2002 (Table 1). Women represented 62% of TIA cases and were more likely to be older (mean age 79.7±7.7 vs. 77.8±7.3) and of Black race (P<0.0001 for each). Women were more likely to have been hospitalized two or more times in the prior year, but less likely to have three or more comorbidites using the Deyo score. They had lower rates of cancer, COPD, and diabetes, but higher rates of hypertension and CHF (P<0.0001 for each). Men had a higher prevalence of cardiovascular risk factors, including diabetes and smoking history as well as prior AMI and cardiovascular procedures than women. Discharge disposition varied by sex, with a higher percentage of women discharged to skilled nursing facilities or other location, and a greater percentage of men discharged home (P<0.0001).
TABLE 1.
Characteristics of Medicare Beneficiaries Hospitalized for TIA
| Total (N=122,063) |
Women (N=76,108) |
Men (N=45,955) |
||
|---|---|---|---|---|
| % | % | % | P-value* | |
| Age (years; mean ± SD) | 79.0 ± 7.6 | 79.7 ± 7.7 | 77.8 ± 7.3 | <.0001 |
| 65–74 | 30.2 | 27.1 | 35.4 | <.0001 |
| 75–84 | 44.8 | 44.3 | 45.6 | |
| 85+ | 25.0 | 28.6 | 19.0 | |
| Race | <.0001 | |||
| White | 85.7 | 85.0 | 86.9 | |
| Black | 9.8 | 10.6 | 8.5 | |
| Other | 2.4 | 2.3 | 2.4 | |
| Hispanic | 2.2 | 2.1 | 2.2 | |
| Source of Admission | <.0001 | |||
| Emergency Department | 78.6 | 78.3 | 79.1 | |
| Skilled Nursing Facility | 0.7 | 0.8 | 0.5 | |
| Other | 20.7 | 20.9 | 20.4 | |
| Hospitalizations in Past Year ≥ 2 | 15.6 | 16.0 | 14.8 | <.0001 |
| Deyo Score ≥ 3 | 16.4 | 15.1 | 18.6 | <.0001 |
| Medical History & Comorbidity | ||||
| Cancer | 2.5 | 1.8 | 3.6 | <.0001 |
| Dementia | 9.9 | 10.6 | 8.8 | <.0001 |
| COPD | 18.4 | 16.9 | 20.9 | <.0001 |
| Ischemic Stroke | 11.4 | 11.6 | 11.2 | 0.0697 |
| Diabetes | 26.5 | 25.6 | 27.9 | <.0001 |
| Smoking | 6.2 | 4.8 | 8.6 | <.0001 |
| Hypertension | 65.4 | 68.6 | 60.2 | <.0001 |
| AMI | 9.2 | 7.5 | 12.1 | <.0001 |
| CHF | 10.3 | 10.6 | 9.9 | <.0001 |
| Atrial Fibrillation | 15.8 | 15.2 | 16.9 | <.0001 |
| Prior CABG | 7.3 | 4.3 | 12.2 | <.0001 |
| Prior PTCA | 2.7 | 2.1 | 3.7 | <.0001 |
| Discharge Disposition | <.0001 | |||
| Home | 70.6 | 68.0 | 74.9 | |
| Skilled Nursing Facility | 13.5 | 15.3 | 10.6 | |
| In-Hospital Death | 0.3 | 0.2 | 0.3 | |
| Other | 15.6 | 16.5 | 14.3 | |
| Length of Stay (days; mean ± SD)† | 3.4 ± 2.6 | 3.5 ± 2.6 | 3.3 ± 2.6 | <.0001 |
P-value is for difference between men and women using t-tests for continuous variables and chi-squared statistics for categorical variables.
Excludes 2,174 patients due to in-hospital death, transfer to another acute care facility, and length of stay >61 days.
Unadjusted 30-day readmission rates were similar for women and men for all-cause readmission (10.6% vs. 10.6%) and recurrent TIA (1.1% vs. 1.1%), but women had lower rates of stroke (1.3% vs. 1.8%; P<0.0001) and CAD hospitalizations (0.5% vs. 0.7%; P<0.0001). At one year, women had higher rates of all-cause readmission (48.8% vs. 47.6%; P=0.0002) and TIA (5.3% vs. 4.9%; P<0.0001), but lower readmission rates for stroke (5.9% vs. 6.9%; P<0.0001), and CAD (4.5% vs. 5.8%; P<0.0001). Mortality rates were lower for women at 30-days (1.4% vs. 1.8%; P<0.0001) and one year (12.9% vs. 14.7%; P<.0001) as compared with men.
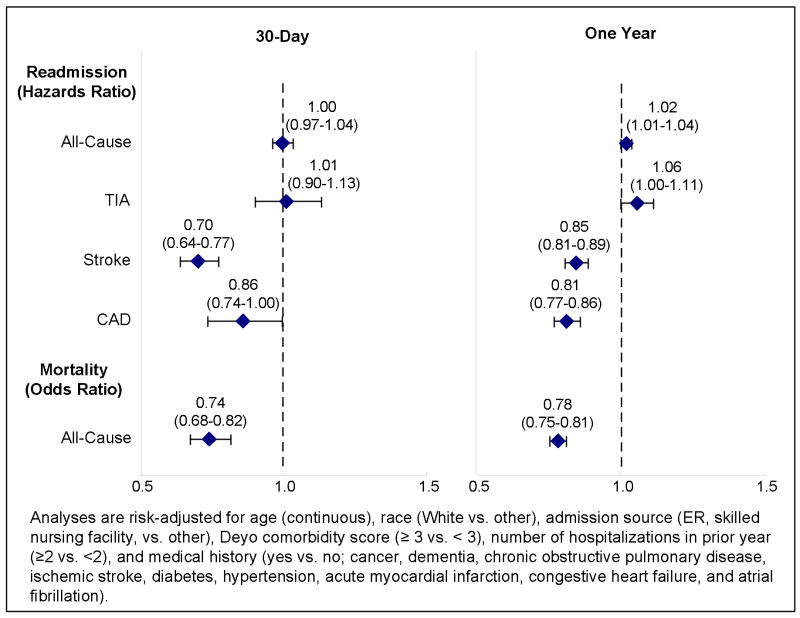
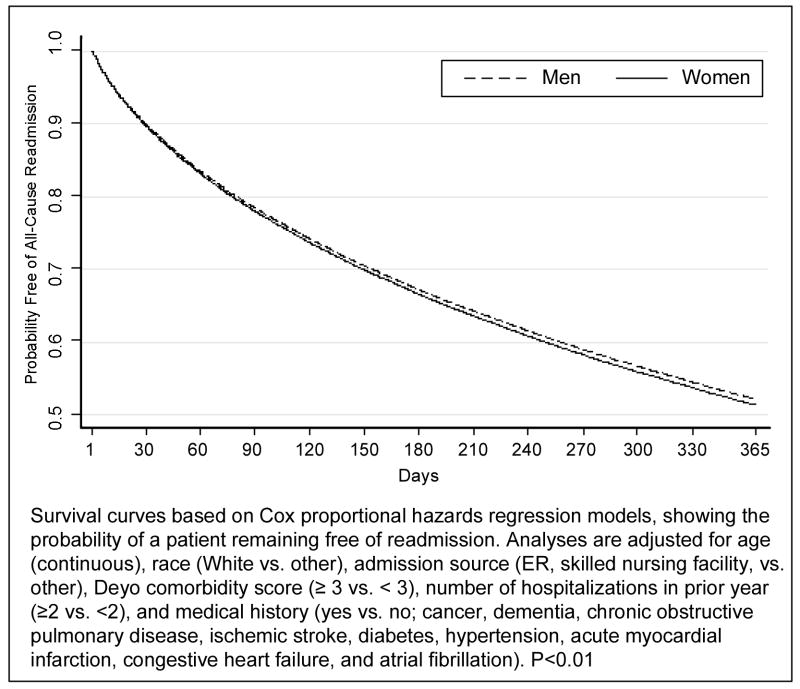
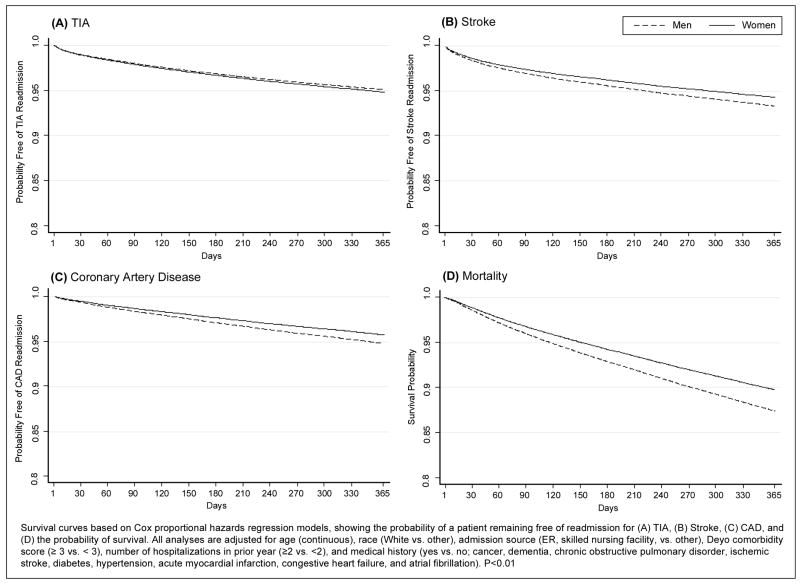
In risk-adjusted analyses, there were no sex-related differences in all-cause readmission (Hazards Ratio [HR]=1.00, 95% Confidence Interval [CI] 0.97–1.04), or hospitalizations for TIA (HR=1.01, CI 0.90–1.13), but women had lower risk of stroke (HR=0.70, CI 0.64–0.77), CAD events (HR=0.86, CI 0.74–1.00), and death (Odds Ratio [OR]=0.74, 0.68–0.82) at 30 days (Figure 1). At one year, women had a slightly higher risk of recurrent TIA relative to men (HR=1.06, CI 1.00–1.11; P<0.05), but lower risk of stroke (HR=0.85, CI 0.81–0.89), CAD events (HR=0.81, CI 0.77–0.86), and death (OR=0.78, CI 0.75–0.81). The risk of adverse events including hospitalization for TIA, stroke, cardiovascular events, all-cause readmission and mortality in the first year after TIA in women and men are shown in Figures 2–3. Men had a higher risk of hospital admission for stroke, CAD, and death compared to women throughout the 365-day period with increasing differences over time.
Figure 1.
Risk-Adjusted Readmission and Mortality, Women Compared to Men
Figure 2.
Risk-Adjusted 365-Day Survival Curves of All-Cause Hospital Readmission
Figure 3.
Risk-Adjusted 365-Day Survival Curves for TIA, Stroke, and Coronary Artery Disease Readmission, and Mortality
Discussion
Among elderly fee-for-service Medicare beneficiaries who were hospitalized for a TIA, women had a lower risk of subsequent hospitalization for major vascular events and death within the first year as compared with men. Women were 30% less likely to have a stroke, 14% less likely to have a cardiac event, and 26% less likely to die within the first 30 days, even after adjustment for comorbid conditions, including pre-existing CHD. Within one year, women were 15% less likely to have a stroke, 19% less likely to have a cardiac event, and 22% less likely to die. Women had a marginally higher risk of recurrent TIA (6%) within one year, which was not evident within the first month.
Our findings are consistent with two European studies from the Dutch TIA Trial, which included 3,127 patients with TIA or minor ischemic stroke.7, 15 The first study reported an increased combined risk of vascular death, stroke, or myocardial infarction for men (HR=1.6, 95% CI 1.3–2.0), and a marginally higher risk of fatal or nonfatal stroke (HR=1.3, 1.0–1.8) over an average 2.6-year follow-up.15 The second, more recent study, reported a higher 10-year risk of vascular death, stroke, or myocardial infarction (HR=1.38, 1.22–1.56) as well as fatal or nonfatal stroke (HR=1.31, 1.07–1.59) for men.7 Our findings extend these results by providing data on outcomes following TIA for recurrent TIA, stroke, and other cardiovascular events, as well as mortality using a more contemporary, national population of elderly TIA patients in the United States.
Several studies reported no sex-related differences in outcomes following TIA.4, 8, 16–19, 21 Many of these were based on international populations,17, 19, 21 had relatively small samples,8, 17–19, 21 were not representative of the national elderly population,4, 8, 16, 18 combined TIA and mild stroke events,16 included patients less than 65 years,4, 8, 17–19, 21 and provided only unadjusted rates by sex.4, 17–19, 21 Moreover, studies have generally focused on stroke risk,4, 17–19, 21 with few analyzing cardiac risk16 or mortality8 following TIA. A study of Michigan Medicare beneficiaries discharged with TIA or stroke found a non-significant lower stroke risk (inclusive of TIA) for women in adjusted analysis (HR=0.83, 0.64–1.07).16 We found an opposite risk profile for men and women; women had a lower stroke risk, but a slightly higher risk of recurrent TIA than men. Some prior studies may have masked these sex effects by combining TIA and stroke outcomes.
There are a number of plausible explanations for the observed sex-based differences in outcomes after TIA. First, they may be due to etiological differences in TIA. Men may have a higher proportion of TIAs caused by large-artery atherosclerosis (LAA), which is associated with greater stroke risk.6 Lower event rates among women could also be attributable to differences in care seeking behaviors, initial diagnostic evaluation, or in the receipt of and/or compliance with secondary prevention measures. Studies of TIA patients, however, found no sex-related differences in recognition of TIA,24 nor were there differences in care-seeking behavior.25 It is unlikely that women receive more aggressive diagnostic evaluations. In fact, women receive comparable care or may be less likely to receive acute therapies and discharge medications than men.26–30 Finally, women may be hospitalized more often for conditions such as migraine that could potentially be misclassified as a TIA. A study that validated ICD-9 TIA diagnosis with review by a neurologist, however, found that fewer than 6% were attributable to these other causes, although this was not analyzed by sex.4 Use of ICD-9 codes for diagnosis of TIA is reasonably valid, with positive predictive values between 70% and 90%,31–34 but data are not available to compare the validity of these codes by sex. Two European studies evaluated the validity of international administrative data for diagnosis of ischemic stroke and did not find a sex-based difference.33, 34
In our sample, the overall 30-day stroke risk of 1.5% is lower than cited in other studies,7, 8, 18, 21, 35–39 whereas our estimate of 30-day mortality is more consistent with previous work.7, 18, 21, 38 There are no data directly comparable with our study as we used national Medicare administrative data. The relatively lower stroke rate could be due to demographic differences of our cohort; Medicare patients are on average older and consist of a higher percentage of women, who we found to have lower short-term stroke risk after TIA. This may also be due to our use of administrative discharge codes for case selection and outcome ascertainment. The majority of other studies employed neurologist review to verify TIA cases, identified events through patient follow-up interviews, and/or included non-admitted patients.7, 8, 18, 21, 35–39 For these reasons, findings from our national study likely underestimate the true risk in the community, but accurately reflect the utilization of hospital resources following hospital admission for TIA among fee-for-service Medicare beneficiaries in the U.S.
We found that the risk of recurrent stroke is almost three times higher than the risk of cardiovascular events within the first 30 days following TIA, but the rates became roughly equivalent by one year. Similarly, Brown et al reported that an ischemic stroke following a stroke or TIA is more common as the first recurrent event than an ischemic cardiac event.16 Other studies have also found that cardiovascular events tend to rival stroke events over longer periods of follow-up.3, 7, 40
The present study has a number of limitations. First, assessment of comorbid conditions is dependent on the accuracy of recorded secondary administrative codes, but we would not expect sex-based differences in data coding. There are additional unmeasured factors, such as clinical data on neurologic symptoms, symptom duration, or etiology, and test results that are not part of the administrative record. Medicare data does not contain information on medication utilization; therefore, we are unable to address potential differences in secondary stroke care by sex, although current research indicates that women are not likely to receive more aggressive care than men.26–29 The index TIA cases and vascular outcomes were ascertained using ICD-9 codes and were restricted to hospitalized events. Two population-based studies reported that 82–86% of TIA cases presented at the emergency department, 8, 18 with 79% of these patients admitted to the hospital. 8, 18 Positive predictive values for the selected codes for TIA, ischemic stroke, and CAD are relatively high,41, 42 and evidence suggests these do not vary by sex.33, 34
Patients hospitalized with TIA represent a high-risk population for stroke, cardiovascular events, and all-cause readmission. Almost one in ten patients will be rehospitalized within the first month, and half are rehospitalized within the first year. Furthermore, women have better outcomes following hospitalization for TIA than men, even after adjusting for demographic and clinical factors. These short-term sex-based differences persist for the first year following TIA. Our analysis has the advantage of providing information about prognosis following TIA based on a 100% national FFS Medicare population with a broad range of vascular outcomes. Further investigation of the underlying causes for these sex-based differences may help improve care and outcomes for both men and women following TIA, and will be critical in planning for the future health care needs of these patients.
TABLE 2.
Rates of Readmission and Mortality Following Hospitalization for TIA
| 30-Day |
One-Year |
|||||||
|---|---|---|---|---|---|---|---|---|
| Total (N=122,063) | Women (N=76,108) | Men (N=45,955) | Total (N=122,063) | Women (N=76,108) | Men (N=45,955) | |||
| % | % | % | P-value* | % | % | % | P-value* | |
| Readmission† | ||||||||
| All-cause | 10.6 | 10.6 | 10.6 | 0.7740 | 48.3 | 48.8 | 47.6 | 0.0002 |
| Recurrent TIA | 1.1 | 1.1 | 1.1 | 0.8636 | 5.2 | 5.3 | 4.9 | <.0001 |
| Stroke | 1.5 | 1.3 | 1.8 | <.0001 | 6.3 | 5.9 | 6.9 | <.0001 |
| CAD | 0.6 | 0.5 | 0.7 | 0.0010 | 5.0 | 4.5 | 5.8 | <.0001 |
| Mortality | 1.5 | 1.4 | 1.8 | <.0001 | 13.6 | 12.9 | 14.7 | <.0001 |
P-value is for difference between men and women using chi-squared statistics.
Censored for 2,064 patients due to hospital transfers and in-hospital deaths.
Acknowledgments
Funding: The Centers for Medicare & Medicaid Services reviewed and approved the use of its data for this work, and approved submission of the manuscript; this approval is based on data use only, and does not represent a Centers for Medicare & Medicaid Services endorsement of or comment on the manuscript content. The project described was supported by Grant Number R01NS043322 from the National Institute of Neurological Disorders and Stroke. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Neurological Disorders and Stroke or the National Institutes of Health.
Footnotes
Conflicts of Interest Disclosures
There are no conflicts of interest to report.
References
- 1.Rosamond W, Flegal K, Furie K, Go A, Greenlund K, Haase N, Hailpern SM, Ho M, Howard V, Kissela B, Kittner S, Lloyd-Jones D, McDermott M, Meigs J, Moy C, Nichol G, O’Donnell C, Roger V, Sorlie P, Steinberger J, Thom T, Wilson M, Hong Y. Heart disease and stroke statistics--2008 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2008;117:e25–146. doi: 10.1161/CIRCULATIONAHA.107.187998. [DOI] [PubMed] [Google Scholar]
- 2.Wu CM, McLaughlin K, Lorenzetti DL, Hill MD, Manns BJ, Ghali WA. Early risk of stroke after transient ischemic attack: a systematic review and meta-analysis. Arch Intern Med. 2007;167:2417–2422. doi: 10.1001/archinte.167.22.2417. [DOI] [PubMed] [Google Scholar]
- 3.Howard G, Evans GW, Crouse JR, 3rd, Toole JF, Ryu JE, Tegeler C, Frye-Pierson J, Mitchell E, Sanders L. A prospective reevaluation of transient ischemic attacks as a risk factor for death and fatal or nonfatal cardiovascular events. Stroke. 1994;25:342–345. doi: 10.1161/01.str.25.2.342. [DOI] [PubMed] [Google Scholar]
- 4.Johnston SC, Gress DR, Browner WS, Sidney S. Short-term prognosis after emergency department diagnosis of TIA. JAMA. 2000;284:2901–2906. doi: 10.1001/jama.284.22.2901. [DOI] [PubMed] [Google Scholar]
- 5.Lavallee PC, Meseguer E, Abboud H, Cabrejo L, Olivot JM, Simon O, Mazighi M, Nifle C, Niclot P, Lapergue B, Klein IF, Brochet E, Steg PG, Leseche G, Labreuche J, Touboul PJ, Amarenco P. A transient ischaemic attack clinic with round-the-clock access (SOS-TIA): feasibility and effects. Lancet Neurol. 2007;6:953–960. doi: 10.1016/S1474-4422(07)70248-X. [DOI] [PubMed] [Google Scholar]
- 6.Purroy F, Montaner J, Molina CA, Delgado P, Ribo M, Alvarez-Sabin J. Patterns and predictors of early risk of recurrence after transient ischemic attack with respect to etiologic subtypes. Stroke. 2007;38:3225–3229. doi: 10.1161/STROKEAHA.107.488833. [DOI] [PubMed] [Google Scholar]
- 7.van Wijk I, Kappelle LJ, van Gijn J, Koudstaal PJ, Franke CL, Vermeulen M, Gorter JW, Algra A. Long-term survival and vascular event risk after transient ischaemic attack or minor ischaemic stroke: a cohort study. Lancet. 2005;365:2098–2104. doi: 10.1016/S0140-6736(05)66734-7. [DOI] [PubMed] [Google Scholar]
- 8.Kleindorfer D, Panagos P, Pancioli A, Khoury J, Kissela B, Woo D, Schneider A, Alwell K, Jauch E, Miller R, Moomaw C, Shukla R, Broderick JP. Incidence and short-term prognosis of transient ischemic attack in a population-based study. Stroke. 2005;36:720–723. doi: 10.1161/01.STR.0000158917.59233.b7. [DOI] [PubMed] [Google Scholar]
- 9.Albers GW, Hart RG, Lutsep HL, Newell DW, Sacco RL. AHA Scientific Statement. Supplement to the guidelines for the management of transient ischemic attacks: A statement from the Ad Hoc Committee on Guidelines for the Management of Transient Ischemic Attacks, Stroke Council, American Heart Association. Stroke. 1999;30:2502–2511. doi: 10.1161/01.str.30.11.2502. [DOI] [PubMed] [Google Scholar]
- 10.Feinberg WM, Albers GW, Barnett HJ, Biller J, Caplan LR, Carter LP, Hart RG, Hobson RW, 2nd, Kronmal RA, Moore WS, et al. Guidelines for the management of transient ischemic attacks. From the Ad Hoc Committee on Guidelines for the Management of Transient Ischemic Attacks of the Stroke Council of the American Heart Association. Circulation. 1994;89:2950–2965. doi: 10.1161/01.cir.89.6.2950. [DOI] [PubMed] [Google Scholar]
- 11.Johnston SC, Nguyen-Huynh MN, Schwarz ME, Fuller K, Williams CE, Josephson SA, Hankey GJ, Hart RG, Levine SR, Biller J, Brown RD, Jr, Sacco RL, Kappelle LJ, Koudstaal PJ, Bogousslavsky J, Caplan LR, van Gijn J, Algra A, Rothwell PM, Adams HP, Albers GW. National Stroke Association guidelines for the management of transient ischemic attacks. Ann Neurol. 2006;60:301–313. doi: 10.1002/ana.20942. [DOI] [PubMed] [Google Scholar]
- 12.Nguyen-Huynh MN, Johnston SC. Evaluation and management of transient ischemic attack: an important component of stroke prevention. Nat Clin Pract Cardiovasc Med. 2007;4:310–318. doi: 10.1038/ncpcardio0889. [DOI] [PubMed] [Google Scholar]
- 13.Sacco RL, Adams R, Albers G, Alberts MJ, Benavente O, Furie K, Goldstein LB, Gorelick P, Halperin J, Harbaugh R, Johnston SC, Katzan I, Kelly-Hayes M, Kenton EJ, Marks M, Schwamm LH, Tomsick T. Guidelines for prevention of stroke in patients with ischemic stroke or transient ischemic attack: a statement for healthcare professionals from the American Heart Association/American Stroke Association Council on Stroke: co-sponsored by the Council on Cardiovascular Radiology and Intervention: the American Academy of Neurology affirms the value of this guideline. Stroke. 2006;37:577–617. doi: 10.1161/01.STR.0000199147.30016.74. [DOI] [PubMed] [Google Scholar]
- 14.Rothwell PM, Giles MF, Chandratheva A, Marquardt L, Geraghty O, Redgrave JN, Lovelock CE, Binney LE, Bull LM, Cuthbertson FC, Welch SJ, Bosch S, Alexander FC, Silver LE, Gutnikov SA, Mehta Z. Effect of urgent treatment of transient ischaemic attack and minor stroke on early recurrent stroke (EXPRESS study): a prospective population-based sequential comparison. Lancet. 2007;370:1432–1442. doi: 10.1016/S0140-6736(07)61448-2. [DOI] [PubMed] [Google Scholar]
- 15.Predictors of major vascular events in patients with a transient ischemic attack or nondisabling stroke. The Dutch TIA Trial Study Group. Stroke. 1993;24:527–531. doi: 10.1161/01.str.24.4.527. [DOI] [PubMed] [Google Scholar]
- 16.Brown DL, Lisabeth LD, Roychoudhury C, Ye Y, Morgenstern LB. Recurrent stroke risk is higher than cardiac event risk after initial stroke/transient ischemic attack. Stroke. 2005;36:1285–1287. doi: 10.1161/01.STR.0000165926.74213.e3. [DOI] [PubMed] [Google Scholar]
- 17.Correia M, Silva MR, Magalhaes R, Guimaraes L, Silva MC. Transient ischemic attacks in rural and urban northern Portugal: incidence and short-term prognosis. Stroke. 2006;37:50–55. doi: 10.1161/01.STR.0000195209.26543.8f. [DOI] [PubMed] [Google Scholar]
- 18.Lisabeth LD, Ireland JK, Risser JM, Brown DL, Smith MA, Garcia NM, Morgenstern LB. Stroke risk after transient ischemic attack in a population-based setting. Stroke. 2004;35:1842–1846. doi: 10.1161/01.STR.0000134416.89389.9d. [DOI] [PubMed] [Google Scholar]
- 19.Ois A, Gomis M, Rodriguez-Campello A, Cuadrado-Godia E, Jimenez-Conde J, Pont-Sunyer C, Cuccurella G, Roquer J. Factors associated with a high risk of recurrence in patients with transient ischemic attack or minor stroke. Stroke. 2008;39:1717–1721. doi: 10.1161/STROKEAHA.107.505438. [DOI] [PubMed] [Google Scholar]
- 20.Rothwell PM, Giles MF, Flossmann E, Lovelock CE, Redgrave JN, Warlow CP, Mehta Z. A simple score (ABCD) to identify individuals at high early risk of stroke after transient ischaemic attack. Lancet. 2005;366:29–36. doi: 10.1016/S0140-6736(05)66702-5. [DOI] [PubMed] [Google Scholar]
- 21.Tsivgoulis G, Spengos K, Manta P, Karandreas N, Zambelis T, Zakopoulos N, Vassilopoulos D. Validation of the ABCD score in identifying individuals at high early risk of stroke after a transient ischemic attack: a hospital-based case series study. Stroke. 2006;37:2892–2897. doi: 10.1161/01.STR.0000249007.12256.4a. [DOI] [PubMed] [Google Scholar]
- 22.Hill ME, Rosenwaike I. The Social Security Administration’s Death Master File: the completeness of death reporting at older ages. Soc Secur Bull. 2001;64:45–51. [PubMed] [Google Scholar]
- 23.Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45:613–619. doi: 10.1016/0895-4356(92)90133-8. [DOI] [PubMed] [Google Scholar]
- 24.Giles MF, Flossman E, Rothwell PM. Patient behavior immediately after transient ischemic attack according to clinical characteristics, perception of the event, and predicted risk of stroke. Stroke. 2006;37:1254–1260. doi: 10.1161/01.STR.0000217388.57851.62. [DOI] [PubMed] [Google Scholar]
- 25.Howard VJ, Lackland DT, Lichtman JH, McClure LA, Howard G, Wagner L, Pulley L, Gomez CR. Care seeking after stroke symptoms. Ann Neurol. 2008;63:466–472. doi: 10.1002/ana.21357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Di Carlo A, Lamassa M, Baldereschi M, Pracucci G, Basile AM, Wolfe CD, Giroud M, Rudd A, Ghetti A, Inzitari D. Sex differences in the clinical presentation, resource use, and 3-month outcome of acute stroke in Europe: data from a multicenter multinational hospital-based registry. Stroke. 2003;34:1114–1119. doi: 10.1161/01.STR.0000068410.07397.D7. [DOI] [PubMed] [Google Scholar]
- 27.Gargano JW, Wehner S, Reeves M. Sex differences in acute stroke care in a statewide stroke registry. Stroke. 2008;39:24–29. doi: 10.1161/STROKEAHA.107.493262. [DOI] [PubMed] [Google Scholar]
- 28.Holroyd-Leduc JM, Kapral MK, Austin PC, Tu JV. Sex differences and similarities in the management and outcome of stroke patients. Stroke. 2000;31:1833–1837. doi: 10.1161/01.str.31.8.1833. [DOI] [PubMed] [Google Scholar]
- 29.Kapral MK, Fang J, Hill MD, Silver F, Richards J, Jaigobin C, Cheung AM. Sex differences in stroke care and outcomes: results from the Registry of the Canadian Stroke Network. Stroke. 2005;36:809–814. doi: 10.1161/01.STR.0000157662.09551.e5. [DOI] [PubMed] [Google Scholar]
- 30.Reid JM, Dai D, Gubitz GJ, Kapral MK, Christian C, Phillips SJ. Gender differences in stroke examined in a 10-year cohort of patients admitted to a Canadian teaching hospital. Stroke. 2008;39:1090–1095. doi: 10.1161/STROKEAHA.107.495143. [DOI] [PubMed] [Google Scholar]
- 31.Ellekjaer H, Holmen J, Kruger O, Terent A. Identification of incident stroke in Norway: hospital discharge data compared with a population-based stroke register. Stroke. 1999;30:56–60. doi: 10.1161/01.str.30.1.56. [DOI] [PubMed] [Google Scholar]
- 32.Kokotailo RA, Hill MD. Coding of stroke and stroke risk factors using international classification of diseases, revisions 9 and 10. Stroke. 2005;36:1776–1781. doi: 10.1161/01.STR.0000174293.17959.a1. [DOI] [PubMed] [Google Scholar]
- 33.Leone MA, Capponi A, Varrasi C, Tarletti R, Monaco F. Accuracy of the ICD-9 codes for identifying TIA and stroke in an Italian automated database. Neurol Sci. 2004;25:281–288. doi: 10.1007/s10072-004-0355-8. [DOI] [PubMed] [Google Scholar]
- 34.Tolonen H, Salomaa V, Torppa J, Sivenius J, Immonen-Raiha P, Lehtonen A. The validation of the Finnish Hospital Discharge Register and Causes of Death Register data on stroke diagnoses. Eur J Cardiovasc Prev Rehabil. 2007;14:380–385. doi: 10.1097/01.hjr.0000239466.26132.f2. [DOI] [PubMed] [Google Scholar]
- 35.Gladstone DJ, Kapral MK, Fang J, Laupacis A, Tu JV. Management and outcomes of transient ischemic attacks in Ontario. CMAJ. 2004;170:1099–1104. doi: 10.1503/cmaj.1031349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hill MD, Yiannakoulias N, Jeerakathil T, Tu JV, Svenson LW, Schopflocher DP. The high risk of stroke immediately after transient ischemic attack: a population-based study. Neurology. 2004;62:2015–2020. doi: 10.1212/01.wnl.0000129482.70315.2f. [DOI] [PubMed] [Google Scholar]
- 37.Johnston SC, Rothwell PM, Nguyen-Huynh MN, Giles MF, Elkins JS, Bernstein AL, Sidney S. Validation and refinement of scores to predict very early stroke risk after transient ischaemic attack. Lancet. 2007;369:283–292. doi: 10.1016/S0140-6736(07)60150-0. [DOI] [PubMed] [Google Scholar]
- 38.Ricci S, Celani MG, La Rosa F, Vitali R, Duca E, Ferraguzzi R, Paolotti M, Seppoloni D, Caputo N, Chiurulla C, et al. A community-based study of incidence, risk factors and outcome of transient ischaemic attacks in Umbria, Italy: the SEPIVAC study. J Neurol. 1991;238:87–90. doi: 10.1007/BF00315687. [DOI] [PubMed] [Google Scholar]
- 39.Sciolla R, Melis F. Rapid identification of high-risk transient ischemic attacks: prospective validation of the ABCD score. Stroke. 2008;39:297–302. doi: 10.1161/STROKEAHA.107.496612. [DOI] [PubMed] [Google Scholar]
- 40.Heyman A, Wilkinson WE, Hurwitz BJ, Haynes CS, Utley CM, Rosati RA, Burch JG, Gore TB. Risk of ischemic heart disease in patients with TIA. Neurology. 1984;34:626–630. doi: 10.1212/wnl.34.5.626. [DOI] [PubMed] [Google Scholar]
- 41.Goldstein LB. Accuracy of ICD-9-CM coding for the identification of patients with acute ischemic stroke: effect of modifier codes. Stroke. 1998;29:1602–1604. doi: 10.1161/01.str.29.8.1602. [DOI] [PubMed] [Google Scholar]
- 42.Tirschwell DL, Longstreth WT., Jr Validating administrative data in stroke research. Stroke. 2002;33:2465–2470. doi: 10.1161/01.str.0000032240.28636.bd. [DOI] [PubMed] [Google Scholar]