Abstract
We recently found that simvastatin can modulate the nuclear factor-κB (NF-κB) activation pathway, but whether other statins have similar effects to those of simvastatin is unknown. Therefore, we evaluated the effect six different statins on TNF-induced NF-κB activation in human myeloid leukemia cells. We then determined whether the combination of statins and standard chemotherapeutic agents could overcome chemoresistance and augment apoptosis. Of the six statins evaluated, only the natural statins (simvastatin, mevastatin, lovastatin, and pravastatin), not the synthetic statins (fluvastatin and atorvastatin), inhibited TNF-induced NF-κB activation. Simvastatin suppressed the NF-κB activation and potentiated the apoptosis induced by doxorubicin, paclitaxel, and 5-fluorouracil. These results suggest that different statins behave differently from one another and that they may be useful in overcoming chemoresistance.
Keywords: Simvastatin, nuclear factor-kappaB, chemotherapeutic agents, apoptosis
1. Introduction
Statins may be the most important family of cholesterol-lowering drugs to emerge in the 21st century [1]. Statins primarily inhibit 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase, which is needed to produce cholesterol through the mevalonate pathway.
Recent evidence suggests that statins have pleiotropic effects and thus may be useful in the treatment of diseases such as cancer [2–6]. Indeed, statins have been found to have anticancer activity in various cancer cell types, including colorectal [7], colon [8], bladder [9], prostate [10], and gastrointestinal [11] cancer, although they do not significantly reduce the risk for breast, prostatic, colorectal, or lung cancer [12]. Browning et al [13] suggested that statins are not associated with short-term cancer risk, but longer latency effects are possible.
Recently, we reported that simvastatin can potentiate the TNF-induced apoptosis through down-regulation of nuclear factor-κB (NF-κB) regulated antiapoptotic gene products [14]. NF-κB activation has been associated with tumor cell proliferation, invasion, angiogenesis, and metastasis through its regulation of various gene products [15]. Thus, NF-κB suppression in cancer cells may be useful in the prevention and treatment of cancer [16]. Inducible drug resistance has emerged as a substantial obstacle to effective cancer therapy, and NF-κB activation may play a role in the development of chemoresistance 16[17]. In fact, chemotherapeutic agents themselves can activate NF-κB, which leads to tumor cells’ eventual resistance to therapy [17]. NF-κB activation has been associated with paclitaxel, doxorubicin, and 5-fluorouracil resistance in tumor cells [18–20].
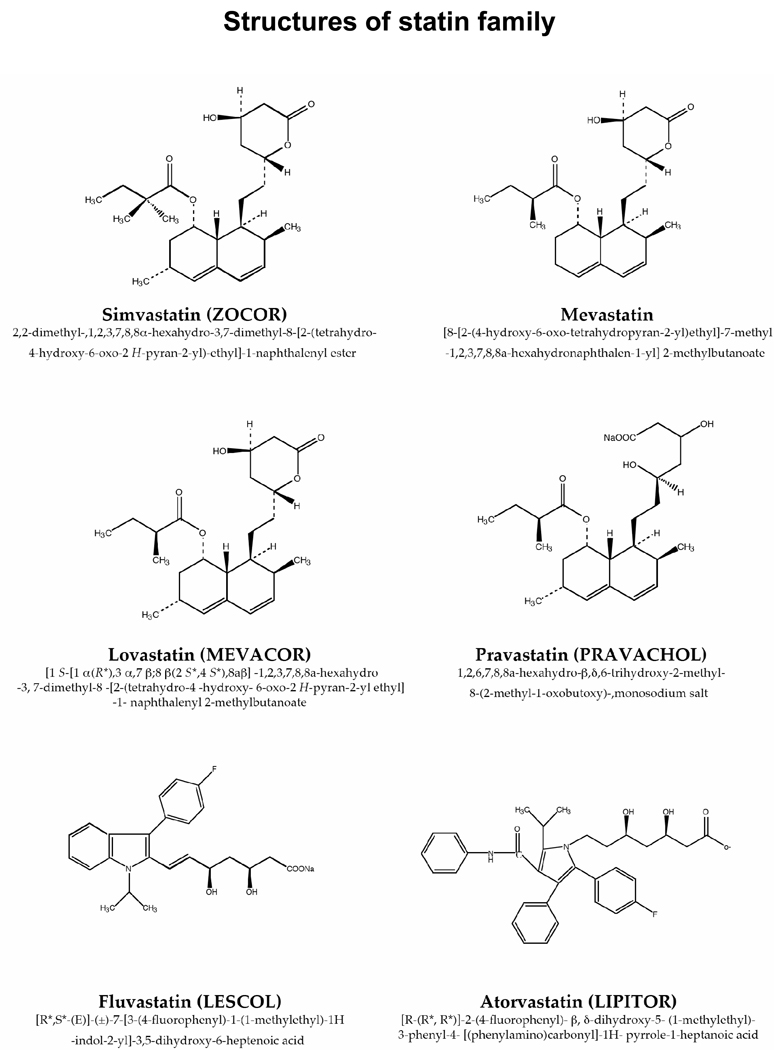
Lovastatin, mevastatin, simvastatin (a methyl derivative of lovastatin), and pravastatin are natural statins, isolated from fermented red yeast rice; fluvastatin, atorvastatin, cerivastatin, rosuvastatin, and pitavastatin are synthetic compounds. Natural and synthetic statins have different biologic characteristics (Fig. 1); whether they have similar potency against NF-κB and can potentiate the effects of chemotherapeutic agents is not understood. Therefore, we examined the ability of six statins to suppress TNF-induced NF-κB activation and if this inhibition overcomes chemoresistance and enhances apoptosis in human myeloid leukemia cells. The six statins varied in their ability to suppress NF-κB activation, and simvastatin suppressed chemotherapeutic agent-induced NF-κB activation, leading to potentiation of apoptosis.
Fig. 1.
Structures of the natural (lovastatin, mevastatin, simvastatin, and pravastatin) and synthetic (fluvastatin, atorvastatin) statins used.
2. Materials and Methods
2.1. Materials
All statins were obtained from LKT Laboratories, Inc. (St. Paul, MN). A 50 mM solution of statins was prepared in 100% dimethyl sulfoxide, stored as small aliquots at −20°C, and then diluted as needed in cell culture medium. Bacteria-derived recombinant human tumor necrosis factor (TNF), purified to homogeneity with a specific activity of 5 × 107 U/mg, was kindly provided by Genentech (South San Francisco, CA). Penicillin, streptomycin, Iscove’s modified Dulbecco’s medium, and fetal bovine serum were obtained from Invitrogen (Grand Island, NY). Paclitaxel, doxorubicin, and 5-fluorouracil were obtained from Sigma-Aldrich (St. Louis, MO).
2.2. Cell lines
Human myeloid leukemia KBM-5 cells were obtained from American Type Culture Collection (Manassas, VA). KBM-5 cells were cultured in Iscove’s modified Dulbecco’s medium supplemented with 15% fetal bovine serum. Media were also supplemented with 100 U/mL of penicillin and 100 µg/mL of streptomycin.
2.3. NF-κB activation
We performed an electrophoretic mobility shift assay (EMSA), essentially as previously described [21]. In brief, nuclear extracts prepared from TNF-treated cells (1 × 106/mL) were incubated with 32P-end-labeled 45-mer double-stranded NF-κB oligonucleotides (15 µg of protein with 16 fmol of DNA) from the human immunodeficiency virus long terminal repeat 5′-TTGTTACAA GGGACTTTC CGCTG GGGACTTTC CAGGGAGGCGTGG-3′ (boldface indicates NF-κB-binding sites) for 30 minutes at 37°C, and the DNA-protein complex that formed was separated from free oligonucleotides on 6.6% native polyacrylamide gels. A double-stranded mutated oligonucleotide, 5-TTGTTACAA CTCACTTTC CGCTG CTCACTTTC CAGGGAGGCGTGG-3′, was used to determine the specificity of NF-κB binding to DNA. The specificity of binding was also evaluated by competition with the unlabeled oligonucleotide. The dried gels were visualized with a Storm 820 phosporimager, and radioactive bands were quantified using Imagequant software (Amersham, Piscataway, NJ).
2.4. Cytotoxicity assay
The effect of simvastatin on the cytotoxic effects of chemotherapeutic reagents was determined by the MTT uptake method, as previously described [22]. In brief, 5000 cells were incubated with simvastatin for 12 hours in triplicate on a 96-well plate and then treated with various concentrations of reagents for 24 hours at 37°C. Thereafter, an MTT solution was added to each well. After a 2-hour incubation at 37°C, extraction buffer (20% SDS and 50% dimethylformamide) was added, the cells were incubated overnight at 37°C, and the optical density was measured at 570 nm using a 96-well multiscanner (MRX Revelation; Dynex Technologies, Chantilly, VA).
2.5. Live/dead assay
To measure apoptosis, we used the live/dead assay (Molecular Probes, OR), which determines intracellular esterase activity and plasma membrane integrity. This assay uses calcein, a polyanionic dye, which is retained in live cells and provides green fluorescence [14]. It also uses the ethidium monomer dye (red fluorescence), which can enter cells only through damaged membranes and bind to nucleic acids but is excluded by the intact plasma membrane of live cells.
In brief, 1 × 106 cells were incubated with 5 µM simvastatin for 12 hours and then treated with 100 nM paclitaxel, 100 nM doxorubicin, and 5 µM 5-fluorouracil for 24 hours at 37°C. Cells were stained with the live/dead reagent (5 µM ethidium homodimer and 5 µM calcein-AM) and then incubated at 37°C for 30 minutes. Cells were analyzed under a fluorescence microscope (Labophot-2; Nikon, Tokyo, Japan). The % values were derived by counting of the red and green cell numbers manually.
3. Results
The structures of the six statins (Fig. 1) suggest that natural statin molecules are more similar to each other than composed to synthetic molecules. The conditions used to investigate their effects on the NF-κB pathway had no effect on cell viability (data not shown).
3.1. Only natural statins suppressed TNF-induced NF-κB activation
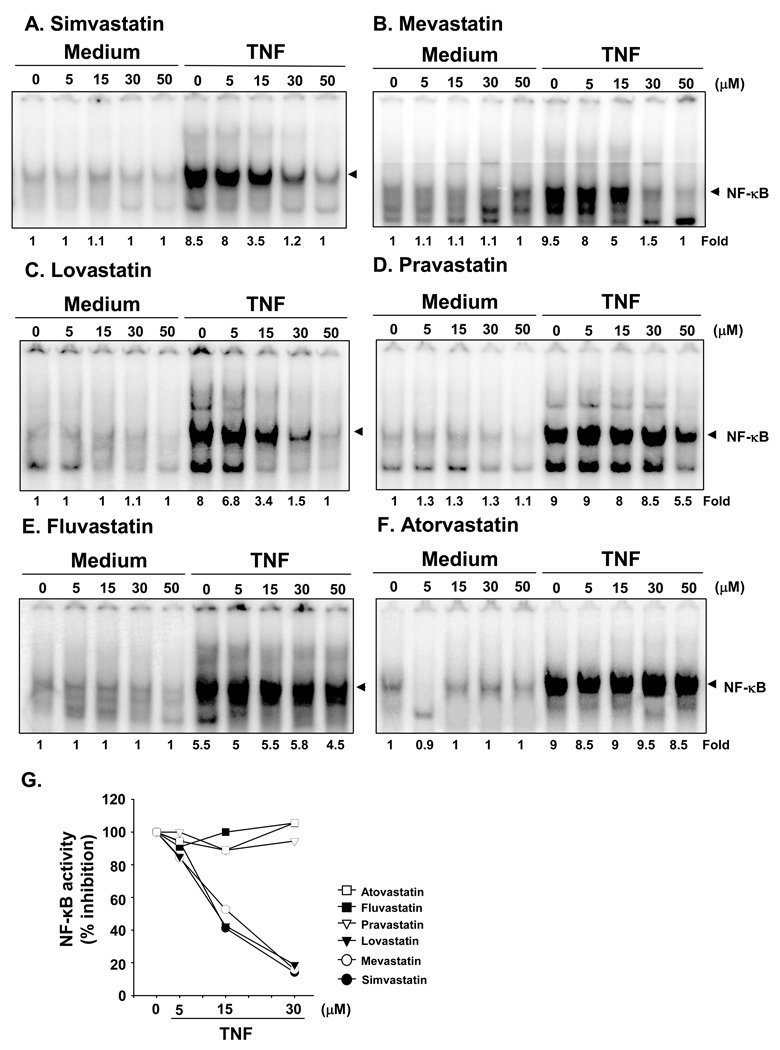
KBM-5 cells were pretreated with different doses of statins for 12 hours and then treated with 0.1 nM TNF for 30 minutes to activate NF-κB. Natural statins (Fig. 2, panels A–D) inhibited TNF-induced NF-κB activation; synthetic statins (panels E and F) did not. Among the natural statins, simvastatin was the most active, and pravastatin was the least active. Therefore, in all subsequent studies, only simvastatin was used.
Fig. 2.
(A–F). Statins differ in their ability to suppress NF-κB activation in KBM-5 cells. KBM-5 cells (1 × 106/mL) were preincubated with the indicated concentrations of statins for 12 hours at 37°C and then treated with 0.1 nM TNF for 30 minutes. Nuclear extracts were prepared and tested for NF-κB activation as described in Materials and Methods. (G). Quantitative analysis of NF-κB inhibitory effect of different statins.
3.2. Simvastatin suppressed NF-κB activation induced by chemotherapeutic agents
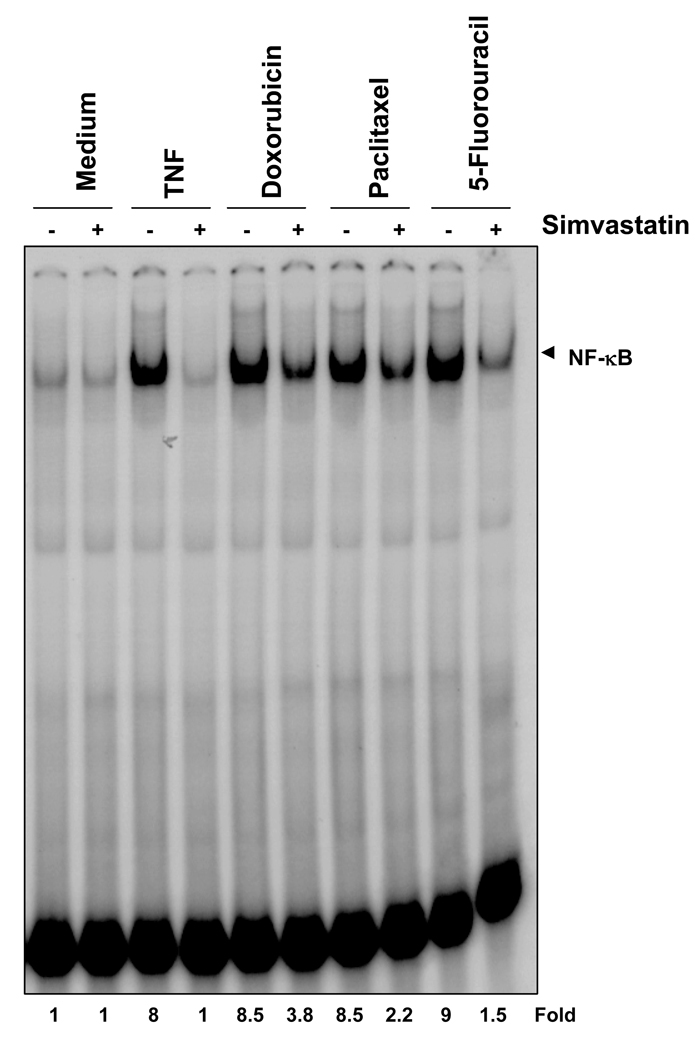
Whether chemotherapeutic agent-induced NF-κB activation could be modulated by statins. KBM-5 cells were pretreated with simvastatin for 12 hours and then with doxorubicin, paclitaxel, or 5-fluorouracil. As shown in Fig. 3, all three chemotherapeutic agents activated NF-κB, and simvastatin treatment suppressed this activation.
Fig. 3.
Simvastatin suppresses chemotherapeutic agent-induced NF-κB activation in KBM-5 cells. KBM-5 cells (1 × 106/mL) were preincubated with 50 µM simvastatin for 12 hours at 37°C and then treated with TNF (0.1 nM) for 30 minutes, doxorubicin (2 µM) for 6 hours, paclitaxel (100 µM) for 8 hours, and 5-fluorouracil (100 µM) for 6 hours. Nuclear extracts were prepared and tested for NF-κB activation as described in Materials and Methods.
3.3. Simvastatin enhanced the cytotoxic effects of doxorubicin, paclitaxel, and 5-fluorouracil
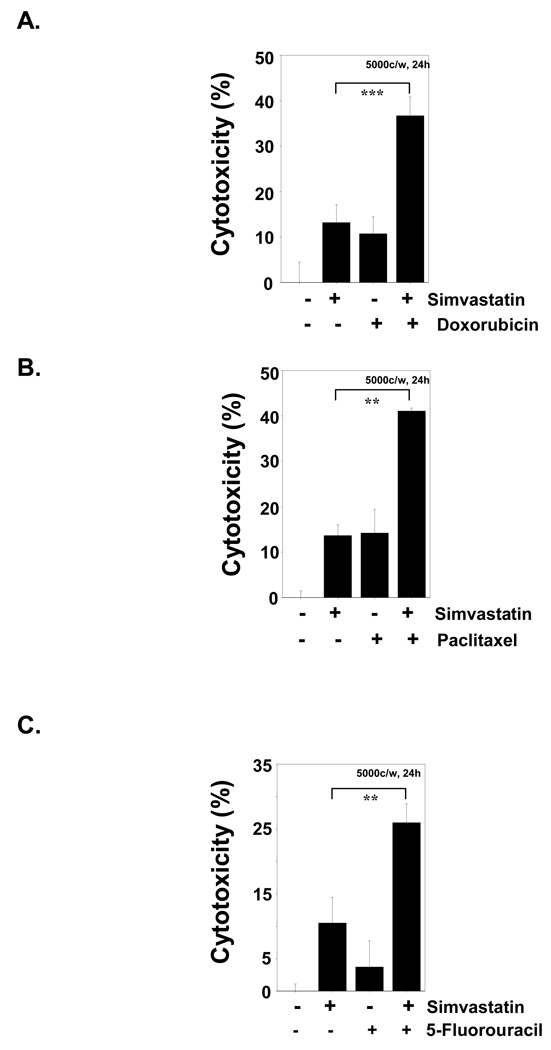
Because NF-κB activation has been shown to suppress apoptosis [23], we determined whether simvastatin modulated chemotherapeutic agent-induced apoptosis in KBM-5 cells using the MTT assay. Simvastatin synergistically enhanced the cytotoxic effects of paclitaxel, doxorubicin, and 5-fluorouracil (Fig. 4A–C).
Fig. 4.
Simvastatin enhances cytotoxicity induced by chemotherapeutic agents. KBM-5 cells (5000 cells/0.1 mL) were incubated at 37°C with (A), 100 nM doxorubicin; (B), 100 nM paclitaxel; (C), 5 µM 5-fluorouracil in the presence and absence of 5 µM simvastatin as indicated for 24 hours, and viable cells were assayed using the MTT reagent. The results are expressed as mean cytotoxicity ± standard deviation from triplicate cultures. Determinations were made in triplicate. Data represent the mean of three measurements ± SD. *** p <0.001, ** p <0.01.
3.4. Simvastatin potentiated the apoptotic effects of doxorubicin, paclitaxel, and 5-fluorouracil
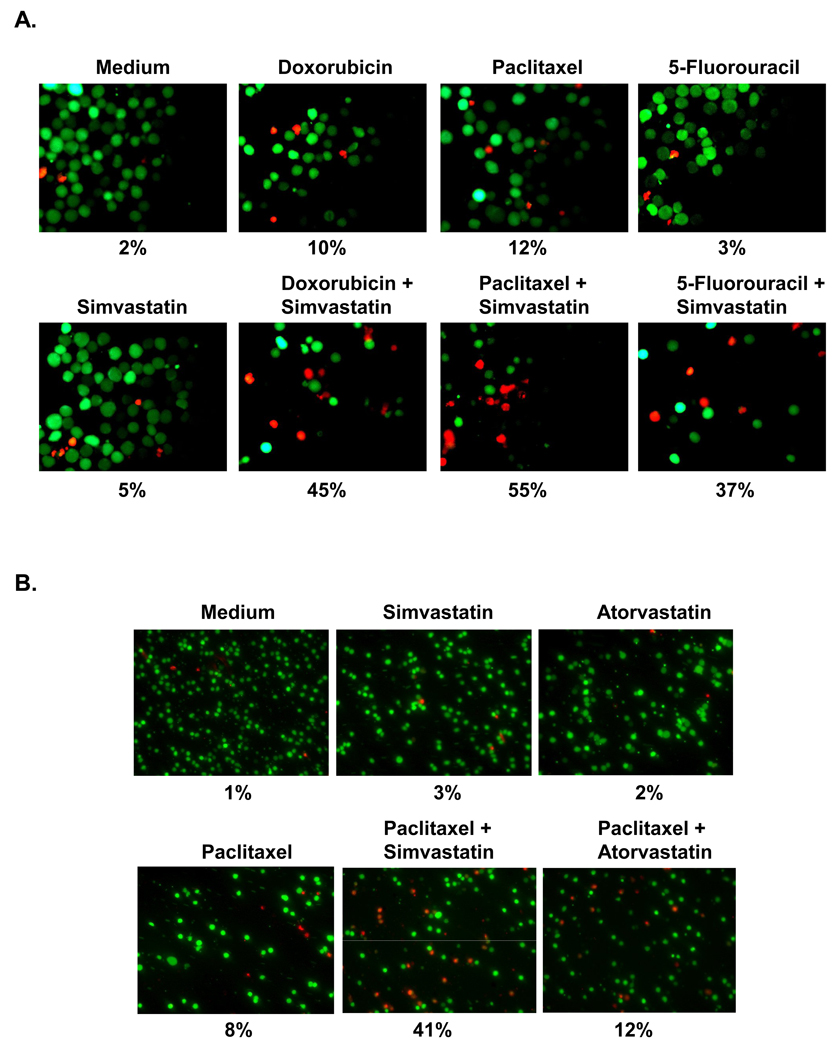
We next performed the live/dead assay and found that simvastatin upregulated paclitaxel-induced apoptosis (from 12% to 55%), doxorubicin-induced apoptosis (10% to 45%), and 5-fluorouracil-induced apoptosis (3% to 37%) (Fig. 5A). The results of these assays suggest that simvastatin enhances the apoptotic effects of chemotherapeutic agents. We also investigated whether atorvastatin enhances paclitaxel-induced apoptosis. In agreement with NF-κB activity, atorvastatin did not significantly enhance the apoptotic effect of paclitaxel (Fig. 5B).
Fig. 5.
(A). Simvastatin potentiates apoptotic effect induced by chemotherapeutic agents. KBM-5 cells (1 × 106/mL) were incubated with chemotherapeutic agents, alone or in combination with simvastatin, as indicated above, for 24 hours. Cell death was determined by the calcein-AM-based live/dead assay, as described in Materials and Methods. (B). Atorvastain has no effect on paclitaxel induced apoptosis. KBM-5 cells (1 × 106/mL) were incubated with paclitaxel, alone or in combination with atorvastatin, as indicated above, for 24 hours. Cell death was determined by the calcein-AM-based live/dead assay, as described in Materials and Methods. Experiments were performed in triplicate. Data are from one representative experiment.
4. Discussion
In present study, we first investigated the effect of various statins on TNF and chemotherapeutic agents induced the NF-κB activation and apoptosis in myeloid leukemia cells. Only natural statins (simvastatin, mevastatin, lovastatin, and pravastatin) blocked TNF-induced NF-kB activation; synthetic statins did not. Simvastatin also suppressed doxorubicin-, paclitaxel-, and 5-fluorouracil-induced NF-kB activation in human myeloid leukemia cells. Simvastatin also potentiated the apoptosis induced by TNF and these chemotherapeutic agents.
Our results indicate that only statins derived from fungal fermentation inhibited NF-κB activation. Synthetic statins, fluvastatin and atorvastatin did not. Why lovastatin, simvastatin, mevastatin, and pravastatin suppressed NF-κB activation and fluvastatin and atorvastatin did not is unclear; Lovastatin, mevastatin, and simvastatin contain a lactone ring (Fig. 1), which may be essential for suppressing NF-κB activation. In contrast, fluvastatin and atorvastatin are acidic in nature. The Ki for 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase of lovastatin, simvastatin, and pravastatin are 0.6 nM, 0.12 nM, and 2.3 nM, respectively [24], which may also explain the difference in their activities.
Lovastatin in combination with TNF has been reported to inhibit proliferation of both murine melanoma and leukemia cells [25]. That mevastatin can was found to suppress TNF-induced NF-κB activation is in agreement with the results of an earlier report study in endothelial cells [26]. Hilgendorff and colleagues [27] determined the effects of different statins on lipopolysaccharide-induced NF-κB activation in human monocytes; and reported that atorvastatin was the most effective in at blocking NF-κB activation, followed by simvastatin, pravastatin, lovastatin, and fluvastatin. Why these results differ so substantially from ours is not clear, it could relate to the inducer and cells used. Hilgendorff and colleagues [27] found that fluvastatin inhibited NF-κB activation by only 5%; this result is similar to ours. Chemotherapeutic agents have been shown to induce NF-κB activation through the upregulation of anti-apoptotic gene products that leads to chemoresistance [17]. In this study, we found that doxorubicin, paclitaxel, and 5-fluorourcil were potent inducers of NF-κB. This NF-kB activation, however, was abrogated by simvastatin. The combination of simvastatin and standard chemotherapeutic agents resulted in enhanced cytotoxic effects and potentiated apoptosis in tumor cells. Thus, simvastatin by the virtue of its NF-κB inhibitory effect can sensitize the cells to these drugs and hence overcome chemoresistance. Under identical conditions, atorvastatin did not have any substantial effect on NF-κB activation and apoptosis. We previously demonstrated that, concurrent with downregulation of gene expression, apoptosis induced by TNF is potentiated by simvastatin [14]. This provides a novel opportunity to exploit statins, not only in the prevention but also the treatment of cancer.
Overall, our results provide novel insights into statins’ role in overcoming chemoresistance through the modulation of NF-κB. Considering an extensive experience on the safety of statins in human subjects, Statins may be a novel approach for the treatment of various cancers.
Acknowledgments
We thank Ann Sutton for her careful review of this manuscript.
Dr. Aggarwal is the Ransom Horne, Jr., Professor of Cancer Research. This study was supported by the Clayton Foundation for Research (B.B.A.) and by the Cancer center core grant from the National Institutes of Health.
The abbreviations used are
- NF-κB
nuclear factor-κB
- TNF
tumor necrosis factor α
- EMSA
electrophoretic mobility shift assay.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Pawelczyk T, Kloszewska I, Sobow T. Statins—“21st century aspirin”? Pol Merkuriusz Lek. 2005;19:111–114. [PubMed] [Google Scholar]
- 2.Miida T, Hirayama S, Nakamura Y. Cholesterol-independent effects of statins and new therapeutic targets: ischemic stroke and dementia. J Atheroscler Thromb. 2004;11:253–264. doi: 10.5551/jat.11.253. [DOI] [PubMed] [Google Scholar]
- 3.Gonyeau MJ. Statins and osteoporosis: a clinical review. Pharmacotherapy. 2005;25:228–243. doi: 10.1592/phco.25.2.228.56954. [DOI] [PubMed] [Google Scholar]
- 4.Brower V. Of cancer and cholesterol: studies elucidate anticancer mechanisms of statins. J Natl Cancer Inst. 2003;95:844–846. doi: 10.1093/jnci/95.12.844. [DOI] [PubMed] [Google Scholar]
- 5.Haffner SM. Statin therapy for the treatment of diabetic dyslipidemia. Diabetes Metab Res Rev. 2003;19:280–287. doi: 10.1002/dmrr.393. [DOI] [PubMed] [Google Scholar]
- 6.Ray JG, Mamdani M, Tsuyuki RT, Anderson DR, Yeo EL, Laupacis A. Use of statins and the subsequent development of deep vein thrombosis. Arch Intern Med. 2001;161:1405–1410. doi: 10.1001/archinte.161.11.1405. [DOI] [PubMed] [Google Scholar]
- 7.Coogan PF, Smith J, Rosenberg L. Statin use and risk of colorectal cancer. J Natl Cancer Inst. 2007;99:32–40. doi: 10.1093/jnci/djk003. [DOI] [PubMed] [Google Scholar]
- 8.Agarwal B, Bhendwal S, Halmos B, Moss SF, Ramey WG, Holt PR. Lovastatin augments apoptosis induced by chemotherapeutic agents in colon cancer cells. Clin Cancer Res. 1999;5:2223–2229. [PubMed] [Google Scholar]
- 9.Hoffmann P, Roumeguere T, Schulman C, van Velthoven R. Use of statins and outcome of BCG treatment for bladder cancer. N Engl J Med. 2006;355:2705–2707. doi: 10.1056/NEJMc062714. [DOI] [PubMed] [Google Scholar]
- 10.Platz EA, Leitzmann MF, Visvanathan K, Rimm EB, Stampfer MJ, Willett WC, et al. Statin drugs and risk of advanced prostate cancer. J Natl Cancer Inst. 2006;98:1819–1825. doi: 10.1093/jnci/djj499. [DOI] [PubMed] [Google Scholar]
- 11.Bhuket TP, Higgins PD. Drug insight: statins and gastrointestinal cancer. Nat Clin Pract Gastroenterol Hepatol. 2006;3:552–562. doi: 10.1038/ncpgasthep0603. [DOI] [PubMed] [Google Scholar]
- 12.Coogan PF, Rosenberg L, Strom BL. Statin use and the risk of 10 cancers. Epidemiology. 2007;18:213–219. doi: 10.1097/01.ede.0000254694.03027.a1. [DOI] [PubMed] [Google Scholar]
- 13.Browning DR, Martin RM. Statins and risk of cancer: a systematic review and metaanalysis. Int J Cancer. 2007;120:833–843. doi: 10.1002/ijc.22366. [DOI] [PubMed] [Google Scholar]
- 14.Ahn KS, Sethi G, Aggarwal BB. Simvastatin potentiates TNF-α-induced apoptosis through the down-regulation of NF-κB-dependent antiapoptotic gene products: role of IκBα kinase and TGF-beta-activated kinase-1. J Immunol. 2007;178:2507–2516. doi: 10.4049/jimmunol.178.4.2507. [DOI] [PubMed] [Google Scholar]
- 15.Ahn KS, Aggarwal BB. Transcription factor NF-κB: a sensor for smoke and stress signals. Ann N Y Acad Sci. 2005;1056:218–233. doi: 10.1196/annals.1352.026. [DOI] [PubMed] [Google Scholar]
- 16.Garg A, Aggarwal BB. Nuclear transcription factor-kappaB as a target for cancer drug development. Leukemia. 2002;16:1053–1068. doi: 10.1038/sj.leu.2402482. [DOI] [PubMed] [Google Scholar]
- 17.Bharti AC, Aggarwal BB. Nuclear factor-kappa B and cancer: its role in prevention and therapy. Biochem Pharmacol. 2002;64:883–888. doi: 10.1016/s0006-2952(02)01154-1. [DOI] [PubMed] [Google Scholar]
- 18.Sethi G, Aggarwal BB. Role of NF-kappaB and NF-kappaB-regulated gene products in chemoresistance and radioresistance. Curr Cancer Ther Rev. 2006;2:115–125. [Google Scholar]
- 19.Patel NM, Nozaki S, Shortle NH, Bhat-Nakshatri P, Newton TR, Rice S, et al. Paclitaxel sensitivity of breast cancer cells with constitutively active NF-kappaB is enhanced by IkappaBalpha super-repressor and parthenolide. Oncogene. 2000;19:4159–4169. doi: 10.1038/sj.onc.1203768. [DOI] [PubMed] [Google Scholar]
- 20.Singh RP, Mallikarjuna GU, Sharma G, Dhanalakshmi S, Tyagi AK, Chan DC, et al. Oral silibinin inhibits lung tumor growth in athymic nude mice and forms a novel chemocombination with doxorubicin targeting nuclear factor kappaB-mediated inducible chemoresistance. Clin Cancer Res. 2004;10:8641–8647. doi: 10.1158/1078-0432.CCR-04-1435. [DOI] [PubMed] [Google Scholar]
- 21.Chaturvedi MM, Mukhopadhyay A, Aggarwal BB. Assay for redox-sensitive transcription factor. Methods Enzymol. 2000;319:585–602. doi: 10.1016/s0076-6879(00)19055-x. [DOI] [PubMed] [Google Scholar]
- 22.Ahn KS, Sethi G, Aggarwal BB. Embelin, an inhibitor of X chromosome-linked inhibitor-of-apoptosis protein, blocks nuclear factor-kappaB (NF-kappaB) signaling pathway leading to suppression of NF-kappaB-regulated antiapoptotic and metastatic gene products. Mol Pharmacol. 2007;71:209–219. doi: 10.1124/mol.106.028787. [DOI] [PubMed] [Google Scholar]
- 23.Giri DK, Aggarwal BB. Constitutive activation of NF-kappaB causes resistance to apoptosis in human cutaneous T cell lymphoma HuT-78 cells. Autocrine role of tumor necrosis factor and reactive oxygen intermediates. J Biol Chem. 1998;273:14008–14014. doi: 10.1074/jbc.273.22.14008. [DOI] [PubMed] [Google Scholar]
- 24.Wong WW, Dimitroulakos J, Minden MD, Penn LZ. HMG-CoA reductase inhibitors and the malignant cell: the statin family of drugs as triggers of tumor-specific apoptosis. Leukemia. 2002;16:508–519. doi: 10.1038/sj.leu.2402476. [DOI] [PubMed] [Google Scholar]
- 25.Sora MK, Kruszewski AA, Stoklosa T, Czyzyk J, Lasek W, Malejczyk J, Jakóbisiak M. Synergistic antiproliferative activity of tumor necrosis factor alpha (TNF-alpha)and lovastatin. Arch Immunol Ther Exp (Warsz) 1994;42:269–274. [PubMed] [Google Scholar]
- 26.Rasmussen LM, Hansen PR, Nabipour MT, Olesen P, Kristiansen MT, Ledet T. Diverse effects of inhibition of 3-hydroxy-3-methylglutaryl-CoA reductase on the expression of VCAM-1 and E-selectin in endothelial cells. Biochem J. 2001;360:363–370. doi: 10.1042/0264-6021:3600363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hilgendorff A, Muth H, Parviz B, Staubitz A, Haberbosch W, Tillmanns H, et al. Statins differ in their ability to block NF-kappaB activation in human blood monocytes. Int J Clin Pharmacol Ther. 2003;41:397–401. doi: 10.5414/cpp41397. [DOI] [PubMed] [Google Scholar]