Abstract
Most in vivo electrophysiological studies of substantia nigra have used rats. With the recent proliferation of the use of mice for in vitro neurophysiological studies because of the availability of various genetically modified strains to identify the roles of various channels and proteins in neuronal function, it is crucial to obtain data on in vivo responses in mice to verify that the in vitro results reflect functioning of systems comparable with those that have been well studied in rat.
Inhibitory responses of rat nigral dopaminergic neurons by stimulation of afferents from striatum, globus pallidus, or pars reticulata have been shown to be mediated predominantly or exclusively by GABAA receptors. This is puzzling given the substantial expression of GABAB receptors and the ubiquitous appearance of GABAB synaptic responses in rat dopaminergic neurons in vitro. In the present study, we studied electrically evoked GABAergic inhibition in nigral dopaminergic neurons in C57BL/6J mice. Stimulation of the three major GABAergic inputs elicited stronger and longer-lasting inhibitory responses than those seen in rats. The early inhibition was GABAA mediated, whereas the later component, absent in rats, was GABAB mediated and selectively enhanced by GABA uptake inhibition. Striatal-evoked inhibition exhibited a slower onset and a weaker initial component compared with inhibition from globus pallidus or substantia nigra pars reticulata. These results are discussed with respect to differences in the size and neuronal density of the rat and mouse brain and the different sites of synaptic contact of the synapses from the three GABAergic afferents.
Keywords: substantia nigra, dopaminergic, inhibition, striatonigral, pallidonigral, pars reticulata
Introduction
Midbrain dopaminergic neurons have long been recognized to play an essential role in the execution of voluntary movement and in the pathophysiology of Parkinson's disease (Hornykiewicz, 1998), to be involved in schizophrenia and in mechanisms of action and addiction to of drugs of abuse (Diana and Tepper, 2002; Kauer and Malenka, 2007), as well as to underlie critical aspects of higher cognitive functions including some types of motor learning, sensorimotor integration, and signaling reward-related aspects of sensory stimuli (Aosaki et al., 1994; Schultz, 1998, 2007).
Most of the afferents to the substantia nigra are GABAergic, and at least 70% of the synapses formed on nigral dopaminergic neurons are GABAergic (Ribak et al., 1976; Bolam and Smith, 1990). Most of the GABAergic innervation arises from the striatum (Grofová and Rinvik, 1970; Hattori et al., 1973a,b; Somogyi et al., 1981; Bolam and Smith, 1990), the globus pallidus (GP) (Hattori et al., 1975; Smith and Bolam, 1990), and the local axon collaterals of the substantia nigra pars reticulata projection neurons (SNr) (Deniau et al., 1982; Grofova et al., 1982; Nitsch and Riesenberg, 1988; Hajós and Greenfield, 1993, 1994; Tepper et al., 1995; Mailly et al., 2003) (for recent review, see Tepper and Lee, 2007).
Neurons in substantia nigra pars compacta and pars reticulata express GABAA and GABAB receptors (Bowery et al., 1987; Nicholson et al., 1992; Charara et al., 2000; Boyes and Bolam, 2003), and nigral neurons respond to stimulation of GABAA and/or GABAB receptors with hyperpolarization and/or inhibition of firing in vivo (Grace and Bunney, 1979; Erhardt et al., 2002) or in vitro (Pinnock, 1984; Lacey et al., 1988; Gulácsi et al., 2003). However, although local electrical stimulation with high-frequency trains in vitro evokes both GABAA and GABAB IPSPs/IPSCs (Sugita et al., 1992; Johnson and North, 1992; Cameron and Williams, 1993; Hajós and Greenfield, 1993, 1994; Häusser and Yung, 1994; Saitoh et al., 2004), in early studies, striatal stimulation in vivo produced inhibition and IPSPs that appeared to be mediated exclusively by GABAA receptors (Nakamura et al., 1979; Grace and Bunney, 1985). Subsequent studies showed that inhibition resulting from stimulation of striatal, pallidal, or pars reticulata afferents was substantially or completely blocked by local application of GABAA antagonists but was not attenuated at all by selective GABAB antagonists, suggesting mediation predominantly or exclusively by GABAA receptors (Tepper et al., 1995; Paladini et al., 1999). The reasons for these differences remained unexplained.
During recent in vivo recordings from dopaminergic neurons in wild-type controls for transgenic R6/2 mice (Shah et al., 2005), we observed that striatal stimulation produced significantly longer-lasting inhibition than comparable stimulation in rats (Paladini et al., 1999), suggesting the possibility of a GABAB response. Therefore, we decided to reinvestigate the roles of GABAA and GABAB receptors and the effects of GABA uptake blockade in afferent-induced inhibition of dopaminergic neurons in vivo in mice.
Materials and Methods
General surgery.
All procedures were performed in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by the Rutgers Animal Care and Facilities Committee. Thirty-five male C57BL6/J mice, obtained from The Jackson Laboratory and weighing between 20 and 30 g at the time of recording, were used as subjects. Mice were housed two per cage in plastic shoebox cages at 21°C on a 12 h light/dark cycle with food and water available ad libitum.
Mice were anesthetized with chloral hydrate (350 mg/kg, i.p.) and installed into a stereotaxic frame. Heart rate and respiration rate were monitored, and supplemental injections of chloral hydrate were given ∼1 h through an indwelling intraperitoneal catheter to maintain an even level of anesthesia. All wound margins and point of contact between the animal and the stereotaxic apparatus were infiltrated with lidocaine solution (2%). Body temperature was maintained at 37°C.
The surgery, extracellular recording, and local injection of drugs have been described previously (Paladini et al., 1999). Briefly, after subcutaneous injection of 5% bupivacaine with epinephrine, the scalp was removed and small burr holes were drilled over the appropriate coordinates, for striatum [anterior (A), 0.9 mm from bregma; lateral (L), 2.3 mm from midline], GP (A, −0.4 mm from bregma; L, 1.8 mm), and ventral thalamus (VT) (A, −1.0 mm from bregma; L, 1.2) for antidromic activation of SNr projection neurons (Tepper et al., 1995). Two stimulating electrodes were implanted in two different regions in each mouse. A 2 mm hole for insertion of the recording microelectrode was drilled above substantia nigra at the following coordinates: A, 1.3 mm from lambda; L, 1.2 from midline.
Stimulation, recording, and drug infusion.
Bipolar stimulating electrodes consisting of two stainless steel enamel-coated wires (California Fine Wire) ∼100 μm in diameter with a tip separation of ∼100 μm were lowered to the appropriate depths in striatum (−2.5 mm from surface), GP (−3.5 mm), and thalamus (−3.5 mm) and affixed in place with cyanoacrylate glue and dental cement. Constant current electrical stimuli were generated with Winston A-65 timer and SC-100 constant-current stimulus isolation units (Winston Electronics). Stimuli consisted of single monophasic square wave pulses ranging in intensity from 100 to 700 μA with durations of 100 μs at a rate of 1.0 Hz. Train stimuli consisted of five 100–500 μA, 100-μs-duration pulses delivered over 50 ms (100 Hz). Trains were delivered at 1.0 Hz. At the end of the experiment, stimulating sites were marked with a small lesion made by passing a 1.0 mA direct current through the stimulating electrode.
Recording electrodes were made from 2.0 mm outer diameter capillary tubing (WPI) using a Narishige PE-2 vertical pipette puller. The electrodes, whose tips were broken under microscopic control to yield final tip diameter of 1.0–2.0 μm, typically possessed resistances between 5 and 15 MΩ in vivo. Barrels for micropressure ejection of drugs were pulled from 1 mm outer diameter borosilicate glass tubing (Fisher Scientific), and their tips were broken back under microscopic control to final diameters of 8–10 μm. The barrels were then heated and bent to ∼30° of vertical shaft. Two pressure ejection pipettes were aligned with the recording electrode shaft with their tips 50–70 μm behind the tip of recording electrode under microscopic control and affixed with cyanoacrylate glue. Before the experiment, the recording electrode was filled with 1 m NaCl, and pressure ejection barrels were filled with GABA antagonists: picrotoxin (PTX) (400–1000 μm in 0.9% saline solution; formerly Research Biochemicals, now Sigma) or CGP55845A (CGP) [(2S)-3-[(15)-1-(3,4-dichlorophenyl)ethyl]amino-2-hydroxypropyl)(phenylmethyl)phosphinic acid] (500–1000 μm in 0.9% saline solution; Tocris Cookson).
Single-unit extracellular recordings were amplified with a Neurodata IR183 preamplifier and displayed on a Tektronix 5113A storage oscilloscope. Data were digitized at 40 kHz with a CED Micro1401 Mk II (Cambridge Electronic Design) and acquired on a personal computer running Spike2 software (Cambridge Electronic Design) and stored for later off-line analysis. Drugs were applied locally through the side barrels by pressure ejection for a period of 100–500 ms at 20–40 psi with a Picospritzer (General Valve Corporation). Drugs were applied in a counterbalanced manner after predrug data were collected. The onset of drug effects was typically within 1–3 s, and drug effects lasted for ∼15–30 min. Although no attempt was made to reverse the effects of the GABA receptor antagonists pharmacologically in these experiments, previous studies using the same method of pressure application showed that the local pressure application of GABA agonists was reversible during subsequent pressure application of appropriate antagonists (Paladini and Tepper, 1999; Paladini et al., 1999).
In all experiments, data were obtained from only one neuron per track to avoid residual drug effects. In some cases, GABA receptor antagonists were applied to up to three neurons from one animal, with recording tracks separated by at least 500 μm. For evoked responses, stimulation intensities were adjusted for each stimulating region. Striatal and pallidal afferents to substantia nigra dopaminergic neurons were activated by electrically stimulating within those nuclei, whereas SNr afferents to dopaminergic neurons were monosynaptically activated by electrically stimulating the ipsilateral thalamus thereby inducing antidromic activation selectively of a large population of pars reticulata GABAergic projection neurons. This excitation propagates into the local axon collaterals that synapse onto dopaminergic neurons (Tepper et al., 1995, 2003). For the sake of brevity, this will be referred to as SNr stimulation, although it should be understood that the stimulating electrode is in the ventral thalamus, and the SNr projection neurons are antidromically activated.
Initial currents were ∼100 μA and were gradually increased until a clear and reliable inhibitory response was obtained, being careful to keep the stimulating current below the minimal amplitude that evoked antidromic responses for each neuron. After reaching this point, at least 5 min of evoked inhibition were recorded to establish the stability of the response. Then the drugs were administered, and, after 1 min of recording of spontaneous activity to define alterations in firing rate and firing patterns, stimulation was applied again using the same current intensities that were used during baseline sampling. For single-pulse stimulation, 100–200 sweeps were typically recorded before and again after drug application, whereas for train stimulation typically 40–100 sweeps were obtained before and after drug application. We tested the effect of two GABAergic antagonists: picrotoxin, a GABAA receptor antagonist, and CGP55845A, a selective GABAB receptor antagonist. Typically two to five pressure applications were applied per drug. All drugs were dissolved in physiological saline at high concentration, stored in aliquots at − 20°C, and used within 1 month. The final concentrations of these solutions were adjusted before the experiment. The effective concentrations of these drugs were determined empirically in previous studies (Celada et al., 1999; Paladini and Tepper, 1999; Paladini et al., 1999).
In some experiments, the effects of intraperitoneal injection of the specific GAT-1 blocker NO-711 [1-(2-(((diphenylmethylene)amino)oxy)ethyl)-1,2,4,6-tetrahydro-3-pyridinecarboxylic acid hydrochloride] (1 mg/kg; Tocris Cookson) (Borden et al., 1994) on spontaneous activity and afferent-induced inhibition were explored, whereas in others (n = 7), local pressure application of nipecotic acid (1 mm; Aldrich) (Krogsgaard-Larsen, 1980). In both cases, the hope was that this would increase electrically evoked synaptic levels of GABA enough to overflow the synapse and activate extrasynaptic GABAB receptors (Scanziani, 2000).
Histology.
At the end of each experiment, the mouse was injected with lethal dose of chloral hydrate, perfused transcardially with saline followed by 10% Formalin. The brains were removed, postfixed in Formalin, cut into 50 μm coronal sections on a freezing microtome, and stained with neutral red to enable histological verification of recording and stimulating electrode sites.
Data analysis.
Data were analyzed off-line using Spike2. Peristimulus time histograms (PSTH) and autocorrelograms were constructed from samples of spontaneous spike trains of 1000–2000 spikes. Firing patterns were classified as pacemaker, random, or bursty from the autocorrelograms as described previously (Tepper et al., 1995). PSTHs (400–1000 ms duration with 2 ms bins) were constructed for each cell for the predrug baseline state and after administration of each of the antagonists. Stimulus-induced inhibition and the effects of local drug application were quantified by a Spike2 script written to consider the onset of inhibition as beginning with the first of at least three consecutive bins that fell outside the range of mean ± 1 SD of the average firing rate during the baseline control period. The offset of inhibition was similarly defined as beginning with the first of at least three consecutive bins that was within the range of mean ± 1 SD. Percentage inhibition was calculated as the mean firing rate during the entire period of inhibition as defined above.
Data were analyzed by using one- and two-way ANOVA with Prism (GraphPad Software), followed with Bonferroni's post hoc tests to compare individual group means. Differences were considered to be significant if p < 0.05. All numerical values are expressed as mean ± SEM.
Results
Data were obtained from 110 spontaneously active substantia nigra dopaminergic neurons that were recorded from 35 mice. All cells reported were located within the substantia nigra pars compacta as confirmed by postmortem histological analysis of electrode tracks. All data reported here were obtained from cells that met well established criteria for electrophysiological identification of substantia nigra dopaminergic neurons in extracellular recordings in vivo in anesthetized rats, including low spontaneous firing rates (5.2 ± 0.2 spikes/s; n = 75) in a regular, random, or bursty firing pattern (Fig. 1D–F), an extracellularly recorded waveform >2 ms in duration (Fig. 1B) that often exhibited a notch on the initial positive component (Fig. 1C) corresponding to an initial segment–somatodendritic break, and/or long-latency antidromic responses from striatum (10.9 ± 0.6 ms; 23 of 50 neurons, 46%) or GP (6.8 ± 0.5 ms; 22 of 55 neurons, 40%) consisting mostly of initial segment-only spikes. Based on estimated straight-line distances from the striatal and pallidal stimulating sites and the nigral recording location, these correspond to conduction velocities of 0.42 m/s for the nigrostriatal pathway and 0.47 m/s for the nigropallidal pathway, both in excellent agreement with data previously obtained in rat. Example recordings from mouse dopaminergic neurons, illustrated in Figure 1, confirm and extend previous observations in mice (Sanghera et al., 1984; Trulson and Trulson, 1987) and show that these neurons display basic electrophysiological characteristics that are indistinguishable from those previously reported in anesthetized rats in vivo (Deniau et al., 1978; Guyenet and Aghajanian, 1978; Grace and Bunney, 1983; Tepper et al., 1984; Paladini et al., 1999).
Figure 1.
Representative extracellular single-unit recordings from dopaminergic neurons in vivo in mouse substantia nigra. A, Ten consecutive sweeps showing antidromic responses of a nigral neuron from striatal stimulation. Note that approximately half of the responses consist of the initial segment spike only (green traces), and that, although the initial segment latency is extremely constant, the somatodendritic component of the antidromic spike exhibits considerable latency variability (black traces). A collision occurs in the red trace. B, Ten consecutive spontaneous spikes (high-pass filtered) superimposed from a neuron firing in the pacemaker mode exhibit the long-duration waveform of nigrostriatal dopaminergic neurons commonly seen in rats. C, Five superimposed spontaneous spikes (high-pass filtered and 10 consecutive spikes averaged) from a neuron firing in the bursting mode exhibits the prominent initial segment–somatodendritic break (arrow) sometimes seen in spontaneous spikes as well as in antidromic responses. D–F, Samples of spontaneous activity from control neurons showing that mouse nigrostriatal neurons exhibit the same three patterns of firing seen in rat dopaminergic neurons: pacemaker (D), random (E), and bursty (F). Each top panel consists of 10 s of spontaneous activity with the corresponding autocorrelogram shown below. Autocorrelograms constructed from 750–100 spontaneous spikes. Bin width, 5 ms. G, Twenty-five consecutive sweeps superimposed to show a typical inhibitory response of a nigrostriatal neuron to single-pulse striatal stimulation (400 μA). Note the long latency to the onset of inhibition, the incomplete nature of the spike suppression, and the long-lasting inhibition. H, Twenty-five consecutive sweeps superimposed to show a typical inhibitory response of a nigrostriatal neuron to single-pulse stimulation of GP (500 μA). Note the rapid onset of the inhibition and the complete suppression of firing during the inhibitory period that is shorter than that evoked from striatum.
Responses of substantia nigra dopaminergic neurons to activation of striatal, pallidal, and SNr GABAergic inputs
The periods of poststimulus activity used for the computation of duration and reduction of firing during the inhibitory responses were defined over the same time range regardless of whether we used single pulses or trains. Five neurons that responded antidromically at the stimulation intensities used to elicit orthodromic inhibition (100–600 μA) were excluded from additional analysis because of the possibility of contamination by the long-lasting spike afterhyperpolarization characteristic of these neurons (Grace and Bunney, 1983; Kita et al., 1986; Tepper et al., 1987). To obtain measures of firing rate that would be comparable between the control period and that after administration of antagonists, we defined the inhibition within the first 100 ms poststimulus as the early inhibitory response (EIR). Inhibition after 100 ms was defined as the late inhibitory response (LIR). Although arbitrary in some respects, the 100 ms cutoff for the EIR and LIR was based on a first pass inspection of our control data as well as a knowledge of the onset latencies and durations reported previously for GABAA- and GABAB-mediated IPSPs in substantia nigra by others (Grace and Bunney, 1985; Hajós and Greenfield, 1993, 1994; Häusser and Yung, 1994)
Inhibitory responses evoked by stimulation of neostriatum, GP, or SNr were occasionally followed by postinhibitory excitation and sometimes by secondary episodes of inhibition at with extremely long-onset latencies (e.g., 375–400 ms) (see Figs. 2A2,B2, 4A1). Onset latencies were analyzed for the EIR but not for the LIR because the onset of the latter often overlapped the EIR as in the examples in Figure 2, A1, A2, and B2.
Figure 2.
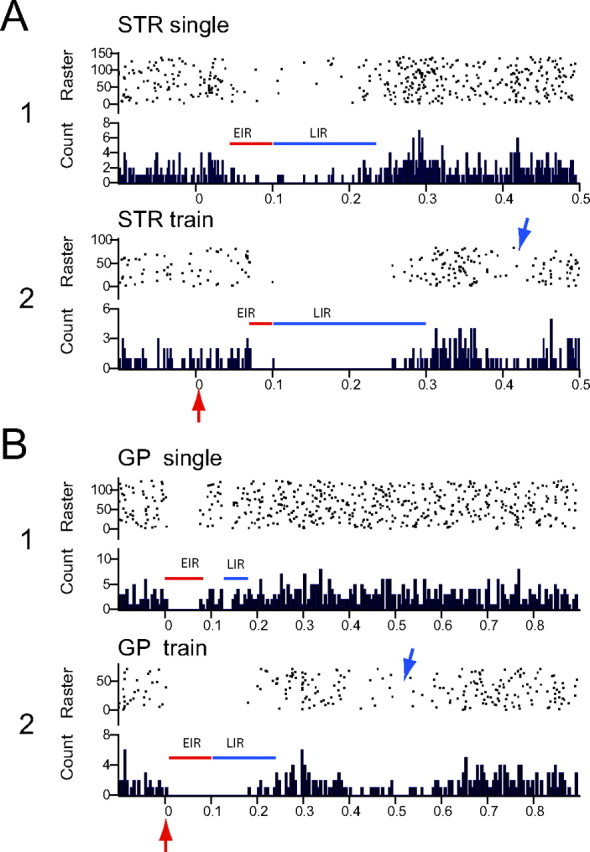
A, B, Effects of single-pulse stimulation and short trains of pulses (arrows) delivered to striatum (A; STR) and GP (B) on the spontaneous activity of one typical mouse nigral dopaminergic neuron in vivo. Note different timescales for striatal and pallidal responses. Inhibitory responses between time 0 and 100 ms were termed the early inhibitory responses (red lines), whereas those with onset >100 ms were termed the late inhibitory responses (blue lines). Note that the EIR evoked from striatal stimulation has a delayed onset and, in the case of single-pulse stimulation in A1, the firing is not completely suppressed. Concurrently, in both A1 and A2, there is a pronounced LIR that, in the case of train stimulation, comprises complete suppression of firing. In contrast, in response to GP stimulation, the EIR exhibits a very short-onset latency and comprises complete suppression of firing. In response to single-pulse stimulation in B1, the LIR is brief, but, after train stimulation, the LIR is of longer duration and also comprises complete suppression of firing. Blue arrows indicate examples of a secondary, very long-latency inhibition occasionally observed. In this and all subsequent PSTHs, the stimulus is delivered at time 0 (red arrow). Each PSTH consists of 75–100 trials. Bin width, 2 ms.
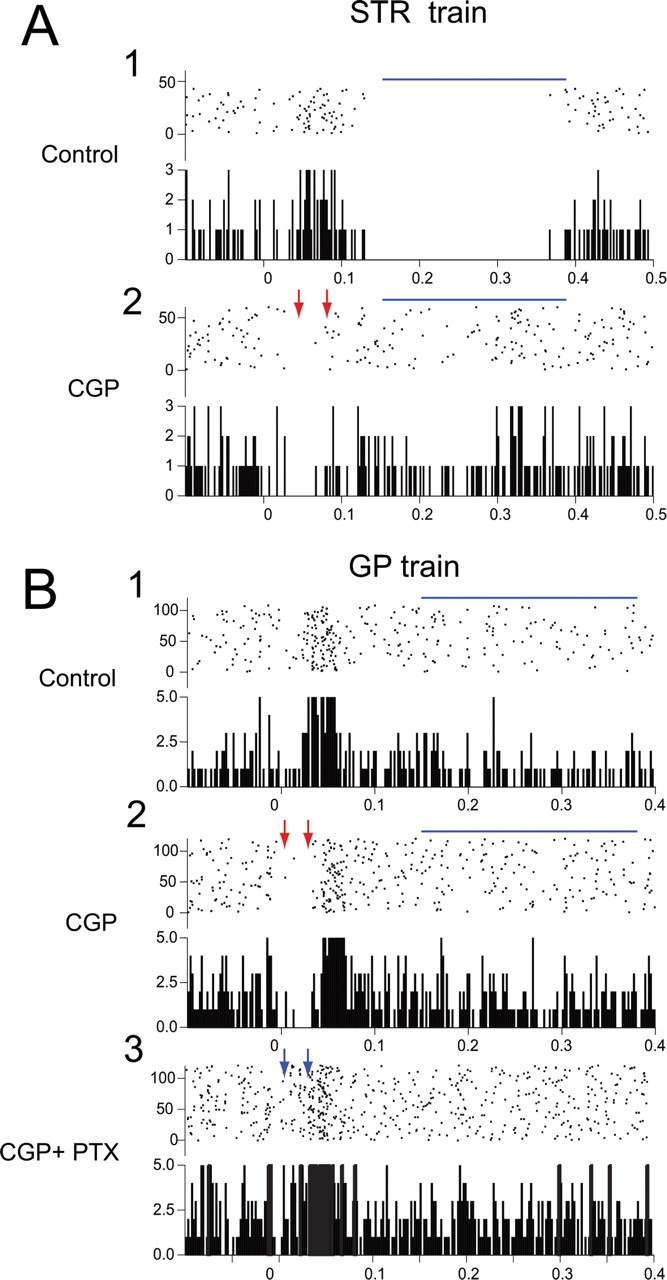
Figure 4.
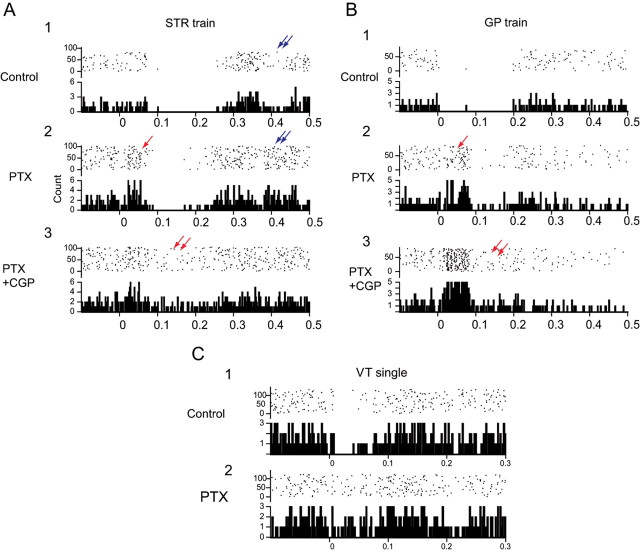
Typical PSTHs illustrating the effects of local blockade of GABAA and/or GABAB receptors on stimulation-induced inhibition. A1, Striatal train stimulation elicits an EIR and an LIR. Note delayed period of inhibition at ∼400 ms (double blue arrows). A2, Local application of PTX blocks the EIR, unmasking a brief excitation (red arrow; for explanation, see Results) and eliminates the delayed inhibition (double blue arrows) but does not affect the LIR. Note that the overall firing rate is increased. A3, Subsequent simultaneous application of PTX and CGP greatly attenuates the LIR (double red arrows), and the EIR remains blocked. Note that the overall firing rate is greater than in control but less than with PTX alone in A2. B1, A brief train to GP with same parameters as in A evokes near-complete suppression of firing during both the EIR and LIR. B2, Local application of PTX completely blocks the EIR, leaving the same excitation as seen in A2 (red arrow) but the LIR remains intact. B3, Subsequent combined application of PTX and CGP eliminates both the LIR (double red arrow) and the EIR. C1, SNr stimulation produces a short-latency EIR exhibiting complete suppression of activity. C2, Application of PTX completely abolishes the EIR. Note the absence of the excitatory response that is evoked by striatal or pallidal stimulation during GABAA receptor blockade. Stimulus is delivered at time 0. Each PSTH consists of 75–100 trials. Bin width, 2 ms. VT, Ventral thalamus.
Responses to striatal stimulation
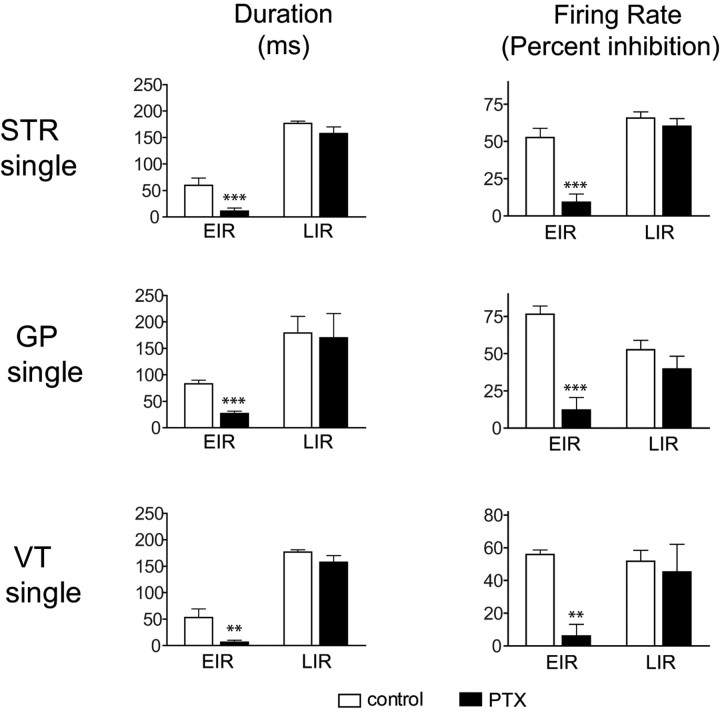
Responses to single-pulse striatal stimulation were characterized in most neurons by an incomplete inhibition in which the neuron continued to fire but at a substantially lower rate than during the prestimulus baseline period (see Figs. 1G, 2A1, 4A). Occasionally, the stimulation resulted in complete suppression of firing. The onset latency for the EIR was variable, ranging from 6 to 80 ms (mean, 29.1 ± 3.5 ms). The mean duration of the striatal-evoked EIR was 68.1 ± 3.7 ms, during which spontaneous activity was suppressed by 56.9 ± 4.2% compared with the prestimulus level. A majority of dopaminergic neurons (34 of 44, 77%) also exhibited the LIR, examples of which are shown in Figures 2A and 4A. The mean duration of the LIR was 156.7 ± 11.5 ms and, during the LIR firing, was reduced by 50.9 ± 4.5%.
A high-frequency train of pulses (five pulses at 100 Hz, 100–500 μA, 100 μs) delivered to striatum produced qualitatively similar results as single-pulse stimulation. An EIR was induced in 44 (100%) neurons with an onset latency of 19.7 ± 3.7 ms and a duration of 61.8 ± 3.6 ms, during which firing was reduced by 48.7 ± 3.8%. Similar to single-pulse stimulation, the great majority of neurons (41 of 44, 93%) also exhibited an LIR that was significantly longer in duration (235.7 ± 17.9 ms; t = 3.652; p < 0.001) and exhibited a greater reduction in firing (by 61.7 ± 3.5%; t = 2.763; p < 0.05) compared with single-pulse stimulation. Population means of these data are plotted in Figure 3.
Figure 3.
Summary graphs of the EIR and LIR of dopaminergic neurons to single-pulse and train stimulation of the striatum (STR), GP, and SNr. A, Both single-pulse and train stimulation of striatum typically evoked the EIR and the LIR, with a small proportion of cells responding with the EIR only. Train stimulation significantly increased the duration and the strength of the LIR compared with single stimuli. B, More than 70% of the neurons responded to single-pulse GP stimuli with EIR-only responses, whereas train stimulation elicited both the EIR and the LIR. Train stimulation in GP is extraordinarily effective at increasing the duration and strength of the evoked inhibition. C, Stimulation of SNr has a profile similar to that of GP in which a large proportion of neurons respond to single-pulse stimuli only with EIR. However, train stimulation evoked the LIR in 100% of the small sample of neurons tested. Train effects on duration and strength of inhibition were not significantly different from single pulse, probably because of the small n. *p < 0.05 and ***p < 0.001 compared with single pulse. STR, n = 37 neuron; GP, n = 44 neurons; and SNr, n = 3 neurons. VT, Ventral thalamus.
Responses to pallidal stimulation
Stimulation of the GP evoked a short-latency EIR in all dopaminergic neurons tested (n = 58 cells). Three of these neurons were excluded from additional statistical analysis, one because it exhibited an antidromic response and two others because data on all parameters with both stimulus protocols could not be obtained. Typical examples of an inhibitory response evoked by single-pulse GP stimulation are shown in Figures 1H and 2B. The EIR exhibited a short onset latency (5.3 ± 0.9 ms), an average duration of 85.2 ± 3.6 ms, during which firing was suppressed by 81.2 ± 3.6% compared with the prestimulus control period. In contrast to striatal-evoked inhibition, pallidal-evoked inhibition always contained an epoch of complete firing suppression (see Figs. 2B, 4B, 10B) that is reflected in a greater mean percentage inhibition (Fig. 3). Also in contrast to striatal stimulation, single-pulse stimulation of GP evoked an LIR in only 26% of neurons (14 of 54 cells) versus 77% (34 of 44) for striatal stimulation. The mean duration of the LIR was 134.2 ± 26.5 ms, during which the firing rate was suppressed by 19.8 + 5.3% of the prestimulus level.
Figure 10.
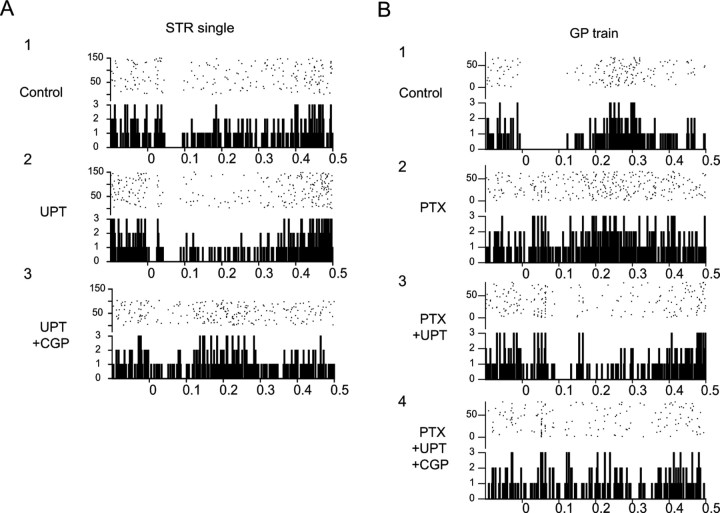
Blockade of GABA uptake with systemic administration of NO-711 (UPT) clearly enhances the LIR. A1, Baseline data show a clear EIR to single-pulse striatal stimulation with little or no LIR apparent. A2, After local application of UPT, the EIR is marginally enhanced, but now there is also a very clear LIR. A3, Combined application of UPT and CGP has a minimal attenuating effect on the EIR but completely blocks the increased LIR induced by uptake blockade. B1, Baseline trace showing complete suppression of firing during the EIR in response to train stimulation from GP. B2, Local application of PTX completely abolishes all inhibition. B3, Subsequent administration of NO-711 reveals a previously unseen, very pronounced LIR. B4, Subsequent application of CGP in the presence of PTX and GABA uptake blockade completely abolishes the LIR.
Stimulation of GP with a train of pulses produced an EIR in all 54 neurons tested with a 8.2 ± 2.4 ms onset latency and a duration of 76.6 ± 4.5 ms during which firing was reduced by 77.2 ± 2.8%, similar to the effects of single-pulse stimulation. However, in marked contrast to single-pulse stimulation, train stimulation evoked an LIR in virtually all neurons (48 of 54, 89% vs 14 of 54, 26%). The train-induced LIR exhibited a significantly longer duration (295.9 ± 20.2; p < 0.001) and a greater depression in firing (60.3 ± 3.3%; p < 0.001) compared with single-pulse GP stimulation.
Responses to SNr stimulation
Thalamic stimulation selectively activates GABAergic inputs to dopaminergic neurons from the axon collaterals of pars reticulata projection neurons through antidromic activation of GABAergic nigrothalamic neurons in rats (Tepper et al., 1995; Paladini et al., 1999). To be certain that we could successfully antidromically activate nigrothalamic neurons in mouse, in some animals extracellular recordings were made from SNr GABAergic neurons during thalamic stimulation. We found that thalamic stimulation produced a short-latency antidromic response in seven of nine recorded pars reticulata neurons (latency, 3.7 ± 0.2 ms), similar to responses observed previously in rats (data not shown).
Also similar to results reported in rats, antidromic activation of SNr projection neurons with single-pulse stimuli produced a short-latency EIR in all tested dopaminergic neurons (n = 17) with a 3.8 ± 0.8 ms onset latency, a duration of 68.9 ± 6.6 ms, and a suppression of firing by 52.7 ± 7.3%. Five of 17 cells also exhibited an LIR. The mean duration of the LIR was 140.8 ± 26.3 ms, and firing during the LIR was depressed by 39.1 ± 10.7%.
Train stimulation of VT induced the EIR in all four neurons tested with an onset latency of 3.3 ± 1.7 ms and a duration of 72.5 ± 6.1 ms, during which firing rate was reduced by 41.4 ± 6.6%. All neurons also exhibited a longer duration of LIR (198.8 ± 10.1 ms) and a greater reduction in firing (58.8 ± 5.5%) to train than single pulses, although the differences did not reach statistical significance perhaps because of the small number of neurons tested.
Comparison of inhibitory responses elicited by different stimulating sites
ANOVA revealed a significant effect of stimulating site on the onset latency (F(2,154) = 35.7; p < 0.0001), duration (F(2,154) = 4.71; p < 0.02), and percentage inhibition (F(2,154) = 17.00; p < 0.0001) of the EIR. Subsequent post hoc tests showed that this was attributable to significantly shorter latencies for EIR inhibition evoked from GP (5.1 ± 0.7 ms; t = 7.82; p < 0.001) and VT (3.5 ± 0.8; t = 5.52; p < 0.001) than for those evoked from striatum (24.7 ± 2.6 ms). The EIR duration was significantly longer (t = 3.0; p < 0.01) for GP stimulation (79.1 ± 2.3 ms) than for striatal stimulation (68.1 + 3.1 ms) but did not differ significantly from that for VT stimulation (68.9 ± 6.2). The strength of the EIR evoked by GP stimulation (79.1 ± 2.3% depression) was also significantly greater (t = 5.3; p < 0.001) than that evoked by striatal stimulation (57.0 ± 3. 4%) but did not differ from that evoked by antidromic activation of SNr neurons (52.7 ± 7.1%). The stimulus site did not significantly affect the LIR.
Comparison of inhibitory responses elicited by different stimulating protocols
Comparison of single-pulse stimulation versus train stimulation pooled across stimulating sites revealed that train stimulation produced greater percentage inhibition than single pulses (F(1,47) = 21.24; p < 0.0001) and longer duration of inhibition (F(1,47) = 14.94; p < 0.0004) of the LIR but had no significant effect on either parameter of the EIR. For striatal stimulation, Bonferroni's post hoc analysis showed that there was a significant stimulus protocol difference for both duration of the LIR (t = 3.652; p < 0.001) and percentage inhibition (t = 2.67; p < 0.05). For GP stimulation, the two stimulus protocols also significantly differed for both percentage inhibition (t = 6.44; p < 0.001) and duration (t = 6.23; p < 0.001) of the LIR.
Effect of local GABAA blockade on inhibitory responses to striatal, pallidal, and thalamic activation
Blockade of GABAA receptors with the GABAA antagonist picrotoxin attenuated or completely eliminated the EIR induced by stimulation of all three afferent inputs as shown for three representative neurons in Figure 4 and for the population as a whole in Figure 5. ANOVA showed that local application of picrotoxin significantly attenuated both the percentage (F(1,68) = 117.6; p < 0.0001) and the duration of the inhibition (F(1,68) = 163.4; p < 0.0001) of the EIR evoked by single-pulse and train stimulation of STR, GP, or SNr but had no effect on the LIR as shown in Figure 5. The reduction or elimination of the suppression in firing rate and the shortening of the duration of the inhibition by picrotoxin was statistically significant for all individual comparisons of the EIR elicited by single-pulse or train stimulation of striatum, GP, or SNr (t = 3.35–12.02; p < 0.01–0.001). The short-latency excitation seen in Figure 4, A and B, after picrotoxin is typical and probably reflects the effects of monosynaptic and/or polysynaptic glutamatergic afferents excited by striatal or GP stimuli whose effects are usually masked by potent GABAergic inhibition when GABAA receptors are not blocked (Tepper et al., 1995; Paladini et al., 1999).
Figure 5.
Summary histograms displaying the effects of local application of PTX on the duration and firing rate inhibition of the EIR and LIR evoked by single-pulse stimulation of striatum, GP, and SNr. Note that the duration of the EIR and the magnitude of the inhibition of the EIR are significantly reduced, but there is no significant effect on the LIR, indicating that the EIR is predominantly or exclusively GABAA-mediated whereas the LIR is not. *p < 0.05 and ***p < 0.001 compared with baseline. VT, Ventral thalamus.
The very long-latency inhibition is also apparent in Figure 4A1 (double blue arrows) and can be seen to be abolished by subsequent picrotoxin application along with the EIR, whereas the LIR remains unaffected. We suspect that this is some kind of network-level phenomenon because the very long-onset latency and GABAA mediation would rule out a monosynaptic response. The origin could not be determined in the present studies, and this response is not considered further in this report.
Similar to effects observed in rats after local application of bicuculline (Tepper et al., 1995; Paladini et al., 1999), in a number of neurons, application of picrotoxin unmasked a short-latency excitatory response to striatal, pallidal, or thalamic stimulation that overlapped and replaced the EIR seen under control conditions as is evident for both neurons shown in Figure 4 (single red arrows in A and B). Furthermore, the repeated secondary episodes of inhibitions that were often induced by single-pulse/or train stimulation (Fig. 2B, 4A) were also eliminated by picrotoxin administration (Fig. 4A1,A2). In summary, local blockade of GABAA receptors with picrotoxin predominantly affected the EIR but has only a minimal effect on the LIR.
Effect of local GABAB blockade on inhibitory responses to striatal, pallidal, and thalamic activation
In marked contrast to its lack of ability to attenuate stimulus-evoked inhibition in rat dopaminergic neurons, local application of CGP55845A produced a clear attenuation of the LIR evoked by stimulation of striatum, GP, and SNr in mice. As shown previously, brief trains of stimuli significantly increased the amplitude and duration of the LIR as well as the number of neurons exhibiting the LIR to GP or SNr stimulation (up to 89%). The LIR was significantly and selectively attenuated by local application of CGP55845A. An ANOVA confirmed that duration (F(1,94) = 111.1; p < 0.0001) and percentage inhibition of firing (F(1,94) = 163.7; p < 0.0001) of the LIR were significantly attenuated by blockade of GABAB receptors by CGP55845A in the majority of dopaminergic neurons. In contrast, attenuating effects on the EIR were essentially absent as shown in the population summary in Figure 6.
Figure 6.
Summary histograms displaying the effects of local application of CGP on mean ± SEM duration and magnitude of the EIR and LIR evoked by train pulse stimulation of striatum and GP and single-pulse stimulation of SNr. Neither the duration or the magnitude of inhibition of the EIR is affected, but there is significant reduction of both duration and depression in firing rate from all three sites in the LIR, indicating that the LIR contains a significant GABAB-mediated component whereas the EIR does not. **p < 0.01 and ***p < 0.001 compared with baseline. VT, Ventral thalamus.
Presynaptic effects of GABAB receptor blockade on inhibitory responses to striatal, pallidal, and SNr activation
In addition to attenuation of the LIR [an effect not previously observed in rats (Paladini and Tepper, 1999; Tepper et al., 1995)], local application of CGP55845A often resulted in augmentation or unmasking of the EIR as a result of blocking presynaptic inhibitory GABAB autoreceptors on the terminals of GABAergic afferents (for recent review, see Misgeld et al., 2007), as reported previously (Paladini et al., 1999). The augmentation of inhibitory responses commonly consisted of modest increases in the strength and/or the duration of the EIR. This effect occurred predominantly in neurons that showed a relatively weak or absent EIR under predrug control conditions, as in both examples in Figure 8. CGP55845A-induced augmentation of the EIR occurred in 55% of neurons after single-pulse GP stimulation and in 36% of neurons after train GP stimulation, and in 22% of neurons after single-pulse striatal stimulation and 7% of neurons after train stimulation of striatum. Although the result was prominent in some neurons as shown in the two examples in Figure 8, statistical analysis of the entire population revealed that difference in the means between predrug condition and CGP55845A administration was not statistically significant for strength of inhibition and only of borderline significance for duration of inhibition (F(1,134) = 3.53; p = 0.058).
Figure 8.
Presynaptic effect of GABAB receptor blockade increases EIR but blocks LIR in two representative neurons. A1, Control trace showing a modest EIR but a strong LIR (blue line) to striatal train stimulation. A2, Local application of CGP greatly increases the strength and duration of the EIR (red arrows) but at the same time almost completely abolishes the LIR (blue line). B1, Control trace in a second neuron showing relatively minimal EIR with incomplete suppression of activity and a more prominent LIR to GP train stimulation. B2, Local application of CGP greatly increases the strength and duration of the EIR, unmasking a period of complete suppression of spiking (red arrows) but also completely abolishing the LIR. B3, Subsequent application of both CGP and PTX abolishes the unmasked EIR (blue arrows).
Effect of local GABAA and GABAB receptor blockade on inhibitory responses to striatal, pallidal, and SNr activation
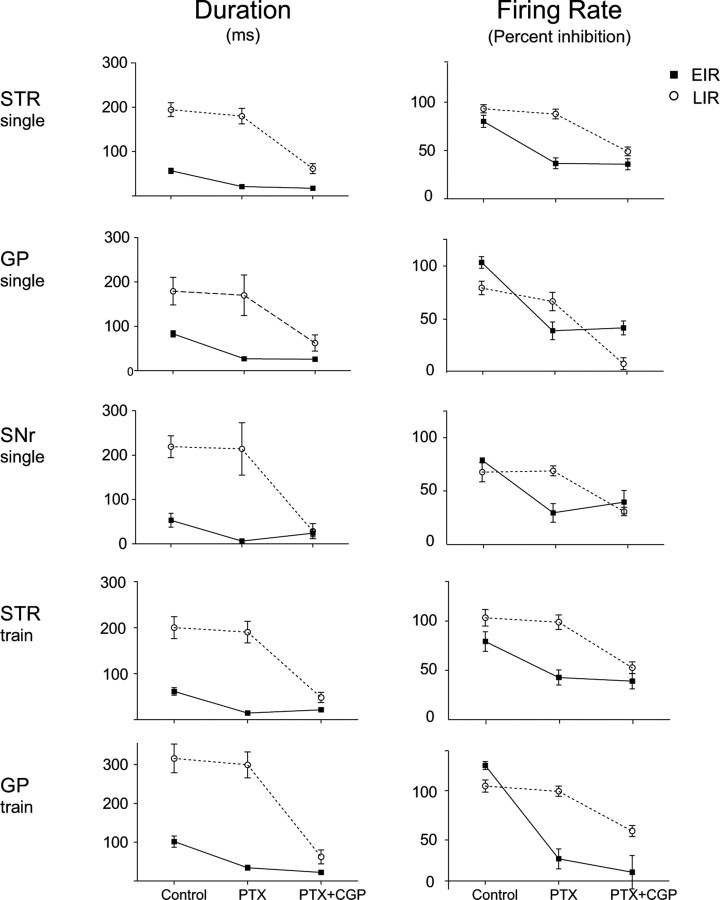
Local application of both picrotoxin and CGP55845A resulted in a substantial reduction or complete elimination of both the EIR and LIR induced by stimulation of STR (43 cells), GP (53 cells), or SNr (9 cells) as illustrated for two typical neurons in Figure 4 and for the entire population in Figure 7. ANOVA of data pooled across stimulus sites showed that reductions in magnitude and duration of the EIR and LIR were statistically significant (EIR percentage inhibition, F(1,100) = 123.6, p < 0.001; LIR percentage inhibition, F(1,68) = 210.4, p < 0.001; EIR duration, F(1,100) = 178.2, p < 0.001; LIR duration, F(1,68) = 149.9, p < 0.001).
Figure 7.
Summary of the effects of local application of PTX and CGP on the EIR and LIR. Means ± SEM duration and magnitude of inhibition (percentage inhibition, right) for both EIR (black circles, solid line) and LIR (white circles, dashed line) are shown for control condition (Control), after local administration of PTX, and after application of PTX together with CGP (PTX+CGP). STR, Striatum.
The effects of GABAA and combined GABAA and GABAB receptor antagonists broken down by stimulus site and stimulation protocol shown in Figures 3 and 5–7 clearly indicate that the EIR is predominantly a result of GABAA receptor activation, whereas the LIR depends predominantly on GABAB receptor activation, independent of the stimulus site or stimulus protocol.
Effect of local GABAA or GABAB receptor blockade on spontaneous activity
As in rats (Paladini and Tepper, 1999), local application of the GABAA antagonist picrotoxin produced a significant increase in the spontaneous firing rate of mouse dopaminergic neurons from 4.6 ± 0.3 to 6.2 ± 0.3 spikes/s (n = 41; t = 5.35; p < 0.001). Local CGP55845A application also produced a more modest but still statistically significant effect on spontaneous activity, causing a decrease in mean firing rates from 5.4 ± 0.3 to 4.7 ± 0.2 spikes/s (n = 62; t = 3.335; p < 0.002). Concurrent or sequential application of both picrotoxin and CGP55845A produced a significant increase in the spontaneous firing rate in mouse dopaminergic neurons from 5.2 ± 0.2 to 6.2 ± 0.3 spikes/s (n = 50; t = 4.111; p < 0.001), as illustrated in Figure 9A.
Figure 9.

Effects of GABAA and GABAB antagonists on spontaneous activity of mouse nigral dopaminergic neurons. A, Under control conditions, mouse dopaminergic neurons fire spontaneously at 4.6 spikes/s, approximately the same rate as in rats. Local application of PTX significantly increased firing rate to 6.2 spikes/s. Conversely, CGP produced a modest but statistically significant decrease in firing rate from 5.4 to 4.7 spikes/s, whereas combined application of CGP and PTX was similar to the effects of PTX alone and significantly increased spontaneous firing rate from 5.2 to 6.2 spikes/s. B, PTX also exerts dramatic effects on firing pattern, shifting the distribution to predominantly bursty firing and essentially eliminating the pacemaker mode. CGP exerts the opposite effect, decreasing burst firing and more than doubling the proportion of neurons firing in the pacemaker mode. Combined application of PTX and CGP produces effects qualitatively similar to those of PTX alone but with smaller proportional shifts in firing pattern. Numbers below the bars indicate the number of neurons. **p < 0.01; ***p < 0.001 (Bonferroni's t test); 111p < 0.001; 1p < 0.01 (χ2 test).
In addition, just as in rats, the most prominent effects of GABAergic antagonists on spontaneous activity were on the pattern rather then the rate of firing, as shown in Figure 9B. Blockade of GABAA receptors exerted a dramatic effect on the pattern of firing in most dopaminergic neurons, leading to a more than tripling of the number of cells firing in the bursty mode but greatly decreasing the incidence of random firing and nearly eliminating the pacemaker mode as shown in Figure 9 (χ2 = 16.2; p < 0.0004). GABAB receptor blockade by CGP55845A caused a modest but statistically significant change in the distribution of firing patterns (χ2 = 7.18; p < 0.03), decreasing the proportion of neurons exhibiting burst or irregular firing and increasing the proportion of neurons exhibiting regular pattern or pacemaker-like activity, whereas simultaneous blockade of both GABAA and GABAB receptors shifted the firing pattern toward bursty firing similar to the effect of GABAA blockade alone but not quite as strongly, because the proportion of cells that shifted to the bursty mode was only 48% compared with >60% after picrotoxin alone, as shown in Figure 9B.
Effects of GABA uptake blockers
It has been suggested previously that activation of postsynaptic GABAB receptors in substantia nigra and elsewhere may require greater GABA synaptic concentrations than activation of GABAA receptors (Fritschy et al., 1999; Paladini et al., 1999; Isaacson, 2000; Scanziani, 2000) because of their preferential localization at extrasynaptic sites (Boyes and Bolam, 2003). Blocking GABA uptake by the GABA transporter (GAT) ought to increase synaptic levels and spillover, thereby increasing GABA receptor activation by following activation of synaptic inputs. We tested this with local pressure application of the nonspecific GAT blocker nipecotic acid (Krogsgaard-Larsen, 1980) and the intraperitoneal application of the specific GAT-1 blocker NO-711 (Borden et al., 1994). Because there was no difference between the action of locally injected nipecotic acid and systemically introduced NO-711 on spontaneous and evoked activity of the dopaminergic neurons, the data were pooled and are reported as the effects of uptake blockade.
There was no significant difference in spontaneous firing rates under control conditions compared with those during blockade of GABA uptake (5.8 ± 0.5 vs 5.6 ± 0.5 spikes/s). However, GAT blockade had a slight tendency to regularize the firing pattern. The number of cells firing in the pacemaker mode increased from 25 to 69%, whereas the number of randomly and bursty firing neurons decreased from 54 to 16% and from 23 to 15%, respectively. Analysis with χ2 revealed that this change was of borderline statistical significance (p = 0.503).
We examined the effect of GABA uptake inhibition on evoked inhibition in 27 dopaminergic neurons. GAT blockade produced only a modest increase in EIR duration to striatal stimuli (88.0 ± 10.5 vs 74.4 ± 11.3 ms; n = 12), and a greater reduction of firing rate in the poststimulus inhibitory period (−69. 9 ± 7.7 vs −48.3 ± 9.5%; n = 12). With GP-evoked responses, blockade of GABA uptake had no effect on duration (94.3 ± 4.6 vs 85.7 ± 3.5; n = 15) or magnitude (−82.34 ± 4.62 vs 83.99 ± 4.66%; n = 15) of the EIR. None of these comparisons was statistically significant.
In contrast, blockade of GABA uptake significantly affected striatal- and pallidal-evoked LIR. Striatal stimuli caused a slightly stronger depression in firing rates under uptake blockade (−65.2 ± 6.2 vs −40.9 ± 12.2%; p < 0.05) and a significantly longer duration of inhibition (200.2 ± 21.7 vs 128.0 ± 28.7 ms; p < 0.05). A typical example is shown in Figure 10. For GP-evoked responses, blockade of GABA uptake produced a significantly larger magnitude of inhibition in firing in the LIR (−60.0 ± 7.3 vs 19.2 ± 8.3%; p < 0.001) and longer duration of inhibition (217.08 ± 2 6.64 vs 100.0 ± 24.6 ms; p < 0.01) as shown in Figure 10.
Some neurons that exhibited only an EIR in response to striatal or pallidal stimulation under control conditions manifest the LIR after GAT blockade. For striatal stimulation, the proportion of neurons exhibiting the LIR increased from 73 to 89%, and, after GP stimulation, the proportion of neurons exhibiting LIR increased from 31 to 80%.
To determine whether NO-711-induced augmentation of the evoked inhibition was mediated by stimulation of GABAB receptors, we tested the ability of locally applied CGP55845A to attenuate or eliminate the effects of previous uptake blockade on electrically evoked inhibition. The GABAB antagonist did not affect either the magnitude (−73.1 ± 6.8 vs −79.9 ± 4.6%) or the duration (92.1 ± 8.2 vs 95.3 ± 6.8 ms) of the striatal- or GP-evoked EIR under uptake blockade. In contrast, application of CGP-55845 significantly reduced the strength (F(1,26) = 76.9; p < 0.0001) and the duration (F(1,26) = 109.1; p < 0.0001) of the LIR evoked from striatal and GP. The GABAB antagonist reduced the magnitude of the striatal-evoked LIR (from −65.2 ± 6.2 to −19.05 ± 7.33% of prestimulus firing; p < 0.001) and also decreased the duration of the inhibition from 200.2 ± 21.7 to 57.67 ± 10.83 ms; p < 0.001). Identical effects were seen for the GP-induced LIR in which GABAB blockade reduced the magnitude of inhibition from −60.0 ± 7.3 to −7.6 ± 7.3% (p < 0.001) and the duration from 217.1 ± 26.6 to 16.5 ± 8.0 ms (p < 0.01).
Discussion
Activation of GABAergic afferents in vivo in mice elicits both GABAA- and GABAB-mediated inhibition
The principal findings in this study were as follows. First, in contrast to rats, electrical stimulation of striatum, GP, or SNr in mice consistently elicited a long-lasting inhibition that was composed of GABAA and GABAB components. Although very low-intensity stimulation could occasionally elicit an EIR alone, even in these cases, the EIR was longer in duration that that seen in rats. The EIR was consistently and selectively blocked by picrotoxin, whereas the LIR was unaffected by picrotoxin but was attenuated or eliminated by CGP55845A. Thus, the EIR is mediated predominantly or exclusively by activation of GABAA receptors, whereas the LIR is mediated predominantly or exclusively by activation of GABAB receptors. Second, blockade of GABA uptake enhanced selectively the LIR component of the evoked inhibition. Finally, analysis of the strength and duration of the evoked inhibition revealed significant differences among the inhibitory responses elicited by striatal, pallidal, or SNr stimulation.
What accounts for the expression of the GABAB-mediated in LIR in mice but not in rats?
Single-pulse or train stimulation of striatum, GP, or SNr in mice evoked both a GABAA-mediated EIR and a GABAB-mediated LIR. In contrast, in previous studies in rats using even stronger stimuli, only the GABAA-mediated EIR could be evoked (Nakamura et al., 1979; Paladini et al., 1999). The expression of the LIR in mice is the reason for the longer-duration inhibition evoked from striatum, GP, and SNr and its GABAB sensitivity.
It is possible that different anesthetics used in this and the previous studies contributed to these differences because urethane and chloral hydrate have very different effects on GABAergic transmission (Sceniak and Maciver, 2006; Lu et al., 2008). However, this is unlikely to be the only or even the principal cause for the differences observed between the present and previous results. Most importantly, striatonigral IPSPs and/or inhibition in previous studies in chloral hydrate-anesthetized rats also appear to have been attributable to GABAA-receptor stimulation (Guyenet and Aghajanian, 1978; Grace and Bunney, 1985). In addition, in a study using striatal stimulation in chloral hydrate-anesthetized rats (Dai and Tepper, 1998), we failed to note any difference between striatal-evoked inhibition of dopaminergic neurons in this preparation and in others in which we used urethane anesthesia (Tepper et al., 1990, Trent and Tepper, 1991).
A more likely possibility is the smaller size of the mouse brain. A rat brain weighs approximately five times as much as a mouse brain (Bishop and Wahlsten, 1999). The packing density of neurons in the brain varies inversely with the size of the brain (Tower, 1954) and, at least in neocortex, scales to a −⅓ power law against total cortical volume (Changizi, 2001; Harrison et al., 2002). This would cause identical amplitude electrical stimuli that stimulate equivalent volumes of tissue to activate much larger numbers of neurons and/or axons as well as a greater proportion of neuronal elements in that volume. Field stimulation of cortex in vitro evoked ∼80% more norepinephrine release from mouse than rat slices, whereas stimulation of striatum released ∼300% more dopamine from mouse than rat slices (Scholze et al., 2007), indicating that the increase in number and proportion of stimulated elements in mouse versus rat translates into similar increases in release and extracellular levels of transmitters. It is also interesting to note that striatal-evoked inhibition is of relatively long duration in the neonatal rat whose brain is similar in size to that of a mouse but decreases through postnatal development as brain size increases (Tepper et al., 1990). In mice, even single-pulse stimulation presumably results in levels of GABA in the terminal regions sufficient to allow for diffusion away from the synapse and activation of extrasynaptic GABAB receptors. This results in the recruitment of the long-latency, longer-duration GABAB response.
This explanation is further supported by the observation that train stimulation promoted the appearance of a CGP-55845-sensitive LIR, even in dopaminergic neurons that responded to single-pulse stimuli with only a brief EIR and little or no LIR under control conditions. In addition, GABAB IPSP/IPSCs observed in vitro often requires high-frequency trains for elicitation (Lacey et al., 1988; Johnson and North, 1992; Hajós and Greenfield, 1993; Häusser and Yung, 1994; Saitoh et al., 2004). These data further support the idea that the major reason for the shorter-duration inhibitions seen in rats in vivo is that the stimuli evoke relatively low levels of GABA in the rat nigral terminal fields that result in predominantly or exclusively GABAA activation, whereas the same stimuli evoke higher levels of GABA in mice that are able to activate extrasynaptic GABAB receptors.
Presynaptic effects of GABAB blockade in substantia nigra
Although unable to block or attenuate the EIR, local application of CGP55845A often enhanced the strength and/or duration of the EIR, just as in rats (Paladini et al., 1999) in which this facilitation was blocked by subsequent local application of GABAA antagonists. Consistent with these findings, CGP55845A caused a slight inhibition of firing rate and a shift of firing pattern away from bursting, as it did in rats (Paladini and Tepper, 1999).
These effects are almost certainly due to increased postsynaptic GABAA receptor stimulation attributable to increased release of GABA from afferents after terminal autoreceptor blockade. Striatonigral afferents to have been shown to express presynaptic GABAB receptors (Boyes and Bolam, 2003, 2007), and GABAergic IPSP/IPSCs are greatly reduced in amplitude by selective GABAB agonists in dopaminergic neurons in vitro (Seabrook et al., 1990; Radnikow et al., 2001; Misgeld, 2004; Saitoh et al., 2004; Misgeld et al., 2007).
Tonic activation of GABAA receptors on nigral neurons has been inferred to occur in vivo and in vitro in rats by the powerful effects of GABAA receptor antagonists on firing rate and pattern (Rick and Lacey, 1994, Tepper et al., 1995; Paladini and Tepper, 1999; present results). In contrast, in mice, as in rats, there does not appear to be a significant GABAB-mediated postsynaptic tone on the dopaminergic (Tepper et al., 1995; Paladini and Tepper, 1999; present results) or GABAergic (Rick and Lacey, 1994) nigral neurons (but see Erhardt et al., 2002), although such a tone is clearly present at the GABAB autoreceptors on the GABAergic afferents.
Effects of GABA uptake blockade on spontaneous activity and stimulus-evoked inhibition
Inhibition of GABA uptake increased the magnitude and duration of the LIR and in several cases made manifest an LIR when there was none before. Both effects were blocked by CGP55845A but not by picrotoxin, consistent with the selective blockade of the LIR by GABAB antagonists shown previously. The selective facilitation of the LIR by uptake blockade is likely attributable to increased extracellular levels of GABA allowing for greater diffusion of GABA to extrasynaptic GABAB receptors.
GABA uptake blockade also produced a very slight but statistically nonsignificant decrease in spontaneous firing rate as well as a modest increase in the proportion of neurons firing in the pacemaker mode and a concomitant decrease in the proportion of neurons firing in the bursty and random modes. These results are also very similar to the presynaptic effects of CGP55845A and are also likely attributable to a slight increase in tonic GABAA receptor stimulation. However, a direct potentiating effect of NO-711 on GABAA receptor binding is also possible (Shen and Johnson, 2001).
Differences in inhibition evoked by activation of striatal, pallidal, and nigral GABAergic afferents
In the present study, single-pulse striatal-induced inhibition was characterized by a relatively slow onset and incomplete suppression of spiking but at the same time elicited a relatively strong LIR. In contrast, activation of GP or SNr afferents produced a short-latency EIR often characterized by complete suppression of activity, yet only approximately one-third of these cells also exhibited an LIR. The LIR differences could be attributable to enriched expression of GABAB receptors in the more distal dendrites in which the majority of the striatal afferents synapse. There is also evidence that different GABAergic inputs are preferentially associated with GABAA or GABAB receptors (Sugita et al., 1992; Cameron and Williams, 1993)
The differences in onset latency are probably attributable to the pathway conduction times. The striatonigral fibers are the slowest conducting of the three main GABAergic inputs (Deniau et al., 1978; Guyenet and Aghajanian, 1978; Ryan et al., 1986; Celada et al., 1999), and the striatonigral pathway is by far the longest of the three, likely accounting for the longer onset latency from striatal stimulation compared with inhibition evoked from either GP or SNr.
The differences in strength of inhibition, conversely, may be accounted for in large part on the basis of synaptic location and number of synapses made. The majority of the striatonigral afferents synapse onto the relatively distal dopaminergic dendrites. In contrast, pallidonigral axons and inputs from SNr neurons give rise to large boutons that make a significant fraction of their contacts proximally, with individual axons making repeated contacts, sometimes forming pericellular baskets around dopaminergic somata (Smith and Bolam, 1990; von Krosigk et al., 1992; Tepper et al., 2002). These are more electrotonically favored locations and would be expected to result in more potent early inhibition compared with that evoked from striatum and also may play role in the rapid onset and typically complete suppression of firing that characterizes pallidal- and SNr-evoked inhibition. In neocortical neurons, GABAB receptor stimulation produces larger conductance increases on distal dendrites than at the soma (Takigawa and Alzheimer, 1999). A similar differential distribution of GABAA and GABAB receptors in nigral neurons could account for the delayed inhibition resulting from striatal stimulation compared with the early and potent inhibition arising from GP and SNr. These differences suggest that pallidal and SNr afferents may be more important for eliciting brief, transient inhibition, whereas striatal inputs may be more important for controlling the excitability of dopaminergic neurons over longer timescales.
Conclusions
Spontaneous firing rates, firing patterns, and antidromic response properties of mouse nigrostriatal neurons are essentially identical to those characterized previously in rats. As in the rat, mouse dopaminergic neurons exhibit tonic activation of postsynaptic GABAA but not GABAB receptors under basal conditions. In marked contrast to rats, however, electrical stimulation of GABAergic inputs in mice evokes stronger and longer-duration inhibitory responses, the late component of which is GABAB mediated. The expression of the GABAB response may be attributable to the smaller size and higher neuronal packing density in the mouse brain allowing for similar stimuli to lead to much higher terminal concentrations of GABA that overflow the synapse and activate extrasynaptic GABAB receptors.
Footnotes
This work was supported in part by National Institutes of Health Grant NS034865 and Rutgers University.
References
- Aosaki T, Graybiel AM, Kimura M. Effect of the nigrostriatal dopamine system on acquired neural responses in the striatum of behaving monkeys. Science. 1994;265:412–415. doi: 10.1126/science.8023166. [DOI] [PubMed] [Google Scholar]
- Bishop KM, Wahlsten D. Sex and species differences in mouse and rat forebrain commissures depend on the method of adjusting for brain size. Brain Res. 1999;815:358–366. doi: 10.1016/s0006-8993(98)01088-9. [DOI] [PubMed] [Google Scholar]
- Bolam JP, Smith Y. The GABA and substance P input to dopaminergic neurones in the substantia nigra of the rat. Brain Res. 1990;529:57–78. doi: 10.1016/0006-8993(90)90811-o. [DOI] [PubMed] [Google Scholar]
- Borden LA, Murali Dhar TG, Smith KE, Weinshank RL, Branchek TA, Gluchowski C. Tiagabine, SK&F 89976-A, CI-966, and NNC-711 are selective for the cloned GABA transporter GAT-1. Eur J Pharmacol. 1994;269:219–224. doi: 10.1016/0922-4106(94)90089-2. [DOI] [PubMed] [Google Scholar]
- Bowery NG, Hudson AL, Price GW. GABAA and GABAB receptor site distribution in the rat central nervous system. Neuroscience. 1987;20:365–383. doi: 10.1016/0306-4522(87)90098-4. [DOI] [PubMed] [Google Scholar]
- Boyes J, Bolam JP. The subcellular localization of GABAB receptor subunits in the rat substantia nigra. Eur J Neurosci. 2003;18:3279–3293. doi: 10.1111/j.1460-9568.2003.03076.x. [DOI] [PubMed] [Google Scholar]
- Boyes J, Bolam JP. Localization of GABA receptors in the basal ganglia. Prog Brain Res. 2007;160:229–243. doi: 10.1016/S0079-6123(06)60013-7. [DOI] [PubMed] [Google Scholar]
- Cameron DL, Williams JT. Dopamine D1 receptors facilitate transmitter release. Nature. 1993;366:344–347. doi: 10.1038/366344a0. [DOI] [PubMed] [Google Scholar]
- Celada P, Paladini CA, Tepper JM. GABAergic control of rat substantia nigra dopaminergic neurons: role of globus pallidus and substantia nigra pars reticulata. Neuroscience. 1999;89:813–825. doi: 10.1016/s0306-4522(98)00356-x. [DOI] [PubMed] [Google Scholar]
- Changizi MA. Principles underlying mammalian neocortical scaling. Biol Cybern. 2001;84:207–215. doi: 10.1007/s004220000205. [DOI] [PubMed] [Google Scholar]
- Charara A, Heilman TC, Levey AI, Smith Y. Pre- and postsynaptic localization of GABAB receptors in the basal ganglia in monkeys. Neuroscience. 2000;95:127–140. doi: 10.1016/s0306-4522(99)00409-1. [DOI] [PubMed] [Google Scholar]
- Dai M, Tepper JM. Do silent dopaminergic neurons exist in rat substantia nigra in vivo? Neuroscience. 1998;85:1089–1099. doi: 10.1016/s0306-4522(97)00615-5. [DOI] [PubMed] [Google Scholar]
- Deniau JM, Hammond C, Riszk A, Feger J. Electrophysiological properties of identified output neurons of the rat substantia nigra (pars compacta and pars reticulata): evidences for the existence of branched neurons. Exp Brain Res. 1978;32:409–422. doi: 10.1007/BF00238711. [DOI] [PubMed] [Google Scholar]
- Deniau JM, Kitai ST, Donoghue JP, Grofova I. Neuronal interactions in the substantia nigra pars reticulata through axon collaterals of the projection neurons. An electrophysiological and morphological study. Exp Brain Res. 1982;47:105–113. doi: 10.1007/BF00235891. [DOI] [PubMed] [Google Scholar]
- Diana M, Tepper JM. Electrophysiological pharmacology of mesencephalic dopaminergic neurons. In: Chiara GD, editor. Handbook of experimental pharmacology. Berlin: Springer; 2002. pp. 1–61. [Google Scholar]
- Erhardt S, Mathé JM, Chergui K, Engberg G, Svensson TH. GABAB receptor-mediated modulation of the firing pattern of ventral tegmental area dopamine neurons in vivo. Naunyn Schmiedebergs Arch Pharmacol. 2002;365:173–180. doi: 10.1007/s00210-001-0519-5. [DOI] [PubMed] [Google Scholar]
- Fritschy JM, Meskenaite V, Weinmann O, Honer M, Benke D, Mohler H. GABAB-receptor splice variants GB1a and GB1b in rat brain: developmental regulation, cellular distribution and extrasynaptic localization. Eur J Neurosci. 1999;11:761–768. doi: 10.1046/j.1460-9568.1999.00481.x. [DOI] [PubMed] [Google Scholar]
- Grace AA, Bunney BS. Paradoxical GABA excitation of nigral dopaminergic cells: indirect mediation through reticulata inhibitory neurons. Eur J Pharmacol. 1979;59:211–218. doi: 10.1016/0014-2999(79)90283-8. [DOI] [PubMed] [Google Scholar]
- Grace AA, Bunney BS. Intracellular and extracellular electrophysiology of nigral dopaminergic neurons. 1. Identification and characterization. Neuroscience. 1983;10:301–315. doi: 10.1016/0306-4522(83)90135-5. [DOI] [PubMed] [Google Scholar]
- Grace AA, Bunney BS. Opposing effects of striatonigral feedback pathways on midbrain dopamine cell activity. Brain Res. 1985;333:271–284. doi: 10.1016/0006-8993(85)91581-1. [DOI] [PubMed] [Google Scholar]
- Grofová I, Rinvik E. An experimental electron microscopic study on the striatonigral projection in the cat. Exp Brain Res. 1970;11:249–262. doi: 10.1007/BF01474385. [DOI] [PubMed] [Google Scholar]
- Grofova I, Deniau JM, Kitai ST. Morphology of the substantia nigra pars reticulata projection neurons intracellularly labeled with HRP. J Comp Neurol. 1982;208:352–368. doi: 10.1002/cne.902080406. [DOI] [PubMed] [Google Scholar]
- Gulácsi A, Lee CR, Sík A, Viitanen T, Kaila K, Tepper JM, Freund TF. Cell type-specific differences in chloride-regulatory mechanisms and GABAA receptor-mediated inhibition in rat substantia nigra. J Neurosci. 2003;23:8237–8246. doi: 10.1523/JNEUROSCI.23-23-08237.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyenet PG, Aghajanian GK. Antidromic identification of dopaminergic and other output neurons of the rat substantia nigra. Brain Res. 1978;150:69–84. doi: 10.1016/0006-8993(78)90654-6. [DOI] [PubMed] [Google Scholar]
- Hajós M, Greenfield SA. Topographic heterogeneity of substantia nigra neurons: diversity in intrinsic membrane properties and synaptic inputs. Neuroscience. 1993;55:919–934. doi: 10.1016/0306-4522(93)90308-3. [DOI] [PubMed] [Google Scholar]
- Hajós M, Greenfield SA. Synaptic connections between pars compacta and pars reticulata neurones: electrophysiological evidence for functional modules within the substantia nigra. Brain Res. 1994;660:216–224. doi: 10.1016/0006-8993(94)91292-0. [DOI] [PubMed] [Google Scholar]
- Harrison KH, Hof PR, Wang SS. Scaling laws in the mammalian neocortex: does form provide clues to function? J Neurocytol. 2002;31:289–298. doi: 10.1023/a:1024178127195. [DOI] [PubMed] [Google Scholar]
- Hattori T, Fibiger HC, McGeer PL, Maler L. Analysis of the fine structure of the dopaminergic nigrostriatal projection by electron microscopic autoradiography. Exp Neurol. 1973a;41:599–611. doi: 10.1016/0014-4886(73)90053-8. [DOI] [PubMed] [Google Scholar]
- Hattori T, McGeer PL, Fibiger HC, McGeer EG. On the source of GABA-containing terminals in the substantia nigra. Electron microscopic autoradiographic and biochemical studies. Brain Res. 1973b;54:103–114. doi: 10.1016/0006-8993(73)90037-1. [DOI] [PubMed] [Google Scholar]
- Hattori T, Fibiger HC, McGeer PL. Demonstration of a pallido-nigral projection innervating dopaminergic neurons. J Comp Neurol. 1975;162:487–504. doi: 10.1002/cne.901620406. [DOI] [PubMed] [Google Scholar]
- Häusser MA, Yung WH. Inhibitory synaptic potentials in guinea-pig substantia nigra dopamine neurones in vitro. J Physiol. 1994;479:401–422. doi: 10.1113/jphysiol.1994.sp020305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hornykiewicz O. Biochemical aspects of Parkinson's disease. Neurology. 1998;51:S2–S9. doi: 10.1212/wnl.51.2_suppl_2.s2. [DOI] [PubMed] [Google Scholar]
- Isaacson JS. Spillover in the spotlight. Curr Biol. 2000;10:R475–R477. doi: 10.1016/s0960-9822(00)00551-0. [DOI] [PubMed] [Google Scholar]
- Johnson SW, North RA. Two types of neurone in the rat ventral tegmental area and their synaptic inputs. J Physiol. 1992;450:455–468. doi: 10.1113/jphysiol.1992.sp019136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kauer JA, Malenka RC. Synaptic plasticity and addiction. Nat Rev Neurosci. 2007;8:844–858. doi: 10.1038/nrn2234. [DOI] [PubMed] [Google Scholar]
- Kita T, Kita H, Kitai ST. Electrical membrane properties of rat substantia nigra compacta neurons in an in vitro slice preparation. Brain Res. 1986;372:21–30. doi: 10.1016/0006-8993(86)91454-x. [DOI] [PubMed] [Google Scholar]
- Krogsgaard-Larsen P. Inhibitors of the GABA uptake systems. Mol Cell Biochem. 1980;31:105–121. doi: 10.1007/BF00240816. [DOI] [PubMed] [Google Scholar]
- Lacey MG, Mercuri NB, North RA. On the potassium conductance increase activated by GABAB and dopamine D2 receptors in rat substantia nigra neurones. J Physiol. 1988;401:437–453. doi: 10.1113/jphysiol.1988.sp017171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu J, Nelson LE, Franks N, Maze M, Chamberlin NL, Saper CB. Role of endogenous sleep-wake and analgesic systems in anesthesia. J Comp Neurol. 2008;508:648–662. doi: 10.1002/cne.21685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mailly P, Charpier S, Menetrey A, Deniau JM. Three-dimensional organization of the recurrent axon collateral network of the substantia nigra pars reticulata neurons in the rat. J Neurosci. 2003;23:5247–5257. doi: 10.1523/JNEUROSCI.23-12-05247.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misgeld U. Innervation of the substantia nigra. Cell Tissue Res. 2004;318:107–114. doi: 10.1007/s00441-004-0918-2. [DOI] [PubMed] [Google Scholar]
- Misgeld U, Drew G, Yanovsky Y. Presynaptic modulation of GABA release in the basal ganglia. Prog Brain Res. 2007;160:245–259. doi: 10.1016/S0079-6123(06)60014-9. [DOI] [PubMed] [Google Scholar]
- Nakamura S, Iwatsubo K, Tsai CT, Iwama K. Cortically induced inhibition of neurons of rat substantia nigra (pars compacta) Jpn J Physiol. 1979;29:353–357. doi: 10.2170/jjphysiol.29.353. [DOI] [PubMed] [Google Scholar]
- Nicholson LF, Faull RL, Waldvogel HJ, Dragunow M. The regional, cellular and subcellular localization of GABAA/benzodiazepine receptors in the substantia nigra of the rat. Neuroscience. 1992;50:355–370. doi: 10.1016/0306-4522(92)90429-6. [DOI] [PubMed] [Google Scholar]
- Nitsch C, Riesenberg R. Immunocytochemical demonstration of GABAergic synaptic connections in rat substantia nigra after different lesions of the striatonigral projection. Brain Res. 1988;461:127–142. doi: 10.1016/0006-8993(88)90731-7. [DOI] [PubMed] [Google Scholar]
- Paladini CA, Tepper JM. GABAA and GABAB antagonists differentially affect the firing pattern of substantia nigra dopaminergic neurons in vivo. Synapse. 1999;32:165–176. doi: 10.1002/(SICI)1098-2396(19990601)32:3<165::AID-SYN3>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- Paladini CA, Celada P, Tepper JM. Striatal, pallidal, and pars reticulata evoked inhibition of nigrostriatal dopaminergic neurons is mediated by GABAA receptors in vivo. Neuroscience. 1999;89:799–812. doi: 10.1016/s0306-4522(98)00355-8. [DOI] [PubMed] [Google Scholar]
- Pinnock RD. Hyperpolarizing action of baclofen on neurons in the rat substantia nigra slice. Brain Res. 1984;322:337–340. doi: 10.1016/0006-8993(84)90129-x. [DOI] [PubMed] [Google Scholar]
- Radnikow G, Titz S, Mades S, Bäurle J, Misgeld U. γ-aminobutyric acid B autoreceptors in substantia nigra and neostriatum of the weaver mutant mouse. Neurosci Lett. 2001;299:81–84. doi: 10.1016/s0304-3940(01)01496-3. [DOI] [PubMed] [Google Scholar]
- Ribak CE, Vaughn JE, Saito K, Barber R, Roberts E. Immunocytochemical localization of glutamate decarboxylase in rat substantia nigra. Brain Res. 1976;116:287–298. doi: 10.1016/0006-8993(76)90906-9. [DOI] [PubMed] [Google Scholar]
- Rick CE, Lacey MG. Rat substantia nigra pars reticulata neurones are tonically inhibited via GABAA, but not GABAB, receptors in vitro. Brain Res. 1994;659:133–137. doi: 10.1016/0006-8993(94)90872-9. [DOI] [PubMed] [Google Scholar]
- Ryan LJ, Young SJ, Groves PM. Substantia nigra stimulation evoked antidromic responses in rat neostriatum. Exp Brain Res. 1986;63:449–460. doi: 10.1007/BF00237469. [DOI] [PubMed] [Google Scholar]
- Saitoh K, Isa T, Takakusaki K. Nigral GABAergic inhibition upon mesencephalic dopaminergic cell groups in rats. Eur J Neurosci. 2004;19:2399–2409. doi: 10.1111/j.0953-816X.2004.03337.x. [DOI] [PubMed] [Google Scholar]
- Sanghera MK, Trulson ME, German DC. Electrophysiological properties of mouse dopamine neurons: in vivo and in vitro studies. Neuroscience. 1984;12:793–801. doi: 10.1016/0306-4522(84)90171-4. [DOI] [PubMed] [Google Scholar]
- Scanziani M. GABA spillover activates postsynaptic GABAB receptors to control rhythmic hippocampal activity. Neuron. 2000;25:673–681. doi: 10.1016/s0896-6273(00)81069-7. [DOI] [PubMed] [Google Scholar]
- Sceniak MP, Maciver MB. Cellular actions of urethane on rat visual cortical neurons in vitro. J Neurophysiol. 2006;95:3865–3874. doi: 10.1152/jn.01196.2005. [DOI] [PubMed] [Google Scholar]
- Scholze P, Orr-Urtreger A, Changeux JP, McIntosh JM, Huck S. Catecholamine outflow from mouse and rat brain slice preparations evoked by nicotinic acetylcholine receptor activation and electrical field stimulation. Br J Pharmacol. 2007;151:414–422. doi: 10.1038/sj.bjp.0707236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz W. Predictive reward signal of dopamine neurons. J Neurophysiol. 1998;80:1–27. doi: 10.1152/jn.1998.80.1.1. [DOI] [PubMed] [Google Scholar]
- Schultz W. Multiple dopamine functions at different time courses. Annu Rev Neurosci. 2007;30:259–288. doi: 10.1146/annurev.neuro.28.061604.135722. [DOI] [PubMed] [Google Scholar]
- Seabrook GR, Howson W, Lacey MG. Electrophysiological characterization of potent agonists and antagonists at pre- and postsynaptic GABAB receptors on neurones in rat brain slices. Br J Pharmacol. 1990;101:949–957. doi: 10.1111/j.1476-5381.1990.tb14186.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah F, Abercrombie ED, Tepper JM. Electrophysiological modifications of nigral dopaminergic neurons in R6/2 Huntington's disease transgenic mice. Soc Neurosci Abstr. 2005;31:300–310. [Google Scholar]
- Shen KZ, Johnson SW. Potentiation of GABAA receptor agonists by GABA uptake inhibitors in the rat ventral midbrain. Eur J Pharmacol. 2001;428:1–7. doi: 10.1016/s0014-2999(01)01218-3. [DOI] [PubMed] [Google Scholar]
- Smith Y, Bolam JP. The output neurones and the dopaminergic neurones of the substantia nigra receive a GABA-containing input from the globus pallidus in the rat. J Comp Neurol. 1990;296:47–64. doi: 10.1002/cne.902960105. [DOI] [PubMed] [Google Scholar]
- Somogyi P, Bolam JP, Totterdell S, Smith AD. Monosynaptic input from the nucleus accumbens–ventral striatum region to retrogradely labelled nigrostriatal neurones. Brain Res. 1981;217:245–263. doi: 10.1016/0006-8993(81)90002-0. [DOI] [PubMed] [Google Scholar]
- Sugita S, Johnson SW, North RA. Synaptic inputs to GABAA and GABAB receptors originate from discrete afferent neurons. Neurosci Lett. 1992;134:207–211. doi: 10.1016/0304-3940(92)90518-c. [DOI] [PubMed] [Google Scholar]
- Takigawa T, Alzheimer C. G protein-activated inwardly rectifying K+ (GIRK) currents in dendrites of rat neocortical pyramidal cells. J Physiol. 1999;517:385–390. doi: 10.1111/j.1469-7793.1999.0385t.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tepper JM, Lee CR. GABAergic control of substantia nigra dopaminergic neurons. Prog Brain Res. 2007;160:189–208. doi: 10.1016/S0079-6123(06)60011-3. [DOI] [PubMed] [Google Scholar]
- Tepper JM, Nakamura S, Young SJ, Groves PM. Autoreceptor-mediated changes in dopaminergic terminal excitability: effects of striatal drug infusions. Brain Res. 1984;309:317–333. [PubMed] [Google Scholar]
- Tepper JM, Sawyer SF, Groves PM. Electrophysiologically identified nigral dopaminergic neurons intracellularly labeled with HRP: light-microscopic analysis. J Neurosci. 1987;9:2794–2806. doi: 10.1523/JNEUROSCI.07-09-02794.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tepper JM, Trent F, Nakamura S. Postnatal development of the electrical activity of rat nigrostriatal dopaminergic neurons. Brain Res Dev Brain Res. 1990;54:21–33. doi: 10.1016/0165-3806(90)90061-3. [DOI] [PubMed] [Google Scholar]
- Tepper JM, Martin LP, Anderson DR. GABAA receptor-mediated inhibition of rat substantia nigra dopaminergic neurons by pars reticulata projection neurons. J Neurosci. 1995;15:3092–3103. doi: 10.1523/JNEUROSCI.15-04-03092.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tepper JM, Celada P, Iribe Y, Paladini CA. Afferent control of nigral dopaminergic neurons—the role of GABAergic inputs. In: Graybiel AM, editor. The basal ganglia VI, Advances in behavioral biology. New York: Kluwer Academic/Plenum; 2003. pp. 641–651. [Google Scholar]
- Tower DB. Structural and functional organization of mammalian cerebral cortex; the correlation of neurone density with brain size; cortical neurone density in the fin whale (Balaenoptera physalus L.) with a note on the cortical neurone density in the Indian elephant. J Comp Neurol. 1954;101:19–51. doi: 10.1002/cne.901010103. [DOI] [PubMed] [Google Scholar]
- Trent F, Tepper JM. Dorsal raphe stimulation modifies striatal-evoked antidromic invasion of nigral dopaminergic neurons in vivo. Exp Brain Res. 1991;84:620–630. doi: 10.1007/BF00230974. [DOI] [PubMed] [Google Scholar]
- Trulson ME, Trulson TJ. Recording of mouse ventral tegmental area dopamine-containing neurons. Exp Neurol. 1987;96:68–81. doi: 10.1016/0014-4886(87)90169-5. [DOI] [PubMed] [Google Scholar]
- von Krosigk M, Smith Y, Bolam JP, Smith AD. Synaptic organization of GABAergic inputs from the striatum and the globus pallidus onto neurons in the substantia nigra and retrorubral field which project to the medullary reticular formation. Neuroscience. 1992;50:531–549. doi: 10.1016/0306-4522(92)90445-8. [DOI] [PubMed] [Google Scholar]