Abstract
Lung mesenchyme is a critical determinant of the shape and size of the lung, the extent and patterning of epithelial branching, and the formation of the pulmonary vasculature and interstitial mesenchymal components of the adult lung. Fibroblast growth factor 9 (FGF9) is a critical regulator of lung mesenchymal growth; however, upstream mechanisms that modulate the FGF mesenchymal signal and the downstream targets of mesenchymal FGF signaling are poorly understood. Here we have identified a robust regulatory network in which mesenchymal FGF signaling regulates β-Catenin mediated WNT signaling in lung mesenchyme. By conditionally inactivating β-Catenin in lung mesenchyme, we show that mesenchymal WNT-β-Catenin signaling is essential for lung development and acts to regulate the cell cycle G1 to S transition and the FGF responsiveness of mesenchyme. Together, both FGF and WNT signaling pathways function to sustain mesenchymal growth and coordinate epithelial morphogenesis during the pseudoglandular stage of lung development.
Keywords: Fibroblast growth factor 9 (FGF9), Fibroblast growth factor receptor (FGFR), Wnt2a, Wnt7b, β-Catenin, Lung development, Mesenchyme
Introduction
Mouse lung development is initiated on embryonic day 9.5 (E9.5) with the formation of two endodermal buds off the ventrolateral foregut. Lung buds invade the surrounding splanchnic mesoderm and overlying mesothelium to establish a lung primordium initially composed of three tissue layers, epithelium (endoderm), mesoderm and mesothelium (Hogan, 1999). As the lung buds grow and enter the pseudoglandular stage of development (E9.5–16), the mesenchyme forms two morphologically and molecularly distinct layers, the sub-mesothelial layer and the sub-epithelial layer (White et al., 2006). These mesenchymal layers express unique intercellular signaling molecules, such as WNT2a and FGF10 in the sub-mesothelial layer and Noggin in the sub-epithelial layer (Bellusci et al., 1996; Mailleux et al., 2005; Weaver et al., 2003; White et al., 2006). Growth and morphogenesis of the lung require intercellular signaling interactions between all primary cell layers of the lung (Cardoso and Lu, 2006; Shannon and Hyatt, 2004; Warburton et al., 2005).
Fgf9 is expressed in the outermost layer of the lung, the mesothelium, and in lung epithelium, and has been identified as a key factor that signals to mesenchyme to regulate proliferation, differentiation and the expression of other factors that in turn regulate epithelial development (Colvin et al., 1999; Colvin et al., 2001). Mesenchymal forms of FGF receptors 1 and 2 have been shown to mediate the FGF9 signal (White et al., 2006). Epithelial FGFs, such as FGF9, often regulate the expression of mesenchymal FGFs and other signaling molecules that in turn regulate epithelial and mesenchymal development. In a screen of candidate genes that are expressed in lung mesenchyme and that could be regulated by mesenchymal FGF signaling, Wnt2a was found to be significantly down-regulated in lungs of mice lacking Fgf9 (Fgf9−/−) (see Fig. 1). This suggested a link between FGF and WNT-β-Catenin signaling in lung mesenchyme and that regulation of WNT-β-Catenin signaling may be responsible for some of the lung phenotypes observed in Fgf9−/− embryos.
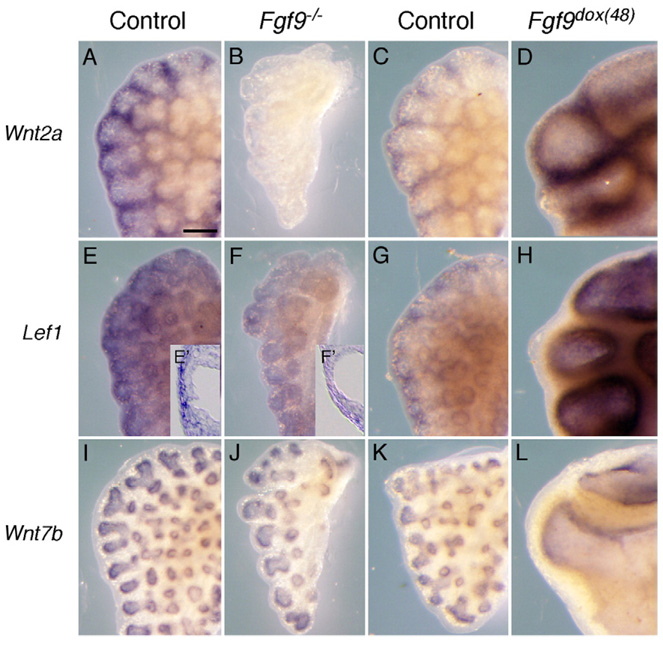
Fig. 1.
FGF9 regulates WNT signaling in lung mesenchyme. (A–H) Whole-mount in situ hybridization showing Wnt2a (A–D) and Lef1 (E–H) expression in E13.5 lung. All tissue pairs were hybridized and developed together in the same tube. Comparison of control (A) and Fgf9−/− (B) lungs shows an absence of Wnt2a expression in lung mesenchyme in Fgf9−/− tissue. In contrast, induced overexpression of FGF9 in lung epithelium from E11.5 to E13.5 (Fgf9dox(48)) (D) results in increased Wnt2a expression compared with the control lungs (C). Comparison of control (E) and Fgf9−/− (F) shows decreased expression of Lef1 in Fgf9−/− lung mesenchyme. Comparison of control (G) and Fgf9dox(48) (H) lungs shows increased expression in FGF9 overexpressing mesenchyme. Cryo-sections (inset) of Lef1 whole-mount in situ hybridization stained control (E′) and Fgf9−/− lungs (F′) reveal decreased Lef1 expression localized in the sub-mesothelial mesenchyme. Color development in panels C, D, G, H were for shorter periods of time compared to panels A, B, E, F. (I–L) Wnt7b expression levels in lung epithelium was not affected in either FGF9 loss of function (Fgf9−/−, J) or gain of function (Fgf9dox(48), L) lungs compared with the controls (I, K). Scale bar in panel A, 200 µm.
WNT signaling is essential for embryonic patterning and cell fate determination (Moon, 2005). At least three intracellular signaling pathways mediate WNT signaling, the WNT-β-Catenin pathway, the WNT/Ca2+ pathway and the planar cell polarity (PCP) pathway. WNT-β-Catenin signaling through frizzled (FZ) receptors and low-density lipoprotein receptor-related protein (LRP) 5 and 6 co-receptors lead to activation of disheveled (DVL), inhibition of GSK3β, and stabilization and cytosolic accumulation of β-Catenin. Increased cytosolic levels of β-Catenin lead to its nuclear translocation and to the formation of active transcription factor complexes of β-Catenin with members of the T Cell Factor (LEF1, TCF1, TCF3, TCF4) transcription factor family, PITX2 and SOX17 (Brantjes et al., 2002; Briata et al., 2003; Kioussi et al., 2002; Vadlamudi et al., 2005; Zorn et al., 1999). Downstream targets of WNT-β-Catenin signaling include the cell cycle regulator, CyclinD1, and transcription factors, N-myc, Lef1 and Pitx2 (Hovanes et al., 2001; Kioussi et al., 2002; Shu et al., 2005; Tetsu and McCormick, 1999). In embryonic lung, nuclear β-Catenin, LEF1 and PITX2 have been detected in the epithelium and in adjacent mesenchyme (Hjalt et al., 2000; Mucenski et al., 2003; Tebar et al., 2001). Similarly, TOPGAL and BATGAL, WNT-β-Catenin reporter genes that detect a subset of sites of WNT-β-Catenin activity, are active in lung epithelium and in adjacent mesenchyme (De Langhe et al., 2005; Maretto et al., 2003; Okubo and Hogan, 2004; Pongracz and Stockley, 2006; Shu et al., 2005).
WNT-β-Catenin signaling has been investigated in lung epithelium through conditional inactivation of β-Catenin or overexpression of the WNT signaling antagonist dickkopf1 (DKK1). Both approaches resulted in defects in distal lung bud formation, possibly due to disruption of FGF10-FGFR2b signaling (Mucenski et al., 2003; Shu et al., 2005). Three Wnts (Wnt2a, Wnt2b, Wnt7b) that typically signal through the WNT-β-Catenin pathway are expressed during lung development. Inactivation of Wnt7b, which is expressed in distal lung epithelium, results in severe lung hypoplasia due to defects in branching morphogenesis, cell proliferation, epithelial differentiation and loss of vascular smooth muscle integrity (Shu et al., 2002). Wnt2a is highly expressed in the distal mesenchyme (Bellusci et al., 1996; Monkley et al., 1996) but Wnt2a targeted mice were reported to have normal lung development (Monkley et al., 1996). Wnt2b (formerly called Wnt13) is also expressed in lung mesenchyme during early stages of lung development (Katoh et al., 1996; Katoh et al., 2000; Zakin et al., 1998). This leaves open the possibility of functional redundancy between these two related and similarly expressed genes. Two Wnts (Wnt5a, Wnt11) that typically signal through non-canonical pathways are expressed in lung epithelium and mesenchyme. Wnt5a is expressed in lung epithelium and adjacent mesenchyme and inactivation of Wnt5a leads to over-branching of the epithelial airway and thickening of the mesenchymal interstitium (Li et al., 2005, 2002). Wnt11 is expressed both in the lung epithelium and mesenchyme, but its function during lung development is not clear (Lako et al., 1998).
Although WNT-β-Catenin signaling is clearly important for lung epithelial development, its role in lung mesenchyme has not been directly examined. Here we identify an essential role for mesenchymal WNT-β-Catenin signaling by conditionally inactivating β-Catenin specifically in lung mesenchyme. We furthermore identify a reciprocal signaling loop in lung mesenchyme in which mesothelial/epithelial to mesenchymal FGF signals regulate Wnt2a expression and WNT-β-Catenin signaling, and mesenchymal WNT-β-Catenin signaling regulates FGFR1 and FGFR2 expression and, consequently, the level of FGF signaling. Disruption of any component of this signaling network results in defects in lung mesenchymal development and consequent defects in epithelial morphogenesis.
Materials and methods
Mouse strains
All mouse strains, including Fgf9+/−, β-Cateninf/f, Dermo1-Cre, TRE-Fgf9-IRES-eGfp, SPC-rtTA, Fgfr1f/f, Fgfr2f/f (f indicates a floxed allele) and Rosa26 reporter (R26R), have been previously described (Brault et al., 2001; Colvin et al., 2001; Soriano, 1999; Tichelaar et al., 2000; Trokovic et al., 2003; White et al., 2006; Yu et al., 2003). Fgfr1/2Dermo1 conditional knockout mice (Dermo1Cre/+, Fgfr1f/f, Fgfr2f/f) and Fgf9dox(48) mice (SPC-rtTA, TRE-Fgf9-IRES-eGfp) were made as described (Perl et al., 2002; White et al., 2006). The β-Cateninf/f strain was acquired through the Jackson Laboratory (Bar Harbor, ME). For conditional inactivation of β-Catenin in lung mesenchyme, mice were generated with the genotype, Dermo1Cre/+, β-Cateninf/f (referred to as β-CateninDermo1) by mating Dermo1Cre/+, β-Cateninf/+ mice with β-Cateninf/f. Control mice were of the genotype Dermo1Cre/+; Dermo1Cre/+, β-Cateninf/+; β-Cateninf/f; or β-Cateninf/+, all of which were phenotypically identical to wild type mice. All loss of function mice were maintained on a mixed 129SV/J-C57B6/J background. Transgenic strains, used for gain of function experiments, were maintained on the FVB background.
Analyses of mouse embryos, histology and immunohistochemistry
To induce FGF9 expression in vivo, the doxycycline-inducible epithelial transcriptional activator (SP-C-rtTA) was used to induce a tetracycline responsive transgene driving FGF9 (Perl et al., 2002; White et al., 2006). Doxycycline chow (Bio-Serv Inc., 300 mg/kg green pellets) was administered to pregnant female mice for 48 h prior to embryo isolation (Fgf9dox(48)).
Embryo tissues were collected in ice-cold PBS, fixed in 4% PFA overnight at 4 °C, washed with PBS, photographed and embedded in paraffin prior to sectioning at 5 µm. For histology, slides were stained with hematoxylin and eosin (H&E). For immunohistochemistry, paraffin-embedded or cryo-sections were rehydrated and treated with 0.3% hydrogen peroxide in methanol for 15 min to suppress the endogenous peroxidase activity. Antigen retrieval was achieved by microwaving the sections in 10 mM citrate buffer for 5 min followed by gradual cooling to room temperature. Sections were incubated overnight at 4 °C with the following primary antibodies: PCNA (sc-56, Santa Cruz Biotechnology Inc, 1:100); β-Catenin (610153, BD Transduction Laboratories, 1:200); LEF1 (sc-8591, Santa Cruz Biotechnology Inc, 1:100); BrdU (347580, Becton Dickinson Immunocytometry Systems, 1:200); phospho-histone H3 (pHH3, H9908, Sigma, 1:100); FGFR2 (sc-122, Santa Cruz Biotechnology Inc, 1:100); CyclinD1 (#2926, Cell Signaling Technology, 1:100); phospho-ERK (M8159, Sigma, 1:100), and TTF-1 (M3575, DAKO Cytomation, 1:100), visualized using Broad Spectrom (AEC) Kit (95-9743, Zymed Laboratories Inc.) or Vectastain® Elite ABC (AEC) kit (PK-4005, Vector Laboratories).
For BrdU analysis, pregnant females were injected with BrdU at 0.1 mg/kg body weight, 2 h prior to harvest. Embryos were collected in ice-cold PBS, processed and sectioned as above.
All staining patterns are representative of at least three embryos.
Cell death analysis
Paraffin or cryo-sectioned slides, prepared as described above, were assayed (TUNEL) using the In Situ Cell Death Detection Kit (Roche Applied Science). Slides were mounted with DAPI containing Vectashield® mounting medium (H-1200, Vector Laboratories) for fluorescent detection or counterstained with hematoxylin (MHS-16, Sigma) for AEC detection and then photographed. All staining patterns are representative of at least three embryos.
Whole-mount in situ hybridization
In situ probes were from the following sources: Lef1, Wnt2a (A. McMahon), Wnt7b (F. Long); Fgf10 (B. Hogan); Spry2; Fgfr1 (S. Werner); N-Myc (E. Morrisey). Probes were synthesized and labeled with a kit from Roche Applied Science. Whole-mount in situ hybridization was performed as described (Colvin et al., 2001). Following color reaction and methanol dehydration, tissues were photographed and then cryo-sectioned (6 µm), mounted on slides and re-photographed. In situ hybridizations of tissue sections were performed as previously described (Colvin et al., 1999). All staining patterns are representative of at least three embryos.
Whole-mount LacZ staining
Lungs were dissected in ice-cold PBS and fixed with 0.5% glutaraldehyde in PBT (PBS, 0.1% Tween-20) overnight at 4 °C. Tissues were washed in PBT twice for 10 min prior to incubation with LacZ staining solution (2 mM MgCl2, 35 mM potassium ferrocyanide, 35 mM potassium ferricyanide, 1 mg/ml X-Gal in PBT) at room temperature in the dark. Following adequate color reaction, tissues were again washed twice in PBT for 10 min each to stop the reaction. Samples were then soaked in 30% sucrose overnight, photographed and then embedded and frozen in OTC for cryosectioning (6 µM). Sections were dried for ~3 h at room temperature, washed with PBS and mounted. All staining patterns are representative of at least three embryos.
Lung organ cultures
Lung explant cultures were performed as described (White et al., 2006). E11.5 control, Fgf9−/− and β-CateninDermo1 embryonic lungs were dissected and cultured on Transwell filters (Costar, Corning) for 48 h at 37 °C, 5% CO2. Mouse FGF9 protein (PeproTech Inc.) was used at a concentration of 2.5 ng/ml in DMEM supplemented with 2 µg/ml heparin. LiCl was used at a concentration of 20 mM (Dean et al., 2005). To quantify mesenchymal thickness, explants were photographed and mesenchymal thickness was measured using Canvas X software. Data shown is representative of at least three independent experiments.
RNA isolation, cDNA synthesis, and qRT-PCR analysis
E14.5 embryonic lung mRNA was isolated using the RNeasy kit (Qiagen Inc.) following manufacturer's instructions. cDNA was synthesized using the SuperScript II first-strand cDNA synthesis kit (Invitrogen). Quantitative RT-PCR was performed on an ABI 7500 machine using TaqMan® probes for Gapdh and Fgfr2. Amplification and analysis were performed according to the manufacturer's instructions. All reactions were normalized to Gapdh. Results were graphed as relative expression compared with control, where control was scaled to 1.
Construction of a Fgfr2 promoter-luciferase reporter
A mouse genomic DNA fragment extending 6.8 kb 5′ and 533 bp 3′ to the Fgfr2 transcription initiation site was excised from BAC DNA (RP23-466 J2) with BamH1 and subcloned into the pGL3 basic vector (Promega Inc.) to generate pGL3-Fgfr2p. This fragment contains Fgfr2 exon 1 and part of intron 1. The pCIG-dominant active β-Catenin vector (0.2 µg) or control vector (pCIG) (Megason and McMahon, 2002) was co-transfected into HEK293 cells with pGL3-Fgfr2p and pCMV-β-Gal (Promega) using Fugene 6 (Roche Applied Science). Luciferase assay was performed 48 h after transfection using the Luciferase Assay System (Promega). All data were normalized to β-Gal activity. Data represents four independent experiments. Positive controls (not shown), using the TOPFLASH reporter, demonstrated activation of WNT signaling.
Results
FGF9 regulates WNT signaling in lung mesenchyme
Wnt2a is prominently expressed in lung mesenchyme and has been shown to signal through the WNT-β-Catenin pathway (Bellusci et al., 1996; Karasawa et al., 2002). Examination of E13.5 lung tissue from mice lacking FGF9 (Fgf9−/− mice) demonstrated a complete absence of Wnt2a expression in lung mesenchyme (Figs. 1A, B). By contrast, induced overexpression of FGF9 in lung epithelium from E11.5 to E13.5 (Fgf9dox(48)) (White et al., 2006) resulted in increased Wnt2a expression (Figs. 1C, D). To determine if WNT-β-Catenin signaling was regulated by FGF9 in lung mesenchyme, we examined the expression of Lef1, a transcriptional target of the WNT-β-Catenin pathway that is expressed in lung mesenchyme (Porfiri et al., 1997; Tebar et al., 2001). In Fgf9−/− mice, Lef1 expression was reduced in lung mesenchyme at E13.5, whereas in mice overexpressing Fgf9 in lung epithelium from E11.5 to 13.5, Lef1 expression was increased in the sub-epithelial mesenchymal domain (Figs. 1E–H). Immunohistochemical detection of LEF1 revealed a similar pattern and regulation in response to gain and loss of expression of Fgf9 (Supplemental Fig. 1). To determine whether FGF9 signaling has any effect on an epithelial Wnt, we examined the expression of the WNT-β-Catenin pathway ligand, Wnt7b (Shu et al., 2002). Expression of Wnt7b was not affected in either FGF9 loss of function or gain of function mouse models (Figs. 1I–L). These data suggest that Wnt2a may contribute to WNT-β-Catenin signaling in lung mesenchyme and is a candidate for a downstream target of FGF9 signaling.
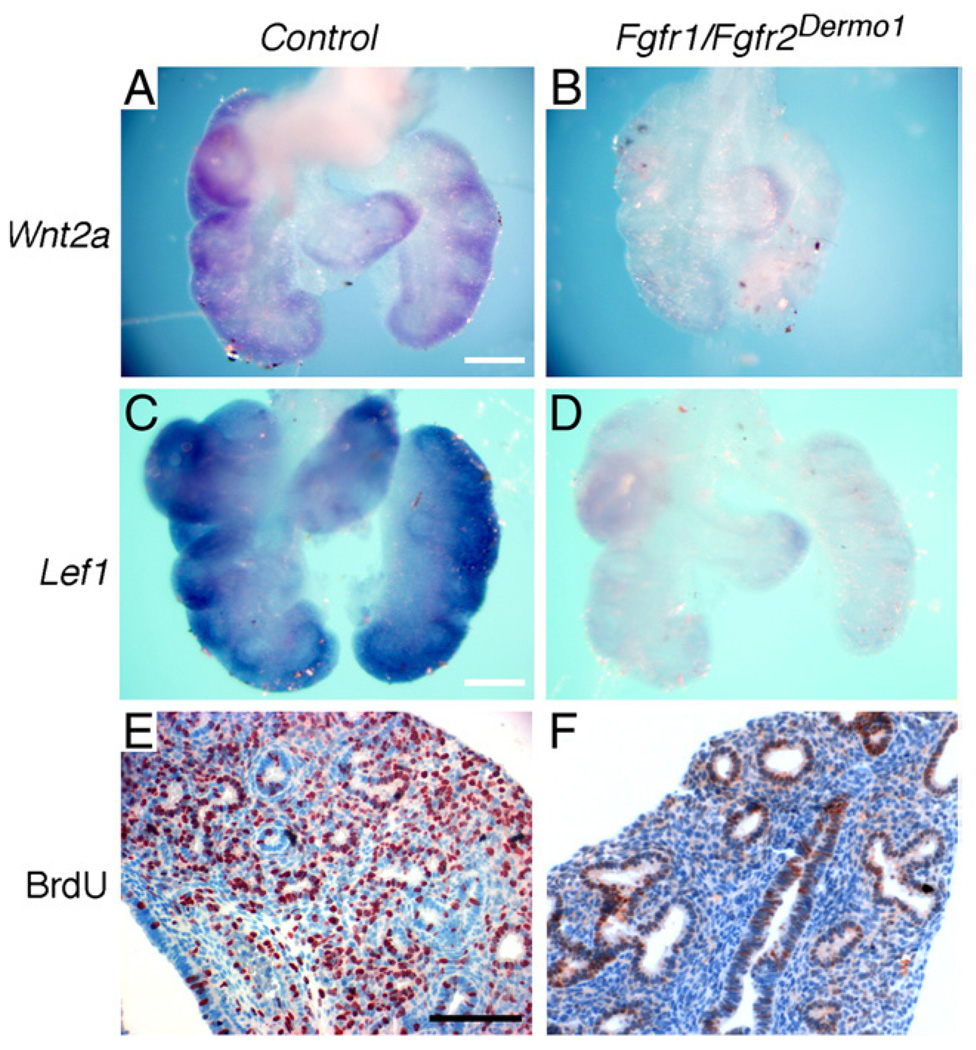
FGF receptors 1 and 2 have been shown to act redundantly to mediate the FGF9 signal in lung mesenchyme (White et al., 2006).We thus hypothesized that inhibition of mesenchymal FGF receptors should also result in decreased mesenchymal WNT-β-Catenin signaling. To test this hypothesis directly, we conditionally inactivated floxed alleles of both Fgfr1 and Fgfr2 in the lung mesenchyme with Dermo1-Cre and then examined the expression of Wnt2a and Lef1. Dermo1-Cre is active in lung mesenchyme and mesothelium, but not epithelium. By E10.5, Dermo1-Cre can efficiently activate the ROSA26 reporter allele (R26R) and β-galactosidase activity throughout lung mesenchyme and mesothelium (see Fig. 3J below). Consistent with our previous report (White et al., 2006), Dermo1cre/+, Fgfr1f/f, Fgfr2f/f (hereafter referred to as Fgfr1/2Dermo1) lungs were smaller than control lungs beginning at E12.5. Whole-mount in situ hybridization of E12.5 Fgfr1/2Dermo1 lungs showed reduced Wnt2a (Figs. 2A, B) and Lef1 expression in lung mesenchyme (Figs. 2C, D), similar to what was observed in Fgf9−/− mice. Consistent with decreased FGFR signaling, mesenchymal proliferation was decreased at E14.5 (Figs. 2E, F). Together, these data suggest that FGFR signaling in lung mesenchyme modulates the level of mesenchymal WNT-β-Catenin signaling in lung mesenchyme.
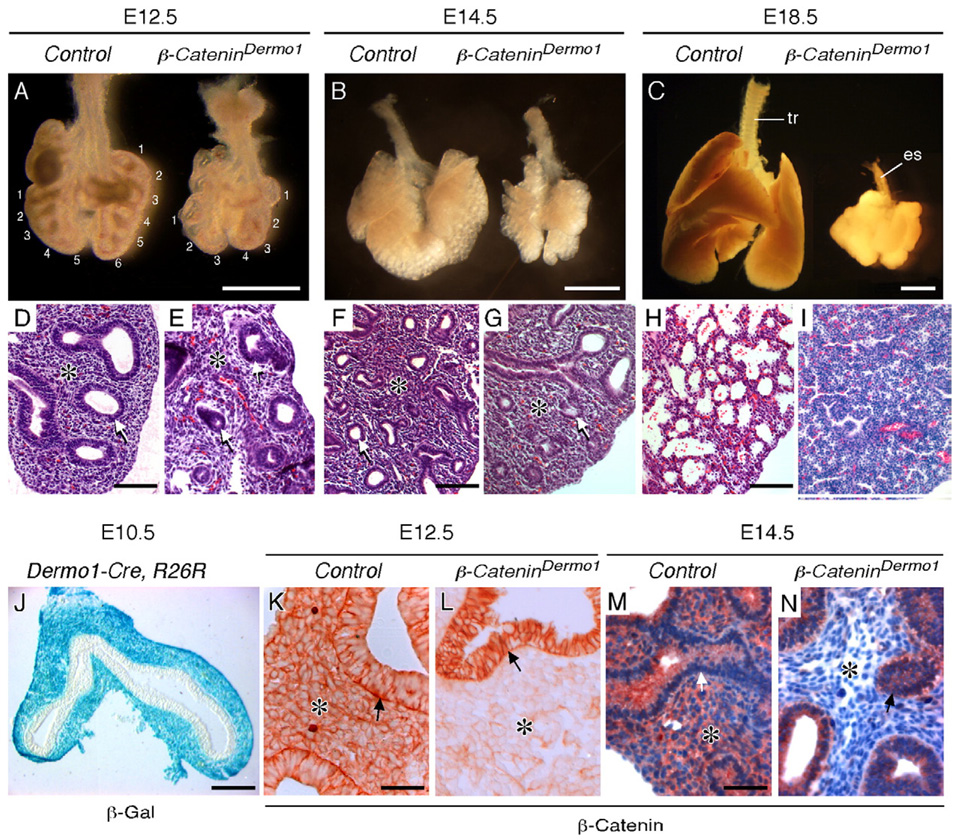
Fig. 3.
Mesenchymal β-Catenin is essential for embryonic lung development. (A–C) Anterior views of gross dissections of control (Dermo1-Cre, β-Cateninf/+) and β-CateninDermo1 lungs at E12.5 (A), E14.5 (B) and E18.5 (C). Note the decreased number of epithelial branches (numbers) but normal number and orientation of lobes at E12.5. tr, trachea; es, esophagus. (D–I) H&E stained histologic sections of lungs at the same stages shown in panels A–C. At E12.5 (E) and E14.5 (G), β-CateninDermo1 lung tissue had fewer epithelial branches and decreased distal mesenchyme compared with controls (D, F). At E18.5 (I), β-CateninDermo1 lung tissue contained small epithelial tubes surrounded by dense disorganized mesenchyme, whereas control lungs (H) had developed numerous primitive alveoli surrounded by blood vessels. (J) Cryo-section of a Dermo1-Cre, Rosa26R lung at E10.5 showing mesenchymal cell-specific β-Gal staining indicating Cre activity throughout embryonic mesenchyme and mesothelium, but not epithelium. (K–N) Immunohistochemistry detection of β-Catenin showing that β-CateninDermo1 lungs have greatly reduced staining in lung mesenchyme as early as E12.5 (L) and complete absence of mesenchymal protein by E14.5 (N), compared with control lungs (K, M). Epithelial expression appeared unaffected at these stages of development. No counterstain was used in panels K and L. Panels M and N were counterstained with hematoxylin. Asterisk indicates mesenchyme; arrow indicates epithelium. Scale bars: panel A, 0.25 mm; panels B and C, 1 mm; panels D, F, H, 100 µm; panel J, 200 µm; panels K, M, 50 µm.
Fig. 2.
Mesenchymal FGFR signaling regulates mesenchymal WNT-β-Catenin signaling and mesenchymal proliferation. (A–D) WNT-β-Catenin signaling in control and Fgfr1/2Dermo1 lung mesenchyme. Whole-mount in situ hybridization of Wnt2a (A, B) and Lef1 (C, D) expression showing down-regulation in Fgfr1/2Dermo1 lung mesenchyme (B, D) compared with controls (Dermo1cre/+, Fgfr1+/f, Fgfr2+/f) (A, C) at E14.5. (E, F) Cell proliferation assessed by BrdU labeling showing decreased labeling in mesenchyme and epithelium of Fgfr1/2Dermo1 lungs (F) compared to control lungs (E) at E14.5. Scale bars: panels A and C, 200 µm; panel E, 100 µm.
Mesenchymal β-Catenin is essential for embryonic lung development
To directly investigate the role of mesenchymal WNT-β-Catenin signaling in embryonic lung development, β-Catenin (β-Catenin f/f) (Brault et al., 2001) was conditionally targeted in lung mesenchyme with Dermo1-Cre (Yu et al., 2003). Mice of the genotype Dermo1Cre/+, β-Cateninf/f, hereafter referred to as β-CateninDermo1, showed impaired lung growth as early as E12.5 (Fig. 3A). Control mice of the genotype Dermo1Cre/+, β-Cateninf/+ or Dermo1+/+, β-Cateninf/f were phenotypically normal (Fig. 3A and data not shown). β-CateninDermo1 lungs had a normal number and orientation of lobes but fewer epithelial branches and decreased mesenchyme. In some cases, lobes were misshapen and bent. By E14.5, the lungs were less than half of the normal size and failed to grow during the remainder of embryonic development (Fig. 3B). By E18.5, β-CateninDermo1 lungs were small and compact, lacked smooth borders, and the trachea had completely degenerated (Fig. 3C). β-CateninDermo1 mice died at birth due to respiratory failure. Histologic sections of E12.5 and E14.5 β-CateninDermo1 lung tissue revealed decreased epithelial branching and decreased distal mesenchyme, but otherwise normal appearing mesenchymal and epithelial compartments (Figs. 3D–G). At E18.5, control lungs had clear airspace development, accompanied by thinning of the mesenchyme and close approximation of fetal capillaries to flattened epithelial cells. By contrast, β-CateninDermo1 lung tissue showed poorly organized peripheral lung tissue without well-formed air sacs (Figs. 3H, I).
To examine the efficiency and specificity of β-Catenin inactivation, immunohistochemistry was used to localize β-Catenin protein. Control lung tissue showed uniform staining for β-Catenin in both epithelium and mesenchyme, while β-CateninDermo1 lungs showed greatly reduced staining in lung mesenchyme as early as E12.5 (Figs. 3K, L) and complete absence of mesenchymal protein at E14.5 (Figs. 3M, N) and E18.5 (Supplemental Fig. 2). Epithelial β-Catenin expression appeared unchanged throughout lung development. To determine whether mesenchymal WNT signaling was reduced as a consequence of inactivation of β-Catenin, expression of the WNT-β-Catenin regulated genes, Lef1, N-Myc and CyclinD1 (see below), was examined. Expression of Lef1 was decreased in β-CateninDermo1 lung mesenchyme at E12.5 (Figs. 4A–D). Immunohistochemistry revealed an absence of LEF1 protein in lung mesenchyme at E12.5 in β-CateninDermo1 lungs (Figs. 4E, F). These data demonstrate down-regulation of mesenchymal WNT-β-Catenin signaling following inactivation of β-Catenin in lung mesenchyme. Interestingly, Wnt2a expression was also reduced in β-CateninDermo1 lungs (Figs. 4G, H), suggesting the presence of a feedback loop downstream of β-Catenin that regulates WNT-β-Catenin signaling (see below).
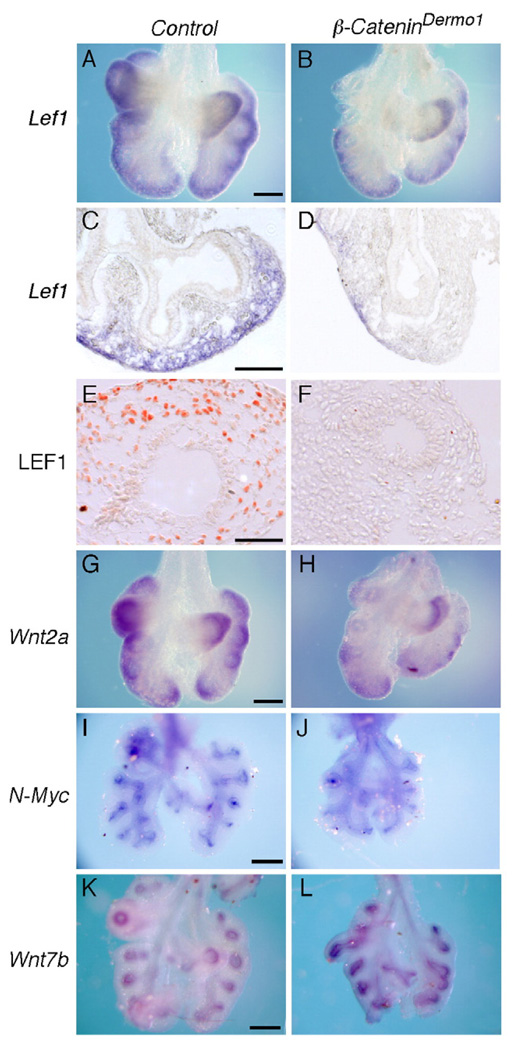
Fig. 4.
Mesenchymal and epithelial WNT-β-Catenin signaling following conditional inactivation of β-Catenin in lung mesenchyme. (A–D) Expression of Lef1 in control (A, C) and β-CateninDermo1 lung mesenchyme (B, D) at E12.5. A,B are whole-mount in situ hybridizations. Panels C, D are cryo-sections of the tissue in panels A and B. (E, F) Immunohistochemical detection of LEF1 showing decreased protein in mesenchyme at E12.5 in β-CateninDermo1 lungs (F) compared with control lungs (E). (G, H) Whole-mount in situ hybridization showing reduced Wnt2a expression in β-CateninDermo1 lung mesenchyme (H) compared with control (G). (I, J) Whole-mount in situ hybridization showing that N-Myc expression in lung epithelium was not affected by inactivating β-Catenin in lung mesenchyme (J) compared with control (I). (K, L) Whole-mount in situ hybridization showing that Wnt7b expression in lung epithelium was increased in β-CateninDermo1 lung (L) compared with control (K). Scale bars: panels A, G, I, K, 200 µm; panels C and E, 50 µm.
To determine whether lung epithelial WNT-β-Catenin signaling was affected by inactivation of β-Catenin in lung mesenchyme, N-Myc expression was examined. N-Myc is expressed in lung epithelium and is a gene known to be regulated by epithelial WNT-β-Catenin signaling (Shu et al., 2005). In situ hybridization analysis showed that, at E12.5, N-Myc expression was not significantly affected by inactivation of lung mesenchymal β-Catenin (Figs. 4I, J). Additionally, Wnt7b, a WNT ligand expressed in lung epithelium that signals through canonical WNT-β-Catenin pathways, showed slightly increased expression in β-CateninDermo1 lung epithelium compared to wild type controls (Figs. 4K, L).
Mesenchymal β-Catenin is required for proliferation and survival of lung mesenchymal and epithelial cells
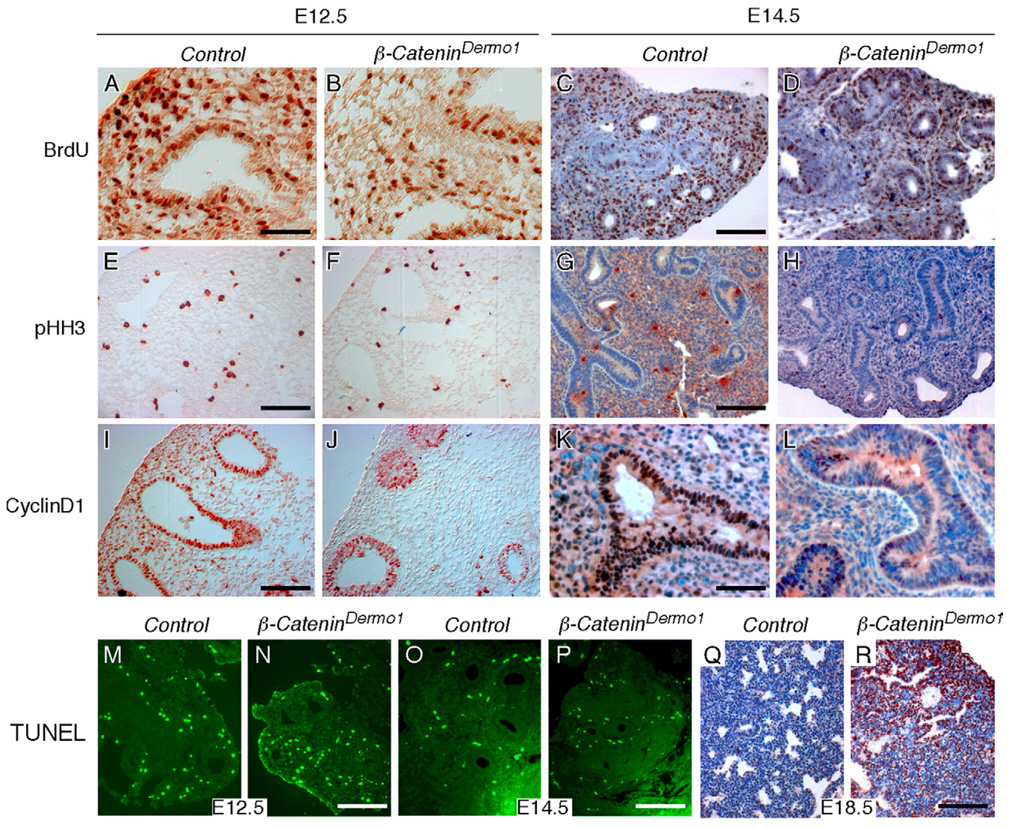
The smaller size of β-CateninDermo1 lungs between E12.5 and E14.5, and the failure to grow after E14.5, suggested that inactivation of β-Catenin may lead to decreased proliferation or increased cell death in lung mesenchyme and may secondarily affect growth and survival of lung epithelium. We assessed cell proliferation by examining expression of proliferating cell nuclear antigen (PCNA), as well as incorporation of bromodeoxyuridine (BrdU). PCNA labels all cycling cells, while BrdU specifically labels cells that are within S phase during the labeling period. At E12.5 and E14.5, BrdU labeling was significantly decreased in β-CateninDermo1 lung tissue compared to control (Figs. 5A–D). Quantitation revealed decreased proliferation within both the epithelial and mesenchymal compartments (Table 1). To further examine cell cycle progression, we examined the level of phosphor-histone H3 (pHH3), a marker expressed during G2/M, by immunohistochemistry. At E12.5, pHH3 labeling was significantly decreased in β-CateninDermo1 lung mesenchyme, but not in epithelium(Figs. 5E, F, Table 1). By contrast, at E14.5, pHH3 labeling was significantly decreased in β-CateninDermo1 lung in both mesenchyme and epithelium (Figs. 5G, H, Table 1). Surprisingly, despite severe hypoplasia of β-CateninDermo1 lung tissue, there was an apparent increase in the PCNA labeling index (number of PCNA-positive nuclei/total nuclei) in both the mesenchymal and epithelial compartments at E14.5 (Table 1, Supplemental Fig. 3), suggesting an arrest in the cell cycle at the G1/S boundary and accumulation of PCNA-positive, G1/S arrested cells. Together, the data suggest that removal of mesenchymal β-Catenin results in an initial significant reduction in the proliferation rate of mesenchymal cells and an apparent secondary defect in epithelial proliferation.
Fig. 5.
Mesenchymal β-Catenin is required for proliferation and survival of lung mesenchymal and epithelial cells. (A–D) BrdU labeling was significantly decreased in β-CateninDermo1 lung tissue at E12.5 (B) and E14.5 (D) compared to control lungs (A, C). (E–H) Immunostaining showing decreased phospho-histone H3 (pHH3) labeling in β-CateninDermo1 lung tissue at E12.5 (F) and E14.5 (H) compared with the controls (E, G). (I–L) Immunostaining showing decreased Cyclin D1 expression in β-CateninDermo1 lung mesenchyme at E12.5 (J) and E14.5 (L) compared with controls (I, K). Note that in epithelium, decreased Cyclin D1 expression was only observed at E14.5 (L). (M–R) Cell death assessed by TUNEL labeling in β-CateninDermo1 embryonic lungs at E12.5 (M, N), E14.5 (O, P) and E18.5 (Q, R). No increased cell death was detected in β-CateninDermo1 lung tissue at E12.4 and E14.5 (M–P). At E18.5, massive apoptosis was apparent throughout β-CateninDermo1 lung epithelium and mesenchyme (R) compared to control (Q). Scale bars: panels A and K, 50 µm; panels C, E, G, I, 100 µm; panels N, P, R, 200 µm.
Table 1.
Quantification of proliferation markers in control and β-CateninDermo1 lungs
| E12.5 (n=3)a | E14.5 (n=3)a | |||||||
|---|---|---|---|---|---|---|---|---|
| Epithelium | Mesenchyme | Epithelium | Mesenchyme | |||||
| Control | CKO | Control | CKO | Control | CKO | Control | CKO | |
| PCNA | 474±10b | 481±11 | 471±18 | 477±9 | 172±26 | 337±16* | 212±15 | 365±10* |
| BrdU | 394±26 | 286±25* | 356±35 | 219±34* | 215±5 | 141±9* | 187±19 | 140 ±15* |
| p-HH3 | 12±3 | 9±1 | 25±3 | 14±2* | 16±2 | 1±1* | 23±2 | 1±1* |
| CyclinD1 | 483±12 | 345±4# | 87±8 | 21±7* | 417±10 | 224±18* | 80±9 | 26±6* |
| p-ERK | 7±4 | 4±4 | 5±1 | 1±1# | 26±7 | 8±3# | 17 ± 4 | 7 ±3# |
CKO, β-CateninDermo1 conditional knockout.
p<0.01
p<0.05, Student's t test.
Number of embryos analyzed. Two–three sections per embryo were counted.
Cell index is defined as the number of positive epithelial or mesenchymal nuclei/500 nuclei counted.
D type Cyclins are required for progression through the cell cycle (Baldin et al., 1993) and their expression is controlled by extracellular growth factors, including members of the WNT and FGF family (Issack and Ziff, 1998; Lobjois et al., 2004). At E12.5 and E14.5, Cyclin D1 expression was reduced in β-CateninDermo1 lung mesenchyme and epithelium (Figs. 5I–L, Table 1). The decrease in epithelium appeared greater at E14.5 (Figs. 5K, L).
Cell death was examined by TUNEL labeling. At E12.5 and E14.5, no significant increase in cell death was detected in β-CateninDermo1 lung tissue (Figs. 5M–P). However, at E18.5, massive apoptosis was apparent throughout lung epithelium and mesenchyme (Figs. 5Q, R). These data indicate that mesenchymal WNT-β-Catenin signaling is either directly required for cell survival after E14.5, that proper WNT-β-Catenin signaling at earlier stages is necessary for lung viability at later times in development, or that other phenotypes affect lung survival at later stages of development. Further studies were therefore focused on earlier developmental stages (E12.5–E14.5) to identify more direct functions of WNT-β-Catenin signaling in lung mesenchyme.
Taken together, these data demonstrate that mesenchymal WNT-β-Catenin signaling is essential for maintaining lung mesenchymal proliferation. This could be a direct consequence of WNT regulation of cell cycle genes or an indirect effect mediated through regulation of other signaling pathways. Additionally, non-autonomous effects on lung epithelial proliferation suggest loss of a mesenchymal-derived signal to epithelial cells.
Epithelial identity and signaling in β-CateninDermo1 lung
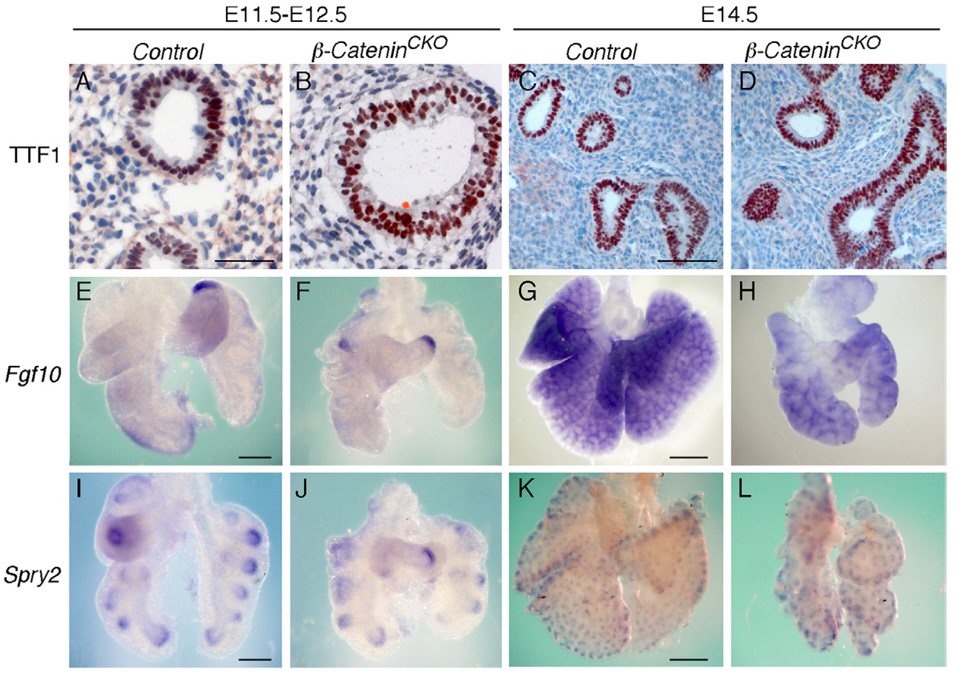
To further examine lung epithelial function in β-CateninDermo1 lung, Thyroid Transcription Factor 1 (TTF1) and Surfactant Protein-C (SP-C) expression was examined. TTF1 is essential for epithelial cell development (Cardoso and Lu, 2006) and SP-C is expressed in distal lung epithelium (Weaver et al., 1999). Immunostaining for TTF1 demonstrated expression in epithelial cell nuclei at near normal levels at E12.5 and E14.5 in β-CateninDermo1 lung (Figs. 6A–D), similar to what was observed in Fgfr1/2Dermo1 lung (data not shown). Expression of SP-C was reduced in β-CateninDermo1 lung at E12.5 (data not shown).
Fig. 6.
Deficiency of lung mesenchymal WNT-β-Catenin signaling does not affect epithelial FGF signaling. (A–D) Immunostaining shows comparable TTF1 expression in the epithelium of control (A, C) and β-CateninDermo1 lung (B, D) at E12.5 and E14.5. (E–H) Whole-mount in situ hybridization at E12.5 (E, F) and E14.5 (G, H) showing comparable levels of Fgf10 in control (E, G) and β-CateninDermo1 lungs (F, H). (I–L) Whole-mount in situ hybridization shows comparable levels of Spry2 expression at E12.5 (I, J) and E14.5 (K, L) in control (I, K) and β-CateninDermo1 lung (J, L). Scale bars: in panels A and C, 50 µm; in panels E and I, 200 µm; in panels G and K, 500 µm.
FGF10 is an essential factor for lung epithelial branching. Focal Fgf10 expression is observed in mesenchyme at the distal tip of the prospective lung lobes starting at ~E10.0 and in distal mesenchyme adjacent to budding airway epithelium at later stages (Bellusci et al., 1997). Expression patterns of Fgf10 were examined to determine whether altered FGF10 expression could account for some of the epithelial phenotypes observed in β-CateninDermo1 lung. At E12.0 and E14.5, control and β-CateninDermo1 lungs exhibited comparable expression patterns and levels of Fgf10 in mesenchyme distal to budding airways (Figs. 6E–H). To examine FGF signaling in lung epithelium, Sprouty 2 (Spry2) expression was examined. Spry2 is one of the earliest targets to be induced in the lung epithelium in response to FGF10 (Mailleux et al., 2001) and is a negative regulator of the FGF signaling pathway. Comparison of Spry2 expression showed no significant difference in control and β-CateninDermo1 lungs at E12.5 and E14.5 (Figs. 6I–L), further suggesting that FGF10-FGFR2b signaling is not a primary target of mesenchymal WNT-β-Catenin signaling.
WNT-β-Catenin signaling regulates FGF receptor expression and function in lung mesenchyme
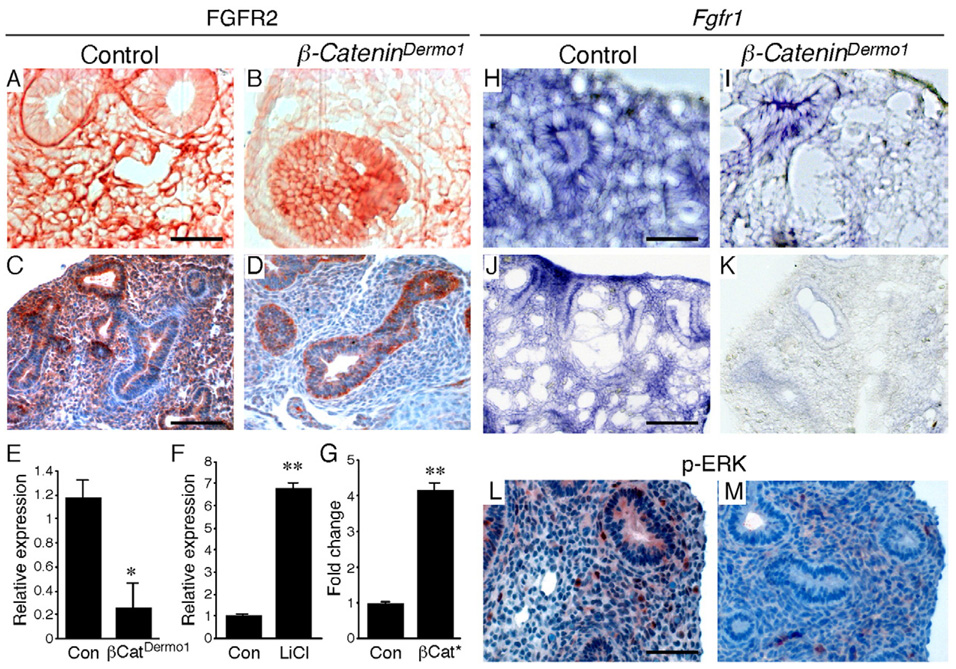
Mesenchymal proliferation is decreased in Fgf9−/−, Fgfr1/2Dermo1 and in β-CateninDermo1 lung tissue. We hypothesized that the proliferation defect in β-CateninDermo1 lung mesenchyme might be mediated in part through regulation of FGF signaling. Fgfr1 and FGFR2 expression was therefore examined. Immunostaining of E12.5 lung showed decreased expression of FGFR2 in β-CateninDermo1 lung mesenchyme but not in epithelium (Figs. 7A, B). At E14.5, FGFR2 expression was not detectable in lung mesenchyme but still was expressed at normal levels in distal epithelium (Figs. 7C, D).
Fig. 7.
WNT-β-Catenin signaling regulates FGF receptor expression in lung mesenchyme. (A–D) Immunostaining of E12.5 and E14.5 lung showing FGFR2 expression in control lung mesenchyme and epithelium (A, C) and the absence of FGFR2 expression in lung mesenchyme but not epithelium in β-CateninDermo1 lung (B, D). (E) Quantitative-RT-PCR detection of Fgfr2 expression showing decreased expression in E14.5 β-CateninDermo1 lung (βCatDermo1) compared to control lung (Con). (F) Quantitative-RT-PCR detection of Fgfr2 expression showing increased expression of E11.5 lung explants treated with 20 mM LiCl for 48 h compared to untreated control explants. (G) Fgfr2 promoter-luciferase reporter gene showing increased activity in response to co-transfection of HEK293 cells with a dominant active β-Catenin expression plasmid (βCat*), compared to control cells transfected with the pCIG plasmid. *, p<0.05; **, p<0.001 (Student's t test). Error bars show SEM. (H–K) In situ hybridization (cryo-section of whole-mount) of E12.5 (H, I) and E14.5 (J, K) lungs showing Fgfr1 expression in control mesenchyme and epithelium (H, J) and reduced Fgfr1 expression in β-CateninDermo1 lung mesenchyme and epithelium (I, K). (L, M) Immunostaining showing decreased expression of p-ERK at E14.5 in β-CateninDermo1 lung (M) compared with the control lung (L). Scale bars: panels A, C, H, J, L, 50 µm.
To further examine the regulation of lung mesenchymal Fgfr2 expression by mesenchymal WNT-β-Catenin signaling, we examined expression of Fgfr2 in E14.5 lung tissue from control and β-CateninDermo1 mice by quantitative RT-PCR. In the absence of mesenchymal WNT-β-Catenin signaling, Fgfr2 showed 4.6 fold lower expression (Fig. 7E). To further test the ability of WNT-β-Catenin signaling to regulate Fgfr2 gene expression, wild type lung explants were treated with 20 mM LiCl for 48 h. LiCl inhibits GSK3β and stabilizes and activates cytosolic β-Catenin (Klein and Melton, 1996). Quantitative RT-PCR showed that in the presence of LiCl, Fgfr2 expression was increased 6.8 fold (Fig. 7F). Finally, 6.8 kb of Fgfr2 genomic sequence, located 5′ to the transcription initiation site, was cloned into a luciferase expression plasmid, pGL3 basic. Co-transfection with pCIG-dominant active β-catenin, containing a constitutively active form of β-Catenin, resulted in a 4.6 fold increase in luciferase activity relative to controls (co-transfection with pCIG) (Fig. 7G).
Fgfr1 mRNA expression was examined by whole-mount in situ hybridization. Fgfr1 showed reduced expression in lung mesenchyme at E12.5 (Figs. 7H, I) and E14.5 (Figs. 7J, K). Epithelial expression was also decreased at E14.5 in β-CateninDermo1 lungs (Fig. 7K). Consistent with decreased FGFR expression and decreased mesenchymal proliferation, p-ERK expression was decreased throughout β-CateninDermo1 lungs at E14.5 (Figs. 7L, M, Table 1).
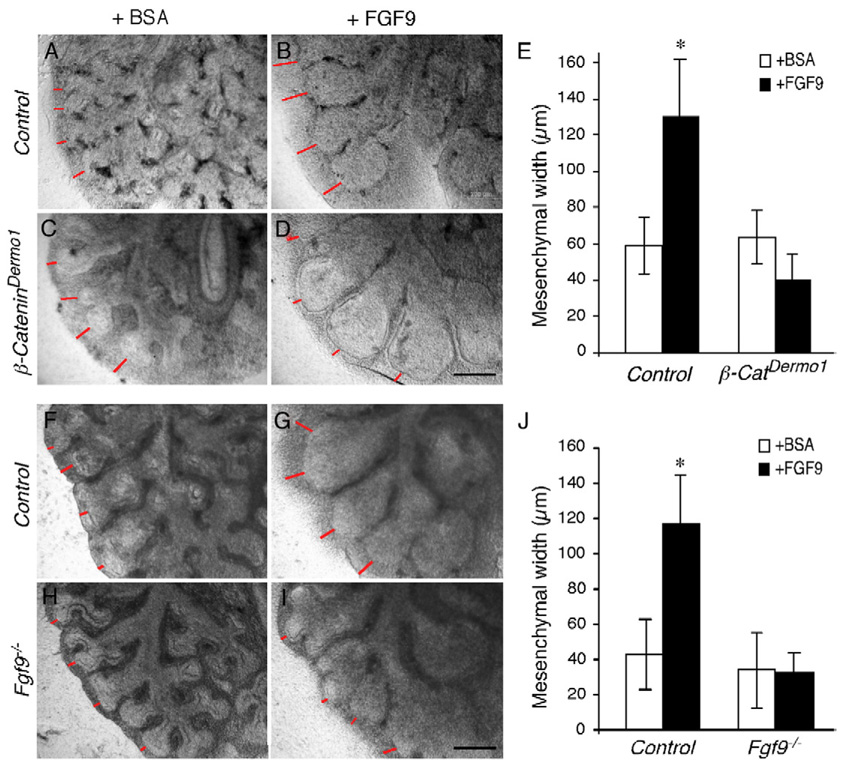
If decreased mesenchymal FGFR expression in β-CateninDermo1 lung was functionally important, one would predict that β-CateninDermo1 lungs would have impaired responsiveness to FGF ligands that primarily signal to mesenchymal FGFRs. To test this, lung explant cultures from control and β-CateninDermo1 lungs were treated with BSA or FGF9 (Figs. 8A–E). Control lung explants responded to FGF9 with mesenchymal hyperplasia, as we have previously reported (White et al., 2006) (Figs. 8A, B). Measurement of mesenchymal thickness in control lung explants showed a 2.8 fold (p<0.01) increase in response to FGF9 (Fig. 8E). In contrast, β-CateninDermo1 lung explants showed no significant mesenchymal response to FGF9, and the sub-mesothelial mesenchymal layer remained thin, similar to BSA treated explants (Figs. 8C–E). Of note, epithelium in both control and β-CateninDermo1 lungs showed a dilation in response to FGF9 (Fig. 8B, D), consistent with the possibility that FGF9 may also signal directly to lung epithelium (del Moral et al., 2006).
Fig. 8.
β-CateninDermo1 and Fgf9−/− lung mesenchyme have a decreased response to FGF9. (A–D) Lung explant cultures from control (A, B) and β-CateninDermo1 lungs (C, D) were treated with BSA (A, C) or FGF9 (B, D). Control lung explants responded to FGF9 (B), but not to BSA (A), with mesenchymal hyperplasia. In contrast, β-CateninDermo1 lungs showed a similar lack of response to both BSA (C) and FGF9 (D). (E) Quantitation of mesenchymal growth in response to BSA and FGF9 (n=3, *p<0.01). (F–I) Lung explant cultures from control (F, G) and Fgf9−/− lungs (H, I) were treated with BSA (F, H) or FGF9 (G, I). Control lung explants responded to FGF9 (G), but not to BSA (F), with mesenchymal hyperplasia. In contrast, Fgf9−/− lungs showed a similar lack of response to both BSA (H) and FGF9 (I). (J) Quantitation of mesenchymal growth in response to BSA and FGF9 (n=3, *p<0.01). Scale bars: D, I, 200 µm.
Another prediction that arises from this regulatory network model is that, in the absence of FGF9, the feedback loop that maintains WNT-β-Catenin signaling and Fgfr expression should degrade and lung explants from Fgf9−/− embryos should not be able to be rescued by treatment with FGF9. Consistent with this prediction, Fgf9−/− lung explants showed no mesenchymal response to FGF9 (Figs. 8H–J), while control mesenchyme showed a 2.7 fold increase in mesenchymal thickness in response to FGF9 (Figs. 8F, G, J). Epithelium from both control and Fgf9−/− explants showed epithelial dilation in response to FGF9 (Figs. 8F–I).
Discussion
We have identified a robust regulatory circuit in which epithelial to mesenchymal FGF signaling regulates the expression of a Wnt ligand and the level of WNT-β-Catenin signaling, and in which mesenchymal WNT-β-Catenin signaling regulates the expression of FGF receptors and the level of mesenchymal FGF signaling (Fig. 9). The only external (non-mesenchymal) factor that has thus far been identified that can modulate this signaling system is FGF9, which is produced by the mesothelial lining of the lung and by airway epithelium, and potentially WNT7b, which is produced in airway epithelium. The primary output of this system appears to be regulation of mesenchymal proliferation, but clearly other mesenchymal factors that regulate lung epithelial proliferation, survival and differentiation must also act downstream of FGF and/or β-Catenin.
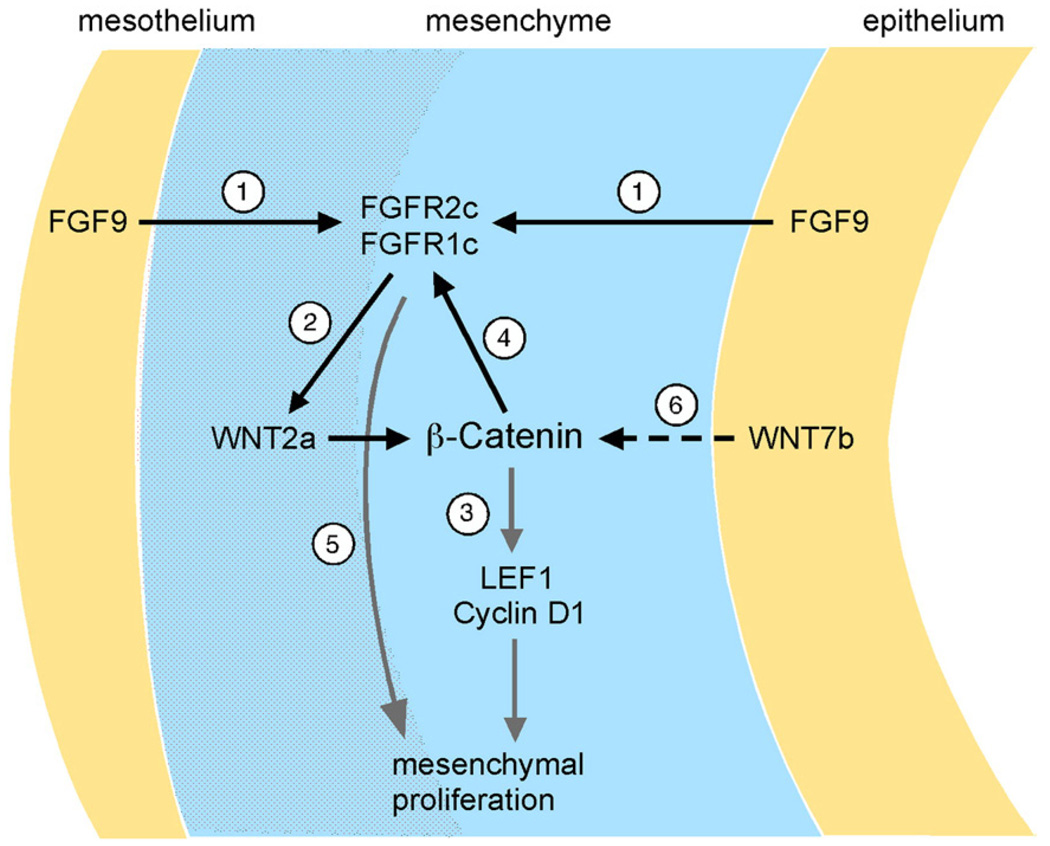
Fig. 9.
Molecular pathways by which WNT-β-Catenin and FGF regulate lung mesenchymal development. (1) At early stages of lung development (E10–E12), FGF9 is produced by lung mesothelium and epithelium and signals to mesenchymal FGFR1c and FGFR2c to maintain mesenchymal proliferation (Colvin et al., 2001; White et al., 2006). In the absence of Fgf9, expression of the mesenchymal WNT-β-Catenin pathway ligand, Wnt2a, and target gene, Lef1, was down-regulated. When FGF9 was overexpressed in airway epithelium, Wnt2a, and the β-Catenin target gene, Lef1, were both up-regulated. This suggests a signaling pathway in which mesenchymal FGFR activation regulates Wnt2a expression (2) and mesenchymal WNT-β-Catenin signaling. (3) Inactivation of mesenchymal β-Catenin resulted in decreased expression of Lef1 mRNA and protein, decreased expression of CyclinD1 and decreased mesenchymal proliferation, suggesting that mesenchymal WNT signaling could mediate, in part, FGF regulated mesenchymal proliferation. (4) Inactivation of mesenchymal β-Catenin also resulted in decreased expression of FGFR1 and FGFR2 in lung mesenchyme, suggesting that feedback through FGFR signaling may contribute to the ability of mesenchymal WNT signaling to regulate proliferation (5) and Wnt2a expression (2). (6) Wnt7b is expressed in airway epithelium and likely signals to mesenchyme to regulate WNT-β-Catenin signaling. Red stippled area represents the Wnt2a expression domain in the sub-mesothelial mesenchymal compartment.
Two predictions can be derived from the FGF–WNT interaction model (Fig. 9). First: down-regulation or inhibition of any component of this system should diminish the output of the entire system. Second: up-regulation of any component of the system should reinforce the entire system. In vitro organ culture experiments and in vivo genetic experiments support the circular and self-sustaining nature of this signaling network. For example, lung tissue lacking mesenchymal β-Catenin was predicted and shown to down-regulate mesenchymal responsiveness to FGF9. Similarly, loss of mesenchymal FGF signaling resulted in diminished WNT-β-Catenin signaling, loss of Fgfr expression and inability of the tissue to be rescued by treatment with FGF9. However, phenotypic differences resulting from loss of either mesenchymal β-Catenin or mesenchymal FGFR1 and FGFR2 indicate that other factors must also feed into this signaling circuit to sustain mesenchymal viability. Apparent differences could also be due to variation in the timing or efficiency of Cre-mediated inactivation of β-Catenin and Fgfrs 1 and 2, versus complete loss of mesenchymal FGF signaling in Fgf9−/− lungs. In FGF loss of function models (Fgf9−/− and Fgfr1/2Dermo1), the lung becomes atrophic by birth but does not exhibit widespread cell death (Colvin et al., 2001; White et al., 2006). By contrast, β-CateninDermo1 lungs exhibit widespread apoptosis by E18.5. These observations provide further evidence that these two pathways are not equivalent and that β-Catenin signaling may have a more important role in mesenchymal cell survival. Furthermore, some intrinsic or extrinsic signal(s) likely maintains low-level mesenchymal β-Catenin signaling and cell viability in the absence of FGF signaling. This is supported by the observed reduction, but not elimination, of WNT-β-Catenin activity (Lef1 expression) in Fgf9−/− lung mesenchyme. However, besides FGF9 and WNT7b, factors that regulate mesenchymal WNT-β-Catenin signaling have not been identified.
Wnt7b is expressed in lung epithelium (Pepicelli et al., 1998; Weidenfeld et al., 2002). Wnt7b−/− embryos have reduced lung mesenchymal proliferation and defects in lung vascular smooth muscle (Shu et al., 2002). It is, therefore, likely that WNT7b signals directly to adjacent sub-epithelial mesenchyme and acts synergistically or redundantly with WNT2a (Fig. 9). Wang et al. (2005) have shown that WNT7b can activate FZD1, 4 and 7 and that these WNT receptors are expressed in lung mesenchyme and in vascular smooth muscle precursors; however, direct targets of WNT-β-Catenin signaling were not examined in lung mesenchyme of Wnt7b−/− mice. Supporting the idea that WNT7b could regulate mesenchymal WNT-β-Catenin activity independent of FGF9, we showed that the expression of Wnt7b was increased in Fgf9−/− lungs. Thus, WNT7b is likely to be a second independent external factor that can modulate the mesenchymal WNT-β-Catenin–FGFR regulatory circuit.
Lef1, a target of WNT-β-Catenin signaling, appears to be uniformly expressed throughout distal mesenchyme. This suggests that Wnt2a, which is expressed in sub-mesothelial mesenchyme, might support both autocrine signaling to sub-mesothelial mesenchyme and paracrine signaling to sub-epithelial mesenchyme. Although a lung mesenchymal phenotype was not reported for Wnt2a−/− mice (Monkley et al., 1996) and the original knockout line was not saved, a second, recently constructed line of Wnt2a−/− mice shows decreased lung mesenchymal proliferation (E. Morrisey, personal communication). Additionally, Wnt7b−/− mice show decreased lung mesenchymal proliferation (Shu et al., 2002). These data support a potential role for these ligands acting together in the regulation of mesenchymal WNT-β-Catenin signaling. To demonstrate this in vivo, future epistasis studies will be required in which Wnt2a and Wnt7b double heterozygous mice are examined.
To address the contribution of WNT-β-Catenin signaling in lung epithelium, several labs have conditionally inactivated β-Catenin in epithelium or overexpressed the WNT antagonist, DKK, in epithelium (De Langhe et al., 2005; Mucenski et al., 2003; Shu et al., 2005). These studies showed that WNT-β-Catenin signaling regulates the expression of N-Myc, Bmp4 and Fgfr2b in lung epithelium and regulates the extent of branching morphogenesis. Down-regulation of epithelial Fgfr2b could account for the observed defects in branching morphogenesis in these mice. Importantly, the identity of the WNT ligand(s) that regulates epithelial WNT-β-Catenin signaling is not known; however, in addition to activating FZD1, 4 and 7 in lung mesenchyme, WNT7b can activate FZD10, which is expressed in lung epithelium (Wang et al., 2005). A possible autocrine role for WNT7b in epithelium is supported by observed defective epithelial differentiation in Wnt7b−/− lung tissue (Shu et al., 2002).
In summary, FGF9, and most likely WNT7b, are two ligands that can independently signal from mesothelial (FGF9) and epithelial (FGF9 and WNT7b) cells to lung mesenchyme to regulate growth. The positive feedback loop within the mesenchymal compartment (Wnt2a, FGFR1, FGFR2 and β-Catenin) allows input from both FGF and WNT signaling systems to modulate the output of the entire system, thus providing a mechanism to tightly regulate mesenchymal lung development and, indirectly, epithelial morphogenesis.
Supplementary Material
Supplementary data associated with this article can be found, in the online version, at doi:10.1016/j.ydbio.2008.04.009.
Acknowledgments
We thank J. Partanen for the Fgfr1f/f mouse line. We thank C. Smith and G. Schmid for animal husbandry and genotyping. This work was funded by the Washington University Cardiovascular Pharmacology Training Grant T32 HL07873 (ACW), the Digestive Diseases Research Core Center Grant P30 DK052574 (transgenic mouse production) and a grant from the March of Dimes Foundation (1FY06-339).
References
- Baldin V, et al. Cyclin D1 is a nuclear protein required for cell cycle progression in G1. Genes Dev. 1993;7:812–821. doi: 10.1101/gad.7.5.812. [DOI] [PubMed] [Google Scholar]
- Bellusci S, et al. Evidence from normal expression and targeted misexpression that bone morphogenetic protein (Bmp-4) plays a role in mouse embryonic lung morphogenesis. Development. 1996;122:1693–1702. doi: 10.1242/dev.122.6.1693. [DOI] [PubMed] [Google Scholar]
- Bellusci S, et al. Fibroblast growth factor 10 (FGF10) and branching morphogenesis in the embryonic mouse lung. Development. 1997;124:4867–4878. doi: 10.1242/dev.124.23.4867. [DOI] [PubMed] [Google Scholar]
- Brantjes H, et al. TCF: Lady Justice casting the final verdict on the outcome of Wnt signalling. Biol. Chem. 2002;383:255–261. doi: 10.1515/BC.2002.027. [DOI] [PubMed] [Google Scholar]
- Brault V, et al. Inactivation of the beta-catenin gene by Wnt1-Cre-mediated deletion results in dramatic brain malformation and failure of craniofacial development. Development. 2001;128:1253–1264. doi: 10.1242/dev.128.8.1253. [DOI] [PubMed] [Google Scholar]
- Briata P, et al. The Wnt/beta-catenin–>Pitx2 pathway controls the turnover of Pitx2 and other unstable mRNAs. Mol. Cell. 2003;12:1201–1211. doi: 10.1016/s1097-2765(03)00407-6. [DOI] [PubMed] [Google Scholar]
- Cardoso WV, Lu J. Regulation of early lung morphogenesis: questions, facts and controversies. Development. 2006;133:1611–1624. doi: 10.1242/dev.02310. [DOI] [PubMed] [Google Scholar]
- Colvin JS, et al. Genomic organization and embryonic expression of the mouse fibroblast growth factor 9 gene. Dev. Dyn. 1999;216:72–88. doi: 10.1002/(SICI)1097-0177(199909)216:1<72::AID-DVDY9>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- Colvin JS, et al. Lung hypoplasia and neonatal death in Fgf9-null mice identify this gene as an essential regulator of lung mesenchyme. Development. 2001;128:2095–2106. doi: 10.1242/dev.128.11.2095. [DOI] [PubMed] [Google Scholar]
- De Langhe SP, et al. Dickkopf-1 (DKK1) reveals that fibronectin is a major target of Wnt signaling in branching morphogenesis of the mouse embryonic lung. Dev. Biol. 2005;277:316–331. doi: 10.1016/j.ydbio.2004.09.023. [DOI] [PubMed] [Google Scholar]
- Dean CH, et al. Canonical Wnt signaling negatively regulates branching morphogenesis of the lung and lacrimal gland. Dev. Biol. 2005;286:270–286. doi: 10.1016/j.ydbio.2005.07.034. [DOI] [PubMed] [Google Scholar]
- del Moral PM, et al. Differential role of FGF9 on epithelium and mesenchyme in mouse embryonic lung. Dev. Biol. 2006;293:77–89. doi: 10.1016/j.ydbio.2006.01.020. [DOI] [PubMed] [Google Scholar]
- Hjalt TA, et al. The Pitx2 protein in mouse development. Dev. Dyn. 2000;218:195–200. doi: 10.1002/(SICI)1097-0177(200005)218:1<195::AID-DVDY17>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- Hogan BL. Morphogenesis. Cell. 1999;96:225–233. doi: 10.1016/s0092-8674(00)80562-0. [DOI] [PubMed] [Google Scholar]
- Hovanes K, et al. Beta-catenin-sensitive isoforms of lymphoid enhancer factor-1 are selectively expressed in colon cancer. Nat. Genet. 2001;28:53–57. doi: 10.1038/ng0501-53. [DOI] [PubMed] [Google Scholar]
- Issack PS, Ziff EB. Altered expression of helix-loop-helix transcriptional regulators and cyclin D1 in Wnt-1-transformed PC12 cells. Cell Growth Differ. 1998;9:837–845. [PubMed] [Google Scholar]
- Karasawa T, et al. Frizzled-9 is activated by Wnt-2 and functions in Wnt/beta-catenin signaling. J. Biol. Chem. 2002;277:37479–37486. doi: 10.1074/jbc.M205658200. [DOI] [PubMed] [Google Scholar]
- Katoh M, et al. Cloning, expression and chromosomal localization of Wnt-13, a novel member of the Wnt gene family. Oncogene. 1996;13:873–876. [PubMed] [Google Scholar]
- Katoh M, et al. Alternative splicing of the WNT-2B/WNT-13 gene. Biochem. Biophys. Res. Commun. 2000;275:209–216. doi: 10.1006/bbrc.2000.3252. [DOI] [PubMed] [Google Scholar]
- Kioussi C, et al. Identification of a Wnt/Dvl/beta-Catenin–>Pitx2 pathway mediating cell-type-specific proliferation during development. Cell. 2002;111:673–685. doi: 10.1016/s0092-8674(02)01084-x. [DOI] [PubMed] [Google Scholar]
- Klein PS, Melton DA. A molecular mechanism for the effect of lithium on development. Proc. Natl. Acad. Sci. U. S. A. 1996;93:8455–8459. doi: 10.1073/pnas.93.16.8455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lako M, et al. Isolation, characterisation and embryonic expression of WNT11, a gene which maps to 11q13.5 and has possible roles in the development of skeleton, kidney and lung. Gene. 1998;219:101–110. doi: 10.1016/s0378-1119(98)00393-x. [DOI] [PubMed] [Google Scholar]
- Li CG, et al. Wnt5a participates in distal lung morphogenesis. Dev. Biol. 2002;248:68–81. doi: 10.1006/dbio.2002.0729. [DOI] [PubMed] [Google Scholar]
- Li C, et al. Wnt5a regulates Shh and Fgf10 signaling during lung development. Dev. Biol. 2005;287:86–97. doi: 10.1016/j.ydbio.2005.08.035. [DOI] [PubMed] [Google Scholar]
- Lobjois V, et al. Specific regulation of cyclins D1 and D2 by FGF and Shh signaling coordinates cell cycle progression, patterning, and differentiation during early steps of spinal cord development. Dev. Biol. 2004;273:195–209. doi: 10.1016/j.ydbio.2004.05.031. [DOI] [PubMed] [Google Scholar]
- Mailleux AA, et al. Evidence that SPROUTY2 functions as an inhibitor of mouse embryonic lung growth and morphogenesis. Mech. Dev. 2001;102:81–94. doi: 10.1016/s0925-4773(01)00286-6. [DOI] [PubMed] [Google Scholar]
- Mailleux AA, et al. Fgf10 expression identifies parabronchial smooth muscle cell progenitors and is required for their entry into the smooth muscle cell lineage. Development. 2005;132:2157–2166. doi: 10.1242/dev.01795. [DOI] [PubMed] [Google Scholar]
- Maretto S, et al. Mapping Wnt/beta-catenin signaling during mouse development and in colorectal tumors. Proc. Natl. Acad. Sci. U. S. A. 2003;100:3299–3304. doi: 10.1073/pnas.0434590100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Megason SG, McMahon AP. A mitogen gradient of dorsal midline Wnts organizes growth in the CNS. Development. 2002;129:2087–2098. doi: 10.1242/dev.129.9.2087. [DOI] [PubMed] [Google Scholar]
- Monkley SJ, et al. Targeted disruption of the Wnt2 gene results in placentation defects. Development. 1996;122:3343–3353. doi: 10.1242/dev.122.11.3343. [DOI] [PubMed] [Google Scholar]
- Moon RT. Wnt/beta-catenin pathway. Sci. STKE cm1 2005. 2005 doi: 10.1126/stke.2712005cm1. [DOI] [PubMed] [Google Scholar]
- Mucenski ML, et al. beta-Catenin is required for specification of proximal/distal cell fate during lung morphogenesis. J. Biol. Chem. 2003;278:40231–40238. doi: 10.1074/jbc.M305892200. [DOI] [PubMed] [Google Scholar]
- Okubo T, Hogan BL. Hyperactive Wnt signaling changes the developmental potential of embryonic lung endoderm. J. Biol. 2004;3:11. doi: 10.1186/jbiol3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pepicelli CV, et al. Sonic hedgehog regulates branching morphogenesis in the mammalian lung. Curr. Biol. 1998;8:1083–1086. doi: 10.1016/s0960-9822(98)70446-4. [DOI] [PubMed] [Google Scholar]
- Perl AK, et al. Conditional gene expression in the respiratory epithelium of the mouse. Transgenic Res. 2002;11:21–29. doi: 10.1023/a:1013986627504. [DOI] [PubMed] [Google Scholar]
- Pongracz JE, Stockley RA. Wnt signalling in lung development and diseases. Respir. Res. 2006;7:15. doi: 10.1186/1465-9921-7-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porfiri E, et al. Induction of a beta-catenin-LEF-1 complex by wnt-1 and transforming mutants of beta-catenin. Oncogene. 1997;15:2833–2839. doi: 10.1038/sj.onc.1201462. [DOI] [PubMed] [Google Scholar]
- Shannon JM, Hyatt BA. Epithelial-mesenchymal interactions in the developing lung. Annu. Rev. Physiol. 2004;66:625–645. doi: 10.1146/annurev.physiol.66.032102.135749. [DOI] [PubMed] [Google Scholar]
- Shu W, et al. Wnt7b regulates mesenchymal proliferation and vascular development in the lung. Development. 2002;129:4831–4842. doi: 10.1242/dev.129.20.4831. [DOI] [PubMed] [Google Scholar]
- Shu W, et al. Wnt/beta-catenin signaling acts upstream of N-myc, BMP4, and FGF signaling to regulate proximal-distal patterning in the lung. Dev. Biol. 2005;283:226–239. doi: 10.1016/j.ydbio.2005.04.014. [DOI] [PubMed] [Google Scholar]
- Soriano P. Generalized lacZ expression with the ROSA26 Cre reporter strain. Nat. Genet. 1999;21:70–71. doi: 10.1038/5007. [DOI] [PubMed] [Google Scholar]
- Tebar M, et al. Expression of Tcf/Lef and sFrp and localization of beta-catenin in the developing mouse lung. Mech. Dev. 2001;109:437–440. doi: 10.1016/s0925-4773(01)00556-1. [DOI] [PubMed] [Google Scholar]
- Tetsu O, McCormick F. Beta-catenin regulates expression of cyclin D1 in colon carcinoma cells. Nature. 1999;398:422–426. doi: 10.1038/18884. [DOI] [PubMed] [Google Scholar]
- Tichelaar JW, et al. Conditional expression of fibroblast growth factor-7 in the developing and mature lung. J. Biol. Chem. 2000;275:11858–11864. doi: 10.1074/jbc.275.16.11858. [DOI] [PubMed] [Google Scholar]
- Trokovic R, et al. FGFR1 is independently required in both developing mid- and hindbrain for sustained response to isthmic signals. EMBO J. 2003;22:1811–1823. doi: 10.1093/emboj/cdg169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vadlamudi U, et al. PITX2, beta-catenin and LEF-1 interact to synergistically regulate the LEF-1 promoter. J. Cell Sci. 2005;118:1129–1137. doi: 10.1242/jcs.01706. [DOI] [PubMed] [Google Scholar]
- Wang Z, et al. Wnt7b activates canonical signaling in epithelial and vascular smooth muscle cells through interactions with Fzd1, Fzd10, and LRP5. Mol. Cell. Biol. 2005;25:5022–5030. doi: 10.1128/MCB.25.12.5022-5030.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warburton D, et al. Molecular mechanisms of early lung specification and branching morphogenesis. Pediatr. Res. 2005;57:26R–37R. doi: 10.1203/01.PDR.0000159570.01327.ED. [DOI] [PubMed] [Google Scholar]
- Weaver M, et al. Bmp signaling regulates proximal-distal differentiation of endoderm in mouse lung development. Development. 1999;126:4005–4015. doi: 10.1242/dev.126.18.4005. [DOI] [PubMed] [Google Scholar]
- Weaver M, et al. Tissue interactions pattern the mesenchyme of the embryonic mouse lung. Dev. Biol. 2003;258:169–184. doi: 10.1016/s0012-1606(03)00117-9. [DOI] [PubMed] [Google Scholar]
- Weidenfeld J, et al. The WNT7b promoter is regulated by TTF-1, GATA6, and Foxa2 in lung epithelium. J. Biol. Chem. 2002;277:21061–21070. doi: 10.1074/jbc.M111702200. [DOI] [PubMed] [Google Scholar]
- White AC, et al. FGF9 and SHH signaling coordinate lung growth and development through regulation of distinct mesenchymal domains. Development. 2006;133:1507–1517. doi: 10.1242/dev.02313. [DOI] [PubMed] [Google Scholar]
- Yu K, et al. Conditional inactivation of FGF receptor 2 reveals an essential role for FGF signaling in the regulation of osteoblast function and bone growth. Development. 2003;130:3063–3074. doi: 10.1242/dev.00491. [DOI] [PubMed] [Google Scholar]
- Zakin LD, et al. Structure and expression of Wnt13, a novel mouse Wnt2 related gene. Mech. Dev. 1998;73:107–116. doi: 10.1016/s0925-4773(98)00040-9. [DOI] [PubMed] [Google Scholar]
- Zorn AM, et al. Regulation of Wnt signaling by Sox proteins: XSox17 alpha/beta and XSox3 physically interact with beta-catenin. Mol. Cell. 1999;4:487–498. doi: 10.1016/s1097-2765(00)80200-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary data associated with this article can be found, in the online version, at doi:10.1016/j.ydbio.2008.04.009.