Abstract
Lipopolysaccharide (LPS), derived from Gram-negative bacteria, is a major cause of acute lung injury and respiratory distress syndrome. Pulmonary surfactant is secreted as a complex mixture of lipids and proteins onto the alveolar surface of the lung. Surfactant phospholipids are essential in reducing surface tension at the air-liquid interface and preventing alveolar collapse at the end of the respiratory cycle. In the present study, we determined that palmitoyl-oleoyl-phosphatidylglycerol and phosphatidylinositol, which are minor components of pulmonary surfactant, and synthetic dimyristoylphosphatidylglycerol regulated the inflammatory response of alveolar macrophages. The anionic lipids significantly inhibited LPS-induced nitric oxide and tumor necrosis factor-α production from rat and human alveolar macrophages and a U937 cell line by reducing the LPS-elicited phosphorylation of multiple intracellular protein kinases. The anionic lipids were also effective at attenuating inflammation when administered intratracheally to mice challenged with LPS. Binding studies revealed high affinity interactions between the palmitoyl-oleoyl-phosphatidylglycerol and the Toll-like receptor 4-interacting proteins CD14 and MD-2. Our data clearly identify important anti-inflammatory properties of the minor surfactant phospholipids at the environmental interface of the lung.
Lipopolysaccharide (LPS),3 derived from Gram-negative bacteria, is a potent stimulator of inflammation (1, 2). LPS molecules are engaged by the plasma LPS-binding protein (LBP) (3) and transferred to CD14, a glycosylphosphatidylinositol-anchored protein, abundantly expressed on macrophages. LPS responses are dependent on the peripherally associated plasma membrane protein MD-2 (4) and the membrane-spanning complex formed by Toll-like receptor (TLR) 4 (5), through which signaling is propagated. TLRs activate four intracellular protein kinase cascades, the IκB kinase (IKK)/NF-kB transcription factor cascade, the extracellular signal-regulated kinase (ERK), c-Jun N-terminal kinase (JNK), and p38 mitogen-activated protein kinase (MAPK) cascades, leading to the induction of many key cytokine genes that are essential for the innate immune response (6–8).
Pulmonary surfactant is a lipoprotein complex secreted by alveolar type 2 cells that reduces surface tension at the air-liquid interface of the alveolar compartment of the lung (9–12). Approximately 10% of surfactant is composed of proteins, including the hydrophilic surfactant proteins A and D (SP-A and SP-D) and the hydrophobic proteins, SP-B and SP-C (13). SP-A and SP-D are now recognized to play important roles in innate immunity (14, 15), and these proteins directly interact with various microorganisms and pathogen-derived components (16). Moreover, by associating with cell surface pattern recognition receptors, SP-A and SP-D regulate inflammatory cellular responses such as the release of LPS-induced proinflammatory cytokines (14, 17). At least one important function of SP-A and SP-D is to suppress the inflammatory response of the lung to LPS.
By weight, ∼90% of surfactant consists of lipids. The major component is phosphatidylcholine (PC) (70–80%), of which nearly 70% is dipalmitoylphosphatidylcholine (DPPC). Pulmonary surfactant also contains variable amounts of phosphatidylglycerol (PG) (7–18%), phosphatidylinositol (PI) (2–4%), and phosphatidylethanolamine (PE) (2–3%) (18). In humans, PG is composed almost exclusively of unsaturated molecular species (19, 20). The PG concentration in the extracellular compartment of the human lung is estimated to be as high as 3 mg/ml (21), and this is the only location in mammals that such levels occur. The functions of the minor phospholipid and the neutral lipid components of surfactant are largely unclear and require further study.
Previous work has provided some evidence that specific phospholipids can modulate inflammation. Oxidized phospholipid inhibits LPS-induced inflammatory responses in human umbilical vein endothelial cells (22). Dioleoylphosphatidylglycerol (DOPG) inhibits phospholipase A2 secretion via a down-regulation of NF-kB activation in guinea pig macrophages (23). Wang et al. (24) expressed CD14 in a macrophage cell line and demonstrated that these cells acquired PI binding activity, which was dependent upon the presence of LBP. The PI binding property correlated with PI-dependent inhibition of cell activation by LPS, and both LPS and PI binding were inhibited by treatment of the cells with anti-CD14 antibodies. Hashimoto et al. (25) identified an unusual 1-O-alkenyl PG from Treponema species that suppresses LPS-mediated macrophage activation. Unsaturated PGs, caridolipin and PI, were shown in the same study to be as effective as 1-O-alkenyl PG. The action of the acidic phospholipids was LBP-dependent and was concluded to involve both LBP and CD14. Mueller et al. (26) also identified LBP as the major target for the antagonistic action of PG, PI, and cardiolipin upon LPS-mediated activation of macrophages.
LPS is a major cause of acute lung injury (ALI) and acute respiratory distress syndrome (ARDS) (27, 28). In addition to LPS, some oxidized lipid species, induced by multiple pathogenic and chemical insults to the lungs, can act as LPS mimetics and activate the TLR4 system to induce ALI (29). ALI/ARDS is a life-threatening condition in which inflammation of the lungs and accumulation of fluid in the alveoli leads to low blood oxygen levels. Over a period of 25 years, the annual incidence of ALI/ARDS is 335,000, with 147,000 deaths per year (28). The mortality rate of ALI/ARDS has not significantly changed over the last decade. In the present study, we examined the anti-inflammatory effect of surfactant phospholipids upon LPS-induced inflammation in rodent and human macrophages. The purpose of this investigation was to determine 1) new details about the molecular specificity of anionic lipid antagonism of LPS action, 2) the influence of surfactant components upon the antagonistic actions of anionic lipids in vitro and in vivo, and 3) the direct interactions between the anionic lipids and the CD14, TLR4, and MD-2 components that participate in LPS activation of target cells. Our results provide evidence that anionic surfactant lipids play an important role in regulating pulmonary inflammation in response to LPS by specific interactions with the CD14 and MD-2 components of the TLR4 signaling pathway. The actions of the anionic surfactant lipids as LPS antagonists could have significant potential for treatment of inflammatory lung diseases such as ALI.
EXPERIMENTAL PROCEDURES
Cells and Reagents
LPS (0111:B4) purified from Escherichia coli was purchased from Sigma-Aldrich. The lipids PC, PG, sphingomyelin (SM), PE, phosphatidylserine (PS), and PI were purchased from Avanti Polar Lipids (Alabaster, AL). TNF-α was from Genzyme (Cambridge, MA). Rabbit polyclonal anti-p46 JNK, rabbit polyclonal anti-p38, mouse monoclonal phospho-specific p46-p54 JNK antibodies, and rabbit polyclonal anti-mitogen-activated protein kinase phosphatase (MKP)-1 antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Rabbit polyclonal phospho-specific p42 ERK, rabbit polyclonal anti-p42 ERK, phospho-specific p38 MAPK, rabbit polyclonal anti-IκBα, and phospho-specific IκBα antibodies were obtained from Cell Signaling Technology (Beverly, MA). [3H]Leucine was from PerkinElmer Life Sciences. Recombinant human CD14 and mouse anti-CD14 monoclonal antibodies, biG2 and biG14, were purchased from Cell Sciences Inc. (Canton, MA). Mouse anti-CD14 monoclonal antibody MEM-18 was purchased from Exbio (Vestec, Czech Republic). Mouse anti-His antibody and horseradish peroxidase-conjugated mouse anti-V5 antibody were obtained from Invitrogen. Mouse IgG1 isotype control, mouse monoclonal anti-human CD14 antibody, and sheep anti-human CD14 polyclonal antibody were purchased from R&D systems (Minneapolis, MN). The macrophage-like cell line U937 (CRL-1593.2) was obtained from the American Type Culture Collection (Manassas, VA). The cells were maintained in endotoxin-free RPMI 1640 medium from Cambrex (East Rutherford, NJ) with 10% heat-inactivated bovine growth serum (Hyclone, Logan, UT).
Isolation of Rat and Human Alveolar Macrophages
Rat alveolar macrophages were isolated from bronchoalveolar lavage fluid (BALF) of Sprague-Dawley rats. The macrophages were plated at 5 × 105 cells/well in 24-well plates (Falcon) in RPMI 1640 medium containing 10% bovine growth serum and allowed to adhere for 2 h prior to treatment with agonists and antagonists. Human alveolar macrophages were isolated from BALF obtained from healthy volunteers using protocols reviewed and approved by the National Jewish Medical Research Center Institutional Review Board (IRB) and the University of Colorado General Clinical Research Center. The macrophages were plated at 5 × 104 cells/well in 96-well plates (Falcon) in RPMI 1640 medium containing 10% bovine growth serum and allowed to adhere for 48 h prior to the addition of agonists and antagonists.
Induction of TNF-α Secretion
U937 cells (1.3 × 105/well in 96-well plates) were induced to differentiate by treatment with 10 nm phorbol myristate acetate for 48 h followed by recovery for 24 h in RPMI 1640 medium containing 10% bovine growth serum. Rat alveolar macrophages (5 × 105/well in 24-well plates) were incubated for 2 h after isolation. Phospholipids were added to the cultures 30 min before adding LPS. After LPS addition, cultures were incubated for 6 h at 37 °C. At the end of the incubation period, the medium was collected and assayed for TNF-α concentrations using an ELISA kit. For all batches of LPS used in these studies, we verified that the induction of inflammatory mediator production was neutralized by treatment with 10 μg/ml polymyxin B. In additional studies, we also verified that the antagonistic actions of anionic lipids were equally effective against both LPS and synthetic KDO2-lipid A.
Preparation of Surfactant Lipids
Surfactant lipids were isolated from the bronchoalveolar lavage of Sprague-Dawley rats, 28 days after intratracheal instillation of 25 mg of silica (∼125 mg/kg). Initially, the surfactant was purified by the method of Hawgood et al. (30) using NaBr density gradient centrifugation. The purified surfactant was extracted with butanol (31) and segregated into butanol-soluble and -insoluble material. The butanol-soluble surfactant lipids were recovered by drying under vacuum and resuspending in chloroform. The phospholipid content was determined by the method of Rouser et al. (32), and the mixture was stored at −20 °C. Prior to use, an aliquot of surfactant lipids was initially dried under nitrogen and subsequently hydrated in 20 mm Tris (pH 7.4), 150 mm NaCl buffer at 37 °C for 1 h. Finally, the surfactant lipids were probe-sonicated in 5–30-s bursts with 1 min of cooling between bursts to make a vesicle preparation for use in experiments.
Analysis of Nitric Oxide Accumulation
Nitric oxide (NO) accumulation in cell culture supernatants was determined as reported previously (33). Briefly, rat alveolar macrophages were stimulated with LPS (10 ng/ml) or LPS plus phospholipids for 24 h. Culture supernatants (usually 100 μl) were combined with an equal volume of Griess reagent (33), and the samples were incubated at room temperature for 10 min before the absorbance was quantified at 550 nm. With the use of a standard curve, the nmol of NO produced were determined and normalized to the total cell number in each sample.
Analysis of Cytokine Production
Human and mouse TNF-α ELISA kits were purchased from Invitrogen. Mouse KC and MIP-2 Quantikine kits were purchased from R&D Systems. Measurements of these cytokines were according to the manufacturers' protocols.
Measurement of MAPK, IκBα, and MKP-1
Monolayers of unstimulated or stimulated macrophages were lysed on ice with 250 μl of ice-cold lysis buffer (50 mm Tris·HCl buffer, pH 8.0, containing 137 mm NaCl, 10% (v/v) glycerol, 1% (v/v) Nonidet P-40, 1 mm NaF, 10 μg/ml leupeptin, 10 μg/ml aprotinin, 2 mm Na3VO4, and 1 mm phenylmethylsulfonyl fluoride) (34). Insoluble nuclear material was removed by centrifugation at 14,000 × g for 10 min at 4 °C, and the resultant supernatants were collected. Aliquots of 15 μg of lysate protein were subjected to SDS-PAGE and transferred onto nitrocellulose membranes (35). The blots were then washed in Tris-Tween-buffered saline (20 mm Tris-HCl buffer, pH 7.6, containing 137 mm NaCl and 0.05% (v/v) Tween 20), blocked with 5% (w/v) nonfat dry milk for 1 h, and probed according to the method described by Towbin et al. (35) with phospho-specific antibodies to p46-p54 JNK, p42/p44 ERK, and p38 MAPK, or IκBα or with a polyclonal MKP-1 or IκBα antibodies in 5% (w/v) bovine serum albumin dissolved in Tris-Tween-buffered saline. Bound primary antibodies were detected by enhanced chemiluminescence (ECL Plus, Amersham Biosciences) with the use of horseradish peroxidase-conjugated secondary anti-rabbit or anti-mouse antibody. To determine loading of proteins between samples, the membranes were probed with rabbit polyclonal p46 JNK, p42/p44 ERK, and p38 MAPK antibodies.
Administration of LPS and Phospholipids in Vivo
Female BALB/c mice from 6 to 8 weeks of age were obtained from Jackson Laboratories (Bar Harbor, ME). Experiments were conducted under a protocol approved by the Institutional Animal Care and Use Committee of the National Jewish Medical and Research Center. Liposomes were formed using a LiposofastTM (Avestin; Ottawa, Canada), which produces unilamellar liposomes of 100 nm of diameter, and then mixed with an aqueous solution containing LPS. The mixture of LPS and phospholipids was sprayed into murine trachea using a MicroSprayerTM aerosolizer (PennCentury, Philadelphia, PA) under isoflurane anesthesia. Delivery by MicroSprayerTM has been shown to result in lung deposition fractions of more than 93% in primates (36). For collection of BALF, lethally anesthetized mice were lavaged via the trachea with 1 ml of Hanks' balanced salt solution (Invitrogen). The volume of collected BALF was measured in each sample, and the number of leukocytes was counted (Coulter Counter; Beckman Coulter). Differential cell counts were determined from at least 300 cells on cytocentrifuged preparations (Cytospin; Shandon Ltd., Runcorn, Cheshire, UK). Slides were stained with modified Wright-Giemsa (Hema; Protocol, Swedesboro, NJ), and the cell populations were differentiated by standard hematologic procedures. Cytokine levels in the BALF or in the supernatants of cultured airway macrophages were measured using ELISA kits.
Soluble Extracellular Domain of TLR4, CD14, and MD-2
A soluble form of the extracellular domain of TLR4 (sTLR4) consists of the putative extracellular sequence (Met1–Lys631) and a His6 epitope tag at its C-terminal end. sTLR4 and MD-2 cDNAs were described previously (37). sTLR4-His6 was subcloned into pcDNA3.1(+) (Invitrogen). MD-2-V5-His6 that contains the C-terminal fusion V5 epitope tag and His6 epitope tag was generated by using PCR and subcloned into pcDNA3.1D/V5-His-TOPO (Invitrogen). A control protein, yeast PstB2-V5-His6 that contains the C-terminal fusion V5 tag epitope and His6 tag epitope, was generated by using PCR and subcloned into the baculovirus vector pVL1392 (38). The epitope-tagged cDNA constructs for sTLR4 and MD-2 were subcloned into PVL1392, and in addition to PstB2, they were independently expressed using a baculovirus-insect cell expression system according to the methods described by O'Reilly et al. (39). The sTLR4 protein, MD-2 protein, sCD14 protein, and PstB2 protein were purified using an epitope affinity column of nickel-nitrilotriacetic acid beads (Qiagen, Valencia, CA) and elution with 50–200 mm imidazole, by the method described previously (40).
Binding of CD14 and MD-2 to Phospholipids
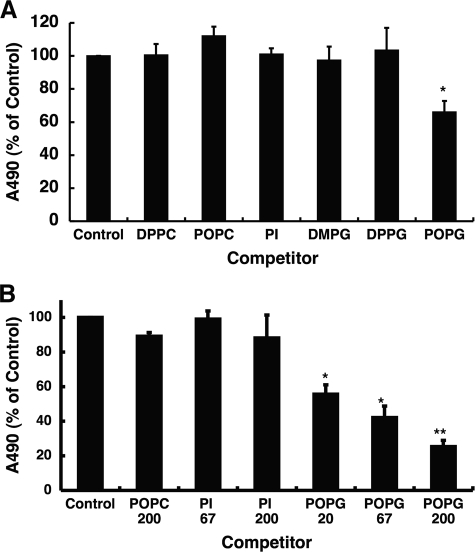
Phospholipids (1.25 nmol) in 20-μl aliquots of ethanol were pipetted onto 96-well half-area plates (Corning Inc., Corning, NY), and the solvent was evaporated using a warm air blower. After nonspecific binding was blocked with 20 mm Tris buffer (pH 7.4) containing 0.15 m NaCl, 5 mm CaCl2 or 2 mm EGTA, and 5% (w/v) bovine serum albumin (buffer A), various concentrations of human CD14 or MD-2 in 25 μl of buffer A were added and incubated at 37 °C for 1 h. The wells were then washed with 20 mm Tris buffer (pH 7.4) containing 0.15 m NaCl and 5 mm CaCl2 or 2 mm EGTA (buffer B), and 1 μg/ml anti-human CD14 IgG or anti-His antibody (50 μl/well) in buffer A was added and incubated overnight at 4 °C followed by incubation with horseradish peroxidase-labeled anti-mouse IgG (1:5000) for 1 h. After washing the wells with buffer B, the peroxidase reaction was finally performed using o-phenylenediamine as a substrate. The binding of CD14 or MD-2 to phospholipids was detected by measuring absorbance at 490 nm.
Binding of LPS to CD14
LPS (2 μg) in 20-μl aliquots of ethanol was pipetted onto a 96-well plate, and the solvent was evaporated using a warm air blower. After the nonspecific binding was blocked with buffer A, mixtures of CD14 (1 μg/ml) and phospholipid liposomes (20 μg/ml) in buffer A, which were preincubated at 37 °C for 1 h, were added and further incubated at 37 °C for 1 h. The amount of bound CD14 was detected using the method described above.
Phospholipid Inhibition of MD-2 and sTLR4 Interaction
Preparations of sTLR4 (100 ng) in aliquots of 20 μl of buffer B were pipetted onto a 96-well plate, and the solvent was evaporated using a warm air dryer. After the nonspecific binding was blocked with buffer A, mixtures of MD-2 (1 μg/ml) and varying concentrations of phospholipid liposomes in buffer A were added and incubated at 37 °C for 1 h. The amount of bound MD-2 was detected using specific antibodies directed against the V5-epitope of the recombinant protein.
Statistical Analysis
All results were expressed as mean ± S.E. Analysis of variance was used to determine the levels of significant difference between all groups. Groups were compared by unpaired two-tailed t test. The p value for significance was set at 0.05.
RESULTS
POPG and PI Inhibit LPS-induced Production of Proinflammatory Cytokines
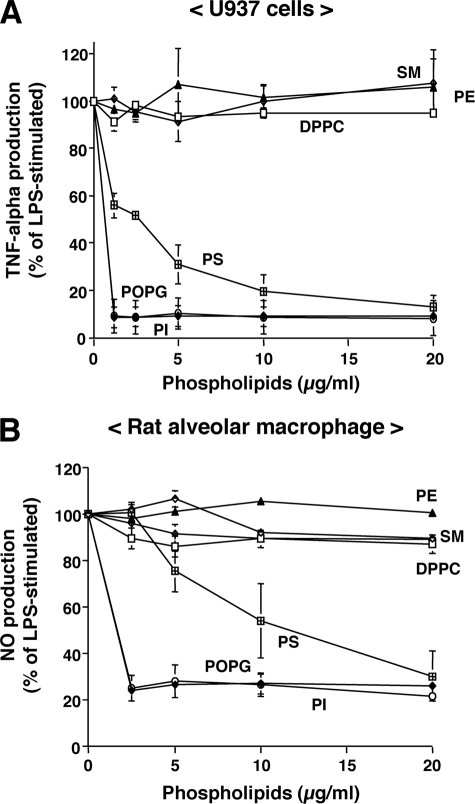
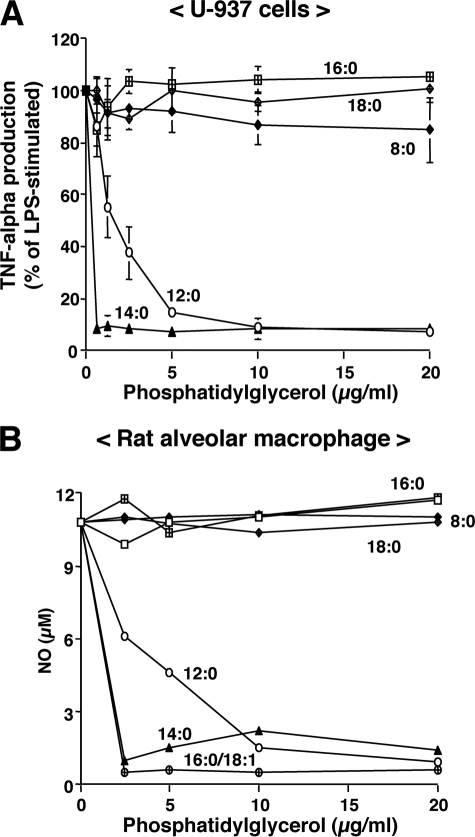
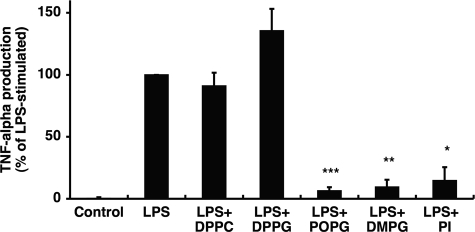
In our initial studies, we investigated the ability of purified lipids normally present as minor components of pulmonary surfactant to modulate LPS-induced cytokine secretion. Macrophages were stimulated with LPS in the presence or absence of purified phospholipids (Fig. 1). Culture supernatants were collected, and TNF-α production by U937 cells and NO production by rat alveolar macrophages were determined. Palmitoyl-oleoyl-phosphatidylglycerol (POPG) and PI significantly attenuated TNF-α and NO production in a concentration-dependent manner with the maximal inhibitory effect ≤ 2.5 μg of phospholipids/ml. Another anionic phospholipid, PS, was less effective than PI and POPG. In contrast, the aminophospholipids and sphingolipids DPPC, PE, and SM had no significant effect on TNF-α or NO production. The major molecular species of PG in humans is POPG, whereas rodent surfactant contains a mixture of disaturated and unsaturated PG. We next examined the effect of saturation and acyl chain length of PGs on the inhibition of LPS-induced inflammation. As shown in Fig. 2, disaturated PGs containing two palmitic (16:0), stearic (18:0), or octanoic (8:0) fatty acids failed to antagonize LPS-induced TNF-α or NO production. However, PGs with two myristic (14:0) fatty acids were as potent as POPG as antagonists of LPS. PGs with two lauric (12:0) fatty acids were also modest antagonists of LPS-induced cytokine production. Although we did not have the reagents to compare different molecular species of PI for LPS antagonism, the PIs that we used were unsaturated with the major form containing 16:0 and 18:2 fatty acids. From the above findings, we conclude that unsaturated PGs and PIs and selected saturated PGs act as potent antagonists of LPS action upon macrophages.
FIGURE 1.
Anionic phospholipids inhibit inflammatory mediator production induced by LPS. Liposomes composed of SM, PE, DPPC, PS, POPG, and PI were formed by bath sonication for 30 min at room temperature. LPS (10 ng/ml) and different concentrations of phospholipids were added to monolayer cultures of differentiated U937 cells (A) or primary rat alveolar macrophages (B). At 6 h after stimulation, media were collected, and secreted TNF-α levels were determined in U937 cultures. NO production was determined 24 h after stimulating rat alveolar macrophages. LPS stimulation in the absence of phospholipid was set as 100%. The data shown are the means ± S.E. from three separate experiments with duplicate samples in each experiment. The average TNF-α production upon LPS stimulation was (8.0 ± 0.54 ng/ml). The average NO production upon LPS stimulation was 12.17 ± 0.27 μm.
FIGURE 2.
The inhibitory effect of phosphatidylglycerols on LPS-induced inflammatory mediator production is molecular species-specific. PG liposomes were formed by bath sonication for 30 min at room temperature. LPS (10 ng/ml) and different concentrations of PG were added to monolayer cultures of differentiated U937 cells (A) or rat alveolar macrophages (B). Media TNF-α measurements were performed 6 h after stimulation. Media NO measurements were performed 24 h after stimulation. LPS stimulation without PG was set at 100%. The molecular species of PG shown on the graph are: 8:0, dioctanoyl-phosphatidylglycerol; 12:0, dilauroyl-phosphatidylglycerol (DLPG); 14:0, DMPG; 16:0, DPPG; 18:0, distearoyl-phosphatidylglycerol; and 16:0/18:1, POPG. The data shown are the means ± S.E. from three separate experiments with duplicate samples in each experiment. The average TNF-α production upon LPS stimulation was 11.3 ± 0.7 ng/ml. The average NO production upon LPS stimulation was 10.1 ± 0.6 μm.
POPG Antagonism of LPS-induced Inflammation Occurs in the Context of Surfactant Phospholipids
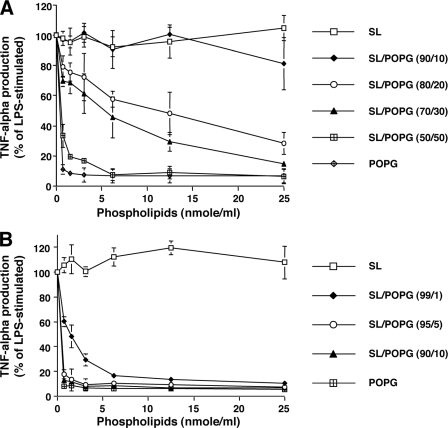
The POPG present in the alveolar compartment is in a lipid-rich environment with concentrations of total phospholipids of 15–30 mg/ml (21), and we examined whether these other lipids can interfere with LPS antagonism. In one set of experiments, POPG was added to organic solvent extracts of surfactant using a method that ensured ideal mixing of all components, and liposomes were prepared by sonication. In a second set of experiments, vesicles composed of surfactant lipids and vesicles composed of POPG were prepared separately and then combined. In this latter situation, there will be two populations of vesicles, one containing randomly mixed surfactant lipids and a second containing pure POPG. The results in Fig. 3A reveal that surface dilution and randomization of POPG within a single vesicle significantly diminishes the potency of the lipid as an antagonist of LPS action. To approximate the activity of POPG alone, the randomized POPG must now constitute nearly 50% of the total lipid present in a surfactant lipid-containing vesicle. In contrast to the results in Fig. 3A, the data presented in Fig. 3B demonstrate that admixture of pure POPG vesicles and randomized surfactant lipid vesicles has essentially no effect upon the activity of POPG as an LPS antagonist, measured by TNF-α production. This result also indicates that the combination of pure POPG vesicles with surfactant lipid vesicles does not result in significant fusion and intermixing of lipids between vesicle bilayers and suggests that introduction of POPG into the lung may potently antagonize LPS action.
FIGURE 3.
Homotypic PG containing liposomes are most effective in antagonizing LPS action in the presence of surfactant lipids. Surfactant lipid (SL) and POPG were dried under nitrogen and hydrated at 37 °C for 1 h. A, surfactant lipid and POPG were mixed in organic solvents prior to drying and hydrating, and subsequently, liposomes were produced. B, surfactant lipid and POPG were made as independent populations of liposomes that were subsequently mixed prior to macrophage treatment. 10 ng/ml LPS and different concentrations of liposome mixtures were added to monolayer cultures of differentiated U937 cells. 6 h after stimulation, media were collected, and TNF-α production was determined. LPS stimulation without phospholipid was set as 100%. The data shown are the means ± S.E. from three separate experiments with duplicate samples in each experiment. The average TNF-α production upon LPS stimulation was 7.0 ± 0.2 ng/ml.
POPG Inhibits the LPS-induced Phosphorylation of MAPK and IκBα and Expression of MKP-1
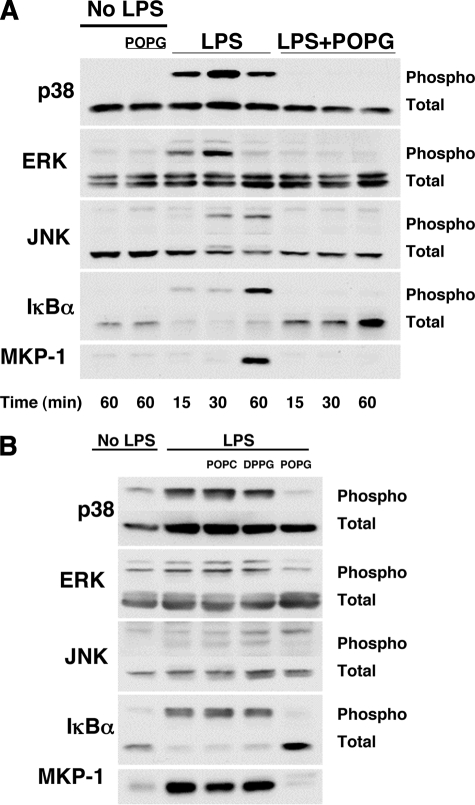
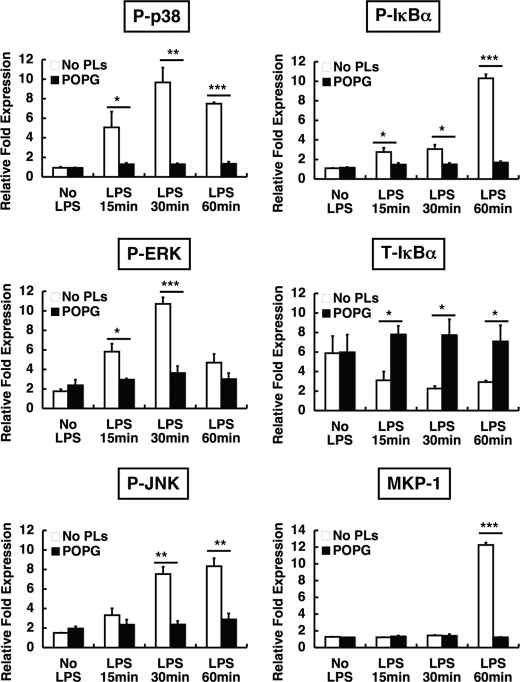
We next investigated the influence of phospholipids upon the intracellular signaling pathways of LPS-induced TNF-α secretion. In these studies, we used concentrations of antagonistic phospholipids of 200 μg/ml, which is ∼10% of the levels present in pulmonary surfactant. Host cells recognize many specific microbial components through Toll-like receptors that mediate immune responses. On alveolar macrophages, LPS binds to membrane CD14 and a TLR4-MD2 complex. The signals from TLR4 are transmitted through MyD88 and TRAF6 (41) to IκBα or MAPKs such as ERK, JNK, and p38. These signals regulate transcription factors and induce proinflammatory cytokine production. In experiments summarized in Fig. 4, differentiated U937 cells were stimulated with LPS in the absence or presence of POPG, and cell lysates were electrophoresed and immunoblotted (Fig. 4A). Significant increases in phosphorylated forms of p38, p42 ERK, and JNK, as well as IκBα, were detected between 15 and 60 min after LPS treatment. LPS treatment also reduced the steady state levels of IκBα due to protein degradation. Treatment of cells with POPG in addition to LPS eliminated the phosphorylation of p38, p42 ERK, JNK, and IκBα and also abrogated the reduction in the steady state levels of IκBα. In addition to inducing phosphorylation of MAPKs and IκBα, LPS induced synthesis of MKP-1 that functions to turn off MAPKs signaling (42, 43). The POPG treatment blocked the synthesis of new MKP-1, indicating that the lipid is likely to act upstream of MAPK activation rather than downstream of the process by induction of MKP-1.
FIGURE 4.
POPG inhibits LPS-induced MAPK and IκBα phosphorylation and MKP-1 expression. A, POPG liposomes (200 μg/ml) were added to monolayer cultures of differentiated U937 cells that received either no treatment or 10 ng/ml LPS. After incubating for the indicated times, cells were lysed using buffer containing detergent, protease inhibitors, and phosphatase inhibitors. Aliquots with 15 μg of protein from lysates were separated by SDS-PAGE and transferred onto nitrocellulose membranes. The amount of phosphorylation was detected using phospho-specific antibodies to p38 MAPK, p42/p44 ERK, p46-p54 JNK, and phosphorylated IκBα. To determine equal loading of proteins between samples, the membranes were probed with rabbit polyclonal p46 JNK, p42/p44 ERK, p38 MAPK, and IκBα antibodies. The expression of MKP-1 was detected with a polyclonal MKP-1 antibody. B, POPC and DPPG liposomes (200 μg/ml) were compared with POPG liposomes (200 μg/ml) as antagonists of LPS activation of cells using the same conditions as described for panel A. The time of analysis following LPS exposure for p38, ERK, and JNK was 30 min, and that for IκBα and MKP-1 was 60 min.
The quantification of Western blotting results from multiple experiments performed as shown in Fig. 4A is presented in Fig. 5. The POPG treatment significantly reduces p38, ERK, JNK, and IκBα phosphorylation in LPS-stimulated cells to values nearly equivalent to untreated cells. The expression of MKP1 was also reduced to control levels by POPG treatment. In addition, the total amount of IκBα present in the cells remained constant when LPS-treated cells were also given POPG. This latter result demonstrates that POPG prevents degradation of IκBα that occurs subsequent to LPS treatment alone. Experiments identical to those described in the legends for were performed to directly compare the antagonism of LPS activation of U937 cells by POPG, POPC, and dipalmitoylphosphatidylglycerol (DPPG) (Fig. 4B). The cells treated with POPG and LPS reproduced the results shown in Figs. 4A and 5. The cells treated with LPS and POPC or DPPG failed to show any significant antagonism of LPS activation of p38, p42/44, JNK, IκBα, or MKP-1 expression. Thus, the inhibition of LPS-induced signaling pathways is dependent upon both the specific acyl chains present in the PG molecule and the specific polar substituent, which defines the class of phospholipid.
FIGURE 5.
Quantification of POPG inhibition of LPS-induced signaling. Western blot analysis as described in the legend for Fig. 4 was performed three or four times on separate samples, and the intensity of phospho-p38 (P-p38), phospho-IκBα (P-IκBα), phospho-ERK (P-ERK), phospho-JNK (P-JNK), total IκBα (T-IκBα), and MKP-1 was calculated using NIH Image J1.34 software. Significance is as follows: *, p < 0.05, **, p < 0.01, ***, p < 0.001 when compared between LPS treatment and LPS plus POPG treatment. No PLs, no phospholipids.
We also conducted control experiments to test whether POPG treatment had a general toxic effect upon U937 cells. Protein synthesis was measured by determining [3H]leucine incorporation into trichloroacetic acid precipitable material in the presence and absence of 200 μg/ml POPG. No changes in protein synthesis occurred over a 6-h period in POPG-treated U937 cells when compared with untreated cells in three independent experiments. Additional studies were performed to examine whether POPG pleiotropically inhibited signaling by macrophages. In these studies, macrophages were treated with TNF-α (10 ng/ml), and the degradation of IκBα was measured. POPG treatment of TNF-α-stimulated macrophages failed to alter IκBα degradation when compared with stimulated cells without POPG treatment. Collectively, these studies indicate that the actions of the anionic lipids upon LPS signaling are specific and not attributable to generalized suppression of cell growth or signaling processes. Additional experiments were performed examining the activity of POPG as an antagonist of flagellin-mediated TLR5 activation on BEAS2B cells and CG-rich oligonucleotide (CpG)-mediated TLR9 activation of RAW 264.7 cells. Results from three independent experiments revealed that POPG had no significant effect upon interleukin 8 production from BEAS2B cells (flagellin treatment = 5.5 ± 0.9 ng/ml; flagellin plus POPG = 6.3 ± 0.2 ng/ml) or TNF-α production from RAW 264.7 cells (CpG treatment = 9.2 ± 1.7 ng/ml; CpG plus POPG = 7.4 ± 1.9 ng/ml). These latter findings clearly demonstrate that the POPG effects are relatively specific for CD14·TLR4·MD-2 related activation of responsive cells.
Anionic Phospholipids Antagonize LPS Activation of Human Alveolar Macrophages
We next examined the lipid antagonism of primary human alveolar macrophages, isolated by bronchoalveolar lavage, and challenged with 10 ng/ml LPS for 6 h. The results presented in Fig. 6 demonstrate that POPG, dimyristoylphosphatidylglycerol (DMPG), and PI markedly attenuate the inflammatory response to LPS of freshly isolated human alveolar macrophages. In contrast, DPPG and DPPC had no significant effect upon the human alveolar macrophage response to LPS. These results demonstrate that human alveolar macrophages are susceptible to regulation of their inflammatory response by resident anionic surfactant phospholipids and the synthetic lipid DMPG.
FIGURE 6.
POPG, DMPG, and PI antagonize the effects of LPS on primary human alveolar macrophages. Human alveolar macrophages were isolated from healthy volunteer BALF and plated onto a 96-well plate. 2 days after plating, 10 ng/ml LPS and 20 μg/ml phospholipids were added to monolayer cultures of human alveolar macrophages. 6 h after stimulation, media were collected, and TNF-α production was determined by ELISA. LPS stimulation without phospholipid was set at 100%. The data shown are the means ± S.E. from three separate experiments with duplicate samples in each experiment. The average TNF-α secretion after LPS stimulation was 30.7 ± 15.1 ng/ml. Significance is as follows: *, p < 0.05, **, p < 0.01, ***, p < 0.001 when compared with LPS stimulation in the absence of POPG.
POPG Inhibits LPS-induced Proinflammatory Cytokine Production in Vivo
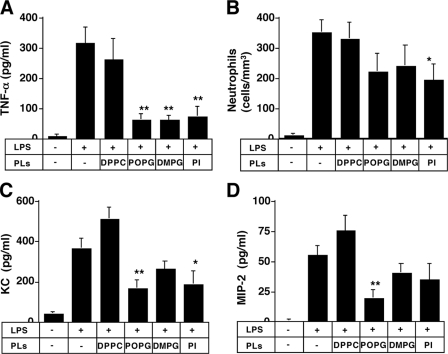
We next examined whether POPG could act to attenuate LPS effects in mice. Phospholipid liposomes were formed using a LiposofastTM pressure filtration apparatus. Mixtures of LPS and phospholipids were sprayed into the trachea of mice using a MicroSprayerTM positioned at the vocal cords. At 18 h after stimulation, the lungs of mice were lavaged (Fig. 7), and TNF-α, neutrophil infiltration, the mouse interleukin-8 equivalent, KC, and MIP-2 were measured in the recovered lavage fluid (44). These inflammatory mediators are important prognostic determinants of ALI/ARDS in humans (45). LPS-induced TNF-α was ∼300 pg/ml and was unaffected by DPPC instillation. In contrast, POPG, DMPG, and PI significantly attenuated the TNF-α secretion in the lung. These results clearly indicate that the intratracheally administered POPG, DMPG, and PI can reduce the inflammation in the lung in vivo. These results correlate well with in vitro results. LPS stimulation also induced the infiltration of neutrophils. POPG, DMPG, and PI, but not DPPC, modestly attenuated the LPS-induced neutrophil infiltration. DMPG, PI, and especially POPG attenuated the KC and MIP-2 secretion in BALF. These findings predict that the antagonistic phospholipids will inhibit the secretion of interleukin-8 from human alveolar macrophages in vivo.
FIGURE 7.
Anionic phospholipids (PLs) modulate lung inflammation induced by intratracheally administered LPS. A mixture of LPS (1 μg) and phospholipids (30 μg) in 20 μl of PBS was sprayed into murine trachea using a MicroSprayerTM aerosolizer. At 18 h after stimulation, lungs were lavaged via the trachea. TNF-α production (A) was determined by ELISA. The number of leukocytes was counted, and differential cell counts (B) were determined from at least 300 cells on cytocentrifuged preparations. Mouse KC (C) and MIP-2 (D) secretion were determined using Quantikine kits (R&D Systems). The data shown are the means ± S.E. from six to eight mice. Significance is as follows: *, p < 0.05, **, p < 0.01, when compared between LPS and LPS plus POPG.
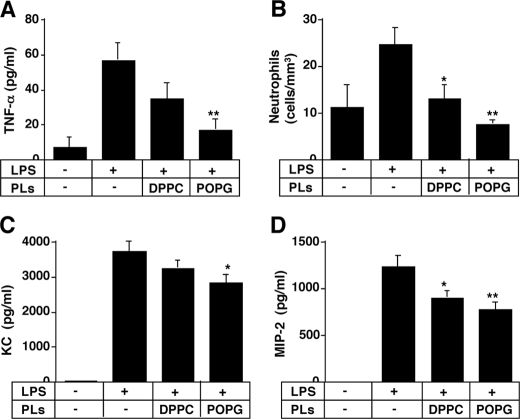
Next, we examined the effect of phospholipids in a non-pulmonary sepsis model in mice. LPS (50 μg/200 μl) was injected into mice via the tail vein, and phospholipids were administered intratracheally at the same time. 3 h after stimulation, BALF was collected (Fig. 8). Intratracheally administered POPG, but not DPPC, significantly inhibited the TNF-α secretion in BALF, indicating that this anionic lipid has an anti-inflammatory effect for sepsis originating outside the lung. POPG administered via the trachea effectively inhibited the infiltration of neutrophils in BALF when compared with DPPC. POPG also significantly attenuated the KC and MIP-2 levels in BALF, although the magnitude of this effect was not very large. These results suggest that POPG may be useful for attenuating lung inflammation caused by sepsis, originating at a non-pulmonary site.
FIGURE 8.
Anionic phospholipids (PLs) modulate lung inflammation induced by intravenously administered LPS. Phospholipids were dried under nitrogen and hydrated, and liposomes were formed using a LiposofastTM. The phospholipids (50 μg) in 20 μl of PBS were sprayed into murine trachea using a MicroSprayerTM aerosolizer. At the same time, LPS (50 μg) in 200 μl of PBS was intravenously administered to mice. 3 h after stimulation, lungs were lavaged via the trachea. TNF-α production (A) was determined by ELISA. The number of leukocytes was counted, and differential cell counts (B) were determined from at least 300 cells on cytocentrifuged preparations. Mouse KC (C) and MIP-2 (D) levels were determined using Quantikine kits (R&D System). The data shown are the means ± S.E. for six to eight mice. Significance is as follows: *, p < 0.05, **, p < 0.01, when compared between LPS and LPS plus POPG.
CD14 Binds POPG and PI in a Concentration-dependent Manner
LPS mainly binds to CD14 on cell surfaces and is subsequently transferred to an MD-2·TLR4 complex on the same membrane to initiate signaling. We prepared recombinant forms of the extracellular domains of CD14 and TLR4 and soluble MD-2 as shown in Fig. 9A. We first investigated whether CD14 is a target for phospholipid interaction that antagonizes LPS action. The anionic surfactant lipids POPG and PI, when adsorbed as a solid phase to microtiter wells, strongly bound to CD14 in a concentration-dependent manner (Fig. 9B). The binding of CD14 to the zwitterionic surfactant lipid, DPPC, was ∼30% of the levels attained for POPG binding. The CD14-lipid interactions were not attenuated by EGTA, and CaCl2 was not required for the binding. These results demonstrate that POPG and PI can directly bind CD14, and these interactions are of higher affinity than those with DPPC. These binding interactions for the anionic phospholipids are consistent with the effect of these same lipids upon inflammatory mediator production. The molecular species of PG were also evaluated for their direct binding interactions with CD14. POPG and DMPG exhibited the strongest direct binding interactions with CD14. However, CD14 also bound to DPPG to nearly the same extent as POPG and DMPG (Fig. 10A). These results clearly indicate that anti-inflammatory anionic phospholipids can bind strongly to CD14. However, some lipids without demonstrable anti-inflammatory effect will also directly bind CD14.
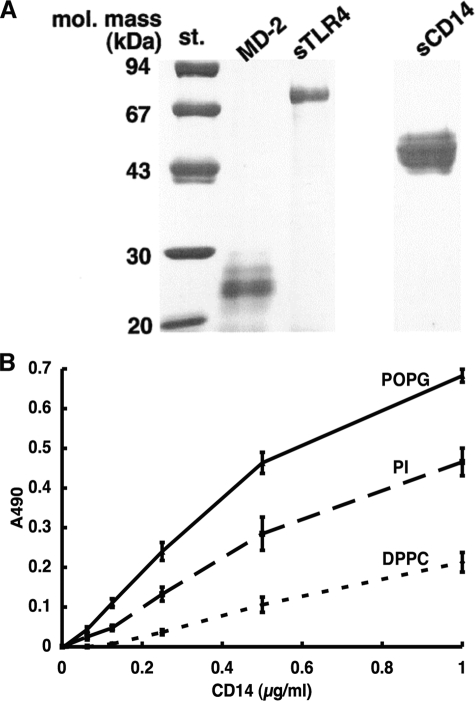
FIGURE 9.
CD14 binds to solid phase lipids. A, affinity-purified preparations (2 μg each) of the extracellular domains of CD14 (sCD14) and TLR4 (sTLR4) and the full-length MD-2 were analyzed by gel electrophoresis under reducing and denaturing conditions. The electrophoretic gels were stained with Coomassie Blue. mol. mass st., molecular mass standards. B, phospholipids (1.25 nmol) in 20 μl of ethanol were placed onto microtiter wells, and the solvent was evaporated. Nonspecific binding was blocked with 20 mm Tris buffer (pH 7.4) containing 0.15 m NaCl, 5 mm CaCl2 (in the upper panel), or 2 mm EGTA (in the lower panel) and 5% (w/v) bovine serum albumin (buffer A). Varying concentrations of human CD14 in buffer A were added and incubated at 37 °C for 1 h. The binding of CD14 to phospholipids was detected using anti-CD14 monoclonal antibody as described under “Experimental Procedures.” The data shown are the means ± S.E. from three separate experiments with duplicate samples in each experiment.
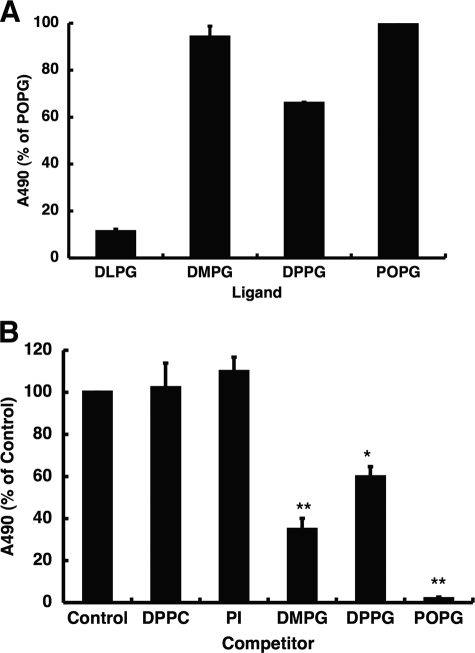
FIGURE 10.
PG Inhibits CD14 binding to solid phase LPS. A, various types of PG were coated onto microtiter plates and incubated with CD14 (1 μg/ml) at 37 °C for 1 h. The binding of CD14 to PG was detected using anti-CD14 monoclonal antibody, and the ELISA-based absorbance of CD14 bound to POPG was defined as 100%. Types of PG shown on the graph are: dilauroylphosphatidylglycerol (DLPG), DMPG, DPPG, and 16:0/18:1 POPG. B, LPS (2 μg) in 20 μl of ethanol was placed onto microtiter wells, and the solvent was evaporated. After blocking the nonspecific binding with buffer A, the mixture of CD14 (1 μg/ml) and phospholipid liposomes (20 μg/ml) in buffer A, which was preincubated at 37 °C for 1 h, was added and incubated at 37 °C for 1 h. The binding of CD14 to LPS was detected using anti-CD14 monoclonal antibody. The ELISA-based absorbance of CD14 bound to LPS was defined as 100%. The data shown are the means ± S.E. from three separate experiments with duplicate samples in each experiment. *, p < 0.05, **, p < 0.01, when compared with LPS-CD14 binding in the absence of phospholipids.
POPG Blocks the Interaction of CD14 with LPS
Another method to evaluate CD14-lipid interaction is to perform competition experiments in which CD14 binding to solid phase LPS is subjected to competition using fluid phase phospholipid liposomes. This series of experiments is described in Fig. 10B. Two lipids that function as potent LPS antagonists in vitro and vivo, DMPG and POPG, are the most effective inhibitors of CD14 binding to solid phase LPS. DPPG, which is inactive as an LPS antagonist, weakly competes for CD14 binding to LPS. These latter findings are consistent with earlier findings about PG antagonism of LPS activation of macrophages. Paradoxically, PI, which is a potent LPS antagonist, fails to compete for CD14 binding to solid phase LPS. These latter results strongly suggest that PI and POPG do not act by identical mechanisms in producing LPS antagonism.
POPG and PI Bind to CD14 at the LPS Binding Site
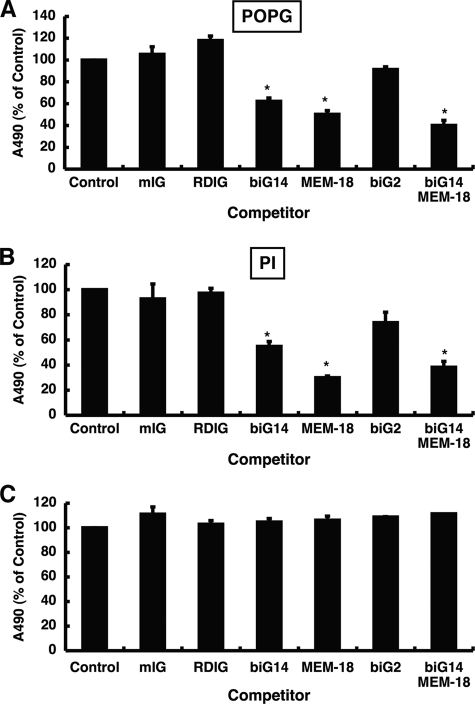
CD14 has four LPS binding sites located at the N terminus of the protein (46). Monoclonal antibodies biG14 and MEM-18 recognize amino acids 39–44 and amino acids 57–64, respectively, that constitute part of the LPS binding site. The biG14 and MEM-18 antibodies are also proven inhibitors of LPS binding to CD14. Another antibody, biG2, recognizes amino acids 147–152, which are not part of the LPS binding site, and biG2 ligation does not inhibit LPS binding to CD14. The epitope for another antibody, RDIg, has not been determined but appears to recognize a site distinct from that used for LPS binding. Solution of the crystal structure of mouse CD14 at a resolution of 2.5 Å provides evidence that LPS binds a defined pocket in the protein (47). The biG14 and MEM-18 binding sites are close to the pocket and predicted to sterically occlude LPS binding. In contrast, the biG2 site when ligated by antibody should not interfere with LPS binding. We examined the action of the above described monoclonal antibodies to determine the relationship between the LPS binding site and the anionic phospholipid binding site on CD14.
In experiments, shown in Fig. 11, the CD14 was preincubated with specific monoclonal antibodies, and the effect of this interaction upon the recognition of solid phase phospholipid by CD14 was measured. The CD14 bound to the solid phase was detected using anti-CD14 polyclonal antibody. As shown in Fig. 11A, the monoclonal antibodies biG14 and MEM-18 significantly reduced the CD14 binding to solid phase POPG by 40–60%, whereas other antibodies that did not recognize the LPS binding site (mouse IG, RDIg, and biG2) failed to significantly alter CD14 recognition of POPG. The addition of biG14 and MEM-18 antibodies together gave slightly higher inhibition of CD14 binding to lipid than either antibody alone. Nearly identical results were obtained when PI was used as the solid phase ligand as shown in Fig. 11B, with the monoclonal antibodies inhibiting the binding reaction by 40–70%. Interestingly, none of the antibodies tested inhibited the binding of CD14 to DPPG (data not shown). Thus, the site of interaction between CD14 and POPG and PI is different from the site of interaction with DPPG. In Fig. 11C, we conducted control experiments with solid phase CD14 to show that ligation of the protein by RDIg, biG14, MEM-18, and biG2 does not attenuate the binding of anti-CD14 polyclonal antibody. Thus, the loss of polyclonal antibody detection of CD14 reflects a reduction in interaction of the protein with phospholipids and is not due to monoclonal antibody interference with polyclonal antibody recognition.
FIGURE 11.
Monoclonal antibodies specific for the LPS binding site inhibit CD14 interaction with POPG and PI. POPG (A) or PI (B) were coated onto microtiter plates. After blocking the nonspecific binding with buffer A, the mixture of CD14 (1 μg/ml) and monoclonal antibodies or isotype control IgG (50 μg/ml) in buffer A, which was preincubated at 37 °C for 1 h, was added and incubated at 37 °C for 1 h. The binding of CD14 to phospholipids was detected using sheep anti-CD14 polyclonal antibody, and the ELISA-based absorbance of CD14 bound to phospholipid was defined as 100%. The data shown are the means ± S.E. from three separate experiments with duplicate samples in each experiment. *, p < 0.05, when compared with CD14 binding in the absence of monoclonal antibody. mIG, mouse Ig. C, CD14 (2 μg) was coated onto microtiter plates, and nonspecific binding was blocked with buffer A. Monoclonal antibodies or isotype control IgG (50 μg/ml) in buffer A were added and incubated at 37 °C for 1 h. The CD14 was detected using sheep anti-CD14 polyclonal antibody, and the ELISA-based absorbance of solid phase CD14 alone was defined as 100%.
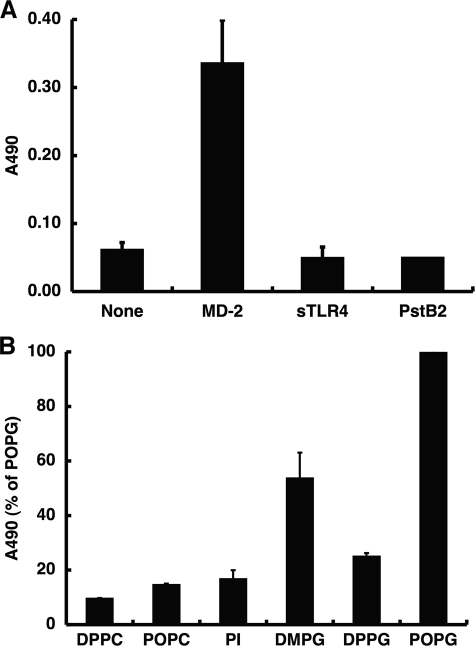
POPG Binds to MD-2 and Blocks the Interaction of MD-2 with sTLR4
TLR4 requires MD-2 for CD14-dependent cellular response to LPS. It is known that LPS binds to CD14 and MD-2 but not TLR4. Next, we examined whether phospholipids directly interact with MD-2 or TLR4. Recombinant MD-2, sTLR4, and the yeast protein PstB2, all with a His6 epitope tag, were expressed using the baculovirus-insect cell expression system (Fig. 9A). Solid phase POPG strongly bound to MD-2 but not to sTLR4 or the control epitope-tagged protein PstB2 (Fig. 12A). The lipid recognition specificity of MD-2 was evaluated using PI, two molecular species of PC, and three molecular species of PG (Fig. 12B). Relative to POPG, only DMPG showed significant binding (∼50% of the POPG value) to MD-2. Neither saturated nor unsaturated PC, unsaturated PI, nor DPPG showed any significant binding to MD-2.
FIGURE 12.
MD-2 preferentially binds POPG. A, POPG (1.25 nmol) was placed onto microtiter wells, and the solvent was evaporated. After blocking nonspecific binding with buffer A, MD-2, sTLR4, and PstB2 (1 μg/ml) in buffer A were added and incubated at 37 °C for 1 h. The binding of recombinant proteins to POPG was detected using anti-His antibody. B, phospholipids (1.25 nmol) were placed onto microtiter wells, and the solvent was evaporated. After blocking, MD-2 (1 μg/ml) in buffer A was added and incubated at 37 °C for 1 h. The binding of MD-2 to phospholipids was detected using anti-His antibody. The ELISA-based absorbance of MD-2 bound to POPG was defined as 100%. The data shown are the means ± S.E. from three separate experiments each with duplicate determinations.
We next probed the influence of lipids upon the interactions between MD-2 and TLR4. The extracellular domain of TLR4 was adsorbed onto microtiter wells, and the direct binding of MD-2 was measured by ELISA using a monoclonal antibody directed against a V5 epitope on the protein. At low levels of lipid competitor added as liposomes, only POPG interfered with the MD-2·TLR4 interaction (Fig. 13A), producing 40% inhibition. In Fig. 13B, the concentration of lipid competitors was varied up to 200 μg/ml, and only POPG showed any significant inhibition (∼75%) of the MD-2-TLR4 interaction. The action of POPG as an inhibitor increased with increasing concentration of the lipid between 20 and 200 μg/ml. These results clearly demonstrate that another site of action of POPG occurs between MD-2 and TLR4. These results further indicate that PI and POPG have non-identical mechanisms of interaction with the innate immune system that result in suppression of inflammation.
FIGURE 13.
POPG disrupts MD-2 interaction with TLR4. sTLR4 (100 ng) was adsorbed onto microtiter wells. After blocking nonspecific binding with buffer A, the mixture of MD-2 (1 μg/ml) and phospholipid liposomes (20 μg/ml) (A) or different concentrations of phospholipids (B) in buffer A, which was preincubated at 37 °C for 1 h, was added and incubated at 37 °C for 2 h. The binding of MD-2 to sTLR4 was detected using horseradish peroxidase-conjugated anti-V5 monoclonal antibody. The ELISA-based absorbance of MD-2 binding without phospholipids was defined as 100%. The data shown are the means ± S.E. from three separate experiments each with duplicate determinations. *, p < 0.05, **, p < 0.01, when compared with MD-2-sTLR4 binding in the absence of phospholipids.
DISCUSSION
The present study provides strong evidence that POPG and PI, which are minor components of pulmonary surfactant, effectively inhibit LPS-induced inflammatory responses by U937 cells, primary rat alveolar macrophages, and primary human alveolar macrophages. POPG and PI block LPS-induced phosphorylation of MAPKs and IκBα. These anionic lipids also prevent LPS-induced degradation of IκBα and MKP-1 expression, consistent with the conclusion that these lipids antagonize LPS signaling from TLR4. These findings identify an intrinsic system within the lung that suppresses inflammation and protects the delicate alveolar compartment from damage. The action of POPG and PI appears complementary to that of the pulmonary surfactant collectins, SP-A and SP-D, that also function to suppress inflammation within the lung (15). By maintaining a basal suppressive state within the conducting and gas exchange regions of the organ, the lung remains largely unresponsive to low level exposure to airborne particulate matter that contains LPS. This type of suppressive state ensures that the alveolar epithelium at the interface with the external environment is not chronically inflamed as a consequence of repeated minor exposure to inflammatory stimuli.
The LPS antagonism of POPG and PI is specific insofar as other phospholipids such as PC, PE, and SM are without effect. In comparison with POPG and PI, another anionic phospholipid, PS, is only a weak antagonist of LPS action. Even within the class of PGs, there is specificity of action. DPPG and DSPG failed to antagonize LPS action upon macrophages, and among shorter chain saturated PGs, only DMPG acted as an effective antagonist. Human surfactant is highly enriched in POPG but contains no DMPG. Thus, these studies also identify DMPG as a novel synthetic antagonist of LPS action.
Supplementation of human surfactant lipids with POPG, DMPG, and PI liposomes produces LPS antagonism both in vitro and in vivo. The in vitro experiments demonstrate that segregated populations of POPG liposomes are the most effective antagonists of LPS action. It is not yet known whether POPG can exist in segregated domains within the surfactant monolayer or within the alveolar hypophase present in the lung. However, biophysical studies provide good evidence that DPPC can exist in distinct domains in the surfactant layer, and thus, it is plausible that POPG could also be present in segregated domains (48). In our studies, the highest levels of POPG or PI used are only 10% of the levels found in the alveolar compartment of the lung. Thus, a relatively small population of segregated anionic surfactant lipid, within the lung, would be sufficient to suppress TLR4 activation. The studies performed with intratracheal administration of POPG, DMPG, and PI, in mice, provide strong evidence that these lipids can effectively attenuate lung inflammation in vivo and are consistent with a model in which the lipid remains in a segregated state following its introduction into the lung.
Several previous studies have identified a role for the anionic phospholipids, PG, PI, and caridolipin, as ligands for LBP and antagonists of LPS activation of TLR4-expressing cells (24–26). Our data are entirely consistent with these previous studies with respect to cellular responses. However, our work now provides clear evidence for the in vivo effectiveness of the anionic lipids within the lung and the direct interactions of these lipids with molecules other than LBP. In this report, we now present clear evidence for direct and specific interactions between CD14, PI, and PG. These binding interactions were concentration-dependent and saturable and of higher affinity than those with DPPC.
The crystal structure of mouse CD14 demonstrates that the protein has LPS binding pockets at its N terminus (47). Four LPS binding regions have been identified within the N-terminal 65 residues of CD14 (49). Monoclonal antibodies biG14 and MEM-18 bind to regions corresponding to the third and fourth pockets and block LPS binding. Both POPG and PI strongly bind to CD14, and the monoclonal antibodies biG14 and MEM-18 compete for the binding of CD14 to these lipids. These data demonstrate significant overlap between the LPS and anionic surfactant phospholipid binding sites. From single-residue mutation experiments, charge reversal mutations within binding regions 3 and 4 had the greatest effect on LPS binding (49). Because the hydrophilic portion of LPS is also negatively charged, the anionic phospholipids may compete with LPS by interfering with charge-dependent interactions with CD14. Additional experiments will be necessary to precisely map the amino acids required for POPG binding. Kim et al. (47) suggested that the hydrophobic portion of LPS binds to the first and second N-terminal pockets because these are the only hydrophobic surfaces large enough to accommodate acyl portions of LPS. POPG and DMPG bind to CD14 and antagonize the actions of LPS. In contrast DPPG, dilauroyl-PG and dioctanoyl-PG fail to antagonize LPS action. The molecular species specificity of PG action demonstrates that fatty acid structure is also an important determinant of the interaction of phospholipids with the hydrophobic pockets in CD14.
LPS also binds to MD-2 without a requirement for either LBP or CD14 (46). MD-2 binds to the extracellular TLR4 domain, and a complex of MD-2 and TLR4, but not TLR4 alone, can interact with LPS (37). POPG binds to MD-2 with high affinity. Interestingly, the interaction of POPG with MD-2 inhibits the binding of the protein to TLR4 and subsequently antagonizes LPS action. The interaction between POPG and MD-2 is specific because PI and PC fail to bind the protein. Previous protein sequence analysis has identified MD-2 as a protein related to fungal PG and PI binding and transfer proteins (50). According to Gioannini et al. (51) the most efficient response to endotoxin occurs when it is sequentially transferred from LBP to CD14 and finally MD-2 to engage TLR4-dependent intracellular signaling. PI interferes with the interactions between LPS and CD14, but POPG acts at multiple steps of protein-LPS recognition. Thus, POPG appears more broadly directed at multiple pattern recognition proteins.
The physical interactions of POPG with LBP, CD14, and MD-2 are consistent with these associations playing a major role in suppressing inflammation. In the context of these experiments, we also examined whether POPG could directly bind and sequester LPS. In these studies, POPG liposomes were mixed with sonicated dispersions of LPS for 1 h at 37 °C, and subsequently, the liposomes were removed from solution by centrifugation, and the resultant pellet and supernatants were measured for LPS content. In three independent experiments, we found that POPG liposomes (200 μg/ml) added at a 105 molar excess relative to LPS (10 ng/ml) failed to remove any significant amount of the LPS from solution, with control recovery of supernatant LPS = 100% and recovery of supernatant LPS after mixture and centrifugation with POPG liposomes = 131.2 ± 38.9%. Thus, the mechanism of action of POPG is by direct binding to LBP, CD14, and MD-2.
Collectively, our findings demonstrate that the anionic pulmonary surfactant lipids can play a crucial role in suppressing inflammatory responses in the delicate alveolar compartments of the lung. The lung appears uniquely poised between suppression and activation of inflammatory responses, with the basal homeostatic condition favoring suppression. This suppression depends on the lipids, PG and PI, and the pulmonary collectins, SP-A and SP-D. The presence of multiple surfactant components with these activities provides a means of expanding the repertoire of pathogen-derived proinflammatory components that can be antagonized during routine daily exposure to airborne microparticulates. The net result appears to maintain the lung in a quiescent state until a critical threshold is reached that finally allows inflammation to proceed. The findings that anionic surfactant phospholipids regulate the innate immune system and directly interact with receptors are important for understanding the fundamental mechanisms of host defense in the lung and raise the possibility that exogenous supplementation with anionic lipids may provide an effective means of controlling excessive inflammation.
A few clinical studies have made connections between surfactant PG content and disease. In idiopathic pulmonary fibrosis, ARDS, and asthma, several groups reported decreased unsaturated PG in surfactant (18, 52–57). The issues of cause and effect in the above diseases remain unclear, but any condition that results in reduced levels of unsaturated PG pools within the lung is likely to increase the susceptibility to inflammatory processes elicited through TLR4 activation.
In summary, our findings identify an important role for the minor acidic lipids of pulmonary surfactant in suppressing inflammation within the alveolar compartment of the lung that is induced by activation of TLR4. The site of action of PG and PI is at the cell surface of macrophages and perhaps other cells where the recognition of LPS by TLR4 is disrupted. The direct high affinity binding of POPG to CD14 and MD-2 constitutes a major mechanism by which this lipid suppresses TLR4 activation by LPS.
Supplementary Material
This work was supported, in whole or in part, by National Institutes of Health Grants PHL 073907 and GCRC M01-RR00051.
This article was selected as a Paper of the Week.
- LPS
- lipopolysaccharide
- LBP
- LPS-binding protein
- PC
- phosphatidylcholine
- PE
- phosphatidylethanolamine
- PG
- phosphatidylglycerol
- PI
- phosphatidylinositol
- PS
- phosphatidylserine
- SM
- sphingomyelin
- DMPG
- dimyristoylphosphatidylglycerol
- DOPG
- dioleoylphosphatidylglycerol
- DPPC
- dipalmitoylphosphatidylcholine
- DPPG
- dipalmitoylphosphatidylglycerol
- POPC
- 1-palmitoyl-2-oleoyl phosphatidylcholine
- POPG
- palmitoyl-oleoyl-phosphatidylglycerol
- TNF
- tumor necrosis factor
- TLR
- Toll-like receptor
- ERK
- extracellular signal-regulated kinase
- JNK
- c-Jun N-terminal kinase
- MAPK
- mitogen-activated protein kinase
- MKP
- MAPK phosphatase
- SP
- surfactant protein
- ALI
- acute lung injury
- ARDS
- acute respiratory distress syndrome
- BALF
- bronchoalveolar lavage fluid
- ELISA
- enzyme-linked immunosorbent assay
- NO
- nitric oxide
- KC
- keratinocyte-derived cytokine.
REFERENCES
- 1.O'Brien A. D., Rosenstreich D. L., Scher I., Campbell G. H., MacDermott R. P., Formal S. B. (1980) J. Immunol. 124, 20–24 [PubMed] [Google Scholar]
- 2.Ulevitch R. J., Tobias P. S. (1995) Annu. Rev. Immunol. 13, 437–457 [DOI] [PubMed] [Google Scholar]
- 3.Wright S. D., Ramos R. A., Tobias P. S., Ulevitch R. J., Mathison J. C. (1990) Science 249, 1431–1433 [DOI] [PubMed] [Google Scholar]
- 4.Nagai Y., Akashi S., Nagafuku M., Ogata M., Iwakura Y., Akira S., Kitamura T., Kosugi A., Kimoto M., Miyake K. (2002) Nat. Immunol. 3, 667–672 [DOI] [PubMed] [Google Scholar]
- 5.Poltorak A., He X., Smirnova I., Liu M. Y., Van Huffel C., Du X., Birdwell D., Alejos E., Silva M., Galanos C., Freudenberg M., Ricciardi-Castagnoli P., Layton B., Beutler B. (1998) Science 282, 2085–2088 [DOI] [PubMed] [Google Scholar]
- 6.Takeda K., Kaisho T., Akira S. (2003) Annu. Rev. Immunol. 21, 335–376 [DOI] [PubMed] [Google Scholar]
- 7.Medzhitov R. (2001) Nat. Rev. Immunol. 1, 135–145 [DOI] [PubMed] [Google Scholar]
- 8.Barton G. M., Medzhitov R. (2003) Science 300, 1524–1525 [DOI] [PubMed] [Google Scholar]
- 9.Pattle R. E. (1955) Nature 175, 1125–1126 [DOI] [PubMed] [Google Scholar]
- 10.Clements J. A. (1957) Proc. Soc. Exp. Biol. Med. 95, 170–172 [DOI] [PubMed] [Google Scholar]
- 11.King R. J., Klass D. J., Gikas E. G., Clements J. A. (1973) Am. J. Physiol. 224, 788–795 [DOI] [PubMed] [Google Scholar]
- 12.Dobbs L. G., Mason R. J. (1978) Am. Rev. Respir. Dis. 118, 705–733 [DOI] [PubMed] [Google Scholar]
- 13.Kuroki Y., McCormack F. X., Ogasawara Y., Mason R. J., Voelker D. R. (1994) J. Biol. Chem. 269, 29793–29800 [PubMed] [Google Scholar]
- 14.Sano H., Kuroki Y. (2005) Mol. Immunol. 42, 279–287 [DOI] [PubMed] [Google Scholar]
- 15.Wright J. R. (2005) Nat. Rev. Immunol. 5, 58–68 [DOI] [PubMed] [Google Scholar]
- 16.Lawson P. R., Reid K. B. (2000) Immunol. Rev. 173, 66–78 [DOI] [PubMed] [Google Scholar]
- 17.Sano H., Sohma H., Muta T., Nomura S., Voelker D. R., Kuroki Y. (1999) J. Immunol. 163, 387–395 [PubMed] [Google Scholar]
- 18.Veldhuizen R., Nag K., Orgeig S., Possmayer F. (1998) Biochim. Biophys. Acta 1408, 90–108 [DOI] [PubMed] [Google Scholar]
- 19.Schmidt R., Meier U., Markart P., Grimminger F., Velcovsky H. G., Morr H., Seeger W., Günther A. (2002) Am. J. Physiol. Lung Cell Mol. Physiol. 283, L1079–1085 [DOI] [PubMed] [Google Scholar]
- 20.Wright S. M., Hockey P. M., Enhorning G., Strong P., Reid K. B., Holgate S. T., Djukanovic R., Postle A. D. (2000) J. Appl. Physiol. 89, 1283–1292 [DOI] [PubMed] [Google Scholar]
- 21.Lewis J. F., Jobe A. H. (1993) Am. Rev. Respir. Dis. 147, 218–233 [DOI] [PubMed] [Google Scholar]
- 22.Bochkov V. N., Kadl A., Huber J., Gruber F., Binder B. R., Leitinger N. (2002) Nature 419, 77–81 [DOI] [PubMed] [Google Scholar]
- 23.Wu Y. Z., Medjane S., Chabot S., Kubrusly F. S., Raw I., Chignard M., Touqui L. (2003) Am. J. Respir. Crit. Care Med. 168, 692–699 [DOI] [PubMed] [Google Scholar]
- 24.Wang P. Y., Kitchens R. L., Munford R. S. (1998) J. Biol. Chem. 273, 24309–24313 [DOI] [PubMed] [Google Scholar]
- 25.Hashimoto M., Asai Y., Ogawa T. (2003) J. Biol. Chem. 278, 44205–44213 [DOI] [PubMed] [Google Scholar]
- 26.Mueller M., Brandenburg K., Dedrick R., Schromm A. B., Seydel U. (2005) J. Immunol. 172, 1091–1096 [DOI] [PubMed] [Google Scholar]
- 27.Atabai K., Matthay M. A. (2002) Thorax 57, 452–458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rubenfeld G. D., Caldwell E., Peabody E., Weaver J., Martin D. P., Neff M., Stern E. J., Hudson L. D. (2005) N. Engl. J. Med. 353, 1685–1693 [DOI] [PubMed] [Google Scholar]
- 29.Imai Y., Kuba K., Neely G. G., Yaghubian-Malhami R., Perkmann T., van Loo G., Ermolaeva M., Veldhuizen R., Leung Y. H., Wang H., Liu H., Sun Y., Pasparakis M., Kopf M., Mech C., Bavari S., Peiris J. S., Slutsky A. S., Akira S., Hultqvist M., Holmdahl R., Nicholls J., Jiang C., Binder C. J., Penninger J. M. (2008) Cell 133, 235–249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hawgood S., Benson B. J., Hamilton R. L., Jr. (1985) Biochemistry 24, 184–190 [DOI] [PubMed] [Google Scholar]
- 31.Kuroki Y., Mason R. J., Voelker D. R. (1988) J. Biol. Chem. 263, 3388–3394 [PubMed] [Google Scholar]
- 32.Rouser G., Siakotos A. N., Fleischer S. (1966) Lipids 1, 85–86 [DOI] [PubMed] [Google Scholar]
- 33.Ding A. H., Nathan C. F., Stuehr D. J. (1988) J. Immunol. 141, 2407–2412 [PubMed] [Google Scholar]
- 34.Hibi M., Lin A., Smeal T., Minden A., Karin M. (1993) Genes Dev. 7, 2135–2148 [DOI] [PubMed] [Google Scholar]
- 35.Towbin H., Staehelin T., Gordon J. (1979) Proc. Natl. Acad. Sci. U.S.A. 76, 4350–4354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Flotte T. R., Laube B. L. (2001) Chest 120, Suppl. 3, 124S–131S [DOI] [PubMed] [Google Scholar]
- 37.Hyakushima N., Mitsuzawa H., Nishitani C., Sano H., Kuronuma K., Konishi M., Himi T., Miyake K., Kuroki Y. (2004) J. Immunol. 173, 6949–6954 [DOI] [PubMed] [Google Scholar]
- 38.Wu W. I., Routt S., Bankaitis V. A., Voelker D. R. (2000) J. Biol. Chem. 275, 14446–14456 [DOI] [PubMed] [Google Scholar]
- 39.O'Reilly D. R., Miller L. K., Luckow V. A. (1992). in Baculovirus Expression Vectors: A Laboratory Manual, pp. 109–215, W. H. Freeman and Company, New York [Google Scholar]
- 40.Sano H., Chiba H., Iwaki D., Sohma H., Voelker D. R., Kuroki Y. (2000) J. Biol. Chem. 275, 22442–22451 [DOI] [PubMed] [Google Scholar]
- 41.Akira S., Takeda K. (2004) Nat. Rev. Immunol. 4, 499–511 [DOI] [PubMed] [Google Scholar]
- 42.Chen P., Li J., Barnes J., Kokkonen G. C., Lee J. C., Liu Y. (2002) J. Immunol. 169, 6408–6416 [DOI] [PubMed] [Google Scholar]
- 43.Zhao Q., Shepherd E. G., Manson M. E., Nelin L. D., Sorokin A., Liu Y. (2005) J. Biol. Chem. 280, 8101–8108 [DOI] [PubMed] [Google Scholar]
- 44.Wuyts A., Haelens A., Proost P., Lenaerts J. P., Conings R., Opdenakker G., Van Damme J. (1996) J. Immunol. 157, 1736–1743 [PubMed] [Google Scholar]
- 45.Meduri G. U., Kohler G., Headley S., Tolley E., Stentz F., Postlethwaite A. (1995) Chest 108, 1303–1314 [DOI] [PubMed] [Google Scholar]
- 46.Viriyakosol S., Kirkland T. N. (1995) J. Biol. Chem. 270, 361–368 [DOI] [PubMed] [Google Scholar]
- 47.Kim J. I., Lee C. J., Jin M. S., Lee C. H., Paik S. G., Lee H., Lee J. O. (2005) J. Biol. Chem. 280, 11347–11351 [DOI] [PubMed] [Google Scholar]
- 48.Nag K., Perez-Gil J., Ruano M. L., Worthman L. A., Stewart J., Casals C., Keough K. M. (1998) Biophys. J. 74, 2983–2995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cunningham M. D., Shapiro R. A., Seachord C., Ratcliffe K., Cassiano L., Darveau R. P. (2000) J. Immunol. 164, 3255–3263 [DOI] [PubMed] [Google Scholar]
- 50.Inohara N., Nuñez G. (2002) Trends Biochem. Sci. 27, 219–221 [DOI] [PubMed] [Google Scholar]
- 51.Gioannini T. L., Teghanemt A., Zhang D., Coussens N. P., Dockstader W., Ramaswamy S., Weiss J. P. (2004) Proc. Natl. Acad. Sci. U.S.A. 101, 4186–4191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Honda Y., Tsunematsu K., Suzuki A., Akino T. (1988) Lung 166, 293–301 [DOI] [PubMed] [Google Scholar]
- 53.Saydain G., Islam A., Afessa B., Ryu J. H., Scott J. P., Peters S. G. (2002) Am. J. Resp. Crit. Care Med. 166, 839–842 [DOI] [PubMed] [Google Scholar]
- 54.McCormack F. X., King T. E., Jr., Voelker D. R., Robinson P. C., Mason R. J. (1991) Am. Rev. Respir. Dis. 144, 160–166 [DOI] [PubMed] [Google Scholar]
- 55.Hite R. D., Seeds M. C., Bowton D. L., Grier B. L., Safta A. M., Balkrishnan R., Waite B. M., Bass D. A. (2005) Am. J. Physiol. Lung Cell Mol. Physiol. 288, L610–617 [DOI] [PubMed] [Google Scholar]
- 56.Markart P., Ruppert C., Wygrecka M., Colaris T., Dahal B., Walmrath D., Harbach H., Wilhelm J., Seeger W., Schmidt R., Guenther A. (2007) Thorax 62, 588–594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Schmidt R., Meier U., Yabut-Perez M., Walmrath D., Grimminger F., Seeger W., Günther A. (2001) Am. J. Respir. Crit. Care Med. 163, 95–100 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.