Abstract
A hallmark of protective immunity during Mycobacterium tuberculosis (M. tb) infection is the regulated secretion of pro-inflammatory and regulatory cytokines. Suppressors of Cytokine Signaling (SOCS) are key regulators of cytokine secretion and function. In this study we investigated regulation of Toll-like receptor 2 (TLR2) and dendritic cell-specific ICAM-3 grabbing non-integrin receptor 1 (DC-SIGNR1)-mediated SOCS1 expression in DCs during M. tb infection. We show that, compared with TLR2, stimulating DC-SIGNR1 on DCs induces higher SOCS1 expression and lower interleukin-12 production. Co-stimulating DC-SIGNR1 and TLR2 differentially regulates SOCS1 expression depending on the relative concentration of their ligands. Stimulating DC-SIGNR1 with M. tb infection increases SOCS1 expression, while stimulating TLR2 with M. tb infection reduces SOCS1 expression. Knockdown of SOCS1 in DCs by siRNA enhances interleukin-12 transcription and protein expression upon DC-SIGNR1 stimulation. Raf-1 and Syk differentially regulate TLR2- and DC-SIGNR1-mediated SOCS1 expression. In addition, DC-SIGNR1 shows greater association with SOCS1 when compared with TLR2. Interestingly, compared with healthy asymptomatic individuals, peripheral blood mononuclear cells of patients with active tuberculosis disease showed higher expression of SOCS1, which was reduced following chemotherapy. Similarly, stimulating DC-SIGNR1 on DCs from M. tb-infected TLR2−/− mice enhanced SOCS1 expression that was reduced following chemotherapy. Further, knockdown of SOCS1 in mouse DCs or human peripheral blood mononuclear cells resulted in increased killing of virulent M. tb. These results indicate that TLR2 and DC-SIGNR1 differentially regulate SOCS1 expression during M. tb infection. This in turn regulates M. tb survival by governing key cytokine expression.
A major prerequisite toward development of vaccine(s) or therapeutic regimens for tuberculosis is a thorough understanding of the immune responses initiated following infection of macrophages and DCs by Mycobacterium tuberculosis (M. tb)2 (1–4). A key factor toward this is cross-regulation of cytokine secretion by different cells of the immune system (5–7). One of the molecules that regulate cytokine expression and cytokine receptor-mediated gene expression in various cells of the immune system is a family of closely related proteins called Suppressors of Cytokine Signaling (SOCS). There are currently eight members of SOCS proteins (SOCS1–7 and CIS (also called SOCS8)), with SOCS1 being the most characterized (8). All eight SOCS family members have a central Src homology 2 domain, an N-terminal domain of variable length, and a 40-amino acid motif at the C terminus known as the SOCS box. Although SOCS molecules exhibit sequence homology, particularly in the SOCS box and Src homology 2 domains, CIS and SOCS2, SOCS1 and SOCS3, SOCS4 and SOCS5, and SOCS6 and SOCS7 have marked pairwise homology across the entire protein sequence. SOCS molecules bind to the cytoplasmic tails of cytokine receptors and block their activation. This consequently leads to a block in the effects of the cytokine in question and leads to skewing of responses.
Although macrophages serve as the long term hosts for mycobacteria, DCs phagocytose mycobacteria and mycobacterial antigens, and these interactions are crucial for the development of protective immunity (9–11). As a result DC function and phenotype are modified following bacterial uptake. A number of surface receptors, e.g. Toll-like receptor (TLR) 2, mannose receptor, CD11b (Mac-1), CD11c, and DEC-205 have been implicated in mycobacterial recognition by DCs, (12–14).
One of the C-type lectin receptors extensively characterized with respect to mycobacteria is DC-specific ICAM-3 grabbing non-integrin (DC-SIGN). DC-SIGN has been reported to be the major receptor on DCs for M. tb (15). DC-SIGN was previously reported as the receptor for HIV gp120 (16). However, it is now demonstrated that DC-SIGN serves as a receptor for a plethora of pathogens, including viruses (e.g. Ebola, Dengue, and HIV), bacteria (e.g. M. tb and Salmonella), and parasites (Leishmania and Plasmodium) (16). Although immature DCs also express high levels of mannose receptor, CD11b, and CD11c, neutralizing DC-SIGN with specific antibodies inhibited the interaction of DCs with both Mycobacteria bovis BCG and mannosylated lipoarabinomannan (manLAM), a major sugar molecule expressed on the cell surface by virulent Mycobacterium sp., by >80%. On the other hand neutralizing mannose receptor had no significant effect. This indicated that DC-SIGN binds manLAM in a specific manner.
In the mouse, the “DC-SIGN” locus is much more complex than in humans and encompasses seven genes coding for SIGNR1–5 and SIGNR7–8 proteins and one pseudogene coding for SIGNR6 protein (17). Of these only one gene coding for mouse DC-SIGN is highly expressed on DCs but not in macrophages and lymphocytes. Subsequently, Caminschi et al. (18) reported the functional comparison of mouse DC-SIGN also known as CIRE with human DC-SIGN and showed that, like human DC-SIGN, CIRE or mouse-DC-SIGN bound mannosylated residues but did not bind pathogens known to interact with human DC-SIGN, including Leishmania mexicana, cytomegalovirus, HIV, and lentiviral particles bearing the Ebola virus glycoprotein. This indicated that the two molecules displayed fine specificities with respect to binding partners from pathogens. Studies by Powlesland et al. (19) also indicated differential binding specificities of mouse DC-SIGN homologs to various ligands. These authors also showed that mouse DC-SIGN, which they called SIGNR5 and human DC-SIGN, displayed similar binding ability to mannose.
manLAM on the other hand has been demonstrated to bind to many DC-SIGN homologs such as DC-SIGNR1 (CD209b) and L-SIGN on both mouse and human cells (20) indicating that this key ligand is important for binding of virulent M. tb to mouse cells as well. In fact mice lacking DC-SIGNR1 induce stronger T cell responses to M. tb (21) indicating an inhibitory role for manLAM in T cell activation. In addition many M. tb components have been identified as stimulators of TLRs. These include the 19-kDa lipoprotein and peptidoglycan that act on TLR2 (22) and the CpG repeat of non-methylated DNA that acts on TLR9 (23). Interestingly, the interactions of M. tb vis-à-vis TLR and DC-SIGN has shown interesting correlates. Although interactions with TLR result in activation of DCs characterized by high IL-12 secretion, interactions with DC-SIGN prevent DC activation by blocking NF-κB activation (24). For example, TLR9 has been shown to regulate Th1 responses to M. tb in cooperation with TLR2 (25, 26). However, similar studies comparing the activities of TLR2 and DC-SIGNR1 in the mouse system have not been investigated as yet.
In light of the above, in this study, we investigated the interplay between SOCS1, DC-SIGNR1, and TLR2 during M. tb infection and the consequences thereof. We show that stimulating DC-SIGNR1 on DCs induces expression of SOCS1 and low IL-12 secretion, whereas stimulating TLR2 results in moderate SOCS1 expression and high IL-12 levels. Importantly, DC-SIGNR1 displays greater recruitment of SOCS1 when compared with TLR2. Knockdown of SOCS1 using siRNA not only results in higher IL-12 expression following DC-SIGNR1 ligation, but also results in effective killing of intracellular M. tb. Patients with active disease show increased expression of SOCS1 that reduce following chemotherapy. These results suggest that differential regulation of SOCS1 expression and recruitment by TLR2 and DC-SIGNR1 might result in opposite effects from the two receptors that have a bearing on the survival of M. tb.
EXPERIMENTAL PROCEDURES
Animals and Human Studies
All experiments were conducted following approval from the institutional animal and human ethics committees. Female BALB/c mice 4–6 weeks of age kept in a pathogen-free environment were used. For some experiments TLR2 knock-out (TLR2−/−) mice in B6 background were used. Following informed consent venous blood was drawn from PPD+ healthy volunteers or patients in the age group of 15–60 years freshly diagnosed with active TB and after 2 months of chemotherapy. Further, either HIV+ or immunocompromised or pregnant women or individuals with a history of TB were excluded from the study.
Materials
Antibodies to SOCS1, DC-SIGN, DC-SIGNR1, TLR2, glyceraldehyde-3-phosphate dehydrogenase, and siRNAs against mouse and human SOCS1 were from Santa Cruz Biotechnologies. siRNAs to Raf-1 and Syk were obtained from Dharmacon. Fluorescein isothiocyanate-tagged antibodies to IFN-γR, IL-12R, or IL-10R and enzyme-linked immunosorbent assay kits were from BD Biosciences, San Diego, CA. M. tb H37Rv mannosylated manLAM was obtained from Tuberculosis Research Materials and Vaccine Testing, Colorado State University. Details of its preparation can be viewed at on the web. TLR2 ligand Pam3Csk4 was purchased from Invivogen. Acetylated Histone 3 ChIP kits were from Upstate Biotechnology, Inc. Lake Placid, NY.
Generation and Enrichment of DCs and Enrichment of PBMCs
DCs were differentiated with GM-CSF as described before (27–30). Briefly, bone marrow from the tibias and femurs of BALB/c mice were flushed out, and lymphocytes and I-A+ cells were depleted following magnet-assisted cell sorting. Cells were cultured in RPMI 1640 medium containing 10% fetal calf serum, 0.05 m 2-mercaptoethanol, 1 mm sodium pyruvate, plus 15 ng/ml GM-CSF. Human PBMCs were enriched from heparinized blood by Ficoll-Paque ™ Plus density gradient centrifugation. Splenic DCs from wild-type or TLR2−/− mice were enriched essentially as described earlier. Briefly, spleen from 6–8 mice were pooled and cut into small fragments. From this B and T cells were removed by incubation with CD19- and CD90-coated magnet-assisted cell sorting microbeads (Miltenyi). From the resulting population CD11c+ DCs were enriched following positive selection with anti-CD11c antibody-coated magnet-assisted cell sorting microbeads.
Stimulation of Cells
Unless otherwise mentioned DCs and PBMCs were stimulated with 1 μg/ml Pam3Csk4 or 5 μg/ml manLAM, and this concentration was considered as high concentration based on extensive dose titrations (data not shown). In experiments where DCs were co-stimulated with both the ligands, the concentrations employed were 0.5 μg/ml Pam3Csk4 and 2.5 μg/ml manLAM, and these concentrations were considered as low.
Infection of Cells with M. tb H37Rv
DCs and PBMCs were infected with M. tb H37Rv at 1 multiplicity of infection for different times. Cells were processed either for Western blotting or immunoprecipitation as described below. Alternatively, cells were processed for monitoring colony forming units.
Transfection of DCs with siRNA
Transfection of DCs with siRNA was carried out as recently described (30). 5 × 106/ml bone marrow precursors were transfected with 60 pmol of siRNA against SOCS1 for 72 h using the Hiperfect transfection reagent (Qiagen) in Opti-MEM medium (Invitrogen). GM-CSF was added 5 h following transfection, and incubation continued for 72 h for DC differentiation. Similarly, 2.5 × 106/ml PBMCs were transfected with SOCS1 siRNA. Knockdown was verified by reverse transcription-PCR, following which, cells were either stimulated with Pam3Csk4 or manLAM or infected with M. tb. Control siRNAs from Santa Cruz Biotechnology (catalogue # sc-37007) and siRNAs to three Plasmodium falciparum genes Berghepain 1 (BP1), PF10_0348 (P. f DBLMSP), and PF10_0351 custom synthesized by Dharmacon were used as nonspecific controls.
Analyses of SOCS1 Levels
SOCS1 levels were monitored by Western blotting. At the end of incubation, cells were chilled on ice and washed once with ice-cold phosphate-buffered saline and lysed in buffer containing 10 mm HEPES (pH 7.9), 10 mm KCl, 0.1 mm EDTA, 0.1 m EGTA, 0.5% Nonidet P-40, and 2 μg/ml each of aprotinin, leupeptin, and pepstatin. The suspension was centrifuged at 13,000 rpm for 2 min at 4 °C. The supernatant was designated as the cytoplasmic extract. 25 μg of cytoplasmic extract were then resolved on 10% SDS-PAGE and subsequently transferred onto nitrocellulose membrane (Hybond C pure, Amersham Biosciences). The blots were then probed with antibodies to SOCS1 followed by horseradish peroxidase-labeled secondary antibodies. Further, a parallel set of samples was run separately on SDS-PAGE and probed for glyceraldehyde-3-phosphate dehydrogenase as loading control. The blots were later developed by chemiluminescence using the Luminol reagent.
Immunoprecipitation
Following incubation, cytoplasmic extracts were prepared as described above. The extracts were precleared with Protein G-agarose beads, and protein concentration was normalized between samples. The precleared extracts were then incubated with either anti-DC-SIGN or anti-DC-SIGNR1, or anti-TLR2 antibodies absorbed to Protein G-agarose beads, and incubated overnight at 4 °C. The beads were pelleted and thoroughly washed several times with lysis buffer. The beads were boiled in SDS-PAGE sample buffer and resolved on 10% SDS-PAGE followed by Western blotting against SOCS1 as described above.
Flow Cytometry
Cells were stained for surface levels of IFN-γR, IL-12R, or IL-10R using fluorescein isothiocyanate-tagged antibodies and analyzed by flow cytometry on FACSCalibur (BD Biosciences). Surface levels of DC-SIGNR1 or TLR2 on DCs were monitored following biotinylation of anti-DC-SIGNR1 or anti-TLR2 antibody and subsequent incubation with DCs, followed by incubation with streptavidin phycoerythrin, respectively. The data were plotted using CellQuest Pro software.
Measurement of Cytokines
Cytokines in the culture supernatants were measured by employing a sandwich enzyme-linked immunosorbent assay as described previously (29, 30). The samples were diluted to obtain absorbance in the linear range of the standards. In addition, cytokines were also measured using the Luminex liquichip platform strictly following the manufacturer's instructions.
Infection and Drug Treatment of Mice
Infection and drug treatment of mice were carried out as recently described (29). Groups of naïve mice (4 mice/group) were infected with 1 × 106 M. tb H37Rv via the tail vein. Following 7 days of infection mice were treated with oral administration of isoniazid (25 mg/kg body weight), ethambutol (100 mg/kg body weight), and rifampicin (20 mg/kg body weight) essentially as described elsewhere (31). A repeat dose was given 7 days after and on every alternate day until the mice were sacrificed.
Statistics
Two-tailed Student's t test was carried out to obtain p values. Values of p < 0.05 were considered as significant.
RESULTS
Stimulation of TLR2 and DC-SIGNR1 Induces SOCS1 Expression
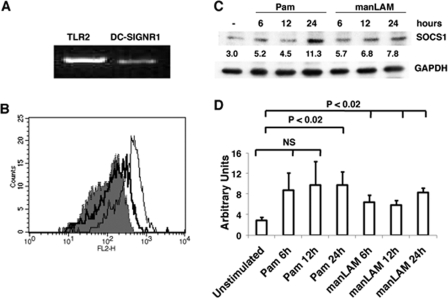
To begin with we investigated the levels of SOCS1 expression following stimulation of DC-SIGNR1 and TLR2 on mouse bone marrow-differentiated DCs. In lieu of the expression of different homologs of DC-SIGN and related molecules in mouse cells (32–34), at the onset, we first confirmed that in our hands DCs differentiated from mouse bone marrow with GM-CSF indeed express DC-SIGNR1 at both transcript and surface protein levels (Fig. 1, A and B). The expression levels were, however, much lower when compared with TLR2.
FIGURE 1.
Mouse bone marrow differentiated DCs express TLR2 and DC-SIGNR1 that when stimulated induce SOCS1 expression. A, reverse transcription-PCR of TLR2 and DC-SIGNR1 mRNA enriched from DCs differentiated from bone marrow with GM-CSF. B, histograms depicting the relative surface levels of DC-SIGNR1 (thick line) and TLR2 (thin line) on DCs differentiated from bone marrow with GM-CSF. The shaded histogram depicts staining with streptavidin-phycoerythrin. For C, DCs were stimulated with 1 μg/ml Pam3Csk4 (Pam) or 5 μg/ml manLAM for the indicated times. Post-incubation, cytoplasmic extracts were Western blotted for SOCS1. Numbers below the SOCS1 blot indicate relative intensities of the bands. Data from one of three independent experiments are shown. For D, densitometric values of the three SOCS1 blots were employed to graphically represent SOCS1 levels in different treatments. Bars represent mean ± S.D. of the three independent experiments. NS indicates means between indicated groups were not significant at p > 0.05.
Next, we stimulated TLR2, with Pam3Csk4, a well characterized TLR2 ligand (35) and stimulated DC-SIGNR1 with manLAM from M. tb H37Rv (15, 16, 21) and monitored the expression of SOCS1. As shown in Fig. 1C, stimulation with either Pam3Csk4 or manLAM induced the expression of SOCS1. Fig. 1D schematically depicts the average of three experiments based on densitometric scanning of the blots. Significant differences were observed between unstimulated and Pam3Csk4 stimulated cells at 24 h (p < 0.02), but not in Pam3Csk4-stimulated cells at 6 or 12 h. However, significant differences were observed between unstimulated and manLAM-stimulated cells (p < 0.02) at all time points. The collated results of all the blots thus indicate that, although stimulation of either TLR2 or DC-SIGNR1 induces the expression of SOCS1, DCSIGNR1 is a more potent inducer of SOCS1 than TLR2. This is based on both the kinetics of statistically significant expression, and because the expression level of TLR2 is much higher than DC-SIGNR1, quantitatively also, DC-SIGNR1 induces higher expression of SOCS1 than TLR2.
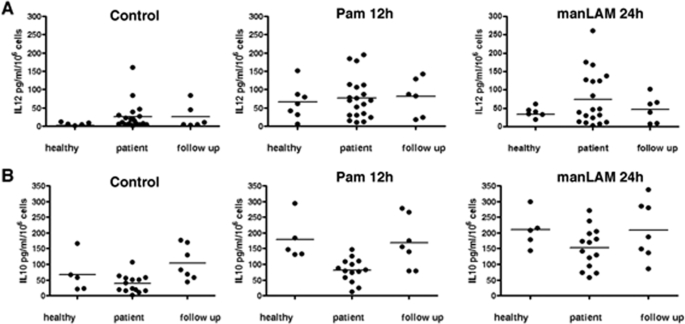
We next investigated the expression of IL-12p40 and IL-10 following stimulation with Pam3Csk4 and manLAM. In comparison to Pam3Csk4 stimulation, manLAM stimulation resulted in lower IL-12p40 (supplemental Fig. 1A). Further, Pam3Csk4 stimulation resulted in low levels of IL-10 expression. In contrast, IL-10 levels were undetectable upon stimulation with manLAM (supplemental Fig. 1B). In addition, the expression of a number of pro-inflammatory cytokines and chemokines such as IL-6, IP-10, MCP-1, MIP-2, and RANTES (regulated on activation normal T cell expressed and secreted) was also higher upon Pam3Csk4 stimulation when compared with manLAM stimulation (supplemental Fig. 1C).
Co-stimulation of TLR2 and DC-SIGNR1 Differentially Induces SOCS1
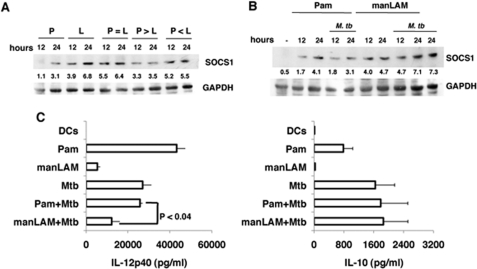
We next investigated whether co-stimulating TLR2 and DC-SIGNR1 would modulate SOCS1 in DCs. To this end, we employed conditions wherein the two receptors would be differentially ligated depending upon the concentration of their respective ligands. So as to achieve measureable additive or suppressive effects, we used different concentrations of Pam3Csk4 and manLAM to stimulate TLR2 and DC-SIGNR1 based on extensive dose titrations (data not shown). As shown in Fig. 2A, individually stimulating DC-SIGNR1 or TLR2 using low concentrations of manLAM and Pam3Csk4, respectively, resulted in higher SOCS1 expression from DC-SIGNR1 when compared with TLR2. Once again, because DCs expressed higher levels of TLR2 than DC-SIGNR1, this indicated that DC-SIGNR1 stimulation indeed induced higher SOCS1 expression when compared with TLR2 stimulation. Next, upon co-stimulating the two receptors using a high concentration of Pam3Csk4 and a low concentration of manLAM (Fig. 2A, p > L (manLAM)), SOCS1 expression was reduced by ∼50% when compared with manLAM alone. In contrast, when the concentration of Pam3Csk4 was low and the concentration of manLAM was high (p < L) or under conditions wherein both concentrations were low (p = L), SOCS1 expression was similar to that obtained with low concentration of DC-SIGNR1 alone. These results indicated that expression of SOCS1 is differentially regulated following simultaneous ligation of TLR2 and DC-SIGNR1, with the latter having a dominating effect over TLR2 in regulating SOCS1 expression. These results have important bearings on M. tb infection wherein multiple receptors are likely to be simultaneously triggered.
FIGURE 2.
Co-stimulating TLR2 and DC-SIGNR1 or with M. tb infection differentially modulates SOCS1 expression. A, DCs were stimulated with 0.5 μg/ml Pam3Csk4 alone (P) or 2.5 μg/ml manLAM (L) alone or co-stimulated with either equal concentrations of both (P = L) for indicated times. Alternatively, DCs were stimulated with 1 μg/ml Pam3Csk4 and 2.5 μg/ml manLAM (P > L), or with 0.5 μg/ml Pam3Csk4 and 5 μg/ml manLAM (P < L) for the indicated times. Post-incubation, cytoplasmic extracts were Western blotted for SOCS1. B, DCs were either stimulated with 1 μg/ml Pam3Csk4 alone (Pam) or 5 μg/ml manLAM alone or co-infected with 1 multiplicity of infection M. tb H37Rv for the indicated times. Post-incubation, cytoplasmic extracts were Western blotted for SOCS1 levels. Numbers below the SOCS1 blot indicate relative intensities of the bands. Data from one of three experiments are shown. C, IL-12p40 and IL-10 levels in the culture supernatants from B at 24-h post-stimulation. Data are the mean ± S.D. of three independent experiments.
Stimulation of TLR2 or DC-SIGNR1 with M. tb Infection Induces Differential Expression of SOCS1 and IL-12
We extended the above observations and stimulated DCs with Pam3Csk4 or manLAM along with simultaneous infection with M. tb and monitored SOCS1 expression. As shown in Fig. 2B, M. tb infection along with Pam3Csk4 (TLR2) stimulation resulted in a significant reduction of SOCS1 expression when compared with stimulation of either Pam3Csk4 alone or M. tb alone. We further confirmed that M. tb infection did not significantly alter the expression of TLR2 on DCs (supplemental Fig. 2). This indicated that the reduction in SOCS1 levels upon stimulating TLR2 along with M. tb infection was not a result of reduced expression of TLR2. In contrast, no reduction in SOCS1 levels was obtained upon manLAM (DC-SIGNR1) stimulation along with M. tb infection, and levels were similar to that obtained with M. tb alone. These results indicated that, although TLR2 displays negative cooperativity with M. tb for SOCS1 expression, DC-SIGNR1 displays positive cooperativity with M. tb for SOCS1 expression. Further, although stimulation of TLR2 alone induced higher IL-12p40 levels when compared with either DC-SIGNR1 or M. tb infection (Fig. 2C), co-stimulation of TLR2 with M. tb infection resulted in a reduction in IL-12p40 levels when compared with M. tb infection alone. Likewise, stimulation of DC-SIGNR1 with M. tb infection also reduced IL-12p40 levels. However, the reduction in IL-12p40 levels was much more (p < 0.04) upon stimulating DC-SIGNR1 along with M. tb infection. These results indicated that, although stimulation of TLR2 alone would result in maximum IL-12 production, simultaneous ligation of other receptors following M. tb infection could attenuate signals from TLR2 thus reducing IL-12 expression. In contrast, stimulation of DC-SIGNR1 along with M. tb infection would amplify signals that result in further reduction in IL-12 expression. No significant changes were observed in IL-10 levels indicating that IL-10 levels are by and large not regulated during co-stimulation of the above receptors.
Knockdown of SOCS1 in DCs Induces Higher IL-12 from DC-SIGNR1
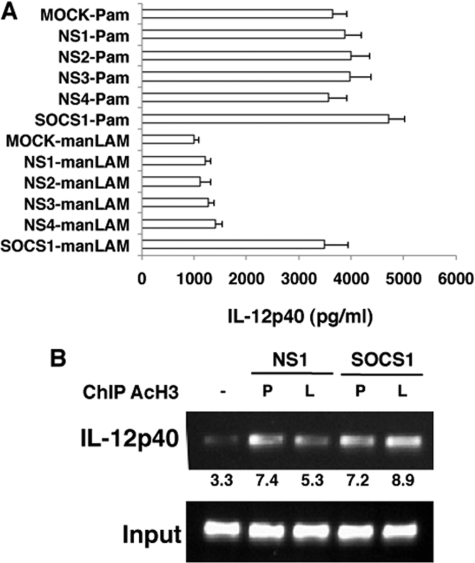
Because stimulation of DC-SIGNR1 resulted in increased SOCS1 expression and lower IL-12p40 expression, we next investigated, if reduced IL-12 expression was a result of higher SOCS1 expression. To this end, we knocked down SOCS1 expression in DCs with specific siRNAs and monitored IL-12p40 levels following stimulation of DC-SIGNR1 and TLR2. As shown in Fig. 3A, knockdown of SOCS1 in DCs indeed resulted in higher IL-12p40 expression following DC-SIGNR1 stimulation. In fact the levels were nearly similar to those obtained following TLR2 stimulation. Expectedly, IL-12p40 levels were further increased, albeit marginally, in these DCs following TLR2 stimulation. We next confirmed the same at the transcriptional level by monitoring the acetylation of histone H3 at the IL-12p40 promoter between position −121 to −131 (a region that has been functionally characterized to be important for IL-12 expression) (36), by chromatin immunoprecipitation in DCs, following knockdown of SOCS1. As shown in Fig. 3B, compared with DC-SIGNR1 stimulation, Pam3Csk4 (TLR2) stimulation displayed increased transcription at the IL-12p40 promoter as evident from increased pull down of acetylated histone. However, knockdown of SOCS1 led to a significant increase in the amplified product upon manLAM (DC-SIGNR1) stimulation, indicating regulation of IL-12p40 at the transcriptional level. This indicated that lower production of IL-12 from DC-SIGNR1 could be a result of higher SOCS1 expression.
FIGURE 3.
Knockdown of SOCS1 results in higher IL-12p40 expression from DC-SIGNR1. A, DCs were transfected with siRNAs against SOCS1 and later stimulated with 1 μg/ml Pam3Csk4 (Pam) or 5 μg/ml manLAM for 12 h. MOCK represents no siRNAs (vehicle control), whereas NS1 represents silencing with nonspecific control siRNAs procured from Santa Cruz Biotechnologies. NS2 to NS4 represent silencing with nonspecific siRNAs to P. falciparum genes Berghepain 1 (BP1), PF10_0348 (P. f DBLMSP), and PF10_0351, respectively, custom synthesized by Dharmacon. IL-12p40 levels in culture supernatants were measured by enzyme-linked immunosorbent assay. Data from one of three independent experiments are shown. B, chromatin immunoprecipitation analysis of acetylated histone H3 (AcH3) from the IL-12p40 promoter in SOCS1-silenced or NS1-silenced DCs stimulated with 1 μg/ml Pam3Csk4 (P) or 5 μg/ml manLAM (L) for 12 h. Data from one of three experiments are shown.
Raf-1 and Syk Regulate SOCS1 Induction from DC-SIGNR1
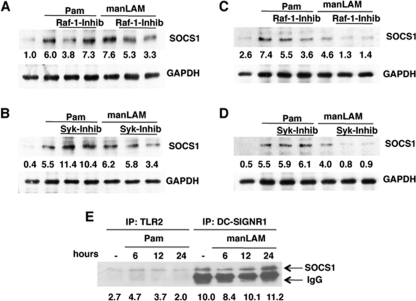
We next investigated mechanism of SOCS1 regulation by DC-SIGNR1 and TLR2. Although, signal transduction pathways from DC-SIGN homologs in either mouse or human cells are not fully characterized, it is reported that human DC-SIGN modulates IL-10 expression from TLR4 that involved activation of the serine and threonine kinase Raf-1 (37). In fact stimulation of human DC-SIGN alone by manLAM activated Raf-1 in the absence of TLR4 co-stimulation. Activated Raf-1 increased acetylation of the p65 subunit of NF-κB that resulted in higher IL-10 from TLR4. Further the same study also pointed to a role of the Src kinase Syk in the above signaling cascade. Therefore, we next investigated whether Raf-1 and/or Syk would regulate SOCS1 in mouse DCs following stimulation of TLR2 or DC-SIGNR1. To that end we monitored SOCS1 expression in DCs by the two receptors following Raf-1 and Syk inhibition. As shown in Fig. 4 (A and B), inhibiting either Raf-1 or Syk significantly inhibited SOCS1 induction by DC-SIGNR1 in a dose-dependent manner indicating a role for Raf-1 and Syk in SOCS1 expression. In contrast, no significant change in SOCS1 expression was apparent following TLR2 stimulation when Raf-1 was inhibited. However, Syk inhibition resulted in a 2-fold increase in SOCS1 expression upon TLR2 stimulation, indicating that Syk played contrasting roles in SOCS1 expression from the two receptors. To confirm that the effects of the biochemical inhibitors were specific, we investigated the same using specific siRNAs against Raf-1 and Syk. As shown in supplemental Fig. 3, similar results were obtained when siRNAs were employed against Raf-1 and Syk. Knockdown of Raf-1 or Syk inhibited SOCS1 expression from DC-SIGNR1. In contrast Raf-1 inhibition did not have any significant effect on SOCS1 expression following TLR2 stimulation, whereas knockdown of Syk enhanced SOCS1 expression following TLR2 stimulation. These results confirmed that the results obtained with biochemical inhibitors were specific to Raf-1 and Syk. Next, to see if the above regulation was dependent or independent of TLR4, we carried out similar experiments with TLR4−/− mice. As shown in Fig. 4 (C and D), inhibiting Raf-1 indeed down-regulated the expression of SOCS1 from DC-SIGNR1. Further, like Raf-1, Syk also positively regulated SOCS1 expression from DC-SIGNR1, because inhibiting Syk significantly inhibited SOCS1 expression. This indicated that both Raf-1 and Syk regulated SOCS1 from DC-SIGNR1 independent of TLR4 signaling. Interestingly, Raf-1 also inhibited SOCS1 expression from TLR2, although the inhibition was less than that observed in DC-SIGNR1 while Syk played an insignificant role. This pointed to a role for Raf-1 in SOCS1 expression from TLR2 in the absence of TLR4.
FIGURE 4.
DC-SIGNR1 induces SOCS1 expression via Raf-1 and Syk and SOCS1 associates with DC-SIGNR1. A and B, SOCS1 levels in DCs stimulated with 1 μg/ml Pam3Csk4 (Pam) or 5 μg/ml manLAM for 24 h in the presence or absence of 5 μm and 10 μm GW5074 a Raf-1 inhibitor (Raf-1-inhib) or 5 μg/ml and 10 μg/ml piceatannol, a Syk inhibitor (Syk-inhib). For C and D, DCs from TLR4−/− mice were processed as in A and B. For E, either TLR2 or DC-SIGNR1 were immunoprecipitated from DCs following stimulation with 1 μg/ml Pam3Csk4 (Pam) or 5 μg/ml manLAM for the indicated times and Western blotted for SOCS1. Numbers below the SOCS1 blot indicate relative intensities of the bands. Data from one of three independent experiments are shown.
DC-SIGNR1 Display Increased Association with SOCS1
SOCS1 has been shown to associate with a number of cytoplasmic molecules in addition to cytokine receptors (8). Because DC-SIGNR1 stimulation increased IL-12p40 expression following knockdown of SOCS1, we investigated whether SOCS1 showed any association with DC-SIGNR1 and TLR2. To this end we immunoprecipitated TLR2 and DC-SIGNR1 in resting and Pam3Csk4- and manLAM-stimulated DCs and immunoblotted for SOCS1. As shown in Fig. 4E DC-SIGNR1 showed higher association with SOCS1 when compared with TLR2. These results together with the results in Fig. 3 strongly indicated that lower IL-12 levels from DC-SIGN could be a result of its increased association with and induced expression of SOCS1.
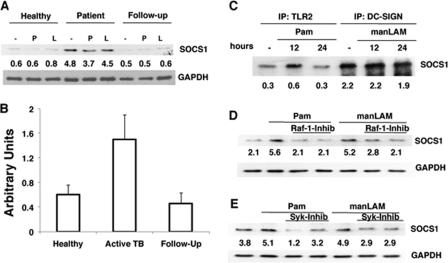
PBMCs of Patients with Active TB Disease Express Higher SOCS1
We next extended the above observations to human cells in the context of M. tb infection. We first confirmed that DC-SIGN is expressed in PBMCs (data not shown). Next, we monitored SOCS1 expression in PBMCs of PPD+ healthy asymptomatic individuals, patients with active TB disease, and the same patients following 2 months of chemotherapy. Twelve patients consented to give blood samples for the study, and 8 patients consented to be bled again following 2 months of therapy. Fig. 5A depicts a representative profile from one of many similar profiles. PBMCs of patients with active TB disease showed much higher expression of SOCS1 when compared with healthy individuals. However, following 2 months of chemotherapy, SOCS1 levels were reduced to those observed in healthy individuals. A schematic of SOCS1 levels in all the healthy controls, patients with active TB, and their follow-ups screened based on densitometric scans of the blots is depicted in Fig. 5B. These results indicate that SOCS1 expression positively correlated with the severity of the TB disease. Further, similar to DC-SIGNR1, SOCS1 in human PBMCs also showed increased association with DC-SIGN, when compared with TLR2 (Fig. 5C) pointing to a similar mechanism of down-regulation of pro-inflammatory responses from DC-SIGN. Furthermore, like DC-SIGNR1, SOCS1 expression by DC-SIGN in human PBMCs was also regulated by Raf-1 and Syk (Fig. 5, D and E), wherein inhibition of either Raf-1 or Syk resulted in a significant inhibition of SOCS1 expression upon either TLR2 or DC-SIGN stimulation. These results indicated that DC-SIGN-mediated regulation of SOCS1 was similar in both mouse and human cells. However, in contrast to mouse DCs, Syk positively regulated SOCS1 expression from TLR2.
FIGURE 5.
Patients with active TB disease express high SOCS1 that is associated with DC-SIGN and regulated by Raf-1 and Syk. A, SOCS1 levels in PBMCs of healthy individual or patient with active TB disease and the same patient after 2 months of chemotherapy (Follow-up) following stimulation with 1 μg/ml Pam3Csk4 (P) or 5 μg/ml manLAM (L) for 24 h. One of eight similar blots is shown. For B, densitometric values of SOCS1 blots of all the healthy controls, patients, and follow-ups were employed to graphically represent SOCS1 levels in different cohorts. Bars represent mean ± S.D. of SOCS1 levels in each cohort irrespective of the treatment employed. C, PBMCs from a patient with active TB disease were simulated with 1 μg/ml Pam3Csk4 (Pam) or 5 μg/ml manLAM for 24 h. Cytoplasmic extracts were immunoprecipitated with either anti-TLR2 or anti-DC-SIGN antibody and Western blotted for SOCS1. D and E, SOCS1 levels in PBMCs of healthy individual stimulated with 1 μg/ml Pam3Csk4 (Pam) or 5 μg/ml manLAM for 24 h in the presence or absence of 5 μm and 10 μm GW5074 a Raf-1 inhibitor (Raf-1-inhib) or 5 μg/ml and 10 μg/ml piceatannol, a Syk inhibitor (Syk-inhib). One of three independent experiments is shown.
TLR2 and DC-SIGN Modulate Expression of Cytokines and Cytokine Receptors
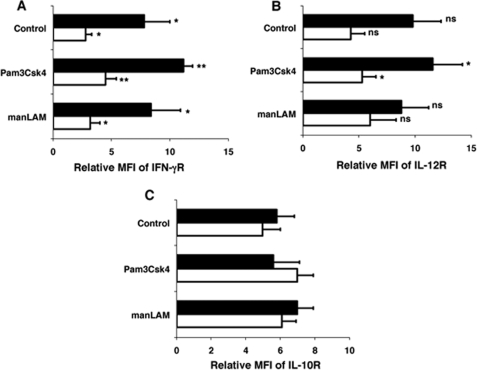
Because the levels of cytokines and their receptors are modulated at different stages of M. tb infection (38) we next investigated the roles played by TLR2 and DC-SIGN to this end. As shown in Fig. 6, TLR2 stimulation resulted in higher IL-12p40 expression in all three cohorts when compared with DC-SIGN stimulation. This could result from increased association of SOCS1 with DC-SIGN when compared with TLR2. On the other hand stimulation of DC-SIGN induced significantly higher IL-10 expression in all the three cohorts, especially in patients with active disease when compared with TLR2 stimulation. Further, it has been reported that PBMCs of healthy individuals express higher IFN-γ receptor when compared with PBMCs from patients with active TB disease (38). We therefore investigated if TLR2 and DC-SIGN would modulate the levels of IFN-γ, IL-12, and IL-10 receptors. As shown in Fig. 7, PBMCs from healthy individuals expressed higher IFN-γ and IL-12 receptor levels than TB patients that were significantly increased following TLR2 stimulation, but not upon DC-SIGN stimulation. IL-10 receptor levels were by and large not different between any groups or treatment. These results demonstrate that TLR2 and DC-SIGN play a role in mediating the levels of pro-inflammatory cytokines and their receptors that might be modulated by their differential associations with SOCS1.
FIGURE 6.
TLR2 stimulation induces high IL-12p40 expression in PBMCs of healthy individuals and TB patients. IL-12p40 (A) and IL-10 (B) levels in culture supernatants of PBMCs from healthy individuals, patients with active TB disease and patients after 2 months of chemotherapy (follow up) following stimulation with 1 μg/ml Pam3Csk4 for 12 h (Pam 12h) or 1 μg/ml manLAM for 24 h. Control depicts levels in unstimulated PBMCs. Each spot represents data from a single individual. Bars in the graphs indicate median within the samples.
FIGURE 7.
TLR2 stimulation increases IL-12 receptor levels on PBMCs. Surface levels of IFN-γ receptor (IFN-γR (A)), IL-12β1 receptor (IL-12R (B)), and IL-10 receptor (IL-10R (C)) in PBMCs of healthy individuals (closed bars) and patients with active TB disease (open bars) stimulated with 1 μg/ml Pam3Csk4 or 5 μg/ml manLAM for 24 h. Control depicts levels in unstimulated PBMCs. Data are represented as Relative Mean Fluorescence Intensity (MFI). Bars represent mean ± S.D. of five healthy and five TB patients. In A: *, p < 0.05 (for unstimulated PBMCs of healthy versus patients and manLAM-stimulated cells of healthy versus patients) and **, p < 0.01 (for Pam3Csk4-stimulated cells of healthy versus patients). In B, *, p < 0.05 (for Pam3Csk4-stimulated cells of healthy versus patients), ns depicts differences in the mean are not significant.
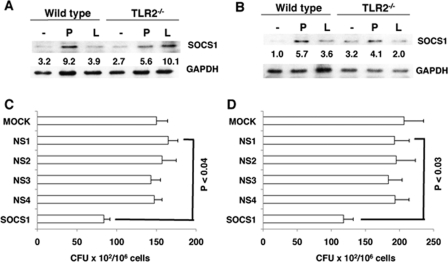
DC-SIGNR1 Stimulation Induces Higher SOCS1 Expression in TLR2−/− DCs
We next extended the observations on human cells to TLR2−/− mice. It is well established that TLR2−/− mice are more susceptible to M. tb infection than wild-type mice (39, 40) and would thus simulate an active TB disease phenotype of human patients. We therefore, investigated SOCS1 expression in splenic DCs from M. tb-infected TLR2−/− mice and also from infected and drug-treated mice. As shown in Fig. 8A, stimulating DC-SIGNR1 in TLR2−/− DCs induced a 2-fold higher SOCS1 expression when compared with TLR2 stimulation. The weak increase in SOCS1 expression following stimulation of TLR2−/− DCs with Pam3Csk4 could result from engagement of TLR1 by Pam3Csk4. It has been reported that Pam3Csk4 binds to both TLR2 and TLR1, although with a much higher affinity to TLR2 (35). Nevertheless, increased SOCS1 expression following DC-SIGNR1 stimulation in TLR2−/− DCs indicated that, with an increase in the severity of the disease, SOCS1 showed higher expression via DC-SIGNR1. Significantly, SOCS1 expression from DC-SIGNR1 in DCs from drug cured TLR2−/−-infected mice was reduced to background levels (Fig. 8B). These results are in concurrence with the results observed in human patients, wherein SOCS1 expression was higher during TB disease and come down following chemotherapy.
FIGURE 8.
DCs from M. tb-infected TLR2−/− mice show high SOCS1 expression upon DC-SIGNR1 stimulation and knockdown of SOCS1 kills intracellular M. tb. A, SOCS1 levels in splenic DCs from M. tb H37Rv-infected wild-type and TLR2−/− mice following stimulation with 1 μg/ml Pam3Csk4 (P) or 5 μg/ml manLAM (L) for 24 h. For B, wild-type and TLR2−/− mice were infected with M. tb H37Rv for 2 weeks followed by drug treatment. SOCS1 levels in splenic DCs were later monitored as in A. One of three experiments is shown. C and D, M. tb H37Rv colony forming units in mouse DCs (C) and human PBMCs (D) following knockdown of SOCS1 with specific siRNAs. MOCK represents no siRNA (vehicle control), while NS1 represents silencing with nonspecific control siRNAs procured from Santa Cruz Biotechnologies. NS2 to NS4 represent silencing with nonspecific siRNAs to P. falciparum genes Berghepain 1 (BP1), PF10_0348 (P. f DBLMSP), and PF10_0351, respectively, custom synthesized by Dharmacon. Data are the mean ± S.D. of three independent experiments.
Knockdown of SOCS1 Results in Increased Killing of M. tb in DCs and PBMCs
In the light of the above data it was relevant to investigate the effects of SOCS1 knockdown on the survival of M. tb in DCs and PBMCs. siRNA-mediated knockdown of SOCS1 resulted in a significant reduction in M. tb colony forming units in both mouse DCs (Fig. 8C, p < 0.04) and human PBMCs (Fig. 8D, p < 0.03), when compared with nonspecific controls. These results emphasize the negative role of SOCS1 in mediating protective responses to M. tb.
DISCUSSION
It is now recognized that stimulation of specific receptors on DCs (as also on other cell types) effectively determines the way an immune response is tailored to a given pathogen. This is true for pathogens such as M. tb, which has successfully devised numerous ways to evade protective responses mounted by a highly evolved immune system (7). This includes down-regulation of cellular activation and antigen presentation pathways by specific antigens resulting in suppressor responses (22). Over the years we have also highlighted the role of M. tb antigens in modulating DC function. These antigen-activated DCs not only down-regulate pro-inflammatory responses to M. tb (Ref. 24 and reviewed in Ref. 42) but also serve as depots for mycobacterial survival by modulating key intracellular signaling molecules. Conditioning DCs with appropriate cytokines and chemokines resulted in mounting of protective responses and the clearance of an established M. tb infection in mice (29).
With respect to DCs, TLR2 and DC-SIGN are the two receptors that play crucial roles with contrasting outcomes (24). This is more true for human DCs, because not much information is known in mouse cells owing to the complex nature of DC-SIGN related molecules expressed in mouse cells (17–21). Major outcomes of TLR2 stimulation are the activation of NF-κB, and the consequent expression of pro-inflammatory cytokines such as tumor necrosis factor-α and IL-12p40. IL-12p40 plays a key role in mediating type 1 (Th1) responses from DCs, a crucial factor that determines resistance or susceptibility to M. tb infection and disease (7, 25, 26). On the other hand, DC-SIGN stimulation results in blockade of TLR-mediated NF-κB activation and IL-12 expression. However, the mechanisms that mediate these differential effects are not yet outlined, possibly owing to the fact that signal transduction pathways from DC-SIGN and its mouse homologs are not yet fully characterized.
A key regulator of cytokine production and activation and in turn the regulator of Th1/Th2 responses is SOCS. The role of SOCS molecules during inflammation and infection are well characterized. SOCS1−/− neonates are hyper-responsive to lipopolysaccharide, and very sensitive to lipopolysaccharide-induced lethality. In addition, BCG infection induces the production of SOCS1 and SOCS3 in mouse macrophages (43). Further, trehalose 6,6′-dimycolate/cord factor, a major component of the M. tb cell wall, induces expression of SOCS and inhibits IFN-γ-stimulated phosphorylation of signal transducers and activators of transcription 1.
In the light of the above, in this study, we investigated the role of SOCS1 in DC activation by TLR2 and DC-SIGNR1, because it has been shown that DC-SIGNR1 negatively regulates Th1 responses to M. tb (21). We show that, although stimulation of TLR2 or DC-SIGNR1 induces SOCS1 expression, DC-SIGNR1 induces SOCS1 with faster kinetics. Additionally, because DC-SIGNR1 levels are much lower when compared with TLR2 levels, the data indicated that, in effect, DC-SIGNR1 induces higher SOCS1 expression. This was further confirmed when the two receptors were co-stimulated with different concentrations of their ligands, wherein SOCS1 was expressed at higher levels when DC-SIGNR1 was the dominant trigger. More importantly, triggering the two receptors in the context of M. tb infection, to simulate a physiological scenario, resulted in a positive synergy between DC-SIGNR1 and M. tb for SOCS1 expression. In contrast SOCS1 expression was lowered when TLR2 was triggered along with M. tb infection. The relative levels of IL-12, a key cytokine that polarizes DCs to induce Th1 responses also negatively correlated with SOCS1 expression. This was confirmed when knockdown of SOCS1 using siRNA resulted in enhanced IL-12 transcription and translation following DC-SIGNR1 stimulation. This clearly indicated that DC-SIGNR1 induces suppressor responses during M. tb infection via enhanced expression of SOCS1.
Importantly, SOCS1 was observed to associate with DC-SIGNR1, whereas its association with TLR2 was minimal. This indicated that it is not only the relative expression of SOCS1 by the two triggers that regulates IL-12 production; it is also the subcellular localization that plays an important role. In effect it also indicated that a major blockade in signaling of DC-SIGNR1 toward IL-12 expression was mediated by its association with SOCS1. Although the inhibitory effects of DC-SIGNR1 during M. tb infection have been well known, the one or more precise mechanisms by which these effects are mediated are not fully understood. We show that one of the mechanisms by which these effects could be modulated is by regulation of SOCS1 expression that subsequently leads to regulation of IL-12 production, a crucial cytokine for mounting protective pro-inflammatory responses from DCs. Several mechanisms have been proposed for the suppression of cytokine production by SOCS1 (43). A direct effect of SOCS1 on the TLR-NF-κB pathway has been proposed. SOCS1 binds to the p65 subunit of NF-κB and facilitates ubiquitylation and degradation of p65 (44). SOCS1 also binds to tyrosine phosphorylated MAL (myeloid differentiation primary-response gene 88 (MyD88)-adaptor-like protein, also known as TIRAP) through its interaction with Bruton's tyrosine kinase and induces ubiquitylation and degradation of MAL, thereby leading to the suppression of MAL-dependent p65 phosphorylation and transactivation of NF-κB (45). In addition to the NF-κB pathway, SOCS1 also regulates the stress-activated MAPKs, Jun N-terminal kinase (JNK), and p38 by binding to apoptosis signal-regulating kinase 1, which is an upstream activator of both the JNK and p38 MAPK cascades (41).
It has been demonstrated that Raf-1 is activated following stimulation of DC-SIGN with manLAM (37). Activated Raf-1 regulates IL-10 expression from TLR4 that further involves the Src tyrosine kinase Syk and the transcription factor NF-κB. Our results on mouse DCs provide an additional pathway wherein Raf-1 and Syk regulate DC-SIGNR1-mediated expression of SOCS1 that in turn regulates IL-12 expression. Importantly, this pathway functions in the absence of TLR signaling indicating that DC-SIGNR1 on its own could suppress pro-inflammatory signals via SOCS1.
We next extended the above results to human cohorts and showed that SOCS1 expression increased with the severity of TB disease such that patients with active TB disease displayed higher SOCS1 levels. More importantly, with the clearance of infection following chemotherapy, SOCS1 levels returned to baseline. The profiles of cytokines and their receptors on PBMCs also correlated well with SOCS1 expression and disease phenotype. This clearly indicated that SOCS1 played a crucial role in modulating immune responses to M. tb infection at different stages. We simulated the human results in a mouse model using TLR2−/− mice that are highly susceptible to M. tb infection and could represent the TB disease phenotype observed in human patients. In concurrence with the human data, DC-SIGNR1 stimulation resulted in higher SOCS1 expression in TLR2−/− DCs from M. tb-infected mice. Significantly, DCs from infected and drug-cured mice did not enhance SOCS1 expression following DC-SIGNR1 stimulation. This clearly indicated that with the severity of disease as represented in patients with active TB disease, the expression of SOCS1 is increased following DC-SIGN stimulation.
The above results add support to the hypothesis proposed by van Kooyk and Geijtenbeek (16), wherein at initial stages of infection when the pathogen load is low, TLR2 triggering induces protective immunity and prevents the development of active TB disease. Following increased bacterial burden and the development of active disease, as a result of HIV infection or other factors (7), soluble manLAM secreted from infected macrophages triggers DC-SIGN to induce suppressor responses that favor the pathogen that includes increased secretion of IL-10 and low production of IL-12. Our results presented in this study indicate that increased expression of SOCS1 could be one of the suppressor molecules that contribute toward induction of these responses via regulation of IL-12 and IL-12 receptor levels. Our data on increased IL-12 production following knockdown of SOCS1 by RNA interference, and the resultant killing of M. tb inside DCs as well as PBMCs adds support to the hypothesis and establishes the negative role of SOCS1 during M. tb infection and disease.
Put together, the results presented in the study have identified a novel mechanism by which the contrasting effects of TLR2 and DC-SIGNR1 on DCs during M. tb infection are regulated. This includes regulation of SOCS1 expression and its subcellular localization. The subsequent regulation of cytokine and cytokine receptor expression during various stages of M. tb infection and disease as a result of high expression of SOCS1 further points to a negative role played by this molecule during M. tb infection.
Supplementary Material
Acknowledgment
We thank Dr. Ruslan Medzhitov at Yale University School of Medicine for the kind gift of TLR2 and TLR4 knockout mice.
This work was supported by grants from the Dept. of Biotechnology, Ministry of Science and Technology, Government of India (to K. N.).

The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. 1–3.
- M. tb
- Mycobacterium tuberculosis
- DC-SIGN
- DC-specific ICAM-3 grabbing non-integrin
- DC-SIGNR1
- DC-SIGN-related molecule 1
- SOCS
- suppressors of cytokine signaling
- manLAM
- mannosylated lipoarabinomannan
- PBMC
- peripheral blood mononuclear cell
- MAL
- myeloid differentiation primary-response gene 88 (MyD88)-adaptor-like protein, also known as TIRAP
- TLR
- Toll-like receptor
- DC
- dendritic cell
- HIV
- human immunodeficiency virus
- IL-12
- interleukin-12
- siRNA
- small interference RNA
- TB
- tuberculosis
- IFN
- interferon
- GM-CSF
- granulocyte macrophage-colony stimulating factor
- R
- receptor
- Th1, -2
- type 1 and 2
- MAPK
- mitogen-activated protein kinase
- JNK
- c-Jun NH2-terminal kinase.
REFERENCES
- 1.World Health Organization (2005) World Health Organization Fact Sheet, Geneva, Switzerland [Google Scholar]
- 2.Kaufmann S. H. (2000) Nat. Med. 6, 955–960 [DOI] [PubMed] [Google Scholar]
- 3.Fine P. E. (1995) Lancet 346, 1339–1345 [DOI] [PubMed] [Google Scholar]
- 4.Hess J., Schaible U., Raupach B., Kaufmann S. H. (2000) Adv. Immunol. 75, 1–88 [DOI] [PubMed] [Google Scholar]
- 5.Manabe Y. C., Bishai W. R. (2000) Nat. Med. 6, 1327–1329 [DOI] [PubMed] [Google Scholar]
- 6.Foote S. (1999) Nat. Genet. 21, 345–346 [DOI] [PubMed] [Google Scholar]
- 7.Flynn J. L., Chan J. (2001) Annu. Rev. Immunol. 19, 93–129 [DOI] [PubMed] [Google Scholar]
- 8.Alexander W. S. (2002) Nat. Rev. Immunol. 2, 410–416 [DOI] [PubMed] [Google Scholar]
- 9.Jiao X., Lo-Man R., Guermonprez P., Fiette L., Dériaud E., Burgaud S., Gicquel B., Winter N., Leclerc C. (2002) J. Immunol. 168, 1294–1301 [DOI] [PubMed] [Google Scholar]
- 10.Tian T., Woodworth J., Sköld M., Behar S. M. (2005) J. Immunol. 175, 3268–3272 [DOI] [PubMed] [Google Scholar]
- 11.Tsuji S., Matsumoto M., Takeuchi O., Akira S., Azuma I., Hayashi A., Toyoshima K., Seya T. (2000) Infect. Immun. 68, 6883–6890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schlesinger L. S. (1993) J. Immunol. 150, 2920–2930 [PubMed] [Google Scholar]
- 13.Prigozy T. I., Sieling P. A., Clemens D., Stewart P. L., Behar S. M., Porcelli S. A., Brenner M. B., Modlin R. L., Kronenberg M. (1997) Immunity 6, 187–197 [DOI] [PubMed] [Google Scholar]
- 14.Nigou J., Zelle-Rieser C., Gilleron M., Thurnher M., Puzo G. (2001) J. Immunol. 166, 7477–7485 [DOI] [PubMed] [Google Scholar]
- 15.Tailleux L., Schwartz O., Herrmann J. L., Pivert E., Jackson M., Amara A., Legres L., Dreher D., Nicod L. P., Gluckman J. C., Lagrange P. H., Gicquel B., Neyrolles O. (2003) J. Exp. Med. 197, 121–127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.van Kooyk Y., Geijtenbeek T. B. (2003) Nat. Rev. Immunol. 3, 697–709 [DOI] [PubMed] [Google Scholar]
- 17.Park C. G., Takahara K., Umemoto E., Yashima Y., Matsubara K., Matsuda Y., Clausen B. E., Inaba K., Steinman R. M. (2001) Int. Immunol. 13, 1283–1290 [DOI] [PubMed] [Google Scholar]
- 18.Caminschi I., Corbett A. J., Zahara C., Lahoud M., Lucas K. M., Sofi M., Vremec D., Gramberg T., Pöhlmann S., Curtis J., Handman E., van Dommelen S. L., Fleming P., Degi-Esposti M. A., Shortman K., Wright M. (2006) Int. Immunol. 18, 741–753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Powlesland A. S., Ward E. M., Sadhu S. K., Guo Y., Taylor M. E., Drickamer K. (2006) J. Biol. Chem. 281, 20440–20449 [DOI] [PubMed] [Google Scholar]
- 20.Koppel E. A., Ludwig I. S., Hernandez M. S., Lowary T. L., Gadikota R. R., Tuzikov A. B., Vandenbroucke-Grauls C. M., van Kooyk Y., Appelmelk B. J., Geijtenbeek T. B. (2004) Immunobiology 209, 117–127 [DOI] [PubMed] [Google Scholar]
- 21.Wieland C. W., Koppel E. A., den Dunnen J., Florquin S., McKenzie A. N., van Kooyk Y., van der Poll T., Geijtenbeek T. B. (2007) Microbes Infect. 9, 134–141 [DOI] [PubMed] [Google Scholar]
- 22.Noss E. H., Pai R. K., Sellati T. J., Radolf J. D., Belisle J., Golenbock D. T., Boom W. H., Harding C. V. (2001) J. Immunol. 167, 910–918 [DOI] [PubMed] [Google Scholar]
- 23.Hemmi H., Takeuchi O., Kawai T., Kaisho T., Sato S., Sanjo H., Matsumoto M., Hoshino K., Wagner H., Takeda K., Akira S. (2000) Nature 408, 740–745 [DOI] [PubMed] [Google Scholar]
- 24.Kaufmann S. H., Schaible U. E. (2003) J. Exp. Med. 197, 1–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dik W. A., Pike-Overzet K., Weerkamp F., de Ridder D., de Haas E. F., Baert M. R., van der Spek P., Koster E. E., Reinders M. J., van Dongen J. J., Langerak A. W., Staal F. J. (2005) J. Exp. Med. 201, 1715–1723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.von Meyenn F., Schaefer M., Weighardt H., Bauer S., Kirschning C. J., Wagner H., Sparwasser T. (2006) Immunobiology 211, 557–565 [DOI] [PubMed] [Google Scholar]
- 27.Latchumanan V. K., Singh B., Sharma P., Natarajan K. (2002) J. Immunol. 169, 6856–6864 [DOI] [PubMed] [Google Scholar]
- 28.Sinha A., Singh A., Satchidanandam V., Natarajan K. (2006) J. Immunol. 177, 468–478 [DOI] [PubMed] [Google Scholar]
- 29.Salam N., Gupta S., Sharma S., Pahujani S., Sinha A., Saxena R. K., Natarajan K. (2008) PLoS ONE 3, e2869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gupta S., Salam N., Srivastava V., Singla R., Behera D., Khayyam K. U., Korde R., Malhotra P., Saxena R., Natarajan K. (2009) PLoS ONE 4, e5305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nikonenko B. V., Samala R., Einck L., Nacy C. A. (2004) Antimicrob. Agents Chemother. 48, 4550–4555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Smith A. L., Ganesh L., Leung K., Jongstra-Bilen J., Jongstra J., Nabel G. J. (2007) J. Exp. Med. 204, 421–430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Geijtenbeek T. B., Groot P. C., Nolte M. A., van Vliet S. J., Gangaram-Panday S. T., van Duijnhoven G. C., Kraal G., van Oosterhout A. J., van Kooyk Y. (2002) Blood 100, 2908–2916 [DOI] [PubMed] [Google Scholar]
- 34.Gramberg T., Caminschi I., Wegele A., Hofmann H., Pöhlmann S. (2006) Virology 345, 482–491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.de Jong M. A., de Witte L., Oudhoff M. J., Gringhuis S. I., Gallay P., Geijtenbeek T. B. (2008) J. Clin. Invest. 118, 3440–3452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ma X., Neurath M., Gri G., Trinchieri G. (1997) J. Biol. Chem. 272, 10389–10395 [DOI] [PubMed] [Google Scholar]
- 37.Gringhuis S. I., den Dunnen J., Litjens M., van Het Hof B., van Kooyk Y., Geijtenbeek T. B. (2007) Immunity 26, 605–616 [DOI] [PubMed] [Google Scholar]
- 38.Singhal A., Jaiswal A., Arora V. K., Prasad H. K. (2007) Infect. Immun. 75, 2500–2510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Drennan M. B., Nicolle D., Quesniaux V. J., Jacobs M., Allie N., Mpagi J., Frémond C., Wagner H., Kirschning C., Ryffel B. (2004) Am. J. Pathol. 164, 49–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fremond C. M., Yeremeev V., Nicolle D. M., Jacobs M., Quesniaux V. F., Ryffel B. (2004) J. Clin. Invest. 114, 1790–1799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.He Y., Zhang W., Zhang R., Zhang H., Min W. (2006) J. Biol. Chem. 281, 5559–5566 [DOI] [PubMed] [Google Scholar]
- 42.Latchumanan V. K., Balkhi M. Y., Sinha A., Singh B., Sharma P., Natarajan K. (2005) Tuberculosis 85, 377–383 [DOI] [PubMed] [Google Scholar]
- 43.Yoshimura A., Naka T., Kubo M. (2007) Nat. Rev. Immunol. 7, 454–465 [DOI] [PubMed] [Google Scholar]
- 44.Ryo A., Suizu F., Yoshida Y., Perrem K., Liou Y. C., Wulf G., Rottapel R., Yamaoka S., Lu K. P. (2003) Mol. Cell 12, 1413–1426 [DOI] [PubMed] [Google Scholar]
- 45.Mansell A., Smith R., Doyle S. L., Gray P., Fenner J. E., Crack P. J., Nicholson S. E., Hilton D. J., O'Neill L. A., Hertzog P. J. (2006) Nat. Immunol. 7, 148–155 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.