Abstract
Macrophages participate pivotally in the pathogenesis of many chronic inflammatory diseases including atherosclerosis. Adiponectin, a vasculoprotective molecule with insulin-sensitizing and anti-atherogenic properties, suppresses pro-inflammatory gene expression in macrophages by mechanisms that remain incompletely understood. This study investigated the effects of adiponectin on major pro-inflammatory signaling pathways in human macrophages. We demonstrate that pretreatment of these cells with adiponectin inhibits phosphorylation of nuclear factor κB inhibitor (IκB), c-Jun N-terminal kinase (JNK), and p38 mitogen-activated protein kinase (MAPK), induced by either lipopolysaccharide (LPS) or tumor necrosis factor (TNF) α, as well as STAT3 phosphorylation induced by interleukin-6 (IL6). Antagonism of IL10 by either neutralizing antibodies or siRNA-mediated silencing did not abrogate the anti-inflammatory actions of adiponectin, indicating that the ability of adiponectin to render human macrophages tolerant to various pro-inflammatory stimuli does not require this cytokine. A systematic search for adiponectin-inducible genes with established anti-inflammatory properties revealed that adiponectin augmented the expression of A20, suppressor of cytokine signaling (SOCS) 3, B-cell CLL/lymphoma (BCL) 3, TNF receptor-associated factor (TRAF) 1, and TNFAIP3-interacting protein (TNIP) 3. These results suggest that adiponectin triggers a multifaceted response in human macrophages by inducing the expression of various anti-inflammatory proteins that act at different levels in concert to suppress macrophage activation.
Adipose tissue, long considered a lipid storage depot, has now gained recognition as an endocrine organ that produces various bioactive molecules with local and systemic functions, collectively known as adipokines (1, 2). Among them, adiponectin has emerged as a key vasculoprotective molecule with insulin-sensitizing, anti-inflammatory, and anti-atherogenic properties (3–5). Numerous (but not all) clinical studies have correlated hypoadiponectinemia with incidence of coronary artery disease, insulin resistance, type 2 diabetes, and hypertension. Experimental studies have demonstrated anti-inflammatory and anti-atherogenic properties of adiponectin by showing that its in vivo overexpression reversed abnormal neointimal thickening in adiponectin-deficient mice, alleviated atherosclerotic lesions in apolipoprotein E-deficient mice, and improved endothelial vasodilator dysfunction and hypertension in obese mice. Cell-based studies demonstrated various potentially anti-atherogenic functions of adiponectin in the major cell types found in atheroma: endothelial cells, smooth muscle cells, and macrophages (3–5).
Adiponectin circulates in the plasma at concentrations of 3–30 μg/ml, forming three major oligomeric complexes with distinct biological functions: trimer, hexamer, and high molecular mass form (3–5). A bioactive proteolytic product that includes the adiponectin C1q-like globular domain also exists in plasma, albeit at very low concentrations (6), and in cell culture medium conditioned by THP-1 or U937 cells stimulated with phorbol esters (7).
Macrophages contribute critically to the pathogenesis of many chronic inflammatory processes including atherogenesis, and thus comprise key targets for the anti-inflammatory action of adiponectin. Adiponectin inhibits lipopolysaccharide (LPS)2-induced pro-inflammatory gene expression in pig and human macrophages, rat Kupffer cells, and RAW264.7 cells by mechanisms that remain incompletely understood but that involve suppression of LPS-induced nuclear factor κB (NFκB) activation (8–11). Adiponectin induces expression of interleukin-10 (IL10), an immunomodulatory cytokine with potent anti-inflammatory activity, in leukocytes (12, 13). Park et al. (14) recently showed that IL10 generated after treating RAW 264.7 cells with globular adiponectin figures essentially in rendering macrophages tolerant to LPS.
We have recently reported that full-length adiponectin inhibits expression of T-lymphocyte-active CXC chemokine receptor 3 (CXCR3) chemokine ligands in human macrophages stimulated by LPS, a process that involves inhibition of interferon (IFN) regulatory factor 3 (IRF3) activation (15). The present study investigated in detail the effects of adiponectin on signaling pathways elicited by the potent pro-inflammatory stimulants LPS, TNFα, and IL6 in human macrophages, and addressed in particular the role of IL10 as a potential mediator of adiponectin function. Our results indicate that adiponectin-induced anti-inflammation in primary human macrophages occurs primarily independently of IL10 and likely involves the concerted action of a group of adiponectin-induced anti-inflammatory molecules that include A20, suppressor of cytokine signaling (SOCS) 3, B-cell CLL/lymphoma (BCL) 3, and TNF receptor-associated factor (TRAF) 1.
EXPERIMENTAL PROCEDURES
Reagents
Human recombinant adiponectin expressed in HEK293 cells was purchased from BioVendor. It contained less than 40 pg endotoxin/ml as determined by the chromogenic Limulus amebocyte assay (Cape Cod, Falmouth, MA). TNFα, IL6, and IL10 were from R&D. LPS from Escherichia coli serotype 055: B6 was from Calbiochem. Polymyxin B was from Sigma. Antibodies to phospho-NFκB inhibitor (IκΒ) α (Ser32), c-Jun N-terminal kinase (JNK), phospho-JNK (Thr183/Tyr185), p38 mitogen-activated protein kinase (MAPK), phospho-p38 MAPK (Thr180/Tyr182), β-actin, signal transducer, and activation of transcription (STAT) 3, and phospho-STAT3 (Tyr705) were from Cell Signaling Technology; anti-IL10, anti-TLR4, IgG isotype controls, and anti-A20 antibodies were from eBioscience; anti-BCL3 and anti-TRAF1 antibodies were from Santa Cruz Biotechnology; and anti-SOCS3 antibody was from Immuno-Biological Laboratories.
Isolation and Treatment of Human Macrophages
Human monocytes were isolated from freshly prepared leukocyte concentrates and differentiated to macrophages as described (16). To test the suppressive effects of adiponectin, macrophages were incubated in RPMI 1640 containing 1% human serum with or without various concentrations of adiponectin for 18 h, followed by the addition of 5 ng/ml LPS, 50 ng/ml TNFα, or 5 ng/ml IL6 for the indicated time. To test the effects of adiponectin on gene expression, macrophages were incubated in RPMI 1640 containing 1% human serum with or without 10 μg/ml adiponectin for the indicated time.
RNA Isolation and Reverse Transcription-Quantitative PCR (RT-qPCR)
Total RNA was isolated using an RNeasy kit (Qiagen) and reverse-transcribed by Superscript II (Invitrogen). Quantitative PCR was performed in a MyiQ Single-Color Real-Time PCR system using SYBR Green I (Bio-Rad). The mRNA levels of the various genes tested were normalized to 18S as an internal control. The primer sequences were: TNFα, 5′-ATCTACTCCCAGGTCCTCTTCAA-3′ and 5′-GCAATGATCCCAAAGTAGACCT-3′; IL6, 5′-AGTGAGGAACAAGCCAGAGCTGTCCAGATG-3′ and 5′-AATCTGAGGTGCCCATGCTACATTTGCCGA-3′; 18S, 5′-ATGGCCGTTCTTAGTTGGTG-3′ and 5′-GAACGCCACTTGTCCCTCTA-3′; IFN-inducible protein 10 (IP10), 5′-GAGCCTCAGCAGAGGAACC-3′ and 5′-GAGTCAGAAAGATAAGGCAGC-3′; IL10, 5′-GATGCCTTCAGCAGAGTGAA-3′ and 5′-GCAACCCAGGTAACCCTTAAA-3′; SOCS3, 5′-TGCGCCTCAAGACCTTCAG-3′ and 5′-GAGCTGTCGCGGATCAGAAA-3′; BCL3, 5′-CACCGAGTGCCAAGAAACC-3′ and 5′-CACCATGCTAAGGCTGTTGTT-3′; A20, 5′-AAGCTGTGAAGATACGGGAGA-3′ and 5′-CGATGAGGGCTTTGTGGATGAT-3′; TRAF1, 5′-AAGGGAGCTAGCCAGAGGAC-3′ and 5′-GTCCTGCCATCCTAACCAGA-3′; TNFAIP3-interacting protein 3 (TNIP3), 5′-AAACTTCCCAATCCCAGTTGAAC-3′ and 5′-GGGTGGGCAATACATCTGTTTT-3′.
cDNA Microarray Screening
Macrophages from four independent donors were treated with or without 10 μg/ml adiponectin for 24 h as described above. 10 μg of total RNA were tested for quality on agarose gels and subjected to microarray screening on Affymetrix HG U133 Plus 2.0 chips. Criteria for differential regulation were set as >2-fold increase or decrease at a probability value of <0.05.
Immunoblots
Whole cell lysates from 25,000 cells were fractionated on 4–12% gradient SDS-PAGE gels (Invitrogen) and transferred to polyvinylidene difluoride membranes. After blocking with 5% defatted milk and incubating with the appropriate antibodies, membranes were developed using a chemiluminescence reagent (PerkinElmer Life Sciences).
RNA Interference
Macrophages were transfected with a pool of IL10-specific small interfering RNA (siRNAs) duplexes (Dharmacon) at 50 nm using Lipofectamine 2000 (Invitrogen) following instructions provided by the manufacturer. A pool of non-targeting siRNA duplexes was used as a control. Experiments were conducted 48 h after transfection.
RESULTS
Adiponectin Suppresses LPS-induced Pro-inflammatory Cytokine Expression in Human Macrophages
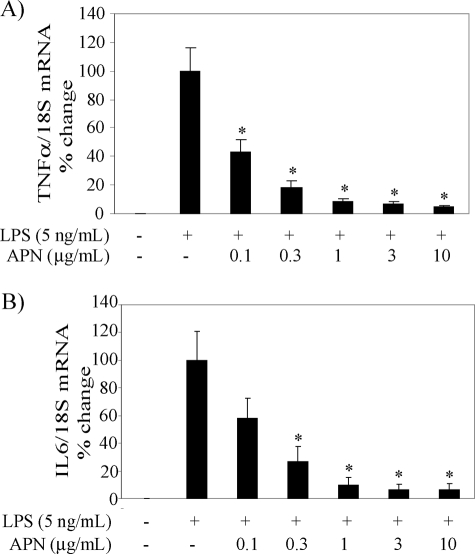
Engagement of Toll-like receptor (TLR) 4 with LPS triggers the activation of two signaling pathways: a myeloid differentiation primary response protein 88 (MyD88)-dependent pathway, which leads to the production of TNFα, IL6, and other pro-inflammatory cytokines, and a MyD88-independent pathway that elicits the expression of IFNβ and IFN-inducible genes (17, 18). We have reported recently that adiponectin suppresses the LPS-induced MyD88-independent expression of T-cell chemokines (IP10, monokine induced by IFNγ (Mig), and IFN-inducible T-cell α chemoattractant (I-TAC)) in a concentration-dependent manner in human macrophages (15). As expected, exposure of macrophages to adiponectin for 18 h also attenuated LPS-induced TNFα and IL6 mRNA expression with similar concentration dependence (Fig. 1).
FIGURE 1.
Adiponectin attenuates TNFα and IL6 expression in human macrophages stimulated with LPS. Cells were treated with the indicated concentrations of adiponectin (APN) for 18 h and subsequently stimulated with 5 ng/ml LPS for 6 h. RT-qPCR measured mRNA levels for TNFα and IL6 as described under “Experimental Procedures.” Data are expressed relative to the values of treatment with LPS alone (100%) in means ± S.E. (n = 3). mRNA levels of 18S served as internal control for adjustment between samples. *, p < 0.05 versus LPS.
Adiponectin Abrogates Signaling Pathways Elicited by LPS, TNFα, and IL6 in Human Macrophages
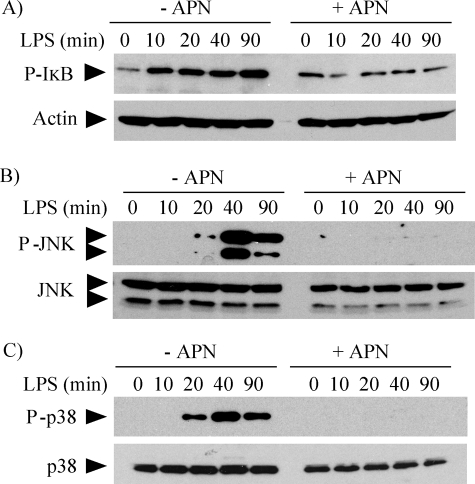
In LPS-stimulated macrophages, the MyD88-dependent expression of pro-inflammatory cytokines requires the phosphorylation and degradation of IκB, which leads to NFκB activation, as well as the phosphorylation and activation of MAPKs (17, 18). Pretreatment of macrophages with 10 μg/ml adiponectin limited LPS-induced IκB, JNK, and p38 phosphorylation (Fig. 2, A, B, and C, respectively).
FIGURE 2.
Adiponectin limits phosphorylation of IκB, JNK, and p38 in human macrophages stimulated with LPS. Cells were incubated without (left lanes) or with (right lanes) 10 μg/ml adiponectin for 18 h and subsequently stimulated with 5 ng/ml LPS for the indicated periods of time. Whole-cell lysates were fractionated by SDS-PAGE and immunoblotted with antibodies to phospho-IκB (A), phospho-JNK (B), or phospho-p38 (C). Actin, total JNK, and total p38 served as loading controls, respectively. Images are representative of three independent experiments on cells from different donors.
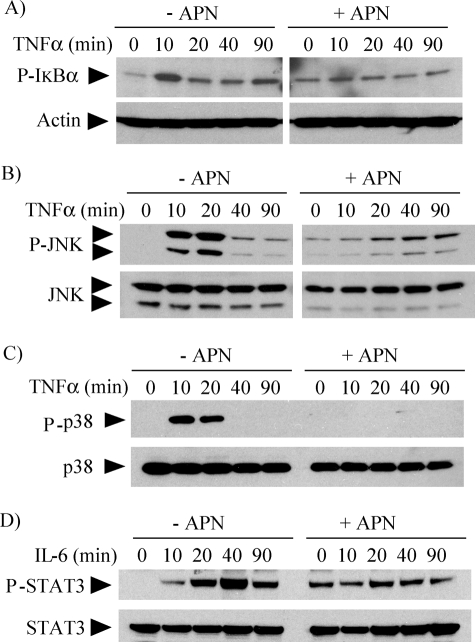
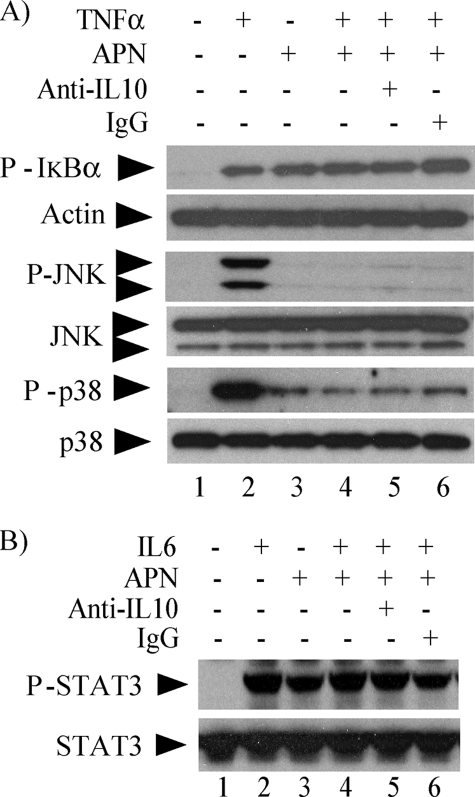
We also examined the action of adiponectin on signaling pathways elicited by TNFα and IL6. TNFα, a potent cytokine that participates in inflammation and immunity, signals through pathways that also involve NFκB and MAPKs (19), whereas IL6, a key regulator of the acute phase response, controls gene transcription through phosphorylation and nuclear translocation of STAT3 (20). Pretreatment of macrophages with 10 μg/ml adiponectin inhibited TNFα-induced IκB (Fig. 3A), JNK (Fig. 3B), and p38 (Fig. 3C) phosphorylation, as well as IL6-induced STAT3 phosphorylation (Fig. 3D). Taken together, these results indicate that adiponectin attenuates multiple pro-inflammatory pathways in human macrophages.
FIGURE 3.
Adiponectin inhibits TNFα- and IL6-induced signaling in human macrophages. Cells were incubated without (left lanes) or with (right lanes) 10 μg/ml adiponectin for 18 h and subsequently stimulated with 50 ng/ml TNFα (A–C) or 5 ng/ml IL6 (D) for the indicated periods of time. Whole-cell lysates were fractionated by SDS-PAGE and immunoblotted with antibodies to phospho-IκB (A), phospho-JNK (B), phospho-p38 (C), or phospho-STAT3 (D). Actin, total JNK, total p38, and total STAT3 served as loading controls, respectively.
The experiments described in Figs. 2, 3, 4, and 5 used primary human macrophages, which characteristically show donor-to-donor variability both in the basal level of phosphorylation of signaling molecules and in the relative intensity of the responses to pro-inflammatory stimuli, but not in phosphorylation kinetics. Pretreatment of cells with adiponectin alone induced sustained levels of phosphorylation of IκB (Figs. 2A, 3A, 4D, and 5A), JNK (Figs. 3B and 5A), p38 (Figs. 4D and 5A), and STAT3 (Figs. 3D and 5B) that also showed donor-to-donor variability, but consistently prevented further phosphorylation beyond the levels acquired during pretreatment.
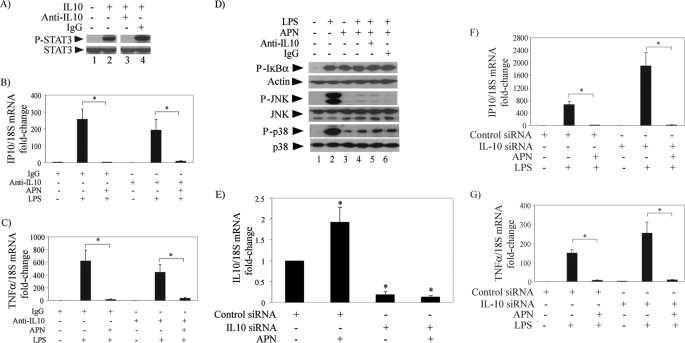
FIGURE 4.
IL10 antagonism does not impair the anti-inflammatory actions of adiponectin in human macrophages stimulated with LPS. A, cells were incubated with no addition (lanes 1 and 2), 10 μg/ml anti-IL10 antibody (lane 3), or control IgG (lane 4) for 18 h and subsequently stimulated with (lanes 2–4) or without (lane 1) 10 ng/ml IL10 for 30 min. Whole-cell lysates were fractionated by SDS-PAGE and immunoblotted with antibodies to phospho-STAT3. Total STAT3 served as loading control. B and C, cells were treated with or without 10 μg/ml adiponectin for 18 h in the presence of anti-IL10 antibody or control IgG as indicated and subsequently stimulated with 5 ng/ml LPS for 6 h. RT-qPCR measured the levels of IP10 (B) and TNFα (C) mRNAs, using 18S as internal control for adjustment between samples. Data are expressed in means ± S.E. (n = 3) relative to the values of samples from cells without adiponectin or LPS treatment. *, p < 0.05. D, cells were incubated with (lanes 3–6) or without (lanes 1 and 2) 10 μg/ml adiponectin for 18 h in the presence of anti-IL10 antibody or control IgG as indicated and subsequently stimulated with 5 ng/ml LPS for 40 min. Whole-cell lysates were fractionated by SDS-PAGE and immunoblotted with antibodies to phospho-IκB (top), phospho-JNK (center), or phospho-p38 (bottom). Actin, total JNK, and total p38 served as loading controls, respectively. E, cells were transfected with IL10-specific or control siRNA as indicated. At 48 h after transfection, cells were incubated with or without 10 μg/ml adiponectin for 6 h. RT-qPCR measured IL10 mRNA levels using 18S as internal control for adjustment between samples. Data are expressed in means ± S.E. (n = 3) relative to the values of samples from cells transfected with control siRNA and left unstimulated. *, p < 0.05 versus control. F and G, cells were transfected with IL10-specific or control siRNA as indicated. At 48 h after transfection, cells were incubated with or without 10 μg/ml adiponectin for 18 h and subsequently stimulated with 5 ng/ml LPS for 6 h. RT-qPCR measured IP10 (F) and TNFα (G) mRNA levels using 18S as internal control for adjustment between samples. Data are expressed in means ± S.E. (n = 3) relative to the values of samples from cells transfected with control siRNA and left unstimulated.*, p < 0.05.
FIGURE 5.
IL10 neutralization does not impair the actions of adiponectin in human macrophages stimulated with TNFα or IL6. A, cells were incubated with (lanes 3–6) or without (lanes 1 and 2) 10 μg/ml adiponectin for 18 h in the presence of anti-IL10 antibody (lane 5) or control IgG (lane 6) and subsequently stimulated with 50 ng/ml TNFα or left unstimulated for 10 min. Whole-cell lysates were fractionated by SDS-PAGE and immunoblotted with antibodies to phospho-IκB (top), phospho-JNK (middle), or phospho-p38 (bottom). Actin, total JNK, and total p38 served as loading controls, respectively. B, cells were incubated with (lanes 3–6) or without (lanes 1 and 2) 10 μg/ml adiponectin for 18 h in the presence of anti-IL10 antibody (lane 5) or control IgG (lane 6) and subsequently stimulated with 5 ng/ml IL6 or left unstimulated for 40 min. Whole-cell lysates were fractionated by SDS-PAGE and immunoblotted with antibodies to phospho-STAT3. Total STAT3 served as loading control.
IL10 Is Not an Essential Mediator of the Anti-Inflammatory Effects Elicited by Adiponectin in Human Macrophages
Recently, Park et al. (14) utilized a neutralizing antibody to IL10 to conclude that this cytokine mediates the desensitization of RAW264.7 cells to LPS elicited by globular adiponectin. As reported previously (12, 13), full-length adiponectin also increased IL10 expression in human macrophages: cell treatment with adiponectin for 24 h elicited the accumulation of 471 ± 190 pg/ml (n = 3) of IL10 protein in the culture media, assessed by an enzyme-linked immunosorbent assay (ELISA). Therefore, we examined the effect of IL10 neutralization on LPS-induced pro-inflammatory cytokine expression and signaling pathways. In our experimental conditions, the anti-IL10 antibody used completely neutralized the action of incubation with 10 ng/ml IL10, assessed by its specific inhibition of IL10-induced STAT3 phosphorylation (Fig. 4A). However, it did not prevent the inhibition of LPS-induced IP10 and TNFα mRNA expression in cells pretreated with adiponectin (Fig. 4, B and C, respectively). Consistently, IL10 neutralization (Fig. 4D, lane 5) did not alter the effect of adiponectin in limiting LPS-induced phosphorylation of IκB, JNK, and p38 to the levels acquired during pretreatment (Fig. 4D, lanes 3 and 4).
We extended the above results by siRNA-mediated IL10 silencing. Transfection of IL10-specific siRNA reduced the expression of IL10 mRNA by 80–90% both in untreated and adiponectin-treated macrophages (Fig. 4E) but did not prevent the inhibition of LPS-induced IP10 or TNFα mRNA expression in cells pretreated with adiponectin (Fig. 4, F and G, respectively).
We also used the IL10-neutralizing antibody to investigate the role of this cytokine in adiponectin-induced inhibition of TNFα and IL6 signaling. As shown above for LPS-induced signaling, IL10 neutralization did not reverse the limitation of TNFα-induced phosphorylation of IκB, JNK, or p38 (Fig. 5A) or of IL6-induced phosphorylation of STAT3 (Fig. 5B) by pretreatment of cells with adiponectin. Collectively, these results indicate that the mechanisms by which adiponectin renders human macrophages tolerant to various pro-inflammatory stimuli do not require IL10.
Adiponectin Induces Expression of Various Anti-inflammatory Genes in Human Macrophages
To probe further anti-inflammatory pathways elicited by adiponectin, we examined the effects of this adipokine on gene expression by transcriptional profiling of macrophages treated with 10 μg/ml adiponectin for 24 h. Compared with controls, adiponectin significantly augmented the expression of 174 genes, including the pro-inflammatory cytokines TNFα and IL6 as previously described for globular adiponectin (14, 21), and attenuated the expression of 19 transcripts by a factor of >10. Importantly, a systematic search for genes with well-established anti-inflammatory properties revealed that adiponectin significantly augmented the expression of A20, SOCS3, BCL3, TRAF1, and TNIP 3.
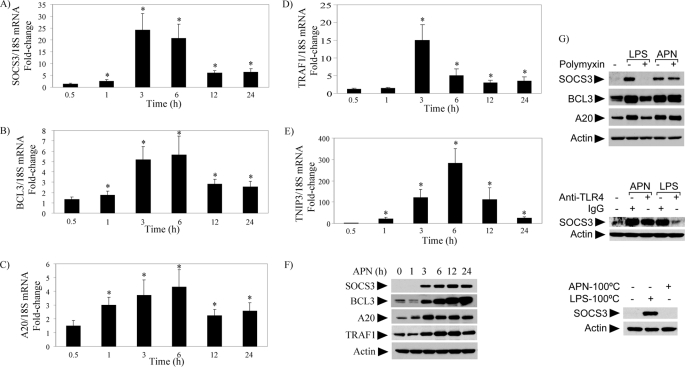
To validate these cDNA microarray data, RT-qPCR tested the time-dependent induction of these mRNAs in adiponectin-stimulated macrophages, showing that they reach maximal levels of expression at 3–6 h after the addition of adiponectin (Fig. 6, A–E). Immunoblot experiments detected the induction and sustained expression of A20, SOCS3, BCL3, and TRAF1 at the protein level (Fig. 6F). Pretreatment of macrophages with polymyxin B (Fig. 6G, top) under conditions that block the action of LPS did not inhibit the expression of these proteins. Consistently, cell pretreatment with neutralizing antibodies to TLR4 attenuated LPS-induced but not adiponectin-induced SOCS3 expression (Fig. 6G, middle). Additionally, a 20-min preincubation at 100 °C abrogated adiponectin-induced but not LPS-induced SOCS3 expression (Fig. 6G, bottom), corroborating that these effects did not stem from endotoxin contamination. Collectively, these results suggest that adiponectin exerts a multifactorial anti-inflammatory action in human macrophages.
FIGURE 6.
Adiponectin induces expression of various anti-inflammatory molecules in human macrophages. Human macrophages were incubated with 10 μg/ml adiponectin for the indicated periods of time. In A–E, RT-qPCR measured the levels of the indicated mRNAs, using 18S as internal control for adjustment between samples. Data are expressed in means ± S.E. (n = 3) relative to the values of samples from vehicle-treated cells at the respective time points. *, p < 0.05. In F, whole-cell lysates were fractionated by SDS-PAGE and immunoblotted with the indicated antibodies, using actin as loading control. In G, cells were pretreated with 1 μg/ml polymyxin (top) or 25 μg/ml of either anti-TLR4 antibodies or control IgG (middle), followed by stimulation with 5 ng/ml LPS or 10 μg/ml adiponectin for 3 h. In G (bottom), adiponectin or LPS were subjected to a 20-min pretreatment at 100 °C before addition to the cells and incubation for 3 h. Whole-cell lysates were fractionated by SDS-PAGE and immunoblotted with the indicated antibodies, using actin as loading control.
DISCUSSION
The current study extends our previous work (15) exploring the mechanisms by which adiponectin limits pro-inflammatory activation in human macrophages. Our results revealed that exposure of these cells to concentrations of adiponectin compatible with physiological plasma levels in humans inhibited LPS-induced cytokine production, as well as the main signaling pathways induced by LPS and the potent pro-inflammatory cytokines TNFα and IL6. Treatment of macrophages with adiponectin alone induced sustained levels of phosphorylation of IκB, JNK, p38, and STAT3, but prevented further activation of these signaling molecules upon addition of pro-inflammatory agonists (Figs. 2–4). These findings provide a mechanistic basis for previous observations suggesting that adiponectin may induce some degree of inflammatory activation that likely mediates tolerance to further treatment with pro-inflammatory stimuli (14, 21).
Our data show that adiponectin limits the activation of NFκB, JNK, and p38 signaling pathways triggered by TNFα in human macrophages (Fig. 3). In contrast, in human endothelial cells adiponectin attenuates TNFα-induced NFκB activation without interfering with JNK or p38 signaling (22, 23), highlighting the cell-specific nature of adiponectin action. Adiponectin suppresses LPS- and TNFα-induced activation of JNK, a critical mediator of insulin resistance in obesity and diabetes, conditions that correlate with hypoadiponectinemia (1, 24). In obesity, the accumulation of free fatty acids, putative TLR4 ligands, and pro-inflammatory mediators such as TNFα disrupts insulin signaling by triggering JNK-mediated serine phosphorylation and inactivation of insulin receptor substrate-1 (1, 25).
Adiponectin induces expression of IL10 in leukocytes (12, 13). Park et al. showed that antibody neutralization of IL10 abrogates the desensitization of RAW 264.7 cells to LPS elicited by globular adiponectin, in support of a model whereby IL10 constitutes an essential mediator of adiponectin anti-inflammatory actions (14). In the current study, a combined approach that used antibody neutralization and siRNA-mediated silencing of IL10 demonstrated the dispensability of this cytokine for the suppressive effects of full-length adiponectin in primary human macrophages. The reasons for the discrepancy between our work and that by Park et al. (14) remain unclear, but the different experimental preparations used in these two studies offers the simplest explanation: primary human macrophages treated with full-length adiponectin expressed in HEK293 cells (this study) versus a continuous mouse cell line treated with globular adiponectin expressed in E. coli (14). The source of recombinant adiponectin may have particular relevance because the bacterially expressed protein lacks post-translational modifications characteristic of adiponectin expressed in eukaryotes, such as lysine glycosylation and hydroxylation, and fails to form high molecular weight multimers (26, 27). The biological relevance of globular adiponectin remains controversial because of its very low levels in circulation. On the other hand, Fruebis et al. (6) proposed that full-length adiponectin circulates in plasma as an inactive precursor that undergoes limited proteolysis to generate the biologically active C-terminal globular fragment. Both full-length and globular adiponectin exert numerous beneficial effects in various animal and cell-based experiments (4, 5), but few studies have shown side-by-side comparisons of the activities of both isoforms. Of note, the overexpression of either full-length or globular adiponectin in apolipoprotein E-deficient mice ameliorates atherosclerosis (28, 29).
Our transcriptional profiling analysis demonstrated that adiponectin induces expression of a host of genes that encode proteins with well-characterized anti-inflammatory functions that likely mediate the desensitization of macrophages to pro-inflammatory stimuli. A20, a cytoplasmic protein with ubiquitin-editing functions, inhibits the NFκB and IRF3 signaling pathways, and thus attenuates TLR- or TNFα-induced signals (30–33). BCL3, a nuclear IκB family member, interacts with NFκB p50 homodimers to attenuate LPS-induced TNFα expression in macrophages (34, 35). TRAF1, an adaptor protein, inhibits NFκB signaling through its association with various TNF receptor family members and cytoplasmic signaling proteins (36, 37). SOCS3, a tyrosine kinase inhibitor, suppresses IL6-induced Janus kinase (JAK)-STAT3 signaling (38–41). TNIP3, a recently identified A20-binding protein, attenuates NFκB activation (42). These adiponectin-induced proteins, and possibly others as yet unidentified, possess diverse biological functions and likely act in a concerted manner to inhibit pro-inflammatory signaling at multiple levels. Indeed, siRNA-mediated reduction of the expression of these proteins, individually or in pairs, did not abrogate the inhibitory effect of adiponectin on LPS-induced pro-inflammatory cytokine expression (results not shown).
Macrophages play a key role in the inflammatory processes that lead to progression and complications of atherosclerosis and many other chronic diseases. Our findings highlight the importance of adiponectin, an anti-atherogenic protein, in controlling macrophage activation in response to pro-inflammatory stimuli. Complete comprehension of the vast repertoire of vasculoprotective actions by adiponectin will require a detailed investigation of adiponectin-induced signaling pathways.
Acknowledgments
We thank Elissa Simon-Morrisey and Gihan Suliman for technical assistance and Joan Edgett for editorial assistance. The authors have no conflicting financial interests to disclose.
This work was supported, in whole or in part, by National Institutes of Health NHLBI Grants R01-HL034636 (to P. L.) and R01-HL0831554 (to M. L. I.). This work was also supported by grants from the Donald W. Reynolds Foundation.
- LPS
- lipopolysaccharide
- NFκB
- nuclear factor κB
- IL10
- interleukin-10
- IFN
- interferon
- TNFα
- tumor necrosis factor α
- IL6
- interleukin-6
- SOCS3
- suppressor of cytokine signaling 3
- BCL3
- B-cell CLL/lymphoma 3
- TRAF1
- TNF receptor-associated factor 1
- IκBα
- NFκB inhibitor α
- JNK
- c-Jun N-terminal kinase
- MAPK
- mitogen-activated protein kinase
- STAT3
- signal transducer and activation of transcription 3
- RT-qPCR
- reverse transcription-quantitative PCR
- siRNA
- small interfering RNA
- TLR
- Toll-like receptor
- MyD88
- myeloid differentiation primary-response protein 88
- IP10
- IFN-inducible protein 10.
REFERENCES
- 1.Rocha V. Z., Libby P. (2009) Nat. Rev. Cardiol. 6, 399–409 [DOI] [PubMed] [Google Scholar]
- 2.Scherer P. E. (2006) Diabetes 55, 1537–1545 [DOI] [PubMed] [Google Scholar]
- 3.Goldstein B. J., Scalia R. G., Ma X. L. (2009) Nat. Clin. Pract. Cardiovasc. Med. 6, 27–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Okamoto Y., Kihara S., Funahashi T., Matsuzawa Y., Libby P. (2006) Clin. Sci. 110, 267–278 [DOI] [PubMed] [Google Scholar]
- 5.Zhu W., Cheng K. K., Vanhoutte P. M., Lam K. S., Xu A. (2008) Clin. Sci. 114, 361–374 [DOI] [PubMed] [Google Scholar]
- 6.Fruebis J., Tsao T. S., Javorschi S., Ebbets-Reed D., Erickson M. R., Yen F. T., Bihain B. E., Lodish H. F. (2001) Proc. Natl. Acad. Sci. U.S.A. 98, 2005–2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Waki H., Yamauchi T., Kamon J., Kita S., Ito Y., Hada Y., Uchida S., Tsuchida A., Takekawa S., Kadowaki T. (2005) Endocrinology 146, 790–796 [DOI] [PubMed] [Google Scholar]
- 8.Thakur V., Pritchard M. T., McMullen M. R., Nagy L. E. (2006) Am. J. Physiol. Gastrointest. Liver Physiol. 290, G998–1007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wulster-Radcliffe M. C., Ajuwon K. M., Wang J., Christian J. A., Spurlock M. E. (2004) Biochem. Biophys. Res. Commun. 316, 924–929 [DOI] [PubMed] [Google Scholar]
- 10.Yamaguchi N., Argueta J. G., Masuhiro Y., Kagishita M., Nonaka K., Saito T., Hanazawa S., Yamashita Y. (2005) FEBS Lett. 579, 6821–6826 [DOI] [PubMed] [Google Scholar]
- 11.Yokota T., Oritani K., Takahashi I., Ishikawa J., Matsuyama A., Ouchi N., Kihara S., Funahashi T., Tenner A. J., Tomiyama Y., Matsuzawa Y. (2000) Blood 96, 1723–1732 [PubMed] [Google Scholar]
- 12.Kumada M., Kihara S., Ouchi N., Kobayashi H., Okamoto Y., Ohashi K., Maeda K., Nagaretani H., Kishida K., Maeda N., Nagasawa A., Funahashi T., Matsuzawa Y. (2004) Circulation 109, 2046–2049 [DOI] [PubMed] [Google Scholar]
- 13.Wolf A. M., Wolf D., Rumpold H., Enrich B., Tilg H. (2004) Biochem. Biophys. Res. Commun. 323, 630–635 [DOI] [PubMed] [Google Scholar]
- 14.Park P. H., McMullen M. R., Huang H., Thakur V., Nagy L. E. (2007) J. Biol. Chem. 282, 21695–21703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Okamoto Y., Folco E. J., Minami M., Wara A. K., Feinberg M. W., Sukhova G. K., Colvin R. A., Kihara S., Funahashi T., Luster A. D., Libby P. (2008) Circ. Res. 102, 218–225 [DOI] [PubMed] [Google Scholar]
- 16.Isoda K., Folco E., Marwali M. R., Ohsuzu F., Libby P. (2008) Atherosclerosis 198, 307–312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Akira S., Takeda K. (2004) Nat. Rev. Immunol. 4, 499–511 [DOI] [PubMed] [Google Scholar]
- 18.Barton G. M., Medzhitov R. (2003) Science 300, 1524–1525 [DOI] [PubMed] [Google Scholar]
- 19.Baud V., Karin M. (2001) Trends Cell Biol. 11, 372–377 [DOI] [PubMed] [Google Scholar]
- 20.Naugler W. E., Karin M. (2008) Trends Mol. Med. 14, 109–119 [DOI] [PubMed] [Google Scholar]
- 21.Tsatsanis C., Zacharioudaki V., Androulidaki A., Dermitzaki E., Charalampopoulos I., Minas V., Gravanis A., Margioris A. N. (2005) Biochem. Biophys. Res. Commun. 335, 1254–1263 [DOI] [PubMed] [Google Scholar]
- 22.Kobashi C., Urakaze M., Kishida M., Kibayashi E., Kobayashi H., Kihara S., Funahashi T., Takata M., Temaru R., Sato A., Yamazaki K., Nakamura N., Kobayashi M. (2005) Circ. Res. 97, 1245–1252 [DOI] [PubMed] [Google Scholar]
- 23.Ouchi N., Kihara S., Arita Y., Okamoto Y., Maeda K., Kuriyama H., Hotta K., Nishida M., Takahashi M., Muraguchi M., Ohmoto Y., Nakamura T., Yamashita S., Funahashi T., Matsuzawa Y. (2000) Circulation 102, 1296–1301 [DOI] [PubMed] [Google Scholar]
- 24.Hirosumi J., Tuncman G., Chang L., Görgün C. Z., Uysal K. T., Maeda K., Karin M., Hotamisligil G. S. (2002) Nature 420, 333–336 [DOI] [PubMed] [Google Scholar]
- 25.Aguirre V., Uchida T., Yenush L., Davis R., White M. F. (2000) J. Biol. Chem. 275, 9047–9054 [DOI] [PubMed] [Google Scholar]
- 26.Richards A. A., Stephens T., Charlton H. K., Jones A., Macdonald G. A., Prins J. B., Whitehead J. P. (2006) Mol. Endocrinol. 20, 1673–1687 [DOI] [PubMed] [Google Scholar]
- 27.Wang Y., Xu A., Knight C., Xu L. Y., Cooper G. J. (2002) J. Biol. Chem. 277, 19521–19529 [DOI] [PubMed] [Google Scholar]
- 28.Okamoto Y., Kihara S., Ouchi N., Nishida M., Arita Y., Kumada M., Ohashi K., Sakai N., Shimomura I., Kobayashi H., Terasaka N., Inaba T., Funahashi T., Matsuzawa Y. (2002) Circulation 106, 2767–2770 [DOI] [PubMed] [Google Scholar]
- 29.Yamauchi T., Kamon J., Waki H., Imai Y., Shimozawa N., Hioki K., Uchida S., Ito Y., Takakuwa K., Matsui J., Takata M., Eto K., Terauchi Y., Komeda K., Tsunoda M., Murakami K., Ohnishi Y., Naitoh T., Yamamura K., Ueyama Y., Froguel P., Kimura S., Nagai R., Kadowaki T. (2003) J. Biol. Chem. 278, 2461–2468 [DOI] [PubMed] [Google Scholar]
- 30.Boone D. L., Turer E. E., Lee E. G., Ahmad R. C., Wheeler M. T., Tsui C., Hurley P., Chien M., Chai S., Hitotsumatsu O., McNally E., Pickart C., Ma A. (2004) Nat. Immunol. 5, 1052–1060 [DOI] [PubMed] [Google Scholar]
- 31.Liew F. Y., Xu D., Brint E. K., O'Neill L. A. (2005) Nat. Rev. Immunol. 5, 446–458 [DOI] [PubMed] [Google Scholar]
- 32.Saitoh T., Yamamoto M., Miyagishi M., Taira K., Nakanishi M., Fujita T., Akira S., Yamamoto N., Yamaoka S. (2005) J. Immunol. 174, 1507–1512 [DOI] [PubMed] [Google Scholar]
- 33.Song H. Y., Rothe M., Goeddel D. V. (1996) Proc. Natl. Acad. Sci. U.S.A. 93, 6721–6725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kuwata H., Watanabe Y., Miyoshi H., Yamamoto M., Kaisho T., Takeda K., Akira S. (2003) Blood 102, 4123–4129 [DOI] [PubMed] [Google Scholar]
- 35.Wessells J., Baer M., Young H. A., Claudio E., Brown K., Siebenlist U., Johnson P. F. (2004) J. Biol. Chem. 279, 49995–50003 [DOI] [PubMed] [Google Scholar]
- 36.Bradley J. R., Pober J. S. (2001) Oncogene 20, 6482–6491 [DOI] [PubMed] [Google Scholar]
- 37.Carpentier I., Beyaert R. (1999) FEBS Lett. 460, 246–250 [DOI] [PubMed] [Google Scholar]
- 38.Croker B. A., Krebs D. L., Zhang J. G., Wormald S., Willson T. A., Stanley E. G., Robb L., Greenhalgh C. J., Förster I., Clausen B. E., Nicola N. A., Metcalf D., Hilton D. J., Roberts A. W., Alexander W. S. (2003) Nat. Immunol. 4, 540–545 [DOI] [PubMed] [Google Scholar]
- 39.Lang R., Pauleau A. L., Parganas E., Takahashi Y., Mages J., Ihle J. N., Rutschman R., Murray P. J. (2003) Nat. Immunol. 4, 546–550 [DOI] [PubMed] [Google Scholar]
- 40.Sasaki A., Yasukawa H., Suzuki A., Kamizono S., Syoda T., Kinjyo I., Sasaki M., Johnston J. A., Yoshimura A. (1999) Genes Cells 4, 339–351 [DOI] [PubMed] [Google Scholar]
- 41.Yasukawa H., Ohishi M., Mori H., Murakami M., Chinen T., Aki D., Hanada T., Takeda K., Akira S., Hoshijima M., Hirano T., Chien K. R., Yoshimura A. (2003) Nat. Immunol. 4, 551–556 [DOI] [PubMed] [Google Scholar]
- 42.Wullaert A., Verstrepen L., Van Huffel S., Adib-Conquy M., Cornelis S., Kreike M., Haegman M., El Bakkouri K., Sanders M., Verhelst K., Carpentier I., Cavaillon J. M., Heyninck K., Beyaert R. (2007) J. Biol. Chem. 282, 81–90 [DOI] [PubMed] [Google Scholar]