Abstract
S-(2-Succinyl)cysteine (2SC) is formed by reaction of the Krebs cycle intermediate fumarate with cysteine residues in protein, a process termed succination of protein. Both fumarate and succination of proteins are increased in adipocytes cultured in high glucose medium (Nagai, R., Brock, J. W., Blatnik, M., Baatz, J. E., Bethard, J., Walla, M. D., Thorpe, S. R., Baynes, J. W., and Frizzell, N. (2007) J. Biol. Chem. 282, 34219–34228). We show here that succination of protein is also increased in epididymal, mesenteric, and subcutaneous adipose tissue of diabetic (db/db) mice and that adiponectin is a major target for succination in both adipocytes and adipose tissue. Cys-39, which is involved in cross-linking of adiponectin monomers to form trimers, was identified as a key site of succination of adiponectin in adipocytes. 2SC was detected on two of seven monomeric forms of adiponectin immunoprecipitated from adipocytes and epididymal adipose tissue. Based on densitometry, 2SC-adiponectin accounted for ∼7 and 8% of total intracellular adiponectin in cells and tissue, respectively. 2SC was found only in the intracellular, monomeric forms of adiponectin and was not detectable in polymeric forms of adiponectin in cell culture medium or plasma. We conclude that succination of adiponectin blocks its incorporation into trimeric and higher molecular weight, secreted forms of adiponectin. We propose that succination of proteins is a biomarker of mitochondrial stress and accumulation of Krebs cycle intermediates in adipose tissue in diabetes and that succination of adiponectin may contribute to the decrease in plasma adiponectin in diabetes.
The accumulation of sugar and lipid-derived chemical modifications on proteins is associated with the etiology of several age-related diseases, including diabetes and its complications (1, 2). The irreversible adducts formed, termed advanced glycation/lipoxidation end products (AGE/ALEs),2 accumulate over time on long lived proteins, such as collagens, affecting the solubility, elasticity, and proteolytic digestibility of the protein (3). AGE/ALEs are considered important mediators of the pathogenesis of diabetic complications through engagement of scavenger receptors, such as RAGE (receptor for AGE) and activation of proinflammatory signaling pathways (4). To date, the study of AGE/ALEs has focused mainly on modification of lysine and arginine residues in proteins by reactive carbonyl intermediates formed during metabolism or autoxidation of carbohydrates and lipids (2, 5). However, free cysteine is more abundant on intracellular proteins and, because of its greater nucleophilicity, is a more likely target for chemical modification by intracellular electrophiles.
We recently identified S-(2-succinyl)-cysteine (2SC), a cysteine modification formed by a Michael addition reaction between the Krebs cycle intermediate fumarate and free sulfhydryl groups on proteins (6). This reaction, in which a thioether bond is formed, is described as succination of protein in order to distinguish it from succinylation, which leads to formation of amide, ester, or thioester bonds. 2SC was detected in human serum albumin and skin collagen and was increased in skeletal muscle protein and urine of diabetic rats. We also identified glyceraldehyde-3-phosphate dehydrogenase as one protein that is significantly modified by 2SC in skeletal muscle, resulting in the decrease in specific activity of glyceraldehyde-3-phosphate dehydrogenase in muscle of diabetic rats (7). We have proposed that 2SC may accumulate as a result of mitochondrial nutrient “flooding” because of an excess of carbohydrate and lipid fuels in diabetes and may be a biomarker of mitochondrial stress in disease.
To gain further insight into the role of succination in the regulation of metabolism, we studied the maturation of 3T3-L1 fibroblasts to adipocytes, an in vitro system in which fumarate and other Krebs cycle intermediates increase severalfold during adipogenesis in high (30 mm) glucose) medium (8, 9). Adipogenesis under these conditions is associated with a substantial increase in oxidative stress as a result of mitochondrial superoxide production (10). We also observed a ≥5-fold increase in fumarate and a ≥10-fold increase in intracellular 2SC accumulation during adipogenesis and identified several of the major proteins modified by 2SC (9). This set of proteins included cytoskeletal proteins, enzymes, heat shock and chaperone proteins, regulatory proteins, and a fatty acid-binding protein, suggesting that succination may have wide ranging effects on the structure of the cytoskeleton and the regulation of metabolism.
The adipocyte is increasingly recognized not only for its role in triglyceride storage but also as an active endocrine organ, secreting hormones and cytokines that orchestrate key metabolic processes in tissues, such as heart, liver, and muscle. All of the adipokines work as part of a greater metabolic regulatory network. Adiponectin and leptin are considered positive regulators of energy intake and expenditure, whereas resistin, interleukin-6, tumor necrosis factor-α, and PAI-1 are implicated in the development of inflammation and insulin resistance. Imbalances in adipokine metabolism are central to adipocyte dysfunction and the ensuing events leading to insulin resistance and diabetes (11, 12).
Adiponectin has received particular attention as the most abundant adipokine, circulating at high levels in human blood. It is an ∼30-kDa glycoprotein that associates intracellularly into trimeric, hexameric (also known as low molecular weight (LMW)), and other high molecular weight (HMW) complexes consisting of 18–36 monomers (13, 14). The various molecular weight species differentially stimulate their target tissues; trimeric adiponectin stimulates muscle fatty acid oxidation through activation of AMP-activated protein kinase, whereas HMW forms act to enhance insulin-mediated inhibition of gluconeogenesis in the liver (15, 16). Plasma adiponectin concentration is reduced in diabetes, in general, as is the ratio of HMW forms to total adiponectin (16).
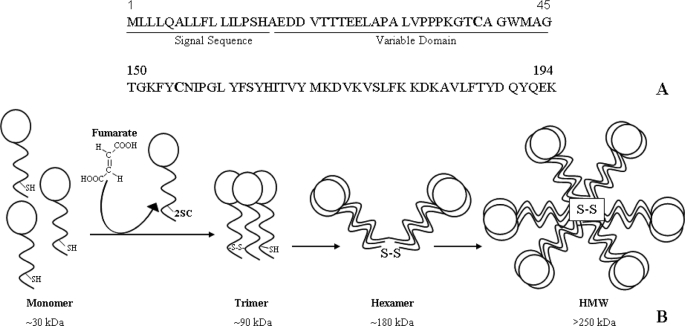
The N-terminal hypervariable domain of adiponectin contains a single cysteine residue followed by a collagenous region containing several conserved lysine and proline residues. Several of these lysines and prolines are subject to modification by hydroxylation and/or glycosylation (17, 18). The cysteine at position 39 in mouse adiponectin is involved in the formation of the oligomeric species of adiponectin through disulfide bonding of monomers and trimers (Fig. 1). Cys-39 is critical for the generation of all higher order complexes, since its mutation to serine inhibits the formation of both trimer and larger species. The only other cysteine present in adiponectin is located in the C-terminal globular domain, and crystallographic studies indicate that it is unlikely to be involved in disulfide bonding of oligomers (14). In this study, we show that adiponectin is a major target of succination in both 3T3-L1 adipocytes and adipose tissue of diabetic (db/db) mice, that Cys-39 is a major site of cysteine succination, and that succinated adiponectin is neither incorporated into polymeric forms in the cell nor secreted from the cell. We propose that succination of adiponectin may contribute to the decrease in plasma adiponectin in diabetes.
FIGURE 1.
Structure of adiponectin. Two cysteines are highly conserved in adiponectin monomer: one in the hypervariable region adjacent to the N terminus (Cys-39) and the other in the C-terminal globular head domain (Cys-155) (A). Adiponectin monomers associate into trimers through disulfide bonding, and trimers associate through disulfide bonds to form LMW and HMW multimers, which are secreted from the adipocyte. Succination of Cys-39 blocks incorporation of adiponectin monomer into trimer and higher molecular weight secreted forms of the protein (B).
EXPERIMENTAL PROCEDURES
Materials
Unless otherwise noted, all chemicals were purchased from Sigma. [U-13C3,15N]cysteine and D8-lysine were from Cambridge Isotope Laboratories (Woburn, MA). 2SC and [U-13C3,15N]2SC were synthesized as previously described (6). Polyacrylamide gels, polyvinylidene difluoride membranes, ECL chemiluminescent substrate, and precast gels were purchased from Bio-Rad. Immobiline IPG strips and ampholytes for isoelectric focusing were purchased from GE Healthcare. Sypro Ruby gel stain was purchased from Molecular Probes, Inc. (Eugene, OR). Bovine pancreatic DNase I was obtained from Pierce. 2-Succinylcysteamine and anti-2SC polyclonal antibody were prepared as described previously (9). 3T3-L1 murine fibroblasts were obtained from the American Type Culture Collection (Manassas, VA) and cultured as described previously (9).
Animals
Type 2 diabetic male db/db mice (Leprdb) and their lean littermates (Leprdb/+) were purchased at 3, 9, and 14 weeks of age from Jackson Laboratories (Bar Harbor, ME). All animals were acclimatized for 1 week, and then experiments were conducted according to the guidelines of the University of South Carolina Institutional Animal Care and Use Committee. Prior to sacrifice, blood was collected from the tail vein. The mice were anesthetized by subcutaneous injection with a mixture of ketamine/xylazine/acepromazine, perfused with 5 ml of physiological saline, and then tissues were collected and snap-frozen.
Protein Extraction from Adipose Tissue
Adipose tissue (0.5 g) was added to 500 μl of ice-cold radioimmunoprecipitation assay buffer (50 mm Tris-HCl, 150 mm NaCl, 1 mm EDTA, 1% Triton X-100, 0.1% SDS, 0.5% sodium deoxycholate, pH 7.4) in the presence of protease inhibitors and sheared 10 times with a 21-gauge needle. The lysate was sonicated using a microprobe at 2 watts on a model 100 sonic dismembrator (Fisher) for 30 s, and the insoluble debris was removed by centrifugation at 5000 × g for 10 min in a Heraeus Biofuge 13 microcentrifuge (Thermo Fisher Scientific, Waltham, MA). A thick lipid layer was visible on the surface, and the infranatant containing soluble proteins was removed using a Hamilton syringe. The infranatant was sonicated for a further 30 s and then allowed to sit on ice for 1 h. The sample was centrifuged again at 10,000 × g for 10 min. The infranatant was removed and vortexed with 9 volumes of ice-cold acetone. After sitting on ice for 15 min, the precipitated protein was centrifuged at 2000 × g for 10 min. The aqueous acetone, containing residual lipids and fumarate, was removed, and the samples were resuspended in 500 μl of radioimmunoprecipitation assay buffer. Removal of fumarate at this point is essential, because fumarate reacts with protein during boiling in radioimmunoprecipitation assay buffer in preparation for PAGE analysis; this leads to an artifactual increase in succination of proteins.
Protein and Triglyceride Analyses
Protein concentration in cell and tissue extracts was measured by the Lowry assay (19). Average protein yields were 1–3% wet weight of adipose tissue. Triglycerides were measured in adipocyte lysates using the Thermo DMA triglyceride assay kit and standard (Thermo Fisher Scientific), according to the manufacturer's instructions.
Gas Chromatography-MS Analysis of 2SC and Fumarate in Adipose Tissue
For measurement of 2SC, adipose tissue protein (2 mg) was hydrolyzed in 1 ml of 6 m HCl for 24 h at 110 °C. The N,O-trifluoroacetyl methyl ester derivative of 2SC was prepared and analyzed by multiple reaction monitoring gas chromatography-MS/MS using a TSQ 7000 triple quadrupole mass spectrometer (Thermo Fisher Scientific), as described previously (9). Fumarate was measured in aliquots (1 mg of protein) of adipose tissue protein, according to the method of Tanaka et al. (20), as described previously (9).
Immunoprecipitation of Proteins
Adiponectin was immunoprecipitated from aliquots of adipose tissue lysate containing equal amounts of adiponectin, measured by an enzyme-linked immunosorbent assay (R&D Systems, Minneapolis, MN). Samples were diluted into 300 μl of radioimmunoprecipitation assay buffer and then precleared by incubation with 10 μl of Protein G-agarose (Pierce) ultralink beads on a Roto-Torque (Cole-Parmer, Vernon Hills, IL) for 30 min at room temperature. After centrifugation at 8000 × g for 2 min in a Heraeus Biofuge 13 microcentrifuge (Thermo Fisher Scientific), the supernatant was removed and then incubated with 5 μg of anti-adiponectin antibody (R&D Systems) on the Roto-Torque for 2 h at room temperature. Protein G-agarose beads (20 μl) were added, followed by incubation on the Roto-Torque for 1 h at room temperature. The beads were then washed three times in 50 mm phosphate buffer, pH 7.4, followed by centrifugation at 8000 × g for 5 min to recover the agarose beads. The agarose beads from the immunoprecipitation were suspended in either reducing or non-reducing SDS buffers prior to electrophoresis and immunoblotting by one-dimensional SDS-PAGE or in rehydration buffer in preparation for isoelectric focusing for two-dimensional PAGE.
Western Immunoblotting
Adipocyte protein lysate (50 μg) or adiponectin immunoprecipitates were diluted to 30 μl in deionized water prior to adding 6 μl of Laemmli loading buffer (21) containing 5% β-mercaptoethanol. The samples were boiled for 10 min at 95 °C and then loaded onto 12.5% Tris-HCl polyacrylamide gels and electrophoresed at 200 V for 55 min. In the case of the non-denaturing gels, the samples were mixed with a nonreducing buffer (1% SDS, 5% glycerol, 10 mm Tris-HCl, pH 6.8) in the absence of mercaptoethanol and then loaded directly onto a 12.5% gel without boiling. The separated proteins were next semi-dry blotted to a polyvinylidene difluoride membrane at 20 V for 45 min, and the membranes were blocked overnight in 5% nonfat dry milk. The membrane was probed for 1 h with 0.5 μg of polyclonal anti-2SC antibody dissolved in 1% nonfat dry milk. Blots were washed in 20 mm Tris-HCl, pH 7.3, containing 0.05% Tween 20, followed by the addition of goat anti-rabbit horseradish peroxidase-labeled secondary antibody (Zymed Laboratories Inc., Carlsbad, CA). After a final series of washes, 2SC immunoreactivity was detected using ECL chemiluminescent substrate exposed to Pierce CL-XPosure film (Pierce).
Two-dimensional Polyacrylamide Gel Electrophoresis
Isoelectric focusing of adipocyte and adipose tissue protein was performed on an Ettan IPGPhor II system (GE Healthcare). Adipocyte samples (700 μg of protein for 24 cm (pH 4.5–5.5 gels) or 100 μg for 11 cm (pH 4–7 gels)) or adiponectin immunoprecipitates were dissolved in rehydration buffer (7 m urea, 2 m thiourea, 2% CHAPS, 1% IPG buffer, and 50 mm dithiothreitol). The samples were loaded in the IPGphor strip holder prior to the addition of the Immobiline IPG strips and were allowed to rehydrate overnight for 18 h prior to isoelectric focusing at 21 °C according to the following step programs: for 11-cm strips, linear at 500 V for 1 h, gradient to 1000 V for 1 h, gradient to 6000 V for 2.5 h, and gradient to 6000 V for 1.4 h; for 24-cm strips, linear at 200 V for 1 h, linear at 500 V for 1 h, linear at 1000 V for 45 min, linear at 2000 V for 45 min, and gradient to 3500 V for 28 h. After electrophoresis, the strips were equilibrated in 15 ml of equilibration buffer I (6 m urea, 0.4 m Tris-HCl, pH 8.8, 30% glycerol (v/v), 2% SDS (w/v), 1% dithiothreitol) and allowed to shake gently for 15 min at 21 °C. The solution was then decanted and replaced with 15 ml of equilibration buffer II (6 m urea, 0.4 m Tris-HCl, pH 8.8, 30% glycerol (v/v), 2% SDS (w/v), 1% iodoacetamide) and incubated with gentle shaking for an additional 15 min. Proteins were electrophoresed on a Bio-Rad Criterion system (11-cm strips) or an Ettan Dalt 6 system (24-cm strips) (GE Healthcare) and fixed with 7.5% acetic acid: 10% methanol solution for 1 h prior to overnight staining with Sypro Ruby gel stain. The stained gels were imaged on a Storm multilaser scanner (GE Healthcare). A duplicate gel was transferred onto polyvinylidene difluoride membrane and fixed as above prior to staining for 15 min with Sypro Ruby blot stain. The blot was washed in water and imaged in the same manner as the gel. The blot was then washed in 150 mm Tris-HCl, pH 6.8, for 10 min prior to blocking and immunostaining with anti-2SC antibody, as described above.
Protein Identification by Mass Spectrometry
The excised bands from one-dimensional gels of immunoprecipitated adiponectin were processed for MS analysis according to the method of Kinter and Sherman (22). Briefly, the protein was alkylated with iodoacetamide, followed by proteolytic digestion in 50 mm ammonium bicarbonate buffer with 2 pmol of Promega sequencing grade modified trypsin (Promega, Madison, WI). The digested samples were analyzed on a nano-LC system (LC Packings, Sunnyvale, CA) coupled to an ESI-linear ion trap mass spectrometer (LTQ; Thermo Finnigan). A 75-μm C18 reverse-phase LC column was used with a 60-min gradient from 2% acetonitrile in 0.1% formic acid solution (solvent A) to 70% solvent A and 30% solvent B (2% water in acetonitrile containing 0.1% formic acid). The LTQ-MS was operated in data-dependent MS/MS analysis mode with an inclusion list and excluded all ions below 500 counts. The inclusion list was used to target unmodified (native), carbamidomethylated and succinated tryptic peptides of interest for CID-MS/MS analysis. The MS/MS data were sequenced manually to identify the peptides with modifications. The MS/MS data were also searched with the Thermo Finnigan Bioworks browser (Thermo Fisher Scientific) to identify the proteins and modifications in the peptides.
Statistical Analysis
Densitometry on Western blotting images was performed using Image J software (National Institutes of Health, Bethesda, MD). Data are summarized throughout as means ± S.E. and are plotted using Sigma Plot 8 software (Systat Software, Inc., Point Richmond, CA). Statistical analyses were performed using InStat (GraphPad Software, San Diego, CA). Differences between groups were analyzed using the unpaired two-tailed t test.
RESULTS
Adiponectin Is Succinated in 3T3-L1 Adipocytes Cultured in High Glucose Medium
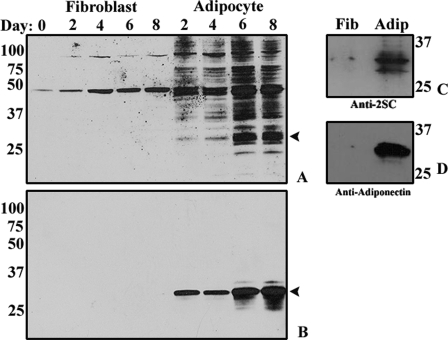
In previous work (9), we have described the kinetics of 2SC formation in proteins of 3T3-L1 adipocyte matured in 30 mm glucose medium. The increase in succinated proteins in adipocytes is illustrated by Western blotting (Fig. 2A, right lanes). In contrast, only two 2SC-labeled proteins were detected in undifferentiated fibroblasts (Fig. 2A, left lanes). The strong band at ∼32 kDa in Fig. 2A suggested that adiponectin, a major adipocytokine, might also be a major succinated protein in adipocytes. As shown in Fig. 2B, when the blots were stripped and then reprobed with anti-adiponectin antibody, the location of adiponectin in Fig. 2B corresponded exactly with the dark protein band in Fig. 2A; the broadening of the band with time in Fig. 2B probably results from microheterogeneity of glycoforms of the protein. To confirm succination of adiponectin, equal amounts of protein from lysates of fibroblasts and adipocytes, cultured in 30 mm glucose for 8 days, were immunoprecipitated with anti-adiponectin antibody. The immunoprecipitates were electrophoresed on a denaturing gel and immunostained with anti-2SC antibody, revealing the presence of multiple bands from 28–34 kDa (Fig. 2C) in the adipocyte sample. The blot was stripped and reprobed with anti-adiponectin, also yielding a strong band (Fig. 2D), confirming the modification of adiponectin by fumarate in adipocytes. Only traces of adiponectin or succinated adiponectin were detectable in undifferentiated fibroblasts (Fig. 2, C and D).
FIGURE 2.
2SC increases in proteins during maturation of adipocytes. A, total cell protein (30 μg) from fibroblasts (lanes 1–5) or adipocytes (lanes 6–9) cultured in 30 mm glucose for up to 8 days was fractionated by SDS-PAGE and then immunoblotted and stained with polyclonal anti-2SC, as described under “Experimental Procedures.” A single major band was detected in fibroblast lysates, whereas adipocytes yielded a time-dependent increase in at least 30 protein bands, representing succinated proteins. B, gels from A were stripped and reprobed with anti-adiponectin antibody. C, in order to examine adiponectin modification, fibroblasts and adipocytes were grown for 8 days in 30 mm glucose. Total cell lysates (500 μg of protein) were immunoprecipitated with anti-adiponectin antibody and analyzed by one-dimensional PAGE. Fibroblasts (left) and adipocytes (right) were probed with anti-2SC antibody. D, gels from C were stripped and reprobed with anti-adiponectin antibody. The numbers indicate molecular weights of marker proteins. Data are from a representative experiment (n = 3).
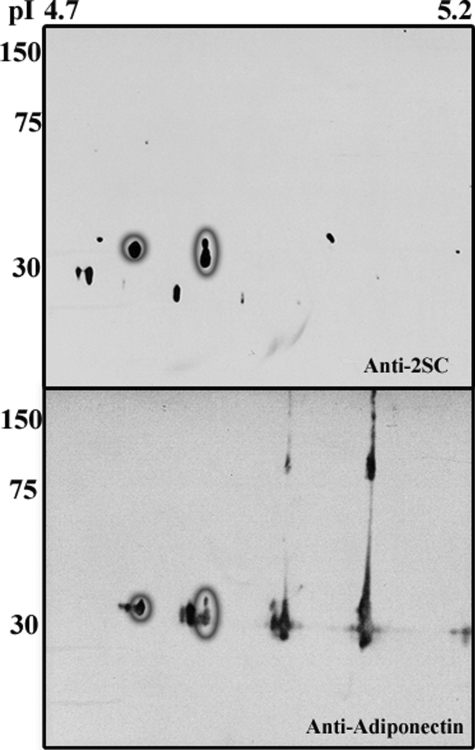
Analysis of an adipocyte whole cell lysate by two-dimensional electrophoresis using narrow range (pH 4.5–5.5) isoelectric focusing revealed a number of prominent 2SC immunoreactive spots around the expected pI and molecular weight range for adiponectin (Fig. 3, top and bottom). The blot was stripped and reprobed with anti-adiponectin, revealing a precise overlap of two of the 2SC and adiponectin spots (circled in Fig. 3, top and bottom); these spots were shifted toward an acidic pI, consistent with the addition of a negatively charged succinate group. Notably, 2SC staining was restricted to monomeric forms of adiponectin and was not present in trimer or HMW isoforms (Fig. 3, top versus bottom). Densitometric analysis indicated that ∼8% of total adiponectin in Fig. 3 (bottom) was present as the two succinated isoforms. Residual dimeric and larger forms of adiponectin were present despite the strong denaturing conditions employed for two-dimensional PAGE. Concentrations of dithiothreitol greater than 100 mm were required to fully denature adiponectin; however, these conditions were incompatible with the isoelectric focusing.
FIGURE 3.
Identification of 2SC-adiponectin in adipocyte proteins. Adipocyte proteins (600 μg of total cell lysate) were analyzed by two-dimensional PAGE on 24-cm gels with a pH range of 4.5–5.5. The section of interest (pI 4.7–5.2) containing adiponectin is shown. Gels were probed with anti-2SC antibody. The brief exposure (1 min) in this experiment shows the most abundant succinated proteins in adipocytes within the labeled molecular weight and pI range (top). The gels were stripped and reprobed with anti-adiponectin (bottom), illustrating that two of the prominent spots in the top (circled) correspond to anionic species of adiponectin in the bottom (circled). The molecular weight markers shown indicate the presence of 2SC on monomeric adiponectin only. The blot is representative of n = 3 experiments.
Detection of 2SC on Cys-39 Adiponectin from 3T3-L1 Adipocytes
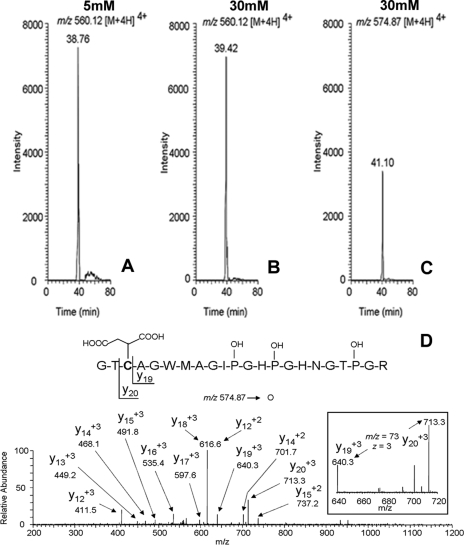
Adiponectin from murine 3T3 adipocytes cultured in low and high glucose medium was immunoprecipitated, reduced with iodoacetamide, and electrophoresed on one-dimensional gels for analysis by mass spectrometry. The adiponectin band was excised and digested with trypsin, and the cysteine-containing peptide corresponding to residues 37–58 (GTCAGWMAGIPGHPGHNGTPGR) of adiponectin was detected by ESI-MS/MS. All three prolines in this peptide are reported to be hydroxylated (18), and we also found that the triply hydroxylated form was the dominant peptide in adiponectin immunoprecipitated from adipocytes. The carbamidomethylated peptide, resulting from reaction with iodoacetamide (m/z 560.12 [M + 4H]4+), was detected at similar intensities in both 5 and 30 mm glucose adipocytes when equal amounts of peptide were injected (Fig. 4, A and B). The succinated peptide (m/z 574.87 [M + 4H]4+), was observed only in adiponectin from cells grown in 30 mm glucose (Fig. 4C). The relative area counts of the succinated/carbamidomethylated peptide (116,000 versus 472,080) suggest ∼25% succination of adiponectin in cells grown in high glucose medium. This is higher than the estimate from densitometry (Fig. 3; ∼8%) but could result from differences in the efficiency of ionization of the native and modified peptides. Sequencing of the succinated peptide (Fig. 4D) confirmed modification of adiponectin by fumarate at Cys-39 (Fig. 4C). The transition from y19 to y20, indicated in the inset, shows the +3 charge state for the residue mass of cysteine (103.1) with a fumarate-derived succinate adduct. Despite several experiments with trypsin and other proteases, we were unable to detect a peptide containing Cys-155 in adiponectin isolated from cells grown in low or high glucose medium.
FIGURE 4.
LC-MS/MS identification of Cys-39 as a prominent site of succination of adiponectin. Adiponectin from 3T3 adipocytes cultured in either 5 or 30 mm glucose was immunoprecipitated and electrophoresed on one-dimensional gels. The isolated adiponectin bands were excised from the gel, alkylated with iodoacetamide, and digested with trypsin, and the peptides were analyzed by high pressure liquid chromatography-ESI-MS/MS. A–C, extracted ion chromatograms for either the carbamidomethylated cysteine-containing peptide (residues 37–58; GTCAGWMAGIPGHPGHNGTPGR) of adiponectin from cells grown in 5 or 30 mm glucose, respectively (A and B), or the succinated peptide from cells grown in 30 mm glucose (C). The masses indicated in each frame correspond to the +4 charge state for the peptide. D, MS/MS sequencing data obtained from fragment ions derived from m/z 574.87 [M + 4H]4+, demonstrating that Cys-39 is the site of succination on adiponectin adipocytes grown in 30 mm glucose. OH-P, hydroxyproline residues in the peptide.
Succinated Adiponectin Is Not Secreted from Adipocytes
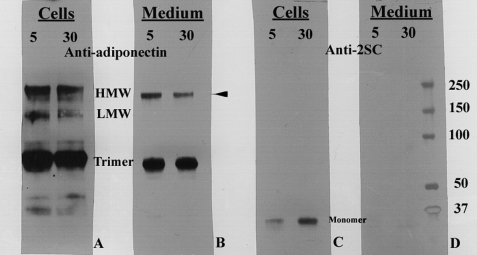
Based on enzyme-linked immunosorbent assay quantification, equal amounts of adiponectin from whole cell lysates or the medium of adipocytes cultured in 5 or 30 mm glucose were immunoprecipitated and analyzed by non-reducing SDS-PAGE (Fig. 5). The intracellular and extracellular adiponectin profiles were similar for cells matured in low and high glucose; however, the fraction of HMW form in cells and medium and HMW form in the medium was consistently lower in cells grown in high glucose medium (summarized in the legend to Fig. 5). The intensity of the monomer isoforms of adiponectin was unaffected by glucose concentration, but these changes could be obscured by effects of succination on turnover of the monomer. Duplicate blots probed with anti-2SC antibody demonstrated the presence of 2SC only on the monomeric form of adiponectin within the cells (Fig. 5C), whereas 2SC was not detected on any adiponectin isoforms secreted into the growth medium (Fig. 5D). A small amount of 2SC was detected on adiponectin from adipocytes matured in 5 mm glucose (Fig. 5C), as reported previously (9), but this was evidently below the threshold for detection by mass spectrometry (Fig. 4). These observations indicate that succination not only inhibits incorporation of adiponectin monomer into higher molecular weight isoforms, consistent with the essential role of Cys-39 in polymerization of the protein, but also suggest that succination impairs the efficiency of assembly of the higher molecular weight isoforms. As we have noted previously (9), succination also targets chaperone proteins, which may affect pathways of assembly and secretion of adiponectin.
FIGURE 5.
Succinated adiponectin is present in cells but is not secreted into the medium of adipocytes grown in high glucose medium. Equal amounts of adiponectin were immunoprecipitated from the cell lysates or growth medium of adipocytes cultured in 5 or 30 mm glucose. The adiponectin was electrophoresed on a non-denaturing gel and stained with anti-adiponectin (A and B) or with anti-2SC (C and D). Quantitative analysis (n = 4) indicated that there was a 16% decrease (p < 0.05) in the relative intensity of the HMW isoform and a 12% decrease (p < 0.05) in the LMW isoform of adiponectin in cells grown in 30 mm Glc (A) and a 17% decrease in the HMW isoform (p < 0.01) in the medium of these cells (B). The LMW isoform was not detectable in the cell medium. 2SC was detected only on adiponectin monomer within the cells (C), whereas succinated adiponectin was not detectable in any of the secreted isoforms of adiponectin in the cell medium (D).
2SC Is Present in the Adipose Tissue of db/db Mice
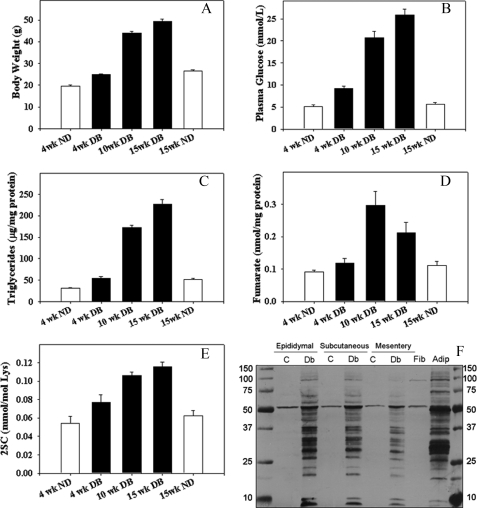
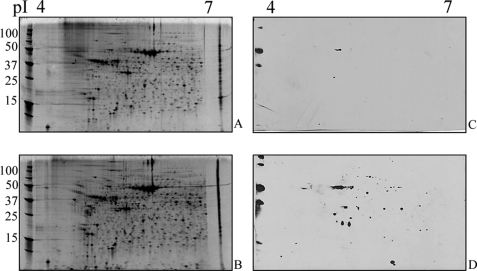
In order to examine succination of adiponectin and other proteins in vivo, we studied the db/db (leptin receptor knock-out) mouse model of type 2 diabetes. These animals become obese and begin to develop diabetes by 4 weeks of age (Fig. 6, A–C). The increases in body weight (adipose tissue mass), glucose, and triglycerides were accompanied by a parallel increase in fumarate in epididymal adipose tissue (Fig. 6D); fumarate increased by 2-fold at 10 weeks in db/db mice and then declined by ∼50% at 15 weeks of age. The decrease in fumarate at 15 versus 10 weeks parallels similar observations during the late stage of adipogenesis in 3T3-L1 fibroblasts (9) and may result from mitochondrial drop-out or oxidative damage in older db/db mice (23). 2SC increased in concert with the increase in fumarate, yielding an ∼2.5-fold increase in 2SC at 15 weeks in db/db mice, compared with lean controls (Fig. 6E). As shown in Fig. 5F, 2SC was detected on a number of proteins in adipose tissue from diabetic mice, and similar proteins appeared to be stained in epididymal, subcutaneous, and mesenteric fat from 15-week-old animals; many of the 2SC-labeled proteins are similar to the profile observed for mature 3T3-L1 adipocytes (Fig. 6F, right column). Fig. 7, A and B, shows Sypro-stained blots of epididymal adipose tissue on a pH 4–7 range gel, demonstrating a broad range of 2SC-modified proteins. An anti-2SC blot revealed a single 2SC-protein at ∼50 kDa in tissue from adipose tissue from control mice (Fig. 7C), identified as β-tubulin (data not shown). In total, the diabetic tissues revealed ∼60 spots that stained positively for 2SC (Fig. 7D).
FIGURE 6.
Fumarate and 2SC-proteins increase in epididymal adipose tissue with progression of diabetes in db/db mice. Various parameters were measured in db/db mice at 4, 10, and 15 weeks of age and compared with control, non-obese animals at 4 and 15 weeks of age. A, body weight. B, the increase in glycemia parallels the increase in body weight. C, the increase in triglyceridemia parallels the increase in body weight. D, fumarate concentration increases during development of diabetes in db/db mice. E, 2SC increases in proteins during development of diabetes. F, adipose tissue protein (50 μg) from representative diabetic mice (DB) and matching controls (ND) at 15 weeks was analyzed by one-dimensional PAGE and probed with anti-2SC antibody, as described under “Experimental Procedures.” Similar proteins are modified by 2SC in epididymal (Epi), subcutaneous (Sub), and mesenteric (Mes) adipose tissue of diabetic rats and were similar to those detected in 3T3 adipocytes (Adip) grown in 30 mm glucose. Staining patterns for protein from adipose tissue of control, non-diabetic (ND) mice resembled the pattern of succination in fibroblasts (Fib), in that only one protein, recently identified as tubulin, was significantly modified. M, marker lane on the far right and left. Data are for a representative experiment and are mean ± S.E., n = 3.
FIGURE 7.
Epididymal fat in db/db mice is rich in succinated proteins. Total epididymal fat protein from control and diabetic mice was analyzed by broad pH range two-dimensional PAGE (pH 4–7) and then stained with Sypro dye (A and B) or blotted with anti-2SC antibody (C and D). One succinated protein was identified in non-diabetic controls (C), whereas >60 proteins were detected in epididymal fat protein from diabetic mice (D).
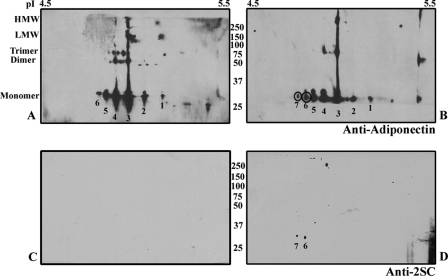
To compare the modification of adiponectin in adipocytes (Fig. 3) and adipose tissue, adiponectin was immunoprecipitated from control and diabetic epididymal adipose tissue, analyzed by two-dimensional PAGE, and probed with anti-adiponectin antibody (Fig. 8, A and B). The immunoblots were stripped and reprobed with anti-2SC antibody (Fig. 8, C and D), indicating the presence of 2SC in two acidic isoforms (circled) of the protein. Based on relative staining intensities of the primary numbered isoforms in Fig. 8B, ∼7% of total adiponectin is modified by 2SC in 15-week-old db/db mice. Comparison of the gels in Figs. 3 and 8 indicates that adiponectin was similarly modified in adipocytes and adipose tissue, consistent with the similar ambient glucose concentrations, 30 mm for adipocytes and ∼27 mm for adipose tissue in db/db mice (Fig. 6B).
FIGURE 8.
Adiponectin is succinated in epididymal adipose tissue of db/db mice. Equal amounts of adiponectin were immunoprecipitated from epididymal adipose tissue from control and db/db mice, analyzed by two-dimensional PAGE, and then probed with anti-adiponectin antibody (top) or stripped and reprobed with anti-2SC antibody. A and B, adiponectin is detectable as multiple isoforms in both control (A) and diabetic mice (B). C and D, 2SC-adiponectin (two spots) is detectable only in diabetic (D) but not control mice (C). Note that isoform 7 is only present in db/db mice, that the intensity of isoform 6 is increased in B versus A, and that 2SC is absent from any of the polymeric isoforms.
Succination Inhibits Incorporation of Adiponectin Monomer into Polymeric and Secreted Isoforms of the Protein in Vivo
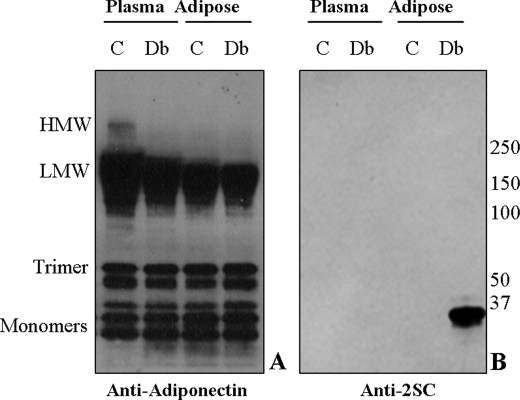
To determine if modification by 2SC influenced oligomerization of adiponectin in vivo, we examined intracellular adiponectin on non-reducing gels. Equal amounts of adiponectin from epididymal adipose tissue of 15-week-old control and db/db mice were analyzed by non-reducing PAGE and stained with anti-adiponectin (Fig. 9A). As indicated in the legend to Fig. 9, there was a significant increase in the fraction of monomer + trimer isoforms of adiponectin in plasma and a similar trend in adipose tissue of db/db versus control animals. When the blot was stripped and reprobed with anti-2SC, 2SC-adiponectin monomer was detected in tissue from diabetic animals but was not detectable in controls (Fig. 9B, last two lanes). The fact that 2SC-adiponectin was not detectable in adiponectin immunoprecipitated from plasma of diabetic mice (Fig. 9B, first two lanes) and that secretion of the HMW isoforms is decreased in the diabetic animal suggests that succination inhibited incorporation of adiponectin into polymeric, secreted forms of the protein, consistent with observations in adipocytes in vitro (Fig. 5).
FIGURE 9.
Succination inhibits incorporation of adiponectin monomer into polymeric forms of adiponectin in epididymal adipose tissue of db/db mice. Equal amounts of adiponectin were immunoprecipitated from the epididymal adipose tissue of 15-week-old control (C) and diabetic (Db) mice. Immunoblots were stained with anti-adiponectin (A) or anti-2SC (B). Quantitative analysis (n = 3) of the isoform distribution indicated that there was a 10% increase in monomer + trimer isoforms in diabetic, compared with control plasma (n = 3, p < 0.05) and a similar (8%, n = 3) but not statistically significant trend in adipose tissue of diabetic mice. Immunoblotting for 2SC demonstrated that succinated adiponectin was not detectable in plasma (B, lanes 1 and 2), whereas 2SC was found only on the monomeric form of adiponectin within adipose tissue (B, lanes 3 and 4).
DISCUSSION
Succination of Adipocyte and Adipose Tissue Proteins
In studies on adipocytes grown in high glucose medium (9) (Fig. 5) and on adipose tissue from type 2 diabetic (db/db) mice (Fig. 7), we have detected ∼60 succinated proteins by two-dimensional PAGE. In contrast, only two spots of succinated protein, now known to be isoforms of tubulin,3 were detected in adipocytes grown in normal (5 mm) glucose concentration or in adipose tissue of non-diabetic mice. The data in Fig. 6E (and in earlier studies on adipocytes grown in high glucose medium (9)) indicate that 2SC accumulates over time (Figs. 2A and 6F). In both adipocytes (9) and db/db mice, the extent of succination of total adipocyte proteins increases in response to increasing extracellular glucose concentration, suggesting that 2SC may be useful as a biomarker of glucotoxicity. We have reported similar increases in fumarate and succination of protein in skeletal muscle of streptozotocin-induced type 1 diabetic rats (7). Whether changes in fumarate concentration and succination of protein are observed in other tissues or in prediabetic states, such as obesity, insulin resistance, or metabolic syndrome, is uncertain at this point. However, the close relationship between the gradual increase in plasma glucose and triglyceride, fumarate, and succination of proteins in the db/db mouse (Fig. 6) suggests that 2SC may be an early indicator of the metabolic changes that lead to overt diabetes.
Since the increase in 2SC is derived from an underlying increase in intracellular fumarate concentration, we have proposed that succination of protein is a biomarker of mitochondrial stress in diabetes (7, 9). Interestingly, in both adipocytes grown in high glucose medium (10) and in the tissues of diabetic mice, there is evidence of oxidative stress of mitochondrial origin (i.e. increased production of superoxide by mitochondria) (24, 25). Thus, 2SC, which is a product of oxidation of cysteine residues by fumarate, may serve as a biomarker of glucose- and or lipid-induced mitochondrial and oxidative stress in diabetes. These stresses may then lead to mitochondrial dysfunction and drop-out, which develops with the progress of diabetes in the db/db mouse (23). Indeed, a decrease in mitochondrial number, with corresponding low levels of respiration and fatty acid oxidation, has been observed in adipocytes of 12-week-old db/db mice (26). Recent studies also suggest that hypertrophic adipocytes may become increasingly hypoxic (26, 27), which would lead to accumulation of NADH and then to accumulation of fumarate and other intermediates through feedback inhibition of Krebs cycle NAD(H)-dependent dehydrogenases. Overall, these data suggest that the accumulation of fumarate and formation of 2SC may represent early biomarkers of mitochondrial stress in the diabetic state, whereas the decline in fumarate at later stages of diabetes may reflect the development of severe mitochondrial damage or loss of mitochondria.
Succination of Adiponectin
The identification of 2SC on adipocyte proteins led us to investigate succination of the highly conserved Cys-39 residue on murine adiponectin, which is involved in oligomerization and intracellular transport of the protein. When adiponectin was immunoprecipitated from adipocytes or adipose tissue of diabetic mice and analyzed by two-dimensional PAGE, it was detected as a series of isoforms that differed in isoelectric point, possibly because of differences in posttranslational modifications, such as sialylation (17, 28, 29). In both adipocytes and adipose tissue, the two most anionic isoforms were identified as 2SC-adiponectin by probing duplicate blots with anti-2SC antibody. Based on densitometry, 2SC-adiponectin accounted for 7–8% of total adiponectin in tissue and cells, respectively, whereas mass spectrometric analysis of adiponectin from adipocytes suggested a higher (∼25%) extent of succination of the protein. Experiments are in progress to gain a more comprehensive qualitative and quantitative analysis of the succinated proteome in epididymal adipose tissue by LC/MS/MS. In other studies, we have shown that ∼20% of glyceraldehyde-3-phosphate dehydrogenase is succinated in skeletal muscle of streptozotocin-induced diabetic rats (7). In both adipose tissue and muscle of diabetic animals, the level of 2SC substantially exceeds the levels of the AGE/ALEs, Nϵ-(carboxymethyl)lysine and Nϵ-(carboxyethyl)lysine, indicating that succination is an abundant, perhaps the most abundant, nonenzymatic modification of tissue proteins in diabetes.
In both the adipocyte model system and in diabetic mice, the succinated forms of adiponectin were detected only inside the cell (Fig. 5, C and D) or in adipose tissue itself (Fig. 9B), not in the adipocyte culture medium or in plasma of control or diabetic mice. These results indicate that succination interferes with incorporation of adiponectin monomer into higher molecular weight, secreted forms of the protein. Recent studies on the post-translational regulation of adiponectin metabolism indicate that its secretion is regulated by a process of “thiol-mediated protein retention” through binding to an intracellular protein, ERp44 (30). Although ∼50% of newly synthesized adiponectin is freely secreted from cells (31), this thiol-dependent mechanism ensures that a fraction is retained in oligomerized trimer form, ready to be secreted in response to metabolic signals. Succination of Cys-39 would limit the formation of disulfide-bonded trimer and other HMW forms by reducing the effective monomer concentration, possibly contributing to decreased secretion of adiponectin in diabetes. Two other well known endoplasmic reticulum chaperones, protein-disulfide isomerase and BiP, are also associated with the early stages of the adiponectin secretory pathway (32). This is noteworthy, since both protein-disulfide isomerase and BiP were previously identified as succinated proteins in 3T3 adipocytes (9). Thus, although 2SC-adiponectin accounts for <10% of intracellular adiponectin (based on densitometry), it is unlikely to be the sole factor causing the decrease in HMW isoforms in plasma in diabetes. In addition to effects on adiponectin, the increased production of succinated proteins may also challenge pathways of protein turnover in the endoplasmic reticulum, leading to ER stress and the unfolded protein response, further exacerbating metabolic derangements in adipose tissue in diabetes.
In conclusion, increased mitochondrial fumarate and succination of adipose tissue proteins are driven by elevated glucose concentration both in vitro and in vivo. The sensitivity to glucose, or glucotoxicity, indicates that excessive nutrient supply results in severe mitochondrial stress. We use the word stress here, rather than dysfunction, deliberately, because we consider the mitochondrial response to excess substrates (i.e. flooding by carbohydrate (and lipid) fuels) as a normal response. Thus, the accumulation of unneeded fuels and adequate ATP leads to increased NADH concentration and oxidizable substrates, including fumarate, in the mitochondrion and subsequently, by facilitated transport, throughout the cell and then to an increase in succination of protein. Further studies will be required to determine the extent to which succination of proteins contributes to the pathogenesis of diabetes and its complications.
Acknowledgment
We thank the mass spectrometry facility at the Medical University of South Carolina for the use of mass spectrometry resources.
This work was supported, in whole or in part, by National Institutes of Health, NIDDK, Grant DK-19971.
N. Frizzell, M. Rajesh, M. J. Jepson, R. Nagai, J. A. Carson, S. R. Thorpe, and J. W. Baynes, manuscript in preparation.
- AGE
- advanced glycation end product
- ALE
- advanced lipoxidation end product
- 2SC
- S-(2-succinyl)cysteine
- HMW
- high molecular weight
- LMW
- low molecular weight
- MS
- mass spectrometry
- CHAPS
- 3-[(3-cholamidopropyl)dimethylammonio]-1-propanesulfonic acid
- LC
- liquid chromatography
- ESI
- electrospray ionization.
REFERENCES
- 1.Thorpe S. R., Baynes J. W. (2003) Amino Acids 25, 275–281 [DOI] [PubMed] [Google Scholar]
- 2.Alt N., Carson J. A., Alderson N. L., Wang Y., Nagai R., Henle T., Thorpe S. R., Baynes J. W. (2004) Arch. Biochem. Biophys. 425, 200–206 [DOI] [PubMed] [Google Scholar]
- 3.Monnier V. M., Sell D. R. (2006) Rejuvenation Res. 9, 264–273 [DOI] [PubMed] [Google Scholar]
- 4.Bierhaus A., Humpert P. M., Morcos M., Wendt T., Chavakis T., Arnold B., Stern D. M., Nawroth P. P. (2005) J. Mol. Med. 83, 876–886 [DOI] [PubMed] [Google Scholar]
- 5.Monnier V. M., Sell D. R., Genuth S. (2005) Ann. N.Y. Acad. Sci. 1043, 567–581 [DOI] [PubMed] [Google Scholar]
- 6.Alderson N. L., Wang Y., Blatnik M., Frizzell N., Walla M. D., Lyons T. J., Alt N., Carson J. A., Nagai R., Thorpe S. R., Baynes J. W. (2006) Arch. Biochem. Biophys. 450, 1–8 [DOI] [PubMed] [Google Scholar]
- 7.Blatnik M., Frizzell N., Thorpe S. R., Baynes J. W. (2008) Diabetes 57, 41–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lai R. K., Goldman P. (1992) Metabolism 41, 545–547 [DOI] [PubMed] [Google Scholar]
- 9.Nagai R., Brock J. W., Blatnik M., Baatz J. E., Bethard J., Walla M. D., Thorpe S. R., Baynes J. W., Frizzell N. (2007) J. Biol. Chem. 282, 34219–34228 [DOI] [PubMed] [Google Scholar]
- 10.Lin Y., Berg A. H., Iyengar P., Lam T. K., Giacca A., Combs T. P., Rajala M. W., Du X., Rollman B., Li W., Hawkins M., Barzilai N., Rhodes C. J., Fantus I. G., Brownlee M., Scherer P. E. (2005) J. Biol. Chem. 280, 4617–4626 [DOI] [PubMed] [Google Scholar]
- 11.Ahima R. S. (2006) Obesity Suppl. 5, 242S–249S [DOI] [PubMed] [Google Scholar]
- 12.Ronti T., Lupattelli G., Mannarino E. (2006) Clin. Endocrinol. 64, 355–365 [DOI] [PubMed] [Google Scholar]
- 13.Tsao T. S., Tomas E., Murrey H. E., Hug C., Lee D. H., Ruderman N. B., Heuser J. E., Lodish H. F. (2003) J. Biol. Chem. 278, 50810–50817 [DOI] [PubMed] [Google Scholar]
- 14.Pajvani U. B., Du X., Combs T. P., Berg A. H., Rajala M. W., Schulthess T., Engel J., Brownlee M., Scherer P. E. (2003) J. Biol. Chem. 278, 9073–9085 [DOI] [PubMed] [Google Scholar]
- 15.Fruebis J., Tsao T. S., Javorschi S., Ebbets-Reed D., Erickson M. R., Yen F. T., Bihain B. E., Lodish H. F. (2001) Proc. Natl. Acad. Sci. U.S.A. 98, 2005–2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pajvani U. B., Hawkins M., Combs T. P., Rajala M. W., Doebber T., Berger J. P., Wagner J. A., Wu M., Knopps A., Xiang A. H., Utzschneider K. M., Kahn S. E., Olefsky J. M., Buchanan T. A., Scherer P. E. (2004) J. Biol. Chem. 279, 12152–12162 [DOI] [PubMed] [Google Scholar]
- 17.Wang Y., Lam K. S., Chan L., Chan K. W., Lam J. B., Lam M. C., Hoo R. C., Mak W. W., Cooper G. J., Xu A. (2006) J. Biol. Chem. 281, 16391–16400 [DOI] [PubMed] [Google Scholar]
- 18.Richards A. A., Stephens T., Charlton H. K., Jones A., Macdonald G. A., Prins J. B., Whitehead J. P. (2006) Mol. Endocrinol. 7, 1673–1687 [DOI] [PubMed] [Google Scholar]
- 19.Lowry O. H., Rosebrough N. J., Farr A. L., Randall R. J. (1951) J. Biol. Chem. 193, 265–275 [PubMed] [Google Scholar]
- 20.Tanaka A., Nose N., Watanabe A. (1980) J. Chromatogr. 194, 21–31 [DOI] [PubMed] [Google Scholar]
- 21.Laemmli U. K. (1970) Nature 227, 680–685 [DOI] [PubMed] [Google Scholar]
- 22.Kinter M., Sherman N. E. (2000) Protein Sequencing and Identification Using Tandem Mass Spectrometry, 1st Ed., pp. 147–165, John Wiley & Sons, Inc., New York [Google Scholar]
- 23.Choo H. J., Kim J. H., Kwon O. B., Lee C. S., Mun J. Y., Han S. S., Yoon Y. S., Yoon G., Choi K. M., Ko Y. G. (2006) Diabetologia 49, 784–791 [DOI] [PubMed] [Google Scholar]
- 24.Moien-Afshari F., Ghosh S., Elmi S., Rahman M. M., Sallam N., Khazaei M., Kieffer T. J., Brownsey R. W., Laher I. (2008) Diabetologia 51, 1327–1337 [DOI] [PubMed] [Google Scholar]
- 25.Rosca M. G., Mustata T. G., Kinter M. T., Ozdemir A. M., Kern T. S., Szweda L. I., Brownlee M., Monnier V. M., Weiss M. F. (2005) Am. J. Physiol. Renal. Physiol. 289, F420–F430 [DOI] [PubMed] [Google Scholar]
- 26.Hosogai N., Fukuhara A., Oshima K., Miyata Y., Tanaka S., Segawa K., Furukawa S., Tochino Y., Komuro R., Matsuda M., Shimomura I. (2007) Diabetes 56, 901–911 [DOI] [PubMed] [Google Scholar]
- 27.Pasarica M., Sereda O. R., Redman L. M., Albarado D. C., Hymel D. T., Roan L. E., Rood J. C., Burk D. H., Smith S. R. (2009) Diabetes 58, 718–725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Peake P. W., Hughes J. T., Shen Y., Charlesworth J. A. (2007) J. Mol. Endocrinol. 39, 45–52 [DOI] [PubMed] [Google Scholar]
- 29.Sato C., Yasukawa Z., Honda N., Matsuda T., Kitajima K. (2001) J. Biol. Chem. 276, 28849–28856 [DOI] [PubMed] [Google Scholar]
- 30.Wang Z. V., Schraw T. D., Kim J. Y., Khan T., Rajala M. W., Follenzi A., Scherer P. E. (2007) Mol. Cell. Biol. 27, 3716–3731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Scherer P. E., Williams S., Fogliano M., Baldini G., Lodish H. F. (1995) J. Biol. Chem. 270, 26746–26749 [DOI] [PubMed] [Google Scholar]
- 32.Wang Z. V., Scherer P. E. (2008) Proc. Natl. Acad. Sci. U.S.A. 105, 18077–18078 [DOI] [PMC free article] [PubMed] [Google Scholar]