Abstract
The structural and catalytic requirements for neutrophil MMP-9 proenzyme (proMMP-9) to induce angiogenesis were investigated using a quantitative angiogenesis model based on grafting of collagen onplants onto the chorioallantoic membrane of chick embryos. Both physiological activation of neutrophil proMMP-9 and proteolytic activity of the generated MMP-9 enzyme were critically dependent on the tissue inhibitor of metalloproteinase (TIMP)-free status of the zymogen. The presence of an intact active site and hemopexin domain were required for full angiogenesis-inducing activity of the MMP-9 enzyme. Timed additions of TIMP-1 to the onplants containing TIMP-free neutrophil proMMP-9 indicated that in vivo activation of the zymogen occurred during the first 24 h after grafting. Within the onplant tissue, MMP-9 activation was accompanied by proteolytic modifications of fibrillar collagen and an influx of host proteins, the rate of which depended on the TIMP-free status of the zymogen. By quantifying the levels of host angiogenic factors, we demonstrated that basic fibroblast growth factor (FGF-2) was a major cytokine becoming bioavailable in the onplant tissue undergoing a neutrophil proMMP-9-mediated angiogenic switch. Inhibition of angiogenesis with specific function-blocking antibodies further indicated an involvement of a FGF-2/FGFR-2 pathway in neutrophil proMMP-9-induced angiogenesis. The enhanced angiogenesis catalyzed by neutrophil MMP-9 appears to evoke also a localized, low threshold level vascular endothelial growth factor (VEGF)/VEGFR-2 pathway, likely functioning in the formation and/or stabilization of blood vessels. That neutrophil proMMP-9, unencumbered by TIMP-1, directly mediates FGF-2-dependent angiogenesis was also demonstrated in our quantitative mouse angiogenesis model employing subcutaneous collagen implants, thus implicating the novel TIMP-free MMP-9/FGF-2/FGFR-2 pathway in proMMP-9-induced angiogenesis in a mammalian setting.
Gelatinase B, or matrix metalloproteinase-9 (MMP-9),3 has been functionally and genetically linked to a number of critical biological functions, including wound healing, inflammation, tumor progression, vascular tissue remodeling, and angiogenesis (1–6). In particular, vascular tissue remodeling and angiogenesis, related histologically and mechanistically, have received a good deal of attention as MMP-9 has been reported to be a major trigger of the angiogenic switch (7). Phenotypic rescue of vasculogenic and angiogenic defects manifested in MMP-9 null mice has suggested a functional link between MMP-9 and the formation, structure, and remodeling of new blood vessels (7–15). The direct contribution of MMP-9 to angiogenesis and vascular performance is thought to involve catalytic activity of the enzyme resulting either in the cleavage of ECM components such as native and denatured collagens (16–18) and processing of various cytokines and chemokines such as CXCL5, CXCL6, and CXCL8 (interleukin-8) (19, 20) or release of angiogenic growth factors such as VEGF (7, 12, 15, 21–24). However, the actual in vivo substrates for MMP-9 pro-angiogenic activity have not been conclusively demonstrated.
At the site of primary tumor formation, the in vivo source of angiogenic MMP-9 also has not been precisely ascribed to a particular cell type. Although a number of different tumor cells express enhanced levels of proMMP-9 and active MMP-9, the presence of tumor-derived MMP-9 does not often directly correlate with the angiogenic potential of the developing tumor (25). Furthermore, other putative sources of MMP-9, i.e. stromal fibroblasts and vascular endothelial cells, do not normally express significant levels of proMMP-9 in the absence of potent transcriptional inducers (3). A potentially rich source of proMMP-9 could be specific inflammatory cells, e.g. infiltrating neutrophils and monocytes, which invariably migrate into pre-angiogenic tissue that has been compromised by different insults such as chronic infection, injury, or tumor-associated inflammation. Among influxing inflammatory cells, the infiltrating neutrophils are the ones which, in the absence of any transcriptional induction, are capable of rapidly releasing the pre-stored proMMP-9 so it is immediately available to exert its vascular remodeling and/or pro-angiogenic action.
Recent studies from Hanahan and co-workers (23, 26) have established a close link between the presence of MMP-9-expressing neutrophils and the onset of the angiogenic switch in developing tumors. The close association of MMP-9 with inflammatory cell-mediated tumor angiogenesis was also demonstrated in tumors where monocyte influx had been greatly diminished by specific blocking reagents and a compensatory neutrophil response was manifested (26). Therefore, inflammatory neutrophils could serve as an immediate and major source of MMP-9 in pre-angiogenic tissue. A question arises, however, as to whether neutrophil-derived MMP-9 itself is uniquely proangiogenic in vivo. By employing a modified chick embryo chorioallantoic membrane (CAM) angiogenesis assay (27), our laboratory demonstrated that human neutrophil proMMP-9 delivered in vivo as purified zymogen is potently angiogenic at low to subnanomolar levels (28). This angiogenic proMMP-9 is released operationally or naturally from human neutrophils in a unique, TIMP-free form, which is in contrast to the TIMP-1-complexed proMMP-9 produced by most other cell types including macrophages and tumor cells (2). It appears to be the TIMP-free nature of neutrophil proMMP-9 that determines its potent proangiogenic potential as this very distinct proMMP-9 form constitutes the major proangiogenic component of the entire human neutrophil-released contents, i.e. granule releasate (28). However, it remained unclear from these earlier studies whether neutrophil proMMP-9 becomes activated and, if activated, when and how MMP-9 enzyme induces angiogenesis mechanistically.
The present study biochemically addresses the above-mentioned issues and demonstrates that neutrophil proMMP-9, as long as it is free of TIMP-1, is activated within the pre-angiogenic tissue during the first 24 h after onplant grafting. Our new findings also demonstrate that the catalytically active, hemopexin domain-containing, full-length enzyme is required for angiogenic induction. Furthermore, the activated MMP-9 induces a differential influx of additional protein components into angiogenic tissue and proteolytically triggers a release of bioactive basic FGF (FGF-2). The released bioavailable FGF-2, acting through its cognate receptor FGFR-2, is the actual inducer of angiogenesis downstream of activated MMP-9. Enhanced angiogenic levels of VEGF do not appear in the MMP-9-treated tissue, but a VEGF/VEGFR pathway may function downstream of the generated FGF-2. Finally, the TIMP-free MMP-9/FGF-2 pathway of angiogenic induction was confirmed to operate not only in the chick CAM assay but also in vivo in a mammalian setting, i.e. in a mouse angiotube assay recently established in our laboratory (27).
EXPERIMENTAL PROCEDURES
Recombinant Proteins and Antibodies
Recombinant wild type (WT), E402A mutant (mutE), and domain deletion variants of human MMP-9 were constructed, isolated, and characterized as previously described (29). Recombinant human FGF-2 and VEGF (VEGF165) were purchased from PeproTech Inc. (Rocky Hill, NJ). Polyclonal rabbit antibody specific to VEGF (sc-507) was from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA). Goat antibodies specific for FGF-2 (AF-233-NA) and VEGFR-2 (AF-357) and monoclonal antibody (mAb) against FGFR-2 (MAB6843) were purchased from R&D Systems (Minneapolis, MN). Function-blocking mAb 7-11C and non-blocking mAb 8-3H and 6-6B, all specific to human MMP-9, were generated in our laboratory (30). Non-immune mouse (015-000-003) and rabbit (011-00-003) IgGs were from Jackson ImmunoResearch Laboratories, Inc. (West Grove, PA), and goat IgG (AB-108-C) was from R&D Systems.
Isolation of Human Neutrophils and Purification of Neutrophil proMMP-9
Neutrophils were isolated from peripheral blood of healthy donors using dextran sedimentation and gradient centrifugation essentially as described (31). Briefly, whole blood was mixed with 6% dextran/saline solution, and erythrocytes were allowed to sediment to the bottom of the tube. The remaining red blood cells in the supernatant were lysed with the buffer containing 0.15 m NH4Cl, 12 mm NaHCO3, and 100 μm Na2EDTA (all from Sigma-Aldrich). After a saline wash, the white blood cells were separated on Histopaque Ficoll 1.077 gradient by centrifugation at 420 × g for 15 min at ambient temperature. The pellet containing neutrophils was resuspended in PBS at 1 × 107 cells/ml. The purity of isolated neutrophils was verified by Hema 3 staining. To induce the release of their granule contents (releasate), the neutrophils were incubated for 1 h at 37 °C with 160 nm phorbol 12-myristate 13-acetate (Sigma-Aldrich) and centrifuged to pellet the cell ghosts and to recover the releasate. To purify MMP-9, neutrophil releasate was incubated at room temperature with gelatin-Sepharose (Amersham Biosciences). After 1–2 h of incubation with rotation, gelatin-Sepharose was pelleted and washed, and bound MMP-9 was eluted with 10% DMSO, PBS. The eluted MMP-9 was dialyzed against PBS in 3.5 kDa molecular mass cutoff dialysis cassettes overnight to remove DMSO. The purified MMP-9 was analyzed by silver staining, gelatin zymography, and Western blotting.
Formation of Neutrophil proMMP-9·TIMP-1 Complexes
The purified proMMP-9 was incubated with excess amounts of TIMP-1 or PBS (control) for 1–2 h at room temperature. The generated neutrophil proMMP-9·TIMP-1 complex was re-isolated by gelatin-Sepharose affinity chromatography, whereas excess TIMP-1 was retained in the flow-through fraction. The purified proMMP-9·TIMP-1 complex was analyzed by silver staining and Western blotting after PAGE under reducing condition. The stoichiometry of the complex was determined from the indicated levels of TIMP-1.
Biochemical Analyses of MMP-9
Purified neutrophil proMMP-9 and proMMP-9·TIMP-1 complex were analyzed by silver staining as described (32). For gelatin zymography, 1–2 ng of MMP-9 purified from neutrophils, recombinant WT, or mutant forms of MMP-9 and MMP-9·TIMP-1 complex were mixed with 5× SDS sample buffer and analyzed on 8% SDS-PAGE gels containing 0.8% gelatin. After electrophoresis, gels were washed twice in 2.5% Triton X-100 for 30 min at room temperature and incubated overnight at 37οC in 50 mm Tris, pH 7.4, 150 mm NaCl, 5 mm CaCl2. Gels were stained with Coomassie Blue G250. Areas devoid of staining corresponded to gelatinolytic bands. Western blot analyses were performed with a mixture of pooled mAbs specific for human MMP-9 (7-11C, 8-3H, and 6-6B) and with a mAb against human TIMP-1 (EMD Biosciences, San Diego, CA). Proteins were separated in 4–20% SDS-PAGE gels and transferred to polyvinylidene difluoride membranes. After blocking with 5% nonfat dry milk, PBS, 0.1% Tween 20, the membranes were incubated with the primary mAb at 1 μg/ml, washed, and incubated with horseradish peroxidase-conjugated goat anti-mouse IgG. Membranes were developed with SuperSignal West Pico Chemiluminescent substrate (Pierce).
Angiogenesis Assay in Chick Embryos
All procedures involving animals were performed in compliance with the protocol approved by Institutional Animal Care and Use Committees of The Scripps Research Institute. CAM angiogenesis assay was performed using shell-free chick embryos as described (27). Briefly, fertilized White Leghorn chicken eggs (Charles River Laboratories, North Franklin, CT) were incubated in a rotating incubator at 38 °C and 85% humidity. At day 3, the contents of the eggs were transferred to sterile plastic weigh boats, covered with a square Petri dish, and placed in a stationary incubator until day 10 when the embryos were grafted with collagen onplants. To prepare the collagen mixture for onplants, 8 volumes of type I rat tail collagen solubilized in acetic acid solution (BD Biosciences) were neutralized with 1 volume of 10× minimum essential medium (BioWhittaker Inc., Walkersville, MD) and 1 volume of 0.1 n NaOH/water to pH 7.2–7.4. The final mixture contained collagen at a concentration of 2 mg/ml and was supplemented with 25 mm HEPES buffer, pH 7.4 (Invitrogen). Pro-angiogenic stimuli, e.g. growth factors, purified neutrophil proMMP-9, recombinant wild type and mutant forms of MMP-9, alone or with function-blocking antibodies were incorporated into collagen mixture at final concentrations indicated in the corresponding Fig. legends. Thirty-μl aliquots of collagen mixtures were distributed over 2 gridded nylon meshes (bottom 4 × 4 mm and top 3 × 3 mm) and allowed to polymerize at 37 °C, generating so-called collagen onplants. The collagen onplants were grafted onto the CAM surface of day-10 chick embryos incubated ex ovo. Angiogenic, i.e. newly formed, blood vessels grown upward into collagen onplants from the pre-existing CAM vasculature were scored 70–92 h after onplant grafting as described (33). Several independent experiments involving 5–6 embryos, each grafted with 5–6 collagen onplants, were performed for each individual variable or time point.
Morphological Analysis of Angiogenic Vasculature in Collagen Onplants
Angiogenesis was induced by neutrophil proMMP-9, FGF-2, and VEGF incorporated into collagen at 2.5, 10, and 15 ng per onplant, respectively. Control onplants were supplemented with no growth factors. After scoring of angiogenesis, collagen onplants with the surrounding CAM were excised and visualized without tissue fixation in the Olympus BX60 microscope equipped with a digital video camera. The digital images of angiogenic vasculature were captured and processed with Adobe Photoshop CS2 software. The diameters of maximally dilated blood vessels were measured and related to the length of a 100-μm bar embedded into the images. The levels of dilation in the angiogenic vessels induced by different growth factors are expressed as average diameters of maximally dilated vessels in corresponding conditions.
Biochemical Analyses of Collagen Onplants after in Vivo Incubation
Collagen onplants were harvested at the indicated time points and extracted with detergent lysis buffer (50 mm Tris, pH 7.4, 150 nm NaCl, 1% Triton X-100, 1 mm EDTA, and protease inhibitors; all from Sigma) and centrifuged. The soluble fraction was analyzed by silver staining, Western blotting, zymography, or ELISA. VEGF and FGF-2 levels in the extracts were quantified with corresponding capture ELISA kits according to the manufacturer's instructions (PeproTech, Rocky Hill, NJ; catalog numbers 900-K10 and 900-K08, respectively).
Analysis of Catalytic Activity of MMP-9 in Collagen/Gelatin Onplants
Gelatin was prepared from neutralized type I collagen by heating at 100 °C for 5 min. Collagen/gelatin onplants (2 mg/ml type I collagen and 0.5 mg/ml gelatin) were supplemented with TIMP-free neutrophil proMMP-9 or neutrophil proMMP-9·TIMP-1 complex. For in vitro analyses, recombinant MMP-3 was additionally incorporated at a 1:10 molar ratio to proMMP-9. For in vivo analyses, the collagen/gelatin onplants containing no MMP-3 were grafted on the CAM of day-10 chick embryos. In both in vivo and in vitro experiments, the onplants were harvested at the indicated time points, extracted with SDS sample buffer, and analyzed by SDS-PAGE zymography and protein staining. Cleavage of gelatin, a preferred substrate of active MMP-9, was monitored by the disappearance of α- and β-gelatin/collagen chains and accumulation of low molecular weight proteins/peptides.
Angiogenesis Assay in Mice
The assays were performed essentially as described (27). Inert silicon tubes were filled with native type I collagen supplemented with tested purified molecules (angiotubes). Angiotubes were surgically inserted under the skin of immunodeficient nude mice (2 per each side of the back). 10–12 days later the mice were sacrificed, and tubes under the skin were exposed and excised. Because newly formed blood-bearing vessels grow into the angiotubes from the preexisting skin vasculature, measuring hemoglobin content was used to determine levels of angiogenesis. The contents of individual angiotubes were lysed with 0.2% Triton X-100, and hemoglobin concentration in the lysates was determined with QuantiChrom hemoglobin assay kit (DIHB-250) according to the manufacturer's instructions (BioAssay Systems, Hayward, CA).
Data Presentation and Statistical Analysis
Angiogenesis data are presented as scatter plots with each point representing angiogenesis index of individual onplants or as bar graphs with the mean ± S.E. representing -fold differences or percentage of inhibition of angiogenesis. -Fold differences were determined as a ratio of angiogenic index observed in an individual onplant over mean of control (1.0) determined for an individual experiment. Statistical differences between groups of data were analyzed by Student's t test using Prism Program (GraphPad Software, San Diego, CA). The differences between the data sets were considered significant for p < 0.05.
RESULTS
Structural and Catalytic Requirements for MMP-9 Ability to Induce Angiogenesis
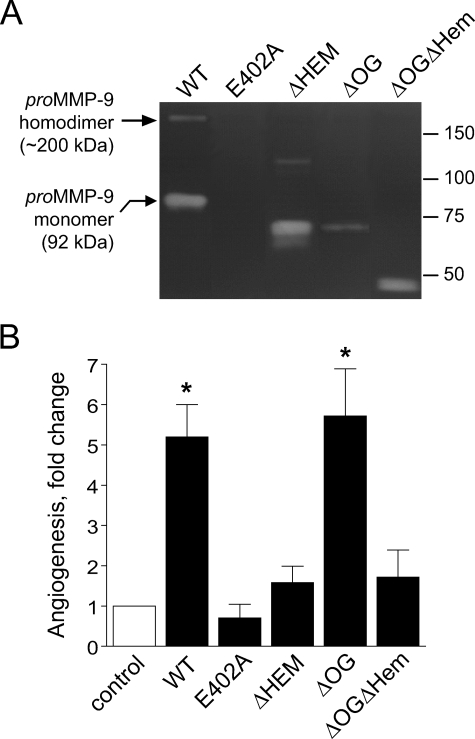
Low levels (0.2–1.0 nm) of purified neutrophil-derived proMMP-9 were previously shown to be highly angiogenic in the chick embryo CAM model (28). To ascertain the overall structural and catalytic requirements for MMP-9 proenzyme to exert its angiogenic capabilities, a number of recombinant mutants and domain variants of human MMP-9 were tested at 1 nm in the in vivo angiogenesis assay (Fig. 1). The mutation of the Glu402 glutamic acid residue to alanine in the catalytic domain of MMP-9 (E402A), rendering the molecule enzymatically inactive (Fig. 1A), completely abrogated the ability of MMP-9 to induce angiogenesis (Fig. 1B). The MMP-9 variant without the hemopexin domain (ΔHem), although maintaining its full gelatinolytic activity, was substantially diminished in its angiogenesis-inducing ability. Interestingly, the variant missing the 64-residue-long linker domain containing all the O-linked glycans (ΔOG), unique for MMP-9, exhibited angiogenesis-inducing activity equal to full-length wild type MMP-9 (WT). Although the gelatinolytic activity of ΔOG-MMP-9 in zymographs is reduced, its native enzymatic activity against soluble substrates has been shown similar to that of WT-MMP-9 (29). When both OG and hemopexin domains of MMP-9 were absent (ΔOGΔHem), the angiogenic capability of MMP-9 was reduced to the low levels manifested by the ΔHem-MMP-9 variant (Fig. 1B). These results demonstrate that the catalytic activity of MMP-9 is required for its angiogenic ability, thus indicating that activation of proMMP-9 must also occur in vivo. Furthermore, these findings demonstrate that the presence of the hemopexin domain is necessary for full angiogenesis-inducing capacity of the MMP-9 enzyme, whereas the MMP-9-distinct OG domain appears unnecessary.
FIGURE 1.
Enzymatic and functional analyses of recombinant forms of human MMP-9. A, zymography analysis of MMP-9 variants. The full-length WT and catalytically inactive mutant (E402A) of MMP-9 and domain-deletion MMP-9 variants lacking the hemopexin domain (ΔHem), the linker domain containing O-linked glycans (ΔOG), and both OG and Hem domains (ΔOGΔHem) were analyzed by gelatin zymography (all at 0.02 pmol/lane). Arrows on the left indicate the positions of homodimer and monomer forms of wild type proMMP-9. Positions of the molecular mass markers in kDa are indicated on the right. B, angiogenic potential of MMP-9 variants. Individual recombinant MMP-9 proteins were added at 1–2 ng per collagen onplant (0.4–0.7 nm). The onplants were grafted on the CAM of day-10 chick embryos, and angiogenic blood vessels were scored within 72–96 h. Data are presented as -fold changes in angiogenesis (means ± S.E. from three independent experiments) over control levels in onplants supplemented with no MMP-9. *, p < 0.01.
Binding of TIMP-1 to Neutrophil ProMMP-9 Negatively Regulates the Angiogenesis-inducing Capability of the Zymogen
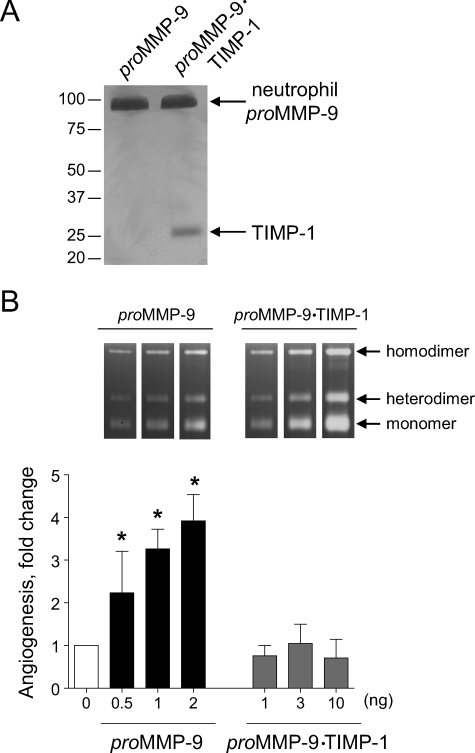
We had previously shown that neutrophil proMMP-9 complexed with TIMP-1 failed to induce angiogenesis when compared with near equivalent molar levels of TIMP-free proMMP-9 (28). However, it remained unclear whether, if added in excess, the proMMP-9·TIMP-1 complex would eventually escape the TIMP-1 restriction and yield MMP-9 capable of inducing angiogenesis. To address this very distinct possibility, a comparative analysis of angiogenic response to increasing doses of TIMP-free proMMP-9 versus proMMP-9 complexed with TIMP-1 was performed (Fig. 2). Silver-stained polyacrylamide gels of the two purified preparations of neutrophil proMMP-9 confirmed the presence of 28-kDa TIMP-1 in the proMMP-9·TIMP-1 complex in equal molar levels to the 92-kDa proMMP-9 zymogen (Fig. 2A). This TIMP-complexed proMMP-9 failed to induce any angiogenesis above control levels even if added at a 20-fold excess (10 ng/onplant) over TIMP-free proMMP-9, which was angiogenic even at a subnanogram level (0.5 ng/onplant) and induced angiogenesis in a dose-dependent manner up to 2 ng/onplant (Fig. 2B). Although the enzymatic signal of the proMMP-9·TIMP-1 complex matched that of the TIMP-free proMMP-9 in gelatin zymographs (insets in Fig. 2B), no induction of angiogenesis occurred when levels of gelatinolytic activity of TIMP-1-complexed proMMP-9 far exceeded those of the TIMP-free proMMP-9. Therefore, the presence or absence of the tightly bound TIMP-1 molecule in the proMMP-9 preparation appears to be the critical determinant of the MMP-9 angiogenic capacity in vivo.
FIGURE 2.
Angiogenesis-inducing capacity of neutrophil proMMP-9 critically depends on TIMP-free status of the zymogen. A, silver staining of neutrophil TIMP-free proMMP-9 and proMMP-9 complexed at 1:1 molar ratio with TIMP-1. Neutrophil proMMP-9 and its stoichiometric complex with TIMP-1 were purified by gelatin-Sepharose affinity chromatography and were visualized by silver staining after SDS-PAGE under reducing conditions. Positions of protein bands corresponding to the monomer of proMMP-9 (92 kDa) and TIMP-1 (28 kDa) are indicated on the right. Positions of molecular mass markers in kDa are indicated on the left. B, angiogenic potential of the neutrophil proMMP-9 and proMMP-9·TIMP-1 complex. Purified proMMP-9 (black bars) and a stoichiometric complex of proMMP-9 with TIMP-1 (gray bars) were incorporated into collagen onplants at increasing concentrations indicated at the bottom of the graph (ng per onplant). Control onplants (open bar; 0 ng) were not supplemented with any additional exogenous factors. The levels of angiogenesis were determined at 70–76 h after grafting onplants onto the CAM of chick embryos. Data are presented as -fold changes in angiogenesis over control levels (means ± S.E. from 2 independent experiments). *, p < 0.05. Inset, zymographs depict gelatinolytic activity of MMP-9 preparations (equivalent of 1 onplant/lane). Positions of the homodimer, heterodimer, and monomer of MMP-9 are indicated on the right.
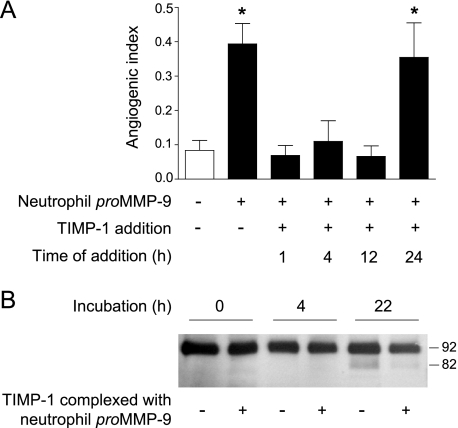
Taking advantage of high affinity binding of TIMP-1 to proMMP-9 and the accessibility of the chick embryo for in vivo intervention, we next addressed when precisely activation of proMMP-9 occurs after grafting of angiogenic onplants (Fig. 3). Timed applications of TIMP-1 into collagen onplants containing TIMP-free neutrophil proMMP-9 completely abrogated induction of angiogenesis over control levels when applied at 1, 4, or 12 h after grafting of onplants, whereas the addition of TIMP-1 at 24 h (Fig. 3A) or later (data not shown) had little or no effect on MMP-9-induced angiogenesis. These data indicate that neutrophil proMMP-9 becomes activated and exerts its catalytic/proteolytic activity within the first 24 h after onplant grafting. That the presence of stoichiometric amounts of TIMP-1 can indeed inhibit processing and activation of neutrophil proMMP-9 in vivo is illustrated in the Western blot time course (Fig. 3B). The processed 82-kDa activated form of MMP-9 was identified at 22 h after grafting collagen onplants supplemented with TIMP-free proMMP-9. In contrast, little to no processed MMP-9 was detected at 22 h in onplants supplemented with equivalent molar levels of proMMP-9·TIMP-1 complex.
FIGURE 3.
TIMP-free neutrophil proMMP-9 exerts angiogenic activity within first 24 h after onplant grafting. A, time-course analysis of TIMP-1-mediated inhibition of angiogenesis. Collagen onplants were supplemented with neutrophil proMMP-9 (2 ng/onplant) and grafted on the CAM. At the indicated time points (h), TIMP-1 was applied at 3× molar excess (+) over neutrophil proMMP-9 (solid bars). Control onplants contained collagen with no MMP-9 (open bar). The data are presented as the means ± S.E. of the angiogenic index determined 77 h after onplant grafting. Presented is one of two independent experiments. *, p < 0.05. B, time-course analysis of the activation of TIMP-free proMMP-9 in collagen onplants. Neutrophil proMMP-9 alone or in a stoichiometric complex with TIMP-1 (+) was incorporated into collagen onplants at 3 nm. At the indicated time periods after grafting on the CAM, the onplants were harvested, and their contents extracted with detergent lysis buffer. The MMP-9 activation in the extracts was analyzed by Western blotting with a mixture of MMP-9-specific mAbs (7-11C, 8-3H, and 6-6B) after SDS-PAGE under reducing conditions. Positions of MMP-9 zymogen (92 kDa) and activated enzyme (82 kDa) are indicated on the right.
TIMP-1 Bound to Neutrophil ProMMP-9 Diminishes Its Activation and Prevents MMP-9 Catalytic Activity in Collagen Onplants
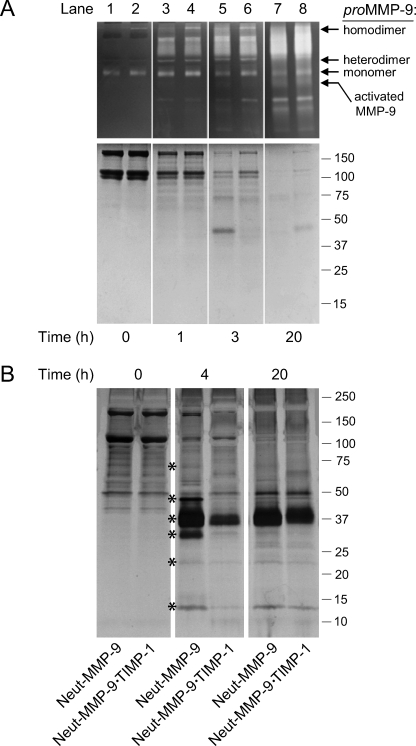
To directly relate the anti-angiogenic role of TIMP-1 complexed to neutrophil proMMP-9 with its ability to prevent or delay the MMP-9 zymogen activation, we have attempted to monitor the actual activation of proMMP-9 and its resulting gelatinolytic activity within the three-dimensional milieu of the collagen onplants. This was accomplished by constructing collagen onplants supplemented with gelatin prepared from the same type I collagen used in onplants. Because gelatin is the preferred substrate of the active MMP-9 gelatinase, any cleavage of gelatin in the mixed onplant served as a sensitive readout for activated MMP-9. Equivalent molar levels of neutrophil proMMP-9 either free of TIMP-1 or stoichiometrically complexed with TIMP-1 were incorporated into the mixed collagen/gelatin onplants. To physiologically activate the proMMP-9, recombinant MMP-3 enzyme was employed and added to all onplants, which were incubated at 37 °C for 2, 4, and 8 h, at which time their contents were extracted with SDS sample buffer and analyzed by both zymography and protein staining (supplemental Fig. 1). The zymographs, illustrating the time course of MMP-3-mediated activation of proMMP-9, clearly indicate that the 82-kDa activated form of MMP-9 appears in the TIMP-free preparation as early as 4 h and is well represented at 8 h. Importantly, the MMP-9·TIMP-1 complex does not manifest this activated form of MMP-9 even after 8 h and only marginally undergoes MMP-3-mediated processing thereafter. When the same onplant extracts were also analyzed by SDS-PAGE, the collagen/gelatin protein bands were slightly diminished in TIMP-free MMP-9 onplants by 4 h and substantially degraded by 8 h, accompanied by the appearance of fragments of lower molecular weight. No such protein changes occurred either by 4 or 8 h of incubation in the onplants supplemented with MMP-9·TIMP-1 complex. These protein-banding modifications in onplants corresponded closely to the proenzyme/active enzyme changes in the gelatin zymography, indicating that the generation of the 82-kDa active form of MMP-9 in tissue-like, three-dimensional collagen gels leads to the coordinate appearance of cleaved collagen/gelatin fragments (supplemental Fig. 1).
To examine the regulatory effects of TIMP-1 on proMMP-9 in actual tissue in vivo, we monitored the activation of zymogen and resulting gelatinolytic activity in collagen/gelatin onplants grafted onto the CAM of live embryos. Because TIMP-free proMMP-9 appears to undergo natural activation in vivo (Fig. 3B), exogenous MMP-3 was not added to the onplants before grafting. The onplants were incubated on the CAM for 1, 3, and 20 h followed by extraction with SDS sample buffer and analyses by zymography and Coomassie Blue staining (Fig. 4A). The proMMP-9 in both TIMP-free and TIMP-1-complexed preparations appeared in the zymographs mainly as the 92-kDa monomers at time 0 and throughout the first 3 h of the time course, after which the monomers become less pronounced (Fig. 4A, top panels). However, host gelatinases, visible as broad >125 kDa lytic zones in the zymographs, appeared to influx into the onplants as early as 1 h and progressively increased with time, whereas the 92-kDa proMMP-9 diminished over time (lanes 3–8). In the protein gels collagen/gelatin protein bands are less intensely stained after 1 h on the CAM compared with time 0, but the collagen chains appear intact (Fig. 4A, bottom, lanes 1–4). By 3 h the collagen/gelatin bands have been cleaved to a significant extent in the onplants containing TIMP-free proMMP-9 with some lower mol mass fragments appearing between 40 and 60 kDa (Fig. 4A, bottom, lane 5). This clearing and fragmentation is much less extensive in onplants supplemented with proMMP-9·TIMP-1 complex (lane 6). After 20 h in vivo, the collagen/gelatin protein bands are almost completely cleared in both sets of onplants (Fig. 4B, bottom, lanes 7 and 8). However, because onplants containing proMMP-9·TIMP-1 complexes do not yield any induced angiogenesis (Fig. 2B), this complete proteolytic clearing of the collagen/gelatin is apparently not the rate-limiting event during induction of angiogenesis. These in vitro and in vivo time courses indicate clearly that demonstrable MMP-9 proteolytic activity is generated differentially in the TIMP-free proMMP-9 angiogenic onplants as compared with the proMMP-9·TIMP-1 complex-containing onplants, and such differential activity appears to be the critical angiogenesis-inducing event.
FIGURE 4.
Activation and proteolytic activity of neutrophil proMMP-9 and influx of host proteins into angiogenic onplants depends on TIMP-free status of the zymogen. A, time course of activation and gelatinolytic activity of neutrophil proMMP-9 and proMMP-9·TIMP-1 complex in vivo. The purified TIMP-free proMMP-9 (lanes 1, 3, 5, and 7) and proMMP-9·TIMP-1 complex (lanes 2, 4, 6, and 8) were incorporated at 3 nm into collagen onplants, which were grafted onto the CAM of chick embryos. At the indicated time intervals (h), the onplants were harvested from the CAM, extracted, processed, and analyzed by gelatin zymography (top panels) and Coomassie staining (bottom panel) of an SDS-PAGE gel run under reducing conditions. Positions of the proMMP-9 homodimer, heterodimer, and monomer and the activated MMP-9 enzyme are indicated on the right of gelatin zymographs. Positions of molecular mass markers in kDa are indicated on the right. B, influx of host proteins into collagen onplants supplemented with neutrophil proMMP-9. Collagen onplants were supplemented with TIMP-free neutrophil proMMP-9 (Neut-MMP-9) or neutrophil proMMP-9 complexed with TIMP-1 (Neut-MMP-9·TIMP-1) at 0.7 nm. At indicated time points (h) after grafting on the CAM, the onplants were harvested and extracted. The onplant extracts were separated by SDS-PAGE under non-reducing conditions, and the protein bands were visualized by silver staining. The positions of host proteins differentially influxed into the onplants containing Neut-MMP-9 versus Neut-MMP-9·TIMP-1 are indicated by asterisks. Positions of molecular weight markers (in kDa) are indicated on the right. White vertical lanes separating individual time points were placed artificially over the images of whole gels for visual purposes (A and B).
MMP-9-dependent Influx of Host Proteins into Angiogenic Onplants
We next determined whether MMP-9-dependent changes of overall protein composition in collagen onplants occur in the course of in vivo angiogenesis. To this end, collagen onplants were supplemented with either neutrophil proMMP-9 naturally free of TIMP-1 (Neut-MMP-9) or neutrophil proMMP-9 complexed with TIMP-1 (Neut-MMP-9·TIMP-1) (Fig. 4B). At various time points after grafting on the CAM, the onplants were harvested, extracted in detergent buffer, and analyzed in silver-stained SDS-PAGE gels. The major protein component of these onplants, i.e. type I native fibrillar collagen, presented in the gels as the 110–120-kDa α chains and the 220–240-kDa β chains, was cleared from the onplants within 20 h of incubation (Fig. 4B). However, there was an active influx of host proteins into the onplants. Importantly, the onplants supplemented with Neut-MMP-9 were differentially influxed with an array of proteins ranging from 15 to 70 kDa. Among those, the protein bands of ∼13, 25, 33, 37, 45, and 65 kDa were distinctly enhanced in the TIMP-free Neut-MMP-9 onplants as compared with onplants supplemented with Neut-MMP-9·TIMP-1 (asterisks in Fig. 4B). However, this differential in the influx of host proteins did not persist, and at later time points the overall protein pattern became almost indistinguishable between the Neut-MMP-9 and Neut-MMP-9·TIMP-1 onplants.
Induction of Angiogenesis by Neutrophil proMMP-9 Involves Released FGF-2
The above-described, SDS gel-based protein analyses demonstrated an apparent influx of host proteins dependent on the TIMP-1 status of MMP-9, suggesting that active MMP-9 may have induced angiogenesis indirectly, i.e. one or a few host proteins influxing in response to TIMP-free MMP-9 may actually function as the direct angiogenesis inducer(s). A full scale microproteomic analysis will have to be carried out by mass spectrometry methods to identify individually the wide array of host (chicken) proteins entering the onplants and will constitute a separate study when the chicken protein data base is fully complete. However, it is known that host angiogenic growth factors such as VEGF and FGF-2 can become bioavailable in tissues undergoing protease-mediated angiogenic switch. Therefore, we have attempted detection and time-dependent analysis of VEGF and FGF-2 within the angiogenic onplants supplemented with neutrophil proMMP-9.
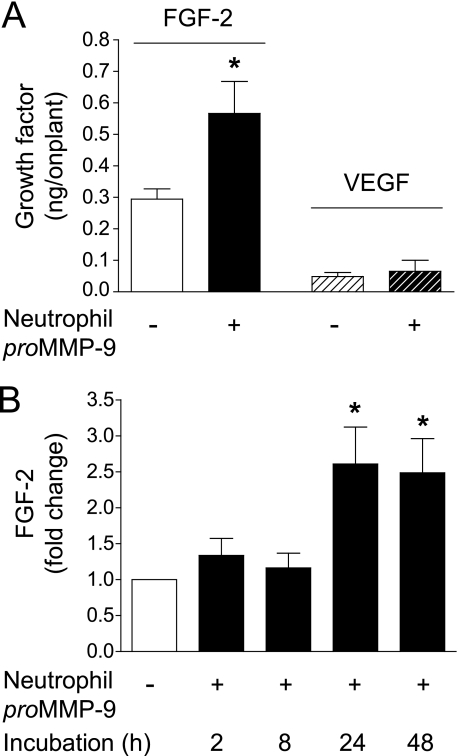
To monitor the appearance and levels of VEGF and FGF-2, we have employed highly sensitive immuno-capture ELISA (Fig. 5). The addition of neutrophil proMMP-9 into the onplants only marginally enhanced the VEGF levels but significantly increased the FGF-2 levels as compared with the no MMP-9 control (Fig. 5A). In separate experiments the efficacy of growth factor extraction from the CAM onplant tissue was shown to be ∼10% that of exogenously added factor; thus, the actual levels of FGF-2 induced in the onplants containing proMMP-9 could be as high as 5–6 ng/onplant. Interestingly, as measured by ELISA, the increased FGF-2 levels were more than 10-fold higher than those of VEGF in MMP-9-supplemented onplants. The levels of VEGF recovered in the onplant extracts (20–40 pg) were often at or below the threshold of detection in the immuno-capture assay, and even taking into account the efficiency of extraction, 200–400 pg of VEGF would be below the physiological levels necessary for angiogenic induction. Therefore, detailed kinetics of VEGF, influxed or generated at lower than threshold levels, was not evaluated. However, the kinetics of MMP-9-dependent appearance of FGF-2 indicate a substantial 2.0–2.5-fold increase in FGF-2 levels between 8 and 24 h after onplant grafting (Fig. 5B), consistent with the time period that proMMP-9 is activated and exerts its catalytic activity.
FIGURE 5.
Increase in bioavailable FGF-2 in MMP-9-supplemented collagen onplants. A, neutrophil proMMP-9 mediates increase of bioavailable FGF-2 in angiogenic onplants. Collagen onplants were supplemented with 2 ng of neutrophil proMMP-9 (+) or buffer control only (−). After 24-h incubation on the CAM, the onplants were harvested and extracted with detergent lysis buffer. The levels of FGF-2 and VEGF were measured in the extracts by a capture ELISA. B, neutrophil proMMP-9 induces time-dependent accumulation of FGF-2. Collagen onplants with (+) or without (−) 2 ng of neutrophil proMMP-9 were harvested at the indicated time points (h) after grafting on the CAM. The onplant extracts were analyzed for the levels of FGF-2 by a specific capture ELISA. *, p < 0.01.
Morphological Characteristics of Neovasculature Induced by Different Angiogenic Factors
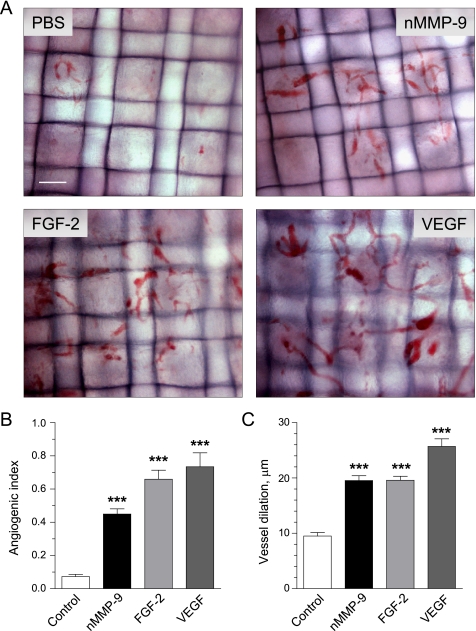
FGF-2 induced by neutrophil proMMP-9 could be an actual angiogenic factor responsible for neovascularization of collagen onplants. Therefore, we compared the efficiency of different pro-angiogenic factors in the CAM angiogenesis model (Fig. 6). Neutrophil MMP-9, FGF-2, and VEGF were supplemented to collagen onplants, which were evaluated 72 h after grafting on the CAM in comparison to control onplants containing no exogenously added growth factors. As could be seen in Fig. 6A, newly formed blood vessels induced by all three factors have similar morphology: they are tortuous and distorted and able to span the length of more than one grid. The vasculature densities in different types of onplants reflected in angiogenic indices are presented in Fig. 6B, confirming that purified FGF-2 and VEGF added at concentrations of 10 and 15 ng/onplant, respectively, are potent angiogenic growth factors. That neutrophil proMMP-9 at 2–4 ng/onplant induced angiogenesis at levels comparable with those induced by exogenous FGF-2 indicates that this MMP is indeed a very potent proangiogenic factor. The visible difference in the angiogenic vasculatures was related mainly to the level of vessel dilation whereby VEGF-induced vessels manifested maximal diameter (on average 25 ± 0.7 μm), whereas proMMP-9- and FGF-2-induced capillaries were of similar diameter in dilated sites (20 ± 0.6 μm versus 20 ± 0.5 μm) (Fig. 6C). However, all angiogenic vessels, including those induced by VEGF, were not obviously leaky as no extravasated erythrocytes were observed around vessels even around the highly dilated capillary structures. Thus, morphological analysis indicates that neutrophil proMMP-9 induces a clear angiogenic vasculature, the morphology of which more closely resembles that induced by FGF-2 than VEGF.
FIGURE 6.
Morphological analysis of angiogenic vasculature induced by different growth factors and neutrophil proMMP-9. Angiogenesis was induced by neutrophil proMMP-9 (nMMP-9), FGF-2, and VEGF incorporated into collagen mixture at 2.5, 10, and 15 ng per onplant, respectively. Control onplants were supplemented with no growth factors. A, morphology of blood vessels in collagen onplants. Angiogenic vasculature was visualized in the plane of the upper mesh, and digital images were captured at original 100× magnification. Bar, 100 μm. B, levels of angiogenesis induced by different pro-angiogenic molecules were determined as angiogenic indices at 72 h after onplants were grafted on the CAM. Presented are cumulative data (means ± S.E.) from 4 independent experiments performed with 4–6 embryos (each carrying 5–7 onplants) per condition. C, blood vessel dilation was determined in the digital images by measuring the diameters of maximally enlarged vessels. Data are the means ± S.E. from a representative experiment. ***, p < 0.001.
FGF-2 and VEGF Pathways Are Involved in Angiogenesis Induced by Neutrophil proMMP-9
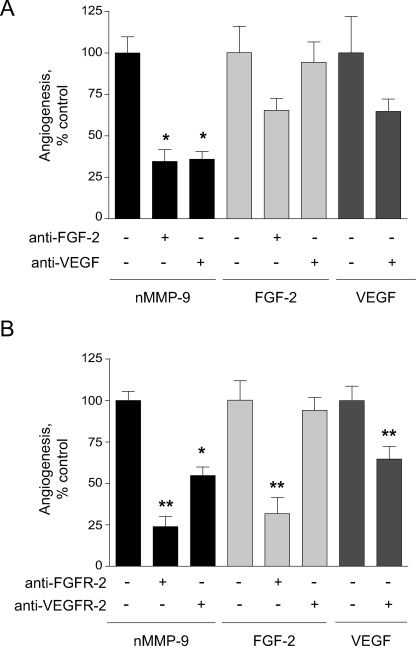
Because FGF-2 was significantly elevated by exogenously added MMP-9, we verified whether angiogenesis catalyzed by MMP-9 was indeed mediated through FGF-2. In addition, because it is known that in mammalian model systems FGF-2-induced angiogenesis requires VEGF signaling (34), we analyzed whether VEGF is also involved in the angiogenesis mediated by MMP-9 in the CAM of the chick embryo. Fig. 7A illustrates efficient inhibition of neutrophil proMMP-9-induced angiogenesis by a function-blocking anti-FGF-2 antibody. The addition of anti-FGF-2 and anti-VEGF antibodies resulted in the substantial inhibition of angiogenesis in response to exogenous FGF-2 and VEGF, respectively (Fig. 7A). However, although anti-VEGF antibody effectively blocked MMP-9-induced angiogenesis, this antibody was not effective in inhibition of FGF-2-induced angiogenesis. These data indicate that although neutrophil proMMP-9 potently enhances the levels of FGF-2, which are likely to be responsible for angiogenesis induction, blocking VEGF also results in a substantial decrease of angiogenesis. However, the total levels of VEGF in the onplants remain at marginal or below threshold levels (Fig. 5).
FIGURE 7.
Neutrophil proMMP-9 induces angiogenesis via FGF-2-mediated pathway. To induce angiogenesis, collagen onplants were supplemented with neutrophil proMMP-9 (nMMP-9, 2 ng) (black bars), FGF-2 (10 ng) (light gray bars), and VEGF (15 ng) (dark gray bars). Additionally, function-blocking anti-FGF-2 or anti-VEGF antibodies (A) and anti-FGFR-2 or VEGFR-2 antibodies (B) were incorporated (+) at 20 μg/ml to probe for the involvement of FGF-2/FGFR-2 and VEGF/VEGFR-2 pathways in MMP-9-induced angiogenesis. Non-immune IgGs were added in control (−/−) onplants. Inhibition of angiogenesis is presented as a percentage of control (100%) in the absence (–/–) of function-blocking antibodies determined from cumulative data from 3 to 9 independent experiments for each condition variant. Data are the means ± S.E. * and **, p < 0.05 and p < 0.01, respectively.
The involvement of the FGF-2 and VEGF pathways in neutrophil proMMP-9-mediated angiogenesis was further corroborated by inhibitory effects of a neutralizing antibody that blocks interaction of the growth factors with their cognate receptors, FGFR-2 and VEGFR-2, respectively (Fig. 7B). Both antibodies blocked MMP-9-induced angiogenesis, although the anti-FGFR-2 antibody was more efficient than anti-VEGFR-2 antibody, which in turn only partially blocked VEGF-induced angiogenesis (Fig. 7B). In addition, the anti-FGFR-2 antibody efficiently blocked angiogenesis induced by human recombinant, TIMP-free MMP-9 added at levels equal in angiogenic potency to neutrophil proMMP-9 (data not shown). Interestingly, inhibition of FGF-2-induced angiogenesis was effective only with anti-FGFR-2, but not anti-VEGFR-2 antibody, suggesting certain differences in signaling pathways involved in angiogenesis induced by exogenously added MMP-9 and FGF-2. Altogether, these results clearly indicate that TIMP-free proMMP-9 induces angiogenesis that involves mainly host FGF-2 generated in an MMP-9-dependent manner and acting through its specific receptor, FGFR-2, but does not exclude an involvement of VEGF/VEGFR-2 signaling pathway likely induced downstream of MMP-9 and FGF-2 and possibly involving a highly localized VEGF, the levels of which are below detection limits of our system.
TIMP-free Neutrophil ProMMP-9 Induces Angiogenesis via FGF-2 in the Mouse Model
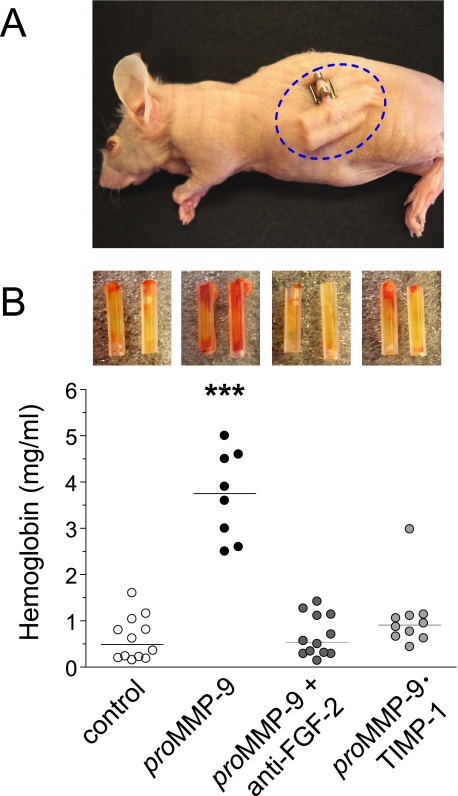
We next attempted to confirm in a mammalian model system that the pro-angiogenic mechanisms of neutrophil proMMP-9 depend on the TIMP-free status of the MMP-9 proenzyme and involve the induction of FGF-2. To this end, we used in vivo angiogenic assays employing collagen-filled silicon tubes (angiotubes) subcutaneously implanted into immunodeficient mice (Fig. 8A). In this assay the angiogenic response to pro-angiogenic growth factors is manifested by the growth of new blood vessels into the open-ended angiotubes (insets in Fig. 8B). The levels of angiogenesis are measured by the amount of hemoglobin extracted from the tubes 10–12 days after implantation. A significant 7–8-fold induction of angiogenesis was observed when angiotubes were filled with collagen supplemented with neutrophil TIMP-free proMMP-9 as compared with control tubes filled with collagen only (Fig. 8B). However, complexing neutrophil proMMP-9 with TIMP-1 almost completely abrogated MMP-9-induced angiogenesis. Furthermore, the addition of anti-FGF-2 antibody to the collagen-MMP-9 mixture prevented any induction of angiogenesis above the no MMP-9 control. Therefore, our major findings implicating the TIMP-free neutrophil MMP-9/FGF-2 angiogenic pathway in the avian model system were quantitatively verified within the mammalian vascular/stromal microenvironment.
FIGURE 8.
Neutrophil TIMP-free MMP-9 induces FGF-2-mediated angiogenesis in a murine angiotube model. A, immunodeficient athymic nu/nu mice were implanted subcutaneously with 4 silicon tubes (2 angiotubes per each flank) filled with collagen containing 3 nm neutrophil proMMP-9 alone or with anti-FGF-2 function-blocking antibody (50 μg/ml) or collagen supplemented with 3 nm neutrophil proMMP-9·TIMP-1 complex. B, after a 10–12-day incubation, the angiotubes were excised and photographed (insets above the corresponding scattergrams), and their hemoglobin contents were measured to determine relative levels of angiogenesis (scattergraph). ***, p < 0.0001 in comparison with all other variants.
DISCUSSION
The unique capability of neutrophil proMMP-9 to function as a rapid and potent angiogenesis inducing factor is mechanistically illustrated in this study. We initially investigated the structural and catalytic requirements for MMP-9 to induce angiogenesis. Through the use of a specific proMMP-9 active site mutant, the catalytic activity of MMP-9 was shown to be necessary for angiogenic induction. This result, although not unexpected, required affirmation as the proMMP-9 molecule contains subdomains such as gelatin binding and hemopexin domains that specifically associate with various cell surface and matrix components modulating cell functions, often independently of the MMP-9 proteolytic potential (3, 29, 35–41). The MMP-9 hemopexin domain, which can modulate substrate specificity and catalytic activity of MMP-9 (3, 42, 43), also appears necessary for angiogenesis as deletion of this domain reduces MMP-9-mediated angiogenesis by 80–90%. The absolute requirement for MMP-9 catalytic activity also implies that the exogenously delivered, MMP-9 zymogen, i.e. the 92-kDa proMMP-9, must undergo enzyme activation in vivo before angiogenic induction. This notion was confirmed by timed additions of the MMP-9 inhibitor, TIMP-1, indicating that neutrophil MMP-9 zymogen indeed undergoes activation in vivo and that prevention of this activation abrogates the angiogenic induction. This additional requirement for zymogen activation is why the unique TIMP-free status endows neutrophil proMMP-9 with the distinct angiogenic capabilities to efficiently function in vivo.
Unencumbered by TIMP-1, the proMMP-9 zymogen undergoes enzyme activation within the initial 24-h time period after grafting, whereas proMMP-9 stoichiometrically complexed to TIMP-1 fails to become activated beyond marginal levels. This critical differential in zymogen activation between the highly angiogenic, TIMP-1-free proMMP-9 and the distinctly non-angiogenic, TIMP-1-complexed proMMP-9 was demonstrated herein both in vitro and in vivo by a number of biochemical analyses. First, only the onplants containing TIMP-free proMMP-9 exhibited a distinct 82-kDa processed form of MMP-9, the appearance of which occurred between 4 and 24 h of in vivo incubation. Second, a clear manifestation of MMP-9 proteolytic/gelatinolytic activity was demonstrated within the actual angiogenic onplants and was closely coordinated with the appearance of the 82-kDa processed form in the same onplants. The catalytic state of the MMP-9 enzyme in the angiogenic onplants was ascertained by incorporating low concentrations of gelatin into the onplants and monitoring the cleavage of this highly preferred substrate of activated MMP-9 over time. Little or no such MMP-9-dependent proteolytic activity was manifested in the onplants that contained proMMP-9 complexed to TIMP-1. These results are significant as the dampening effect of TIMP-1 on proMMP-9 activation, previously shown to occur in solution in vitro (44, 45), for the first time is demonstrated here to operate in vivo as a critical regulatory mechanism involved in angiogenesis.
The coordination between the processing of 92-kDa proMMP-9 to 82-kDa MMP-9 and the appearance of MMP-9 proteolytic activity occurred within the first 24 h of the 70–80-h in vivo angiogenesis. This 24-h window is also the critical time span for initiating angiogenesis induction as dampening proMMP-9 zymogen activation by the addition of TIMP-1 during this time period prevented angiogenesis. Thus, the absence or presence of TIMP-1 bound to proMMP-9 controls the rate and extent of zymogen activation in vivo and, therefore, determines the ensuing proteolytic activity of MMP-9, which in turn directly affects the onset of angiogenesis. By inference, this TIMP-1-centric regulation would also control overall neutrophil-mediated angiogenesis as the bulk of the angiogenic potential of these leukocytes is associated with their secretory granules, and the major angiogenic component of the released neutrophil granules is the TIMP-free proMMP-9 molecule (28).
It is important to point out that many different types of cells such as inflammatory cells, endothelial cells, and stromal cells, which migrate into or reside within pre-angiogenic tissue, can be induced to produce angiogenic levels of proMMP-9. However, most of these cell types, with the exception of neutrophils (2), secrete proMMP-9 complexed with TIMP-1. While screening a large number of malignant and normal cell types after inducing them to synthesize proMMP-9, we and others found that the secreted proMMP-9 invariably was in complex with TIMP-1 (2, 28, 44–49). Our data in this study (Fig. 2) demonstrate that the proMMP-9·TIMP-1 complex is non-angiogenic in vivo even if used at a 20-fold excess compared with TIMP-free proMMP-9. These results suggest that even if cells other than neutrophils are induced to release into the pre-angiogenic tissue elevated levels of proMMP-9, the secreted zymogen will remain stable as a non-angiogenic, inactive proenzyme as long as it is in stoichiometric complex with TIMP-1. In contrast, neutrophils are unique in that they accumulate, store, and then release TIMP-free proMMP-9, which as we have shown herein becomes rapidly activated in the pre-angiogenic onplant tissue. The resulting activated enzyme manifests an apparently uninhibited proteolytic activity within the onplant tissue which is responsible for the ensuing burst of new blood-carrying vessels that arise 40–50 h later.
The identity of the specific proteins that are cleaved by the activated MMP-9 and which directly lead to angiogenic induction is still unresolved. Our proteomic analysis of extracted pre-angiogenic tissue has linked the newly generated MMP-9 activity with specific cleavage and degradation of collagen/gelatin. In addition, we have demonstrated in this study (Fig. 4B) that a number of host proteins/polypeptides rapidly influx into the pre-angiogenic tissue, coordinated with and in direct response to generated MMP-9 activity, suggesting an early host response to activated MMP-9. Specific angiogenic polypeptides influxing into the onplants have thus far not been identified using mass spectrometry/liquid chromatography analyses. However, using a highly sensitive capture ELISA, FGF-2 was identified as a host angiogenic protein, the levels of which were increased in the pre-angiogenic tissue in conjunction with MMP-9 activation. This observed enhancement of FGF-2 levels was shown to be highly relevant since a specific function-blocking antibody to FGF-2 completely inhibited angiogenesis mediated by TIMP-free proMMP-9. Further evidence that release of FGF-2 by activated MMP-9 is a crucial angiogenic-inducing event was indicated by sensitivity to the neutralizing antibody against the specific FGF-2 receptor, anti-FGFR-2. Thus, MMP-9 activity results in an enhancement of bioavailable FGF-2, which then becomes the essential downstream angiogenic inducer acting through its specific receptor, FGFR-2. That activated MMP-9 would enhance the levels of FGF-2 is consistent with the known release of this angiogenic growth factor sequestered within the proteinaceous confines of the stromal extracellular matrix (50, 51). In fact, urokinase-type plasminogen activator-mediated mobilization of endogenous FGF-2 from CAM tissue has been reported and linked circumstantially to enhanced angiogenesis in the chick embryo (52). In particular, urokinase-type plasminogen activator has been demonstrated to induce angiogenesis in vivo via plasmin-dependent degradation of ECM, causing the apparent mobilization of stored endogenous FGF-2 (53). Proteolytic cleavage of matrix components sequestering FGF-2 by active MMP-9 is also consistent with the indicated requirement of the hemopexin domain of MMP-9 for angiogenic induction. This C-terminal non-catalytic domain, often referred to as PEX, can be involved in directing MMP-9 to specific components of the ECM and enhancing the degradative activity of the enzyme to those PEX-directed substrates (37, 40, 42, 43). Furthermore, the peak of MMP-9-mediated enhancement of FGF-2 occurs at ∼24 h, i.e. coinciding with the end of the time period when MMP-9 has exerted its major proteolytic activity and, coordinately, its major angiogenic-inducing activity.
FGF-2 appears to be a major angiogenic factor induced in the collagen onplants by neutrophil proMMP-9 as there was no significant increase in the levels of VEGF. This finding is consistent with previous studies demonstrating that FGF-2 is the growth factor governing angiogenesis in the CAM of chick embryo (54). Interestingly, the angiogenic vessels induced in the collagen onplants by either neutrophil MMP-9 or exogenously added FGF-2 exhibited similar morphology, i.e. were organized in a complex network of distorted capillaries or small blood vessels with numerous dilation areas. In comparison to both FGF-2 and neutrophil proMMP-9, a more pronounced vascular network with more dilated capillaries was induced by exogenous VEGF, consistent with its known vasodilatory effects. Altogether, these data support our idea that neutrophil proMMP-9 mediates angiogenesis via release of ECM-sequestered FGF-2 or induction of FGF-2 production by resident cell types of the CAM or recruited inflammatory cells. However, the findings demonstrating that neutrophil proMMP-9-mediated angiogenesis was sensitive to anti-VEGF mAb indicate that VEGF might be also released or induced by MMP-9 in a local microenvironment in quantities sufficient to contribute to angiogenic induction but not detectable by conventional ELISA or Western blotting. Additionally, the angiogenic network developing in response to neutrophil proMMP-9 was also sensitive to blocking VEGFR-mediated functions as indicated by significant inhibitory effects of anti-VEGFR-2 antibody. This inhibition of angiogenesis would be consistent with the notion of the overall importance of VEGF/VEGFR-2 signaling in survival of endothelial cells not only in established vasculature but especially in newly developing angiogenic networks. It is possible that the source of VEGF may be coming from endothelial cells themselves, stimulated by FGF-2 to express VEGF in a paracrine manner (55, 56). In addition to the endothelial cells, stromal cells at the site of FGF-2-induced angiogenesis have been shown to respond with de novo VEGF expression in Matrigel plug (57) and tumor neovascularization (58) models. Interestingly, when added at quantities exceeding physiological levels, exogenous FGF-2 provides substantial protection for angiogenic blood vessels against the detrimental effects of blocking VEGF or VEGF receptor as shown in our angiogenesis model system (Fig. 7). Overall, our findings appear to implicate the mechanism where neutrophil proMMP-9-mediated angiogenesis involves catalytically induced or released FGF-2, which operates upstream of VEGF/VEGFR. In this regard, a leading position of FGF-2 in a hierarchy of angiogenic factors, including VEGF, has been indicated in numerous studies involving mammalian model systems (34, 51).
There are alternative scenarios to our proposed release of ECM-sequestered FGF-2 by activated MMP-9 (59). It is possible that activated MMP-9 proteolytically induces the influx of various cell types by processing or activating specific cell-attracting cytokines or chemokines. These recruited cells could either import FGF-2 themselves or bring about the release of sequestered FGF-2 via their own proteolytic enzyme systems. The mobilized cells might be additional inflammatory cells or bone marrow-derived progenitor cells or stromal cells such as pericytes and activated fibroblasts. Indeed, MMP-9 has been reported to induce the recruitment of these cell types into specific tissues (2, 9, 12–14, 60). In addition, MMP-9 has been shown to proteolytically process and potentiate specific cell attracting cytokines, including interleukin-8 (3, 19), and to proteolytically release cell recruiting factors, e.g. kit ligand (9, 12, 21). Importantly, these alternative angiogenesis scenarios would still evoke an initial requirement for rapidly activated and unencumbered MMP-9 (i.e. neutrophil proMMP-9) to proteolytically induce cell recruitment. Furthermore, if the recruited cells would import FGF-2 or the means to generate FGF-2, the sensitivity to anti-FGF-2 and anti-FGFR-2 would also be observed, all of which is consistent with our data.
The above proposed alternative scenarios, where MMP-9-dependent cellular intermediates generate enhanced levels of bioavailable FGF-2, are indeed possible and not without a precedent. In the developing CAM of the chick embryo, chorionic ectoderm has been demonstrated to be a major cellular source of FGF-2, eliciting an angiogenic response in the undifferentiated mesodermal blood vessels which in turn synthesize an autocrine supply of FGF-2 necessary for further proliferation and differentiation of endothelial cells (61). However, thus far we have no direct evidence that such intermediary cell types function downstream of activated MMP-9 in our angiogenesis model.
Finally, it is important to emphasize that our proposed angiogenic cascade begins with the delivery to the pre-angiogenic tissue of TIMP-free neutrophil proMMP-9. However, there are numerous pathophysiological processes, including tumor development, injury, and inflammation that could induce angiogenic switching via distinct pathways independent of MMP-9 and FGF-2. If some of these pathophysiological processes involve an initial infiltration of neutrophils, such influx will naturally include the delivery to the tissue of the potent angiogenic inducer that is described in the present study, namely TIMP-free proMMP-9. Depending on the tissue milieu, neutrophil proMMP-9, unencumbered by TIMP-1, will likely become activated and enhance the levels of bioavailable FGF-2, thereby merging into the precise angiogenic pathway proposed herein. This overall cascade constitutes an alternative pathway to the major established angiogenic switching mechanisms. These mechanisms implicate MMPs (including TIMP-1-complexed, poorly activatable MMP-9), deriving from tumor cells, macrophages, endothelial cells, and/or bone marrow-derived progenitor cells and proteolytically generating bioavailable VEGF as the central angiogenic inducer (7, 12, 15, 21–24). Our alternative pathway, centered on degranulating neutrophils, would have a greater potential for the enhanced MMP-9 activity to generate another bioavailable angiogenic growth factor, i.e. FGF-2.
Although our alternative angiogenic switching pathway was elucidated in the chick embryo CAM model, our major findings were verified herein in the mammalian setting where collagen-filled implants (angiotubes) supplemented with pro- or anti-angiogenic moieties were subcutaneously implanted in the mice (Fig. 8). The presented data indicate that in mouse tissue the TIMP-free neutrophil proMMP-9 also functions as a more potent angiogenic inducer than proMMP-9 complexed with TIMP-1 and that such neutrophil proMMP-9-mediated angiogenesis proceeds through an FGF-2 pathway. It is noteworthy that when anti-VEGF therapy in tumor-bearing animals or patients fails, re-activation of tumor angiogenesis often proceeds via FGF-2 (62–64). It remains to be determined whether this reactivated alternative FGF-mediated pathway leads back to the unique neutrophil TIMP-free proMMP-9 molecule as the inducer and/or generator of this additional angiogenic switch.
Supplementary Material
Acknowledgments
We thank Dr. Rafael Fridman for generously providing recombinant human proMMP-9 and TIMP-1. We also thank Chenxing Li and Ilse Van Aelst for excellent technical assistance.
This work was supported, in whole or in part, by National Institutes of Health Grants CA55852 and CA105412 (to J. P. Q.) and Postdoctoral Training Grant 5T32 HL07195-31 (to V. C. A.). This work was also supported by Geconcerteerde OnderzoeksActies GOA/2007/15, Excellentiefinanciering EF/05/015, and the Fund for Scientific Research of Flanders FWO-Vlaanderen (to G. O.).

The on-line version of this article (available at http://www.jbc.org) contains supplemental Fig. 1.
- MMP
- matrix metalloproteinase
- proMMP-9
- MMP-9 proenzyme
- Neut-MMP-9
- neutrophil proMMP-9
- CAM
- chorioallantoic membrane
- FGF-2
- basic fibroblast growth factor
- mAb
- monoclonal antibody
- TIMP
- tissue inhibitor of metalloproteinase
- VEGF
- vascular endothelial growth factor
- WT
- wild type
- ECM
- extracellular matrix
- PBS
- phosphate-buffered saline
- ELISA
- enzyme-linked immunosorbent assay
- OG
- O-linked glycans
- FGFR-2
- FGF receptor 2
- VEGFR-2
- VEGF receptor 2
- Hem
- hemopexin domain.
REFERENCES
- 1.Vu T. H., Werb Z. (ed) (1998) in Matrix Metalloproteinases ( Parks W. C., Mecham R. P. eds) pp. 115–148, Academic Press, San Diego [Google Scholar]
- 2.Opdenakker G., Van den Steen P. E., Dubois B., Nelissen I., Van Coillie E., Masure S., Proost P., Van Damme J. (2001) J. Leukocyte Biol. 69, 851–859 [PubMed] [Google Scholar]
- 3.Van den Steen P. E., Dubois B., Nelissen I., Rudd P. M., Dwek R. A., Opdenakker G. (2002) Crit. Rev. Biochem. Mol. Biol. 37, 375–536 [DOI] [PubMed] [Google Scholar]
- 4.Parks W. C., Wilson C. L., López-Boado Y. S. (2004) Nat. Rev. Immunol. 4, 617–629 [DOI] [PubMed] [Google Scholar]
- 5.Björklund M., Koivunen E. (2005) Biochim. Biophys. Acta 1755, 37–69 [DOI] [PubMed] [Google Scholar]
- 6.van Kempen L. C., de Visser K. E., Coussens L. M. (2006) Eur. J. Cancer 42, 728–734 [DOI] [PubMed] [Google Scholar]
- 7.Bergers G., Brekken R., McMahon G., Vu T. H., Itoh T., Tamaki K., Tanzawa K., Thorpe P., Itohara S., Werb Z., Hanahan D. (2000) Nat. Cell Biol. 2, 737–744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Coussens L. M., Tinkle C. L., Hanahan D., Werb Z. (2000) Cell 103, 481–490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Heissig B., Hattori K., Dias S., Friedrich M., Ferris B., Hackett N. R., Crystal R. G., Besmer P., Lyden D., Moore M. A., Werb Z., Rafii S. (2002) Cell 109, 625–637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huang S., Van Arsdall M., Tedjarati S., McCarty M., Wu W., Langley R., Fidler I. J. (2002) J. Natl. Cancer Inst. 94, 1134–1142 [DOI] [PubMed] [Google Scholar]
- 11.Johnson C., Sung H. J., Lessner S. M., Fini M. E., Galis Z. S. (2004) Circ. Res. 94, 262–268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Heissig B., Rafii S., Akiyama H., Ohki Y., Sato Y., Rafael T., Zhu Z., Hicklin D. J., Okumura K., Ogawa H., Werb Z., Hattori K. (2005) J. Exp. Med. 202, 739–750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jodele S., Chantrain C. F., Blavier L., Lutzko C., Crooks G. M., Shimada H., Coussens L. M., Declerck Y. A. (2005) Cancer Res. 65, 3200–3208 [DOI] [PubMed] [Google Scholar]
- 14.Ahn G. O., Brown J. M. (2008) Cancer Cell 13, 193–205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Du R., Lu K. V., Petritsch C., Liu P., Ganss R., Passegué E., Song H., Vandenberg S., Johnson R. S., Werb Z., Bergers G. (2008) Cancer Cell 13, 206–220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Van den Steen P. E., Proost P., Grillet B., Brand D. D., Kang A. H., Van Damme J., Opdenakker G. (2002) FASEB J. 16, 379–389 [DOI] [PubMed] [Google Scholar]
- 17.Hangai M., Kitaya N., Xu J., Chan C. K., Kim J. J., Werb Z., Ryan S. J., Brooks P. C. (2002) Am. J. Pathol. 161, 1429–1437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hamano Y., Zeisberg M., Sugimoto H., Lively J. C., Maeshima Y., Yang C., Hynes R. O., Werb Z., Sudhakar A., Kalluri R. (2003) Cancer Cell 3, 589–601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Van den Steen P. E., Proost P., Wuyts A., Van Damme J., Opdenakker G. (2000) Blood 96, 2673–2681 [PubMed] [Google Scholar]
- 20.Van Den Steen P. E., Wuyts A., Husson S. J., Proost P., Van Damme J., Opdenakker G. (2003) Eur. J. Biochem. 270, 3739–3749 [DOI] [PubMed] [Google Scholar]
- 21.Yang L., DeBusk L. M., Fukuda K., Fingleton B., Green-Jarvis B., Shyr Y., Matrisian L. M., Carbone D. P., Lin P. C. (2004) Cancer Cell 6, 409–421 [DOI] [PubMed] [Google Scholar]
- 22.Lee S., Jilani S. M., Nikolova G. V., Carpizo D., Iruela-Arispe M. L. (2005) J. Cell Biol. 169, 681–691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nozawa H., Chiu C., Hanahan D. (2006) Proc. Natl. Acad. Sci. U.S.A. 103, 12493–12498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kong D., Li Y., Wang Z., Banerjee S., Sarkar F. H. (2007) Cancer Res. 67, 3310–3319 [DOI] [PubMed] [Google Scholar]
- 25.Deryugina E. I., Quigley J. P. (2006) Cancer Metastasis Rev. 25, 9–34 [DOI] [PubMed] [Google Scholar]
- 26.Pahler J. C., Tazzyman S., Erez N., Chen Y. Y., Murdoch C., Nozawa H., Lewis C. E., Hanahan D. (2008) Neoplasia 10, 329–340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Deryugina E. I., Quigley J. P. (2008) Methods Enzymol. 444, 21–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ardi V. C., Kupriyanova T. A., Deryugina E. I., Quigley J. P. (2007) Proc. Natl. Acad. Sci. U.S.A. 104, 20262–20267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Van den Steen P. E., Van Aelst I., Hvidberg V., Piccard H., Fiten P., Jacobsen C., Moestrup S. K., Fry S., Royle L., Wormald M. R., Wallis R., Rudd P. M., Dwek R. A., Opdenakker G. (2006) J. Biol. Chem. 281, 18626–18637 [DOI] [PubMed] [Google Scholar]
- 30.Ramos-DeSimone N., Moll U. M., Quigley J. P., French D. L. (1993) Hybridoma 12, 349–363 [DOI] [PubMed] [Google Scholar]
- 31.Badwey J. A., Curnutte J. T., Berde C. B., Karnovsky M. L. (1982) Biochem. Biophys. Res. Commun. 106, 170–174 [DOI] [PubMed] [Google Scholar]
- 32.Mortz E., Krogh T. N., Vorum H., Görg A. (2001) Proteomics 1, 1359–1363 [DOI] [PubMed] [Google Scholar]
- 33.Seandel M., Noack-Kunnmann K., Zhu D., Aimes R. T., Quigley J. P. (2001) Blood 97, 2323–2332 [DOI] [PubMed] [Google Scholar]
- 34.Murakami M., Simons M. (2008) Curr. Opin. Hematol. 15, 215–220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Allan J. A., Docherty A. J., Barker P. J., Huskisson N. S., Reynolds J. J., Murphy G. (1995) Biochem. J. 309, 299–306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Olson M. W., Toth M., Gervasi D. C., Sado Y., Ninomiya Y., Fridman R. (1998) J. Biol. Chem. 273, 10672–10681 [DOI] [PubMed] [Google Scholar]
- 37.Cha H., Kopetzki E., Huber R., Lanzendörfer M., Brandstetter H. (2002) J. Mol. Biol. 320, 1065–1079 [DOI] [PubMed] [Google Scholar]
- 38.Fridman R., Toth M., Chvyrkova I., Meroueh S. O., Mobashery S. (2003) Cancer Metastasis Rev. 22, 153–166 [DOI] [PubMed] [Google Scholar]
- 39.Mira E., Lacalle R. A., Buesa J. M., de Buitrago G. G., Jiménez-Baranda S., Gómez-Moutón C., Martínez-A C., Mañes S. (2004) J. Cell Sci. 117, 1847–1857 [DOI] [PubMed] [Google Scholar]
- 40.Björklund M., Heikkilä P., Koivunen E. (2004) J. Biol. Chem. 279, 29589–29597 [DOI] [PubMed] [Google Scholar]
- 41.Redondo-Muñoz J., Ugarte-Berzal E., García-Marco J. A., del Cerro M. H., Van den Steen P. E., Opdenakker G., Terol M. J., García-Pardo A. (2008) Blood 112, 169–178 [DOI] [PubMed] [Google Scholar]
- 42.Roeb E., Schleinkofer K., Kernebeck T., Pötsch S., Jansen B., Behrmann I., Matern S., Grötzinger J. (2002) J. Biol. Chem. 277, 50326–50332 [DOI] [PubMed] [Google Scholar]
- 43.Geurts N., Martens E., Van Aelst I., Proost P., Opdenakker G., Van den Steen P. E. (2008) Biochemistry 47, 2689–2699 [DOI] [PubMed] [Google Scholar]
- 44.Watanabe H., Nakanishi I., Yamashita K., Hayakawa T., Okada Y. (1993) J. Cell Sci. 104, 991–999 [DOI] [PubMed] [Google Scholar]
- 45.Ogata Y., Itoh Y., Nagase H. (1995) J. Biol. Chem. 270, 18506–18511 [DOI] [PubMed] [Google Scholar]
- 46.Moll U. M., Youngleib G. L., Rosinski K. B., Quigley J. P. (1990) Cancer Res. 50, 6162–6170 [PubMed] [Google Scholar]
- 47.Okada Y., Gonoji Y., Naka K., Tomita K., Nakanishi I., Iwata K., Yamashita K., Hayakawa T. (1992) J. Biol. Chem. 267, 21712–21719 [PubMed] [Google Scholar]
- 48.Ramos-DeSimone N., Hahn-Dantona E., Sipley J., Nagase H., French D. L., Quigley J. P. (1999) J. Biol. Chem. 274, 13066–13076 [DOI] [PubMed] [Google Scholar]
- 49.Dolo V., Ginestra A., Cassará D., Ghersi G., Nagase H., Vittorelli M. L. (1999) Ann. N. Y. Acad. Sci. 878, 497–499 [DOI] [PubMed] [Google Scholar]
- 50.Bikfalvi A., Klein S., Pintucci G., Rifkin D. B. (1997) Endocr. Rev. 18, 26–45 [DOI] [PubMed] [Google Scholar]
- 51.Presta M., Dell'Era P., Mitola S., Moroni E., Ronca R., Rusnati M. (2005) Cytokine Growth Factor Rev. 16, 159–178 [DOI] [PubMed] [Google Scholar]
- 52.Ribatti D., Presta M. (2002) J. Cell. Mol. Med. 6, 439–446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ribatti D., Leali D., Vacca A., Giuliani R., Gualandris A., Roncali L., Nolli M. L., Presta M. (1999) J. Cell Sci. 112, 4213–4221 [DOI] [PubMed] [Google Scholar]
- 54.Ribatti D., Urbinati C., Nico B., Rusnati M., Roncali L., Presta M. (1995) Dev. Biol. 170, 39–49 [DOI] [PubMed] [Google Scholar]
- 55.Seghezzi G., Patel S., Ren C. J., Gualandris A., Pintucci G., Robbins E. S., Shapiro R. L., Galloway A. C., Rifkin D. B., Mignatti P. (1998) J. Cell Biol. 141, 1659–1673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kanda S., Miyata Y., Kanetake H. (2004) J. Biol. Chem. 279, 4007–4016 [DOI] [PubMed] [Google Scholar]
- 57.Claffey K. P., Abrams K., Shih S. C., Brown L. F., Mullen A., Keough M. (2001) Lab. Invest. 81, 61–75 [DOI] [PubMed] [Google Scholar]
- 58.Tsunoda S., Nakamura T., Sakurai H., Saiki I. (2007) Cancer Sci. 98, 541–548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.McDonnell K., Bowden E. T., Cabal-Manzano R., Hoxter B., Riegel A. T., Wellstein A. (2005) Lab. Invest. 85, 747–755 [DOI] [PubMed] [Google Scholar]
- 60.Chantrain C. F., Shimada H., Jodele S., Groshen S., Ye W., Shalinsky D. R., Werb Z., Coussens L. M., DeClerck Y. A. (2004) Cancer Res. 64, 1675–1686 [DOI] [PubMed] [Google Scholar]
- 61.Ribatti D., Bertossi M., Nico B., Vacca A., Ria R., Riva A., Roncali L., Presta M. (1998) J. Submicrosc. Cytol. Pathol. 30, 127–136 [PubMed] [Google Scholar]
- 62.Casanovas O., Hicklin D. J., Bergers G., Hanahan D. (2005) Cancer Cell 8, 299–309 [DOI] [PubMed] [Google Scholar]
- 63.Jubb A. M., Oates A. J., Holden S., Koeppen H. (2006) Nat. Rev. Cancer 6, 626–635 [DOI] [PubMed] [Google Scholar]
- 64.Bergers G., Hanahan D. (2008) Nat. Rev. Cancer 8, 592–603 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.