Abstract
Ferredoxin (Fd) is the major iron-containing protein in photosynthetic organisms and is central to reductive metabolism in the chloroplast. The Chlamydomonas reinhardtii genome encodes six plant type [Fe2S2] ferredoxins, products of PETF, FDX2–FDX6. We performed the functional analysis of these ferredoxins by localizing Fd, Fdx2, Fdx3, and Fdx6 to the chloroplast by using isoform-specific antibodies and monitoring the pattern of gene expression by iron and copper nutrition, nitrogen source, and hydrogen peroxide stress. In addition, we also measured the midpoint redox potentials of Fd and Fdx2 and determined the kinetic parameters of their reactions with several ferredoxin-interacting proteins, namely nitrite reductase, Fd:NADP+ oxidoreductase, and Fd:thioredoxin reductase. We found that each of the FDX genes is differently regulated in response to changes in nutrient supply. Moreover, we show that Fdx2 (Em = −321 mV), whose expression is regulated by nitrate, is a more efficient electron donor to nitrite reductase relative to Fd. Overall, the results suggest that each ferredoxin isoform has substrate specificity and that the presence of multiple ferredoxin isoforms allows for the allocation of reducing power to specific metabolic pathways in the chloroplast under various growth conditions.
Ferredoxins are small (∼11,000-kDa), soluble, iron-sulfur cluster-containing proteins with strongly negative redox potentials (−350 to −450 mV) that function as electron donors at reductive steps in various metabolic pathways (1–3). In photosynthetic organisms, the well studied ferredoxin (Fd4; the product of the PETF gene) is the most abundant iron-containing protein in the chloroplast and is central to the distribution of photosynthetically derived reductive power (4).
The most well known Fd-dependent reaction is the transfer of electrons from photosystem I (PSI) to NADPH, catalyzed by Fd:NADP+ oxidoreductase (FNR). The NADPH produced by this reaction donates electrons to the only reductant-requiring step in the Calvin cycle and other steps in anabolic pathways that require NADPH as reductant. In addition, reduced Fd directly donates electrons to other metabolic pathways by interacting with various enzymes in the chloroplast. This includes Fd:thioredoxin reductase (FTR), which converts a light-driven electron signal into a thiol signal that is transmitted to thioredoxins (TRXs) present in the plastid as different types (or different isoforms). Once reduced, TRXs interact with specific disulfide bonds on target enzymes, modulating their activities (5). Other Fd targets include hydrogenase, which is responsible for hydrogen production in anaerobic conditions in green algae; glutamine-oxoglutarate amidotransferase in amino acid synthesis; nitrite and sulfite reductases in nitrate and sulfate assimilation, respectively; stearoyl-ACP Δ9-desaturase in fatty acid desaturation; and phycocyanobilin:Fd oxidoreductase in synthesis of phytochromobilin (6). Fd also functions in non-photosynthetic cells. Here, FNR catalyzes the reduction of Fd by NADPH produced in the oxidative pentose phosphate pathway, enabling Fd-dependent metabolism to occur in the dark (7, 8).
The single-celled green alga, Chlamydomonas reinhardtii is an excellent reference organism for studying both metabolic adaptation to nutrient stress and photosynthesis (9–13). The Chlamydomonas genome encodes six highly related plant type ferredoxin genes (9). Until recently, only the major photosynthetic ferredoxin, Fd (encoded by PETF), which mediates electron transfer between PSI and FNR, had been characterized in detail (14).
Many land plants are known to have multiple ferredoxins. Typically, they are differently localized on the basis of their function. Photosynthetic ferredoxins reduce NADP+ at a faster rate and are localized to the leaves, whereas non-photosynthetic ferredoxins are more efficiently reduced by NADPH and are localized to the roots. Arabidopsis thaliana has a total of six ferredoxin isoforms (15). Of these, two are photosynthetic and localized in the leaves. The most abundant, AtFd2, is involved in linear electron flow, and the less abundant (5% of the ferredoxin pool), AtFd1, has been implicated in cyclic electron flow (16). There is one non-photosynthetic ferredoxin located in the roots, AtFd3, which is nitrate-inducible. This protein has higher electron transfer activity with sulfite reductase in in vitro assays compared with other Arabidopsis ferredoxin isoforms, suggesting in vivo function of AtFd3 in nitrate and sulfate assimilation (15, 17). In addition, there is one evolutionarily distant ferredoxin, AtFd4, of unknown function with a more positive redox potential present in the leaves and two other proteins which are “ferredoxin-like” and uncharacterized (15). Zea mays has four ferredoxin isoforms, two photosynthetic and two non-photosynthetic (18). One of the non-photosynthetic isoforms is specifically induced by nitrite, suggestive of a role in nitrate metabolism (19). A cyanobacterium, Anabaena 7120, has two ferredoxins, vegetative and heterocyst type (by analogy to leaf and root types, respectively). The heterocyst type is present only in cells that have differentiated into nitrogen-fixing cells, indicating that this form may serve to transfer electrons to nitrogenase (20).
We hypothesize that the presence of as many as six ferredoxin isoforms in a single-celled organism like C. reinhardtii allows for the differential regulation of each isoform and therefore the prioritization of reducing power toward certain metabolic pathways under changing environmental conditions. To test this hypothesis, expression of the genes (PETF and FDX2–FDX6) encoding the six ferredoxin isoforms in Chlamydomonas reinhardtii was monitored under various conditions in which well characterized ferredoxin-dependent enzymes are known to be expressed. In addition, we also analyzed the interaction of Fd and Fdx2 with several ferredoxin-interacting proteins, such as NiR, FNR, and FTR, and determined the kinetic parameters of the corresponding reactions.
We found that each of the FDX genes is indeed differently regulated in response to changes in nutrient supply. In the case of FDX2 whose product is most similar to classical Fd, we suggest that it has specificity for nitrite reductase based on its pattern of expression and activity with nitrite reductase.
EXPERIMENTAL PROCEDURES
Growth Conditions
C. reinhardtii strains 17D−, CC124, CC125, CC425 (cw15 arg2), 704 (cw15 nit2+ Nia1:ars) (21), 21gr (CC1690, Nit5−), and 2137 (CC1021) were used in this study. Starter cultures were maintained in the standard Tris acetate-phosphate (TAP) medium at 24 °C at a light intensity of 95 μmol m−2 s−1 light and constant shaking at 180 rpm. For analysis of the effects of ammonium versus nitrate in strain 21gr, nitrogen-free TAP medium was prepared, and 7.5 mm NH4Cl or 4 mm KNO3 was added as a nitrogen source. The establishment of copper deficiency and treatment of cells with nickel were conducted as described previously (22). Hypoxia was achieved by bubbling with nitrogen gas in the dark.5 For iron deficiency experiments, TAP medium was made in acid-washed glassware using trace elements without iron, which was subsequently added to a final concentration of 0.1–200 μm from a solution of iron-EDTA (23). Cultures were inoculated from a starter culture to a final concentration of 105 cells/ml. Cells were collected for RNA and protein isolation at mid-log phase when the culture reached a density of 2–5 × 106 cells/ml.
Chloroplast and Mitochondria Isolation
Chloroplasts and mitochondria were isolated according to procedures described previously (24, 25) from a 4-liter culture of CC425 (Fig. 3A) or strain 704 (Fig. 3, B and C) grown to a cell density of 2 × 106 cells/ml. Cells were collected by centrifugation (4000 × g, 10 min), resuspended in 50 ml of breaking buffer (0.3 m sorbitol, 50 mm Hepes-KOH, pH 7.8, 2 mm EDTA, pH 7.5, 5 mm MgCl2) supplemented with 0.1% bovine serum albumin and 0.5% polyvinylpyrrolidone-40, and broken in a Yeda press (4.5 bars, 30 s). The broken cells were fractionated by centrifugation (1000 × g, 5 min). The supernatant (containing mitochondria) was processed as in Ref. 26 except that the resulting pellet was resuspended in 1 ml of wash buffer (10 mm potassium phosphate, pH 7.2, 0.25 m sorbitol, 1 mm EDTA, pH 7.5), collected by centrifugation, and resuspended in 10 mm sodium phosphate, pH 7.0, and assayed for purity by immunoblot. The pellet fraction (containing chloroplasts) was washed twice in 50 ml of breaking buffer, resuspended in 20 ml, loaded onto a 45/75% (12 ml each) Percoll step gradient, and separated by centrifugation (9300 × g, 20 min). Intact chloroplasts were collected at the 45/75% interface and were washed twice in 200 ml of breaking buffer (1000 × g, 5 min). Isolated chloroplasts were resuspended in 10 mm sodium phosphate, pH 7.0, and assayed for purity by immunoblot.
FIGURE 3.
Localization of Fd, Fdx2, Fdx3, and Fdx6. A, 20 μg of total cellular protein, isolated mitochondria, and chloroplasts was separated on a 15% polyacrylamide gel containing SDS, and the abundance of Fd, Fdx3, and Fdx6 was analyzed by immunoblot. Cox2b and ketoacid reductoisomerase (KARI) abundance were analyzed in order to assess the purity of isolated mitochondria and chloroplasts. B, 40 μg of soluble fraction of total cells, isolated mitochondria, and chloroplasts were separated on a 15% polyacrylamide gel containing SDS and analyzed for the abundance of Fdx2 by immunoblot. C, 20 μg of total cellular protein, isolated mitochondria, and chloroplasts were analyzed as in B to assess the purity of isolated mitochondria and chloroplasts. D, 20 μg of total cellular protein and purified chloroplasts were separated on a 12% polyacrylamide gel containing SDS to assess cytosolic contamination of purified chloroplasts.
Expression and Purification of Recombinant Ferredoxins
Plasmid constructs for expressing recombinant ferredoxins were constructed by standard molecular biology methods using expression vector pET21b and gene-specific primer sets. The N termini of recombinant Fdx2–Fdx6 were predicted based on sequence alignment with the PETF gene product (Fig. 1A). The stop codon of each gene was included, resulting in untagged recombinant protein. Constructs were confirmed by sequencing and co-transformed with pLysRARE (Novagen) into Escherichia coli BL21 (DE3) for expression. Cultures (1 liter) were grown at 30 °C to an A600 of 0.7 prior to the addition of isopropyl 1-thio-β-d-galactopyranoside (0.5 mm) and FeSO4 (50 μm). After 16 h, cells were collected by centrifugation (4400 × g, 5 min, 4 °C) and resuspended in 10 ml of cold 20 mm Tris-HCl, pH 7.4, containing 20 mm NaCl and 2 mm β-mercaptoethanol. Cells were broken by one cycle of freezing and thawing, followed by sonication on ice (Fisher model 550 sonic dismembrator, large tip, 70% intensity, 10 cycles of 1 min on, 1 min off). To completely break the cells, the sample was also passed three times though a French pressure cell operated at 17,000 p.s.i. Insoluble material was removed by centrifugation (110,000 × g, 4 °C, 1 h). The supernatant was treated with DNase I (1 μg/ml) and streptomycin sulfate (0.3% w/v) for 1 h at 4 °C, and the precipitate was removed by centrifugation (48,000 × g, 30 min, 4 °C). The supernatant was dialyzed three times (2, 12, and 3 h) against 2 liters of 20 mm Tricine-NaOH, pH 7.4, plus 2 mm β-mercaptoethanol (Buffer A) containing 0.1 m NaCl and centrifuged again (48,000 × g, 30 min, 4 °C). The supernatant was loaded onto a DE52 anion exchange column (2.6 × 5 cm) equilibrated with Buffer A containing 0.1 m NaCl, and a gradient of NaCl (0.1-1 m in Buffer A, 370 ml) was applied. Reddish colored fractions were collected, and their spectra were recorded. Protein samples were concentrated (Millipore Ultracel YM-3) to 5 ml, and the salt concentration was reduced by the addition of 35 ml of Buffer A. Samples were loaded onto a Q-Sepharose anion exchange column (1.6 × 10 cm) equilibrated with Buffer A containing 0.1 m NaCl, and a gradient of NaCl (0.1-1 m in Buffer A, 180 ml) was applied. Fractions were collected and concentrated as before and loaded onto a Sephacryl S-100 HR gel filtration column (1.6 × 60 cm) equilibrated with Buffer A containing 0.1 m NaCl. All chromatographic runs were performed at a constant flow rate of 1 ml/min.
FIGURE 1.
The Chlamydomonas genome encodes six highly related ferredoxins. A, multiple alignment of six ferredoxins in C. reinhardtii was created using Multalin (available on the World Wide Web). Conserved cysteine residues that coordinate the [Fe2S2] cluster are shown in red. The red arrow indicates N termini used for generation of recombinant protein. The sequences of peptides used to generate antibodies specific to Fd, Fdx3, and Fdx6 are highlighted in yellow. Transit sequences of Fd (14, 63) and Fdx5 (39) are underlined. Conserved, negatively charged residues implicated in interaction of Fd with Fd:NADP+ oxidoreductase, Fd:thioredoxin reductase, nitrite reductase, and glutamate synthase are indicated by a red dot. B, a phylogenetic tree of ferredoxin proteins from A. thaliana, Z. mays, Physcomitrella patens, Ostreococcus tauri, Micromonas pusilla, Volvox carteri, and C. reinhardtii was generated with Phylogeny.fr (available on the World Wide Web). Only the core sequence was used (indicated by wavy lines in A). Chlamydomonas ferredoxins are indicated in blue. Photosynthetic and non-photosynthetic ferredoxins are indicated by green and red boxes, respectively. Bootstrap values are indicated in red. Bar, 0.4 amino acid substitutions/site. C, FDX transcript abundance was determined by RNA-seq in Chlamydomonas strain 2137 (E. Urzica and S. S. Merchant, unpublished results).
For purification of Chlamydomonas Fd, strain CC425 was grown in TAP medium (15 liters) supplemented with 100 μg/ml arginine under a photon flux density of 25 μmol photons m−2 s−1. When the cell density reached 1 × 107 cells/ml, cells were collected by centrifugation (4400 × g, 5 min, 4 °C); resuspended in 200 ml of 20 mm Tricine-NaOH, pH 7.4, 50 mm NaCl; and lysed by two cycles of freezing (−20 °C) and thawing (room temperature), followed by another cycle of freezing and thawing with liquid nitrogen. Insoluble particles were removed by ultracentrifugation (110,000 × g, 4 °C, 1 h), and Fd was purified according to the procedures described for recombinant ferredoxin.
Quantitative Real Time PCR
Total RNA was extracted from Chlamydomonas cells, and quantitative real time PCR was performed as described in Ref. 25 using gene-specific primers with an efficiency of 90–100% (Table 1). Melting curves were performed after the PCR to assess the presence of a unique final product, and the product was sequenced from one reaction to verify that it represented the gene of interest. The data are presented as the -fold change in mRNA abundance, normalized to an endogenous reference gene (CBLP or UBI2), relative to either the sample grown in nutrient-replete TAP medium. The abundance of CBLP or UBI2 did not change under the conditions tested in this work.
TABLE 1.
Ferredoxin primers used for real time PCR
Protein IDs correspond to version 3.0 draft genome. The percentage efficiency of each primer pair is calculated based on the theoretical doubling of product at each cycle. 100% efficiency indicates that the amount of product doubles at every cycle. The primer pairs corresponding to each gene are shown. The upper primer of each pair corresponds to the forward direction, and the lower primer corresponds to the reverse direction. All primer sequences are written 5′ to 3′.
| Gene name | Protein ID | Efficiency | Primer pair |
|---|---|---|---|
| % | |||
| PETF | 147787 | 95 | GTGTCCTTCGTCGCCACTCA |
| GTAGGTGTCAGCGGGGCACT | |||
| FDX2 | 159161 | 99 | ATGGACCTGCCCTACTCGT |
| GTCGCTGCCTTTAGAGCTTG | |||
| FDX3 | 196707 | 95 | AGCAGCACCCTAGCCAGCAC |
| TGATGCCCTTCGACTCCACA | |||
| FDX4 | 196705 | 94 | CTGCCAGGTCAAGACCATTT |
| CTGTCCTCATCCGGTCATCT | |||
| FDX5 | 156833 | 92 | ACCATCCTCACGCACCAG |
| CCCCTCCGTTGCGTGATAAA | |||
| FDX6 | 196703 | 97 | GCCGGTGCACAAGATCAAGA |
| GCAGCTCCAGACCCTTGTCC |
Protein Isolation for Immunoblot Analysis
Chlamydomonas proteins were extracted essentially as described previously (25). Cells were collected by centrifugation at 1000 × g for 5 min, washed in 10 mm sodium phosphate, pH 7.0, resuspended in the same buffer to a concentration of 2 × 108 cells/ml, and stored at −80 °C. For denaturing gel electrophoresis, cells were lysed by freeze/thaw cycling and separated into soluble and membrane components by centrifugation (10,000 × g, 5 min) as described in Ref. 27. The pellet was washed once and resuspended to the same volume as that of the soluble fraction. Protein concentration was determined by the Lowry method against a bovine serum albumin standard.
Antibody Production
Antibodies that were specific to Fd, Fdx2, Fdx3, and Fdx6 were supplied by Agrisera (Umeå, Sweden). The synthetic peptides NH2-CTIQTHQEEALY-COOH (Fd), NH2-CIVILTDQESKL-COOH (Fdx2), NH2-RPEPTMGKGWAELQK-COOH (Fdx3), and NH2-IERDDYALSIANMDE-COOH (Fdx6) used as immunogen (Fig. 1) were designed by Environmental Proteomics (Sackville, Canada).
Immunodetection
Proteins were separated on SDS-containing polyacrylamide gels (15%) and transferred in a semidry blotter onto polyvinylidene difluoride (0.45 μm; Millipore, Bedford, MA) for 90 min under constant current (400 mA) in transfer buffer (25 mm Tris, 192 mm glycine, 20% (v/v) methanol). Membranes were blocked overnight in 5% (w/v) dry milk in TBS (10 mm Tris-HCl, 150 mm NaCl, pH 7.5) with 0.05% (w/v) Tween 20. Membranes were incubated in primary antisera diluted in TBS with 0.05% Tween 20 for 90 min (Fdx5 was incubated for 5 h). Primary antibody dilutions were as follows: Fd, 1:10,000; Fdx2, 1:1000; NiR, 1:500; CF1, 1:10,000; Cox2b, 1:5000; ketoacid reductoisomerase, 1:10,000; Fdx3, 1:1000; Fdx5, 1:1000; Fdx6, 1:1000. After primary antibody treatment, membranes were washed three times in TBS with 0.05% Tween 20 for 5 min. The secondary antibody was alkaline phosphatase-conjugated goat anti-rabbit IgG (Southern Biotechnology Associates) used at 1:3000 or goat anti-rabbit horseradish peroxidase (Pierce) used at 1:25,000. Bound antibody was detected using either the alkaline phosphatase color reaction as described by Sambrook and Russell (28) or the Supersignal West Pico chemiluminescent substrate (Pierce), respectively.
Cyclic Voltammetry
Cyclic voltammetric measurements were performed using a conventional three-electrode system connected to a BAS 100-watt potentiostat (Bioanalytical Systems, West Lafayette, IN). A glass slide coated with indium-doped tin oxide served as the working electrode (SPI Supplies, West Chester, PA), a platinum wire served as the auxiliary, and the reference was an Ag|AgCl electrode in a 3 m NaCl filling solution (Bioanalytical Systems, West Lafayette, IN). All experiments were performed under an N2 atmosphere inside a glove box. The experimental procedure was modeled from one developed by Avila et al. (29). The protein samples were prepared for analysis by overnight dialysis (Slide-A-Lyzer MINI dialysis units; 3500 molecular weight cut-off; Pierce) into 100 mm MOPS buffer, pH 7.0, and were subsequently transferred into the glove box. A cationic electrode-modifying agent, poly-l-lysine, was added to the sample solution. The solution was then diluted with excess buffer to give nominal concentrations of 100 and 300 μm for the sample and poly-l-lysine, respectively. The solution volume in all cases was 400 μl. Over the range of scan rates examined, the peak current varied linearly with the square root of the scan rate, indicative of a diffusion-controlled process occurring at the electrode surface. Chemical reversibility was judged by the ratio of cathodic to anodic peak currents, and the electrochemical reversibility was judged by the separation of cathodic and anodic peak potentials. Spinach ferredoxin was measured as a reference.
Ferredoxin-Nitrite Reductase (NiR)-dependent Nitrite Reductase Activity
Reduction of nitrite to ammonium by NiR was monitored as a function of Fd concentration. Reactions (0.5 ml, 25 °C) contained 50 mm Tris-HCl (pH 7.5), 50 mm NaCl, 0.5 mm NaNO2, 100 nm NiR (from Chlamydomonas (purified according to Ref. 30) or Synechocystis sp. PCC6803),6 ferredoxin, and an NADPH-generating system (500 μm NADPH, 5 mm glucose-6-phosphate, 2 units of glucose-6-phosphate dehydrogenase, and 100 nm Z. mays leaf FNR). Reactions were stopped by dilution of 50 μl of the reaction mixture into 1 ml of water, and the amount of nitrite remaining was determined as in Ref. 8. The Michaelis-Menten constant (Km) values for Fd or Fdx2 were determined using ferredoxin ranging from 1 to 50 μm. Three independent experiments were performed at each ferredoxin concentration, and the Km and kcat values were calculated by non-linear regression using the program Origin.
Ferredoxin-dependent Cytochrome c Reductase Activity
Electron transfer between ferredoxin and FNR was measured indirectly as the reduction of cytochrome c by ferredoxin. Reactions (0.6 ml, 25 °C) containing 50 mm Tris-HCl (pH 7.5), 1 mm MgCl2, 100 mm NaCl, 200 μm cytochrome c, 40 nm Z. mays FNR (32), 50 μm NADPH in the presence of the NADPH-generating system (5 mm glucose-6-phosphate, 1 unit of glucose-6-phosphate dehydrogenase) and ferredoxin. The reduction of cytochrome c was monitored by the increase of absorbance at A550, and rates were calculated using a molar absorption coefficient of 20 mm−1 cm−1. For measurement of C. reinhardtii FNR-cytochrome c reductase activity, reactions (0.5 ml, 25 °C) contained 30 mm Tris-HCl (pH 7.9), 180 μm NADPH, 40 μm horse heart cytochrome c, 6 nm FNR (33), and ferredoxin. The Michaelis-Menten constant (Km) values for Fd or Fdx2 were determined using ferredoxin ranging from 0.5 to 60 μm. Three independent experiments were performed at each ferredoxin concentration, and the Km and kcat values were calculated by non-linear regression using the program Origin.
Activation of NADP-dependent Malate Dehydrogenase in the Reconstituted Fd/TRX Light System
Activation of NADP-dependent malate dehydrogenase (10 μm) by recombinant Arabidopsis TRXf1 (10 μm) was carried out in a reconstituted system composed of thylakoid membranes (34), 2 μm Synechocystis sp. PCC6803 FTR (35), and variable concentrations of ferredoxin in 50 mm HEPES (pH 7.5) containing 1 mm MgCl2 and 0.3 m sorbitol. Reactions were performed at 25 °C under 150 μmol m−2 s−1 illumination, and after a 5-min incubation, aliquots were withdrawn to assay NADP-dependent malate dehydrogenase activity and monitored as described in Ref. 36. Three independent experiments were performed at each ferredoxin concentration.
RESULTS
The Chlamydomonas Genome Encodes Six Ferredoxin Isoforms
The release of the Chlamydomonas genome allowed us to search for genes similar to PETF. Five previously uncharacterized ferredoxin-encoding genes were identified. The cDNAs encoding each ferredoxin were sequenced and named FDX2–FDX6.
The six ferredoxin-encoding genes are highly similar at the primary sequence level. They all contain the motif CX4CX2CXnC and therefore classify as plant type [Fe2S2] ferredoxins (37). The PETF transcript is the most abundant of the ferredoxin-encoding transcripts in cells grown in normal TAP medium, whereas FDX2–FDX6 each contributes less than 1% to the total ferredoxin-encoding gene pool (Fig. 1C). The PETF and FDX2 gene products (Fd and Fdx2, respectively) are the most similar, sharing 82% sequence identity (Fig. 1A). Fdx3 and Fdx6 are the most divergent, containing long N- and C-terminal extensions. In phylogenetic analysis comparing ferredoxin sequences in the green evolutionary lineage, ferredoxin isoforms seemed to cluster together based on functionality rather than by organism (Fig. 1B). Fdx2 clustered with non-photosynthetic root type ferredoxins. Fd and Fdx5 clustered near photosynthetic leaf type ferredoxins, although Fdx5 is much more divergent. Fdx3, Fdx4, and Fdx6 have most likely evolved specialized functions, since they do not cluster with leaf or root type ferredoxins. Furthermore, the occurrence of related ferredoxin isoforms in other plants and algae suggest that these specialized functions are conserved in the plant lineage (Fig. 1B).
Fdx2, -3, -5, and -6 Are Chloroplast-localized
To study the function of each ferredoxin isoform, we sought to localize each protein product, since compartmentalization provides clues to function. Although C. reinhardtii Fd was originally isolated in 1966, the actual gene product of PETF has never been uniquely distinguished and localized (38). Antibodies were generated against antigenic peptides corresponding to unique regions of the predicted protein sequences encoded by PETF, FDX2, FDX3, and FDX6 (Fig. 1A). In order to verify the specificity of each antibody, each ferredoxin isoform was expressed in E. coli, and equal amounts of soluble lysate corresponding to each ferredoxin isoform were analyzed by immunoblot with each antibody. All antibodies showed high selectivity for their respective antigens, and no cross-reaction between ferredoxin species was detected (Fig. 2). This enabled us to determine the subcellular location of Fd, Fdx2, Fdx3, and Fdx6.
FIGURE 2.
Specificity of ferredoxin antibodies. 20 μg of soluble protein extracts from E. coli cells expressing recombinant ferredoxins was separated by denaturing polyacrylamide gel electrophoresis, and the specificity of each ferredoxin antibody was analyzed by immunoblot.
Chloroplasts and mitochondria were purified by density gradient centrifugation (see “Experimental Procedures”). We assessed the purity of each fraction by immunoblot analysis of chloroplast-localized ketoacid reductoisomerase, which was present exclusively in the chloroplast fraction, and Cox2b (cytochrome oxidase), a protein of the inner mitochondrial membrane, which was present only to a small extent in the chloroplast fraction (Fig. 3A). This indicated that the mitochondrial fraction was completely free of chloroplasts, whereas the chloroplast fraction was minimally contaminated with mitochondria. Due to the low abundance of Fdx2 in normal TAP medium (see below), the localization of Fdx2 was determined under different conditions (Fig. 3B). All ferredoxins tested in this work (Fd, Fdx2, Fdx3, and Fdx6) were located in the chloroplast (Fig. 3, A and C). Although each ferredoxin isoform contains a putative organelle-targeting sequence, cytosolic localization is always a formal possibility. Therefore, we assessed the level of cytosolic contamination in the chloroplast fraction by probing for α-tubulin (Fig. 3D). α-tubulin was more abundant in total cells compared with purified chloroplasts. Given that the chloroplast comprises 50% of the cell in Chlamydomonas, this indicates that the purified chloroplasts are minimally contaminated with cytosol. Fdx5 was recently shown by others to also be localized in the chloroplast (39). Therefore, like Fd, the newly identified Fdx2, -3, -5, and -6 function in plastid-based metabolism.
FDX Genes Are Differentially Regulated
In order to potentially distinguish the metabolic pathways for which each ferredoxin isoform might show specificity, we determined the pattern of expression of FDX genes in conditions in which well characterized chloroplast-localized ferredoxin-dependent enzymes are known to be expressed. In particular, we measured the relative abundance of FDX mRNA in conditions of nutrient assimilation, hydrogen production, and peroxide stress. RNA was isolated from Chlamydomonas cells grown in each of these conditions, and relative transcript abundances were determined by real time PCR using gene-specific primers (Table 1).
During the assimilation of nitrate, reducing power from ferredoxin is required for the reduction of nitrite to ammonia by nitrite reductase. The expression of the nitrate assimilation pathway is controlled by the transcription factor, Nit2, which is negatively regulated by ammonia (40). To test whether a specific ferredoxin isoform was involved in nitrate assimilation, cells were grown in ammonium versus nitrate as the sole nitrogen source, and the abundance of each FDX transcript in nitrate-grown cells was compared with ammonium-grown cells. The nitrate reductase structural gene, NIA1, was amplified as a control for operation of the nitrogen signaling system (Fig. 4) (40, 41). The FDX2 transcript was 300–400 times more abundant when the cells were grown in nitrate compared with ammonia, whereas the abundance of all other FDX and PETF transcripts did not change (Fig. 4A). The specific increase of FDX2 in response to nitrate suggests substrate specificity of Fdx2 for NiR. The abundance of Fdx2 polypeptide was increased at least 10-fold in nitrate-grown cells, and NiR was increased at least 15-fold (Fig. 4B). Although classical Fd is still the most abundant of the ferredoxins in this condition (data not shown), the coordinate accumulation of Fdx2 and NiR polypeptides in response to nitrate further supports the hypothesis that NiR is a specific substrate of Fdx2.
FIGURE 4.
FDX2 is induced in nitrate-grown cells. A, RNA was isolated from wild-type Chlamydomonas strain 21gr cells grown in TAP medium containing ammonium or nitrate as a nitrogen source, and FDX expression was analyzed by quantitative real time PCR. Relative expression was normalized to UBI2 expression, and -fold induction was calculated by the 2−ΔΔCT method (64). Amplifications were performed in technical triplicate and averaged. Biological duplicates are shown. Inset, amplification product of NIA1 was visualized on a 1% agarose gel. B, soluble protein extracts of Chlamydomonas strain 21gr grown in TAP medium containing ammonium or nitrate as a nitrogen source were separated on a denaturing polyacrylamide gel containing SDS, and Fdx2 and NiR abundance were analyzed by immunoblot.
Ferredoxin may also have an antioxidant role in the chloroplast. Reduced ferredoxin transfers electrons to TRXs via FTR. After reduction, TRXs interact with multiple targets, including proteins linked to stress responses, such as peroxiredoxins and glutathione peroxidases, which are involved in hydrogen peroxide (H2O2) and alkylhydroperoxide reduction (42, 43). Therefore, to assess whether a ferredoxin isoform might be specific for this antioxidant pathway, cells were treated with 1 mm H2O2 for 2 h. MSD3, encoding an H2O2-inducible manganese-superoxide dismutase, was amplified as a control (25). FDX2 abundance was uniquely increased ∼70-fold in response to H2O2 (Fig. 5A). Since Fdx2 could not be detected in H2O2-treated cells (data not shown), we suggest that Fdx2 is particularly prone to damage by peroxide, and we propose that the up-regulation of FDX2 could be viewed as an attempt to compensate for the loss of Fdx2.
FIGURE 5.
Fdx2 is damaged by H2O2in vivo. A, RNA was isolated from Chlamydomonas strain 17D− grown in TAP medium prior to and 2 h after the addition of 1 mm H2O2. Gene expression was measured by quantitative real time PCR. FDX abundance after 2 h of H2O2 treatment was compared with expression prior to the addition of H2O2. RNA abundance was normalized to CBLP. MSD3 was tested as a control for H2O2 treatment. Two independent experiments are shown. B, soluble protein extracts isolated from Chlamydomonas strain 21gr grown in TAP medium containing NO3− prior to and 1 and 2 h after the addition of 1 mm H2O2 were separated on a 15% denaturing polyacrylamide gel containing SDS, and Fdx2 and Fd abundance were determined by immunoblot.
In order to test this possibility, cells were grown in nitrate to induce Fdx2 expression and then treated with 1 mm H2O2. Protein was isolated prior to and 1 and 2 h after the addition of H2O2 and analyzed for Fdx2 abundance. In some experiments, we saw that after 1 h of exposure to 1 mm H2O2, Fdx2 was decreased and almost completely lost after 2 h (Fig. 5B). Fd and CF1 were also decreased after 2 h (Fig. 5B), but Fdx2 was decreased to a greater extent, supporting the idea of a difference in the physical properties of Fdx2 and Fd, despite their similarities at the primary sequence level. In conclusion, the induction of FDX2 might simply occur in a feedback response to the loss of functional Fdx2.
In anaerobic conditions, ferredoxin provides electrons for hydrogen production via interaction with hydrogenase (44, 45). The FDX5 transcript was induced 200-fold in anaerobically grown cells (Fig. 6A), suggesting that Fdx5 might be the source of electrons for the hydrogenase reaction, but a very recent study does not support this hypothesis (39, 46). Therefore, we considered other pathways that respond to low O2. One such pathway is the Crr1 (copper response regulator) regulon. Certain genes that are activated in oxygen deficiency are also activated in response to copper deficiency (47). These responses are mediated by the transcription factor Crr1 (22, 48). Therefore, we wondered whether FDX5 expression was also controlled by Crr1. Crr1 activates the expression of target genes in hypoxia, in copper deficiency, and in the presence of nickel (48, 49). When we compared the abundance of the FDX5 transcript in RNA isolated from copper-replete versus copper-deficient cells, we noted that the FDX5 transcript was increased in abundance by 4 orders of magnitude in the absence of copper (Fig. 6B). In addition, the transcription of FDX5 was also increased (up to 102-fold) in the presence of nickel (Fig. 6C). The coordinate expression of FDX5 with other targets of Crr1 suggests a role for Fdx5 in Crr1-dependent signaling in copper deficiency. The corresponding protein was almost undetectable in normal TAP medium but was increased at least 20-fold in copper deficiency, further supporting an important role for Fdx5 during copper-deficient metabolism (Fig. 6D).
FIGURE 6.
FDX5 is controlled by copper homeostasis factor Crr1. A, RNA was isolated from wild-type Chlamydomonas strain CC124 grown in the dark in order to induce hypoxia and from cells grown aerobically in the light. Relative abundance of FDX RNAs was determined by quantitative real time PCR. Gene expression was normalized to CBLP. Two independent experiments are shown. B, RNA was isolated from wild-type Chlamydomonas cells grown in the presence and absence of copper. FDX gene expression was analyzed as in A. Two independent experiments are shown. Circles, strain 2137; triangles, strain CC125. C, RNA was isolated from wild-type Chlamydomonas cells grown in the presence and absence of nickel. FDX expression was analyzed as in A. Two independent experiments are shown. Circles, strain 2137; triangles, strain CC125. D, soluble protein extracts of Chlamydomonas strain 2137 grown in TAP medium containing 0 (−Cu) or 2 μm copper (+Cu) were separated on a 15% non-denaturing polyacrylamide gel, and Fdx5 abundance was analyzed by immunoblot. Plastocyanin and cytochrome c6 abundance were analyzed as markers for copper-deficient and copper-replete conditions, respectively.
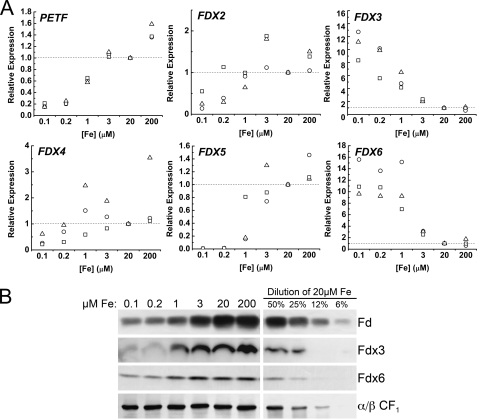
The abundance of ferredoxin, the most abundant iron-containing protein in photosynthetic cells, is a classic marker of iron nutritional state (50, 51). RNA was isolated from cells grown in various concentrations of iron. The PETF transcript was decreased 80% when iron was limited (0.1 μm iron) (Fig. 7A). Interestingly, in contrast, FDX3 and FDX6 were both increased about 10-fold in iron limitation (0.1 μm iron) relative to iron-replete conditions (20 μm iron) (Fig. 7A). In cyanobacteria, ferredoxin is decreased in an iron-sparing response and replaced with flavodoxin, which can substitute in many, although not all, Fd-dependent reactions (52, 53). We previously showed that Fd is indeed a sensitive marker of iron nutrition in Chlamydomonas as well (23), but a substitute flavodoxin is not evident in the genome (as defined by mutual best hit in April 2009). Therefore, we wondered whether iron allocation to ferredoxins might be determined by differential expression of PETF and FDX genes. Unexpectedly, for Fdx3 and Fdx6, the abundance of the corresponding proteins did not match, and in fact, Fdx3 and Fdx6 abundance decreased progressively in iron-deficient and iron-limited cells (Fig. 7B). The observed up-regulation of FDX3 and FDX6 mRNA may therefore serve to compensate for reduced amounts of Fdx3 and Fdx6 in iron-limited cells, analogous to the increase of FDX2 in H2O2-treated cells.
FIGURE 7.
Ferredoxin expression in response to iron nutrition. A, FDX expression was analyzed by quantitative real time PCR in Chlamydomonas strain 17D− cells grown in various concentrations of iron. Relative gene expression was normalized to CBLP. Three independent experiments are shown. B, 20 μg of soluble protein extracts of Chlamydomonas strain 17D− grown in TAP medium containing 0.1, 0.2, 1, 3, 20, or 200 μm iron were separated on a 15% denaturing polyacrylamide gel containing SDS, and Fd, Fdx3, and Fdx6 abundance were analyzed by immunoblot.
Fdx2 Is a More Efficient Electron Donor to NiR than Fd
Since the interaction of ferredoxin with NiR is well characterized (30, 54, 55), we sought to test the hypothesis that Fdx2 is a more specific electron donor to NiR than Fd. Recombinant Fd and Fdx2 were purified from E. coli (see “Experimental Procedures”), and electron transfer activity was measured between Fdx2 and NiR and compared with electron transfer activity of Fd with NiR. When Fd and Fdx2 were assayed with Chlamydomonas NiR, the Michaelis-Menten constants for both ferredoxins were similar (Km ∼25 μm; Table 2); however, the turnover numbers of NiR in the presence of Fdx2 or Fd differed by ∼10-fold (Table 2). Although the reduction of NiR via Fdx2 had a kcat of 14.6 s, the same reaction had a kcat of 1.35 s when Fd was used (Table 2). When we compared the catalytic efficiencies (kcat/Km; Table 2), Chlamydomonas NiR appeared about 10 times more efficient in the presence of Fdx2 than in the presence of Fd. When the same assays were carried out using NiR from the cyanobacteria Synechocystis sp. PCC6803, although the turnover numbers were comparable, the Michaelis-Menten constants differed 2-fold (Table 2). The enzyme had a Km of 17 μm for Fdx2 and a Km of 42 μm for Fd (Table 2). Overall, these values resulted in a 2-fold higher catalytic efficiency of the enzyme in the presence of Fdx2 compared with Fd (Table 2). This indicates that the apparent differences of catalytic efficiencies are decreased with the heterologous enzyme. We can clearly conclude that Fdx2 is a better electron donor to NiR than Fd. These results support the hypothesis that Fdx2 has substrate specificity toward NiR.
TABLE 2.
Kinetic parameters for nitrate reduction catalyzed by NiR from Chlamydomonas or Synechocystis, mediated by Chlamydomonas Fd or Fdx2
Km and kcat were determined by non-linear regression. The values are means ± S.D. of three independent determinations.
| NiR | Fd |
Fdx2 |
||||
|---|---|---|---|---|---|---|
| Km | kcat | kcat/Km | Km | kcat | kcat/Km | |
| μm | s−1 | μm | s−1 | |||
| C. reinhardtii | 29.0 ± 2.9 | 1.35 ± 0.06 | 0.047 | 25.5 ± 3.4 | 14.6 ± 1.8 | 0.573 |
| Synechocystis sp. 6803 | 41.5 ± 6.7 | 666 ± 48 | 13.9 | 17.3 ± 5.2 | 480 ± 57 | 27.7 |
Fd:NADP+ Oxidoreductase (FNR) Has a Lower Km for Fdx2 than for Fd
Since ferredoxin is most well known for its reaction with FNR, we were interested in comparing the kinetics of electron transfer between Chlamydomonas Fd and Fdx2 with homologous FNR. The results of these experiments (summarized in Table 3) demonstrate that although Chlamydomonas FNR shows almost identical kcat in the presence of both ferredoxins, the Km for Fdx2 (Km = 6 μm) was 6 times lower than the Km for Fd (Km = 39 μm), resulting in a 6-fold higher catalytic efficiency (kcat/Km; Table 3). We also determined kinetic parameters of root and leaf forms of FNR from Z. mays, and we did not observe any significant differences either in Km for Fd and Fdx2 or turnover numbers, resulting in almost identical catalytic efficiencies for both FNR forms (Table 3). As observed for NiR, the differences of catalytic efficiencies appeared decreased with heterologous enzymes.
TABLE 3.
Kinetic parameters of Chlamydomonas FNR and leaf type and root type FNR from Z. mays in NADPH-dependent cytochrome c reduction
Km and kcat were determined by non-linear regression. The values are means ± S.D. of three independent determinations.
| FNR | Fd |
Fdx2 |
||||
|---|---|---|---|---|---|---|
| Km | kcat | kcat/Km | Km | kcat | kcat/Km | |
| μm | s−1 | μm | s−1 | |||
| C. reinhardtii | 39.1 ± 4.6 | 252 ± 37 | 6.44 | 6.58 ± 1.33 | 248 ± 23 | 37.7 |
| Z. mays leaf type | 28.4 ± 1.8 | 106 ± 4 | 3.73 | 25.6 ± 3.2 | 104 ± 10 | 4.06 |
| Z. mays root type | 28.5 ± 0.5 | 211 ± 8 | 7.40 | 24.9 ± 4.4 | 254 ± 28 | 10.2 |
Fd Is a More Efficient Electron Donor to FTR than Fdx2
Another ferredoxin-dependent signal transduction pathway is the light-dependent electron transfer from ferredoxin to thioredoxin catalyzed by FTR. This reaction maintains the chloroplastic TRX pool in a reduced state, allowing modulation of several metabolic processes through disulfide-thiol interchanges, depending on light conditions.
In order to determine the affinity of FTR for Fd or Fdx2, we reconstituted a complete light activation system (described under “Experimental Procedures”). In this system, we evaluated the electron transfer from Fd or Fdx2 to FTR by following the thioredoxin-dependent activation of a classical thioredoxin-regulated enzyme, NADP-dependent malate dehydrogenase. The results of these experiments (summarized in Table 4) revealed that 3 times more Fdx2 than Fd was required to achieve 50% activation of malate dehydrogenase (Table 4). This result shows that FTR is probably not a specific target of Fdx2, suggesting that the regulation of the TRX redox pool is under the control of Fd.
TABLE 4.
Half-saturation concentration (S0.5) of Fd or Fdx2 for NADP-MDH activation rate in a reconstituted Fd/thioredoxin light system
The values are means ± S.D. of three independent determinations.
| Ferredoxin | S0.5 |
|---|---|
| μm | |
| Fd | 1.7 ± 0.1 |
| Fdx2 | 8.2 ± 0.5 |
The Midpoint Potential of Fdx2 Is More Positive than That of Fd
The efficiency of electron transfer between ferredoxin and its partner enzymes is dependent not only on the kinetics of the reaction but also on the oxidation-reduction (redox) midpoint potential of the [Fe2S2] cluster of the ferredoxin relative to that of its partner enzyme. Therefore, the redox potentials of Fd and Fdx2 were measured by cyclic voltammetry. The midpoint potential of spinach ferredoxin (−420 mV) was measured as a reference. The midpoint potentials obtained were −398 mV for Fd purified from Chlamydomonas, and −321 mV for recombinant Fdx2 (Fig. 8 and Table 5), indicating that Fdx2 has a narrower range of possible substrates that it can reduce in a thermodynamically favorable reaction than does Fd.
FIGURE 8.
The midpoint potentials of Fd and Fdx2. Cyclic voltammograms were obtained at an indium-doped tin oxide electrode from a solution of 100 mm spinach (S.o.) ferredoxin, Chlamydomonas (C.r.) Fd, or Fdx2 in 100 mm MOPS, pH 7.0, at a sweep rate of 50 mV/s and given versus the Ag/AgCl electrode.
TABLE 5.
Midpoint potential of Fd versus Fdx2
| Ferredoxin | Midpoint Potential |
|---|---|
| Spinach Fd | −420 mV |
| C. reinhardtii Fd | −398 mV |
| Fdx2 | −321 mV |
DISCUSSION
Six Ferredoxins in Chlamydomonas Are Differently Regulated and Fdx2 Exhibits Different Substrate Specificity with Respect to Fd
The Chlamydomonas genome encodes six plant type [Fe2S2] ferredoxin genes, PETF and FDX2–FDX6 (9). Of these, PETF contributes 98% of the total ferredoxin-encoding transcript pool (∼107 mRNA transcripts/μg of total mRNA) in cells grown in TAP medium (Fig. 9). This fraction changes in response to environmental stress or nutrient deficiency (Figs. 4–7) and is most dramatically different in a situation of copper-deficiency, where the FDX5 transcript is nearly as abundant as PETF (Fig. 9).
FIGURE 9.
Change in FDX transcript abundance in response to copper and iron nutrition. FDX transcript abundance was determined by RNA-seq in Chlamydomonas strain 2137 (M. Castruita and S. S. Merchant, unpublished results; E. Urzica and S. S. Merchant, unpublished results). The sizes of the circles are proportional to the relative abundance of the total ferredoxin transcript pool in each condition.
The increase in FDX5 transcript is paralleled by a corresponding increase in the protein. This is highly suggestive of a specific function of Fdx5 in the copper deficiency response. One possibility is that there are changes to the photosynthetic apparatus beyond the replacement of plastocyanin by cytochrome c6 and that Fdx5 supplements Fd (product of PETF) as the acceptor from PSI. Another possibility is that Fdx5 serves as the reductant for the aerobic oxidative cyclase in chlorophyll biosynthesis (56). This di-iron enzyme requires a source of electrons for reduction of the second atom of O2 to H2O, and it is conceivable that a specialized ferredoxin might provide this function. Indeed, it is known that a stromal factor is required in vitro for the cyclase reaction (57). The pattern of expression of FDX5 with regard to O2, copper nutrition, and nickel supplementation is coordinate with the reciprocal expression of CRD1 and CTH1, encoding the two forms of the cyclase (58). The occurrence of two isoforms with perhaps different specificity for Fd versus Fdx5 might be a mechanism for promoting the cyclase reaction in copper-deficient cells. The location of Fdx5 to the chloroplast (39) is not incompatible with either hypothesis.
In the case of Fdx2, we were able to ascribe a role in nitrogen assimilation on the basis of the increased accumulation of both RNA and protein in cells grown in nitrate versus ammonia (Fig. 4). More specifically, kinetic analysis of the nitrite reductase reaction demonstrated a preference for Fdx2 versus Fd as an electron donor, suggesting that Fdx2 is specifically involved in nitrite reduction.
Fdx2 was also a better electron donor toward Chlamydomonas Fd:NADP+ oxidoreductase than Fd. Interestingly, in the case of both nitrite reductase and Fd:NADP+ oxidoreductase assays, the preference for Fdx2 was only evident using a homologous system. When cyanobacterial nitrite reductase and maize Fd:NADP+ oxidoreductase were assayed, there were no significant differences in electron transfer activity between Fdx2 versus Fd. These results emphasize the importance of using homologous enzymes and substrates in reactions involving protein-protein interactions. However, when we assessed substrate specificity of ferredoxins for Synechocystis Fd:thioredoxin reductase, we observed that Fd shows a 3-fold higher electron transfer activity toward Synechocystis Fd:thioredoxin reductase than Fdx2. These results, although obtained using heterologous enzymes, strongly suggest that electrons from photosystem I are preferentially transferred to thioredoxin via Fd:thioredoxin reductase by Fd.
Different Physical Properties of Fdx2 versus Fd
A considerable body of evidence points to the importance of electrostatic interactions in the interaction between ferredoxin and enzymes, such as Fd:NADP+ oxidoreductase, Fd:thioredoxin reductase, nitrite reductase, glutamate synthase, and sulfite reductase (6). Given the acidic isoelectric points of most plant type ferredoxins and the presence of three highly conserved negatively charged regions on the surfaces of these ferredoxins, conserved, negatively charged amino acids are proposed to make important contributions to the interaction between ferredoxin and its target enzymes (6). In the specific case of C. reinhardtii Fd, Jacquot et al. (59) have used a site-directed mutagenesis approach to provide evidence for the importance of a conserved glutamate, Glu91, located in the short C-terminal α-helix (α1) of Fd, and the negatively charged patch, located in the most N-terminal α-helix, which includes Asp25, Glu28, and Glu29, in the interaction of this ferredoxin with Fd:NADP+ oxidoreductase and Fd:thioredoxin reductase. García-Sánchez et al. (60, 61) have also provided evidence, using carbodiimide cross-linking of Fd to nitrite reductase and glutamate synthase in crude C. reinhardtii soluble extracts, that both Glu91 and the α1 charged patch appear to play a role in complex formation between Fd and both of these ferredoxin-dependent enzymes. All four negatively charged residues are conserved only in Fd, Fdx2, and Fdx5 and probably contribute to substrate selectivity in vivo (Fig. 1A).
Despite the high sequence similarity between Fd and Fdx2 (82% identity for mature protein), comparable with the sequence identity between Chlamydomonas and spinach Fd, there are significant differences in physical properties and hence presumably function. For instance, dithionite-reduced Fd is conventionally used in nitrite reductase assays (8, 62), but Fdx2 could not be reoxidized after dithionite reduction (data not shown). Fdx2 is also more sensitive to H2O2 than is Fd (Fig. 5B). Structural modeling predicts differences in surface charge distribution of the two proteins (Fig. 10A), and this is supported by their different electrophoretic mobilities in a non-denaturing gel system (Fig. 10B). Together with the difference in midpoint potential (−398 and −321 mV for Fd and Fdx2, respectively; Table 5), these distinct physical properties probably contribute to the in vivo substrate specificities of the two proteins. We note that the root type non-photosynthetic ferredoxins also have a higher midpoint potential (−330 mV for maize) compared with that of the leaf type (−420 mV for spinach, maize, and Arabidopsis) (15).
FIGURE 10.
Surface charge distribution of Fdx proteins. A, the coordinates of the Chlorella fusca ferredoxin (Protein Data Bank code 1AWD) were used to derive coordinates of the Chlamydomonas ferredoxins (31). The N terminus of Fdx2 was estimated based on homology to the transit sequence of Fd. Electrostatic potentials were calculated in PyMOL (available on the World Wide Web) using the Adaptive Poisson-Boltzmann Solver plugin. Red, negatively charged amino acids; blue, positively charged amino acids. B, 0.5 μg of purified Fd from Chlamydomonas and recombinant Fd and Fdx2 were separated on an 18% non-denaturing polyacrylamide gel.
In the dark, FNR catalyzes the reduction of ferredoxin by NADPH (7). In higher plants, this reaction occurs in roots. The Km of FNR for Fdx2 was 6 times lower than that for Fd. Perhaps the lower Km of FNR for Fdx2 allows for the interaction of the low abundant Fdx2 with FNR in a unicellular organism, especially since Fd is so abundant (Fig. 9). Moreover, its induction by nitrate might favor nitrogen assimilation versus carbon assimilation. Collectively, these results suggest that Fdx2 serves the function of a non-photosynthetic root type ferredoxin, whereas Fd probably plays a major role in photosynthesis, being a more specific electron donor for Fd:thioredoxin reductase.
Acknowledgments
We thank the Kazusa DNA Research Institute for providing cDNA clones, Krishna Niyogi for strain 17D−, Thomas Happe for antibodies against Fdx5, Antonio Márquez for antibodies against NiR, Maryse Block for ketoacid reductoisomerase antibody, and Jim Umen for antibodies against α-tubulin. We are grateful to Janette Kropat and Anja Hemschemeier for protein and RNA samples shown in Fig. 6, to Eugen Urzica and Madeli Castruita for data contributing to Fig. 9, and to Valerie Villareal for help generating protein models shown in Fig. 10. We also thank Myroslawa Miginiac-Maslow, Dudley Page and Anja Hemschemeier for critical comments on the manuscript.
This work was supported by Department of Energy Grants DE-FD02-04ER15529 (to S. S. M.) and DE-FG03-99ER20346 (to D. B. K.), National Science Foundation (NSF) Grant CHE0517080-3 (to P. J. F.), and Agence Nationale de la Recherche Grant ANRJC05-45741 (to M. Z. and S. D. L.).
The nucleotide sequence(s) reported in this paper has been submitted to the Gen-BankTM/EBI Data Bank with accession number(s) DQ36047, DQ360408, DQ360409, DQ360410, and DQ360411.
A. Hemschemeier, unpublished observations.
K. Sekine, Y. Sakakibara, T. Hase, and N. Sato, manuscript in preparation.
- Fd
- ferredoxin
- PSI
- photosystem I
- FNR
- Fd:NADP+ oxidoreductase
- NiR
- nitrite reductase
- TRX
- thioredoxin
- FTR
- Fd:thioredoxin reductase
- TAP
- Tris acetate-phosphate
- MOPS
- 4-morpholinepropanesulfonic acid
- Tricine
- N-[2-hydroxy-1,1-bis(hydroxymethyl)ethyl]glycine.
REFERENCES
- 1.Fukuyama K. (2004) Photosynth. Res. 81, 289–301 [DOI] [PubMed] [Google Scholar]
- 2.Tagawa K., Arnon D. I. (1968) Biochim. Biophys. Acta 153, 602–613 [DOI] [PubMed] [Google Scholar]
- 3.Mortenson L. E., Valentine R. C., Carnahan J. E. (1962) Biochem. Biophys. Res. Commun. 7, 448–452 [DOI] [PubMed] [Google Scholar]
- 4.Merchant S., Sawaya M. R. (2005) Plant Cell 17, 648–663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lemaire S. D., Michelet L., Zaffagnini M., Massot V., Issakidis-Bourguet E. (2007) Curr. Genet. 51, 343–365 [DOI] [PubMed] [Google Scholar]
- 6.Hase T., Schürmann P., Knaff D. B. (2006) in Photosystem I: The Light-Driven Plastocyanin:Ferredoxin Oxidoreductase ( Golbeck J. H. ed) Springer, Dordrecht, The Netherlands [Google Scholar]
- 7.Bowsher C. G., Boulton E. L., Rose J., Nayagam S., Emes M. J. (1992) Plant J. 2, 893–898 [Google Scholar]
- 8.Suzuki A., Oaks A., Jacquot J. P., Vidal J., Gadal P. (1985) Plant Physiol. 78, 374–378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Merchant S. S., Allen M. D., Kropat J., Moseley J. L., Long J. C., Tottey S., Terauchi A. M. (2006) Biochim. Biophys. Acta 1763, 578–594 [DOI] [PubMed] [Google Scholar]
- 10.Rochaix J. D. (2002) FEBS Lett. 529, 34–38 [DOI] [PubMed] [Google Scholar]
- 11.Grossman A. R., Croft M., Gladyshev V. N., Merchant S. S., Posewitz M. C., Prochnik S., Spalding M. H. (2007) Curr. Opin. Plant Biol. 10, 190–198 [DOI] [PubMed] [Google Scholar]
- 12.Moseley J. L., Chang C. W., Grossman A. R. (2006) Eukaryot. Cell 5, 26–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang Z., Shrager J., Jain M., Chang C. W., Vallon O., Grossman A. R. (2004) Eukaryot. Cell 3, 1331–1348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schmitter J. M., Jacquot J. P., de Lamotte-Guéry F., Beauvallet C., Dutka S., Gadal P., Decottignies P. (1988) Eur. J. Biochem. 172, 405–412 [DOI] [PubMed] [Google Scholar]
- 15.Hanke G. T., Kimata-Ariga Y., Taniguchi I., Hase T. (2004) Plant Physiol. 134, 255–264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hanke G. T., Endo T., Satoh F., Hase T. (2008) Plant Cell Environ. 31, 1017–1028 [DOI] [PubMed] [Google Scholar]
- 17.Wang R., Guegler K., LaBrie S. T., Crawford N. M. (2000) Plant Cell 12, 1491–1509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hase T., Mizutani S., Mukohata Y. (1991) Plant Physiol. 97, 1395–1401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Matsumura T., Sakakibara H., Nakano R., Kimata Y., Sugiyama T., Hase T. (1997) Plant Physiol. 114, 653–660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schrautemeier B., Böhme H. (1985) FEBS Lett. 184, 304–308 [Google Scholar]
- 21.Loppes R., Radoux M., Ohresser M. C., Matagne R. F. (1999) Plant Mol. Biol. 41, 701–711 [DOI] [PubMed] [Google Scholar]
- 22.Kropat J., Tottey S., Birkenbihl R. P., Depège N., Huijser P., Merchant S. (2005) Proc. Natl. Acad. Sci. U.S.A. 102, 18730–18735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moseley J. L., Allinger T., Herzog S., Hoerth P., Wehinger E., Merchant S., Hippler M. (2002) EMBO J. 21, 6709–6720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Long J. C., Sommer F., Allen M. D., Lu S. F., Merchant S. S. (2008) Genetics 179, 137–147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Allen M. D., Kropat J., Tottey S., Del Campo J. A., Merchant S. S. (2007) Plant Physiol. 143, 263–277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Eriksson M., Gardestrom P., Samuelsson G. (1995) Plant Physiol. 107, 479–483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Howe G., Merchant S. (1992) EMBO J. 11, 2789–2801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sambrook J., Russell D. W. (2001) Molecular Cloning: A Laboratory Manual, 3rd Ed., pp. A9.39–A9.42, Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY [Google Scholar]
- 29.Avila L., Wirtz M., Bunce R. A., Rivera M. (1999) J. Biol. Inorg. Chem. 4, 664–674 [DOI] [PubMed] [Google Scholar]
- 30.Tripathy J. N., Hirasawa M., Kim S. K., Setterdahl A. T., Allen J. P., Knaff D. B. (2007) Photosynth. Res. 94, 1–12 [DOI] [PubMed] [Google Scholar]
- 31.Kelley L. A., Sternberg M. J. E. (2009) Nat. Protoc. 4, 363–371 [DOI] [PubMed] [Google Scholar]
- 32.Onda Y., Matsumura T., Kimata-Ariga Y., Sakakibara H., Sugiyama T., Hase T. (2000) Plant Physiol. 123, 1037–1045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Decottignies P., Le Maréchal P., Jacquot J. P., Schmitter J. M., Gadal P. (1995) Arch. Biochem. Biophys. 316, 249–259 [DOI] [PubMed] [Google Scholar]
- 34.Droux M., Jacquot J. P., Miginac-Maslow M., Gadal P., Huet J. C., Crawford N. A., Yee B. C., Buchanan B. B. (1987) Arch. Biochem. Biophys. 252, 426–439 [DOI] [PubMed] [Google Scholar]
- 35.Michelet L., Zaffagnini M., Marchand C., Collin V., Decottignies P., Tsan P., Lancelin J. M., Trost P., Miginiac-Maslow M., Noctor G., Lemaire S. D. (2005) Proc. Natl. Acad. Sci. U.S.A. 102, 16478–16483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Issakidis E., Miginiac-Maslow M., Decottignies P., Jacquot J. P., Crétin C., Gadal P. (1992) J. Biol. Chem. 267, 21577–21583 [PubMed] [Google Scholar]
- 37.Bertini I., Luchinat C., Provenzani A., Rosato A., Vasos P. R. (2002) Proteins Struct. Funct. Genet. 46, 110–127 [DOI] [PubMed] [Google Scholar]
- 38.Gorman D. S., Levine R. P. (1966) Plant Physiol. 41, 1643–1647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jacobs J., Pudollek S., Hemschemeier A., Happe T. (2009) FEBS Lett. 583, 325–329 [DOI] [PubMed] [Google Scholar]
- 40.Camargo A., Llamas A., Schnell R. A., Higuera J. J., González-Ballester D., Lefebvre P. A., Fernández E., Galván A. (2007) Plant Cell 19, 3491–3503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fernández E., Schnell R., Ranum L. P., Hussey S. C., Silflow C. D., Lefebvre P. A. (1989) Proc. Natl. Acad. Sci. U. S. A. 86, 6449–6453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Goyer A., Haslekås C., Miginiac-Maslow M., Klein U., Le Marechal P., Jacquot J. P., Decottignies P. (2002) Eur. J. Biochem. 269, 272–282 [DOI] [PubMed] [Google Scholar]
- 43.Dayer R., Fischer B. B., Eggen R. I. L., Lemaire S. D. (2008) Genetics 179, 41–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Happe T., Mosler B., Naber J. D. (1994) Eur. J. Biochem. 222, 769–774 [DOI] [PubMed] [Google Scholar]
- 45.Happe T., Naber J. D. (1993) Eur. J. Biochem. 214, 475–481 [DOI] [PubMed] [Google Scholar]
- 46.Mus F., Dubini A., Seibert M., Posewitz M. C., Grossman A. R. (2007) J. Biol. Chem. 282, 25475–25486 [DOI] [PubMed] [Google Scholar]
- 47.Quinn J. M., Barraco P., Eriksson M., Merchant S. (2000) J. Biol. Chem. 275, 6080–6089 [DOI] [PubMed] [Google Scholar]
- 48.Quinn J. M., Eriksson M., Moseley J. L., Merchant S. (2002) Plant Physiol. 128, 463–471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Quinn J. M., Kropat J., Merchant S. (2003) Eukaryot. Cell 2, 995–1002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hutber G. N., Hutson K. G., Rogers L. J. (1977) FEMS Microbiol. Lett. 1, 193–196 [Google Scholar]
- 51.Sandmann G., Peleato M. L., Fillat M. F., Lázaro M. C., Gómez-Moreno C. (1990) Photosynth. Res. 26, 119–125 [DOI] [PubMed] [Google Scholar]
- 52.Laudenbach D. E., Reith M. E., Straus N. A. (1988) J. Bacteriol. 170, 258–265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.McKay R. M., Geider R. J., LaRoche J. (1997) Plant Physiol. 114, 615–622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hirasawa M., Dose M. M., Kleis-SanFrancisco S., Hurley J. K., Tollin G., Knaff D. B. (1998) Arch. Biochem. Biophys. 354, 95–101 [DOI] [PubMed] [Google Scholar]
- 55.Hirasawa M., Tollin G., Salamon Z., Knaff D. B. (1994) Biochim. Biophys. Acta 1185, 336–345 [DOI] [PubMed] [Google Scholar]
- 56.Tottey S., Block M. A., Allen M., Westergren T., Albrieux C., Scheller H. V., Merchant S., Jensen P. E. (2003) Proc. Natl. Acad. Sci. 100, 16119–16124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rzeznicka K., Walker C. J., Westergren T., Kannangara C. G., von Wettstein D., Merchant S., Gough S. P., Hansson M. (2005) Proc. Natl. Acad. Sci. U.S.A. 102, 5886–5891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Moseley J. L., Page M. D., Alder N. P., Eriksson M., Quinn J., Soto F., Theg S. M., Hippler M., Merchant S. (2002) Plant Cell 14, 673–688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jacquot J. P., Stein M., Suzuki A., Liottet S., Sandoz G., Miginiac-Maslow M. (1997) FEBS Lett. 400, 293–296 [DOI] [PubMed] [Google Scholar]
- 60.García-Sánchez M. I., Díaz-Quintana A., Gotor C., Jacquot J. P., De la Rosa M. A., Vega J. M. (2000) J. Biol. Inorg. Chem. 5, 713–719 [DOI] [PubMed] [Google Scholar]
- 61.García-Sánchez M. I., Gotor C., Jacquot J. P., Stein M., Suzuki A., Vega J. M. (1997) Eur. J. Biochem. 250, 364–368 [DOI] [PubMed] [Google Scholar]
- 62.Wada K., Onda M., Matsubara H. (1989) J. Biochem. 105, 619–625 [DOI] [PubMed] [Google Scholar]
- 63.Stein M., Jacquot J. P., Miginiac-Maslow M. (1993) Plant Physiol. 102, 1349–1350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Livak K. J., Schmittgen T. D. (2001) Methods 25, 402–408 [DOI] [PubMed] [Google Scholar]