Abstract
The chemokine decoy receptor D6 controls inflammatory responses by selective recognition and degradation of most CCR1 to CCR5 agonistic ligands. CCL14 is a homeostatic chemokine present at high concentrations in the serum with a weak agonist activity on CCR1. Under inflammatory conditions, plasmin and UPA-mediated truncation of 8 amino acids generates the potent CCR1/CCR3/CCR5 isoform CCL14(9–74), which is further processed and inactivated by dipeptidyl peptidase IV/CD26 that generates CCL14(11–74). Here we report that D6 efficiently binds both CCL14 and its truncated isoforms. Like other D6 ligands, the biologically active CCL14(9–74) induces adaptive up-regulation of D6 expression on the cell membrane and is rapidly and efficiently degraded. In contrast, the D6-mediated degradation of the biologically inactive isoforms CCL14(1–74) and CCL14(11–74) is very inefficient. Thus, D6 cooperates with CD26 in the negative regulation of CCL14 by the selective degradation of its biologically active isoform. Analysis of a panel of CC chemokines and their truncated isoforms revealed that D6-mediated chemokine degradation does not correlate with binding affinity. Conversely, degradation efficiency is positively correlated with D6 adaptive up-regulation. Sequence analysis indicated that a proline residue in position 2 of D6 ligands is dispensable for binding but crucial for D6 adaptive up-regulation and efficient degradation.
The chemokine system is composed by nearly 50 ligands that exert chemotactic and cytokine-like activities through the binding to 18 G protein-coupled receptors (1–3). Chemokines can be classified according to the number and relative position of cysteine residues in the N terminus into four classes (C, CC, CXC, and CX3C). Alternatively, they may also be classified on the basis of their expression patterns and functions as “inflammatory” (produced only upon activation) and “homeostatic” (expressed in discrete locations in the absence of apparent activating stimuli) (4). In addition to gene expression regulation, post-transcriptional regulatory mechanisms, including chemokine degradation by non-signaling chemokine decoy receptors (5, 6), binding to glycosaminoglycans (7), and posttranslational processing by endogenous peptidases or peptidyl arginine deiminases (8, 9), are emerging mechanisms that fine tune chemokine biological activities and leukocyte recruitment.
Chemokine decoy receptors are a distinct subset of chemokine receptors characterized by their inability to transduce conventional signaling that leads to chemotaxis while supporting efficient degradation of the ligand (10). D6, the best described chemokine decoy receptor (6), is a constitutively internalizing receptor expressed by lymphatic endothelial cells (11), trophoblast cells (12), and, at lower levels, some leukocyte subsets (13). D6 selectively recognizes and degrades most inflammatory CC chemokine agonists of CCR1 to CCR5 (10). We have recently described that D6 is up-regulated on the cell membrane in a ligand concentration-dependent manner in order to optimize its scavenger performance. This adaptive up-regulation represents a rapid and unique posttranscriptional mechanism allowing D6 to control inflammation(14). In vivo experiments with D6−/− mice have demonstrated that its chemokine scavenging activity attenuates the severity of inflammation in different experimental models (12, 15–17) and suppresses inflammation-driven tumor development (18) (19).
Chemokine extracellular processing has complex effects on the chemokine biology, in that it usually results in biological inactivation, but for some chemokines, processing increases the biological activity or receptor specificity rather then resulting in biological inactivation (8, 20), and in some cases truncated molecules even act as chemotaxis antagonists in vitro and in vivo (21, 22). Chemokine processing plays a unique role for a CC chemokine subfamily with an extended N-terminal domain that has to be cleaved in order to activate the molecules (23, 24). This chemokine subfamily includes CCL14, CCL15, and CCL23 in humans, clustered in an identical orientation within a region spanning ∼40 kbp of chromosome 17q11.2. Although they lack murine homologues, the murine CCL6 and CCL9 chemokines have been identified as murine orthologues of the human CCL15 and CCL23, respectively (23). The best known member of this subfamily is CCL14, also named plasmatic hemofiltrate CC chemokine 1 (25) because of its presence at high concentrations in normal human plasma (1.5–10 nm) (25). Although CCL14 shares 46% sequence identity with CCL3 and CCL4, it is a weak CCR1 agonist. Upon removal of the first 8 amino acid residues from the N terminus of CCL14 by urokinase plasminogen activator and/or plasmin, the prochemokine is converted into CCL14(9–74), a potent agonist for CCR1, CCR3, and CCR5 (26–28). Interestingly, the new N terminus is now recognized and processed by the dipeptidyl peptidase IV (CD26), which further cleaves 2 amino acids and generates the biologically inactive CCL14(11–74) variant (29).
To characterize the interplay between these two posttranslational regulatory mechanisms of chemokine activity, in this study we analyzed the D6 interaction properties of full-length and truncated isoforms of this subfamily of CC chemokines. Results indicate that although D6 binds the three CCL14 isoforms, it is able to efficiently degrade only the biologically active one CCL14(9–74) that induces its adaptive up-regulation on the cell membrane. These results prompted us to reexamine recognition versus adaptive up-regulation and ligand degradation by D6 of different CC chemokines and their truncated isoforms. This analysis revealed that a proline residue in position 2 of the N-terminal domain of D6 ligands is dispensable for D6 binding but is required for high efficiency D6-mediated degradation and adaptive up-regulation.
EXPERIMENTAL PROCEDURES
Chemicals, Antibodies, and Cell Culture
Recombinant human chemokines were from R&D Systems (Minneapolis, MN), with the exception of CCL3 and CCL14(9–74), which were purchased from Peprotech (Rocky Hill, NJ). CCL14(11–74) was obtained by incubating CCL14(9–74) with recombinant CD26 (Sigma) in 50 mm Tris, pH 7.5, containing 1 mm EDTA. Truncation was evaluated by desalting a small aliquot on a C4 ZipTip (Millipore, Billerica, MA) and subsequent mass spectrometry on an ion trap Esquire-LC mass spectrometer (Bruker Daltonics). Once all CCL14(9–74) was converted into CCL14(11–74), the reaction was stopped by acidification with trifluoroacetic acid, and CCL14(11–74) was purified by liquid chromatography-mass spectrometry on a C8 Aquapore reverse phase high pressure liquid chromatography column (2.1 × 220 mm; PerkinElmer Life Sciences). Edman degradation on a Procise cLC protein sequencer (Applied Biosystems, Foster City, CA) confirmed the truncation of the N terminus and was used to determine the concentration of CCL14(11–74). CCL8 and CCL8(6–75) were chemically synthesized or expressed in Escherichia coli, as described previously (30).
125I-CCL4 and 125I-CCL2 were from PerkinElmer Life Sciences. Unconjugated and phycoerythrin-conjugated rat anti-human D6 monoclonal antibodies, biotinylated goat anti-human CCL14 antibody, and the Duo Set ELISA4 kit to measure CCL14 were purchased form R&D Systems (Minneapolis, MN). CHO-K1/hD6 and CHO-K1/mD6 transfectants, expressing human and murine D6, respectively, were obtained and cultured as previously described (15).
Binding Assays
CCL4 competitive binding was performed by incubating 3 × 104 CHO-K1/D6 transfectants, seeded in 96-well plates the day before the experiment, with 100 pm 125I-labeled CCL4 in the presence of different concentrations of unlabeled CCL2, CCL4, CCL14, CCL17, or CCL22 in 60 μl of binding buffer (DMEM/F-12, 4 mm HEPES, pH 7.4, and 1% BSA) at 4 °C for 2 h. After incubation, the cell-associated radioactivity was measured. To estimate the Kd (i.e. the equilibrium dissociation constant), CCL4 homologous competitive binding data were analyzed by non-linear regression using the equation of the homologous competitive binding curve (Prism4 software; GraphPad). Inhibition curves were analyzed using the one-site competitive binding equation to estimate the IC50 value, from which the Ki value was then calculated according to the Cheng-Prusoff equation.
Dissociation Kinetics Experiments
For ligand dissociation experiments, 3 × 104 CHO-K1/D6 cells seeded in 96-well plates were first incubated for 3 h at 4 °C with 50 pm 125I-labeled CCL4 in 60 μl of binding buffer (DMEM/F-12, 4 mm HEPES, pH 7.4, and 1% BSA). After incubation with the tracer, the unbound radioligands were removed, and the cells were washed twice with cold binding buffer. Then a 100 nm concentration of either CCL2, CCL4, CCL14, CCL17, or CCL22 was added, and the dissociation of radioactive ligand was followed. The dissociation reactions were measured over a total period of 6 h.
Receptor Internalization and Cell Surface Expression
Antibody internalization experiments were performed to evaluate D6 internalization. CHO-K1/D6 transfectants (5 × 105) were washed with PBS (Biosera, East Sussex, UK) supplemented with 1% BSA (GE Healthcare Europe, Chalfont St. Giles, UK) and stained on ice with 5 μg/ml anti-D6 antibody for 1 h. Labeled cells were washed and then incubated for the indicated times at 37 °C in DMEM/F-12 with 1% BSA to allow receptor internalization. Where indicated, chemokines (116 nm) were added. Samples were then returned to ice, washed, and incubated with anti-rat IgG allophycocyanin for 30 min in fluorescence-activated cell sorting (FACS) buffer (PBS supplemented with 1% BSA and 0.01% NaN3). To evaluate receptor up-regulation on cell the surface, cells (5 × 105) were incubated at 37 °C with the indicated chemokine (100 nm) for different times in DMEM/F-12 with 1% BSA. Cells were then transferred on ice, washed, and labeled with FACS buffer containing 5 μg/ml anti-D6 phycoerythrin-conjugated antibody for 1 h. For all experiments, 1 × 104 events of viable cells were acquired using a FACS Canto flow cytometer and analyzed using the FACS Diva software (BD Biosciences).
Chemokine Scavenging Assay
CHO-K1/D6 transfectants were plated the day before the experiment in 96-well plates at a concentration of 3 × 104 cells/well. Cells were then incubated with a 1.16 nm concentration of the indicated chemokine for increasing times. The supernatant was collected, and remaining intact chemokine was detected by sandwich ELISA or Western blot. To evaluate inhibition of chemokine scavenging, CHO-K1/D6 transfectants were plated as described above and then incubated for 6 h in 60 μl of DMEM/F-12 supplemented with 1% BSA, 0.1 nm 125I-CCL4, and the indicated concentrations of unlabeled chemokines. Proteins in the supernatants were precipitated with 12.5% trichloroacetic acid (Carlo Erba Reagents, Rodano, Italy) at 4 °C for 15 min, and both soluble and insoluble fractions were collected. The radioactivity present in each fraction was measured using a Wizard automatic counter (PerkinElmer Life Sciences). Inhibition of the degradation rate was obtained by data fitting with non-linear regression and interpolation with Michaelis-Menten equation using Prism4 software.
Western Blotting
Scavenging assay was performed on CHO-K1/D6 transfectants with 350 nm of the indicated chemokines. Supernatants were diluted with SDS-PAGE loading buffer (10% glycerol, Tris-HCl, pH 6.8, 10% SDS, 0.05% bromphenol blue, 1 mm β-mercaptoethanol), electrophoresed under reducing conditions on 15% polyacrylamide gels, and electrotransferred onto 0.2-μm nitrocellulose filters (Bio-Rad). Membranes were saturated with 5% nonfat milk in PBS containing 0.1% Tween 20 overnight at 4 °C and then probed with biotinylated goat anti-CCL14 or anti-CCL3 antibody (R&D Systems) for 2 h at room temperature. Membranes were incubated for 1 h with horseradish peroxidase-streptavidin (BioSPA, Milano, Italy). Peroxidase was detected by chemiluminescent horseradish peroxidase substrate (Millipore).
Immunofluorescence and Confocal Microscopy Analysis
CHO-K1/D6 (1 × 105) cells were seeded onto glass dishes in 24-well plates and grown at 37 °C for 18 h. Cells were stimulated for 30 min with the indicated chemokines (100 nm) in DMEM/F-12 supplemented with 1% BSA, fixed with 4% paraformaldehyde for 15 min, permeabilized with 0.3% Triton X-100 (Merck) in PBS for 5 min, and incubated with 10% normal goat serum (Dako, Glostrup, Denmark) for 30 min. Fixed cells were incubated with anti-D6 antibody (R&D Systems) for 2 h at room temperature. After washing three times with 0.05% Tween 20 in PBS (pH 7.4), the coverslips were incubated with AlexaFluor594-conjugated anti-rat antibody for 1 h (Molecular Probes), extensively washed, and incubated with 4′,6-diamidino-2-phenylindole (Invitrogen) for 5 min. Specimens were mounted with FluoSave reagent (Calbiochem), and high resolution images (1024 × 1024 pixels) were acquired sequentially with a ×60 1.4 numerical aperture Plan-Apochromat oil immersion objective by using an FV1000 laser-scanning confocal microscope (Olympus, Hamburg, Germany). Differential interference contrast (Nomarski technique) was also used. Images were assembled and cropped by Photoshop software (Adobe Systems, San Jose, CA).
Statistical Analysis
Data were analyzed by unpaired Student's t test using Prism4 software. Linear regression analysis was performed, and the coefficient of correlation was calculated using Prism4 software.
RESULTS
Differential Interaction of CCL14 and Its Processed Forms with D6
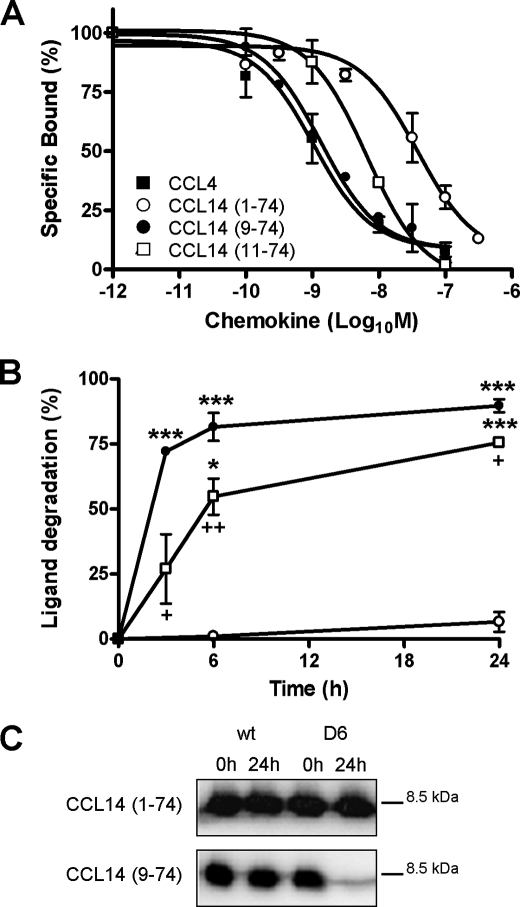
The chemokine decoy receptor D6 binds with high affinity most inflammatory CC chemokine agonists of CCR1 to CCR5. Three CC chemokines, namely CCL14, CCL15, and CCL23, constitute a distinct subfamily activated by proteolytic cleavage of the N terminus during inflammatory conditions. In order to understand if D6 was able to interact with this subfamily of chemokines, binding displacement experiments were performed on CHO-K1/hD6 transfectants using radiolabeled CCL4. Human D6 bound with high affinity the proteolytically activated CCL14(9–74) isoform and with lower affinity the full-length biologically inactive CCL14(1–74) isoform (Fig. 1A). The binding affinity of CCL14(9–74) was in the low nanomolar range, comparable with the one of CCL4 and CCL22, whereas the calculated Ki for CCL14(1–74) was similar to CCL17 (Table 1). Similar results were obtained for murine D6 (Table 1). CCL14(9–74) is subjected to proteolytic inactivation by the dipeptidyl peptidase CD26, which further cleaves the first two amino acids from the CCL14(9–74) N terminus, generating the biologically inactive CCL14(11–74) isoform. D6 also bound CCL14(11–74) (Fig. 1A), with a calculated Ki similar to CCL14(9–74) (Table 1). In order to define the ability of D6 to degrade CCL14 isoforms, scavenging assays were performed. CHO-K1/hD6 transfectants were incubated with 1.16 nm CCL14(1–74), CCL14(9–74), or CCL14(11–74) for increasing times, and the intact chemokine remaining in the supernatant was measured by ELISA. As shown in Fig. 1B, the biologically active CCL14(9–74) was efficiently degraded by D6, whereas CCL14(1–74), although being recognized by D6, was fully recovered in the supernatant even after a 24-h incubation. Finally, the CD26-inactivated CCL14(11–74) isoform was degraded by D6 with significantly reduced efficiency as compared with CCL14(9–74) (T½ = 5.7 ± 0.4 versus 2.3 ± 0.2 h, respectively; p = 0.0016). Because ELISA tests can detect partially degraded chemokines, supernatants derived from CHO-K1 or CHO-K1/hD6 incubated with CCL14(1–74) or CCL14(9–74) for 24 h were analyzed by SDS-PAGE and Western blot. The band corresponding to CCL14(9–74) was not detectable after incubation with CHO-K1/hD6 transfectants, indicating complete degradation of the protein, whereas the band corresponding to CCL14(1–74) was not affected by incubation with either CHO-K1 or CHO-K1/hD6 transfectants, consistent with a lack of degradation by D6 (Fig. 1C). These data identify CCL14 and its processing isoforms as new D6 ligands and indicate that CCL14 processing not only affects its biological properties but also influences its binding affinity for D6. Furthermore, since cleavage of the first 2 amino acids from CCL14(9–74) does not influence D6 binding but significantly impairs D6-mediated degradation, these results also indicate that a direct correlation between ligand affinity and degradation by D6 is not present for CCL14 and its truncated isoforms.
FIGURE 1.
D6 binding and scavenging activities on CCL14 isoforms. A, competitive binding of 125I-hCCL4 to CHO-K1/hD6 transfectants in the presence of different concentrations of unlabeled human CCL4 (■), CCL14(1–74) (○), CCL14(9–74) (●), and CCL14(11–74) (□). B, kinetics of CCL14(1–74) (○), CCL14(9–74) (●), and CCL14(11–74) (□) degradation in human D6 stable cell lines. CHO-K1/hD6 transfectants were incubated with 1.16 nm CCL14(1–74), CCL14(9–74), or CCL14 (11–74) at 37 °C for the indicated periods. Results are 100 minus the percentage of input chemokine remaining in the supernatant as assessed by an ELISA test. C, supernatants derived from CHO-K1 and CHO-K1/hD6 transfectants incubated for 24 h with CCL14(1–74) or CCL14(9–74) were analyzed by SDS-PAGE and Western blot. Data shown in A and B are the mean ± S.E. of at least three independent experiments. Data shown in C represent one representative experiment of three performed. *, p < 0.05; ***, p < 0.0005, chemokine degradation of CHO-K1/hD6 versus CHO-K1 wild type cells (wt). +, p < 0.05; ++, p < 0.005, CHO-K1/hD6 degradation of CCL14(11–74) compared with CCL14(9–74).
TABLE 1.
Binding constants of different chemokines and their processed isoforms for human and murine D6
Heterologous dissociation data obtained using the radiolabeled ligands 125I-labeled human CCL4 or CCL2 (where indicated) were analyzed using the one-site competitive binding equation to estimate the IC50 value, from which the Ki value was then calculated according to the Cheng-Prusoff equation.
| Ligand |
Ki ± S.E. |
|
|---|---|---|
| Human D6 | Murine D6 | |
| nm | ||
| CCL2 | 2.2 ± 0.11a | NDb |
| CCL3 | 17.25 ± 1.48 | ND |
| CCL3(5–68) | 4.68 ± 0.97 | ND |
| CCL3L1 | 1.21 ± 1.48 | ND |
| CCL4 | 1.46 ± 1.11 | 0.16 ± 0.004 |
| CCL8 | 10 ± 0.17a | ND |
| CCL8(6–75) | 20 ± 0.3a | ND |
| CCL14(1–74) | 23.40 ± 3.34 | 6.4 ± 2.83 |
| CCL14(9–74) | 1.53 ± 0.25 | 0.75 ± 0.39 |
| CCL14(11–74) | 6.5 ± 1.2 | ND |
| CCL17 | 17.25 ± 5.02 | ND |
| CCL22 | 0.48 ± 0.21 | ND |
a Data obtained using 125I-labeled human CCL2.
b ND, not detected.
Lack of D6 Interaction with CCL15, CCL23, and Their Truncated Isoforms
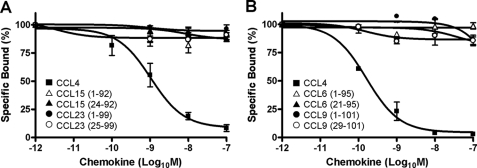
Like CCL14, CCL15 and CCL23 are also produced as prochemokines that undergo proteolytic cleavage of the N terminus during inflammatory conditions to be activated. Differently from CCL14, no binding was detected on CHO-K1/hD6 transfectants for CCL15 and CCL23, both for full-length and truncated isoforms (Fig. 2A). Similarly, CHO-K1/mD6 transfectants were unable to bind both full-length and truncated isoforms of CCL6 and CCL9, the murine CCL15 and CCL23 orthologues (23) (Fig. 2B).
FIGURE 2.
D6 binding activities on CCL15 and CCL23 isoforms. A, competitive binding of 125I-hCCL4 to CHO-K1/hD6 transfectants in the presence of different concentrations of unlabeled human CCL4 (■), CCL15(1–92) ( ), CCL15(24–92) (▲), CCL23(1–99) (●), and CCL23(25–99) (○). B, competitive binding of 125I-hCCL4 to CHO-K1/mD6 cells in the presence of different concentrations of unlabeled human CCL4 (■), CCL6(1–95) (
), CCL15(24–92) (▲), CCL23(1–99) (●), and CCL23(25–99) (○). B, competitive binding of 125I-hCCL4 to CHO-K1/mD6 cells in the presence of different concentrations of unlabeled human CCL4 (■), CCL6(1–95) ( ), CCL6(21–95) (▲), CCL9(1–101) (●), and CCL9(29–101) (○). Data shown are mean ± S.E. of at least three independent experiments.
), CCL6(21–95) (▲), CCL9(1–101) (●), and CCL9(29–101) (○). Data shown are mean ± S.E. of at least three independent experiments.
CCL14(1–74) Is Not a D6 Antagonist
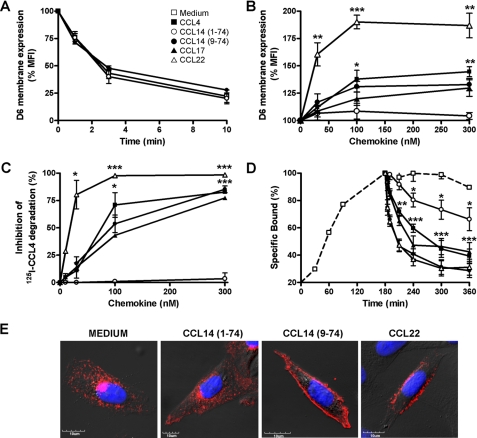
Since CCL14(1–74) is recognized by D6 but not degraded, we asked whether this chemokine isoform might work as a D6 antagonist. To this purpose, we first evaluate whether CCL14(1–74) could modify the constitutive internalization of D6 by performing antibody internalization experiments. CHO-K1/hD6 transfectants were incubated at 4 °C in the presence of a monoclonal antibody to D6 in order to label only receptors on the cell surface and were then returned at 37 °C for increasing times in the presence of CCL14(1–74) or CCL14(9–74). As shown by Fig. 3A, the D6 constitutive internalization rate was not modified by the addition of different CCL14 isoforms, suggesting that these chemokine isoforms, like the other D6 ligands, could not influence the receptor internalization rate. Since inflammatory CC chemokines are able to adaptively up-regulate D6 surface expression levels (14), we investigated whether different CCL14 isoforms could exert a similar effect. As shown in Fig. 3B, although CCL14(9–74) was able to induce a dose-dependent increase of D6 expression to a similar extent as other D6 high affinity ligands, including CCL3L1, CCL4, and CCL17, CCL14(1–74) did not modify D6 surface levels. These data were confirmed by immunofluorescence experiments on CHO-K1/hD6 transfectants (Fig. 3E). CCL14(9–74) like CCL22 was able to induce D6 membrane translocation, whereas CCL14(1–74) did not affect D6 intracellular localization. In order to understand whether CCL14(1–74) might inhibit D6 scavenging activity due to receptor occupancy, CHO-K1/hD6 transfectants were incubated with 0.1 nm 125I-CCL4 together with increasing concentrations of unlabeled chemokines. CCL4 degradation was not modified by CCL14(1–74) addition, whereas co-incubation with CCL14(9–74), CCL17, or CCL22 inhibited CCL4 degradation rate (Fig. 3C). To further investigate the potential inhibitory activity of CCL14(1–74) on D6 scavenging, dissociation curves were performed using radiolabeled CCL4 at the equilibrium (3 h) on CHO-K1/hD6 transfectants and subsequently incubating cells for increasing times with unlabeled chemokines. 125I-CCL4 was fully displaced from the receptor by the homologous unlabeled chemokine (Fig. 3D). Heterologous dissociation reactions indicate that 125I-CCL4 could also be fully displaced from the receptor by unlabeled CCL17, CCL22, and CCL14(9–74), with dissociation curves similar to those observed with homologous dissociation. On the contrary, CCL14(1–74) was unable to fully displace 125I-CCL4, suggesting that it has only partially overlapping binding sites on D6.
FIGURE 3.
CCL14(1–74) is not a D6 antagonist. A, D6 internalization. CHO-K1/hD6 transfectants were incubated at 4 °C for 1 h with anti-D6 antibody. Cells were then incubated at 37 °C for different times in the presence of 116 nm of CCL4 (■), CCL14(1–74) (○), CCL14(9–74) (●), or only medium (□) and then stained with secondary antibody for 1 h at 4 °C. B, D6 membrane up-regulation. CHO-K1/hD6 transfectants were incubated at 37 °C for 1 h with increasing concentrations of CCL4 (■), CCL14(1–74) (○), CCL14(9–74) (●), CCL17 (▲), and CCL22 ( ) and then labeled with αD6-PE antibody. Results are expressed as the percentage of MFI basal D6 expression. C, inhibition of D6-mediated CCL4 scavenging. CHO-K1/hD6 transfectants were incubated for 3 h with 0.1 nm 125I-CCL4 and increasing concentrations of unlabeled CCL4 (■), CCL14(1–74) (○), CCL14(9–74) (●), CCL17 (▲), and CCL22 (
) and then labeled with αD6-PE antibody. Results are expressed as the percentage of MFI basal D6 expression. C, inhibition of D6-mediated CCL4 scavenging. CHO-K1/hD6 transfectants were incubated for 3 h with 0.1 nm 125I-CCL4 and increasing concentrations of unlabeled CCL4 (■), CCL14(1–74) (○), CCL14(9–74) (●), CCL17 (▲), and CCL22 ( ). Results are expressed as 100 minus the percentage of degraded radiolabeled chemokine present in the supernatant. D, 125I-CCL4 dissociation curves. CHO-K1/hD6 transfectants were incubated for 3 h at 4 °C with 50 pm 125I-CCL4, washed to remove unbound radioligand, and incubated for increasing times with 100 nm CCL4 (■), CCL14(1–74) (○), CCL14(9–74) (●), CCL17 (▲), and CCL22 (○) or only medium (□). Results are expressed as 100 minus the percentage of radioligand remaining cell-associated. Data shown in A–D are the mean ± S.E. of at least three independent experiments performed. *, p < 0.05; **, p < 0.005; ***, p < 0.0005, chemokine- versus medium-treated CHO-K1/hD6 transfectants. E, confocal images of immunofluorescence-stained CHO-K1/D6 cells. Shown is one representative experiment of at least three performed of D6 staining after stimulation with the indicated chemokines (100 nm, 30 min). Each panel shows the double staining of D6 (indicated in red) with 4′,6-diamidino-2-phenylindole (indicated in blue).
). Results are expressed as 100 minus the percentage of degraded radiolabeled chemokine present in the supernatant. D, 125I-CCL4 dissociation curves. CHO-K1/hD6 transfectants were incubated for 3 h at 4 °C with 50 pm 125I-CCL4, washed to remove unbound radioligand, and incubated for increasing times with 100 nm CCL4 (■), CCL14(1–74) (○), CCL14(9–74) (●), CCL17 (▲), and CCL22 (○) or only medium (□). Results are expressed as 100 minus the percentage of radioligand remaining cell-associated. Data shown in A–D are the mean ± S.E. of at least three independent experiments performed. *, p < 0.05; **, p < 0.005; ***, p < 0.0005, chemokine- versus medium-treated CHO-K1/hD6 transfectants. E, confocal images of immunofluorescence-stained CHO-K1/D6 cells. Shown is one representative experiment of at least three performed of D6 staining after stimulation with the indicated chemokines (100 nm, 30 min). Each panel shows the double staining of D6 (indicated in red) with 4′,6-diamidino-2-phenylindole (indicated in blue).
The N Terminus Dictates the Interaction and Fate of D6 Ligands
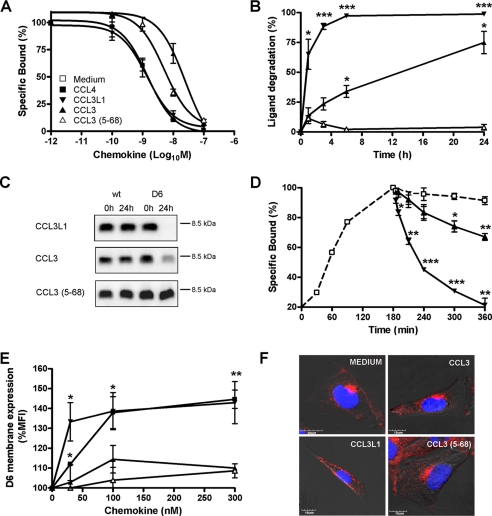
Results reported above indicate that the biological outcome of D6 binding to CCL14 and its processing isoforms is strongly influenced by the N-terminal domain of the ligand. Other chemokine isoforms with a modified N-terminal domain were tested in binding and scavenging assays on CHO-K1/hD6 transfectants to understand whether this also applies to other D6 ligands. CCL3 is a non-allelic isoform of CCL3L1 with reduced agonistic activity on CCR5 and decreased binding affinity for both CCR5 and D6 due to only a few amino acid substitutions, including the presence of a serine instead of a proline residue at position 2 (31). CCL3 displaced 125I-CCL4 with a calculated Ki similar to CCL14(1–74) (Fig. 4A), it was degraded by D6-expressing cells with a significantly reduced efficiency as compared with CCL3L1 (T½ = 6.3 ± 0.3 versus 0.5 ± 0.1 h, respectively; p = 0.0006) (Fig. 4, B and C), it did not induce D6 membrane up-regulation (Fig. 4, E and F), and it only partially displaced previously bound CCL4 (Fig. 4D). These data indicate that the N-terminal proline residue in position 2 of CCL3L1 is required to modify receptor trafficking that leads to rapid chemokine degradation. When the processed isoform of CCL3, CCL3(5–68) (20), was tested, it was found to bind D6 with higher binding affinity, as compared with CCL3 (Fig. 4A and Table 1), but was not degraded by D6-expressing cells (Fig. 4, B and C), did not modify D6 surface expression levels (Fig. 4, E and F), and only partially displaced previously bound CCL4 (Fig. 4D).
FIGURE 4.
D6 exhibits similar binding but different scavenging activities for CCL3L1, CCL3, and CCL3(5–68). A, competitive binding. CHO-K1/hD6 cells were incubated with 125I-hCCL4 and the indicated concentrations of unlabeled human CCL4 (■), CCL3L1 (▼), CCL3 (▲), and CCL3(5–68) ( ). B, scavenging assay. CHO-K1/hD6 cells were incubated with 1.2 nm CCL3L1 (▼), CCL3 (▲), and CCL3(5–68) (
). B, scavenging assay. CHO-K1/hD6 cells were incubated with 1.2 nm CCL3L1 (▼), CCL3 (▲), and CCL3(5–68) ( ) at 37 °C for the indicated periods. Results are indicated as 100 minus the percentage of intact chemokine, measured by ELISA test. C, supernatants derived from CHO-K1 and CHO-K1/hD6 transfectants incubated for 24 h with CCL3L1, CCL3, and CCL3(5–68) were analyzed by SDS-PAGE and Western blot. D, 125I-CCL4 dissociation curves. D6 transfectants were incubated for 3 h at 4 °C with 50 pm 125I-labeled CCL4; then the supernatant was removed and replaced by 100 nm CCL3L1 (▼), CCL3 (▲), CCL3(5–68) (
) at 37 °C for the indicated periods. Results are indicated as 100 minus the percentage of intact chemokine, measured by ELISA test. C, supernatants derived from CHO-K1 and CHO-K1/hD6 transfectants incubated for 24 h with CCL3L1, CCL3, and CCL3(5–68) were analyzed by SDS-PAGE and Western blot. D, 125I-CCL4 dissociation curves. D6 transfectants were incubated for 3 h at 4 °C with 50 pm 125I-labeled CCL4; then the supernatant was removed and replaced by 100 nm CCL3L1 (▼), CCL3 (▲), CCL3(5–68) ( ), or medium (□) for increasing periods. Results are expressed as 100 minus the percentage of radioligand remaining cell-associated. E, D6 membrane up-regulation. CHO-K1/hD6 cells were incubated at 37 °C for 1 h with increasing doses of indicated chemokines and then labeled for FACS analysis with anti-D6-PE antibody. F, confocal images of immunofluorescence-stained CHO-K1/D6 cells. Shown is double staining of D6 (red) with 4′,6-diamidino-2-phenylindole (blue) after stimulation with the indicated chemokines (100 nm, 30 min). Results shown in A, B, D, and E are the mean ± S.E. of at least three independent experiments performed. Results shown in C and F are representative experiments of at least three performed *, p < 0.05; **, p < 0.005; ***, p < 0.0005, chemokine versus medium-treated CHO-K1/D6 transfectants.
), or medium (□) for increasing periods. Results are expressed as 100 minus the percentage of radioligand remaining cell-associated. E, D6 membrane up-regulation. CHO-K1/hD6 cells were incubated at 37 °C for 1 h with increasing doses of indicated chemokines and then labeled for FACS analysis with anti-D6-PE antibody. F, confocal images of immunofluorescence-stained CHO-K1/D6 cells. Shown is double staining of D6 (red) with 4′,6-diamidino-2-phenylindole (blue) after stimulation with the indicated chemokines (100 nm, 30 min). Results shown in A, B, D, and E are the mean ± S.E. of at least three independent experiments performed. Results shown in C and F are representative experiments of at least three performed *, p < 0.05; **, p < 0.005; ***, p < 0.0005, chemokine versus medium-treated CHO-K1/D6 transfectants.
These data indicate that the N-terminal portion of CCL3 is not required for D6 binding. On the contrary, deletion of the first 4 amino acids of CCL3 increased its binding affinity to D6 but completely abolished its degradation. Finally, when CCL8 and its truncated isoform CCL8(6–75) (30) were tested in D6 binding and scavenging experiments, N-terminal truncation of CCL8 did not significantly affect D6 binding (Table 1), but CCL8 (6–75) was degraded with a significantly reduced efficiency as compared with full-length CCL8 (T½ = 6.4 ± 0.4 versus 2.5 ± 0.1 h, respectively; p = 0.003), similar to CCL3 and CCL14(11–74) (data not shown).
Binding, Adaptive Up-regulation, and Degradation of Different D6 Ligands
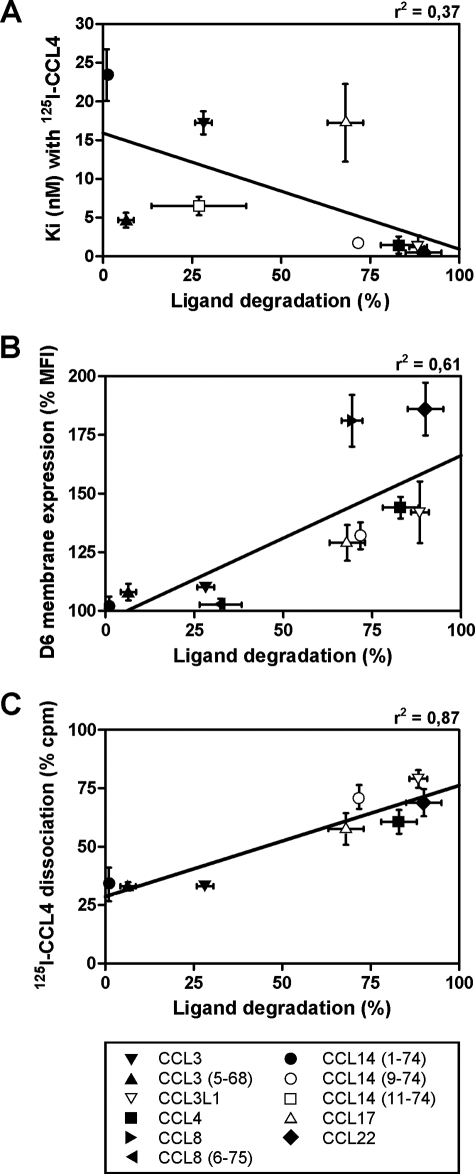
Results reported above indicate that D6 ligands can be classified into three classes according to degradation efficiency: 1) chemokines degraded with high efficiency (including CCL14(9–74) as well as CCL2, CCL3L1, CCL4, and CCL22); 2) chemokines degraded with low efficiency (including CCL14(11–74) and CCL8(6–75) as well as CCL3); and 3) chemokines that are not degraded (including CCL14(1–74) and CCL3(5–68)). For the chemokines evaluated in this study, no direct correlation was found between binding affinities and degradation efficiency (Fig. 5A), indicating that chemokine recognition by D6 does not necessarily result in its efficient degradation. On the other hand, only chemokines that are degraded with high efficiency are also able to modify intracellular D6 traffic, as shown by the positive correlation between chemokine degradation efficiency and D6 adaptive up-regulation on the cell membrane (Fig. 5B). Finally, a strong correlation was found between chemokine degradation efficiency and displacement of CCL4 previously bound to D6 (Fig. 5C), indicating that only chemokines with D6 overlapping binding sites with CCL4 are able to induce their rapid degradation. Interestingly, sequence analysis (Fig. 6) revealed that all members of this class of D6 ligands, with the only exception of CCL17, have a proline residue in position 2, suggesting a relevant role for this amino acid in the induction of D6 adaptive redistribution on the cell membrane that results in efficient chemokine degradation.
FIGURE 5.
Correlations between ligand degradation by D6-expressing cells. Ligand degradation efficiency, calculated as the percentage of chemokine removed from the supernatant after 3 h of incubation with CHO-K1/hD6 cells, has been correlated with the binding affinities (Ki) measured in competition binding with 125I-CCL4 on CHO-K1/hD6 (A), with the percentage of up-regulation of D6 membrane expression after 1 h of chemokine stimulation (B), or with the percentage of 125I-CCL4 dissociation after 3 h of incubation with unlabeled chemokines (C). The lines represent linear regression calculated for the indicated ligands.
FIGURE 6.
Amino acid sequence of employed CC chemokines. Alignment of CC chemokines recognized by D6 was performed using the program ClustalW2 (available on the World Wide Web). Amino acid residues conserved in all aligned sequences are indicated by asterisks; conserved substitutions are indicated by colons; semiconserved substitutions are indicated by dots. Gray boxed chemokines are the ones efficiently scavenged by D6. Conserved cysteine residues are underlined, and proline residues in position 2 are in boldface type.
DISCUSSION
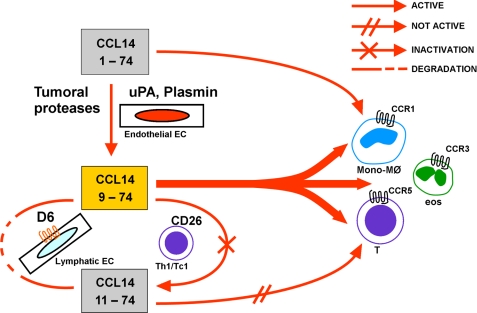
Protease-mediated processing and decoy/scavenger receptors are emerging as key regulatory mechanisms tuning the biological activity of chemokines during inflammatory responses (5, 8). CCL14, CCL15, and CCL23 represent a unique subfamily of chemokines, in that they are classified as constitutive because they circulate at substantial levels in the serum of healthy subjects but act as inflammatory chemokines after cleavage of their unique N-terminal domain (23). This study identifies one of these chemokines, namely CCL14, and its truncated isoforms as new ligands for the chemokine decoy receptor D6. Unexpectedly, although it was recognized with similar affinity, the scavenging activity of D6 for the three CCL14 isoforms is completely different. The full-length biologically inactive CCL14(1–74) is not degraded by D6, whereas D6 degrades the inflammatory chemokine CCL14(9–74) with high efficacy, and its efficacy is significantly reduced for the CD26-inactivated CCL14(11–74) isoform. Thus, the N-terminal portion of CCL14, which controls the biological activity of this chemokine, also plays a key role in its degradation. Removal of the first 8 amino acids is required for CCL14 conversion into a potent inflammatory chemokine but also allows D6-mediated degradation, and further proteolytic cleavage strongly impairs its biological activity and similarly reduces the rate of degradation via D6. D6 may thus be added to the regulatory mechanisms of CCL14 activity. CCL14 is present in the blood as an inactive chemokine. It is proteolytically activated by serine proteases of the fibrinolytic cascade to a potent chemoattractant for CCR1/CCR3/CCR5-expressing cells (26) and is then either further processed by CD26 or degraded by the scavenging receptor D6 (Fig. 7).
FIGURE 7.
Model of CCL14 activation and inactivation. CCL14(1–74) is a homeostatic chemokine present at high concentrations in the serum with a weak activity on CCR1. Under inflammatory conditions, CCL14(1–74) is activated by proteases of the coagulation and fibrinolytic systems that cleave the first 8 N-terminal amino acids and generate the CCL14(9–74) isoform, a strong agonist of CCR1, CCR3, and CCR5. CCL14(9–74) is then inactivated by two distinct mechanisms. The serine protease CD26, expressed by Th1 lymphocytes, removes two N-terminal amino acids and generates the inactive CCL14(11–74) isoform; the chemokine decoy receptor D6, expressed by lymphatic vessels, internalizes and degrades CCL14(9–74) with high efficiency. D6 also binds and degrades CCL14(11–74) but with lower efficiency as compared with CCL14(9–74).
Processing of CCL14(9–74) to CCL14(11–74) strongly affects the efficiency of D6-mediated degradation. This conversion is mediated by CD26, a dipeptidyl peptidase that cleaves specifically after a proline residue present in position 2 of CCL14(9–74). In order to test the relevance of this residue for D6-mediated degradation, we compared CCL3L1 with its non-allelic isoform CCL3, which only differs by a proline-to-serine substitution in position 2 (31), and its truncated isoform CCL3(5–68). As compared with CCL3L1, both CCL3 and CCL3(5–68) showed a significant reduction of D6-mediated degradation. Similarly, the CCL8 truncated isoform CCL8(6–75) retained binding to D6, but it was degraded at a significantly slower rate as compared with CCL8. Collectively, these data indicate that there is no correlation between chemokine binding affinities and their effective degradation by D6 and that a proline residue in position 2 of the N-terminal domain of D6 ligands is required for rapid degradation.
Results presented here describe CCL14(1–74) as the first D6 ligand that is not degraded. CCL14(1–74) also did not affect D6 constitutive internalization or membrane up-regulation; nor did it modify inflammatory degradation of other ligands. For these characteristics, CCL14(1–74) may be considered the first D6 neutral ligand (32). Heterologous dissociation curves indicated that unlabeled CCL14(1–74) only partially displaced previously bound 125I-CCL4 (Fig. 3), suggesting that it interacted with D6 regions that are not available when the receptor is occupied with other ligands or that it had partly overlapping D6 binding sites. The same behavior in dissociation experiments was found for CCL3 and CCL3(5–76). Interestingly, a similar phenomenon was observed for other chemokine receptors, such as CCR1 (33), CXCR2 (34), and the virus-encoded ORF74 and US28 chemokine receptors (32).
Chemokine interaction with their cognate receptors is believed to occur through a first interaction of the rigid loop of the chemokine that follows the second cysteine, which is important for high affinity binding, followed by the interaction of the flexible N terminus of the chemokine preceding the first cysteine, which is essential for the subsequent receptor activation (35). We are here proposing that a similar mechanism may also occur for the chemokine decoy receptor D6, in which chemokines may first bind D6 with their N-loop and then activate their pathway to degradation through the binding of their N-terminal proline to the D6 seven-transmembrane bundle. In the absence of this second step, as in the case of ligands without a proline in position 2, receptor trafficking is not modified, and the D6-dependent degradative activity is strongly reduced.
In conclusion, results presented here indicate that D6 cooperates with CD26/dipeptidyl peptidase IV (29) in the inactivation and degradation of biologically active CCL14 isoforms. Moreover, by using CCL14 and other chemokine isoforms, we have shown that a proline residue in position 2 of D6 ligands is dispensable for receptor binding, but it is required to induce receptor traffic modifications resulting in efficient degradation of inflammatory CC chemokines.
This work was supported by research grants of the European Community (INNOCHEM project 518167), the Ministero dell'Istruzione dell'Università e della Ricerca (Progetti di Ricerca di Interesse Nazionale projects 2002061255 and 2007ENYMAN_003; Fondo per gli Investimenti della Ricerca di Base project RBIN04EKCX), Fondazione Cariplo (NOBEL project), the Centers of Excellence (Credit EF/05/15) of the K.U. Leuven, the Concerted Research Actions of the Regional Government of Flanders, the Fund for Scientific Research of Flanders (FWO-Vlaanderen), and the Interuniversity Attraction Poles Programme, Belgian State, Belgian Science Policy. This work was conducted in the context and with the support of the Fondazione Humanitas per la Ricerca. This work was also supported by the Italian Association for Cancer Research.
- ELISA
- enzyme-linked immunosorbent assay
- DMEM
- Dulbecco's modified Eagle's medium
- BSA
- bovine serum albumin
- FACS
- fluorescein isothiocyanate
- PBS
- phosphate-buffered saline.
REFERENCES
- 1.Charo I. F., Ransohoff R. M. (2006) N. Engl. J. Med. 354, 610–621 [DOI] [PubMed] [Google Scholar]
- 2.Bonecchi R., Galliera E., Borroni E. M., Corsi M. M., Locati M., Mantovani A. (2009) Front. Biosci. 14, 540–551 [DOI] [PubMed] [Google Scholar]
- 3.Murphy P. M., Baggiolini M., Charo I. F., Hébert C. A., Horuk R., Matsushima K., Miller L. H., Oppenheim J. J., Power C. A. (2000) Pharmacol. Rev. 52, 145–176 [PubMed] [Google Scholar]
- 4.Mantovani A. (1999) Immunol. Today 20, 254–257 [DOI] [PubMed] [Google Scholar]
- 5.Mantovani A., Bonecchi R., Locati M. (2006) Nat. Rev. Immunol. 6, 907–918 [DOI] [PubMed] [Google Scholar]
- 6.Locati M., Torre Y. M., Galliera E., Bonecchi R., Bodduluri H., Vago G., Vecchi A., Mantovani A. (2005) Cytokine Growth Factor Rev. 16, 679–686 [DOI] [PubMed] [Google Scholar]
- 7.Johnson Z., Proudfoot A. E., Handel T. M. (2005) Cytokine Growth Factor Rev. 16, 625–636 [DOI] [PubMed] [Google Scholar]
- 8.Mortier A., Van Damme J., Proost P. (2008) Pharmacol. Ther. 120, 197–217 [DOI] [PubMed] [Google Scholar]
- 9.Proost P., Loos T., Mortier A., Schutyser E., Gouwy M., Noppen S., Dillen C., Ronsse I., Conings R., Struyf S., Opdenakker G., Maudgal P. C., Van Damme J. (2008) J. Exp. Med. 205, 2085–2097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bonecchi R., Locati M., Galliera E., Vulcano M., Sironi M., Fra A. M., Gobbi M., Vecchi A., Sozzani S., Haribabu B., Van Damme J., Mantovani A. (2004) J. Immunol. 172, 4972–4976 [DOI] [PubMed] [Google Scholar]
- 11.Nibbs R. J., Kriehuber E., Ponath P. D., Parent D., Qin S., Campbell J. D., Henderson A., Kerjaschki D., Maurer D., Graham G. J., Rot A. (2001) Am. J. Pathol. 158, 867–877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Martinez de la Torre Y., Buracchi C., Borroni E. M., Dupor J., Bonecchi R., Nebuloni M., Pasqualini F., Doni A., Lauri E., Agostinis C., Bulla R., Cook D. N., Haribabu B., Meroni P., Rukavina D., Vago L., Tedesco F., Vecchi A., Lira S. A., Locati M., Mantovani A. (2007) Proc. Natl. Acad. Sci. U.S.A. 104, 2319–2324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McKimmie C. S., Fraser A. R., Hansell C., Gutiérrez L., Philipsen S., Connell L., Rot A., Kurowska-Stolarska M., Carreno P., Pruenster M., Chu C. C., Lombardi G., Halsey C., McInnes I. B., Liew F. Y., Nibbs R. J., Graham G. J. (2008) J. Immunol. 181, 8171–8181 [DOI] [PubMed] [Google Scholar]
- 14.Bonecchi R., Borroni E. M., Anselmo A., Doni A., Savino B., Mirolo M., Fabbri M., Jala V. R., Haribabu B., Mantovani A., Locati M. (2008) Blood 112, 493–503 [DOI] [PubMed] [Google Scholar]
- 15.Martinez de la Torre Y., Locati M., Buracchi C., Dupor J., Cook D. N., Bonecchi R., Nebuloni M., Rukavina D., Vago L., Vecchi A., Lira S. A., Mantovani A. (2005) Eur. J. Immunol. 35, 1342–1346 [DOI] [PubMed] [Google Scholar]
- 16.Di Liberto D., Locati M., Caccamo N., Vecchi A., Meraviglia S., Salerno A., Sireci G., Nebuloni M., Caceres N., Cardona P. J., Dieli F., Mantovani A. (2008) J. Exp. Med. 205, 2075–2084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jamieson T., Cook D. N., Nibbs R. J., Rot A., Nixon C., McLean P., Alcami A., Lira S. A., Wiekowski M., Graham G. J. (2005) Nat. Immunol. 6, 403–411 [DOI] [PubMed] [Google Scholar]
- 18.Nibbs R. J., Gilchrist D. S., King V., Ferra A., Forrow S., Hunter K. D., Graham G. J. (2007) J. Clin. Invest. 117, 1884–1892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vetrano S. B., Sarukhan A., Savino B., Bonecchi R., Correale C., Arena V., Fantini M., Roncalli M., Malesci A., Mantovani A., Locati M., Danese S. (2009) Gut, in press [DOI] [PubMed] [Google Scholar]
- 20.Proost P., Menten P., Struyf S., Schutyser E., De Meester I., Van Damme J. (2000) Blood 96, 1674–1680 [PubMed] [Google Scholar]
- 21.Struyf S., Proost P., Van Damme J. (2003) Adv. Immunol. 81, 1–44 [DOI] [PubMed] [Google Scholar]
- 22.Proost P., Struyf S., Van Damme J. (2006) Biochem. Soc. Trans. 34, 997–1001 [DOI] [PubMed] [Google Scholar]
- 23.Forssmann U., Mägert H. J., Adermann K., Escher S. E., Forssmann W. G. (2001) J. Leukocyte Biol. 70, 357–366 [PubMed] [Google Scholar]
- 24.Berahovich R. D., Miao Z., Wang Y., Premack B., Howard M. C., Schall T. J. (2005) J. Immunol. 174, 7341–7351 [DOI] [PubMed] [Google Scholar]
- 25.Schulz-Knappe P., Mägert H. J., Dewald B., Meyer M., Cetin Y., Kubbies M., Tomeczkowski J., Kirchhoff K., Raida M., Adermann K., Kist A., Reinecke M., Sillard R., Pardigol A., Uguccioni M., Baggiolini M., Forssmann W. G. (1996) J. Exp. Med. 183, 295–299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vakili J., Ständker L., Detheux M., Vassart G., Forssmann W. G., Parmentier M. (2001) J. Immunol. 167, 3406–3413 [DOI] [PubMed] [Google Scholar]
- 27.Blain K. Y., Kwiatkowski W., Zhao Q., La Fleur D., Naik C., Chun T. W., Tsareva T., Kanakaraj P., Laird M. W., Shah R., George L., Sanyal I., Moore P. A., Demeler B., Choe S. (2007) Biochemistry 46, 10008–10015 [DOI] [PubMed] [Google Scholar]
- 28.Detheux M., Ständker L., Vakili J., Münch J., Forssmann U., Adermann K., Pöhlmann S., Vassart G., Kirchhoff F., Parmentier M., Forssmann W. G. (2000) J. Exp. Med. 192, 1501–1508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Forssmann U., Hartung I., Bälder R., Fuchs B., Escher S. E., Spodsberg N., Dulkys Y., Walden M., Heitland A., Braun A., Forssmann W. G., Elsner J. (2004) J. Immunol. 173, 3456–3466 [DOI] [PubMed] [Google Scholar]
- 30.Struyf S., Proost P., Vandercappellen J., Dempe S., Noyens B., Nelissen S., Gouwy M., Locati M., Opdenakker G., Dinsart C., Van Damme J. (2009) Eur. J. Immunol. 39, 843–857 [DOI] [PubMed] [Google Scholar]
- 31.Nibbs R. J., Yang J., Landau N. R., Mao J. H., Graham G. J. (1999) J. Biol. Chem. 274, 17478–17483 [DOI] [PubMed] [Google Scholar]
- 32.Rosenkilde M. M., Schwartz T. W. (2000) Mol. Pharmacol. 57, 602–609 [DOI] [PubMed] [Google Scholar]
- 33.Jensen P. C., Thiele S., Ulven T., Schwartz T. W., Rosenkilde M. M. (2008) J. Biol. Chem. 283, 23121–23128 [DOI] [PubMed] [Google Scholar]
- 34.Ahuja S. K., Murphy P. M. (1996) J. Biol. Chem. 271, 20545–20550 [DOI] [PubMed] [Google Scholar]
- 35.Blanpain C., Doranz B. J., Bondue A., Govaerts C., De Leener A., Vassart G., Doms R. W., Proudfoot A., Parmentier M. (2003) J. Biol. Chem. 278, 5179–5187 [DOI] [PubMed] [Google Scholar]